Abstract
Background. Despite the benefits of antiretroviral therapy (ART), tuberculosis (TB) is the leading cause of mortality among human immunodeficiency virus (HIV)-infected persons in Africa. Nigeria bears the highest TB burden in Africa and second highest HIV burden globally. This long-term multicenter study aimed to determine the incidence rate and predictors of TB in adults in the Harvard/AIDS Prevention Initiative in Nigeria (APIN) and President's Emergency Plan for AIDS Relief (PEPFAR) Nigeria ART program.
Methods. This retrospective evaluation used data collected from 2004 to 2012 through the Harvard/APIN PEPFAR program. Risk factors for incident TB were determined using multivariate Cox proportional hazards regression with time-dependent covariates.
Results. Of 50 320 adults enrolled from 2005 to 2010, 11 092 (22%) had laboratory-confirmed active TB disease at ART initiation, and 2021 (4%) developed active TB after commencing ART. During 78 228 total person-years (PY) of follow-up, the TB incidence rate was 25.8 cases per 1000 PY (95% confidence interval [CI], 24.7–27.0) overall, and it decreased significantly both with duration on ART and calendar year. Risk factors at ART initiation for incident TB included the following: earlier ART enrollment year, tenofovir-containing initial ART regimen, and World Health Organization clinical stage above 1. Time-updated risk factors included the following: low body mass index, low CD4+ cell count, unsuppressed viral load, anemia, and ART adherence below 80%.
Conclusions. The rate of incident TB decreased with longer duration on ART and over the program years. The strongest TB risk factors were time-updated clinical markers, reinforcing the importance of consistent clinical and laboratory monitoring of ART patients in prompt diagnosis and treatment of TB and other coinfections.
Keywords: antiretroviral therapy, HIV, incidence, Nigeria, tuberculosis
Despite a decline in prevalence of human immunodeficiency virus (HIV) from 3.7% in 2003 to 3.2% in 2014, Nigeria has 3.4 million people currently living with HIV, the second highest HIV burden globally [1]. The first national tuberculosis (TB) prevalence survey by the National TB and Leprosy Control Programme (NTBLCP) in 2012 resulted in adjustment of Nigeria's 2013 TB prevalence estimate to 570 000, ranking it the highest TB burden country in Africa [2]. The survey also led to the downward revision of Nigeria's TB case detection rate to 16%, the poorest of all high TB burden countries.
Tuberculosis is the leading cause of mortality among HIV-infected persons in Africa; the relative risk of developing TB is approximately 20 times greater in HIV-infected persons than in HIV-uninfected persons [3]. Although antiretroviral therapy (ART) reduces TB risk in HIV-infected individuals by 67% [4], incidence remains higher among HIV-infected patients receiving ART than among the HIV-uninfected population [5, 6], and HIV/TB-coinfected individuals have an increased risk of mortality [7–9]. Compounding the problem, TB diagnostics lack sensitivity among HIV-infected patients [10] and drug-resistant TB is a growing problem [11].
Through funding from the President's Emergency Plan for AIDS Relief (PEPFAR), the Harvard T.H. Chan School of Public Health collaborated with the AIDS Prevention Initiative in Nigeria (APIN) to support delivery of ART to over 100 000 HIV-positive patients from 2004 to 2012. There have been no multicenter studies assessing the incidence rate of and risk factors for TB among patients receiving ART in Nigeria, and few studies were conducted in other low- and middle-income countries (LMIC). Our objectives were to determine the incidence rate of TB and its temporal trends and to identify demographic and clinical risk factors for incident TB among ART patients.
METHODS
Study Design and Participants
We conducted a retrospective cohort study using electronic data collected as part of routine clinical care by the Harvard/APIN PEPFAR Nigeria program [12]. Human immunodeficiency virus care and treatment conformed to Nigeria's national standards. In the early years of the program, ART was initiated in HIV-infected patients with CD4+ cell count <200 cells/mm3 or with symptoms and CD4+ cell count <350 cells/mm3. In 2010, eligibility criteria expanded to include CD4+ cell count <350 cells/mm3 regardless of symptoms. After ART initiation, patients were scheduled for routine clinical visits at 3 and 6 months and, if stable, every 6 months thereafter. At each visit, TB symptom screening, physical examination, and relevant laboratory tests, including CD4+ cell count, viral load (VL), hematology, and chemistries, were performed, as previously described [13].
We evaluated data from HIV-1-infected, antiretroviral-naive adults (age ≥15 years) who initiated ART on a standard first-line zidovudine (AZT)- or tenofovir (TDF)-containing regimen. Because AZT and TDF were introduced into the program mid-2005, these patients enrolled on ART between May 2005 and November 2010 and had at least 15 months of follow-up observation, with data available through February 2012. Patients who did not provide written informed consent for their data to be used for research were not included. Our final cohort included patients from 30 ART sites in 9 of 36 Nigerian states: Benue, Borno, Enugu, Kaduna, Lagos, Plateau, Ogun, Oyo, and Yobe.
This study was approved by the institutional review board (IRB) at Harvard, and all consent forms were approved by the IRBs at Harvard, APIN, and all affiliated sites.
Tuberculosis Screening and Diagnosis
Tuberculosis management followed the Nigerian national guidelines of the NTBLCP [14]. Upon entry into the program and at ART initiation, all patients underwent TB testing with chest radiography and acid-fast bacilli (AFB) microscopy, which required collection of 3 sputum specimens over 2 consecutive days, assisted by symptom assessment (current cough, night sweats, weight loss, fever, and history of contact with a person with chronic cough). At all subsequent clinic visits, patients were routinely clinically screened for TB, and those with symptoms suggestive of TB were tested with sputum AFB microscopy and chest radiography, as described above. All AFB smear-positive cases were diagnosed as active TB. If smear-negative, the treating clinician made a diagnosis of TB based on clinical symptoms and radiologic and histologic results. Mycobacterial culture was not routinely available. Extrapulmonary TB cases were diagnosed based on general TB and organ-specific symptoms, assisted by sputum AFB testing and histology, as available.
The date corresponding to the first clinical record indicating a positive pulmonary or extrapulmonary TB diagnosis or receipt of TB treatment at a PEPFAR center or an outside TB center (ie, national directly observed treatment, short course [DOTS] center) was designated as the initial TB date. For this analysis, “prevalent TB” was defined as a case with an initial TB date 6 months before or up to 3 months after ART initiation. “Incident TB” was defined as a case with an initial TB date after the first 3 months on ART.
