Abstract
Plasma cells, which secrete auto-antibodies, are considered to be the arch-criminal of autoimmune diseases such as systemic lupus erythematosus, but there are many cytokines involved in inducing the differentiation of B-cell subsets into plasma cells. Here, we emphasize IL-21, which has emerged as the most potent inducer of plasma cell differentiation. In this review, we focused on the promoting effects of IL-21 on plasma cell differentiation and discuss how these effects contribute to B cell-mediated autoimmune disease.
Keywords: Bcl-6, Blimp-1, IL-21, plasma cell, systemic lupus erythematosus
Introduction
Plasma cells (PCs), which act as double-edged swords in the immune system, are the unique source of auto-antibodies in autoimmune diseases such as systemic lupus erythematosus (SLE). Accumulation of auto-reactive PCs and auto-antibodies1,2 has been reported in SLE patients and murine models. Thus, controlling PC differentiation from B cells may be a promising strategy to inhibit autoimmune disease development.
Recently, high IL-21 serum levels were detected in SLE patients and animal models. Studies indicated that IL-21 is important in the pathogenesis of murine lupus.3,4,5,6 All of this evidence indicated that IL-21 may act as a promoter of PC differentiation,7 which results in PC and auto-antibody accumulationand leads to autoimmune disease. Therefore, a better understanding of the function of IL-21 networks in PC differentiation will be helpful in developing treatments to control SLE development.
In the following sections, we review the process of B cells differentiating into PCs, and discuss the relative IL-21 network involved in that process.
PC differentiation: the importance of T cells, cytokines and transcription factors
After completing their early development in the bone marrow, immature B cells migrate to secondary lymphoid organs, whereby they further differentiate into marginal zone or follicular B cells.8,9 When activated in the secondary lymphoid organs, marginal zone B cells expand and differentiate into short-lived PCs, which rapidly undergo apoptosis to exert a rapid and temporary protection of organs.10,11,12 Together, marginal zone B cells and B1-B cells participate in the early immune response against T-independent antigens.13 In contrast, portions of follicular B cells undergo an extra-follicular response and produce short-lived unmutated PCs, which act as an early defense against foreign threats.14,15,16,17,18 Additionally, a portion of follicular B cells migrate to the perimeter of the follicles to form germinal centers (GCs)14,19(Figure 1). In the GC, the follicular B cells differentiate into centroblasts, which proliferate to form the GC dark zone. Moreover, the centroblasts proliferate and undergo somatic hypermutation and class switching. Clones with weak affinity or that auto-react die of apoptosis or experience further rounds of somatic hypermutation in the dark zone.20 The other cells, however, migrate into the light zone where the proliferation speed is decreased. Here, GCs undergo a reaction to increase Ig affinity with the help of T cells to produce either memory B cells or affinity-matured long-lived PCs, (Figure 2) which migrate to specialized niches in the bone marrow that help maintain survival.21 PCs can be divided into two subsets, namely short- and long-lived PCs, according to their life spans.22,23 Both short- and long-lived PCs have critical humoral immunity roles in the defense against foreign pathogens.
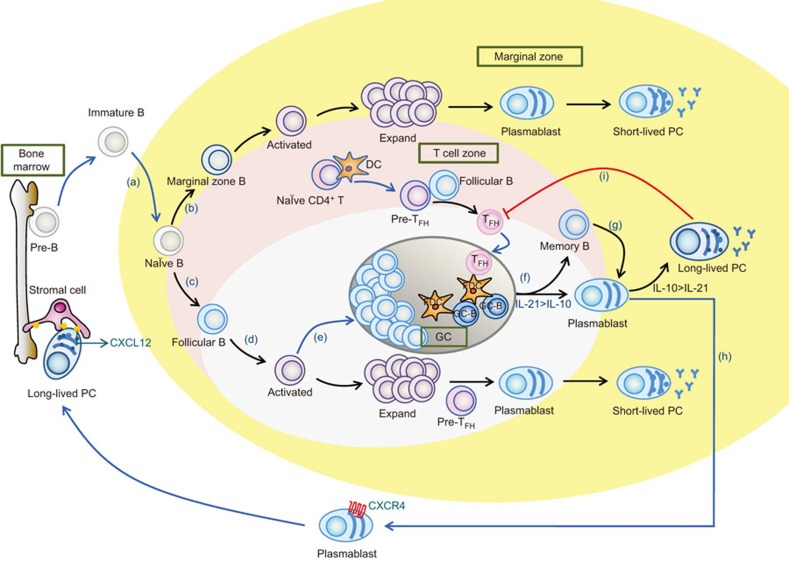
Figure 1.
PC development in lymphoid tissues. (a) Immature B cells leave the bone marrow and migrate to the lymphoid tissues. In the spleen, immature B cells can mature into either marginal zone B cells (b) or follicular B cells (c). (b) When activated at the T/B border, the marginal zone B cells rapidly differentiate into IgM-secreting plasmablasts and short-lived PCs in a T-independent manner to participate in the early immune response. (d) After being activated, follicular B cells with intrinsically higher affinity will preferentially migrate to the extrafollicular area, where they undergo rapid expansion and become short-lived PCs with the help of pre-follicular helper T (pre-TFH) cells. (e) Follicular B cells with lower affinity receptors enter the follicular foci to form the GC. (f) Within the GC, TFH cells continue providing assistance to the B-cell development, maintain the GC reaction, and facilitate the production of long-lived PCs and memory B cells. (g) When an antigen is present for a second time, memory B cells can rapidly differentiate into PCs to help resist an infection. (h) A portion of plasmablasts developed in the GC migrate to the bone marrow under the control of CXCL12 to find survival niches that ensure the steady survival of long-lived PCs. (i) PCs negatively regulate TFH cells. IL-21 has a potent effect on centroblasts differentiating into plasmablasts, and IL-10 has a greater impact on the differentiation of PCs from plasmablasts than does IL-21. GC, germinal center; PC, plasma cell; TFH, follicular helper T.
