Abstract
Purpose
Photoreceptor genesis in the retina requires precise regulation of progenitor cell competence, cell cycle exit, and differentiation, although information around the mechanisms that govern these events currently is lacking. In zebrafish, the basic helix-loop-helix (bHLH) transcription factor NeuroD governs photoreceptor genesis, but the signaling pathways through which NeuroD functions are unknown. The purpose of this study was to identify these pathways, and during photoreceptor genesis, Notch signaling was investigated as the putative mediator of NeuroD function.
Methods
In embryos, genetic mosaic analysis was used to determine if NeuroD functions is cell- or non–cell-autonomous. Morpholino-induced NeuroD knockdown, CRISPR/Cas9 mutation, and pharmacologic and transgenic approaches were used, followed by in situ hybridization, immunocytochemistry, and quantitative RT-PCR (qRT-PCR), to identify mechanisms through which NeuroD functions. In adults, following photoreceptor ablation and NeuroD knockdown, similar methods as above were used to identify NeuroD function during photoreceptor regeneration.
Results
In embryos, NeuroD function is non–cell-autonomous, NeuroD knockdown increases Notch pathway gene expression, Notch inhibition rescues the NeuroD knockdown-induced deficiency in cell cycle exit but not photoreceptor maturation, and Notch activation and CRISPR/Cas9 mutation of neurod recapitulate NeuroD knockdown. In adults, NeuroD knockdown prevents cell cycle exit and photoreceptor regeneration and increases Notch pathway gene expression, and Notch inhibition rescues this phenotype.
Conclusions
These data demonstrate that during embryonic development, NeuroD governs photoreceptor genesis via non–cell-autonomous mechanisms and that, during photoreceptor development and regeneration, Notch signaling is a mechanistic link between NeuroD and cell cycle exit. In contrast, during embryonic development, NeuroD governs photoreceptor maturation via mechanisms that are independent of Notch signaling.
Keywords: photoreceptor development, photoreceptor regeneration, rods, cones, cell cycle
The vertebrate retina is composed of seven cell types, generated in a conserved sequence from a pool of multipotent, mitotic progenitors.1 Several basic helix-loop-helix (bHLH) transcription factors have prominent roles in the retina to regulate cell fate specification,2–5 cell cycle exit, and neuronal differentiation and maturation.6,7 NeuroD is a proneural bHLH transcription factor identified in vivo as a neuronal determination factor,8 and in vitro as a link between cell cycle exit and neuronal differentiation.9 In the mouse retina, NeuroD is required for survival of rods10 and regulates opsin selection among cones.11 In the chick, NeuroD is required for photoreceptor development and survival.12 In medaka fish, NeuroD and Six6 coregulate each other to control amacrine cell and photoreceptor genesis.6 In embryonic zebrafish, neurod is expressed in mitotic photoreceptor progenitors,13,14 and this expression is regulated by the zinc finger protein, Insm1a.15 Within the photoreceptor lineages NeuroD governs cell cycle exit and photoreceptor maturation.7
The Notch pathway mediates cell-to-cell communication through receptor-ligand interactions. Notch receptors are expressed on the cell surface and interact with membrane-bound ligands (e.g., Delta, Jagged), regulating transcription in apposing cells.16,17 In vertebrate retinal development, Notch signaling regulates the balance between neurogenesis and gliogenesis,18–20 maintains progenitors in an undifferentiated, proliferative state,19,21 specifies cell fates, and governs the onset of neurogenesis.22 These events can be regulated in the retina through transcriptional control of Notch signaling molecules. For example, in the chick and mouse, the bHLH transcription factor Ascl1a governs cell cycle exit and differentiation through regulation of the Notch ligand dll,23,24 and in zebrafish the HLH-binding cofactor Id2a regulates the transition from proliferation to differentiation through regulation of Notch ligands and receptors.21
Following retinal damage in zebrafish, Müller glia function as intrinsic stem cells that dedifferentiate, reenter the cell cycle, and produce neurogenic progenitors that regenerate lost neurons.25–33 Recent studies in zebrafish have identified mechanisms that govern the Müller glia response to retinal injury,34–37 but information is lacking around the pathways that govern photoreceptor regeneration from Müller glia–derived progenitor cells. The mechanisms that govern retinal regeneration are expected to largely recapitulate embryonic development,28,29,38 though few molecules have been functionally studied during both events. NeuroD function has not been studied in the adult zebrafish retina, but following photoreceptor ablation neurod is expressed in Müller glia–derived mitotic progenitors,39 suggesting that NeuroD has a role in photoreceptor regeneration.
The goal of the current study is to identify the mechanisms that govern photoreceptor genesis from the pool of multipotent progenitors in the embryo and from stem cell-derived progenitors in the adult, by elucidating the pathways through which NeuroD functions during photoreceptor development and regeneration, respectively. In embryos, reciprocal transplant chimeric analysis shows that for cell cycle exit and photoreceptor maturation, NeuroD function is non–cell-autonomous. Knockdown of NeuroD and CRISPR/Cas9 targeted mutation of neurod prevent cell cycle exit and photoreceptor maturation, and increase expression of Notch pathway molecules. Inhibition of Notch signaling rescues deficiencies in cell cycle exit but not photoreceptor maturation. In adults, NeuroD knockdown prevents cell cycle exit among injury-induced progenitors and photoreceptor regeneration, and this, too, is rescued by Notch inhibition. These data demonstrated a conserved function for NeuroD during photoreceptor genesis and regeneration, and identified Notch signaling as a molecular mechanism that links these events.
Methods
These studies adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the University of Michigan Institutional Animal Care and Use Committee.
NeuroD KnockDown in Embryos
We used AB strain zebrafish (Danio rerio) for developmental experiments and they were purchased from the Zebrafish International Research Center (ZIRC; University of Oregon, Portland, OR, USA). Embryos were collected within 15 minutes of spawning and incubated at 28.5°C on a 14/10-hour light/dark cycle. Morpholino oligonucleotides (MO; Gene Tools, LLC, Philomath, OR, USA) were used to induce NeuroD knockdown. The neurod MO (ATG 5′-TGACTTCGTCATGTCGGAACTCTAG-3′) and neurod MM control MO (5′-TGAGTTGGTCATCTCGCAACTGTAG-3′) have been described previously.7 Morpholino oligonucleotides were diluted in 1 × Danieau buffer40 and 5 ng MOs were injected at the 1 cell stage.
Systemic Labeling With 5-Bromo-2′-Deoxyuridine (BrdU) or 5-Ethynyl-2′-Deoxyuridine (EdU)
Cells in S-phase of the cell cycle were labeled with either BrdU or EdU. Embryos were incubated for 20 minutes in ice-cold (48 hours post fertilization [hpf]) or room-temperature (70 hpf) 10 mM BrdU or 1.5 mM EdU, dissolved in embryo rearing solution containing 15% dimethylsulfoxide (DMSO). For BrdU staining, sections were incubated in 100°C sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) for 30 minutes to denature DNA and cooled at room temperature for 20 minutes. Sections then were subjected to standard immunolabeling as described below. Ethinyl deoxyuridine was visualized using the Click-it EdU kit (Invitrogen, Carlsbad, CA, USA).
Chimeric Analysis
Chimeric mosaic analysis was performed at the shield stage as previously described.41–43 Reciprocal transplants were performed between wild-type (WT) and NeuroD knockdown embryos and larvae were incubated in EdU as described above. To label donor cells, embryos were injected with 5% tetramethyl rhodamine-dextran (D-1816, 10,000 MW; Life Technologies, Carlsbad, CA, USA) at the 1- to 2-cell stage. To evaluate the differentiation of rod photoreceptors, donor cells were taken from Tg(XOPS:GFP) embryos, in which green fluorescent protein (GFP) is driven by promoter elements of Xenopus rhodopsin.44 To evaluate cone differentiation, retinal sections were immunostained for markers of cone photoreceptors as described below.
Mosaic analysis data were collected from larvae at 72 to 75 hpf. Retinas containing a narrow radial clone of donor-derived cells were selected for analysis. Confocal images were collected using a Leica SP5 confocal microscope (Leica, Wetzlar, Germany), and data were taken from 5-μm thick Z-stacks through clones of donor-derived cells. Within each clone, the number of dextran-labeled cells within the outer nuclear layer was counted and scored for the presence of EdU, GFP, or the cone cell marker, Zpr-1. Five clones of donor cells were evaluated per treatment group.
CRISPR/Cas9 Targeted Mutation of neurod
The sgRNA target sequence for neurod (GGACGACGAGGAAGAAGAAGAGG) was identified using ZiFiT software (available in the public domain at zifit.partners.org). Cas9 mRNA and sgRNA were generated according to previously described methods.45 Single cell-stage embryos were injected with 1 nL solution containing 100 pg/nL sgRNA and 150 pg/nL Cas9 mRNA. Embryos were collected for histology and genetic sequencing at 70 hpf, following incubation in EdU as described above. Embryo heads were collected and fixed in 4% buffered paraformaldehyde for histology. For each individual embryo, the hindbrain and tails were digested at 55°C for 3 hours in PCR extraction buffer containing 10 mM Tris-HCl (pH8), 2 mM EDTA, 0.2% Triton X-100, and 200 μg/mL Proteinase K.46 Samples then were heated to 95°C, centrifuged, and stored at −20°C.
T7 Assay for Detection of CRISPR-Induced Insertions or Deletions
The T7 endonuclease assay was conducted according to established protocols (available in the public domain at www.crisprflydesign.org). Briefly, screening primers (F: 5′-GCGCTCTAGTGCATTTCATC-3′, R: 5′-TAGTTCTTGGCTAGTCGGAG) were designed to amplify a 530 bp region around the neurod sgRNA target site. We performed PCR using standard protocols for Platinum Taq-HF polymerase (Invitrogen), using 10 μL PCR extraction buffer (above) as the DNA template. Purified DNA product (200 ng) was used for the analysis, and 10 U T7 endonuclease I (New England Biolabs, Ipswich, MA, USA) was used for the digest. The presence of CRISPR-induced insertions or deletions (indels) was indicated by a double band approximately 250 to 300 bp. For embryos that were positive for indels, the PCR product was subcloned into the pGEM-T Easy vector (Promega, Madison, WI, USA), and 6 to 9 clones were sequenced for each embryo. Mutations were detected using a pairwise blast (National Center for Biotechnology Information [NCBI], Bethesda, MD, USA) against WT DNA and amino acid sequences determined using the ExPASy translate tool (available in the public domain at www.expasy.org).
Inhibition of Notch Signaling With DAPT
The Notch pathway was inhibited with the γ-secretase inhibitor DAPT (N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenylglycine t-Butyl Ester, 565784; Calbiochem, EMD Millipore, Billerica, MA, USA), which inhibits Notch cleavage and activation of the intracellular domain.47 DAPT was diluted in embryo (E3) media for a working concentration of 50 μM (in 0.2% DMSO).21 Embryos were treated from 48 to 70 hpf and exposed to systemic BrdU (described above) for 20 minutes before being killed. Control embryos were treated in E3 with a matched concentration of DMSO only.
To quantify BrdU- and/or EdU-labeled cells, for each 10-μm-thick section, a confocal z-stack was collected through the entire section using a Leica SP5 confocal microscope. Each section contained optic nerve to standardize the retinal location analyzed in each embryo. Cells were quantified in the entire z-stack using the Spots function of Leica Imaris Software with standardized parameters for sensitivity and cell size. Following DAPT treatment in embryos, BrdU-positive cells were counted in one section from one eye of each embryo (n = 8 embryos per treatment). Statistical significance was determined using a Student's t-test.
Transgenic Lines and Conditional Activation of the Notch Pathway
The tol2 pT2KhspGGFF vector (generous gift from Koichi Kawakami, National Institute of Genetics, Mishima, Shizuoka Japan) containing a GFP-Gal4-FF fusion protein (GGFF) downstream of the hsp70 promoter48 was injected into WT zebrafish embryos at the 1-cell stage (∼25–50 pg) along with tol2 transposon mRNA (∼25–50 pg). F0 embryos were screened for GFP expression in the lens at 72 hpf, raised, and outcrossed to WT adults, and F1 embryos were screened in the same way. Hemizygous F1 Tg(hsp70:GGFF) adults then were crossed with hemizygous Tg(UAS:myc-notch1a:intra).49 Embryos were heat-shocked in a 38°C water bath for 1 hour at 52 to 53 hpf, and screened for GFP expression at 69 hpf. GFP-positive embryos were selected for analysis, indicating that they carried the GGFF transgene. Of these, 50% carried Tg(hsp70:GGFF) alone and were used as controls, and 50% carried both transgenes, confirmed post hoc by immunolabeling with anti–c-Myc. Embryos were incubated in 1.5 mM EdU for 20 minutes (described above) immediately before being killed at 70 hpf. For quantification of EdU+ cells in the ONL, confocal z-stacks were collected through one entire section of a single eye per embryo (n = 8 Myc+, n = 5 Myc− [WT]). Only sections passing through the optic nerve were used as a means to standardize the retinal location analyzed. EdU+ cells within the ONL were quantified as described above.
