Abstract
Phagocytes, the physiological compartment in which Leishmania parasites reside, are the main site of action of the drug miltefosine, but the intracellular pharmacokinetics of miltefosine remain unexplored. We developed a bioanalytical method to quantify miltefosine in human peripheral blood mononuclear cells (PBMCs), expanding from an existing high performance liquid chromatography-tandem mass spectrometry method for the quantification of miltefosine in plasma. The method introduced deuterated miltefosine as an internal standard. Miltefosine was extracted from PBMC pellets by addition of 62.5% methanol. Supernatant was collected, evaporated and reconstituted in plasma. Chromatographic separation was performed on a reversed phase C18 column and detection with a triple-quadrupole mass spectrometer. Miltefosine was quantified using plasma calibration standards ranging from 4 to 1000 ng/mL. This method was validated with respect to its PBMC matrix effect, selectivity, recovery and stability. No matrix effect could be observed from the PBMC content (ranging from 0.17 to 26.3 × 106 PBMCs) reconstituted in plasma, as quality control samples were within 3.0% of the nominal concentration (precision less than 7.7%). At the lower limit of quantitation of 4 ng/mL plasma, corresponding to 0.12 ng/106 PBMCs in a typical clinical sample, measured concentrations were within 8.6% of the nominal value. Recovery showed to be reproducible as adding additional pre-treatment steps did not increase the recovery with more than 9%. This method was successfully applied to measure intracellular miltefosine concentrations in PBMC samples from six cutaneous leishmaniasis patients up to one month post-treatment.
1. Introduction
The Leishmania parasite, causative agent of the neglected infectious disease leishmaniasis, resides and replicates within human phagocytes. These cells are therefore the main site of action of the antileishmanial drug miltefosine [1], however, the intracellular pharmacokinetics of the drug are currently unknown. Miltefosine is transported into cells both by passive incorporation in the cellular membranes (non-saturable from 20 to 200 μM/8.2 to 82 μg/mL) and by active carrier-mediated cellular transport (saturable at 50 μM/20.4 μg/mL) [2,3]. In Dutch cutaneous leishmaniasis (CL) patients, the average steady-state plasma concentration, reached only in the last week of treatment during a standard 28-day miltefosine regimen, was 30.8 μg/mL [4]. Within the treatment period, the contribution of the active (saturable) transport is thus substantial and the relative contribution of both transport mechanisms on the intracellular miltefosine accumulation in vivo is expected to vary during treatment. The saturability of the active transport could result in substantial between-subject variability in intracellular miltefosine concentrations.
Resident tissue macrophages are the host cells for intracellular Leishmania survival and replication. Thus, intracellular drug quantification is pivotal to provide a better understanding of the drug disposition within the physiological compartment in which the parasites reside. Intracellular miltefosine concentrations better represent the drug concentrations to which the parasites are exposed and will probably relate more accurately to Leishmania drug susceptibility and pharmacokinetic/ pharmacodynamic relationships than plasma drug concentrations.
We have previously validated an LC/MS–MS assay to measure miltefosine in plasma [5]. Here we expand this method to intracellular measurements. In this assay peripheral blood mononuclear cells (PBMCs) were used as a model to assess intracellular miltefosine accumulation within human leukocytes. The sample pre-treatment was modified and a partial validation was executed. This assay was evaluated using PBMC samples from six Colombian CL patients treated with a miltefosine monotherapy.
2. Methods
2.1. Chemicals and reagents
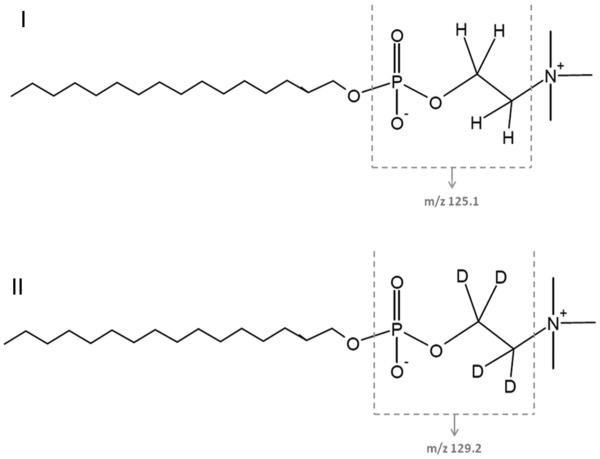
Miltefosine and phosphate buffered saline (PBS) were purchased from Sigma–Aldrich (Zwijndrecht, the Netherlands), and deuterated miltefosine (miltefosine-D4, Fig. 1) from Alsachim (Illkirch Graffenstaden, France). Acetonitrile, methanol and H2O were obtained from Biosolve Ltd. (Valkenswaard, the Netherlands), ammonia 25%, triethylamine and acetic acid 99.8% from Merck (Amsterdam, the Netherlands) and Ficoll from GE Healthcare (Hoevelaken, the Netherlands). Blank Na-EDTA plasma was obtained from Bioreclamations (Baltimore, US).