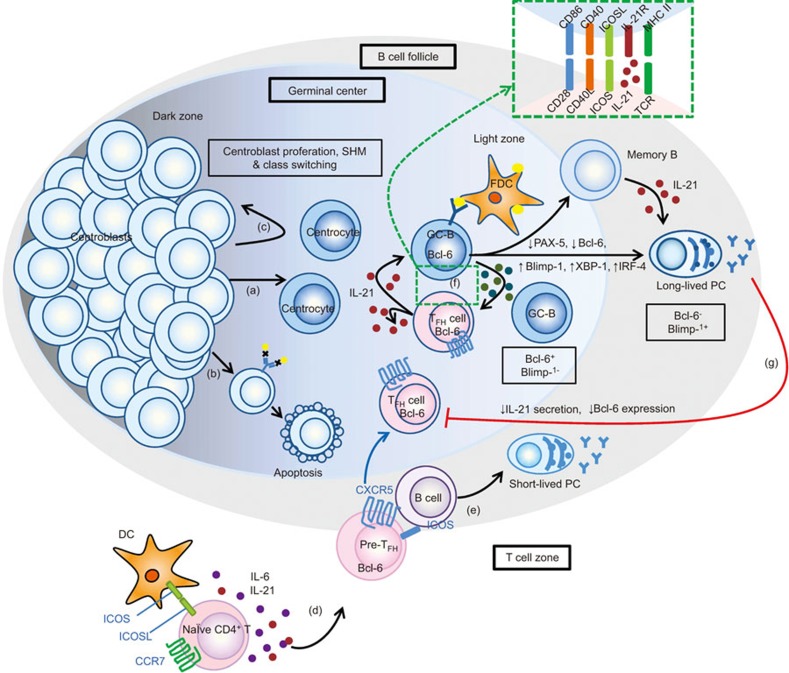
Figure 2.
IL-21 signaling regulates germinal center formation and reactions and maintains TFH cell development. Antigen-activated follicular B cells enter the follicular foci. In the dark zone, they differentiate into centroblasts. (a) The centroblasts proliferate and undergo SHM and class switching. Then, they migrate into the light zone and differentiation is facilitated for increased Ig affinity. In this stage, they are called centrocytes. Clones with weak affinity or with auto-reaction die of apoptosis (b) or experience further rounds of SMH in the dark zone (c). (d) At the same time, in T-cell zone, naive CD4+ T cells are activated after recognizing peptide–MHC class II complexes on DCs and develop into pre-TFH cells with the downregulation of CCR7, upregulation of CXCR5 and increased Bcl-6 expression (under the regulation of IL-6 and IL-21), which allows them to migrate to the B cell follicle. (e) In the extra-follicular area, the pre-TFH cells aid the follicular B cells, which results in the differentiation of short-lived PC. The interaction between T and B cells drives the full development of TFH cells. (f) Within the GC, TFH cells continue supporting the B cell development in the GC through T- and B-cell interactions, such as with CD28/CD86, CD40L/CD40, ICOS/ICOS-L and cytokine interactions (including IL-21). IL-21 secreted by TFH cells can also maintain TFH cell development in an autocrine manner. The GC reaction will result in the formation of memory cells and long-lived PCs, which express high levels of Blimp-1. The process of PC differentiation requires the downregulation of PAX-5 and Bcl-6 as well as Blimp-1, XBP-1 and IRF-4 upregulation. (g) PCs have a negative regulatory effect on TFH cells by shutting down their capacity to secrete IL-21 and decreasing their Bcl-6 expression. Bcl-6, B-cell lymphoma-6; Blimp-1, B lymphocyte induced maturation protein-1; DC, dendritic cell; GC, germinal center; ICOS, inducible costimulator; IRF-4, IFN-induced regulatory factor 4; PAX-5, paired box-5; PC, plasma cell; SHM, somatic hypermutation; TFH, follicular helper T; XBP-1, X-box binding protein-1.
These differentiation events are also partly mediated by T follicular helper (TFH) cells. These cells are a specific T-cell subset with unique surface markers, cytokines, and transcription factors that are different from other CD4+ T-cell subsets. Phenotypically, TFH cells can be characterized by the high expression of chemokine (C–X–C motif) receptor 5 (CXCR-5), programmed death-1, CD40L and inducible costimulator (ICOS). Additionally, B-cell lymphoma 6 (Bcl-6) acts as a transcription factor for TFH cells.24 These markers contribute to the location and function of TFH cells and direct the interaction of TFH cells with B cells to promote a T cell-dependent B-cell response.25 It is now known that TFH cells, localized in the B-cell follicle, are essential for the formation of GCs and the selection of mutated GC B cells. They can interact with B cells through CD28/CD86, CD40L/CD40, ICOS/ICOS-L and instruct their differentiation into memory cells or long-lived PCs (Figure 2). Purified TFH cells that were cocultured with purified tonsil PCs resulted in increased tonsillar PC immunoglobulin production. Additionally, this effect was impaired when TFH cell-derived IL-21 was blocked.26 Additionally, IgE and other immunoglobulin isotypes were observed to be switched under the influence of T cell-derived cytokines.24 For instance, IL-10 was identified as the switch factor for IgG4,27 and IL-21 was shown to be the switch factor for human IgG1 and IgG3.28 The precise control of TFH cell numbers is necessary to produce affinity-matured antibody responses with the absence of self-reactivity.29 Moreover, TFH cell differentiation and functions are precisely regulated in a complex temporospatial manner.30 IL-21 is also an important regulator for the generation of TFH cells in an autocrine manner.31 However, studies32 have shown that IL-21 is required for TFH expansion or persistence, rather than for their appearance, localization or their ability to support GC formation. Furthermore, IL-21 was found to directly impact B-cell function and promote PC development in secondary lymphoid organs.33 Therefore, the critical regulatory functions of IL-21 on GC and TFH cells may provide an inspiration in future PC differentiation studies.
