Abstract
Objective
To evaluate an informed shared decision-making programme (ISDM-P) for people with type 2 diabetes under high fidelity conditions.
Design
Randomised, single-blinded trial with sham control intervention and follow-up of 6 months.
Setting
Single-centre diabetes clinic providing care according to the national disease management programme in Germany.
Participants
154 people with type 2 diabetes without diagnosis of ischaemic heart disease or stroke.
Interventions
The ISDM-P is executed by diabetes educators. Core component is a patient decision aid on the prevention of myocardial infarction supplemented by a 90 min group teaching session. The structurally equivalent control intervention addresses stress issues.
Main outcome measures
Primary outcome was risk comprehension, including realistic expectations about benefits and harms of interventions. It was assessed by a 12-item questionnaire after the teaching session when patients set and prioritise their treatment goals. Key secondary outcome was adherence to treatment goals, operationalised as achievement of individual goals and medication uptake. ISDM-P teaching sessions were video-taped to monitor intervention fidelity.
Results
72 of 77 ISDM-P and 71 of 77 control patients completed the questionnaire (score 0–12). ISDM-P patients achieved higher levels of risk comprehension, mean score 8.25 vs 2.62, difference 5.63 (95% CI 4.82 to 6.44), and realistic expectations (score 0–6), 4.51 vs 0.85, 3.67 (3.23 to 4.11). More ISDM-P patients wished to take statins, 59.2% vs 30.4%, 28.7% (12.9% to 44.5%); more prioritised blood pressure control, 51.4% vs 25.7%, and fewer intensive glucose control, 33.3% vs 60%, p=0.002. More ISDM-P patients achieved their glycated haemoglobin goals, 95.8% vs 85.7%, 10.1% (0.6% to 19.5%). Achievement of prioritised goals and medication uptake were comparable between groups.
Conclusions
The ISDM-P on preventive measures in type 2 diabetes was effective under high fidelity conditions. Involvement of diabetes educators may facilitate implementation of the informed shared decision-making.
Trial registration number
ISRCTN84636255.
Keywords: MEDICAL EDUCATION & TRAINING
Strengths and limitations of this study.
Current teaching programmes for people with type 2 diabetes usually do not provide numerical and comparative risk information. The informed shared decision-making programme (ISDM-P) is innovative in that it includes risk information and initiates shared decision-making as recommended in recent guidelines.
The development of the ISDM-P was theory based and followed the UK MRC framework for the development and evaluation of complex interventions.
This study comprises a rigorously designed randomised controlled trial and qualitative methods to monitor intervention fidelity.
Patients were blinded against the allocation, but it was impossible to keep the diabetes educators and other members of the healthcare teams blinded.
The study was designed as proof-of-concept, which might limit generalisability.
Introduction
Cardiovascular disease is the predominant life-threatening complication associated with type 2 diabetes. An array of behavioural directives is imposed on these patients such as quitting smoking; increasing exercise; reducing weight; and adhering to self-monitoring, dietary and medication prescriptions. Evidence on the efficacy of the recommended measures is variable. Some may even do more harm than good such as the intensive lowering of blood glucose values by polypharmacy.1 Patients frequently feel demotivated and overloaded by the plethora of medical orders. This might contribute to poor long-term adherence even to the most effective preventive interventions such as blood pressure control or use of statins.2–5 Lack of patient involvement in decision-making has been suggested as an important reason for low adherence and limited treatment success.6
Since 2012, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) claim a ‘patient-centred approach’ in the care of people with type 2 diabetes with particular emphasis on shared decision-making (SDM).7 SDM is also strongly recommended in the recent national treatment guideline for type 2 diabetes in Germany.8
SDM is not yet implemented in diabetes care. When we started this project, literature searches on SDM in the context of cardiovascular prevention in type 2 diabetes in 20059 and updates in 200810 retrieved only very few relevant publications. Two of them focused on patient participation in the selection of oral antidiabetic agents or statin treatment.11 12 A recent pragmatic randomised controlled trial on a patient decision aid included general practices in the Netherlands.13 The trial failed to demonstrate an improvement of the primary outcome measure, which was empowerment of people with type 2 diabetes for setting and achieving goals.
As we designed the present project, we had identified several barriers for the implementation of SDM. They included the lack of ready to use evidence-based patient information (EBPI) material, time constraints on the healthcare teams and physician encounters overloaded with complex information.9
We have developed an informed shared decision-making programme (ISDM-P) to address these barriers.10 14 Main components are a decision aid and a corresponding teaching module provided by diabetes educators who teach and support patients to understand risk information and to define a hierarchy of individual treatment goals. In order to enhance practicability, the teaching module was designed as a supplementary session in the patient education programme that was already integrated into the German national disease management programme for type 2 diabetes.15
The ISDM-P is a complex intervention. It includes a number of interdependent components that may interact with contextual factors. The development process of the ISDM-P followed the UK MRC framework for complex interventions.16 Accordingly, development and evaluation of the components encompassed theoretical and empirical groundwork focusing on in-depth understanding of contextual interactions and implementation processes. Details on the underlying theories, design and pilot testing of the decision aid, the teaching curriculum and the trainer modules have been published previously.9 10 14 17 18
The objective of the present study was to assess the efficacy of the whole programme in a randomised controlled trial under high fidelity conditions. We investigated if the ISDM-P leads to higher levels of risk comprehension and realistic expectations concerning benefits and harms of preventive options. In addition, we evaluated if the programme helps patients to define, to prioritise and to achieve individual treatment goals.