Histology, Immunohistochemistry, and In Situ Hybridization
For histology, tissues were fixed overnight at 4°C in phosphate buffered 4% paraformaldehyde, infiltrated with 20% sucrose, then frozen in optical cutting temperature (OCT) medium. Immunostaining and in situ hybridization were performed on 10-μm-thick cross-sections through the central retina in the vicinity of the optic nerve. Immunostaining was performed using previously published protocols.50 The primary and secondary antibodies and dilutions used here were: mouse anti-BrdU 1:100 (347580; BD Biosciences, Franklin Lakes, NJ, USA), mouse anti-PCNA 1:100 (8825; Sigma-Aldrich Corp., St. Louis, MO, USA), Zpr-1 1:200 (anti-Arrestin 3, red-green double cones, ZIRC), Zpr-3 1:200 (anti-Rhodopsin, rods, ZIRC), anti–c-Myc 1:200 (9E10; ThermoFisher Scientific, Halethorp, MD, USA), rabbit anti-GFP (ab290; Abcam, Cambridge, UK), and goat anti-mouse Alexa Fluor 488 and 555 1:500 (Life Technologies). Following immunohistochemistry, when applicable, a TUNEL labeling In situ Cell Death Detection Kit (Roche Diagnostic Corp., Indianapolis, IN, USA) was used to compare cell death between treatment groups.
In situ hybridizations were performed using previously published protocols.13,51 Riboprobes for ascl1a, notch1a, her4, deltaA, and deltaD were generated from PCR products,52 and riboprobes for rhodopsin, pde6c, neurod, crx, nr2e3, and pax6a were generated from full-length cDNA clones. In vitro transcription was performed using the appropriate polymerase and DIG or fluorescein labeling kit (Roche Diagnostic Corp.). Double in situ hybridization for notch1a and dla was performed as described previously.13 For comparisons of relative expression, tissue sections from control and experimental groups were placed on the same slide and developed for identical periods of time.
Reverse Transcriptase Quantitative Real-Time PCR (qRT-PCR)
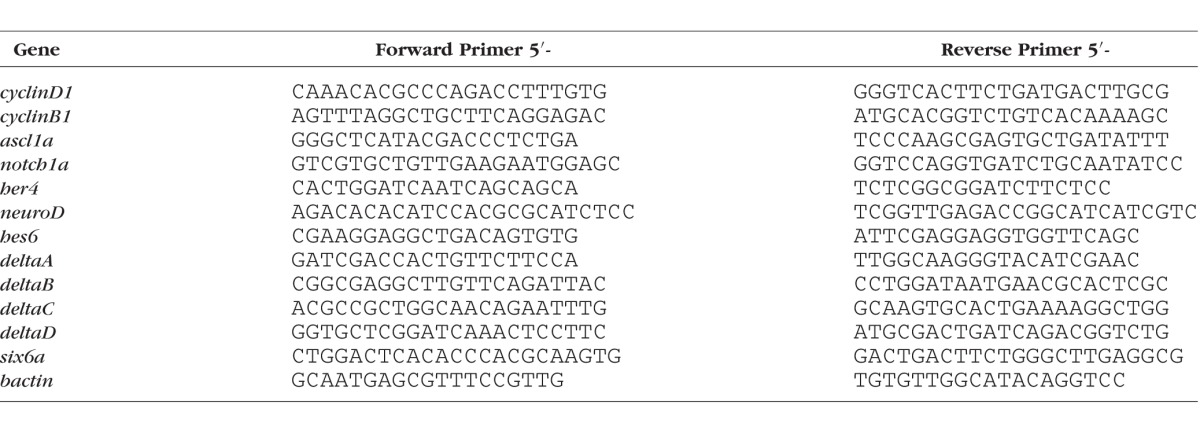
Total RNA was extracted from embryo forebrains and eyes (heads) using the Aurum Total RNA Mini Kit and following the manufacturer's protocol (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Three to five biological replicates were collected for each treatment and each time point (30–50 heads per replicate). Reverse transcription was performed using the Qiagen QuantiTect Reverse Transcription kit according to the manufacturer's instructions (Qiagen, Venlo, The Netherlands). Each biological replicate was run in triplicate using 6 ng cDNA and IQ SYBR Green Supermix (Bio-Rad Laboratories, Inc.) and a Bio-Rad 384 Well Real Time PCR machine. Primer sets are listed in Table 1. Expression was calculated from ΔCT values and normalized to bactin expression using Bio-Rad CFX Manager Software. Fold changes were calculated by dividing normalized expression by control values. Statistical significance was determined using a Student's t-test.
Table 1.
Primer Sets Used for qRT-PCR
Photoreceptor Ablation in Adults
The Tg(neurod:egfp) zebrafish line53 was used for regeneration experiments and was previously shown to be an accurate reporter for endogenous neurod expression.39 Adult fish were housed in recirculating systems at 28.5°C on a 14/10-hour light/dark cycle. To ablate retinal photoreceptors, fish were exposed to bright light as described previously.54–57 For the NeuroD knockdown experiments in which proliferating progenitors and regenerated photoreceptors were counted (see below), albino fish were used to consistently ablate all photoreceptors in the dorsal retina.57 For experiments in which colocalization of EGFP and other molecules were analyzed using immunocytochemistry and in situ hybridization, complete ablation of photoreceptors was not required and, therefore, fully pigmented fish were used. The regeneration response is identical in albino and pigmented fish (Taylor SM, Hitchcock PF, unpublished data, 2014). Before bright light exposure, albino fish were housed in complete darkness for 10 days and pigmented fish for 24 hours. Immediately after dark adaptation, fish were exposed to high intensity light (ca. 100,000 lux) from a mercury arc lamp for 30 minutes and then to constant light (ca. 20,000 lux) for 72 hours.56,57 Finally, to analyze expression of molecules in Müller glia, the Tg(gfap:egfp)58 fish line and EGFP immunocytochemistry were used to identify Müller glia cell bodies and processes. For all experiments, days post lesion (dpl) indicates the number of days following the onset of light exposure.
Adult Morpholino Electroporation
For adult experiments, neurod or standard control MO (5′- CTCTTACCTCAGTTACAATTTATA-3′) were purchased from Gene Tools, LLC with a 3′ lissamine (sulforhodamine B) tag. Lissamine imparts a positive charge on the morpholino to facilitate electroporation into retinal cells59 and provides a fluorescent label to evaluate the effectiveness of the electroporation. Morpholino oligonucleotides were reconstituted to a concentration of 3 mM and 0.5 μL MO solution was injected into the vitreous chamber of the left eye of each fish. Morpholino oligonucleotide injections were performed at 2 dpl, just before the onset of neurod expression in dividing progenitors.39 Immediately after MO injection, eyes were electroporated across the dorsal-ventral axis following previously described protocols,60,61 Briefly, electroporations each consisted of two pulses at 75 V for 50 ms and 1 second between pulses. To evaluate electroporation effectiveness, 10-μm-thick cross-sections were cut through the central retina from each eye, slides were rinsed 3 × 5 minutes with PBS, counterstained with 4′,6-diamidino-2-phenylendole (DAPI), coverslipped and imaged immediately using epifluorescence to visualize lissamine distribution. Eyes with little or no fluorescent lissamine visible in retinal cells or that were damaged by the electroporation were discarded and not subjected to further analysis.
Western Blot Analysis
Protein samples were obtained by pooling five adult retinas in lysis buffer containing protease and phosphatase inhibitors (5872; Cell Signaling Technology, Danvers, MA, USA). Proteins were separated in a 12% SDS-PAGE gel and transferred to a nitrocellulose membrane (Sigma-Aldrich Corp.). The membrane was blocked in 5% nonfat dry milk in PBS with 0.05% Tween-20 for 2 hours and then incubated overnight at 4°C with rabbit anti-NeuroD antibody7 diluted 1:1000 in 2.5% blocking solution. Blots were rinsed with PBS+0.05% Tween-20 and incubated with horseradish peroxidase-conjugated secondary IgG (1:5000) for 1 hour. Bands were visualized using the enhanced chemiluminescence assay (ECL detection system; Amersham Biosciences, Arlington Heights, IL, USA). As loading controls, blots were incubated in stripping buffer (21059; ThermoFisher Scientific) for 5 minutes, then blocked and labeled as described above with anti-βactin (1:5000, Calbiochem). Images were captured using the FluorChem E Imaging System (Bio-Techne, Minneapolis, MN, USA).
Cell Cycle Analysis and Notch Inhibition in Adults
To label cells in S-phase of the cell cycle, at 3 dpl adult fish were injected intraperitoneally (IP) with 4 mM EdU to label proliferating cells. Animals then were returned to normal intensity and cyclical lighting, and were treated by swimming the fish for 24 hours in 5 mM BrdU before being killed at 6 or 7 dpl. To inhibit Notch signaling in adults, DAPT was diluted in aquarium water to 40 μM (in 0.16% DMSO)37,62 and fish were treated with DAPT or DMSO only from 4 to 6 dpl.
To analyze cell cycle exit, for each adult fish, BrdU, EdU, and BrdU/EdU colabeled cells were counted as described above and averaged from 3 nonadjacent sections from a single retina (n = 5–6 fish per treatment). To quantify regenerated photoreceptors in adults, 10-μm-thick sections were processed for in situ hybridization for rhodopsin (rods) or pde6c (cones) and labeled cells were counted manually. For both techniques in adult retinas, labeled cells were counted within a 500-μm length of retina centered between the dorsal margin and optic nerve. Statistical significance was determined using a Student's t-test.
Results
NeuroD Function Is Non–Cell-Autonomous
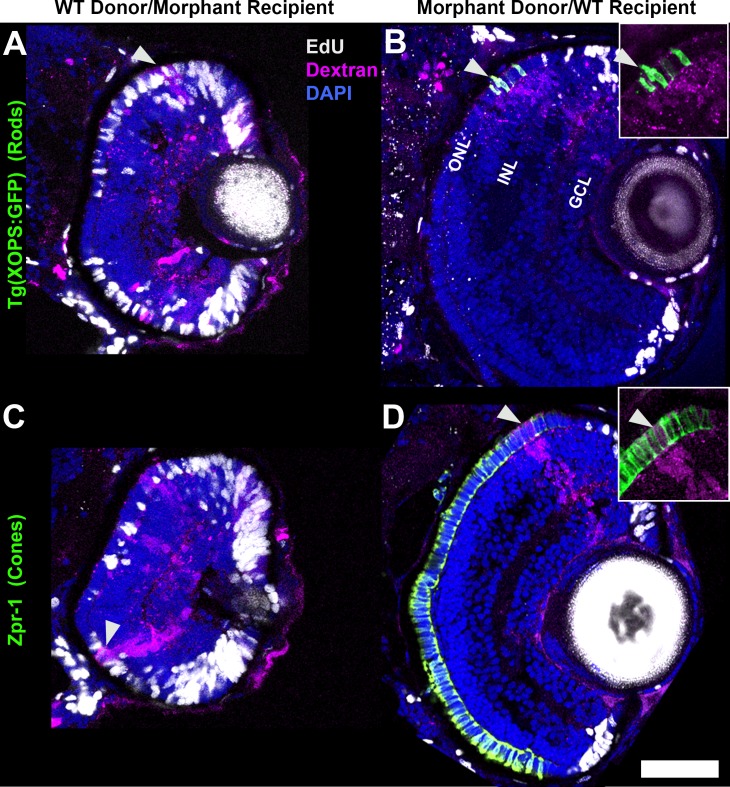
As a first test of the mechanisms by which NeuroD governs photoreceptor genesis and maturation, NeuroD was knocked down by injecting ATG-targeted MO and then cells were transplanted reciprocally between morphant and WT embryos. Dextran-labeled donor cells were analyzed for the presence or absence of EdU and markers of mature photoreceptors in the outer nuclear layer (ONL) of host retinas between 72 and 75 hpf, a time point at which photoreceptor genesis and maturation in the central retina are complete in the WT retina63 but are incomplete following NeuroD knockdown.7 To identify unambiguously mature rod photoreceptors derived from donor embryos, cells were transplanted from the Tg(XOPS:GFP) line, which expresses GFP under the control of the Xenopus rhodopsin promoter (Fadool 2003). To identify mature cone photoreceptors, the Zpr-1 antibody was used to label red and green double cones.64 As a control, WT cells were transplanted into WT hosts, which demonstrated that transplantation had no effect on cell cycle exit or photoreceptor maturation among donor-derived or host cells (Supplementary Fig. S1).