Fig. 1.
Structural formulas of miltefosine (I) and the internal standard miltefosine-D4 (II), indicating the m/z fragments.
2.2. Clinical sample collection and PBMC isolation
Heparin-treated blood samples (10 mL for adults, 3 mL for children) were taken from CL patients (Section 2.7) and centrifuged 10 min at 800 × g at room temperature. All plasma was transferred and stored at −80 °C, while the remaining blood sample was diluted 1:4 in PBS and placed over a Ficoll gradient at a 1:5 Ficoll-to-blood ratio. Samples were centrifuged 15 min at 400 × g at room temperature and the mononuclear leukocyte layer was isolated. Subsequently the cells were washed two times with 10 mL PBS, resuspended in 1 mL PBS and counted on a haemocytometer. Samples were centrifuged at 800 × g, the supernatant was removed, and the PBMC pellet stored at −80 °C. Plasma and PBMC pellets were transported on dry ice to the bioanalytical laboratory and stored at −20 °C until analysis.
The cell pellet was resuspended in 120 μL PBS, after which the cells were lysed by adding 200 μL methanol yielding a total volume of 320 μL 62.5% methanol-PBS (v/v). The sample was vortexed and centrifuged for 5 min at 20,000 × g. The supernatant, referred to as the “PBMC lysate” was transferred to a 1.5 mL tube. Depending on the expected concentration, the PBMC lysate volume transferred varied between 50 μL (expected concentration above upper limit of quantitation, ULOQ) and 280 μL (expected concentration close to lower limit of quantitation, LLOQ). Finally, the PBMC lysate was evaporated under a gentle stream of nitrogen and reconstituted in 250 μL of blank Na-EDTA human plasma. These so-called “reconstituted PBMC samples” were handled as normal plasma samples (Section 2.5).
2.3. Preparation of plasma calibration standards and internal standard solution
Calibration standards were prepared in plasma. A stock solution of 1 mg/mL miltefosine was prepared in methanol-water (1:1, v/v). Calibration standard working solutions were further diluted from this stock solution with methanol–water (1:1, v/v) to final concentrations of 0.08, 0.2, 0.4, 1, 2, 4, 8, 16 and 20 μg/mL.
Calibration standards were freshly prepared before each run by spiking 570 μL of blank Na-EDTA human plasma with 30 μL of working solution, yielding calibration standards of 4, 10, 20, 50, 100, 200, 400, 800 and 1000 ng/mL. Two 250 μL aliquots were prepared per calibration standard and processed for each analytical run.
An internal standard working solution was prepared by dilution of a stock solution of 1 mg/mL deuterated miltefosine (miltefosine-D4) in methanol-water (1:1, v/v) to 4000 ng/mL with methanol-water (1:1, v/v).
2.4. Preparation of PBMC quality control samples
A separate stock solution of 1 mg/mL miltefosine in methanol-water (1:1, v/v) was prepared from an independent weighing for the preparation of quality control (QC) samples. PBMC QC working solutions were diluted from this stock solution with methanolwater (1:1, v/v) to concentrations of 0.6, 15 and 37.5 μg/mL and an LLOQ working solution of 0.2 μg/mL.
To mimic the study samples, QC samples were prepared freshly by spiking 5 μL of working solution to 95 μL of blank PBMC lysate. To prepare blank PBMC lysate, blank PBMCs were isolated from human leukocyte buffy coat (~50 mL, freshly derived from 500 mL whole blood) purchased from Sanquin (Amsterdam, the Netherlands). 200 mL PBS was added to the buffy coat, and 25 mL aliquots of this suspension were each carefully added to 12.5 mL of high density centrifugation medium Ficoll. After a 20 min 550 × g centrifugation at 4 °C (without brake), the interface containing the PBMCs was transferred to a clean tube. Subsequently, the PBMCs of each aliquot were washed with 35 mL PBS and centrifuged at 1500 × g for 5 min at 4 °C (without brake). The supernatant was discarded and the pellet was resuspended in 300 μL PBS. All aliquots were pooled and a cell count was performed with a Cell Dyn Hematology analyzer (Abbott Diagnostics, Lake Forest, IL). After a 3000 × g centrifugation, PBS was removed to adjust the PBMC concentration to approximately 200 × 106 cells per mL PBS, to obtain reconstituted PBMC QC samples containing 7.1 × 106 cells, close to the mean found in patient samples (see Section 2.7). Depending on the final volume of blank PBMCs in PBS, a volume of methanol was subsequently added to obtain PBMC lysate of 62.5% methanol-PBS (v/v).