Methods
The study was a parallel group, two-arm, single-blinded, randomised controlled trial with 6 months of follow-up. A detailed study protocol was published.14 Patients were eligible if they were registered in the German Disease Management Programme (DMP) for type 2 diabetes,19 were 40–69 years old, had glycated haemoglobin (HbA1c) values between 6% and 9%, had no history of ischaemic heart disease (International Classification of Diseases (ICD) I20-I25) or stroke (ICD I63), and had previously participated in structured diabetes education sessions as typically provided within the DMP.20 Patients were excluded if they had proliferative retinopathy, chronic kidney disease stage 3 or higher,21 metastatic cancer, were addicted to alcohol or cared for by a legal guardian. The study took place at the outpatient department of Endocrinology and Metabolic Diseases at the Jena University Hospital, Jena, Germany. The outpatient diabetes clinic provides standard care for a large catchment area. Within the DMP, patients are followed usually quarterly but at least once a year.
Intervention
The ISDM-P is a complex intervention.16 22 23 By definition, complex interventions comprise interdependently acting components essential to their proper functioning. For example, patient teaching and provider training may act interdependently.
The underlying approach of the ISDM-P follows Ajzen's24 theory of planned behaviour which suggests that behaviour is influenced by (1) individual attitudes, (2) subjective social norms such as perceived attitudes of family members or the healthcare team, and (3) perceived and actual individual behaviour control. The most predictive variable of SDM behaviour is intention.25 Subjective social norms and perceived behaviour control are the most frequently identified determinants of a health professional's intention to perform SDM.
As one of the relevant issues, social norms of diabetes educators and possible concerns of physicians were addressed in the curriculum. The provision of evidence-based information aimed at strengthening behaviour control by resolving knowledge deficits and by realigning unrealistic expectations.
Components of the ISDM-P are (1) an evidence-based decision aid for patients on the prevention of heart attack,10 (2) structured patient teaching provided by diabetes educators and (3) a provider training. The decision aid is supposed to be provided 2 weeks before the teaching session. It includes EBPI on heart attack risk, risk factors and different preventive options.10 Other diabetes-related risks, such as stroke or microvascular complications, are also considered. We used UKPDS data on the combined end point for ‘any diabetes-related end point’ to communicate benefits and harms of blood pressure and blood glucose control.4 26 The decision aid is available on request from the corresponding author. The patient teaching module is curriculum based and focuses on the EBPI provided within the decision aid. A single session targets a group of 4–6 patients and is scheduled to take 90 min. Educational elements are illustrating wall charts and worksheets. A magnet board is used for the visualisation of quantity risk with 100 orange and blue game pieces representing people with and without myocardial infarction.
During the teaching session, the diabetes educator guides through the decision-making process. This encompasses assessing each patient's individual heart attack risk, providing outcome probabilities of the available preventive options, and supporting patients to set and prioritise individual goals regarding smoking cessation, glucose control, blood pressure control and statin treatment. At the end of the teaching session, the diabetes educators use specific question cards to check patients’ understanding and to repeat information, if necessary. We conducted four test sessions and subsequent focus groups with a total of 24 participants with type 2 diabetes to pilot the teaching module.
The provider training comprises a training DVD and a training session that includes a demonstration of the patient teaching. The DVD is intended to prepare diabetes educators. It includes objectives and contents of the teaching, basic principles of SDM and an exemplary presentation of specific topics in the patient teaching. The provider training focuses on the EBPI provided within the decision aid and patient teaching. It also addresses the implementation of the patient teaching curriculum and the handling of the media. Piloting showed good overall feasibility.
Comparison
In order to achieve structural equivalence, a sham control intervention was applied. It comprised usual care supplemented with a 90 min teaching module on sports, nutrition and stress issues. Before the teaching session, all participants received a brochure on stress management.27 Diabetes educators were prepared for the teaching session with a brief provider training.
Procedure
In December 2012, two diabetes educators were trained in the ISDM-P by a research fellow of the University of Hamburg (SB). Two additional diabetes educators received the training for the control group (provided by JK). Each of both trainings lasted about 4 hours.
The study was submitted for registration on 22 February 2013. Recruitment of patients started on 12 March 2013. Nevertheless, the registration was classified as retrospective because the date of payment was used as the date of finalised registration. We have notified the registration administrator to correct the classification from retrospective to prospective. We were informed that this was not possible. However, the administrator included an explanation in the registration protocol to testify that following the prospective submission, there were no subsequent changes to the protocol. The recruitment started after initiation of public registration.
The electronic patient records used for DMP documentation were screened for eligible patients. Eligible patients were marked on the records and asked for participation during the next consultation with their physician. Baseline data were extracted from the electronic patient records. After the patients had given informed consent they were randomised into one of the two study groups. Patients received either the decision aid or the brochure on stress management.10 27 An appointment within the next 2–4 weeks for the patient teaching was made. When the appointment failed, a new one was made. In order to ensure that the patients keep the appointment date, they were contacted by phone a few days before. At the end of the teaching session, patients documented their individual preferences on treatment goals regarding smoking cessation, HbA1c level, blood pressure control (systolic blood pressure) and statin treatment on standardised forms. Risk comprehension, including realistic expectations on the prevention of myocardial infarction, was assessed after the teaching session and at 6 months follow-up. At follow-up, patients were asked to bring their medication boxes/pill packages to assess current medication. The overall trial end date was supposed to be December 2013. However, patient recruitment took longer than expected. The last patient was enrolled in June 2014; the last teaching session was conducted in October 2014; follow-up data were completed in March 2015.