When WT cells were transplanted into NeuroD knockdown recipients, 15.3% of donor cells within the ONL were EdU-positive, and none of the donor cells expressed markers of mature rod or cone photoreceptors (Table 2; Figs. 1A, 1C, arrowheads). In the reciprocal experiment, when cells from NeuroD knockdown were transplanted into WT hosts, 0% of the donor cells contained EdU, and all donor cells within the ONL expressed markers of mature rod or cone photoreceptors (Table 2; Figs. 1B, 1D, arrowheads). These experiments demonstrated that cell cycle exit and maturation of transplanted cells are determined exclusively by the host retina, and, therefore, in cells of the photoreceptor lineages, NeuroD function is non–cell-autonomous. This indicates that NeuroD governs cell cycle exit and photoreceptor maturation via mechanisms mediated through cell–cell signaling.
Table 2.
Fate of Donor Cells in NeuroD Knockdown/WT Chimeras
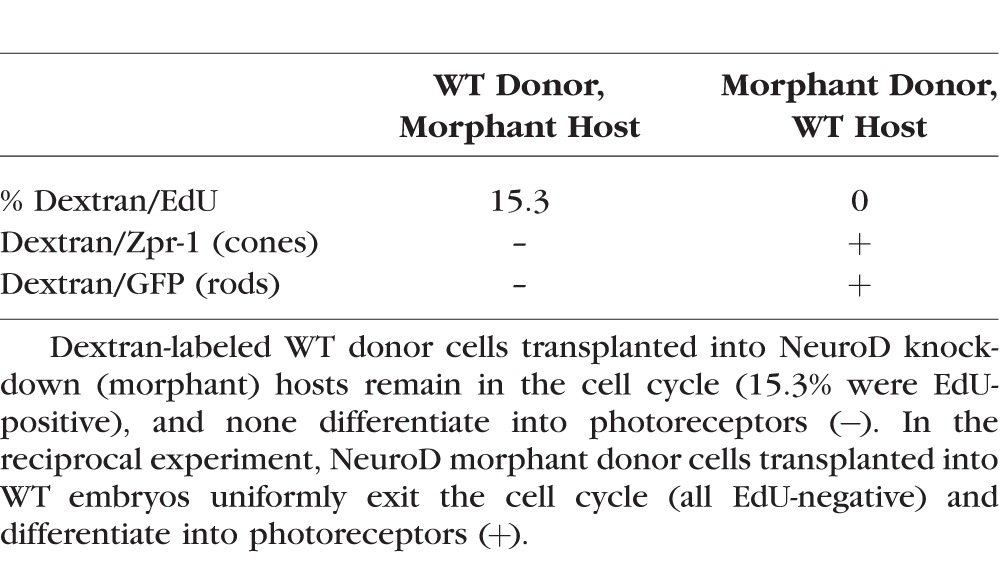
Figure 1.
NeuroD function is non–cell-autonomous. Reciprocal transplant chimeric analysis showing the fates of dextran-labeled donor cells on the ONL. (A) Wild-type donor cells in NeuroD knockdown hosts remain in the cell cycle (EdU-positive) and do not differentiate into rod photoreceptors (arrowhead; Tg[XOPS:GFP]-negative). (B) NeuroD knockdown Tg(XOPS:GFP) donor cells in WT hosts exit the cell cycle (EdU-negative) and differentiate into rods (arrowhead; Tg[XOPS:GFP]-positive). (C) Wild type donor cells in NeuroD knockdown hosts remain in the cell cycle (EdU-positive) and do not differentiate into cones (arrowhead; Zpr-1-negative). (D) NeuroD knockdown donor cells in WT hosts exit the cell cycle (EdU-negative) and differentiate into cones (arrowhead; Zpr-1-positive). GCL, ganglion cell layer. Scale bar: 50 μm.
NeuroD Regulates Expression of Notch Signaling Molecules and Targets of the Notch Pathway
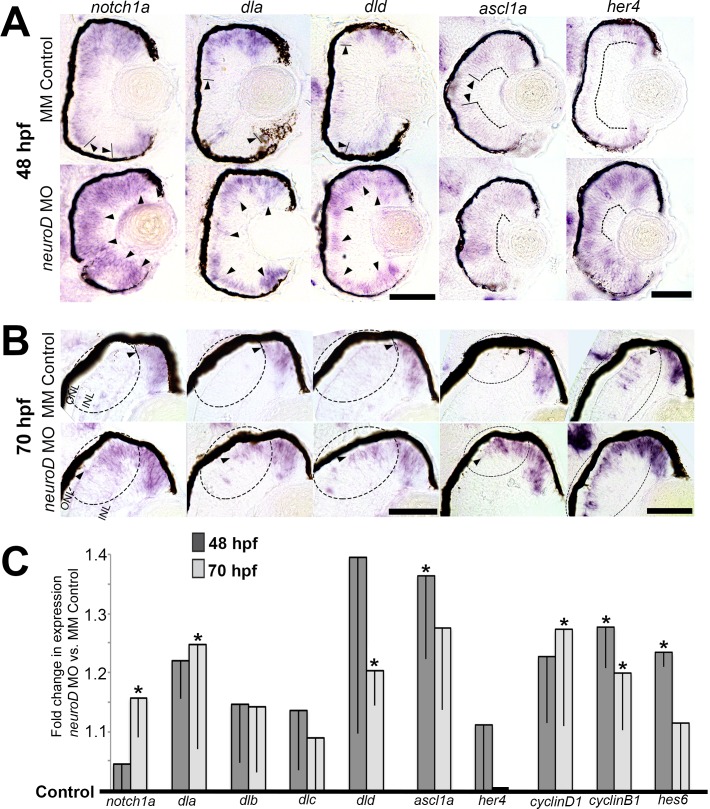
The data demonstrating that NeuroD function is non–cell-autonomous prompted us to investigate intercellular Notch signaling as a mediator of NeuroD function. Two time points were selected for analysis in the embryonic retina: 48 and 70 hpf. The 48-hpf time point allowed analysis of events leading up to the onset of photoreceptor genesis, which, outside the precocious ventral patch,65 begins at approximately 48 hpf. The 70-hpf time point allowed analysis of events occurring between 48 and 70 hpf, corresponding to the onset and completion of photoreceptor genesis and maturation (see prior report63). In situ hybridization showed that in control retinas, the Notch receptor notch1a, the Notch ligands deltaA (dla) and deltaD (dld), and the Notch targets ascl1a and her4 are expressed throughout the central retina at 30 hpf (data not shown), but are downregulated in the central retina by 48 hpf (Fig. 2A, arrowheads) and restricted to the ciliary marginal zone (CMZ) by 70 hpf (Fig. 2B, arrowheads). However, NeuroD knockdown results in the failure of cells to downregulate these genes (Figs. 2A, 2B, arrowheads), and at 70 hpf this is most apparent in the developing ONL adjacent to the dorsal CMZ (Fig. 2B, circled region) where photoreceptor genesis is occurring. Corresponding qRT-PCR data from embryo heads show that at 48 and/or 70 hpf, NeuroD knockdown results in significantly increased expression of notch1a, dla, and dld, as well as several downstream targets of the Notch pathway including cyclinD1, ascl1a, cyclinB1, and hes6. The overall level of her4 expression is not significantly affected by NeuroD knockdown (Fig. 2C), and her4 expression appears to be unaffected in the brain (data not shown). This suggests that NeuroD regulation of her4 is restricted primarily to the retina and suggests the possibility that her4 may be regulated by other mechanisms in addition to the Notch pathway. These data demonstrated that in the developing retina, NeuroD negatively regulates intercellular Notch signaling, thereby negatively regulating downstream targets of the Notch pathway.
Figure 2.
NeuroD negatively regulates Notch signaling molecules and downstream targets of the Notch pathway. (A, B) In situ hybridizations showing the normal retinal expression of neurod compared to notch1a, dla, dld, ascl1a, and her4 at 48 (A) and 70 (B) hpf in control embryos and following NeuroD knockdown. Arrowheads indicate zones of expression; circled regions highlight expression differences at 70 hpf in and around the developing ONL and INL adjacent to the dorsal CMZ. Scale bars: 50 μm. (C) Quantitative RT-PCR data indicating the fold differences in expression between control embryos (black horizontal line) and embryos following NeuroD knockdown at 48 and 70 hpf. *P < 0.05, Student's t-test. Error bars: standard deviation.
NeuroD is Not Required for Photoreceptor Fate Specification or Progenitor Survival
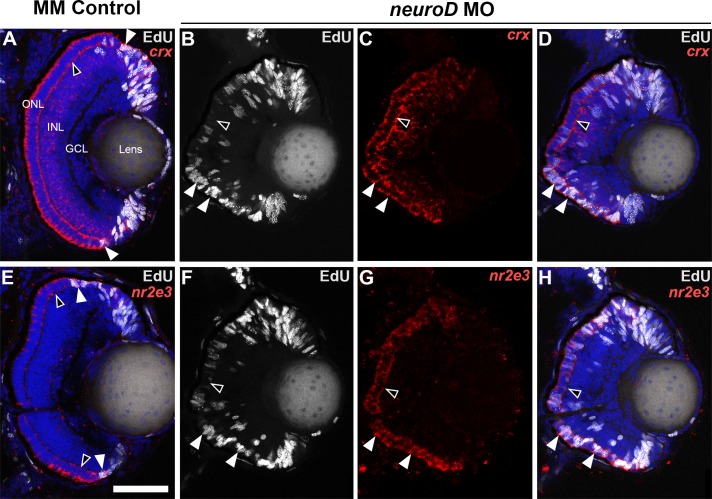
Two questions remain unanswered from previous studies: Is NeuroD required for photoreceptor cell fate specification, and does the small eye phenotype following NeuroD knockdown result from increased apoptosis? To answer the first question, in situ hybridization was used to determine if NeuroD knockdown alters the expression of the canonical markers of the photoreceptor lineages crx and nr2e3. In the developing retina, crx and nr2e3 are expressed in mitotic photoreceptor progenitors, then nr2e3 is downregulated in postmitotic cones.66,67 NeuroD knockdown does not prevent the expression of these genes in either dividing or postmitotic cells (Fig. 3) and, due to the lack of mature cones following NeuroD knockdown, results in nr2e3 expression in a higher number of cells (Fig. 3G). These observations indicate that NeuroD is not required for photoreceptor fate specification. To address the second issue, TUNEL labeling revealed that at 70 hpf, the number of apoptotic cells in the retina does not change following NeuroD knockdown (Supplementary Fig. S2). This indicates that during the temporal window of photoreceptor genesis, NeuroD is not required for the survival of photoreceptor progenitors. This suggests that the reduced eye diameter at 70 hpf resulting from NeuroD knockdown likely is caused by the lack of photoreceptor differentiation rather than cell death.
Figure 3.
During embryonic development, NeuroD knockdown does not affect expression of the photoreceptor lineage markers nr2e3 and crx. In control retinas at 72 hpf, crx (A) and nr2e3 (E) are expressed in postmitotic photoreceptors (black arrowheads) and in EdU+ progenitors (white arrowheads) adjacent to the CMZ that have not yet exited the cell cycle. Likewise, following NeuroD knockdown (neurod MO), crx (B–D), and nr2e3 (F–H) are expressed in postmitotic photoreceptors (black arrowheads) and in EdU+ progenitors (white arrowheads) distributed throughout the ONL Scale bar: 50 μm.