After spiking, the QCs were evaporated under a gentle stream of nitrogen and reconstituted in 250 μL of blank Na-EDTA human plasma. The final miltefosine concentrations of the reconstituted PBMC QCs were 12, 300 and 750 ng/mL (low; QCL, mid; QCM and high; QCH respectively). Considering an average of 7.1 × 106 PBMCs in the reconstituted PBMC samples, this would correspond to concentrations of 0.42, 11 and 26 ng/106 cells.
2.5. Plasma sample preparation and LC-MS/MS analysis
Reconstituted PBMC samples and plasma calibration standards were further prepared as previously described [5] with slight modifications. First, 25 μL of miltefosine-D4 (4000 ng/mL) was added to each 250 μL aliquot, except for double blanks to which 25 μL methanol-water (1:1, v/v) was added. All samples were briefly vortexed and subsequently 700 μL of acetic acid buffer (1 M, pH 4.5) was added. Samples were vortexed and centrifuged 5 min at 23,100 × g at room temperature. The extraction of miltefosine was performed on Bond Elut PH SPE cartridges (Agilent Technologies, Amstelveen, the Netherlands), which were first conditioned with 1 mL acetonitrile and subsequently 1 mL acetic acid buffer (1 M, pH 4.5). Afterwards, samples were loaded on the SPE cartridges and the cartridges were washed with 1 mL methanol–water (1:1, v/v). The analyte was eluted with two times 750 μL of 0.1% (v/v) triethylamine in methanol. The eluate was transferred to a glass autosampler vial and 10 μL was injected on the analytical column.
The chromatographic separation and LC–MS/MS analysis was performed as described previously [5], but the more sensitive API3000 triple-quadrupole mass spectrometer was used, which was equipped with a turbo-ionspray source (AB Sciex, Framingham, MA, USA). Mass spectrometer settings were optimized on the API3000. Miltefosine was monitored at a mass transition of m/z 408.5 to 125.1 and miltefosine-D4 at m/z 412.6 to 129.2.
2.6. Partial validation procedure for PBMC samples
Due to the fact that this method is an extension of an existing previously validated method [5], a partial validation [6] was performed in which the following aspects were investigated.
2.6.1. Matrix effect of PBMC content
The amount of cells in PBMC pellets may vary from sample to sample due to physiological variability in the number of circulating PBMCs and the volume of blood collected. Additionally, PBMC lysate volumes transferred differ depending on the expected miltefosine concentration.
To investigate the potential effect of PBMC content in the plasma matrix on the miltefosine quantification, blank PBMC samples in PBS were prepared containing different PBMC concentrations to yield reconstituted PBMC samples with final PBMC counts of 0.17, 4.0, 6.8, 16 and 26 × 106 cells (a range covering the clinical samples received, see Section 2.7). The cells were lysed with methanol to obtain PBMC lysate of 62.5% methanol-PBS (v/v). The blank PBMC lysate was spiked at the QCL and QCH level in triplicate to yield concentrations of 12.2 and 761 ng/mL miltefosine in the reconstituted PBMC samples. A matrix effect was considered when the measured analyte concentration was outside 85–115% of the nominal concentration.
2.6.2. Selectivity
Double blanks and LLOQ samples (5 μL working solution + 95 μL blank PBMC lysate) were prepared from blank PBMC lysate originating from six different individuals (six batches), to check the specificity and selectivity of this method. For four out of six batches, the signal-to-noise ratio of the LLOQ should be above 5 and the LLOQ samples should be within ±20% of the nominal concentration.
2.6.3. Recovery
One of the challenges in the determination of intracellular drug concentrations is that it is not possible to truly mimic the uptake of the analyte by PBMCs, and therefore it is not possible to quantitatively assess the absolute pre-treatment recovery of miltefosine from PBMCs. Therefore we have investigated whether the relative recovery could be improved by applying different methods to lyse PBMCs. Three patient samples (Section 2.7) were pre-treated with three different methods: (a) the method described in Section 2.2; (b) same as (a), plus 1 hour freeze at −20 °C; (c) same as (b), plus 30 min sonication. Additionally, microbead homogenization was evaluated on eight other patient samples in which the PBMC pellets appeared to not be homogeneously suspended.