Outcome measures
Primary outcome was patients’ comprehension of relevant risk information after the teaching session. As outlined in the study protocol, risk comprehension was operationalised by the level of patient knowledge and understanding of the notion of risk, individual heart attack risk, and probabilities of benefits and harms of preventive treatment options, including realistic expectations.14 We used a 12-item standardised questionnaire with a subdomain of six items on realistic expectations. The questionnaire was designed based on the cognitive domain of Bloom's taxonomy of educational objectives aimed to evaluate different levels of comprehension.28 Each correctly answered item scored one point. We counted missing responses as wrong answers. Item analysis resulted in good test quality (Cronbach's α=0.87 for the total test, and 0.86 for the subdomain of realistic expectations).29 Comprehension, including realistic expectations, was additionally assessed as a secondary outcome measure at 6 months follow-up. To avoid learning effects, comprehension and realistic expectations were not assessed at baseline.14
Secondary outcome measures also comprised adherence to individual and prioritised treatment goals. Operationalisation of these outcomes is outlined in the study protocol.14 We used the following variables: (1) achievement of individual treatment goals regarding the use of statins, levels of office systolic blood pressure and HbA1c, and smoking; (2) achievement of the prioritised treatment goal; (3) medication uptake as reported by patients. The achievement of treatment goals was assessed by comparing statin uptake, office blood pressure values and HbA1c levels at follow-up with the treatment goals that the patients have set and prioritised at the end of the teaching session. Smoking status at follow-up was assessed by using the standardised interview question ‘On how many of the past 30 days did you smoke a cigarette?’ Patients were classified as smokers if the answer was one or more.
Sample size
We assumed patients in the ISDM group to answer 70% of the questionnaire correctly (8 out of 12 questions), compared with 50% in the control group (6 out of 12). Based on an estimated 0.4 SD and striving for 80% power with an 5% α, the study needed 64 participants in each group (total=128) to enable detection of this 20% absolute difference by an independent two-sided t test. By estimating a non-responder/drop-out rate of about 15%, 154 participants needed to be recruited for randomisation.
Randomisation and blinding
Randomisation was performed in permuted blocks of eight patients to ensure close balance of numbers of participants in each group and sufficient numbers of participants (n=4) in each teaching session. Allocation of patients was concealed and independently performed by the Centre for Clinical Studies at the Jena University Hospital. Potential participants were informed about the study aim to compare two approaches of patient information on the prevention of myocardial infarction. Patients were kept blinded to study group allocation, which was validated by asking patients at follow-up ‘What do you think? Did you receive the study intervention or the control intervention?’ Allocation was concealed during data entry and analysis.
Statistical methods
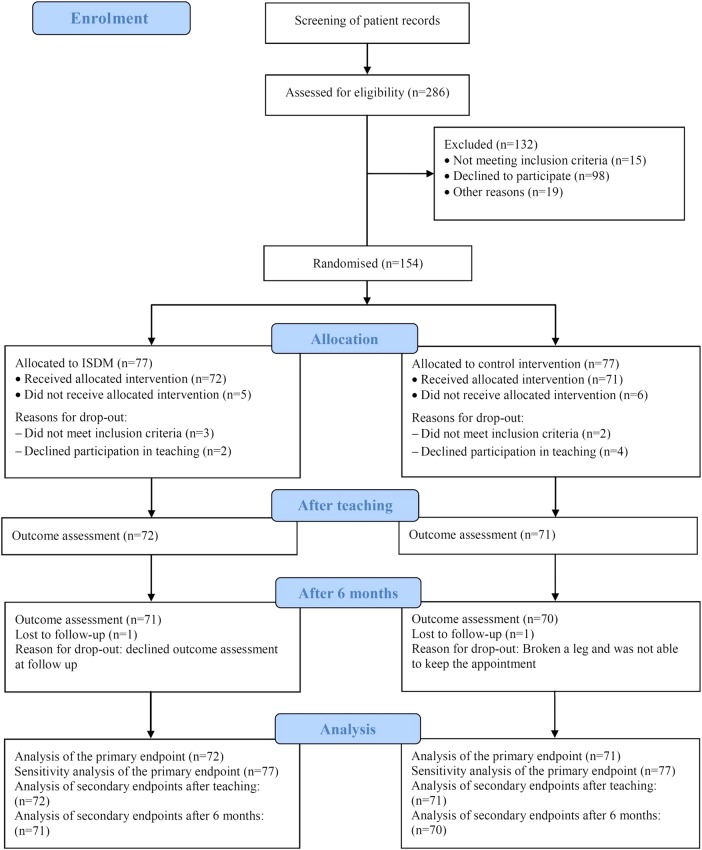
Statistical analyses of primary and secondary outcomes were carried out based on the intention-to-treat principle. A total of 11 patients did not participate in the teaching sessions after randomisation (figure 1). Primary analysis was without imputation of missing values. For sensitivity analysis of the primary end point, we multiplied imputed missing values by the fully conditional specification method using an extensive set of baseline covariates, for example, gender, age, social status, body mass index, diabetes duration, HbA1c and blood pressure.30
Figure 1.
Study flow (ISDM, informed shared decision-making).
Baseline characteristics are described by using means and SD or frequencies.
We used unpaired t tests to compare mean scores of comprehension and realistic expectations. We compared average differences between planned and achieved values of blood pressure and HbA1c using unpaired t tests. We used Fisher's exact tests to assess goal achievement regarding statin choice, office systolic blood pressure and HbA1c (defined as reaching 80–120% of the goal), smoking and the prioritised goal. We used the Mann-Whitney U test to compare medication uptake after the teaching session with 6 months follow-up (increase/unchanged/decrease), and the χ2 test to compare the difference in the prioritised treatment goals between groups.
We conducted predefined additional analyses using Fisher's exact test to assess if risk comprehension was associated with the level of numeracy.14 We used the 1 min Berlin Numeracy Test for general population to assess numeracy: ‘Imagine we are throwing a five-sided die 50 times. On average, out of these 50 throws how many times would this five-sided die show an odd number (1, 3 or 5)?’31 We also used Fisher's exact test to assess if comprehension was associated with the achievement of treatment goals. For that reason, we defined two groups of patients regarding their level of comprehension (sufficient/insufficient). At least nine correctly answered questions were considered as sufficient risk comprehension to make informed choices.32 We used analysis of variance to assess if comprehension was associated with age and Spearman's r correlation coefficient to assess if the comprehension score is associated with social status. We used Mann-Whitney U test to assess if heart attack risk or social status is influencing the achievement of patients’ prioritised treatment goal. We used t test for paired samples to assess differences in comprehension from after teaching to 6 months follow-up in the ISDM group.