CRISPR/Cas9 Targeted Mutation of neurod Phenocopies Morpholino Knockdown and Identically Affects Notch Signaling
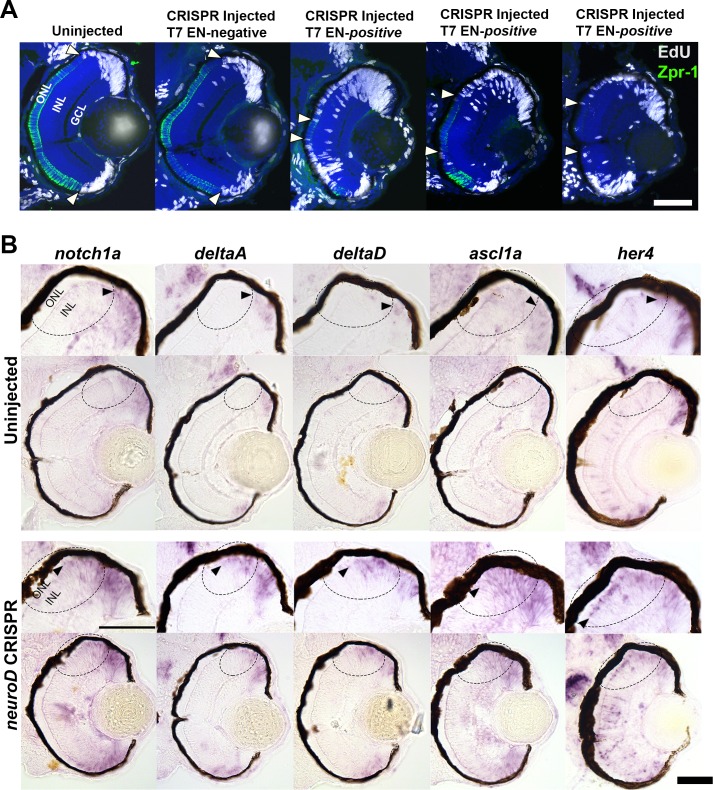
The zebrafish community has raised concerns about potential off-target effects of MOs and cautioned that MOs should be used only if targeted gene mutation produces the same phenotype.68,69 To determine if targeted mutation of neurod results in the same retinal phenotype as NeuroD knockdown, preliminary experiments were undertaken using CRISPR/Cas9 genome editing to create targeted mutations in the neurod gene. To characterize mutations in individual embryos displaying a retinal phenotype, DNA was collected at 70 hpf, PCR amplified across the sgRNA target site and subcloned into pGEM-T Easy (Promega). Six to nine clones were sequenced per embryo, and between one and three indels were identified in each embryo from distinct clones. At least one WT DNA strand was detected in each embryo, indicating mosaicism at the targeted sequences. Mutations ranged from deletions of 1 to 69 nucleotides and/or insertions of 1 to 2 nucleotides, all starting or ending within the center of the sgRNA target sequence. Most indels introduced putative premature stop codons. CRISPR/Cas9-injected embryos that tested negative for insertions/deletions (indels) had normal retinas, identical to WT uninjected embryos (Fig. 4A). Of 14 injected embryos analyzed, 12 (86%) tested positive for indels, and 5 of these (42%) had a retinal phenotype consistent with that observed following NeuroD knockdown using MOs. This phenotype included reduced eye size (Fig. 4A), excess BrdU+ cells in the ONL and paucity of mature photoreceptors (Fig. 4A, cf. Figs. 1A, 1C; arrowheads). NeuroD knockdown and targeted mutation of neurod also produced identical changes to expression of genes in the Notch pathway. At 70 hpf, cells in embryos with neurod indels had increased expression of notch1a, dla, dld, ascl1a, and her4 in the central retina (Fig. 4B, arrowheads and circled regions). The phenotypes combined with the sequencing results suggest that embryos with neurod indels are equivalent to embryos in which NeuroD has been knocked down. These data demonstrated that reductions in NeuroD caused by targeted mutation or morpholino knockdown cause identical retinal phenotypes and increased expression of Notch pathway genes.
Figure 4.
CRISPR/Cas9 targeted mutation of neurod phenocopies NeuroD knockdown and similarly affects Notch signaling. (A) EdU and Zpr-1 (cones) labeling in retinas of uninjected and WT injected (T7 endonuclease [EN]-negative) embryos, compared to retinas of 3 embryos positive for insertions and/or deletions in the neurod gene (T7 endonuclease [EN]-positive). Zpr-3 labeling (rods) was affected identically (data not shown). As a relative comparison of photoreceptor progenitors still in the cell cycle in the central retina, arrowheads indicate the central-most EdU+ cells in the ONL. (B) In situ hybridization showing retinal expression of notch1a, dla, dld, ascl1a, and her4 in embryos with neurod indels compared to uninjected controls. Arrowheads indicate zones of expression; circled regions highlight expression differences in and around the developing ONL and INL adjacent to the dorsal CMZ. Scale bars: 50 μm.
Notch Signaling Governs NeuroD-Mediated Cell Cycle Exit, But Not Photoreceptor Maturation
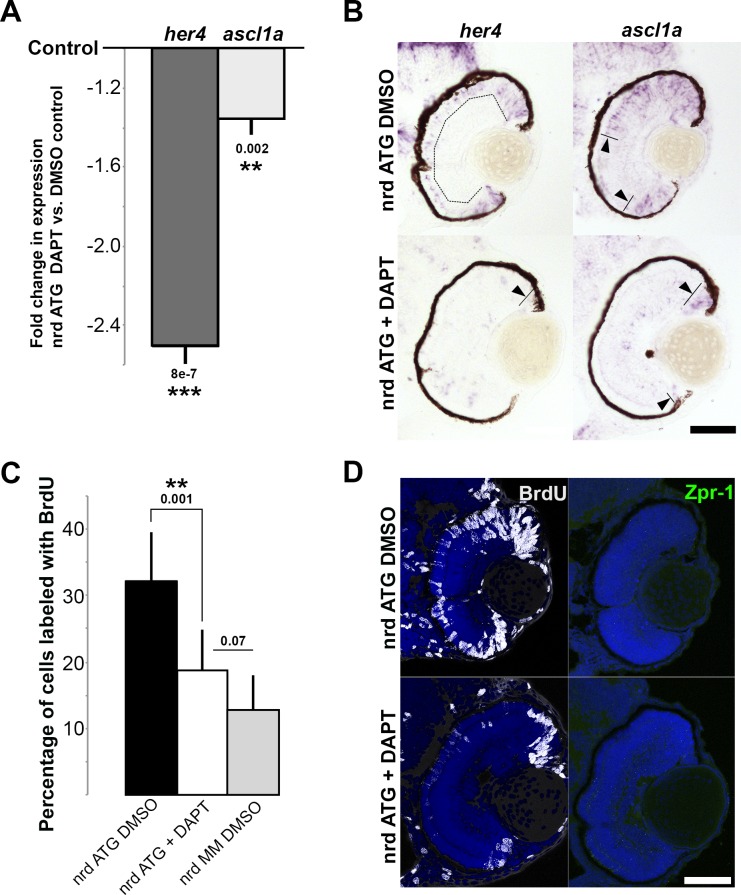
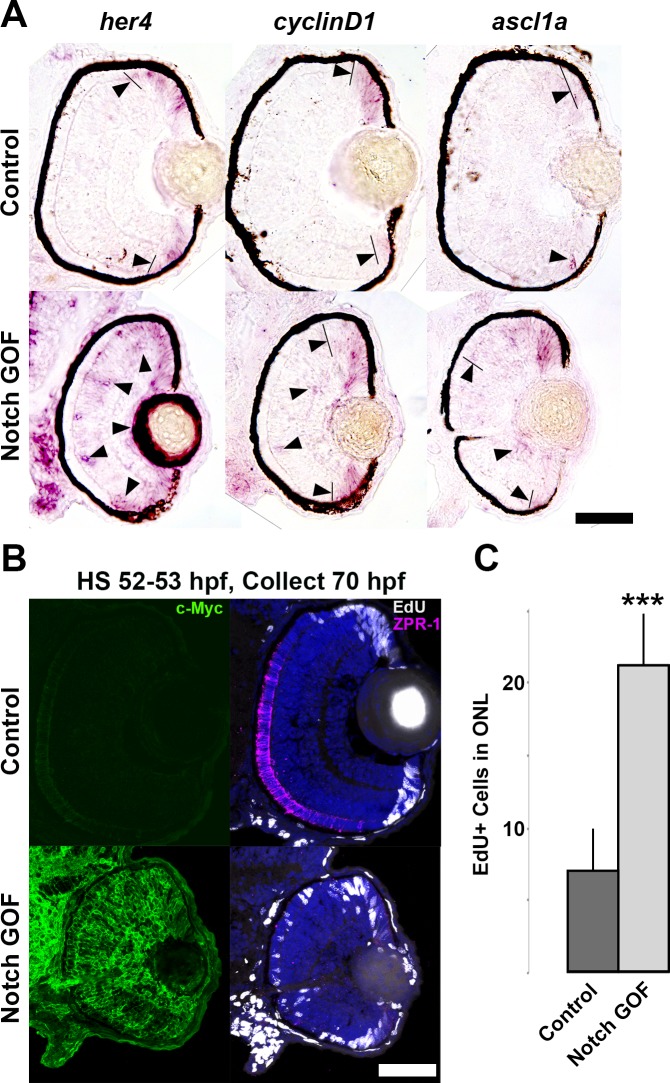
As a test of the hypothesis that Notch signaling mediates NeuroD function, Notch signaling was inhibited by exposing embryos to the gamma secretase inhibitor, DAPT, between 48 and 70 hpf following NeuroD knockdown. DAPT treatment significantly reduced expression of the Notch targets her4 and ascl1a (Fig. 5A), restoring an expression pattern similar to controls (Fig. 5B, cf. Figs. 2A–C; arrowheads). These results confirmed that DAPT treatment inhibited the Notch pathway, and are interpreted to show that NeuroD regulation of her4 and ascl1a is mediated through Notch signaling. Similarly, following NeuroD knockdown, DAPT treatment reduced the number of BrdU-positive progenitors to control levels (Figs. 5C, 5D), demonstrating that in the absence of NeuroD, inhibition of Notch signaling is sufficient to drive photoreceptor progenitors out of the cell cycle. However, DAPT treatment was not sufficient to rescue photoreceptor maturation (Fig. 5D). To determine if the activation of Notch signaling in the presence of the normal expression of NeuroD is sufficient to prevent cycle exit and photoreceptor maturation, Tg(hsp70:GGFF) × Tg(UAS:myc-notch1a:intra) embryos were heat-shocked at 52 to 53 hpf to conditionally activate the Notch pathway. Notch activation (Notch gain-of-function [GOF]) caused substantial upregulation of the direct Notch target gene her4 throughout the lens and retina (Fig. 6A, arrowheads), confirming strong upregulation of Notch pathway activity. Compared to controls, Notch GOF caused modest increases in the expression of cyclinD1 and ascl1a in the central retina (Fig. 6A, arrowheads), resulted in significantly more EdU+ progenitors in the ONL, and caused a marked decrease in photoreceptor maturation (Figs. 6B, 6C). Furthermore, identical to NeuroD knockdown, Notch GOF resulted in reduced eye size (Figs. 6A, 6B) and no increased apoptosis (data not shown). These results demonstrated that conditional activation of the Notch signaling pathway is sufficient to prevent photoreceptor genesis, recapitulating NeuroD knockdown. Together, the Notch inhibition and GOF data are interpreted to show that cell cycle exit among photoreceptor progenitors is governed through repression of Notch signaling by NeuroD, but that NeuroD governs photoreceptor maturation through mechanisms independent of the Notch pathway.
Figure 5.
Inhibition of Notch signaling reduces her4, ascl1a, and ONL cell proliferation following NeuroD knockdown, but does not rescue photoreceptor differentiation. (A) Quantitative RT-PCR data showing fold changes in her4 and ascl1a expression following NeuroD knockdown in embryos treated 48 to 70 hpf with DAPT, compared to control embryos treated with DMSO only (solid black line). (B) In situ hybridization showing retinal expression of her4 and ascl1a following NeuroD knockdown in embryos treated 48 to 70 hpf with DMSO only compared to embryos treated with DAPT. Dashed lines and arrowheads indicate zones of expression. (C) Percentage of retinal cells labeled with BrdU following NeuroD knockdown in embryos treated with DMSO only, compared to embryos treated with DAPT, and with embryos injected with MM control morpholinos treated with DMSO only. (D) BrdU and Zpr-1 (cones) labeling following NeuroD knockdown in embryos treated with DMSO only compared to embryos treated with DAPT; note the absence of cones in both images. Scale bars: 50 μm. Actual P values shown on graphs; **P < 0.005, ***P < 0.0005, Student's t-test. Error bars: standard deviation.
Figure 6.
Conditional activation of the Notch pathway up-regulates Notch target expression and prevents cell cycle exit and photoreceptor genesis. (A) In situ hybridization showing retinal expression of the Notch targets her4, cyclinD1, and ascl1a in control embryos compared to Notch GOF embryos heat-shocked 52-53 hpf. (B) EdU, Zpr-1, and Myc labeling in 70 hpf control (top panels: Tg[hsp:GGFF] only) and Notch GOF (Tg[hsp:GGFF] × Tg[UAS:myc-notch1a:intra]) embryos heat-shocked 52-53 hpf. (C) Quantification of EdU+ cells in the ONL of 70 hpf control embryos (n = 5) compared to Notch GOF embryos (n = 8) heat-shocked 52 to 53 hpf. Scale bars: 50 μm. ***P = 0.00003, Student's t-test. Error bars: standard deviation.
