Abstract
Dengue incidence continues to increase globally and, in the absence of an efficacious vaccine, prevention strategies are limited to vector control. It has been suggested that targeting the most productive breeding sites instead of all water-holding containers could be a cost-effective vector control strategy. We sought to identify and continuously control the most productive Aedes (Stegomyia) breeding site in an endemic urban area in Colombia and followed the subsequent incidence of dengue. In the urban area of Guadalajara de Buga, southwestern Colombia, potential breeding sites inside and outside houses were first characterized, and local personnel trained to assess their productivity based on the pupae/person index. Simultaneously, training and monitoring were implemented to improve the dengue case surveillance system. Entomological data and insecticide resistance studies were used to define the targeted intervention. Then, a quasi-experimental design was used to assess the efficacy of the intervention in terms of the positivity index of the targeted and non- targeted breeding sites, and the impact on dengue cases. Street catch basins (storm drains) were the potential breeding site most frequently found containing Aedes immature stages in the baseline (58.3% of 108). Due to the high resistance to temephos (0% mortality after 24 h), the intervention consisted of monthly application of pyriproxyfen in all the street catch basins (n = 4800). A significant decrease in catch basins positivity for Aedes larvae was observed after each monthly treatment (p < 0.001). Over the intervention period, a reduction in the dengue incidence in Buga was observed (rate ratio 0.19, 95% CI 0.12–0.30, p < 0.0001) after adjusting for autocorrelation and controlling with a neighboring town, Palmira, This study highlights the importance of street catch basins as Aedes breeding sites and suggests that their targeted control could help to decrease dengue transmission in such areas.
Keywords: Dengue, Intervention, Catch basins, Aedes aegypti, Pupae index
1. Introduction
Dengue viruses (DENV), transmitted to humans by Aedes aegypti, are responsible for dengue fever (DF) and severe dengue (SD). The number of infected people each year is unknown but has been estimated at 50–100 million new DF infections occur each year, causing ~500,000 cases of SD and >20,000 deaths (Gubler, 2001, 2002, 2004), and possibly many more (Bath et al., 2013). The incidence of DF and SD is increasing due to the expansion of mosquito populations favored by uncontrolled urban development, inadequate vector control programs (Scott and Morrison, 2010), and global human mobility (WHO, 2006). No vaccine has yet been licensed (Wan et al., 2013),and hence, prevention programs rely on vector control and avoidance of mosquitoes bites. Moreover, vector control is expected to continue after a dengue vaccine is deployed to maximize its impact.
In Colombia, dengue vector control during epidemics focuses on the ultra-low volume (ULV) application of insecticides in houses around reported severe dengue cases, and on the streets of the most affected neighborhoods, together with community campaigns to promote the removal of unused containers in and around houses (Ministerio de la Protección Social, 2012). Routinely, periodic (3 to 4 times per year) entomological surveillance is carried out by sanitation technicians on randomly selected premises. The sanitation technicians record the presence of potential breeding sites and calculate Stegomyia indices. Positive containers that cannot be removed are treated with larvicides, mainly Temephos (Ministerio de la Protección Social, 2012). Although a national integrated vector control strategy is in place, dengue transmission has not decreased in the country. During the biggest outbreak in 2010, dengue incidences reached 666 cases per 100,000 inhabitants, 624 for DF and 41 for SD (SIVIGILA, 2013). The following years the morbidity rates for non-severe cases were 122.8 and 241.9 in 100,000 inhabitants for 2011 and 2012, respectively (SIVIGILA, 2013).
One limitation of the current dengue vector control strategies is that the continuity of the routine entomological surveillance and interventions can be disrupted by short term contracting of personnel. A second limitation is that vector control interventions are based on national guidelines rather than on local environmental and epidemiological characteristics. In order to make informed decisions and improve vector control at local level, it is necessary to have better data collection and analysis (Morrison et al., 2008). For example, most entomological surveillance programs do not distinguish the type of breeding sites and mosquito productivity, variables that are important in designing vector control strategies (Focks, 2003; WHO, 2006). Monitoring the production of late-instar larvae and pupae is putatively more informative than traditional Stegomyia indices but they are not yet routinely used (Alexander et al., 2006; Focks, 2003; Focks and Alexander, 2007; Focks et al., 2000). There is some evidence that identification and targeting the most productive breeding sites can be as effective in lowering entomological indices as targeting all water-holding containers, with lower implementation costs (Tun-Lin et al., 2009). Here, we describe a vector control strategy, its operationalization, the prioritization and control of breeding sites, and the subsequent entomological and epidemiological results, in an endemic town in southwestern Colombia.