Intervention fidelity
ISDM-P teaching sessions were video-taped and analysed to evaluate intervention fidelity and to achieve in-depth understanding of implementation processes.14 33 In order to maintain and optimise the fidelity of teaching and contents, video analysis-based feedback was provided to diabetes educators after the initial sessions. Likewise, issues with the patient teaching of the control group were discussed and resolved after the first sessions. We also assessed the number of patients who had a consultation with a physician after the teaching session.
Results
Baseline characteristics
A total of 154 patients were randomised to either the ISDM group (n=77) or the control group (n=77; figure 1). Baseline characteristics were equally distributed (table 1). Five of 77 patients in the ISDM-P and 6/77 in the control group did not participate in the teaching sessions (figure 1). They did not meet the inclusion criteria (n=5) or declined participation in the teaching session (n=6).
Table 1.
Baseline characteristics of patients
| Characteristic | ISDM group (n=77) | Control group (n=77) |
|---|---|---|
| Women | 36 (46.8) | 36 (46.8) |
| Age, years | 61.8 (6.5) | 61.7 (6.5) |
| Duration of diabetes, years | 13.7 (7.3) | 12.7 (6.6) |
| Systolic blood pressure, mm Hg | 145 (20.0) | 145 (16.5) |
| Diastolic blood pressure, mm Hg | 80 (10.2) | 84 (9.9) |
| Body mass index, kg/m2 | 33.3 (7.1) | 32.7 (7.4) |
| HbA1c, % | 6.9 (0.7) | 7.2 (0.7) |
| Total cholesterol*, mmol/L | 5.4 (1.2) | 4.9 (1.3) |
| HDL-cholesterol†, mmol/L | 1.2 (0.3) | 1.2 (0.3) |
| LDL-cholesterol‡, mmol/L | 3.0 (1.0) | 2.8 (1.1) |
| Smoker | 8 (10.4) | 11 (14.3) |
| Social status score§ | 12.04 (3.8) | 13.08 (3.4) |
| Medication for glucose control | 71 (92.2) | 72 (93.5) |
| Insulin | 46 (59.7) | 44 (57.1) |
| Metformin | 55 (71.4) | 55 (71.4) |
| Sulfonylurea | 10 (13.0) | 11 (14.3) |
| DPP-4 inhibitors | 5 (6.5) | 7 (9.1) |
| Medication for blood pressure control | 66 (85.7) | 67 (87.0) |
| Statin medication | 44 (57.1) | 36 (46.8) |
| Previous participation in teaching sessions for hypertension | 36 (46.8) | 27 (35.1) |
Values are numbers (percentages) or means (SD).
*ISDM n=76, control group n=75.
†ISDM n=75, control group n=75.
‡ISDM n=75, control group n=74.
§ISDM n=75, control group n=72; social status includes educational status, occupational status and income: score 3–21 (lower scores indicating lower social class).47
DPP-4, dipeptidyl peptidase-4; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; ISDM, informed shared decision-making; LDL, low-density lipoprotein.
Primary outcome
A total of 72 patients in the ISDM-P and 71 patients in control group completed the questionnaire (score 0–12) after the teaching session. The mean score for risk comprehension was 8.25 for the ISDM group and 2.62 for control group (mean difference 5.63 (4.82 to 6.44); p<0.001). The mean score of the subdomain realistic expectations (score 0–6) was 4.51 vs 0.85 (mean difference 3.67 (3.23 to 4.11); p<0.001; table 2).
Table 2.
Comprehension and realistic expectations after teaching
| Outcome | ISDM group (n=72) | Control group (n=71) | Difference (95% CI); p Value |
|---|---|---|---|
| Risk comprehension (score 0–12) | 8.25 (2.86) | 2.62 (1.96) | 5.63 (4.82 to 6.44); <0.001 |
| Realistic expectations (score 0–6) | 4.51 (1.61) | 0.85 (0.98) | 3.67 (3.23 to 4.11); <0.001 |
| Sufficient risk comprehension (score 9 or more) | 35 (48.6) | 0 (0) | 48.6% (37.0% to 60.2%); <0.001 |
Values are means (SD) or numbers (percentage).
ISDM, informed shared decision-making.
After imputation of missing values, the mean comprehension score was 8.27 vs 2.81 (difference 5.46 (4.64 to 6.27); p<0.001), and 4.49 vs 0.96 (difference 3.53 (3.09 to 3.97; p<0.001) for realistic expectations, respectively.
Secondary outcomes
At 6 months follow-up, there were small but statistically significant differences between study groups regarding risk comprehension and realistic expectations: 3.68 vs 2.70 (difference 0.98 (0.15 to 1.80); p=0.021), and 1.41 vs 0.90 (difference 0.51 (0.09 to 0.93); p=0.018), respectively (see online supplementary table S1). Again, missing values did not affect results (data not shown).
The treatment goals set by the patients regarding systolic blood pressure, HbA1c levels and smoking cessation did not differ between groups. However, more patients of ISDM-P wished to take statins (difference 28.7% (12.9% to 44.5%); p=0.001; table 3). In order to further explore this finding, we conducted a post hoc analysis. We compared statin prescriptions at baseline with the treatment goal set by the patient after the teaching session (see online supplementary table S2). Among patients who were initially not on statins, more of the ISDM group tended to start taking a statin (5/31 vs 2/38), whereas among patients who were already on statins, fewer of the ISDM group wished to stop statin treatment (3/40 vs 12/31; p=0.001).