The differentiation of activated B cells into PCs is regulated by transcriptional programs and networks that are affected by numerous microenviromental factors. In addition to IL-21, the key transcription factors involved in regulating PC differentiation include B lymphocyte-induced maturation protein-1 (Blimp-12) and the transcriptional repressor Bcl-6. There exists an interesting reciprocal repression capacity between Blimp-1 and Bcl-6. The balance between the Blimp-1 and Bcl-6 axes also involves the participation of other important transcription factors such as paired box-5 (PAX-5), X-box binding protein-1 (XBP-1), and IFN-induced regulatory factor 412,34 (Figures 2 and 3b). Blimp-1 is a crucial promoting transcription factor in PC differentiation35,36 for both short and long-lived PCs.37 Bcl-6, which is expressed in GC B and TFH cells, is required for TFH cell formation and supports GC formation and reactions.18,38,39 Additionally, it blocks PC differentiation and directs GC B-cell fate by suppressing Blimp-1 expression.
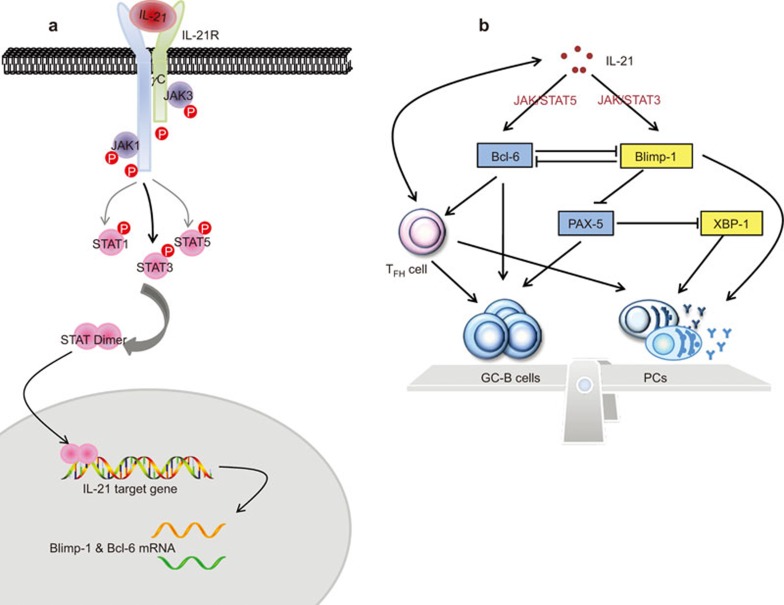
Figure 3.
The IL-21 signaling network involved in PC differentiation. (a) When IL-21 binds to the IL-21R, JAK1 and JAK3 are activated and strongly phosphorylate STAT1 and STAT3 and weakly phosphorylate STAT5 proteins through interactions with IL-21Rα and γc, respectively. Then, the STATs dimerize and are subsequently shuttled to the nucleus, where they bind to their target gene regulatory elements. (b) This signaling event introduces the transcriptional factors that mutually affect GC B-cell development and PC differentiation. IL-21 can induce the expression of Blimp-1 and Bcl-6 through JAK/STAT signaling. These two transcriptional factors mutually repress with each other, and there is a balance between the two. Bcl-6 and PAX-5 are highly active in GC B cells to promote GC B-cell differentiation and repress plasmacytic development. Blimp-1 and XBP-1, however, promote PC differentiation. Additionally, IL-21 acts on TFH cells in an autocrine manner, and Bcl-6 is an important transcription factor in TFH cell development. TFH cells are the primary cells that aid B-cell development in the GC. Moreover, they also assist the differentiation of GC B cells into long-lived PCs, which is similar to their ability to help follicular B cells differentiate into short-lived PCs. Thus, IL-21 also regulates GC B-cell development and PC differentiation through TFH cells. Bcl-6, B-cell lymphoma-6; Blimp-1, B lymphocyte induced maturation protein-1; GC, germinal center; PAX-5, paired box-5; PC, plasma cell; TFH, follicular helper T; XBP-1, X-box binding protein-1.
IL-21 is the most potent PC differentiation inducer
Where does IL-21 come from?