Time Course of neurod Expression in the Adult Regenerating Retina
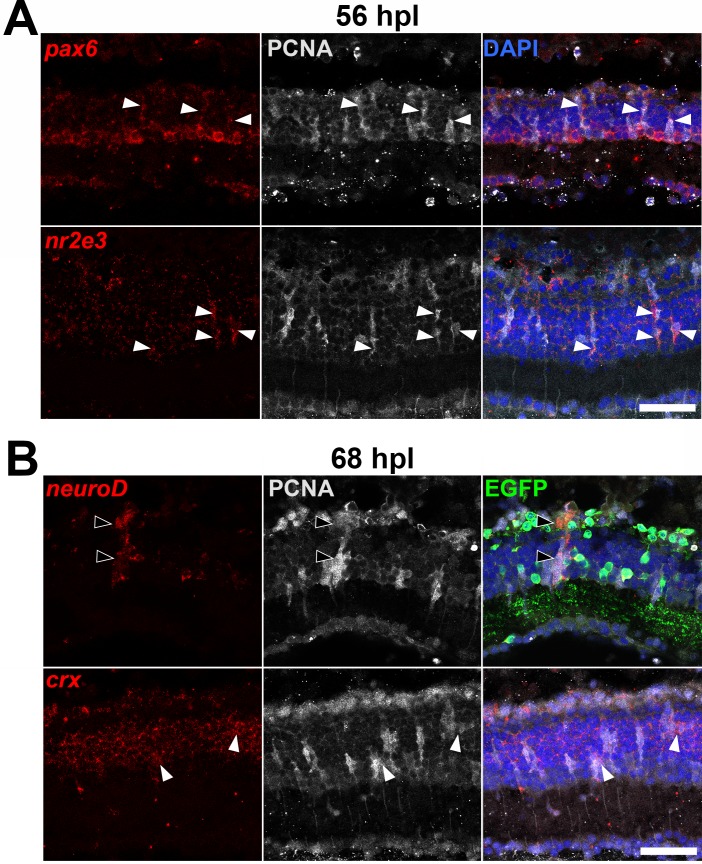
As a first step to understand NeuroD function during photoreceptor regeneration, following photoreceptor ablation with bright light, neurod expression was characterized in relation to pax6a, a marker of dividing progenitors as well as mature amacrine and ganglion cells, and the photoreceptor lineage markers crx and nr2e3. Within 24 to 48 hours after light onset (hpl), a subset of Müller glia divides once, each producing a mitotic daughter cell that subsequently divides multiple times to produce a cluster of neurogenic progenitors.30 Therefore, in the present study, 48 hpl was the earliest time point analyzed for progenitor markers. In situ hybridization and immunohistochemistry showed that the progenitor cell marker pax6a and the photoreceptor lineage marker nr2e3 are expressed in proliferating cell nuclear antigen-positive (PCNA+) cells by 56 hpl (Fig. 7). In comparison, neurod mRNA was first detectable in a subset of PCNA+ progenitors at 68 hpl (Fig. 7), slightly earlier than the 72 hpl time point identified in earlier studies.39 The photoreceptor lineage marker crx is constitutively expressed throughout the inner nuclear layer (INL) and ONL, and also was first noticeably upregulated in a subset of PCNA+ progenitors only after 68 hpl (Fig. 7). NeuroD knockdown did not prevent nr2e3 or crx expression (Supplementary Fig. S3), indicating that during photoreceptor regeneration, NeuroD is not required for photoreceptor fate specification among the Müller glia–derived progenitors.
Figure 7.
Earliest detectable expression of pax6, nr2e3, neurod, and crx in Müller glia–derived progenitors. (A) pax6 and nr2e3 expression is detectable in PCNA+ progenitors as early as 56 hpl (arrowheads). (B) neurod expression is detectable in PCNA+ cells as early as 68 hpl, but in Tg(neurod:egfp) fish, EGFP immunolabeling is not yet detectable in these cells (black arrowheads); crx is constitutively expressed throughout the INL and at 68 hpl is first detectable at higher levels in PCNA+ progenitors (white arrowheads). Scale bars: 50 μm.
At 72 hpl and later, the Tg(neurod:egfp) line is an accurate reporter of endogenous neurod expression39 and showed that at 72 hpl, all BrdU+/EGFP+ (neurod+) photoreceptor progenitors express pax6a, nr2e3, and crx (Supplementary Fig. S4). However, by 7 dpl, when regenerated photoreceptors are exiting the cell cycle and differentiating,29,60 pax6a is downregulated in all photoreceptors while neurod and nr2e3 are downregulated in newly postmitotic cones but continue to be expressed in rods (Supplementary Fig. S4). This pattern persists in the fully regenerated retina at 14 dpl (Supplementary Fig. S5) and suggests a role for NeuroD during maturation and/or maintenance of rods.
Following Photoreceptor Ablation, NeuroD Governs Cell Cycle Exit and Photoreceptor Regeneration
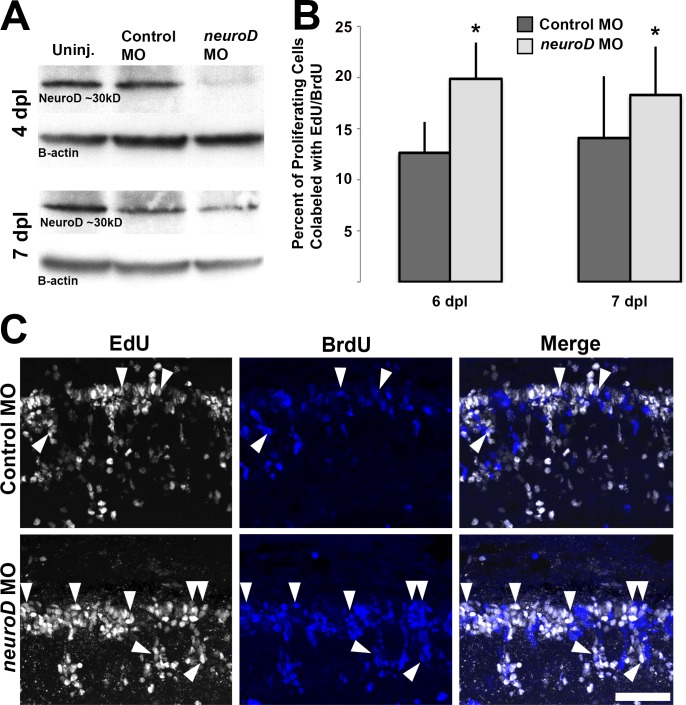
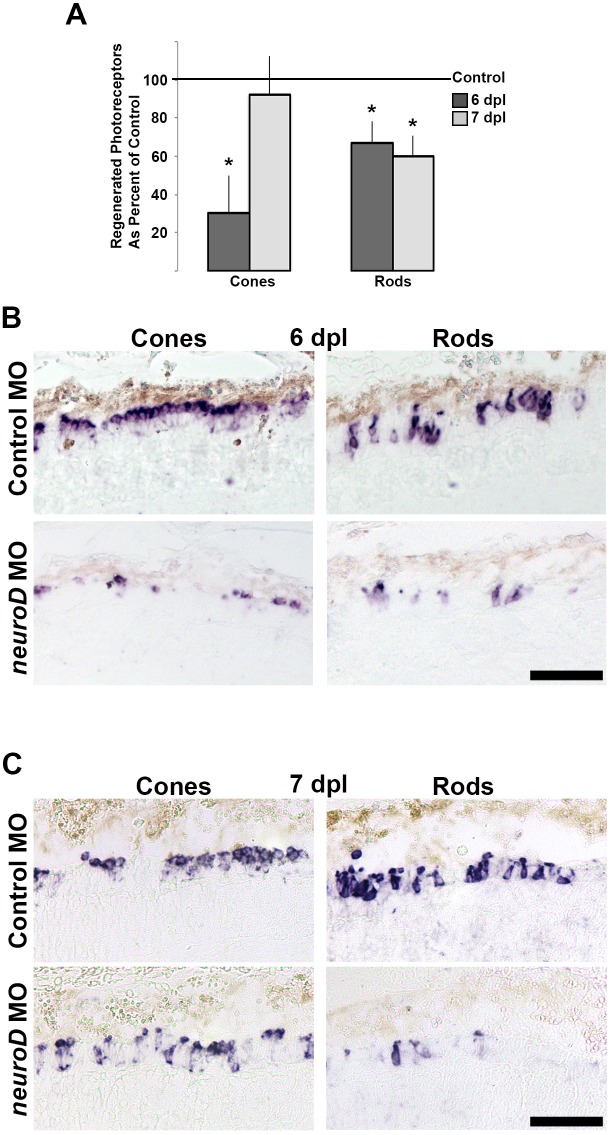
The knowledge that NeuroD governs cell cycle exit among photoreceptor progenitors in the embryonic retina prompted us to determine if NeuroD functions similarly during photoreceptor regeneration. To test this, MOs were used to knock down NeuroD protein and labeling with two different cell cycle markers, EdU and BrdU, was used to analyze cell cycle exit. Morpholinos were injected at 48 hpl, before the onset of neurod expression, to prevent translation of NeuroD protein. Western blots confirmed efficient knockdown of NeuroD protein at 4 dpl (14.5% of control), but recovery of NeuroD protein by 7 dpl (107% of control; Fig. 8A). EdU was administered systemically at 3 dpl, when photoreceptor progenitors are proliferating in the INL,29 and fish were exposed to BrdU from 5 to 6 dpl or from 6 to 7 dpl, when photoreceptor progenitors are in the nascent ONL and normally exiting the cell cycle.29,60 This paradigm differentially labels cells in S-phase of the cell cycle at each time point and the percentage of cells colabeled with EdU and BrdU was used as a proxy for the percentage of cells that remained in the cell cycle between 3 and 6 dpl. Compared to controls at 6 and 7 dpl, NeuroD knockdown resulted in a higher percentage of proliferating cells colabeled with EdU and BrdU (Figs. 8B, 8C). There was a greater difference between control and knockdown retinas at 6 than at 7 dpl (Fig. 8B), perhaps reflecting the recovery of NeuroD by 7 dpl (cf. Fig. 8A). This demonstrates that NeuroD knockdown prevents photoreceptor progenitors from exiting the cell cycle and is interpreted to show that in the injured, regenerating retina, NeuroD governs cell cycle exit among the Müller glia–derived photoreceptor progenitors. To determine if the results described above impact photoreceptor regeneration, in situ hybridization was used at 6 and 7 dpl to label regenerated cones (pde6c) and rods (rhodopsin). At 6 dpl, NeuroD knockdown resulted in fewer regenerated cones and rods (Figs. 9A, 9B); however, by 7 dpl, cone regeneration recovers along with the recovery of NeuroD (Figs. 9A, 9C). These data demonstrated that, following NeuroD knockdown, the failure of progenitors to exit the cell cycle is sufficient to prevent or delay photoreceptor regeneration.
Figure 8.
NeuroD is required for photoreceptor progenitors to exit the cell cycle. (A) Following photoreceptor ablation and morpholino injection/electroporation at 2 dpl, Western blot indicates strong knockdown of NeuroD protein (neurod MO) at 4 dpl compared to control MO or uninjected retinas, but recovery of NeuroD protein by 7 dpl. (B) When fish are injected with EdU at 3 dpl and exposed to BrdU from 5 to 6 or from 6 to 7 dpl, NeuroD knockdown results in a greater percentage of progenitors that are colabeled with EdU and BrdU at 6 and 7 dpl, indicating that fewer cells have exited the cell cycle; Student's t-test, *P < 0.05. (C) Corresponding images show the increase in EdU+/BrdU+ colabeled cells (arrowheads) following NeuroD knockdown. Scale bar: 50 μm.
Figure 9.
NeuroD is required for photoreceptor regeneration. (A) Compared to controls (solid black line), NeuroD knockdown results in reduced rod and cone regeneration by 6 dpl, but only reduced rod regeneration by 7 dpl. Student's t-test, *P < 0.05. Corresponding images at 6 dpl (B) and 7 dpl (C) show in situ hybridizations for cones (pde6c) and rods (rhodopsin). Scale bars: 50 μm.
NeuroD Governs Photoreceptor Regeneration Through Delta-Notch Signaling
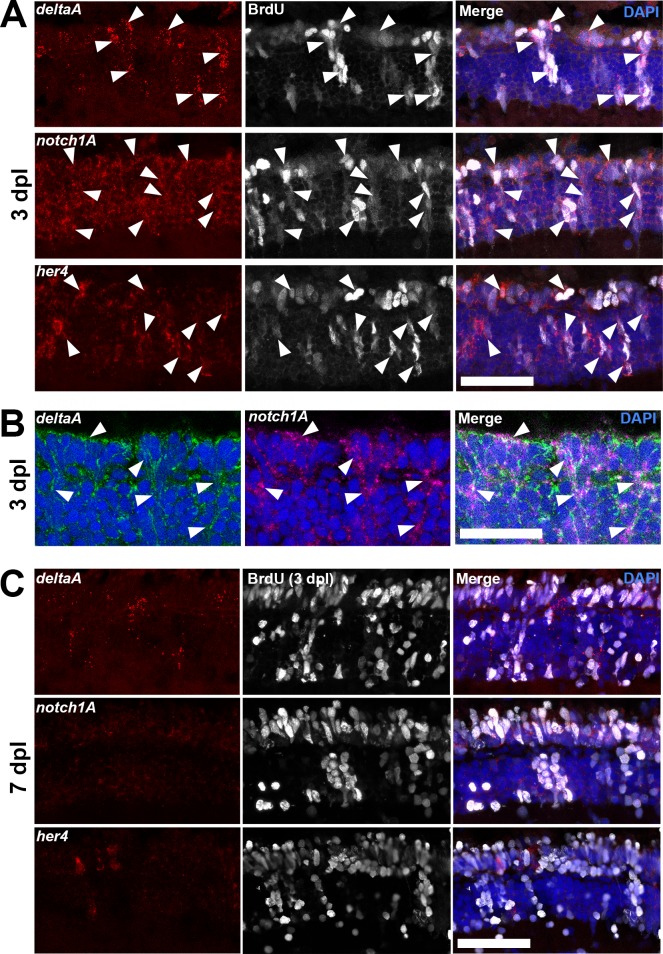
The data showing that NeuroD governs cell cycle exit through Notch signaling in the developing retina led us to investigate Notch signaling as a putative mediator of NeuroD function during photoreceptor regeneration. First, in situ hybridization was used to characterize the timing of Notch pathway gene expression in photoreceptor progenitors. At 72 hpl, deltaA, notch1a, and her4 are coexpressed in BrdU+ cells (Figs. 10A, 10B). By 7 dpl, most progenitors that were labeled with BrdU at 3 dpl have exited the cell cycle (cf. Fig. 8) and no longer express Notch pathway genes (Fig. 10C). These observations showed that within photoreceptor progenitors, Notch pathway gene expression coincides with proliferation and is downregulated during photoreceptor differentiation.