Table 3.
Patients’ treatment goals after teaching
| Treatment goal | ISDM group N=72 |
Control group N=71 |
Difference (95% CI); p Value |
|---|---|---|---|
| Taking statins | 42/71 (59.2) | 21/69 (30.4) | 28.7 (12.9 to 44.5); 0.001 |
| Stop smoking | 4/8 (50.0) | 4/11 (36.4) | 13.6 (−31.2 to 58.5); 0.552 |
| Average group systolic blood pressure, mm Hg | 131 (7.30) | 132 (8.11) | −0.9 (−3.5 to 1.7); 0.419 |
| Average group HbA1c, % | 6.83 (0.49) | 6.76 (0.56) | 0.07 (−0.11 to 0.25); 0.492 |
Values are numbers (percentages) or means (SD).
HbA1c, glycated haemoglobin; ISDM, informed shared decision-making.
Prioritisation of treatment goals differed significantly between groups (see online supplementary table S3). More patients in the ISDM group than in the control group prioritised blood pressure control (51.4% vs 25.7%), whereas fewer patients in the ISDM group prioritised glucose control (33.3% vs 60.0%; p=0.002).
At follow-up, more patients in the ISDM group achieved their HbA1c goals (95.8% vs 85.7%, difference 10.1% (0.6% to 19.5%); p=0.046; table 4). As mean HbA1c levels did not differ between groups at baseline and follow-up, we did an additional post hoc analysis to further explore this observation. Patients in the ISDM group tended to set slightly higher HbA1c goals than patients in the control group (table 3, online supplementary table S4). The resulting mean difference between baseline HbA1c levels and patients’ HbA1c goals was 0.1% for the ISDM group and 0.39% for the control group. The HbA1c level of ISDM patients at follow-up was 0.12% higher than their targeted HbA1c goal, whereas patients of the control group had 0.41% higher HbA1c levels (difference 0.29% (0.06% to 0.53%); p=0.016; see online supplementary table S5).
Table 4.
Achievement of treatment goals at 6 months follow-up
| Outcome | ISDM N=72 |
Control group N=71 |
Difference (95% CI); p Value |
|---|---|---|---|
| Statin | 64/70 (91.4) | 57/68 (83.8) | 7.6% (−3.4% to 18.6%); 0.203 |
| Blood pressure* | 48/71 (67.6) | 49/70 (70.0) | −2.4% (−17.7% to 12.9%); 0.856 |
| HbA1c* | 68/71 (95.8) | 60/70 (85.7) | 10.1% (0.6% to 19.5%); 0.046 |
| Smoking | 4/8 (50.0) | 7/12 (58.3) | −8.3% (−52.9% to 36.2%); 1.000 |
| Prioritised goal | 58/69 (84.1) | 56/64 (87.5) | −3.4% (−15.3% to 8.5%); 0.627 |
Values are numbers (percentages)
*Achievement is defined as reaching a value between 80% and 120% of the defined goal.
HbA1c, glycated haemoglobin; ISDM, informed shared decision-making.
There was no difference with respect to other treatment goals. In both groups, most patients achieved their prioritised goals (84.1% vs 87.5%; table 4).
Medication uptake did not significantly change from baseline to directly after the teaching and to 6 months follow-up (see online supplementary tables S6 and S7).
Additional analyses
A total of 41/143 patients correctly answered the numeracy test with no significant difference between groups. These patients achieved a higher questionnaire score than patients who gave a wrong answer or did not answer at all. After the teaching session, sufficient risk comprehension (score ≥9) was achieved by 35 of 72 patients in the ISDM group and by no one in the control group (table 2). There was no significant difference in the achievement of treatment goals between patients with or without sufficient risk comprehension. There was also no difference between age groups (40–49, 50–59, 60–69 years). Social status did not correlate with risk comprehension score (Spearman's r=0.147, p=0.085). Social status and heart attack risk did not differ between patients achieving or not achieving their prioritised treatment goals. In the ISDM group, the comprehension score decreased over time (mean difference 4.62 (3.91 to 5.33); p<0.001).
Intervention fidelity
Overall, 36 ISDM teaching sessions were given by two diabetes educators. Group size varied between one and four patients. Twenty-four ISDM sessions were video-taped. Analyses did not reveal major barriers. The diabetes educators sufficiently followed the curriculum and adequately used the media (wall charts, worksheets, question cards and a magnetic board). Mean duration was 87 min (range 55–138 min). Using the tool for estimation of the heart attack risk and for calculating the individual benefit from statins were the most time consuming parts of the teaching session. The diabetes educators perceived the ISDM sessions as less interactive as the usual teaching sessions. They modified the page on glucose control of the wall chart to make the discussion more interactive.
In the control group, a total of 34 teaching sessions were conducted. Group size varied between one and five patients. Initially, diabetes educators felt uncomfortable with providing a sham intervention and appeared reluctant regarding the teaching contents for stress coping and the relaxation exercise. The research team discussed these issues with the diabetes educators and deliberated strategies to deal with them.
Blinding of patients was assessed among the last 100 patients (the first 43 patients were inadvertently not asked); 21 of 50 patients in the ISDM-P thought that they received the intervention, and 16 of 50 patients of the control group (Cohen's κ coefficient k=0.10).
According to the protocol, patients who opt for a change in therapy should meet a physician after the teaching session. A total of 21 patients in the ISDM-P and 22 control patients had a consultation with a physician at the diabetes outpatient clinic.
Deviation from the study protocol
When registering the study, we erroneously did not state a previous diagnosis of stroke as an exclusion criterion, although in the decision aid the target group explicitly excludes persons with a history of stroke. Therefore, three randomised patients (2 intervention, 1 control group) with a history of stroke were excluded before the teaching session, but were included in the intention-to-treat sensitivity analysis with imputation of missing values (figure 1). In addition, lower age limit was changed from 45 years at study registration to 40 years with the intention to accelerate patient enrolment.14 However, most patients were older than 50 years.