Data
Clinical variables were collapsed into categories based on relevant thresholds for the regression analysis. Body mass index (BMI) was grouped into 5 World Health Organization (WHO)-defined categories (Table 1). Anemia was defined using WHO-recommended hemoglobin cutoffs: nonanemia (≥12 g/dL for women and ≥13 g/dL for men), mild/moderate anemia (8–11.9 g/dL for women and 8–12.9 g/dL for men), and severe anemia (<8 g/dL) (pregnancy was not differentiated) [15]. Time-updated VLs were categorized as suppressed (≤400 copies/mL) or unsuppressed (>400 copies/mL) throughout follow-up. Using pharmacy prescription electronic refill records, which have been shown to be a valid proxy [16, 17], average percentage ART adherence was calculated as number of days supplied over total days in each time interval, adjusting for leftover medication. Adherence categories were based on previously published conventions: ≥95%, 80%–94.9%, and <80% [18–20].
Table 1.
Frequencies of Sociodemographic and Clinical Characteristics at ART Initiation by TB Status Among ARV-Naive Adults Enrolled in Harvard/APIN PEPFAR ART Program 2005–2010
| Characteristics | TB Status, Patients, No. (%) |
||||
|---|---|---|---|---|---|
| All Patients | No TB During ART | Prevalent TB | Incident TB During ART | P Value | |
| Total | 43 703 (100) | 30 590 (100) | 11 092 (100) | 2021 (100) | – |
| Age, yr | <.001 | ||||
| 15–29 | 11 751 (26.9) | 8716 (28.5) | 2484 (22.4) | 551 (27.3) | |
| 30–34 | 10 044 (23.0) | 7106 (23.2) | 2468 (22.3) | 470 (23.3) | |
| 35–41 | 11 571 (26.5) | 7806 (25.5) | 3234 (29.2) | 531 (26.3) | |
| 42+ | 10 312 (23.6) | 6951 (22.7) | 2894 (26.1) | 467 (23.1) | |
| Sex | <.001 | ||||
| Male | 15 433 (35.3) | 9781 (32.0) | 4928 (44.4) | 724 (35.8) | |
| Female | 28 270 (64.7) | 20 809 (68.0) | 6164 (55.6) | 1297 (64.2) | |
| Education | <.001 | ||||
| None | 7967 (18.5) | 5645 (18.7) | 1926 (17.6) | 396 (19.8) | |
| Primary | 9497 (22.0) | 6398 (21.2) | 2691 (24.6) | 408 (20.4) | |
| Secondary | 15 236 (35.3) | 10 607 (35.1) | 3938 (36.1) | 691 (34.5) | |
| Tertiary | 10 437 (24.2) | 7563 (25.0) | 2367 (21.7) | 507 (25.3) | |
| Employment status | <.001 | ||||
| Nonincome generating | 10 439 (24.1) | 7642 (25.2) | 2289 (20.9) | 508 (25.3) | |
| Laborer/service worker | 30 066 (69.5) | 20 714 (68.4) | 7994 (73.0) | 1358 (67.8) | |
| Manager/professional | 2740 (6.3) | 1933 (6.4) | 669 (6.1) | 138 (6.9) | |
| Marital status | <.001 | ||||
| Single | 8483 (19.6) | 5786 (19.1) | 2315 (21.1) | 382 (19.0) | |
| Married | 25 078 (57.9) | 17 866 (58.9) | 6046 (55.1) | 1166 (58.0) | |
| Separated/divorced | 3824 (8.8) | 2536 (8.4) | 1102 (10.0) | 186 (9.2) | |
| Widowed | 5958 (13.7) | 4169 (13.7) | 1512 (13.8) | 277 (13.8) | |
| HIV risk factor | .008 | ||||
| Heterosexual only | 39 066 (95.6) | 27 443 (95.8) | 9818 (95.1) | 1805 (94.8) | |
| Other/multiple | 1819 (4.4) | 1217 (4.2) | 503 (4.9) | 99 (5.2) | |
| ART enrollment year | <.001 | ||||
| 2005 | 2072 (4.7) | 1325 (4.3) | 598 (5.4) | 149 (7.4) | |
| 2006 | 3964 (9.1) | 2687 (8.8) | 980 (8.8) | 297 (14.7) | |
| 2007 | 6405 (14.7) | 4254 (13.9) | 1741 (15.7) | 410 (20.3) | |
| 2008 | 10 851 (24.8) | 7433 (24.3) | 2881 (26.0) | 537 (26.6) | |
| 2009 | 10 968 (25.1) | 7728 (25.3) | 2832 (25.5) | 408 (20.2) | |
| 2010 | 9443 (21.6) | 7163 (23.4) | 2060 (18.6) | 220 (10.9) | |
| Treatment site type | <.001 | ||||
| Secondary hospital | 4305 (9.9) | 3130 (10.2) | 1013 (9.1) | 162 (8.0) | |
| Tertiary hospital | 39 398 (90.1) | 27 460 (89.8) | 10 079 (90.9) | 1859 (92.0) | |
| Initial ART regimen | <.001 | ||||
| TDF + XTC + EFV | 8415 (19.3) | 3955 (12.9) | 4227 (38.1) | 233 (11.5) | |
| TDF + XTC + NVP | 9058 (20.7) | 6729 (22.0) | 1756 (15.8) | 573 (28.4) | |
| AZT + 3TC + EFV | 4599 (10.5) | 2374 (7.8) | 2074 (18.7) | 151 (7.5) | |
| AZT + 3TC + NVP | 21 631 (49.5) | 17 532 (57.3) | 3035 (27.4) | 1064 (52.6) | |
| WHO clinical stage | <.001 | ||||
| 1 | 8647 (23.7) | 7435 (28.8) | 848 (9.4) | 364 (22.3) | |
| 2 | 9921 (27.2) | 8047 (31.1) | 1341 (14.8) | 533 (32.7) | |
| 3 | 12 805 (35.1) | 7381 (28.6) | 4913 (54.4) | 511 (31.3) | |
| 4 | 5137 (14.1) | 2979 (11.5) | 1936 (21.4) | 222 (13.6) | |
| Hepatitis B | .035 | ||||
| No | 25 229 (83.9) | 18 013 (84.1) | 6037 (83.8) | 1179 (81.5) | |
| Yes | 4857 (16.1) | 3418 (15.9) | 1171 (16.2) | 268 (18.5) | |
| Hepatitis C | .236 | ||||
| No | 28 175 (94.6) | 20 073 (94.4) | 6753 (95.0) | 1349 (94.5) | |
| Yes | 1623 (5.4) | 1185 (5.6) | 359 (5.0) | 79 (5.5) | |
| Body mass index, kg/m2 | <.001 | ||||
| <16 (severely underweight) | 1703 (5.4) | 872 (4.0) | 751 (9.2) | 80 (5.1) | |
| 16–18.49 (underweight) | 4659 (14.8) | 2758 (12.6) | 1644 (20.2) | 257 (16.4) | |
| 18.5–24.99 (normal) | 19 387 (61.4) | 13 631 (62.3) | 4786 (58.9) | 970 (61.8) | |
| 25–29.99 (overweight) | 4577 (14.5) | 3568 (16.3) | 795 (9.8) | 214 (13.6) | |
| ≥30 (obese) | 1238 (3.9) | 1045 (4.8) | 144 (1.8) | 49 (3.1) | |
| CD4+ cell count, cells/µL | <.001 | ||||
| ≤100 | 13 888 (33.4) | 8700 (29.7) | 4502 (43.2) | 686 (35.7) | |
| 101–200 | 16 155 (38.8) | 11 715 (40.0) | 3668 (35.2) | 772 (40.1) | |
| 201–350 | 10 018 (24.1) | 7721 (26.4) | 1882 (18.1) | 415 (21.6) | |
| >350 | 1575 (3.8) | 1157 (3.9) | 368 (3.5) | 50 (2.6) | |
