Identifying the cellular and molecular suppressors of the immune response against tumors is a popular topic in the field of tumor immunology that will provide new targets for the cancer immunotherapy. Suppressing the suppressor is a very attractive approach to enhancing the anti-tumor immune response by reversing the immunosuppression or blocking immune escape in the late stages of cancer progression. It is well known that tumors, especially at the late stage of development, can actively drive the generation of immunosuppressive or regulatory immune cell subtypes and can also induce the massive accumulation of tumor-promoting myeloid immune cells in the tumor microenvironment. For example, myeloid-derived suppressor cells, tumor-associated macrophages (TAMs) and regulatory T cells have been found to be the major tumor-promoting immune cells in the tumor microenvironment.1 Although much work has been conducted to identify the molecular mechanisms of the generation and accumulation of these types of tumor-promoting cells locally in the tumor microenvironment and systemically in the draining lymph nodes and other organs, the precise molecular mechanisms for their mobilization, differentiation, accumulation and function remain to be further investigated. Another key question in this field surrounds the difference and functional relationship between the migrating or recruited immune cells and the tissue-resident immune cells in the tumor microenvironment.
Macrophages are a population of immune cells with a high degree of phenotypic and functional heterogeneity. Macrophages, which are widely distributed in all tissues, play a major role in the host innate immune response against infections; additionally, macrophages are well recognized for their roles in homeostasis, tissue repair and development.2 In the last decade, there have been numerous reports about the relationship between macrophages and tumors. One of the major concerns in this field is the origin and function of TAMs. TAMs can enhance tumor cell proliferation, invasion and metastasis; stimulate angiogenesis; and inhibit the T cell-mediated anti-tumor immune response, thus promoting tumor progression.3,4 Much clinical data show that more accumulation of TAMs in the tumor tissue indicates a poor prognosis for cancer patients.5 However, there are contradicting reports about the cellular origin of TAMs accumulated in the tumor microenvironment and the identification of the master molecule responsible for TAM differentiation from their precursors. In a recent issue of Science, Franklin et al.6 reported that TAMs are phenotypically and functionally different from the traditional M2 TAMs in the mouse model of mammary cancer; the group reported that TAMs are differentiated from CCR2+ inflammatory monocytes and that their differentiation depends on the Notch signaling pathway via the transcription factor RBPJ (Figure 1). These new findings not only enrich our understanding of the origin and function of TAMs, but also propose new pathways for the differentiation of TAMs, thus providing new clues for developing strategies for cancer immunotherapy.
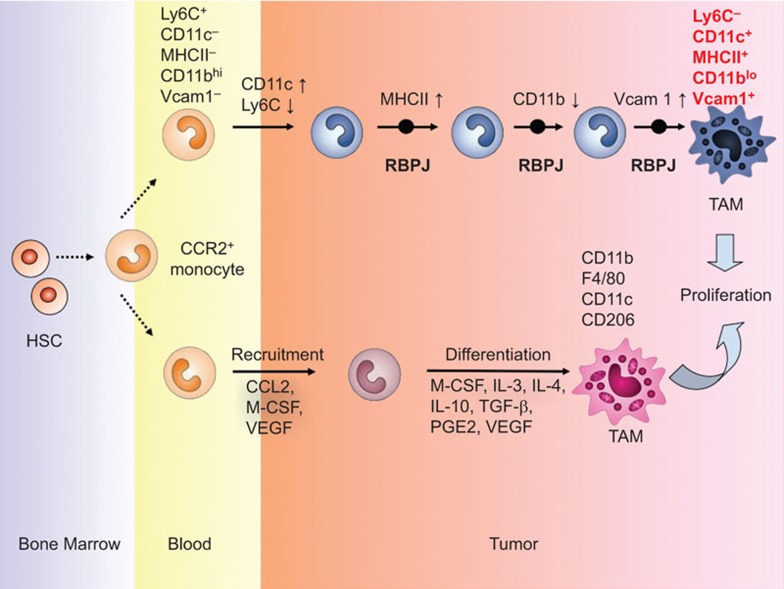
Figure 1.
Origin and differentiation of TAMs. TAMs originate from the circulating Ly6C+CCR2+ monocytes, which are derived from bone marrow hematopoietic stem cells. The inflammatory monocytes are recruited to the tumor tissues and then differentiate into TAMs. At the early stage of differentiation, the inflammatory monocytes exhibit an increase in CD11c and a decrease in Ly6C; this process involves RBPJ-mediated Notch signaling. However, at a relatively late stage of differentiation, RBPJ induces the upregulation of MHC-II and downregulation of CD11b, then the significant upregulation of Vcam1 on the inflammatory monocytes, finally driving the differentiation of the CCR2+ inflammatory monocytes to Ly6C−CD11c+MHCII+CD11bloVcam1+ TAMs that can further proliferate in the tumor tissue and mediate the suppression of T-cell immunity against cancer. TAM, tumor-associated macrophage.