Figure 10.
Notch signaling molecules are expressed in proliferating photoreceptor progenitors. (A) In situ hybridizations show that at 72 hpl, the Notch ligand deltaA, the Notch receptor notch1a, and the direct Notch target her4 are expressed in proliferating (BrdU+) photoreceptor progenitors (arrowheads). (B) Double in situ hybridization shows colocalization of deltaA and notch1a in the same cells (arrowheads). (C) By 7 dpl, deltaA, notch1a, and her4 are no longer expressed in the cells that were proliferating at 3 dpl (labeled with BrdU). Scale bars: 50 μm.
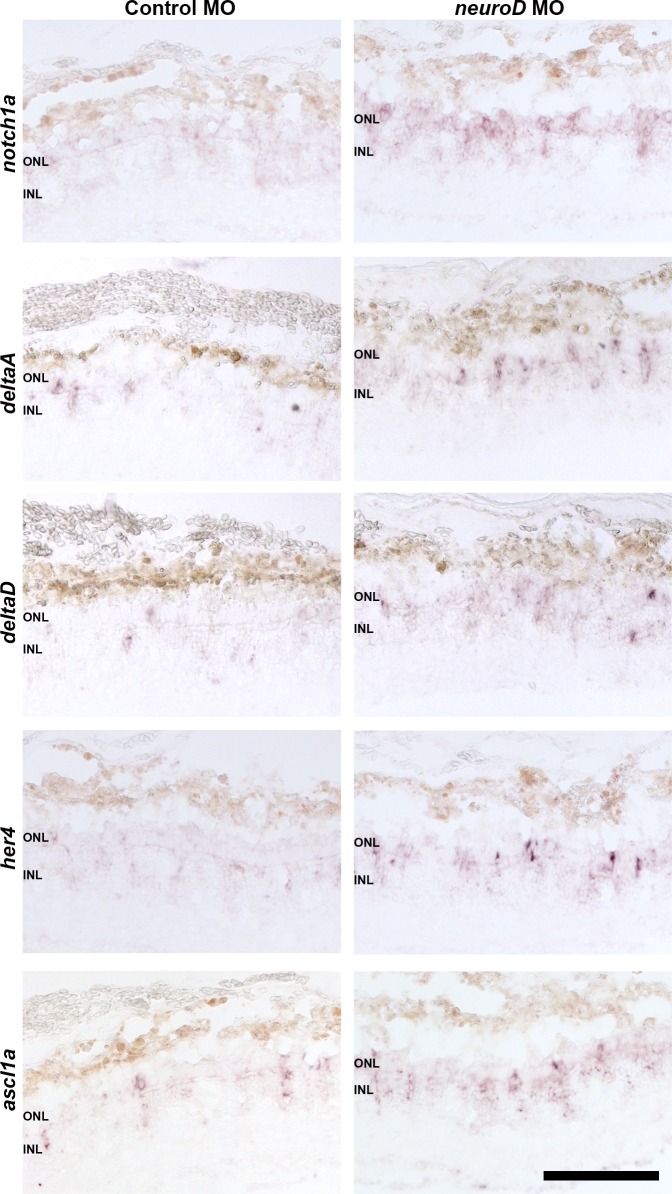
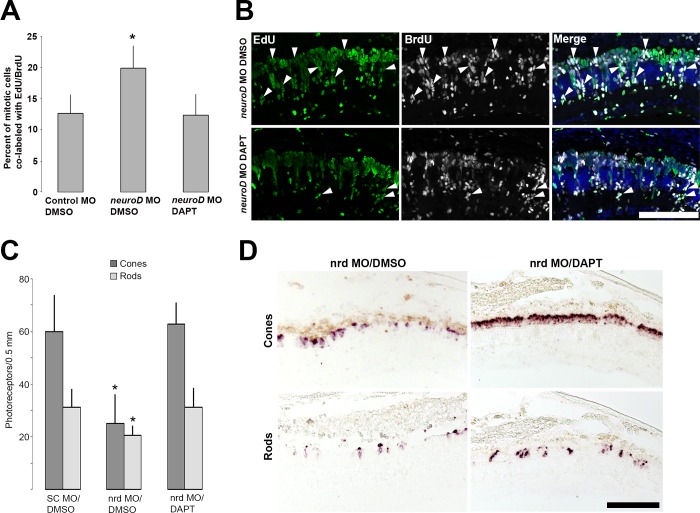
Downregulation of Notch pathway genes, concomitant with increasing neurod expression and cell cycle exit, suggests that Notch signaling and NeuroD may affect the cell cycle in opposing ways. To determine if Notch signaling genes are regulated downstream of NeuroD, in situ hybridization was used to compare expression of the Notch signaling molecules notch1a, deltaA, and deltaD, and the Notch targets her4 and ascl1a, in control and experimental retinas. At 4 dpl, a time point when photoreceptor progenitors are proliferating and few have exited the cell cycle, NeuroD knockdown has no discernable effect on Notch pathway gene expression (data not shown). However, at 6 dpl, when regenerated photoreceptors are normally differentiating (cf. Figs. 8, 9), NeuroD knockdown results in increased expression of Notch signaling genes and Notch targets (Fig. 11). This indicates that NeuroD knockdown prevents the normal downregulation of Delta-Notch signaling among progenitors and that during photoreceptor regeneration NeuroD negatively regulates Delta-Notch signaling. To determine if Notch signaling mediates NeuroD function, following photoreceptor ablation and NeuroD knockdown, fish were treated with the gamma secretase inhibitor, DAPT, from 4 to 6 dpl. Proliferating cells were labeled with EdU (IP injection) at 3 dpl and with BrdU (immersion) at 5 to 6 dpl. Colabeling analysis was performed as described above to determine the proportion of progenitors that remained in the cell cycle. DAPT treatment reduced expression of her4 and ascl1a, confirming that Notch signaling was inhibited (Supplementary Fig. S6), reduced the percentage of progenitors colabeled with EdU and BrdU to control levels (Figs. 12A, 12B) and fully rescued the deficiency in photoreceptor regeneration induced by NeuroD knockdown (Figs. 12C, 12D). These results indicate that inhibiting Notch signaling is sufficient to rescue NeuroD knockdown, and that NeuroD governs photoreceptor regeneration through negatively regulating Notch signaling.
Figure 11.
NeuroD negatively regulates Notch signaling molecules and targets of the Notch pathway. At 6 dpl in both the INL and ONL, in situ hybridizations indicate that NeuroD knockdown results in increased expression of the Notch receptor notch1a, the Notch ligands deltaA and deltaD, and the Notch pathway targets her4 and ascl1a. Scale bar: 50 μm.
Figure 12.
NeuroD governs cell cycle exit and photoreceptor regeneration through Notch signaling. (A) Compared to controls (control MO treated with DMSO vehicle), NeuroD knockdown (neurod MO treated with DMSO vehicle) results in an increased percentage of EdU+/BrdU+ colabeled cells, but inhibition of Notch signaling with the gamma secretase inhibitor DAPT from 4 to 6 dpl (neurod MO, DAPT) restores the percentage of EdU+/BrdU+ colocalized cells to control levels. Student's t-test, *P < 0.05. (B) Corresponding images at 6 dpl show the reduction in EdU+/BrdU+ colabeled cells (arrowheads) in neurod morphants following DAPT treatment. (C) NeuroD knockdown results in fewer regenerated cone and rod photoreceptors, but inhibition of Notch signaling with the gamma secretase inhibitor DAPT from 4 to 6 dpl (neurod MO, DAPT) restores the number of regenerated photoreceptors to control levels. Student's t-test, *P < 0.05. (D) Corresponding images at 6 dpl show in situ hybridizations for cones (pde6c) and rods (rhodopsin). Scale bars: 50 μm.
Discussion
Photoreceptor genesis in the vertebrate retina requires precise regulation of events that determine progenitor fate, regulate the timing of cell cycle exit, and induce differentiation and maturation.1,70–73 During retinal development in zebrafish the bHLH transcription factor NeuroD is expressed in mitotic photoreceptor progenitors and nascent photoreceptors,13,14,39 and within the photoreceptor lineage, NeuroD governs cell cycle exit and photoreceptor maturation.7 Photoreceptor genesis normally is complete by 70 hpf63; however, in the absence of NeuroD, even though cellular laminae form, photoreceptor progenitors fail to exit the cell cycle and mature photoreceptors are absent.7 Following conditional ectopic expression of NeuroD, nearly all dividing cells are forced out of the cell cycle, cyclinD1, cyclinB1, and cyclinE1 are downregulated and cyclin kinase inhibitors, p27 and p57, are upregulated.7 In the adult retina following photoreceptor ablation, neurod is expressed in Müller glia–derived mitotic progenitors.39 This suggests that NeuroD also has a role in photoreceptor regeneration, but this has not been investigated. The goal of the current study was to identify the molecular mechanisms through which NeuroD functions and to mechanistically link NeuroD to the mechanisms that govern the cell cycle during photoreceptor genesis in embryos and photoreceptor regeneration in adults.
In the embryonic retina, genetic mosaic analysis showed that NeuroD regulates cell cycle exit and photoreceptor maturation through non–cell-autonomous mechanisms. This indicates that NeuroD governs photoreceptor genesis and maturation through the regulation of intercellular signaling events. Transcription factors by their nature function cell-autonomously by binding DNA to regulate gene transcription, but they also can function non–cell-autonomously by regulating intercellular signaling molecules.29,36,74–78 This also is true for bHLH factors that regulate intercellular signaling pathways in the retina.23,29,36,76 There also are rare examples of transcription factors functioning as secreted molecules.79–81 In vitro, naked NeuroD protein can be transported through cell membranes and activate transcriptional events.82 However, there is no evidence in vivo for the secretion of NeuroD or any other bHLH transcription factor, and we infer that NeuroD is not secreted in the developing or regenerating zebrafish retina.
Notch signaling was investigated as the putative non–cell-autonomous mediator of NeuroD function and our data were interpreted to show that NeuroD governs cell cycle exit in photoreceptor progenitors through repression of Delta-Notch signaling. Gamma-secretase inhibitors, such as DAPT, effectively inhibit Notch pathway activation in the zebrafish retina and other models18,21,34,37,83; however, it should be noted that gamma-secretase is necessary for other signaling mechanisms, such as through the amyloid precursor protein (APP).83 Therefore, it is possible that some of the effects of DAPT treatment, such as regional specific expression patterns of her4 and ascl1a, result from interference with other pathways in addition to Notch signaling. However, our Notch GOF data in the embryo reciprocate the DAPT data with regard to cell cycle exit and photoreceptor genesis and, therefore, strongly support our conclusions. Our findings are consistent with the conserved role of Notch signaling to prevent differentiation and maintain progenitors in a proliferative state.18–21,84,85 Several studies implicate cyclinD1, cyclinD3, and cdk genes as direct regulatory targets of the Notch signaling pathway, whereby Notch signaling promotes progression from G1 to S phases of the cell cycle.86–89 Notch signaling also indirectly regulates cyclinB1 expression.90 Therefore, transcriptional repression of Notch signaling by NeuroD provides a mechanistic link between NeuroD and cell cycle regulation through cyclinB1 and cyclinD1 (see also the report by Ochocinska and Hitchcock7).