| Viral load, copies/mL | <.001 | ||||
| 0–1000 | 2962 (7.8) | 2174 (8.2) | 655 (6.8) | 133 (7.6) | |
| 1001–10 000 | 5194 (13.7) | 3899 (14.7) | 1108 (11.5) | 187 (10.7) | |
| 10 001–100 000 | 13 370 (35.3) | 9630 (36.3) | 3110 (32.4) | 630 (35.9) | |
| 101 000–1 000 000 | 16 326 (43.1) | 10 792 (40.7) | 4731 (49.3) | 803 (45.8) | |
| Anemia | <.001 | ||||
| No anemia | 8959 (23.1) | 7084 (26.0) | 1485 (15.2) | 390 (21.6) | |
| Mild/moderate anemia | 26 443 (68.1) | 18 397 (67.6) | 6779 (69.2) | 1267 (70.3) | |
| Severe anemia | 3432 (8.8) | 1751 (6.4) | 1535 (15.7) | 146 (8.1) | |
Abbreviations: APIN, AIDS Prevention Initiative in Nigeria; ART, antiretroviral therapy; ARV, antiretroviral; AZT, zidovudine; EFV, efavirenz; HIV, human immunodeficiency virus; NVP, nevirapine; PEPFAR, President's Emergency Plan for AIDS Relief; TB, tuberculosis; TDF, tenofovir; WHO, World Health Organization; XTC, lamivudine or emtricitabine; 3TC, lamivudine.
Body mass index, CD4+ cell count, VL, and hemoglobin at enrollment were the measurements closest to month 0 and examined as fixed variables in bivariate analyses only. Time-updated BMI, CD4+ cell count, VL, and hemoglobin used the recorded values closest to the start of each evaluated time interval. Average percentage adherence during a given time interval was evaluated for associations with incident TB in the subsequent time interval.
Statistical Analysis
All statistical analyses were performed using Stata version 13.1 (StataCorp, College Station, TX). Tuberculosis incidence rates were calculated as the number of new cases of TB divided by total person-years (PY) at risk, and expressed per 1000 PY. Because a TB diagnosis within the first 3 months of ART was defined as a prevalent case, time at risk for incident TB began at month 3, the effective baseline for the risk analysis, and ended on either the date of the first recorded TB diagnosis, for those who developed incident TB, or the date of the last recorded ART pick-up, for those who were never diagnosed with TB during the observation period. Censoring of patients occurred at discontinuation from the program due to death, transfer, withdrawal, or loss to follow-up, or at the end of data collection on February 29, 2012. The Kaplan–Meier estimator of the survivor function was used to generate a TB-free survival curve. Annual prevalent TB rates were calculated as the number of patients with TB at ART initiation divided by the total number of patients initiating ART each year.
Cox proportional hazards models were generated to evaluate risk factors for incident TB during ART. For the time-dependent data set, the first follow-up interval started at month 3 and ended at month 6, and all intervals thereafter were 6 months. The log-rank test was used for bivariate analyses, and variables with P ≤ .20 were considered for inclusion in multivariate Cox models.
For multivariate analyses, treatment site was used as a cluster variable. All variables with P ≤ .05 in the multivariate analyses and additional clinically relevant variables were retained in the final models. Clinically plausible interactions were tested, revealing significant interactions between sex with BMI and sex with anemia, which were included in the final models. The proportionality assumption was tested for each predictor by graphing the survival functions and using Schoenfeld residuals.
Missing data ranged from 0% (for sex, treatment site, enrollment year, initiating ART regimen) to 31.6% (hepatitis C) for baseline characteristics. Imputed baseline variables included age, education, income group, marital status, HIV risk factor, WHO stage, hepatitis B, hepatitis C, and height. Imputed time-updated variables included weight, CD4+ cell count, VL, and hemoglobin. To address potential bias resulting from missing data, 10 imputed datasets were created in which missing values for fixed and time-updated variables were imputed using the “two-fold” fully conditional specification algorithm [21]. Multivariate analyses were performed using both complete cases and following multiple imputations.
RESULTS
A total of 50 320 HIV-1-infected ART-naive adults initiated treatment on a standard first-line AZT- or TDF-containing regimen between May 2005 and November 2010 (Figure 1). Of those patients, 11 092 (22.0%) had active TB disease at ART initiation and were excluded from the incidence rate and risk factor analyses. Of the patients who were TB-negative at ART initiation (39 228), 6617 (16.9%) patients discontinued ART before 3 months, and they also could not be assessed for incident TB. The prevalent TB and discontinued patient groups were compared with included patients, and both groups had higher likelihoods of being male and having regimens containing efavivenz (vs nevirapine) and TDF (vs AZT), advanced WHO clinical stage, lower BMI, CD4+ cell count ≤100 cells/mm3, VL > 100 000 copies/mL, and severe anemia at ART initiation than included patients (Table 1; data for discontinued not shown). In addition, prevalent TB patients were more likely to be older, and discontinued patients more likely to have attained a lower educational level and have initial CD4+ cell count >350 cells/mm3, than included patients.
Figure 1.