In the study protocol, we accidentally defined sufficient comprehension as 8 of 12 correctly answered questions.14 However, we originally intended and actually set a 75% cut-off which corresponds to ≥9 correctly answered questions. Owing to a mistake of the organising study centre at Hamburg University, only the last 100 patients were asked to rate their group allocation.
Discussion
Statement of principal findings
About half of the patients who attended the ISDM-P, but no patient in the control group, demonstrated sufficient comprehension of risk information. The latter is the necessary prerequisite for informed decision-making.32 Priorities and actual preventive decisions of ISDM patients were more in line with scientific evidence than those of control group patients. As a result, most ISDM patients laid emphasis on blood pressure control rather than intensive blood glucose control. HbA1c levels and blood pressure values were already adjusted at low levels with no differences between groups. However, more patients in the ISDM group achieved their HbA1c goals, since they had set slightly higher HbA1c goals after the teaching. While in the ISDM group more patients wished to continue or to start statin treatment, in the control group more patients wished to stop it. Self-reported statin intake did not differ. It remains unclear if the patients really adhered to their statin medication. Nonetheless, the study results indicate that the ISDM-P not only effects comprehension and goal setting, but may also influence behaviour, such as treatment adherence. The ISDM-P was successfully executed by diabetes educators.
Strengths and weaknesses of the study
This study has several strengths. The study intervention has been meticulously developed following the framework for the development and evaluation of complex interventions proposed by the UK MRC.16 This randomised controlled trial was rigorously designed and conducted under high fidelity conditions. Patients were blinded against the allocation by using a sham intervention in the control arm. Alongside to the trial, qualitative methods were utilised to monitor intervention fidelity and to evaluate possible barriers to the implementation. Teaching programmes for people with type 2 diabetes used to focus on blood glucose control and usually do not provide numerical and comparative risk information.15 34–36 Our programme is innovative. It includes risk information and is explicitly designed to initiate SDM as recommended in recent guidelines for the treatment of type 2 diabetes.8 37
Practicability of the ISDM-P has been proven. It may help to overcome time constraint of physicians.
There were also weaknesses. It was impossible to keep the diabetes educators and other members of the healthcare teams blinded. There is no information on the primary end point at baseline. An administration of the questionnaire at baseline, however, would probably have produced learning effects and thus induced bias. The study was designed as proof-of-concept, which might limit generalisability. There were minor deviations from study protocol.
Strengths and weaknesses in relation to other studies, discussing important differences in results
Our findings are in accordance with results of a recent systematic review showing that decision aids can enhance patient involvement in various health and treatment decisions.38 However, sharing decisions between patients and physicians remains uninformed unless evidence-based information is provided and understanding of information is assured.32 39 40 Most decision aids are used as isolated tools to prepare patients for their consultations with the physician or for personal reading after the medical encounter.41 Even if a decision aid includes evidence-based risk information, patients may not be able to comprehend the information without educational support. Without sufficient understanding, decision making is not informed.32
Only very few randomised controlled trials on decision aids and SDM in the context of type 2 diabetes are available. Montori's research group has evaluated a ‘statin choice decision aid’ that focuses on cardiovascular prevention in type 2 diabetes,42 and a ‘diabetes medication choice decision aid’. The latter decision aid provides patient information on antihyperglycaemic drugs, including effects on HbA1c, weight change, risk of hypoglycaemia, the need for blood sugar testing, daily routine and side effects.43 Numerical risk information on cardiovascular outcomes is not provided. Both decision aids are designed to be used by physicians or nurses during consultation with the patient. Both can improve knowledge and reduce decisional conflict.42 43 Indicators of behaviour change, such as self-reported medication adherence, were ambiguous. The ‘statin choice decision aid’ was less successful in primary care than at a specialised centre, when used by endocrinologists.44 In a project called ‘the Patients ANd Decision Aids (PANDAs)’, people with type 2 diabetes received a decision aid on starting insulin or were treated as usual.45 The healthcare team provided the decision aid in the waiting room directly before consultation. Knowledge and realistic expectations improved in the intervention group. Behaviour change was not assessed. Denig et al13 evaluated different formats of a decision aid for people with diabetes within a randomised controlled trial. The primary outcome, patient empowerment in goal setting and achievement did not differ between intervention and control group. Consultations were not objectively evaluated, but the analysis of self-reported questionnaires indicated that the decision aids might not have been appropriately used.
Our ISDM-P was implemented under high fidelity conditions. The provided information was relevant and evidence based; the educators were trained in basic competencies of evidence-based medicine and risk communication. We implemented measures to assure that patients understood the information before initiating the decision-making process.
Up to now there has been no decision aid comprehensively addressing prevention of myocardial infarction in type 2 diabetes by providing patient tailored numerical risk information on clinical outcomes. Discussing all options during patient consultation is impractical and time consuming. We, therefore, developed a group teaching programme to be provided before the consultation by diabetes educators or specialised nurses. Since structured patient education is an essential part of diabetes care, the ISDM-P can be easily implemented.
Implications for clinicians and policymakers
Without sufficient education, risk literacy is still low among patients, diabetes educators and even physicians. Patients and healthcare providers need access to understandable high-quality evidence-based information and education. The ISDM-P can implement these requirements. Its components can also be used to adapt already implemented educational programmes.
Unanswered questions and future research
In a number of studies, the success of SDM interventions is measured by using the construct of ‘informed choice’ as a multidimensional outcome measure.32 Risk knowledge is core part of this construct as it is required to make informed decisions. In our study, no patient in the control group demonstrated sufficient risk knowledge and understanding. Thus, there was no informed decision-making in the control group.