As an important type of immune cells in the tumor microenvironment, macrophages have been demonstrated to be important in tumor progression, depending on the stage of tumor development and the tumor type.7 Generally, TAMs are accepted to promote tumor progression via different mechanisms. Macrophages are a heterogeneous cell population that can be conventionally divided into two subgroups: M1 and M2. M1-type macrophages, which are classically activated, play important roles in the innate response against invading pathogens. M2-type macrophages, which are alternatively activated, play important roles in tissue repair and tumor progression. Previous studies have shown that Notch signaling plays a decisive role in the polarization of M2 macrophages.8,9 A recent study shows that mouse peritoneal macrophages can specifically express the transcription factor Gata6, which can affect the phenotype of macrophages by altering their transcriptome, thus contributing to the inflammatory response and the renewal of macrophages in the inflammatory response.10 Furthermore, the localization of tissue-specific macrophages and the polarization of macrophage function can be determined, at least partially, by this pathway.11 Several cytokines, such as IL-6, have been found to be important in the development and maintenance of the macrophage subsets and their functions in inflammation and tissue homeostasis.12 Much work has been performed to identify the soluble factors, such as chemokines and growth factors, in polarizing macrophage function in tumor progression. For example, the chemokine CCL2 and macrophage colony-stimulating factor can recruit monocytes to the tumor tissue, and then IL-4, IL-10, IL-13 and other cytokines in the tumor microenvironment can induce the monocytes to differentiate into TAMs.13 TAMs have a very weak ability to present antigens but exhibit a very potent ability to promote tumor progression through a variety of mechanisms. However, the detailed mechanisms for the recruitment and tumor-promoting function of TAMs are not fully understood; thus, further investigation is required to identify new targets for cancer immunotherapy.
In this article, Franklin and colleagues utilized the MMTV-PyMT (PyMT) mouse mammary tumor model to investigate the cellular components in CD45+ tumor-infiltrating leukocytes and to attempt to elucidate the three subsets of myeloid cells the roles they play in tumor progression. The authors found that MMTV-PyMT breast tumor growth can actively recruit CCR2+ inflammatory monocytes to the tumor tissue and expand the number of macrophages in the tumor microenvironment. Unexpectedly, they distinguished the TAMs from the ‘breast tissue macrophages' (mammary tissue macrophages, MTMs) using a series of experiments including morphology, flow cytometry and transcriptional analysis. Additionally, they showed that TAMs express the macrophage transcription factor Mafb and the macrophage markers CD64 and MerTK, but not the dendritic cell-specific transcription factors Zbtb46 and Flt3, c-Kit and other markers. Furthermore, using many mouse models that were deficient in different molecules involved in cell migration and differentiation, the authors convincingly showed that TAMs were derived from bone marrow cells and originated from CCR2+ inflammatory monocytes, but independent of pre-dendritic cell. Thus, the authors proposed a new origin of conventional TAMs. Notably, using gene profiling to distinguish TAMs from MTMs, the investigators found that TAMs expressed low levels of the integrin protein CD11b but high levels of Vcam1 following tumor progression. Unexpectedly, TAMs did not express the markers of alternatively activated macrophages, including Mrc1 (CD206), Ym1 and Fizz1, while MTMs did. This observation further shows that the TAMs that the authors discovered in this study are not generated by conversion from MTMs. By analyzing the molecular mechanism for TAM differentiation, the authors revealed that transcription factor RBPJ-mediated Notch signaling pathway is responsible for the TAM differentiation from CCR2+ inflammatory monocytes (Figure 1). Finally, the team demonstrated that the in vivo depletion of TAMs, but not MTMs, can efficiently restore the tumor-infiltrating cytotoxic T-cell response by decreasing the number of immunosuppressive PD-1+GzmB−CD8+ T cells.
TAMs have been verified to promote tumor progression via different mechanisms (Figure 2). TAMs can suppress the CD8+ T-cell immune response against cancer by directly interacting with T cells or by secreting immunosuppressive factors to inhibit T-cell effector function.5,14 This study showed the role of Notch-dependent, monocyte-derived TAMs in tumor progression by disrupting anti-tumor T-cell immunity, adding new insight to the immune tolerance and escape of tumors. However, some concerns still exist regarding the roles of these TAMs in tumor metastasis. Whether these TAMs can promote tumor angiogenesis, or induce the apoptotic resistance of tumor cells, remains to be determined. One important question surrounds the relationship of these TAMs with M2 macrophages or other regulatory immune cells, such as regulatory T cells and myeloid-derived suppressor cells, in tumor immune escape. It remains to be determined what mechanisms TAM deletion drives the restoration of the immunosuppression in the tumor microenvironment by affecting the tumor-promoting functions of other types of immunosuppressive cell subsets. Hypoxia and cell death in the tumor microenvironment may contribute to the induction of tumor immunosuppression.15 Furthermore, many new immunosuppressive cell subsets have been identified in cancer patients.16 Thus, the cellular and molecular mechanism for the generation of TAMs may be more complicated than we expected.