Initially, IL-21 was reported to be produced by activated CD4+ T cells.40 Subsequently, it was shown that Th2 cells and not Th1 cells produce IL-21.41 More recently, it was reported that IL-17-producing CD4+ T cells (Th17) produce higher levels of IL-21 than Th2 cells and that IL-21 acts as an autocrine growth factor for Th17 cells.42,43,44,45 However, in a study that established the intracellular cytokine staining of IL-21, it was observed that a considerable number of IL-21-producing CD4+ T cells were negative for intracellular IL-17A and IL-17F under Th17-polarizing conditions.46 This provided new insight and guidance into the cellular source of IL-21 in activated CD4+ T cells. Even more recent research showed that IL-21 was a potential product associated with Th17 and TFH cells.47 A recent study6 argued strongly that IL-21, which was associated with SLE symptoms in BXSB-Yaa mice, was not a product of Th17 cells but appeared to be generally produced by ICOS+CD4+ T splenic T cells. A similar study defined anatomically distinct extrafollicular cells that were specialized humoral effector T cells akin to TFH cells that regulate PC differentiation via IL-21 production in MRL/MpJ-Faslpr (MRL/lpr) mice.48 Additionally, there is considerable evidence indicating that TFH cells are a robust source of IL-21.31,47
The effects of IL-21 in regulating PC differentiation in mice and humans
IL-21 is involved in the development, differentiation, and death in the late B-cell development stage in mice. It can induce mature B cells to differentiate into Ig-secreting PCs.7 Additionally, in IL-21 receptor (IL-21R)-deficient mice, B-cell development for the most part was normal but the IgG1 was lower after immunization; however, IgE was higher than the wild-type animals.49,50 The former mice were found to have a severely impaired IgG response.49,50 Additionally, MRL/lpr mice that were treated with IL-21R.Fc fusion protein had reduced circulating ds-DNA auto-antibody and total sera IgG1 and IgG2a levels.51
In humans, IL-21 is the major cytokine that induces B-cell activation, PC differentiation, and Ig production. Moreover, it can induce PC differentiation and Ig secretion in human CD19+ peripheral blood and splenic B cells when combined with anti-CD40.52,53 IL-21, however, inhibits anti-IgM and IL-4-induced proliferation.40 Additionally, activation with IL-21 and/or TLR-9 induced CD19+CD27+ memory B cells and CD19+CD38highIgD− PC levels in active SLE patients and healthy controls.4 These studies indicated that T-cell-derived IL-21 may exert different effects on B-cell differentiation depending on the presence of different stimuli during immune responses. Moreover, another study investigated the effect of IL-21 and IL-10 on the different PC development stages in the GC. IL-21 preferentially converted CD77+ centroblasts into CD20−CD38high plasmablasts in the early stage; however, IL-10 had a more potent effect on the terminal differentiation of CD20−CD38high plasmablasts into CD138+ PC in the later stage54 (Figure 1).
The IL-21 mechanism of action
On the one hand, IL-21 acts directly on B cells and controls GC B-cell formation in a B cell-intrinsic fashion.55 IL-21 maintains the expression of Bcl-6 in GC B cells and sustains the GC32,55 (Figure 3b). Many murine model studies have shown that the spontaneous generation of GCs correlated with autoimmune disease development,56,57 which suggests that the GC may be a pathogenic hot spot in autoimmune disease due to its production of auto-PCs and auto-antibodies.58 On the other hand, IL-21, which is produced mainly by TFH cells,59 is also essential for TFH cell development. Events that occur in the GC are all dependent on the assistance of TFH cells, including the maintenance and the activity of the GC. GC dysregulation is often due to the aberrant accumulation of hyperactive and/or dysfunctional TFH cells.60,61 (Figure 3b) IL-21 plays an essential role in TFH cell development, and IL-21-deficient T cells are not able to induce TFH cell development, GC formation or antibody production in K/BxN mice.62 However, an excessive number of TFH cells appears to lower the selection threshold in the GC reaction and allows for the survival of low affinity or self-reactive clones.29 Based on the above, the IL-21 signaling pathway profoundly affects the B-cell response to antigens, maintains GC persistence and function, and promotes PC formation.32,63
Furthermore, IL-21 mediates the differentiation and function of T, B and Natural Killer cell (NK) through binding of its receptor, IL-21R, which consists of a common γ-chain and a cytokine-specific α-chain.64 When IL-21 binds to IL-21R, JAK1 and JAK3 interact with the IL-21Rα- and γ-chains, respectively. Then, STAT1, STAT3 and STAT5 are phosphorylated. These transcription factors contribute to the activation of multiple different downstream genes in B and T cells.65 Additionally, IL-21 has been shown to mainly activate STAT3 signaling and to a lesser extent STAT1 and STAT5 signaling.64 (Figure 3a) STAT5 signaling induces Bcl-6 expression, which blocks PC differentiation and promotes proliferative self-renewal signaling in human B cells.66 STAT3, in contrast, upregulated Blimp-1 gene expression to promote PC differentiation in a murine model.67 These studies demonstrate the ability of IL-21 to upregulate Blimp-1 and Bcl-6 expression. Another study, however, proposed that IL-21 positively regulates Bcl-6 expression through the activation of AP-1 and STAT3.68 Although many studies have investigated the IL-21-induced Bcl-6 expression levels, the mechanism still remains ambiguous.52,69 Studies have shown that in addition to IL-21, IL-10 and IL-6 were also required for PC survival by inducing STAT3 phosphorylation.70 In addition to the JAK/STAT pathway, the MAPK and PI3K pathways are also associated with IL-21 signaling, which were reported to be crucial for IL-21-mediated cell proliferation.65,71,72