Physicians in our study were informed about aims, contents and structure of the ISDM-P, but did not receive special training in SDM. Thus, the ISDM-P may not realign power imbalance between patients and their physicians.46 Core concept of the ISDM-P was that diabetes educators would act as patient coaches. They were supposed to organise a consultation with the physician in case patients want to adjust treatment goals that require changes of drug therapy. However, self-reported statin intake remained unchanged although a relevant proportion of patients in the control group expressed a wish to not continue statin treatment. We have not analysed patient–physician encounters in the present study. We could not validate self-reported adherence to statin treatment.
Based on these findings, we developed two additional components for the ISDM-P: (1) a single page documentation sheet for treatment goals, and (2) a structured SDM training module for physicians. Both are aimed at optimising the consultation in terms of SDM. First, patients document their treatment goals and thereafter, patients and physicians deliberate on these goals. In case the physician deviates from the patient's defined goals, the reasons are documented on the sheet. The patient keeps the original sheet and a copy is stored in the patient's record. We are evaluating the extended ISDM-P within a cluster randomised, controlled trial in the setting of family practices. We are assessing if patients are more adherent to medication when this is prescribed based on informed SDM.29
Footnotes
Contributors: All authors meet the ICMJE criteria for authorship. SB, ML, JK and IM designed the study. TH, NK and UAM are involved in the coordination and management of data acquisition at the study site (Jena University Hospital). SB, ML, JK and IM designed and tested the questionnaire. TL did the statistical planning and analyses of the study. SB and ML wrote the manuscript. IM substantially contributed to the draft of the manuscript. ML, JK, UAM, SB and IM applied for funding. All authors critically revised the manuscript and approved the final version. All authors had full access to data. SB is the guarantor.
Funding: The study was funded by the European Foundation for the Study of Diabetes (EFSD) on behalf of the European Association for the Study of Diabetes (EASD).
Competing interests: None declared.
Ethics approval: The study protocol was approved by the ethics committee of the Jena University Hospital (ref: 3225-08/11). All participants gave informed consent before taking part.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Gerstein HC, Miller ME, Byington RP et al. . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schedlbauer A, Davies P, Fahey T. Interventions to improve adherence to lipid lowering medication. Cochrane Database Syst Rev 2010;(3):CD004371 10.1002/14651858.CD004371.pub3 [DOI] [PubMed] [Google Scholar]
- 3.Nieuwlaat R, Wilczynski N, Navarro T et al. . Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014;11:CD000011 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998;317:703–13. 10.1136/bmj.317.7160.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa J, Borges M, David C et al. . Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials. BMJ 2006;332:1115–24. 10.1136/bmj.38793.468449.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheridan SL, Draeger LB, Pignone MP et al. . A randomized trial of an intervention to improve use and adherence to effective coronary heart disease prevention strategies. BMC Health Serv Res 2011;11:331 10.1186/1472-6963-11-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inzucchi SE, Bergenstal RM, Buse JB et al. . Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–96. 10.1007/s00125-012-2534-0 [DOI] [PubMed] [Google Scholar]
- 8.German Medical Association, National Association of Statutory Health Insurance Physicians, Association of the Scientific Medical Societies. National disease management guidelines programme: typ-2-diabetes mellitus—therapy 2013. http://www.leitlinien.de/nvl/diabetes/therapie (accessed 27 May 2015).
- 9.Lenz M, Kasper J, Mühlhauser I. Searching for diabetes decision aids and related background information. Diabet Med 2006;23:912–16. 10.1111/j.1464-5491.2006.01917.x [DOI] [PubMed] [Google Scholar]
- 10.Lenz M, Kasper J, Mühlhauser I. Development of a patient decision aid for prevention of myocardial infarction in type 2 diabetes—rationale, design and pilot testing. Psychosoc Med 2009;6:Doc05 10.3205/psm000061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montori VM, Breslin M, Maleska M et al. . Creating a conversation: insights from the development of a decision aid. PLoS Med 2007;4:e233 10.1371/journal.pmed.0040233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health Dialog. Living with diabetes. Making lifestyle changes to last a lifetime. Boston: Foundation for Informed Medical Decision Making, 2007. [Google Scholar]
- 13.Denig P, Schuling J, Haaijer-Ruskamp F et al. . Effects of a patient oriented decision aid for prioritising treatment goals in diabetes: pragmatic randomised controlled trial. BMJ 2014;349:g5651 10.1136/bmj.g5651 [DOI] [PubMed] [Google Scholar]
- 14.Buhse S, Heller T, Kasper J et al. . An evidence-based shared decision making programme on the prevention of myocardial infarction in type 2 diabetes: protocol of a randomised-controlled trial. BMC Fam Pract 2013;14:155 10.1186/1471-2296-14-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kronsbein P, Jörgens V, Mühlhauser I et al. . Evaluation of a structured treatment and teaching programme on non-insulin-dependent diabetes. Lancet 1988;2:1407–11. 10.1016/S0140-6736(88)90595-8 [DOI] [PubMed] [Google Scholar]
- 16.Craig P, Dieppe P, Macintyre S et al. . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenz M, Mühlhauser I [Cardiovascular risk assessment for informed decision making. Validity of prediction tools]. Med Klin (Munich) 2004;99:651–61. 10.1007/s00063-004-1097-3 [DOI] [PubMed] [Google Scholar]
- 18.Lenz M, Mühlhauser I. Decision aids in diabetes. In: Edwards A, Elwyn G, eds. Shared decision making in health care: achieving evidence based patient choice. 2nd edn Oxford: Oxford University Press, 2009;285–95. [Google Scholar]
- 19.Busse R. Disease management programs in Germany's statutory health insurance system. Health Aff 2004;23:56–67. 10.1377/hlthaff.23.3.56 [DOI] [PubMed] [Google Scholar]
- 20.German Medical Association, National Association of Statutory Health Insurance Physicians, Association of the Scientific Medical Societies. National guideline type 2 diabetes. Structured educational programmes—long version 2012. http://www.leitlinien.de/nvl/diabetes/schulungsprogramme (accessed 27 May 2015).