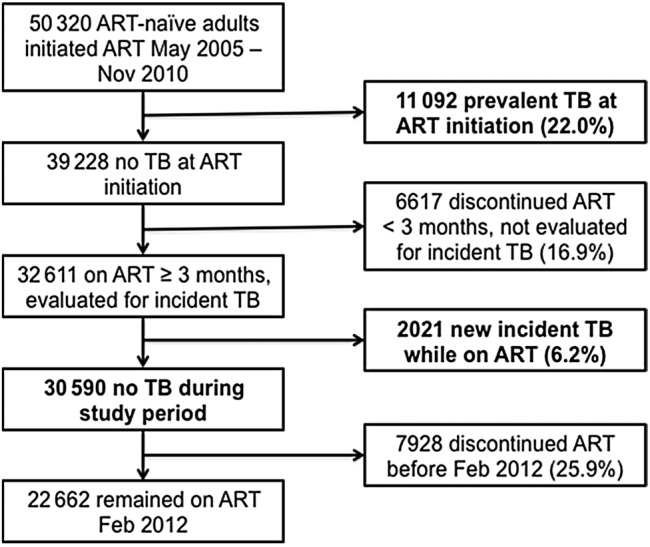
Study population diagram. Abbreviations: ART, antiretroviral therapy; TB, tuberculosis.
Tuberculosis Incidence Rate
Of 32 611 patients included in the incident TB analyses, 2021 (6.2%) developed active incident TB after ART initiation. Of the 30 590 patients who remained TB-free, 7928 (25.9%) discontinued ART before February 29, 2012 due to death, transfer, withdrawal, or loss to follow-up, and contributed person-time at risk for TB until censoring at their last recorded ART pick-up. The median follow-up time since ART initiation for the 32 611 patients was 29.2 months (interquartile range [IQR], 17.8–43.2), contributing a total of 78 228 PY at risk for TB. The overall TB incidence rate was 25.8 cases per 1000 PY on ART (95% confidence interval [CI], 24.7–27.0).
The TB incidence rate decreased with duration on ART, most significantly from Year 1 to 2 (Figure 2). From 45.6 cases per 1000 PY (95% CI, 42.9–48.5) in Year 1, TB incidence declined to 6.8 cases per 1000 PY (95% CI, 3.9–11.7) by Year 6. The probability of TB-free survival for patients who did not have TB at ART enrollment decreased from 0.96 in Year 1 to 0.90 in Year 6 (Figure 3).
Figure 2.
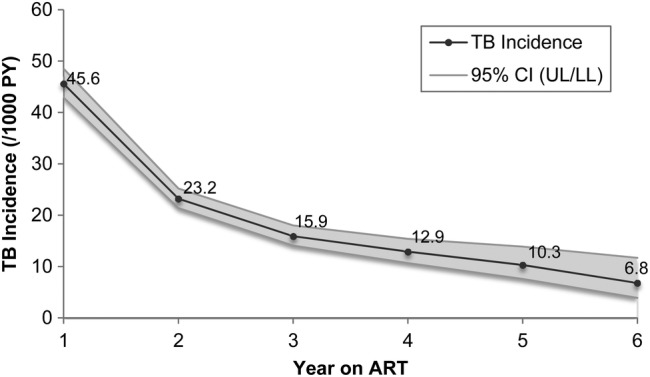
Rate of first incident tuberculosis (TB) occurrence by duration on antiretroviral therapy (ART). Abbreviations: CI, confidence interval; PY, person-years.
Figure 3.
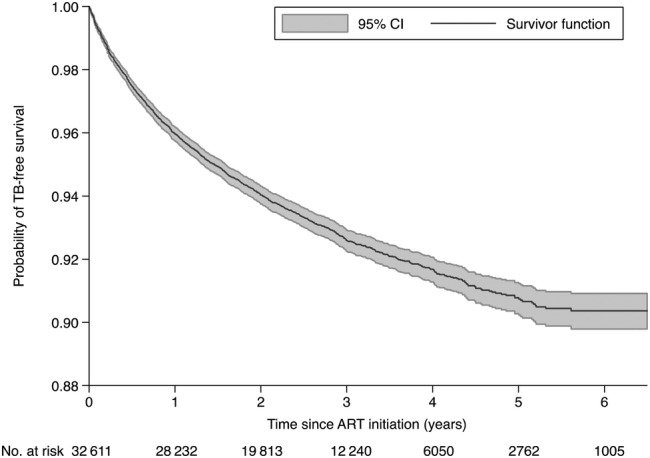
Kaplan-Meier tuberculosis (TB)-free survival curve with 95% confidence interval (CI). Abbreviation: ART, antiretroviral therapy.
The percentage of patients with prevalent TB of total patients initiating ART each calendar year remained relatively steady from 2005 to 2010, averaging 22.6% (range, 7.2%; Figure 4A). In contrast, the annual rate of incident TB during ART for all patients without TB at ART initiation decreased each calendar year from 49.4 cases per 1000 PY (95% CI, 40.8–59.8) in 2006 to 13.6 cases per 1000 PY (95% CI, 12.2–15.1) in 2011 (Figure 4B). These rates were calculated using all incident TB cases and all PYs at risk for TB in each year. Because early ART is associated with higher TB incidence and later ART with reduced TB incidence, the declining incidence rate partially reflects the temporal shift in the program's patient composition from newly enrolled patients who contributed PYs during early ART to increasing proportions of ART-experienced patients who contributed PYs during later ART.
Figure 4.
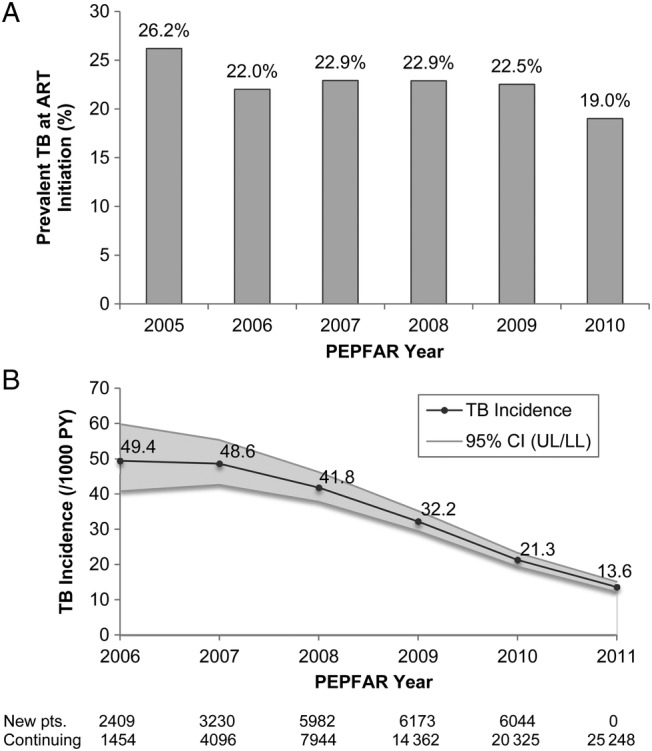
Tuberculosis (TB) rates by the President's Emergency Plan for AIDS Relief (PEPFAR) program year: percentage prevalent TB of patients initiating antiretroviral therapy (ART) 2005–2010 (A) and rate of first incidence TB occurrence during ART 2006–2011 (B). Abbreviations: CI, 95% confidence interval; pts, patients.