Based on the above, the IL-21 signaling pathway activates the JAK/STAT pathway, which is followed by Blimp-1 and Bcl-6 gene expression induction in the nucleus. In other words, IL-21 that is produced by TFH cells acts directly on B cells to maximize the expression of Bcl-6 to promote GC B-cell development and survival. Additionally, it also promotes Blimp-1 expression, which facilitates PC differentiation.73 (Figure 3b) The reciprocal repression between Blimp-1 and Bcl-6 exists during the overall B cell differentiation process. Specifically, these proteins decide whether naïve B cells will differentiate into GC B cells (under the regulation of Bcl-6) or into extra-follicular PCs (under the regulation of Blimp-1). Moreover, Bcl-6 mRNA and protein are highly expressed in GC B cells during the GC reaction. At the end of the GC reaction, Bcl-6 expression is downregulated and Blimp-1 expression is upregulated to facilitate the differentiation into long-lived PCs.74 When the balance favors PC differentiation, Blimp-1 is derepressed from Bcl-6 and suppresses PAX-5; this leads to the XBP-1 de-repression, which promotes PC differentiation. Bcl-6 and PAX-5 promote B-cell proliferation and the GC reaction by repressing Blimp-1 and XBP-1 (Figure 3b). The mutual repression between these two transcriptional factors prevents the unlimited formation of PCs in the GC and prevents the reversion of the PC back into an earlier B-cell stage.75
Studies have shown that mice lacking Bcl-6 contained elevated Blimp-1+ PCs level.67 In humans, reducing Bcl-6 expression by RNA interference resulted in an increase in Blimp-1.76 It is thought that Bcl-6 suppressed PC differentiation because of its negative regulation on Blimp-1.77 However, a study also reported that the inhibitory effect of Bcl-6 on Blimp-1 was not absolute because the elevated Blimp-1 expression was observed in Bcl-6+ B cells exposed to STAT3 activation either directly or via IL-21 stimulation.78 Therefore, the upregulation of Blimp-1 alone was not sufficient for primary human B cells to differentiate into PCs. Additionally, the downregulation of Bcl-6 was also required for the complete PC differentiation process.77 However, Bcl-6 deficient mice that lacked GCs had impaired TFH cell formation, which consequently developed damaged T cell-dependent antibody responses.58 As shown previously, GC reactions produce long-lived PCs and memory cells. Because Bcl-6 is essential for the GC development and reactions, Bcl-6 may also prepare GC B cells for differentiation into long-lived PCs. The promoting or inhibiting effect of Bcl-6 on PC differentiation is dependent on the different stages of B-cell differentiation, and whether the cells enter into the follicular to facilitate the GC reaction or to differentiate into short-lived PC in extrafollicular.
Although the functions of Blimp-1 and Bcl-6 in B cells have been investigated intensively for nearly 15 years, the impact on T cells remained relatively unknown until recently.79 CD4+ T cells play an essential role in helping B cells differentiate into PCs. The balance between Blimp-1 and Bcl-6 expression is also critical for TFH cell differentiation. A series of studies have demonstrated that Bcl-6 is the master regulator for TFH cell development and that Blimp-1 is a critical antagonist of TFH differentiation because of its antagonistic relationship with Bcl-6.38 Specifically, CD4+ T cells can be divided into four subsets: Th1 cells, Th2 cells, Th17 cells and regulatory T cells. These subsets have a powerful ability to clear a given infection or inflammatory state.80 Interestingly, TFH cells express high levels of Bcl-6,81 and the remaining CD4+ T cells (non-TFH cells) possess high Blimp-1 expression levels,38,82,83 which suggests that Bcl-6 versus Blimp-1 expression might decide the direction of CD4+ T cell differentiation. Bcl-6 expression leads to TFH cell differentiation. Once the development of TFH cells is established, these cells assist with GC formation and activity, which also provides an environment for the subsequent differentiation of GC B cells into post-switched PCs (Figure 3b).
Additionally, a growing amount of recent evidence suggests84 that a negative regulatory capacity of PCs to TFH cells also existed (Figure 2). This report showed that TFH cells accumulated in the absence of PCs and that the PCs could decrease IL-21 and Bcl-6 levels in TFH cells. Therefore, we came to the conclusion that a balance between PC and TFH cells exists and maintaining that balance is crucial in humoral immunity.84
In brief, IL-21 can regulate B- and T-cell development by moderating the Blimp-1 and Bcl-6 axis to regulate PC differentiation. On the one hand, IL-21 directly mediates B-cell proliferation and apoptosis in a context-dependent manner, but it also promotes immunoglobulin production and isotype class switching.31,85,86 The effect of IL-21 on PC differentiation is derived from its capacity to increase Blimp-1 expression, whereas the increase of Bcl-6 may prepare GC B cells for their subsequent differentiation into post-switched PCs. On the other hand, IL-21 signaling in CD4+ T cells is essential for the generation and differentiation of TFH cells, which supports PC differentiation and antibody production in GCs.
IL-21/IL-21R and SLE
The effects of IL-21 on B cell differentiation into PCs and on T-cell responses makes IL-21 an attractive candidate for SLE.50,69 In humans, higher serum IL-21 and IL-21 mRNA levels were observed in SLE patients,87,88 and population-based case–control association analyses suggest that allelic variation in the IL-21 gene was a risk factor for SLE.87 In a study evaluating SLE patient autologous mixed CD3+ T- and CD19+ B-lymphocyte cultures, the exogenous addition of IL-21 and/or CpG-ODN2006 caused a significant increase in secreted IgG and the PC proportion reduced following IL-21R.Fc treatment.4
In SLE murine models, the lupus BXSB-Yaa mouse showed increased IL-21 at the transcriptional and serum protein levels compared to wild-type mice69 and the genetic deletion of IL-21R in these mice led to the disappearance of abnormal SLE characteristics, including hypergammaglobulinemia, autoantibody production and renal disease.6 Deregulated production of IL-17 and IL-21 resulted in either a lupus-like disease state or in rheumatoid arthritis-like symptoms in a murine model.89 The sanroque mouse bore a mutation that repressed TFH cell development, which resulted in excessive IL-21 production and lupus-like symptom development.90 MRL/lpr mice treated with IL-21R/Fc displayed reduced autoantibody levels and SLE-like symptoms.91
The downstream genes and proteins of the IL-21 signaling cascade were also altered in SLE patients and murine models. Additionally, elevated Blimp-1 expression, which correlated with increased PC levels, was detected in SLE patients and lupus murine models.92 Moreover, Blimp-1 siRNA reduced serum anti-dsDNA antibody levels by eliminating anti-dsDNA antibody-producing PCs and thus impeded lupus development.76 However, the changes in Bcl-6 expression in autoimmune disease remain disputed. Studies have reported elevated Bcl-6 mRNA levels in the CD4+ T cells of RA patients26 and SLE-prone mice.69 The breakdown of the Blimp-1 and Bcl-6 balance leads to auto-PC accumulation, which tends to cause autoimmune disease.