2. Methodology
2.1. Study site
This study was carried out in the municipality of Guadalajara de Buga, also known simply as Buga, in the Department (state) of Valle del Cauca, located in the southwest of Colombia (3°53′57″N and 76°17′1″W) at an altitude of 900 m. Its urban area includes 97,262 inhabitants, and a total of 32,224 houses grouped in six comunas (districts) (DANE, 2005a). Buga has reported the highest average annual incidence of dengue transmission in Valle del Cauca, at 397 (SD 295) per 100,000 inhabitants from 1996 to 2008 (Secretary of Health of Valle del Cauca personal communication 2008). The principal vector is Ae. aegypti and Ae. albopictus was reported for the first time in 2007 (Secretary of health of Valle del Cauca personal communication 2007). Buga has one of the highest coverage of public services in the region (electricity 99%, water supply 96.8%, and sewage system 93.7%) and high levels of literacy (93% of people five years old or older are able to read) (DANE, 2005a). This city is a road transit point for Buenaventura, the main port on the Colombian Pacific Ocean. Truck drivers contributing to a high potential flux of dengue serotypes, as do the approximately 3 million pilgrims per year visiting the Basilica of “Our Lord of Miracles”. Buga was the site of the first isolation of DENV3 in the southwest of Colombia (Secretary of Health of Valle del Cauca personal communication 2007) and all four dengue serotypes are currently circulating, with cases of severe dengue observed in children and adolescents (7–13 years old) (Secretary of health of Valle del Cauca personal communication 2007).
This study received ethical approval by the institutional review committee for humans of the Centro Internacional de Entrenamiento e Investigaciones Médicas—CIDEIM and approval by the Territorial Council of Health Social Security (CTSSS from its Spanish acronym) and the Secretary of Health of Buga.
2.2. Study design
As part of a quasi-experimental study to promote the community participation in dengue control in Buga conducted from September 2008 to March 2010, an entomological component was implemented in two phases.
Initially, information on human resources available for dengue entomological surveillance and control activities was gathered; the current entomological surveillance process and data collection forms were critically reviewed and re-design; and the sanitation technicians were trained in the new field work methodology. The latter consisted of workshops to review and get acquainted with the revised data collection forms, house sampling strategy and pupae identification. Simultaneously, the research team provided training to the local staff involved in case surveillance at the Secretary of Health in Buga and at the public and private health care centers in the town (Carabali et al., 2013; Zea and Osorio, 2011).
The first phase consisted of the baseline entomological characterization of potential breeding sites inside and outside houses. Additionally, insecticide susceptibility to the principally used insecticides was evaluated. Both studies were used to define the targeted intervention.
In the second phase implemented the entomological intervention. It was evaluated using periodical entomological surveys and weekly dengue cases reports. During the study, the researchers provided continuous training and support to the local dengue case surveillance team to maintain awareness of dengue case identification and high levels of compliance with the dengue reporting processes.
2.3. Entomological baseline (first phase)
The same entomological surveillance methodology was used throughout the study. In the first phase was used to establish baseline information on breeding sites and pupal productivity and develop a vector control strategy carried out by the sanitation technicians. In the second phase, it was used to monitor entomological indices during and after the intervention.
2.3.1. Entomological surveillance inside houses
For the entomological surveillance inside houses the available survey questionnaire was modified to include information such as home address, comuna, the number of adults and children per house, the survey date and the technician’s name. The type of each potential breeding site was recorded and classified as: (1) rooftop water storage tanks; (2) ground level water storage tanks for laundry; (3) tires; (4) steel water storage drums; (5) flower pots; (6) plants in water; (7) bottles and cans; (8) small miscellaneous (<500 mL volume); and (9) large miscellaneous (>500 mL). For each container, the volume of water was estimated and the presence of larvae and/or pupae was recorded. Those with Aedes larvae and/or pupae were recorded as positive. All pupae were identified as Aedes or Culex sp with the help of a magnifier. The numbers of pupae were counted by technicians if fewer than 30. If more, it was classified visually as: (A) 31–50; (B) 51–70; (C) 71–100; (D) >101. The classification ranges for pupae counting were agreed with the technicians during the training activities. Since their job had never required counting pupae, initially they refused to do so because of the amount of anticipated work. However, after carrying out the initial training surveys, they agreed to count until 30 pupae and then use the above scheme. An aquarium net and a white tray were provided to count the pupae.