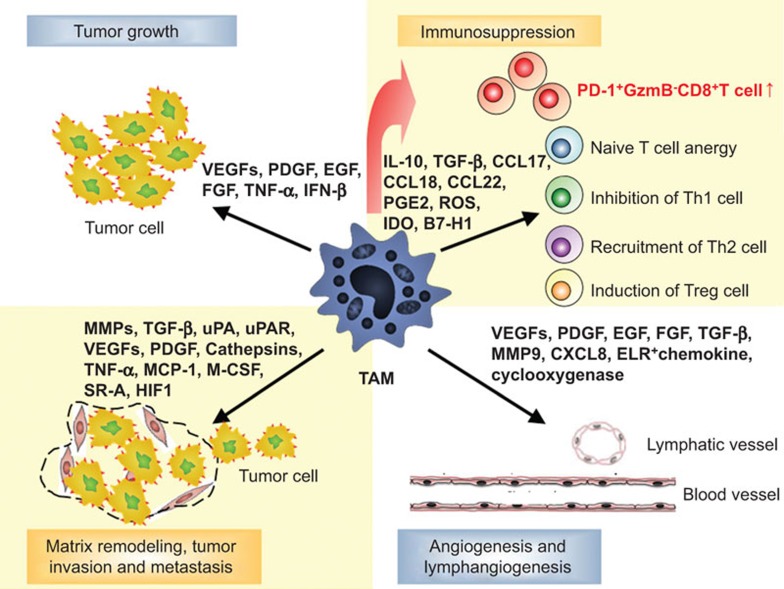
Figure 2.
Multiple functions of TAMs in tumor progression. TAMs can promote tumor progression in a variety of ways. Monocyte-derived, RBPJ-dependent TAMs can induce the expansion of PD-1+GzmB−CD8+ T cells in the tumor tissue to mediate immunosuppression; TAMs can inhibit the proliferation of effector T cells or recruit more Th2 or regulatory T cells to the tumor tissue to suppress the anti-tumor immune response. TAMs can release a large number of angiogenic factors, such as VEGF and PDGF, which can promote tumor angiogenesis. In addition, TAMs can secrete numerous growth factors, MMPs, which can work jointly to promote tumor cell proliferation, invasion and metastasis. MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; TAM, tumor-associated macrophage; VEGF, vascular endothelial growth factor.
The identification of new origins and the molecular profiling of TAMs is of great interest. It remains to be determined how broadly applicable the findings obtained from the mouse mammary cancer model the authors used are. Future questions will surround the phenotypic and functional identification of these TAMs in other non-mammary mouse cancer models. How to translate the basic results from the mouse model to clinical practice and how to link these TAM results to clinical studies will be critical to cancer immunotherapy. These questions require further investigation in the future, and any progress in addressing these questions will benefit the design of powerful cancer immunotherapy.
References
- 1Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14: 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013; 496: 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140: 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014; 41: 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K et al. The cellular and molecular origin of tumor-associated macrophages. Science 2014; 344: 921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Perdiguero EG, Geissmann F. Cancer immunology. Identifying the infiltrators. Science 2014; 344: 801–802. [DOI] [PubMed] [Google Scholar]
- 8Palaga T, Ratanabunyong S, Pattarakankul T, Sangphech N, Wongchana W, Hadae Y et al. Notch signaling regulates expression of Mcl-1 and apoptosis in PPD-treated macrophages. Cell Mol Immunol 2013; 10: 444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res 2010; 70: 4840–4849. [DOI] [PubMed] [Google Scholar]
- 10Rosas M, Davies LC, Giles PJ, Liao CT, Kharfan B, Stone TC et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science 2014; 344: 645–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 2014; 157: 832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol 2014; 15: 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol 2013; 31: 317–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Alderton GK. Tumour immunology: turning macrophages on, off and on again. Nat Rev Immunol 2014; 14: 136–137. [DOI] [PubMed] [Google Scholar]
- 15Wu C, Zhang Y, Jiang Y, Wang Q, Long Y, Wang C et al. Apoptotic cell administration enhances pancreatic islet engraftment by induction of regulatory T cells and tolerogenic dendritic cells. Cell Mol Immunol 2013; 10: 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu Y et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology 2014; 59: 567–579. [DOI] [PubMed] [Google Scholar]