Conclusion, comments and future perspectives
B cells play fundamental roles in supplying protective immunity against infection by differentiating into PCs and secreting antigen-specific antibodies. On the contrary, the dysregulated production of excessive self-reactive antibody quantities can lead to autoimmune diseases, such as SLE. IL-21, secreted mainly by TFH cells, has been proven to promote PC differentiation and antibody secretion. Although various mechanisms exist regarding the effects of IL-21 on PC differentiation, we focused on the IL-21 signaling network that is involved in the Blimp-1/Bcl-6 axis in this review. IL-21 has the capacity to regulate the Blimp-1 and Bcl-6 balance, which is also essential for TFH cell differentiation and GC formation. Subsequently, with the help of TFH cells, short-lived PCs develop and the GC reaction produces long-lived PCs and antibodies. In conclusion, proposed therapies that inhibit the action of IL-21 by either directly targeting IL-21 or indirectly targeting TFH cells or the downstream signaling molecules of the IL-21/IL-21R cascade, such as Blimp-1 or Bcl-6, to block the differentiation of autoreactive B cells into PCs and provide practicable strategies for the treatment of SLE, have been partly evaluated in murine SLE models.6,51,93,94 These findings will also offer a profound foundation and new insight into the development of new SLE drugs.
Acknowledgments
This work was financially supported by the National Nature Science Foundation of China (No. 81173075, 81330081), the Specialized Research Fund for the Doctoral Program of Higher Education, China (No. 20123420110003) and the Anhui Province Nature Science Foundation for the University (No. KJ2011A177).
The authors declare that there are no conflicts of interest.
References
- 1Hostmann A, Jacobi A, Mei H, Hiepe F, Dörner T. Peripheral B cell abnormalities and disease activity in systemic lupus erythematosus. Lupus 2008; 17: 1064–1069. [DOI] [PubMed] [Google Scholar]
- 2Mariño E, Grey ST. B cells as effectors and regulators of autoimmunity. Autoimmunity 2012; 45: 377–387. [DOI] [PubMed] [Google Scholar]
- 3Liu R, Wu Q, Su D, Che N, Chen H, Geng L et al. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Res Ther 2012; 14: R255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Nakou M, Papadimitraki E, Fanouriakis A, Bertsias G, Choulaki C, Goulidaki N et al. Interleukin-21 is increased in active systemic lupus erythematosus patients and contributes to generation of plasma B cells. Clin Exp Rheumatol 2012; 31: 172–179. [PubMed] [Google Scholar]
- 5Terrier B, Costedoat-Chalumeau N, Garrido M, Geri G, Rosenzwajg M, Musset L et al. Interleukin 21 correlates with T cell and B cell subset alterations in systemic lupus erythematosus. J Rheumatol 2012; 39: 1819–1828. [DOI] [PubMed] [Google Scholar]
- 6Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci USA 2009; 106: 1518–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Simard N, Konforte D, Tran AH, Esufali J, Leonard WJ, Paige CJ. Analysis of the role of IL-21 in development of murine B cell progenitors in the bone marrow. J Immunol 2011; 186: 5244–5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol 2013; 131: 959–971. [DOI] [PubMed] [Google Scholar]
- 9Tedder TF. CD19: a promising B cell target for rheumatoid arthritis. Nat Rev Rheumatol 2009; 5: 572–577. [DOI] [PubMed] [Google Scholar]
- 10Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol 2001; 2: 764–766. [DOI] [PubMed] [Google Scholar]
- 11Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunol Rev 2004; 197: 192–205. [DOI] [PubMed] [Google Scholar]
- 12Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol 2005; 5: 230–242. [DOI] [PubMed] [Google Scholar]
- 13Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 2001; 14: 617–629. [DOI] [PubMed] [Google Scholar]
- 14Liu Z, Zou Y, Davidson A. Plasma cells in systemic lupus erythematosus: the long and short of it all. Eur J Immunol 2011; 41: 588–591. [DOI] [PubMed] [Google Scholar]
- 15Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med 2012; 209: 1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Oracki SA, Walker JA, Hibbs ML, Corcoran LM, Tarlinton DM. Plasma cell development and survival. Immunol Rev 2010; 237: 140–159. [DOI] [PubMed] [Google Scholar]
- 17Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med 2006; 203: 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med 2011; 208: 1377–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Nikbakht N, Shen S, Manser T. Cutting edge: macrophages are required for localization of antigen-activated B cells to the follicular perimeter and the subsequent germinal center response. J Immunol 2013; 190: 4923–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol 2009; 9: 845–857. [DOI] [PubMed] [Google Scholar]
- 21Luther SA. Plasma cell precursors: long-distance travelers looking for a home. Immunity 2010; 33: 9–11. [DOI] [PubMed] [Google Scholar]
- 22Bortnick A, Allman D. What is and what should always have been: long-lived plasma cells induced by T cell-independent antigens. J Immunol 2013; 190: 5913–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. Arthritis Res Ther 2004; 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Kemeny DM. The role of the T follicular helper cells in allergic disease. Cell Mol Immunol 2012; 9: 386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Shekhar S, Yang X. The darker side of follicular helper T cells: from autoimmunity to immunodeficiency. Cell Mol Immunol 2012; 9: 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Rodríguez-Bayona B, Ramos-Amaya A, Bernal J, Campos-Caro A, Brieva JA. Cutting edge: IL-21 derived from human follicular helper T cells acts as a survival factor for secondary lymphoid organ, but not for bone marrow, plasma cells. J Immunol 2012; 188: 1578–1581. [DOI] [PubMed] [Google Scholar]