Risk Factors for Incident Tuberculosis
In bivariate analyses, male sex, HIV transmission risk factor other than heterosexual sex, earlier ART enrollment year, TDF-containing initial ART regimen, advanced WHO clinical stage at ART initiation, hepatitis B virus infection at ART initiation, low BMI, low CD4+ cell count, high VL, anemia, and poor average percentage ART adherence were all associated with increased risk of incident TB (Table 2).
Table 2.
Cox Regression Analysis of Demographic and Clinical Variables Associated With Incident TB Among ARV-Naive Adults Enrolled in Harvard/APIN PEPFAR Program 2005–2010
| Bivariate Analysis (N = 32 611) |
Multivariate Analysesa |
|||||
|---|---|---|---|---|---|---|
| Complete Cases (N = 12 996) |
Multiple Imputations (N = 32 611) |
|||||
| Unadjusted HR (95% CI) |
P Value | Adjusted HR (95% CI) |
P Value | Adjusted HR (95% CI) |
P Value | |
| Age, yr | ||||||
| 15–29 | Ref | |||||
| 30–34 | 1.02 (.92–1.12) | .763 | ||||
| 35–41 | 1.05 (.96–1.14) | .276 | ||||
| 42+ | 1.04 (.90–1.21) | .591 | ||||
| Sex | ||||||
| Male | Ref | Ref | Ref | |||
| Female | 0.84 (.76–.93) | .001 | 1.39 (.49–3.98) | .534 | 1.29 (.59–2.81) | .525 |
| Education | ||||||
| None | Ref | |||||
| Primary | 0.90 (.76–1.07) | .239 | ||||
| Secondary | 0.91 (.75–1.10) | .313 | ||||
| Tertiary | 0.89 (.75–1.05) | .176 | ||||
| Employment status | ||||||
| Nonincome-generating | Ref | |||||
| Laborer/service worker | 1.00 (.89–1.12) | .976 | ||||
| Manager/professional | 1.02 (.90–1.15) | .770 | ||||
| Marital status | ||||||
| Single | Ref | |||||
| Married | 0.95 (.86–1.05) | .323 | ||||
| Separated/divorced | 1.10 (.94–1.29) | .239 | ||||
| Widowed | 0.96 (.79–1.17) | .694 | ||||
| HIV risk factor | ||||||
| Heterosexual only | Ref | |||||
| Other/multiple | 1.20 (1.05–1.37) | .009 | ||||
| ART enrollment year | ||||||
| 2005–2007 | Ref | Ref | Ref | |||
| 2008–2010 | 0.68 (.52–.90) | .007 | 0.78 (.62–.98) | .031 | 0.68 (.56–.84) | <.001 |
| Treatment site type | ||||||
| Secondary hospital | Ref | |||||
| Tertiary hospital | 1.08 (.82–1.42) | .592 | ||||
| Initial ART regimen | ||||||
| AZT-based | Ref | Ref | Ref | |||
| TDF-based | 1.31 (1.21–1.42) | <.001 | 1.21 (1.07–1.36) | .002 | 1.24 (1.12–1.37) | <.001 |
| WHO clinical stage | ||||||
| 1 (asymptomatic) | Ref | Ref | Ref | |||
| 2–4 (symptomatic) | 1.40 (1.28–1.53) | <.001 | 1.20 (1.02–1.41) | .029 | 1.18 (1.04–1.33) | .010 |
| Hepatitis B | ||||||
| No | Ref | |||||
| Yes | 1.16 (1.08–1.24) | <.001 | ||||
| Hepatitis C | ||||||
| No | Ref | |||||
| Yes | 0.96 (.81–1.13) | .591 | ||||
| Body mass index at enrollmentb, kg/m2 | ||||||
| <16 (severely underweight) | 1.40 (1.13–1.72) | .002 | – | – | ||
| 16–18.49 (underweight) | 1.36 (1.21–1.52) | <.001 | – | – | ||
| 18.5–24.99 (normal) | Ref | – | – | |||
| 25–29.99 (overweight) | 0.83 (.69–1.01) | .057 | – | – | ||
| ≥30 (obese) | 0.65 (.46–.90) | .011 | – | – | ||
| CD4+ cell count at enrollmentb, cells/µL | ||||||
| ≤100 | Ref | – | – | |||
| 101–200 | 0.82 (.75–.91) | <.001 | – | – | ||
| 201–350 | 0.69 (.61–.77) | <.001 | – | – | ||
| >350 | 0.57 (.44–.73) | <.001 | – | – | ||
| Viral load at enrollmentb, copies/mL | ||||||
| 0–1000 | Ref | – | – | |||
| 1001–10 000 | 0.76 (.57–1.03) | .077 | – | – | ||
| 10 001–100 000 | 1.03 (.80–1.32) | .842 | – | – | ||
| >100 000 | 1.19 (.98–1.44) | .085 | – | – | ||
| Anemia at enrollmentb | ||||||
| No anemia | Ref | – | – | |||
| Mild/moderate anemia | 1.34 (1.18–1.51) | <.001 | – | – | ||
| Severe anemia | 1.75 (1.54–1.98) | <.001 | – | – | ||
| Body mass index, kg/m2 | ||||||
| <16 (severely underweight) | 5.33 (4.08–6.98) | <.001 | 4.12 (2.20–7.72) | <.001 | 3.85 (2.75–5.38) | <.001 |
| 16–18.49 (underweight) | 2.32 (1.92–2.80) | <.001 | 2.14 (1.62–2.81) | <.001 | 2.18 (1.80–2.65) | <.001 |
| 18.5–24.99 (normal) | Ref | Ref | Ref | |||
| 25–29.99 (overweight) | 0.74 (.64–.86) | <.001 | 0.78 (.67–.92) | .003 | 0.67 (.58–.78) | <.001 |
| ≥30 (obese) | 0.63 (.52–.77) | <.001 | 0.46 (.23–.93) | .030 | 0.45 (.27–.75) | .002 |
| CD4+ cell count, cells/µL | ||||||
| ≤100 | Ref | Ref | Ref | |||
| 101–200 | 0.42 (.37–.47) | <.001 | 0.60 (.48–.74) | <.001 | 0.63 (.54–.73) | <.001 |
| 201–350 | 0.25 (.21–.30) | <.001 | 0.38 (.28–.52) | <.001 | 0.44 (.38–.52) | <.001 |
| >350 | 0.19 (.17–.21) | <.001 | 0.32 (.27–.37) | <.001 | 0.35 (.32–.38) | <.001 |
| Viral load status | ||||||
| Undetectable (≤400 copies/mL) | Ref | Ref | Ref | |||
| Detectable (>400 copies/mL) | 1.83 (1.65–2.03) | <.001 | 1.26 (1.15–1.38) | <.001 | 1.25 (1.13–1.37) | <.001 |
| Anemia | ||||||
| No anemia | Ref | Ref | Ref | |||
| Mild/moderate anemia | 1.90 (1.66–2.17) | <.001 | 2.48 (2.02–3.04) | <.001 | 2.06 (1.69–2.51) | <.001 |
| Severe anemia | 5.05 (4.01–6.35) | <.001 | 4.36 (1.98–9.62) | <.001 | 3.88 (2.04–7.37) | <.001 |
| Percentage ART adherence | ||||||
| 95%–100% | Ref | Ref | Ref | |||
| 80%–94.9% | 1.15 (1.02–1.31) | .028 | 1.16 (.91–1.47) | .221 | 1.07 (.91–1.24) | .428 |
| <80% | 1.77 (1.50–2.08) | <.001 | 1.42 (1.21–1.68) | <.001 | 1.28 (1.03–1.58) | .023 |
Abbreviations: APIN, AIDS Prevention Initiative in Nigeria; ART, antiretroviral therapy; ARV, antiretroviral; AZT, zidovudine; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratios; PEPFAR, President's Emergency Plan for AIDS Relief; Ref, reference group; TB, tuberculosis; TDF, tenofovir; WHO, World Health Organization.