Earlier work determined that NeuroD governs photoreceptor genesis and maturation in the developing zebrafish retina.7 However, that study did not determine if NeuroD is required for specification of the photoreceptor lineage or for cell survival. The canonical photoreceptor lineage markers nr2e3 and crx are expressed in mitotic photoreceptor progenitors.66,67,91–93 NeuroD knockdown does not prevent expression of either crx or nr2e3, indicating that NeuroD is not required to specify the photoreceptor lineage. At 70 hpf, NeuroD knockdown also did not alter the number of apoptotic cells in the developing retina, indicating that during the time of normal photoreceptor genesis, NeuroD is not required for survival of photoreceptor progenitors. Our transient knockdown of NeuroD, however, cannot determine if NeuroD is required for survival of photoreceptors or progenitors beyond this time point. In the mouse retina, NeuroD1 is not required for photoreceptor lineage specification, progenitor survival, or photoreceptor genesis, but is required for opsin selection among cones11 and for survival of a subset of mature rod photoreceptors.10 In the mouse hippocampus and olfactory bulb, NeuroD1 is required for survival and maturation of adult-born neurons.94 These observations suggest that NeuroD also might have a role in neuron survival in zebrafish and will be a focus of future work.
The zebrafish community has raised concerns regarding the use of MOs as experimental tools due to the possibility of unidentifiable off-target effects.69 Some have shown that in many cases gene mutation and morpholino-based protein knockdown do not produce the same developmental phenotype.68 These studies conclude, however, that when used appropriately and when morpholino-based knockdown and genetic mutation produce the same phenotype, morpholinos can be effective tools for research. In the present study, morpholino-based knockdown of NeuroD and CRISPR/Cas9-based mutation of neurod produce the same retinal phenotype. Despite the expected cellular mosaicism of the gene disruption in CRISPR/Cas9 injected embryos, nearly half of these embryos have a retinal phenotype consistent with NeuroD knockdown. Both approaches show that NeuroD is required for photoreceptor progenitors to exit the cell cycle and photoreceptors to differentiate, and that NeuroD regulates Notch pathway gene expression. This validates the results from previous studies that investigated NeuroD function,7 provides two independent methods to test NeuroD function and validates the use of morpholinos in the present study.
Among photoreceptor progenitors, NeuroD links cell cycle exit, differentiation, and maturation, but our data indicated that NeuroD regulates these events through separate non–cell-autonomous mechanisms. In the embryo, inhibition of Notch signaling following NeuroD knockdown is sufficient to rescue defects in cell cycle exit, but is not sufficient to restore photoreceptor maturation. This observation differs from Id2a knockdown in which inhibition of Notch signaling rescues defects in cell cycle exit and photoreceptor maturation.21 These disparate results can be explained by the normal retinal expression of neurod following Id2a knockdown (Taylor SM, Hitchcock PF, unpublished data, 2014), suggesting that, independent of Notch signaling, photoreceptor maturation is critically dependent on the normal expression of neurod. These data demonstrate that cell cycle exit and photoreceptor maturation are regulated by NeuroD, but are regulated through different pathways. Our findings are consistent with data showing that, as in the yng/brg1 mutation in the developing retina,43,95 fate specification, cell cycle withdrawal, and differentiation can be uncoupled.
Following photoreceptor ablation in the adult retina, nr2e3 is expressed before neurod and NeuroD knockdown does not prevent either nr2e3 or crx expression. These observations indicated that in the regenerating retina, as in the embryo, NeuroD does not specify the photoreceptor fate. As regenerated photoreceptors differentiate, neurod and nr2e3 are downregulated in cones but continue to be expressed in rods. This suggests a role for NeuroD in terminal differentiation and/or photoreceptor maintenance, independent of Notch signaling. The absence of neurod expression from mature cones has been documented in embryonic and adult retinas,14,39 although recent data indicate rhythmic, circadian expression in mature cones peaking in the light phase and in mature rods peaking in the dark phase.96 Our data were all collected during the light phase, indicating that loss of neurod from newly regenerated cones results from regenerative mechanisms rather than circadian rhythmicity.
Following photolytic lesioning, NeuroD knockdown prevents cell cycle exit and photoreceptor regeneration and results in increased expression of Notch pathway genes. These defects are rescued by Notch inhibition, and this is interpreted to show that, in the light-damaged retina, NeuroD governs cell cycle exit and photoreceptor regeneration through Notch signaling. This shows that Notch signaling prevents cell cycle exit in photoreceptor progenitors, contrasting with the effect of Notch signaling on Müller glia to maintain these cells in a nonproliferative, differentiated state.34 This suggests that Notch signaling might regulate the single, asymmetrical division of Müller glia, causing the parent cell to exit the cell cycle while simultaneously causing the daughter cell to proliferate. This is consistent with the role of Notch in the zebrafish retina to promote gliogenesis in some progenitors while maintaining others in a proliferative state18,20 and the requirement for Notch in the asymmetrical division of progenitors that give rise to glia/neuron sibling pairs in the Drosophila CNS.97 Within 24 to 48 hours after injury, the time frame when Müller glia divide, the Notch pathway genes notch1a, deltaA, and her4 are strongly upregulated in the retina and remain upregulated through 7 dpl.37 We show that by 72 hpl, Notch pathway genes are expressed in all proliferating photoreceptor progenitors. This differs from observations after physical damage to all retinal layers where, 4 days after retinal injury, notch1a was expressed in all BrdU-positive cells while delta genes were “preferentially expressed” in apposing BrdU-negative cells.37 The disparity between our study and that of Wan et al.37 may be due to their approach of damaging all retinal layers, thus generating a more heterogeneous mix of progenitor types and variability in Notch pathway gene expression. We also showed that among photoreceptor progenitors in the developing and regenerating retina, NeuroD negatively regulates ascl1a through repression of Notch signaling. This indicates that in photoreceptor progenitors, Notch signaling positively regulates ascl1a, contrasting with the effect of Notch signaling to negatively regulate ascl1a in Müller glia.37 This suggests that, in photoreceptor progenitors, Ascl1a functions downstream of Notch in the transition from proliferation to differentiation and suggests a mechanism through which Notch signaling may regulate the cell cycle differently in Müller glia and their progeny.
In conclusion, our data demonstrated a conserved role for NeuroD during photoreceptor development and regeneration in which Notch signaling is a mechanistic link between NeuroD and cell cycle exit. We also showed that during embryonic development, NeuroD functions non–cell-autonomously and governs photoreceptor maturation through pathways that do not involve Notch signaling. Future work will focus on identifying the mechanisms, during development and regeneration, that function upstream of NeuroD and through which NeuroD governs photoreceptor maturation.
Supplementary Material
Acknowledgments
The authors thank Ann C. Morris, University of Kentucky, for providing Tg(UAS:myc-notch1a:intra) fish and for scientific consult; Laura Kakuk-Atkins, Dilip Pawar, and Zachary Stamplis for technical assistance; and Christine Crilly for administrative assistance.
Supported by grants from the National Institutes of Health/National Eye Institute ([NIH/NEI], Bethesda, MD, USA), R01 EY07060 (PFH), F32 EY023129 (SMT), T32 EY013934 (SMT), Core Grant P30EYO7003 (University of Michigan), Core Grant P30RY004068 (Wayne State University), NIH EY017753 (JMF), NIH EY020106 (KAD), grants from Fight for Sight and the Midwestern Eye-Bank (PFH), and Research to Prevent Blindness (Wayne State University). Fish lines and reagents provided by ZIRC were supported by NIH-NCRR Grant P40 RR01. The authors alone are responsible for the content and writing of the paper.
Disclosure: S.M. Taylor, None; K. Alvarez-Delfin, None; C.J. Saade, None; J.L. Thomas, None; R. Thummel, None; J.M. Fadool, None; P.F. Hitchcock, None
References
- 1. Bassett EA,, Wallace VA. Cell fate determination in the vertebrate retina. Trends Neurosci. 2012; 35: 565–573. [DOI] [PubMed] [Google Scholar]
- 2. Brzezinski JA,, Kim EJ,, Johnson JE,, Reh TA. Ascl1 expression defines a subpopulation of lineage-restricted progenitors in the mammalian retina. Development. 2011; 138: 3519–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pollak J, Wilken MS, Ueki Y, et al. . ASCL1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development. 2013; 140: 2619–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mao C-A, Cho J-H, Wang J, et al. . Reprogramming amacrine and photoreceptor progenitors into retinal ganglion cells by replacing Neurod1 with Atoh7. Development. 2013; 140: 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akagi T, Inoue T, Miyoshi G, et al. . Requirement of multiple basic helix-loop-helix genes for retinal neuronal subtype specification. J Biol Chem. 2004; 279: 28492–28498. [DOI] [PubMed] [Google Scholar]
- 6. Conte I, Marco-Ferreres R, Beccari L, et al. . Proper differentiation of photoreceptors and amacrine cells depends on a regulatory loop between NeuroD and Six6. Development. 2010; 137: 2307–2317. [DOI] [PubMed] [Google Scholar]
- 7. Ochocinska MJ,, Hitchcock PF. NeuroD regulates proliferation of photoreceptor progenitors in the retina of the zebrafish. Mech Devel. 2009; 126: 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JE,, Hollenberg SM,, Snider L,, Turner DL,, Lipnick N,, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995; 268: 836–844. [DOI] [PubMed] [Google Scholar]
- 9. Farah MH,, Olson JM,, Sucic HB,, Hume RI,, Tapscott SJ,, Turner DL. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development. 2000; 127: 693–702. [DOI] [PubMed] [Google Scholar]
- 10. Morrow EM,, Furukawa T,, Lee JE,, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999; 126: 23–36. [DOI] [PubMed] [Google Scholar]
- 11. Liu H, Etter P, Hayes S, et al. . NeuroD1 regulates expression of thyroid hormone receptor 2 and cone opsins in the developing mouse retina. J Neurosci. 2008; 28: 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yan R-T,, Wang S-Z. Requirement of neuroD for photoreceptor formation in the chick retina. Invest Ophthalmol Vis Sci. 2004; 45: 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hitchcock P,, Kakuk-Atkins L. The basic helix-loop-helix transcription factor neuroD is expressed in the rod lineage of the teleost retina. J Comp Neurol. 2004; 477: 108–117. [DOI] [PubMed] [Google Scholar]
- 14. Ochocinska MJ,, Hitchcock PF. Dynamic expression of the basic helix-loop-helix transcription factor neuroD in the rod and cone photoreceptor lineages in the retina of the embryonic and larval zebrafish. J Comp Neurol. 2007; 501: 1–12. [DOI] [PubMed] [Google Scholar]
- 15. Forbes-Osborne MA,, Wilson SG,, Morris AC. Insulinoma-associated 1a (Insm1a) is required for photoreceptor differentiation in the zebrafish retina. Dev Biol. 2013; 380: 157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hori K,, Sen A,, Artavanis-Tsakonas S. Notch signaling at a glance. J Cell Sci. 2013; 126: 2135–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Artavanis-Tsakonas S,, Rand MD,, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999; 284: 770–776. [DOI] [PubMed] [Google Scholar]
- 18. Bernardos RL,, Lentz SI,, Wolfe MS,, Raymond PA. Notch-Delta signaling is required for spatial patterning and Müller glia differentiation in the zebrafish retina. Dev Biol. 2005; 278: 381–395. [DOI] [PubMed] [Google Scholar]
- 19. Jadhav AP,, Cho S-H,, Cepko CL. Notch activity permits retinal cells to progress through multiple progenitor states and acquire a stem cell property. Proc Natl Acad Sci U S A. 2006; 103: 18998–19003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheer N,, Groth A,, Hans S,, Campos-Ortega JA. An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development. 2001; 128: 1099–1107. [DOI] [PubMed] [Google Scholar]
- 21. Uribe RA,, Kwon T,, Marcotte EM,, Gross JM. Id2a functions to limit Notch pathway activity and thereby influence the transition from proliferation to differentiation of retinoblasts during zebrafish retinogenesis. Dev Biol. 2012; 371: 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perron M,, Harris WA. Determination of vertebrate retinal progenitor cell fate by the Notch pathway and basic helix-loop-helix transcription factors. Cell Mol Life Sci. 2000; 57: 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nelson BR,, Hartman BH,, Ray CA,, Hayashi T,, Bermingham-Mcdonogh O,, Reh TA. Acheate-scute like 1 (Ascl1) is required for normal delta-like (Dll) gene expression and notch signaling during retinal development. Dev Dyn. 2009; 238: 2163–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nelson BR,, Reh TA. Relationship between Delta-like and proneural bHLH genes during chick retinal development. Dev Dyn. 2008; 237: 1565–1580. [DOI] [PubMed] [Google Scholar]
- 25. Bernardos RL,, Barthel LK,, Meyers JR,, Raymond PA. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J Neurosci. 2007; 27: 7028–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fausett BV,, Goldman D. A role for alpha1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006; 26: 6303–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Chem Biol. 2014; 1–12. [DOI] [PMC free article] [PubMed]