- 27Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol 1998; 160: 3555–3561. [PubMed] [Google Scholar]
- 28Pene J, Gauchat JF, Lecart S, Drouet E, Guglielmi P, Boulay V et al. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol 2004; 172: 5154–5157. [DOI] [PubMed] [Google Scholar]
- 29Pratama A, Vinuesa CG. Control of TFH cell numbers: why and how? Immunol Cell Biol 2014; 92: 40–48. [DOI] [PubMed] [Google Scholar]
- 30Yu D, Linterman M. The temporospatial control of Tfh cells. Immunol Cell Biol 2014; 92: 20–21. [DOI] [PubMed] [Google Scholar]
- 31Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 2008; 29: 127–137. [DOI] [PubMed] [Google Scholar]
- 32Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med 2010; 207: 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev 2008; 223: 60–86. [DOI] [PubMed] [Google Scholar]
- 34Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol 2008; 8: 663–674. [DOI] [PubMed] [Google Scholar]
- 35Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol 2010; 11: 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Ochiai K, Muto A, Tanaka H, Takahashi S, Igarashi K. Regulation of the plasma cell transcription factor Blimp-1 gene by Bach2 and Bcl6. Int Immunol 2008; 20: 453–460. [DOI] [PubMed] [Google Scholar]
- 37Angelin-Duclos C, Cattoretti G, Lin KI, Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J Immunol 2000; 165: 5462–5471. [DOI] [PubMed] [Google Scholar]
- 38Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009; 325: 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD et al. Bcl6 mediates the development of T follicular helper cells. Science 2009; 325: 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000; 408: 57–63. [DOI] [PubMed] [Google Scholar]
- 41Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M et al. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon-producing Th1 cells. J Exp Med 2002; 196: 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Fantini MC, Rizzo A, Fina D, Caruso R, Becker C, Neurath MF et al. IL-21 regulates experimental colitis by modulating the balance between Treg and Th17 cells. Eur J Immunol 2007; 37: 3155–3163. [DOI] [PubMed] [Google Scholar]
- 43Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 2007; 448: 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 2007; 448: 480–483. [DOI] [PubMed] [Google Scholar]
- 45Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007; 8: 967–974. [DOI] [PubMed] [Google Scholar]
- 46Suto A, Kashiwakuma D, Kagami S, Hirose K, Watanabe N, Yokote K et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med 2008; 205: 1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol 2008; 26: 741–766. [DOI] [PubMed] [Google Scholar]
- 48Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med 2008; 205: 2873–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity 2002; 16: 559–569. [DOI] [PubMed] [Google Scholar]
- 50Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A et al. A critical role for IL-21 in regulating immunoglobulin production. Science 2002; 298: 1630–1634. [DOI] [PubMed] [Google Scholar]
- 51Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol 2007; 178: 3822–3830. [DOI] [PubMed] [Google Scholar]
- 52Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol 2005; 175: 7867–7879. [DOI] [PubMed] [Google Scholar]
- 53Pène J, Gauchat JF, Lécart S, Drouet E, Guglielmi P, Boulay V et al. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol 2004; 172: 5154–5157. [DOI] [PubMed] [Google Scholar]
- 54Yoon SO, Zhang X, Berner P, Choi YS. IL-21 and IL-10 have redundant roles but differential capacities at different stages of plasma cell generation from human germinal center B cells. J Leukoc Biol 2009; 86: 1311–1318. [DOI] [PubMed] [Google Scholar]
- 55Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med 2010; 207: 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J et al. Interleukin 17–producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol 2007; 9: 166–175. [DOI] [PubMed] [Google Scholar]
- 57Luzina IG, Atamas SP, Storrer CE, Kelsoe G, Papadimitriou JC, Handwerger BS. Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol 2001; 70: 578–584. [PubMed] [Google Scholar]
- 58Zhang X, Ing S, Fraser A, Chen M, Khan O, Zakem J et al. Follicular helper T cells: new insights into mechanisms of autoimmune diseases. Ochsner J 2013; 13: 131–139. [PMC free article] [PubMed] [Google Scholar]
- 59Lüthje K, Kallies A, Shimohakamada Y, Belz GT, Light A, Tarlinton DM et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat Immunol 2012; 13: 491–498. [DOI] [PubMed] [Google Scholar]
- 60Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol 2012; 8: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol 2009; 9: 845–857. [DOI] [PubMed] [Google Scholar]
- 62Block KE, Huang H. The cellular source and target of IL-21 in K/BxN autoimmune arthritis. J Immunol 2013; 191: 2948–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell–B cell collaboration. J Immunol 2007; 179: 5886–5896. [DOI] [PubMed] [Google Scholar]