If the additional yield due to an extra pre-treatment step was less than 15% for at least two thirds of the samples, it was not included in the final pre-treatment method.
2.6.4. Stability in 62.5% methanol-PBS (v/v)
PBMC lysate stability was tested by spiking blank PBMC lysate at QCL and QCH level in triplicate. The samples were stored at −20 °C for 15 days. Stability was considered acceptable when the measured analyte concentration was within 85–115% of the nominal concentration.
2.7. Clinical application
PBMC and plasma samples were collected from January 2012 to October 2013 in an open label non-randomized pharmacokinetic clinical trial of miltefosine in children and adults with CL. This study was approved and monitored by the institutional review board for ethical conduct of research involving human subjects of the Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM), in accordance with national (resolution 8430, República de Colombia, Ministry of Health, 1993) and international (Declaration of Helsinki and amendments, World Medical Association, Fortaleza, Brasil, October 2013) guidelines. Samples were collected nominally on day 1, 15 and 28 of a 1.8–2.5 mg/kg/day 28-day miltefosine regimen, and one, two, three and five months after treatment. Eleven patient samples were used for the bioanalytical validation of the recovery within this method. Subsequently, samples obtained from six patients in this study were included as a clinical validation to show the robustness and applicability of the developed method.
As described in Section 2.2, PBMC samples were reconstituted in 250 μL plasma and quantified using plasma calibration standards, therefore the measured concentrations are in ng/mL plasma. These concentrations were corrected for the dilution factor (DF, Eq. (1)) and multiplied by 320/1000 μL to calculate the total amount of miltefosine in the pellet (Eq. (2)).
Subsequently, this value was converted to amount of miltefosine per 106 cells, based on the sample cell counts (Eq. (3)). The mean number of PBMCs in the reconstituted PBMC sample for the six patients (total samples, n = 25) was 6.8 × 106 PBMCs, with a range from 0.72 to 23 × 106 PBMCs.
The intracellular miltefosine concentration was calculated by using an average volume of 283 fl for a single peripheral blood mononuclear cell [7], equal to 0.000283 mL per 106 cells (Eq. (4), in which "IC" stands for intracellular).
| (1) |
| (2) |
| (3) |
| (4) |
3. Results and discussion
3.1. Matrix effect of PBMC content
Table 1 shows the matrix effect of the different PBMC amounts in the reconstituted PBMC samples. The bias was within 3.0% of the nominal concentration and the coefficient of variation (CV%) within 7.7%. It can be concluded that the PBMC counts expected in clinical practice do not influence the accuracy of the method and that there was no additional matrix effect due to the PBMC content.
Table 1.
Matrix effect of different amounts of PBMCs in reconstituted PBMC sample (n = 3).
| Nominal concentration 12.2 ng/mL |
Nominal concentration 761 ng/mL |
|||||
|---|---|---|---|---|---|---|
| Amount of PBMCs in reconstituted PBMC sample (×106) |
Mean calculated concentration (ng/mL) |
Precision (%CV) |
Bias (%DEV) |
Mean calculated concentration (ng/mL) |
Precision (%CV) |
Bias (%DEV) |
| 0.17 | 12.1 | 3.0 | −0.8 | 738 | 1.2 | −3.0 |
| 4.0 | 12.1 | 2.4 | −1.1 | 756 | 4.3 | −0.6 |
| 6.8 | 12.6 | 6.8 | 3.0 | 749 | 3.5 | −1.6 |
| 16 | 12.1 | 4.5 | −0.5 | 763 | 3.2 | 0.2 |
| 26 | 12.5 | 7.7 | 2.7 | 755 | 0.6 | −0.7 |
3.2. Selectivity
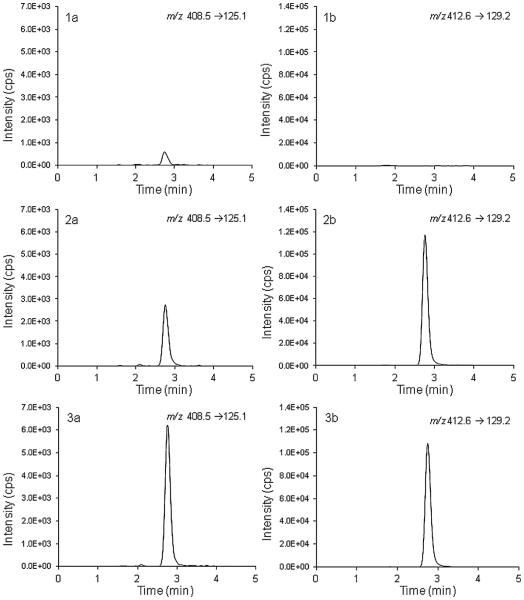