- 21.National Kidney Foundation. KDOQI Clinical practice guideline for diabetes and chronic kidney disease: 2012 update. Am J Kidney Dis 2012;60:850–86. 10.1053/j.ajkd.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 22.Lenz M, Steckelberg A, Mühlhauser I. Patient education programmes and decision aids—evaluation of complex interventions. Av Diabetol 2008;24:443–52. [Google Scholar]
- 23.Mühlhauser I, Berger M. Patient education—evaluation of a complex intervention. Diabetologia 2002;45:1723–33. 10.1007/s00125-002-0987-2 [DOI] [PubMed] [Google Scholar]
- 24.Ajzen I. The theory of planned behaviour. Org Behav Hum Decis Process 1991;50:179–211. 10.1016/0749-5978(91)90020-T [DOI] [Google Scholar]
- 25.Thompson-Leduc P, Clayman ML, Turcotte S et al. . Shared decision-making behaviours in health professionals: a systematic review of studies based on the Theory of Planned Behaviour. Health Expect 2015;18:754–74. 10.1111/hex.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes study (UKPDS) Group. Lancet 1998;352:837–53. 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 27.Vollmer-Rupprecht R. Stress 2013. http://www.tk.de/centaurus/servlet/contentblob/48660/Datei/63352/TK-Broschuere-Der-Stress.pdf (accessed 27 May 2015).
- 28.Anderson LW, Krathwohl DR. A taxonomy for learning, teaching, and assessing: a revision of bloom's taxonomy of educational objectives. New York: Longman, 2001. [Google Scholar]
- 29.Buhse S, Mühlhauser I, Kuniss N et al. . An informed shared decision making programme on the prevention of myocardial infarction for patients with type 2 diabetes in primary care: protocol of a cluster randomised, controlled trial. BMC Fam Pract 2015;16:43 10.1186/s12875-015-0257-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Buuren S, Brand JPL, Groothuis-Oudshoorn CGM et al. . Fully conditional specification in multivariate imputation. J Stat Comput Simul 2006;76:1049–64. 10.1080/10629360600810434 [DOI] [Google Scholar]
- 31.Cokely ET, Galesic M, Schulz E et al. . Measuring risk literacy: the Berlin Numeracy Test. Judgm Decis Mak 2012;7:25–47. [Google Scholar]
- 32.Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expect 2001;4:99–108. 10.1046/j.1369-6513.2001.00140.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellg AJ, Borrelli B, Resnick B et al. . Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443–51. 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- 34.Kulzer B, Hermanns N, Reinecker H et al. . Effects of self-management training in type 2 diabetes: a randomized, prospective trial. Diabet Med 2007;24:415–23. 10.1111/j.1464-5491.2007.02089.x [DOI] [PubMed] [Google Scholar]
- 35.Hermanns N, Kulzer B, Maier B et al. . The effect of an education programme (MEDIAS 2 ICT) involving intensive insulin treatment for people with type 2 diabetes. Patient Educ Couns 2012;86:226–32. 10.1016/j.pec.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 36.Deakin TA, McShane CE, Cade JE et al. . Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;(2):CD003417 10.1002/14651858.CD003417.pub2 [DOI] [PubMed] [Google Scholar]
- 37.Inzucchi SE, Bergenstal RM, Buse JB et al. . Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–9. 10.2337/dc14-2441 [DOI] [PubMed] [Google Scholar]
- 38.Stacey D, Légaré F, Col NF et al. . Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014;1:CD001431 10.1002/14651858.CD001431.pub4 [DOI] [PubMed] [Google Scholar]
- 39.Edwards A, Elwyn G. Shared decision making in health care: achieving evidence based patient choice. 2nd edn Oxford: Oxford University Press, 2009. [Google Scholar]
- 40.Bunge M, Mühlhauser I, Steckelberg A. What constitutes evidence-based patient information? Overview of discussed criteria. Patient Educ Couns 2010;78:316–28. 10.1016/j.pec.2009.10.029 [DOI] [PubMed] [Google Scholar]
- 41.Elwyn G, Frosch D, Volandes AE et al. . Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Making 2010;30:701–11. 10.1177/0272989X10386231 [DOI] [PubMed] [Google Scholar]
- 42.Weymiller AJ, Montori VM, Jones LA et al. . Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch Intern Med 2007;167:1076–82. 10.1001/archinte.167.10.1076 [DOI] [PubMed] [Google Scholar]
- 43.Mullan RJ, Montori VM, Shah ND et al. . The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med 2009;169:1560–8. 10.1001/archinternmed.2009.293 [DOI] [PubMed] [Google Scholar]
- 44.Mann DM, Ponieman D, Montori VM et al. . The Statin Choice decision aid in primary care: a randomized trial. Patient Educ Couns 2010;80:138–40. 10.1016/j.pec.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 45.Mathers N, Ng CJ, Campbell MJ et al. . Clinical effectiveness of a patient decision aid to improve decision quality and glycaemic control in people with diabetes making treatment choices: a cluster randomised controlled trial (PANDAs) in general practice. BMJ Open 2012;2:pii: e001469 10.1136/bmjopen-2012-001469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns 2014;94:291–309. 10.1016/j.pec.2013.10.031 [DOI] [PubMed] [Google Scholar]
- 47.Dulon M, Bardehle D, Blettner M. [Assessing social inequality in microcensus data and German national health examination survey]. Gesundheitswesen 2003;65:629–35. [DOI] [PubMed] [Google Scholar]