a For the multivariate analyses, the final models are presented, which included the interactions between sex with BMI and sex with anemia and accounted for correlation within sites using clustered robust standard errors.
b Not included in multivariate models; instead included the respective time-dependent variables, because all were significant.
It is notable that BMI, CD4+ cell count, VL, and anemia at ART initiation were all significantly associated with incident TB, but their respective time-updated markers all had markedly stronger hazard ratios in bivariate analyses (Table 2). Although it had a significant overall P value from the log-rank test, initial VL was not significant at the P = .05 level in any category.
In the multivariate complete case analysis including interactions, earlier ART enrollment year, TDF-containing initial ART regimen, WHO clinical stage 2–4 (symptomatic) at ART initiation, low BMI, low CD4+ cell count, detectable VL, anemia, and average percentage ART adherence below 80% were significant predictors of incident TB. These findings were corroborated in the multiple imputation model.
DISCUSSION
This is the first multisite, programmatic evaluation of TB incidence and risk factors in ART patients in Nigeria. Few others have evaluated TB incidence in LMIC ART programs. Considering those with significant population size and observation time, a South Africa study (n = 7536) reported an overall incidence rate of 42 cases per 1000 PY over a median follow-up of 21.4 months, and a recent Tanzania study (n = 53 056) reported 44 cases per 1000 PY among ART patients over a median follow-up of 24 months [22, 23]. Our incidence rate of 25.8 cases per 1000 PY over a median follow-up of 29.2 months is conservative because we excluded the first 3 months from time at risk for TB; analyses that included the first 3 months reported the highest TB incidence during this period, often attributed to delayed diagnosis and immune reconstitution inflammatory syndrome [5, 7, 22, 24–27].
As anticipated, TB incidence declined with duration on ART, which likely reflects the concurrent CD4+ cell count increase and immune reconstitution due to ART [5–7, 22, 25–29]. The high overall prevalent TB rate of 22% is attributed to Nigeria's high TB burden, HIV coinfection of our patient population, and our designation of TB diagnoses within 3 months of ART initiation as prevalent cases. Because TB testing with chest radiography and AFB microscopy assisted by symptom screening was prescribed for all patients initiating ART, our case detection rate for prevalent TB is expected to be high despite Nigeria's poor overall case detection rate. The persistently high rate of prevalent TB throughout the observed PEPFAR program years is reflective of the TB epidemic in the broader Nigerian population, in which the national TB prevalence remained relatively static at approximately 325 cases per 100 000 population according to WHO estimates during those years [2]. In contrast, the annual rate of incident TB declined notably by over 70% in the treated ART population from 2006 to 2011. Although WHO's estimates of Nigeria's national TB incidence rates remained steady at approximately 340 cases per 100 000 population per year during the same period [2], the downward trend in the TB incidence rate within the PEPFAR ART program reflects the impact of ART on reducing new TB disease in this treated patient population. During these years, Nigeria implemented a national scale-up of comprehensive HIV care programs, significantly bolstered with PEPFAR support beginning in 2004, along with increased collaboration with the national DOTS program.
Even though males comprised a greater percentage of the prevalent TB subset (44.4%) than of the total study population (35.3%; Table 1), male sex was not a significant risk factor for incident TB among ART patients after controlling for other variables and interactions. Previous studies were divided in their findings: some found male sex was a significant risk factor for incident TB during ART [7, 23, 25–27, 30], and others did not [5, 6, 22, 24, 28]. The WHO reported a global male/female TB notification ratio of 1.6 in 2013 [2]; possible explanations include differences in access to care, diagnosis, exposure, risk behaviors, and physiology [31–34]. Despite higher TB notification rates in men, women may have higher rates of progression from TB infection to disease and mortality [31]. The gender disparities in both TB risks and outcomes are complex and worthy of further investigation.
Previous studies reported that poor immunologic and/or virologic status, low BMI, and anemia were associated with incident TB among ART patients [5–7, 22–30, 35–38]. We found that CD4+ cell counts are significant predictors of TB and that values closer to time of TB diagnosis were stronger predictors of incident TB than baseline CD4+ counts; patients with CD4+ cell counts >350 cells/mm3 had one third the risk of developing TB by their next visit compared with those with CD4+ cell counts ≤100 cells/mm3. Although studies in South Africa and Spain found no independent association between VL and incident TB [5, 28, 37], we found that patients with unsuppressed VL had a 25% higher risk of developing TB than those with suppression. In our study, nutritional status was highly correlated with incident TB: severely underweight patients had 4 times the risk and obese patients had half the risk of developing TB compared with patients of normal weight. Furthermore, patients with severe anemia had 4 times the risk of developing TB by their next visit than those without anemia.