A peak with the same retention time and mass transition as miltefosine was observed both in plasma and reconstituted PBMC double blanks (reconstituted in the same plasma), possibly due to a memory effect. The memory effect was constant over the run and did not decrease in intensity as for the 20 plasma double blanks injected within one run, the memory peak remained at an average signal of 22% of the LLOQ. This memory effect was not observed in the previously validated method [5], but this could possibly be explained by the increased sensitivity as a result of a change in the mass spectrometer. The memory effect was independent of the injected sample and no additional effect was measured after injecting the ULOQ. Reconstitution solvents were tested and found not to be contaminated. For 5 out of the 6 tested batches of PBMCs, the interference in the blanks was an average of 23% of the LLOQ and was thus comparable to the interference found in the plasma of the reconstituted PBMC sample. The interference was constant over the run and therefore the calibration standards corrected for this. The accuracies of the LLOQ samples were within 94.1% and 108.6% of the nominal concentration and were therefore found to be acceptable. Because the six PBMC batches each contained different cell amounts, the highest of the six (8.5 × 106 cells in the reconstituted PBMC sample) was taken as a reference to calculate an LLOQ of 0.12 ng per million cells. It should be mentioned that the LLOQ is dependent on the amount of PBMCs in the reconstituted PBMC sample, as the LLOQ (expressed per million cells) decreases with an increase of the amount of cells isolated. Fig. 2 shows representative chromatograms of a double blank, LLOQ and QCL sample.
Fig. 2.
Representative ion chromatograms of the (1) double blank; (2) LLOQ (4 ng/mL) and (3) QCL (12.2 ng/mL). The left panel (a) shows the miltefosine ion chromatogram and the right panel (b) the internal standard miltefosine-D4 ion chromatogram.
It could be argued that because the memory peak in double blanks is higher than 20% of the LLOQ the selectivity is not sufficient, as the signal to noise ratio is below 5. However, because the miltefosine signal in double blanks is stable between different batches of plasma and PBMC matrices, and because the LLOQ is still measured precisely and accurately, the selectivity of the assay was considered acceptable.
3.3. Recovery
The addition of a freezing or sonication step did not yield an increase in intracellular miltefosine recovery of more than 5%. The increase in miltefosine recovery due to microbead homogenization was less than 9% for seven out of eight patient samples. It was concluded that the method had an acceptable reproducibility regarding the release of miltefosine from PBMCs and therefore these extra steps were not added to the pre-treatment method.
3.4. Stability in 62.5% methanol-PBS (v/v)
QCs spiked in PBMC blank lysate were stable for at least 15 days at −20 °C. The mean measured concentrations were 99.2% and 98.6% of the nominal concentration for QCL and QCH, respectively, with acceptable variation (<3.2%).
3.5. Clinical application
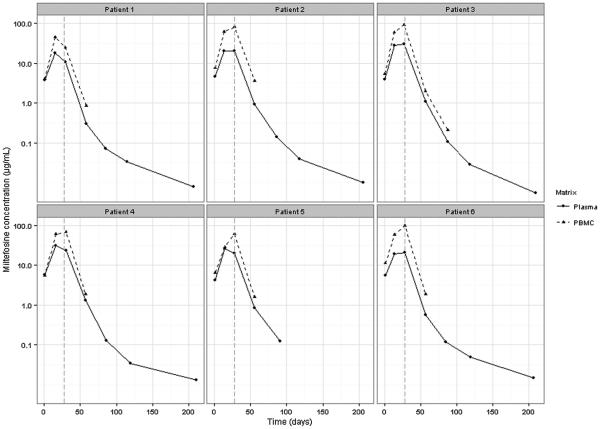
Plasma concentration-time curves and corresponding intracellular concentration-time curves for six CL patients are presented in Fig. 3. The intracellular miltefosine kinetic profile is similar to the kinetic profile of miltefosine plasma concentrations. This is particularly clear for patient 1, who showed a decline in miltefosine concentration between day 14 and day 28 of treatment which is apparent for both plasma and intracellular concentrations. Intracellular miltefosine concentrations were measurable up to one month post-treatment, and for patient 3 even up to two months post-treatment due to the high number of PBMCs isolated. Interestingly, end-of-treatment intracellular miltefosine concentrations are higher than the plasma miltefosine concentrations, indicating that an accumulation of miltefosine takes place in PBMCs.