- 64Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol 2005; 5: 688–698. [DOI] [PubMed] [Google Scholar]
- 65Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol 2008; 26: 57–79. [DOI] [PubMed] [Google Scholar]
- 66Scheeren FA, Naspetti M, Diehl S, Schotte R, Nagasawa M, Wijnands E et al. STAT5 regulates the self-renewal capacity and differentiation of human memory B cells and controls Bcl-6 expression. Nat Immunol 2005; 6: 303–313. [DOI] [PubMed] [Google Scholar]
- 67Reljic R, Wagner SD, Peakman LJ, Fearon DT. Suppression of signal transducer and activator of transcription 3-dependent B lymphocyte terminal differentiation by BCL-6. J Exp Med 2000; 192: 1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68Arguni E, Arima M, Tsuruoka N, Sakamoto A, Hatano M, Tokuhisa T. JunD/AP-1 and STAT3 are the major enhancer molecules for high Bcl6 expression in germinal center B cells. Int Immunol 2006; 18: 1079–1089. [DOI] [PubMed] [Google Scholar]
- 69Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol 2004; 173: 5361–5371. [DOI] [PubMed] [Google Scholar]
- 70Rodríguez-Bayona B, Ramos-Amaya A, López-Blanco R, Campos-Caro A, Brieva JA. STAT-3 activation by differential cytokines is critical for human in vivo-generated plasma cell survival and Ig secretion. J Immunol 2013; 191: 4996–5004. [DOI] [PubMed] [Google Scholar]
- 71Strengell M, Matikainen S, Sirén J, Lehtonen A, Foster D, Julkunen I et al. IL-21 in synergy with IL-15 or IL-18 enhances IFN-γ production in human NK and T cells. J Immunol 2003; 170: 5464–5469. [DOI] [PubMed] [Google Scholar]
- 72Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood 2007; 109: 4135–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73Ding BB, Bi E, Chen H, Yu JJ, Ye BH. IL-21 and CD40 L synergistically promote plasma cell differentiation through upregulation of Blimp-1 in human B Cells. J Immunol 2013; 190: 1827–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74Basso K, Dalla-Favera R. Roles of BCL6 in normal and transformed germinal center B cells. Immunol Rev 2012; 247: 172–183. [DOI] [PubMed] [Google Scholar]
- 75Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol 2005; 5: 230–242. [DOI] [PubMed] [Google Scholar]
- 76Zhou Z, Ren Y, Hu Z. Blimp-1 siRNA inhibits B cell differentiation and prevents the development of lupus in mice. Hum Immunol 2012; 74: 297–301. [DOI] [PubMed] [Google Scholar]
- 77Shaffer A, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 2000; 13: 199–212. [DOI] [PubMed] [Google Scholar]
- 78Diehl SA, Schmidlin H, Nagasawa M, van Haren SD, Kwakkenbos MJ, Yasuda E et al. STAT3-mediated up-regulation of BLIMP1 is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J Immunol 2008; 180: 4805–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol 2008; 26: 133–169. [DOI] [PubMed] [Google Scholar]
- 80Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood 2008; 112: 1557–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol 2008; 26: 741–766. [DOI] [PubMed] [Google Scholar]
- 82Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B et al. Early commitment of naive human CD4+ T cells to the T follicular helper (TFH) cell lineage is induced by IL-12. Immunol Cell Biol 2009; 87: 590–600. [DOI] [PubMed] [Google Scholar]
- 83Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol 2009; 10: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84Pelletier N, McHeyzer-Williams LJ, Wong KA, Urich E, Fazilleau N, McHeyzer-Williams MG. Plasma cells negatively regulate the follicular helper T cell program. Nat Immunol 2010; 11: 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol 2004; 173: 68–78. [DOI] [PubMed] [Google Scholar]
- 86Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 2007; 448: 480–483. [DOI] [PubMed] [Google Scholar]
- 87Sawalha AH, Kaufman KM, Kelly JA, Adler AJ, Aberle T, Kilpatrick J et al. Genetic association of interleukin-21 polymorphisms with systemic lupus erythematosus. Ann Rheum Dis 2008; 67: 458–461. [DOI] [PubMed] [Google Scholar]
- 88Webb R, Merrill JT, Kelly JA, Sestak A, Kaufman KM, Langefeld CD et al. A polymorphism within IL21R confers risk for systemic lupus erythematosus. Arthritis Rheum 2009; 60: 2402–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89Biswas PS, Kang K, Gupta S, Bhagat G, Pernis AB. A murine autoimmune model of rheumatoid arthritis and systemic lupus erythematosus associated with deregulated production of IL-17 and IL-21. Methods Mol Biol 2012; 900: 233–251. [DOI] [PubMed] [Google Scholar]
- 90Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 2005; 435: 452–458. [DOI] [PubMed] [Google Scholar]
- 91Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol 2007; 178: 3822–3830. [DOI] [PubMed] [Google Scholar]
- 92Luo J, Niu X, Liu H, Zhang M, Chen M, Deng S. Up-regulation of transcription factor Blimp1 in systemic lupus erythematosus. Mol Immunol 2013; 56: 574–582. [DOI] [PubMed] [Google Scholar]
- 93McPhee CG, Bubier JA, Sproule TJ, Park G, Steinbuck MP, Schott WH et al. IL-21 is a double-edged sword in the systemic lupus erythematosus-like disease of BXSB.Yaa mice. J Immunol 2013; 191: 4581–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94Rankin AL, Guay H, Herber D, Bertino SA, Duzanski TA, Carrier Y et al. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Fas(lpr/lpr)/J mice. J Immunol 2012; 188: 1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]