To our knowledge, this is the first TB incidence study to show that time-updated ART adherence (<80%) was a continuous significant predictor of TB. Two smaller studies in Mozambique and Nigeria reported an association between adherence evaluated once and incident TB [35, 38], but the large Tanzania study found no association between time-updated adherence with incident TB [23]. Given that ART reduces TB incidence, it is necessary to include time-updated continuous measures of adherence throughout a patient's follow-up in assessing TB risk. Because ART adherence has been correlated with better virologic outcomes [39], poor adherence likely increases susceptibility to TB and other opportunistic infections by impairing virologic suppression and immunologic recovery and inducing development of drug resistance and treatment failure [40]. By incorporating time-updated adherence data into our analyses, we were able to show that monitoring of adherence is crucial for reducing TB risk.
Unlike previous studies, we found significant interactions between sex with BMI and anemia in multivariate analyses. An examination of these interactions revealed that underweight males had higher TB risk than underweight females, and, similarly, anemic males had higher TB risk than anemic females. Thus, although male gender alone was not an independent risk factor for incident TB, being both male and either underweight or anemic increased TB risk, and these subcategories may be driving the apparent gender differential in TB incidence. Sex differences in the role of nutrients, including iron and vitamin D, in the immune response to TB have been suggested as possible explanations for differences in susceptibility, and synergetic links between sex hormones and the immune system have been shown [33, 34]. The previously undetected interactions that we found between sex and nutritional status may provide new epidemiologic evidence of biological differences between males and females in immune response to TB, and these results suggest that nutritional interventions, especially for males, may aid in bolstering TB immunity.
As a large observational, retrospective programmatic evaluation, our analysis has inherent limitations. First, our data were limited by missing information; to address this, we performed a separate multiple imputation analysis, the results of which supported our complete case findings, but which assumes data are missing at random. Despite its limitations, the multiple imputation analysis avoided loss of information and addressed possible differences in the incomplete cases. In addition, although TB testing was done in accordance with the national NTBLCP guidelines, which included AFB testing, radiology, and histology, assisted by symptom screening, the possibility of missing TB cases, including extrapulmonary TB whose diagnosis in resource-limited settings relies primarily on clinical symptoms, and misdiagnosed cases cannot be ignored. Furthermore, because patients may present to the clinic at varying stages of TB disease and reporting of test results may lag, the TB diagnosis date is only a proxy for the time of initiation of active TB disease; we used the earliest TB date based on available records. Finally, our data did not allow us to accurately distinguish true reinfections from recurrences in all cases; therefore, we did not include patients with prevalent TB in the incidence analysis and censored patients who developed incident TB after their first occurrence. Additional analyses, in which DOTS records are accessed, might provide greater resolution of reinfections and recurrences, allowing assessment of reinfections and inclusion of patients with prevalent TB in the incidence analysis to augment our findings.
This study also has notable strengths. To our knowledge, this is the first multicenter assessment of TB incidence and risk factors in ART patients in Nigeria, which has the second highest HIV burden and highest TB burden in Africa. Amongst other studies of TB incidence in patients on ART in LMIC, it remains one of the largest in study population, length of patient observation time, microbiologically confirmed TB, and continual measurements of CD4+ cell count, VL, BMI, anemia, and adherence. There has been notable variation among previous studies in reported risk factors and the nature and degree of risk associations due to differences in sample size, duration, methods, and variables included; the large number of patients in our program and follow-up period of over 6 years (32 611 patients observed for a median of 29.2 months totaling 78 228 PY) contribute to its significant statistical power, providing stronger evidence to support the reports that found significant correlations between immunologic, virologic, and nutritional statuses with incident TB. In addition to baseline markers, we examined a number of relevant clinical risk factors as time-dependent covariates for the entire length of each patient's observation period, which is necessary because these markers, like TB risk, vary over the course of ART, and as they fluctuate, TB risk changes accordingly. In addition, this is the first study to show that poor ART adherence is a continuous significant predictor of incident TB, indicating the value of adherence monitoring as a TB intervention. Finally, despite conflicting reports on gender's association with incident TB, this study is the first to report that gender is not an independent risk factor but an effect modifier in the significant associations of BMI and anemia with TB, suggesting gender differences in nutrition's role in TB immunity.
CONCLUSIONS
In conclusion, we found that although prevalent and incident TB rates were high in this Nigerian ART program, the overall TB incidence rate among treated patients decreased significantly over the observed years. In addition to providing stronger evidence for previously identified risk factors of TB in HIV-infected patients, we have presented novel findings on additional predictors and further elucidated the association between BMI and anemia with TB incidence through examination of interactions with gender. Our findings provide valuable information for programs designing health interventions for identifying high-risk patients, clinical and laboratory monitoring, adherence counseling and monitoring, and targeted early diagnosis and treatment.
Acknowledgments
We are grateful to all of the patients whose data were used in this study. We are also grateful to the clinical, laboratory, and data staff in the Harvard/AIDS Prevention Initiative in Nigeria and President's Emergency Plan for AIDS Relief program whose dedication and effort enabled this work.
Author contributions. P. J. K. provided the concept and oversight for the study. C. A. C. designed the study, conducted the literature search, collected and analyzed the data, and drafted the report with figures. S. T. M. oversaw the data analysis, and, along with G. E. and E. T. T., provided feedback on statistical methods and data interpretation. B. C. provided technical assistance on database methods, and H. E. R., P. A., and P. O. contributed programmatic expertise. All authors reviewed, revised, and approved the final report.