Fig. 3.
Representative concentration-time curves of miltefosine plasma concentrations (solid line) and intracellular miltefosine concentrations (dashed line) for six cutaneous leishmaniasis patients. The vertical grey dashed line indicates the end of treatment.
4. Conclusion and future perspectives
A pre-treatment method, additional to a previously validated method for quantitation of miltefosine in plasma, was successfully developed and validated to measure miltefosine concentrations in isolated PBMCs. The method showed to be reproducible and selective, and the LLOQ was sufficient to quantify intracellular miltefosine concentrations in patient samples up to one month post-treatment. The LLOQ of this assay was 0.12 ng miltefosine per million cells, based on 8.5 × 106 cells in the reconstituted PBMC sample. To allow for quantification of miltefosine for a longer period after treatment, the LLOQ could be further decreased by augmenting the number of isolated PBMCs per sample or by using more sensitive mass spectrometry equipment.
An initial exploration of intracellular miltefosine concentrations in samples from CL patients showed similar kinetic profiles in intracellular and plasma concentrations, and suggests drug accumulation in PBMCs. Since miltefosine is used in the treatment of infection with the intracellular Leishmania parasite, intracellular pharmacokinetic data can enrich our understanding of the exposure-response relationship of this drug. The here presented bioanalytical method can be applied to further establish this relationship.
Acknowledgements
We gratefully acknowledge the patients participating in this study, their families, and community leaders. We thank CIDEIM personnel, Isabel Guasaquillo, Adriana Navas and Angelica Mera for processing of blood samples, and the clinical team in CIDEIM Cali and Tumaco, Jimena Jojoa, Wilson Cortes, Mary Luz Hurtado and Dr. Mabel Castillo for their assistance in patient diagnosis, recruitment, enrollment and follow up. This work received financial support from COLCIENCIAS grant # 2229-519-28930. MMC was a recipient of COLCIENCIAS Young Investigator Award, Contract # 0040-2012. We thank Paladin Labs Inc. for providing adult and pediatric formulations of miltefosine.
References
- [1].Dorlo TPC, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis, J. Antimicrob. Chemother. 2012;67:2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- [2].Ménez C, Buyse M, Farinotti R, Barratt G. Inward translocation of the phospholipid analogue miltefosine across Caco-2 cell membranes exhibits characteristics of a carrier-mediated process. Lipids. 2007;42:229–240. doi: 10.1007/s11745-007-3026-8. [DOI] [PubMed] [Google Scholar]
- [3].Ménez C, Buyse M, Dugave C, Farinotti R, Barratt G. Intestinal absorption of miltefosine: contribution of passive paracellular transport. Pharm. Res. 2007;24:546–554. doi: 10.1007/s11095-006-9170-7. [DOI] [PubMed] [Google Scholar]
- [4].Dorlo TPC, van Thiel PPAM, Huitema ADR, et al. Pharmacokinetics of miltefosine in Old World cutaneous leishmaniasis patients, Antimicrob. Agents Chemother. 2008;52:2855–2860. doi: 10.1128/AAC.00014-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dorlo TPC, Hillebrand MJX, Rosing H, Eggelte TA, de Vries PJ, Beijnen JH. Development and validation of a quantitative assay for the measurement of miltefosine in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;865:55–62. doi: 10.1016/j.jchromb.2008.02.005. [DOI] [PubMed] [Google Scholar]
- [6].US Food and Drug Administration FDA, Guidance for Industry: Bioanalytical Method Validation US Department of Health and Human, Services Food and Drug Administration, and Center for Drug Evaluation and Research. 2001 http://www.fda.gov/downloads/Drugs/Guidances/ucm070107 pdf Available from.
- [7].Simiele M, D’Avolio A, Baietto L, et al. Evaluation of the mean corpuscular volume of peripheral blood mononuclear cells of HIV patients by a coulter counter to determine intracellular drug concentrations, Antimicrob. Agents Chemother. 2011;55:2976–2978. doi: 10.1128/AAC.01236-10. [DOI] [PMC free article] [PubMed] [Google Scholar]