- 28. Gorsuch RA,, Hyde DR. Regulation of Müller glial dependent neuronal regeneration in the damaged adult zebrafish retina. Exp Eye Res. 2014; 123: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lenkowski JR,, Raymond PA. Müller glia: stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res. 2014; 40: 94–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nagashima M,, Barthel LK,, Raymond PA. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development. 2013; 140: 4510–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fimbel SM,, Montgomery JE,, Burket CT,, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007; 27: 1712–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karl MO,, Reh TA. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med. 2010; 16: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hitchcock P,, Ochocinska M,, Sieh A,, Otteson D. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog Retin Eye Res. 2004; 23: 183–194. [DOI] [PubMed] [Google Scholar]
- 34. Conner C,, Ackerman KM,, Lahne M,, Hobgood JS,, Hyde DR. Repressing notch signaling and expressing tnf are sufficient to mimic retinal regeneration by inducing Muller glial proliferation to generate committed progenitor cells. J Neurosci. 2014; 34: 14403–14419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nelson CM,, Ackerman KM,, O'Hayer P,, Bailey TJ,, Gorsuch RA,, Hyde DR. Tumor necrosis factor-alpha is produced by dying retinal neurons and is required for Muller glia proliferation during zebrafish retinal regeneration. J Neurosci. 2013; 33: 6524–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramachandran R,, Zhao X-F,, Goldman D. Insm1a-mediated gene repression is essential for the formation and differentiation of Müller glia-derived progenitors in the injured retina. Nat Cell Biol. 2012; 14: 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wan J,, Ramachandran R,, Goldman D. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev Cell. 2012; 22: 334–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gemberling M,, Bailey TJ,, Hyde DR,, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013; 29: 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thomas JL,, Ochocinska MJ,, Hitchcock PF,, Thummel R. Using the Tg(nrd:egfp)/albino zebrafish line to characterize in vivo expression of neurod. PLoS One. 2012; 7: e29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nasevicius A,, Ekker SC. Effective targeted gene ‘knockdown' in zebrafish. Nat Genet. 2000; 26: 216–220. [DOI] [PubMed] [Google Scholar]
- 41. Carmany-Rampey A,, Moens CB. Modern mosaic analysis in the zebrafish. Methods. 2006; 39: 228–238. [DOI] [PubMed] [Google Scholar]
- 42. Alvarez-Delfin K, Morris AC, Snelson CD, et al. . Tbx2b is required for ultraviolet photoreceptor cell specification during zebrafish retinal development. Proc Natl Acad Sci U S A. 2009; 106: 2023–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Link BA,, Fadool JM,, Malicki J,, Dowling JE. The zebrafish young mutation acts non-cell-autonomously to uncouple differentiation from specification for all retinal cells. Development. 2000; 127: 2177–2188. [DOI] [PubMed] [Google Scholar]
- 44. Fadool JM. Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev Biol. 2003; 258: 277–290. [DOI] [PubMed] [Google Scholar]
- 45. Hwang WY, Fu Y, Reyon D, et al. . Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013; 31: 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Westerfield M. Chapter 9: Molecular methods. : The Zebrafish Book A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: University of Oregon Press; 2000. [Google Scholar]
- 47. Geling A,, Steiner H,, Willem M,, Bally-Cuif L,, Haass C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002; 3: 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kawakami K, Abe G, Asada T, et al. . zTrap: zebrafish gene trap and enhancer trap database. BMC Dev Biol. 2010; 10: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scheer N,, Campos-Ortega JA. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech Devel. 1999; 80: 153–158. [DOI] [PubMed] [Google Scholar]
- 50. Luo J,, Uribe RA,, Hayton S,, Calinescu A-A,, Gross JM,, Hitchcock PF. Midkine-A functions upstream of Id2a to regulate cell cycle kinetics in the developing vertebrate retina. Neural Dev. 2012; 7: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barthel LK,, Raymond PA. Subcellular localization of α-tubulin and opsin mRNA in the goldfish retina using digoxigenin-labeled cRNA probes detected by alkaline phosphatase and HRP histochemistry. J Neurosci Methods. 1993; 50: 145–152. [DOI] [PubMed] [Google Scholar]
- 52. David R,, Wedlich D. PCR-based RNA probes: a quick and sensitive method to improve whole mount embryo in situ hybridizations. Biotech. 2001; 30: 769–775. [PubMed] [Google Scholar]
- 53. Obholzer N, Wolfson S, Trapani JG, et al. . Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J Neurosci. 2008; 28: 2110–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vihtelic TS,, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000; 44: 289–307. [DOI] [PubMed] [Google Scholar]
- 55. Vihtelic TS,, Soverly JE,, Kassen SC,, Hyde DR. Retinal regional differences in photoreceptor cell death and regeneration in light-lesioned albino zebrafish. Exp Eye Res. 2006; 82: 558–575. [DOI] [PubMed] [Google Scholar]
- 56. Taylor S,, Chen J,, Luo J,, Hitchcock P. Light-Induced Photoreceptor Degeneration in the Retina of the Zebrafish. Methods in Molecular Biology. Totowa, NJ: Humana Press; 2012: 247–254. [DOI] [PubMed] [Google Scholar]
- 57. Thomas JL,, Nelson CM,, Luo X,, Hyde DR,, Thummel R. Characterization of multiple light damage paradigms reveals regional differences in photoreceptor loss. Exp Eye Res. 2012; 97: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bernardos RL,, Raymond PA. GFAP transgenic zebrafish. Gene Expr Patterns. 2006; 6: 1007–1013. [DOI] [PubMed] [Google Scholar]
- 59. Thummel R,, Bailey TJ,, Hyde DR. In vivo electroporation of morpholinos into the adult zebrafish retina. J Vis Exp. 2011; 27: e3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Craig SEL,, Thummel R,, Ahmed H,, Vasta GR,, Hyde DR,, Hitchcock PF. The zebrafish galectin Drgal1-l2 is expressed by proliferating Müller glia and photoreceptor progenitors and regulates the regeneration of rod photoreceptors. Invest Ophthalmol Vis Sci. 2010; 51: 3244–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thummel R,, Kassen SC,, Montgomery JE,, Enright JM,, Hyde DR. Inhibition of Müller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev Neurobiol. 2008; 68: 392–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chapouton P, Skupien P, Hesl B, et al. . Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J Neurosci. 2010; 30: 7961–7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stenkamp DL. Neurogenesis in the fish retina. Int Rev Cytol. 2007; 259: 173–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zou J,, Lathrop KL,, Sun M,, Wei X. Intact retinal pigment epithelium maintained by nok is essential for retinal epithelial polarity and cellular patterning in zebrafish. J Neurosci. 2008; 28: 13684–13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schmitt EA,, Dowling JE. Early retinal development in the zebrafish, Danio rerio: light and electron microscopic analyses. J Comp Neurol. 1999; 404: 515–536. [PubMed] [Google Scholar]
- 66. Chen J,, Rattner A,, Nathans J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J Neurosci. 2005; 25: 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shen YC,, Raymond PA. Zebrafish cone-rod (crx) homeobox gene promotes retinogenesis. Dev Biol. 2004; 269: 237–251. [DOI] [PubMed] [Google Scholar]
- 68. Kok FO, Shin M, Ni C-W, et al. . Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell. 2015; 32: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schulte-Merker S,, Stainier DY. Out with the old in with the new: reassessing morpholino knockdowns in light of genome editing technology. Development. 2014; 141: 3103–3104. [DOI] [PubMed] [Google Scholar]
- 70. Agathocleous M,, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Annu Rev Cell Dev Biol. 2009; 25: 45–69. [DOI] [PubMed] [Google Scholar]
- 71. Dyer MA,, Cepko CL. Regulating proliferation during retinal development. Nat Rev Neurosci. 2001; 2: 333–342. [DOI] [PubMed] [Google Scholar]
- 72. Reese BE. Development of the retina and optic pathway. Vision Res. 2011; 51: 613–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Swaroop A,, Kim D,, Forrest D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Chem Biol. 2010; 11: 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Epstein DJ,, McMahon AP,, Joyner AL. Regionalization of Sonic hedgehog transcription along the anteroposterior axis of the mouse central nervous system is regulated by Hnf3-dependent and -independent mechanisms. Development. 1999; 126: 281–292. [DOI] [PubMed] [Google Scholar]
- 75. Nemer G,, Nemer M. Transcriptional activation of BMP-4 and regulation of mammalian organogenesis by GATA-4 and -6. Dev Biol. 2003; 254: 131–148. [DOI] [PubMed] [Google Scholar]
- 76. Ramachandran R,, Zhao X-F,, Goldman D. Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration. Proc Natl Acad Sci U S A. 2011; 108: 15858–15863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xiao YQ,, Freire-de-Lima CG,, Schiemann WP,, Bratton DL,, Vandivier RW,, Henson PM. Transcriptional and translational regulation of TGF-beta production in response to apoptotic cells. J Immunol. 2008; 181: 3575–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhuang L, Hulin J-A, Gromova A, et al. . Barx2 and Pax7 have antagonistic functions in regulation of wnt signaling and satellite cell differentiation. Stem Cells. 2014; 32: 1661–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Beurdeley M, Spatazza J, Lee HHC, et al. . Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci. 2012; 32: 9429–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lesaffre B,, Joliot A,, Prochiantz A,, Volovitch M. Direct non-cell autonomous Pax6 activity regulates eye development in the zebrafish. Neural Dev. 2007; 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nishida A, Furukawa A, Koike C, et al. . Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003; 6: 1255–1263. [DOI] [PubMed] [Google Scholar]
- 82. Noguchi H,, Bonner-Weir S,, Wei FY,, Matsushita M,, Matsumoto S. BETA2/NeuroD protein can be transduced into cells due to an arginine- and lysine-rich sequence. Diabetes. 2005; 54: 2859–2866. [DOI] [PubMed] [Google Scholar]
- 83. Fortini ME. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol. 2002; 3: 673–684. [DOI] [PubMed] [Google Scholar]
- 84. Jadhav AP,, Mason HA,, Cepko CL. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development. 2006; 133: 913–923. [DOI] [PubMed] [Google Scholar]
- 85. Henrique D, Hirsinger E, Adam J, et al. . Maintenance of neuroepithelial progenitor cells by Delta-Notch signalling in the embryonic chick retina. Curr Biol. 1997; 7: 661–670. [DOI] [PubMed] [Google Scholar]
- 86. Joshi I, Minter LM, Telfer J, et al. . Notch signaling mediates G1/S cell-cycle progression in T cells via cyclin D3 and its dependent kinases. Blood. 2009; 113: 1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cohen B, Shimizu M, Izrailit J, et al. . Cyclin D1 is a direct target of JAG1-mediated Notch signaling in breast cancer. Breast Cancer Res Treat. 2009; 123: 113–124. [DOI] [PubMed] [Google Scholar]
- 88. Das D, Lanner F, Main H, et al. . Notch induces cyclin-D1-dependent proliferation during a specific temporal window of neural differentiation in ES cells. Dev Biol. 2010; 348: 153–166. [DOI] [PubMed] [Google Scholar]
- 89. Ronchini C,, Capobianco AJ. Induction of cyclin D1 transcription and CDK2 activity by Notchic: implication for cell cycle disruption in transformation by Notchic. Mol Cell Biol. 2001; 21: 5925–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yu Y, Zhang Y, Guan W, et al. . Androgen receptor promotes the oncogenic function of overexpressed jagged1 in prostate cancer by enhancing cyclin B1 expression via Akt phosphorylation. Mol Cancer Res. 2014; 12: 830–842. [DOI] [PubMed] [Google Scholar]
- 91. Liu Y,, Shen Y,, Rest JS,, Raymond PA,, Zack DJ. Isolation and characterization of a zebrafish homologue of the cone rod homeobox gene. Invest Ophthalmol Vis Sci. 2001; 42: 481–487. [PubMed] [Google Scholar]
- 92. Morris AC,, Scholz TL,, Brockerhoff SE,, Fadool JM. Genetic dissection reveals two separate pathways for rod and cone regeneration in the teleost retina. Dev Neurobiol. 2008; 68: 605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Brockerhoff SE,, Fadool JM. Genetics of photoreceptor degeneration and regeneration in zebrafish. Cell Mol Life Sci. 2011; 68: 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gao Z, Ure K, Ables JL, et al. . Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009; 12: 1090–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gregg RG,, Willer GB,, Fadool JM,, Dowling JE,, Link BA. Positional cloning of the young mutation identifies an essential role for the Brahma chromatin remodeling complex in mediating retinal cell differentiation. Proc Natl Acad Sci U S A. 2003; 100: 6535–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Laranjeiro R,, Whitmore D. Transcription factors involved in retinogenesis are co-opted by the circadian clock following photoreceptor differentiation. Development. 2014; 141: 2644–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Udolph G,, Rath P,, Chia W. A requirement for Notch in the genesis of a subset of glial cells in the Drosophila embryonic central nervous system which arise through asymmetric divisions. Development. 2001; 128: 1457–1466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.