Disclaimers. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. Funders had no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
Financial support. This work was supported by the US Department of Health and Human Services, Health Resources and Services Administration (grant U51HA02522) and the Centers for Disease Control and Prevention through a cooperative agreement with the AIDS Prevention Initiative in Nigeria (grant PS 001058).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.UNAIDS. AIDSinfo. Available at: http://aidsinfo.unaids.org/ Accessed 20 July 2015. [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report 2014. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
- 3.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis 2010; 50:S201–7. [DOI] [PubMed] [Google Scholar]
- 4.Lawn SD, Wood R, De Cock KM et al. . Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis 2010; 10:489–98. [DOI] [PubMed] [Google Scholar]
- 5.Lawn SD, Myer L, Edwards D et al. . Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 2009; 23:1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, Wood R, Kaplan R et al. . Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One 2012; 7:e34156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komati S, Shaw PA, Stubbs N et al. . Tuberculosis risk factors and mortality for HIV-infected persons receiving antiretroviral therapy in South Africa. AIDS 2010; 24:1849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu R, Mills EJ, Beyene J et al. . Impact of tuberculosis on mortality among HIV-infected patients receiving antiretroviral therapy in Uganda: a prospective cohort analysis. AIDS Res Ther 2013; 10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A, Wood R, Kaplan R et al. . Prevalent and incident tuberculosis are independent risk factors for mortality among patients accessing antiretroviral therapy in South Africa. PLoS One 2013; 8:e55824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott AM, Namaambo K, Allen BW et al. . Negative sputum smear results in HIV-positive patients with pulmonary tuberculosis in Lusaka, Zambia. Tuber Lung Dis 1993; 74:191–4. [DOI] [PubMed] [Google Scholar]
- 11.Dinic L, Akande P, Idigbe EO et al. . Genetic determinants of drug-resistant tuberculosis among HIV-infected patients in Nigeria. J Clin Microbiol 2012; 50:2905–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaplin B, Meloni S, Eisen G et al. . Scale-up of networked HIV treatment in Nigeria: creation of an integrated electronic medical records system. Int J Med Inform 2015; 84:58–68. [DOI] [PubMed] [Google Scholar]
- 13.Hamel DJ, Sankale JL, Samuels JO et al. . Building laboratory capacity to support HIV care in Nigeria: Harvard/APIN PEPFAR, 2004–2012. Afr J Lab Med. 2015; 4:Art. #190. Available at: http://dx.doi.org/10.4102/ajlm.v4i1.190. Accessed 20 July 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Federal Ministry of Health Nigeria Department of Public Health. National Tuberculosis and Leprosy Control Programme (NTBLCP), Worker's Manual, Revised 5th Edition. Nigeria: Federal Ministry of Health Nigeria, 2010. Available at: http://www.who.int/hiv/pub/guidelines/nigeria_tb.pdf. Accessed 20 July 2015. [Google Scholar]
- 15.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization, 2011. (WHO/NMH/NHD/MNM/11.1). Available at: http://www.who.int/vmnis/indicators/haemoglobin.pdf. Accessed 20 July 2015. [Google Scholar]
- 16.Grossberg R, Gross R. Use of pharmacy refill data as a measure of antiretroviral adherence. Curr HIV/AIDS Rep 2007; 4:187–91. [DOI] [PubMed] [Google Scholar]
- 17.Sangeda RZ, Mosha F, Prosperi M et al. . Pharmacy refill adherence outperforms self-reported methods in predicting HIV therapy outcome in resource-limited settings. BMC Public Health 2014; 14:1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Low-Beer S, Yip B, O'Shaughnessy MV et al. . Adherence to triple therapy and viral load response. J Acquir Immune Defic Syndr 2000; 23:360–1. [DOI] [PubMed] [Google Scholar]
- 19.Nachega JB, Hislop M, Dowdy DW et al. . Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr 2006; 43:78–84. [DOI] [PubMed] [Google Scholar]
- 20.Weidle PJ, Wamai N, Solberg P et al. . Adherence to antiretroviral therapy in a home-based AIDS care programme in rural Uganda. Lancet 2006; 368:1587–94. [DOI] [PubMed] [Google Scholar]
- 21.Welch CA, Bartlett J, Petersen I. Application of multiple imputation using the two-fold fully conditional specification algorithm in longitudinal clinical data. Stat Med 2014; 33:3725–37. [PMC free article] [PubMed] [Google Scholar]
- 22.Van Rie A, Westreich D, Sanne I. Tuberculosis in patients receiving antiretroviral treatment: incidence, risk factors, and prevention strategies. J Acquir Immune Defic Syndr 2011; 56:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu E, Makubi A, Drain P et al. . Tuberculosis incidence rate and risk factors among HIV-infected adults with access to antiretroviral therapy. AIDS 2015; 29:1391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girardi E, Sabin CA, d'Arminio Monforte A et al. . Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis 2005; 41:1772–82. [DOI] [PubMed] [Google Scholar]
- 25.Brinkhof MW, Egger M, Boulle A et al. . Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin Infect Dis 2007; 45:1518–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermans SM, Kiragga AN, Schaefer P et al. . Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in sub-Saharan Africa. PLoS One 2010; 5:e10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholas S, Sabapathy K, Ferreyra C et al. . Incidence of tuberculosis in HIV-infected patients before and after starting combined antiretroviral therapy in 8 sub-Saharan African HIV programs. J Acquir Immune Defic Syndr 2011; 57:311–8. [DOI] [PubMed] [Google Scholar]
- 28.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS 2005; 19:2109–16. [DOI] [PubMed] [Google Scholar]
- 29.Akanbi MO, Achenbach CJ, Feinglass J et al. . Tuberculosis after one year of combination antiretroviral therapy in Nigeria: a retrospective cohort study. AIDS Res Hum Retroviruses 2013; 29:931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno S, Jarrin I, Iribarren JA et al. . Incidence and risk factors for tuberculosis in HIV-positive subjects by HAART status. Int J Tuberc Lung Dis 2008; 12:1393–400. [PubMed] [Google Scholar]
- 31.Holmes CB, Hausler H, Nunn P. A review of sex differences in the epidemiology of tuberculosis. Int J Tuberc Lung Dis 1998; 2:96–104. [PubMed] [Google Scholar]
- 32.Borgdorff MW, Nagelkerke NJ, Dye C, Nunn P. Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. Int J Tuberc Lung Dis 2000; 4:123–32. [PubMed] [Google Scholar]
- 33.Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Med 2009; 6:e1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nhamoyebonde S, Leslie A. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis 2014; 209:S100–6. [DOI] [PubMed] [Google Scholar]
- 35.Auld AF, Mbofana F, Shiraishi RW et al. . Incidence and determinants of tuberculosis among adults initiating antiretroviral therapy--Mozambique, 2004–2008. PLoS One 2013; 8:e54665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batista J, de Albuquerque Mde F, Maruza M et al. . Incidence and risk factors for tuberculosis in people living with HIV: cohort from HIV referral health centers in Recife, Brazil. PLoS One 2013; 8:e63916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin-Echevarria E, Serrano-Villar S, Sainz T et al. . Development of tuberculosis in human immunodeficiency virus infected patients receiving antiretroviral therapy. Int J Tuberc Lung Dis 2014; 18:1080–4. [DOI] [PubMed] [Google Scholar]
- 38.Musa BM, Musa B, Muhammed H et al. . Incidence of tuberculosis and immunological profile of TB/HIV co-infected patients in Nigeria. Ann Thorac Med 2015; 10:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahana SY, Rohan J, Allison S et al. . A meta-analysis of adherence to antiretroviral therapy and virologic responses in HIV-infected children, adolescents, and young adults. AIDS Behav 2013; 17:41–60. [DOI] [PubMed] [Google Scholar]
- 40.Gardner EM, Burman WJ, Steiner JF et al. . Antiretroviral medication adherence and the development of class-specific antiretroviral resistance. AIDS 2009; 23:1035–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