2.3.1.1. House selection
For logistical purposes it was considered more efficient to assign technicians to operationally defined geographical zones instead of administratively defined comunas. Hence, the town was divided into roughly equal sized zones using a map provided by the Secretary of Municipal Planning. The number of such zones varied according to the number of field technicians available (between 11 and 20). Each zone was randomly assigned to each technician (based on a table of random numbers). To identify the starting point in each zone, its blocks were numbered and then one was selected randomly (using a table of random numbers) and, finally, the house at the southwest corner was chosen to be inspected. After obtaining verbal informed consent of an adult from the selected house, the technician searched for potential breeding sites inside the house. The house inspection took around 15 min, depending on the number of breeding sites found. The inspection continued at every 7th house in a clockwise direction. If a selected house could not be inspected (e.g. no answer or consent declined), the nearest house was surveyed and from this the house selection continued until the sample size was completed. Two members of the research team supervised the technicians for at least ten houses to verify that data collection was done correctly and accurately.
2.3.1.2. Sample size
The sample size was estimated in 185 houses per comuna based on the mean Breteau index with 50% accuracy, assuming a negative binomial distribution following the Krebs method (Krebs, 1999). Specifically we used the following equation: n = (100 × Z1–α/2)2 × ((1/μ) + (1/k))/r2, where α = 0.05 for 95% confidence limits, giving Z = 1.96, μ is the mean, k is the dispersion parameter and r is the percentage accuracy required. Previous data from the study area indicated Breteau index of 10% (μ = 0.1) and k = 0.5, respectively.
2.3.2. Breeding sites outside houses
Catch basins along streets, parks and vacant lots were inspected for potential breeding sites and immature mosquitoes. In the first phase, catch basins were sampled using an aquarium net (8 × 10 cm), at ached to a long handle or a stick. For each catch basin, a sample was obtained from each of the four corners, with a time gap of 5-min between samplings (Giraldo-Calderon et al., 2008). The complete volume of the basins was not examined due to the infeasibility of removing their concrete lids. The immature stages collected were placed in a white plastic tray, larvae and pupae of Aedes and Culex were identified, and the pupae were separated to estimate mosquito production. An average of 20 catch basins was sampled per comuna, based on personnel available. The observed number of Aedes pupae separated in the plastic tray was classified as (A) 1–10, (B) 11–30, (C) 31–50, (D) >50.
2.3.3. Insecticide susceptibility
Aedes larvae were collected from different breeding sites in Buga to evaluate insecticide susceptibility. The field collected Ae. aegypti larvae were colonized at CIDEIM and the adult F1 and F2 were evaluated using the CDC bioassay (Brogdon and McAllister, 1998), with concentrated insecticides from Chem Service® (West Chester, PA, USA). Diagnostic doses and resistance thresholds previously obtained with the susceptible Rockefeller strain, by the group, were used in the CDC bioassays (Ocampo et al., 2011). The insecticide diagnostic doses and resistance threshold were evaluated for the following insecticides: lambdacyhalothrin (6.25 μg/mL—30 min), malathion (100 μg/mL—30 min), and propoxur (12.5 μg/mL—30 min). These insecticides were chosen because malathion and lambdacyhalothrin are the most common insecticides used for adult vector control, and propoxur is highly used in homes. CDC results were expressed as the proportion dead at the threshold time.
Larval bioassays were conducted for the larvicide temephos and the insect growth regulator pyriproxyfen in third instar larvae of Ae. aegypti. Temephos analysis followed WHO guidelines (WHO, 2005) using a diagnostic dose of 0.012 ppm (product from Chem Service®, West Chester, PA, USA) (Ocampo et al., 2011). The temephos results were expressed as the proportion dead 24 h after exposure. The percentage inhibition of adult emergence with pyriproxyfen was analyzed using a concentration of 0.05 mg/mL as previously standardized at CIDEIM (unpublished data). The larvae and pupae mortality was measured until 100% mortality of larvae/pupae or adult emergence was observed (WHO, 2005).
2.4. Intervention (second phase)
Based on the results from the baseline entomological survey, insecticide susceptibility, the level of training of technicians achieved and the number of staff available, we developed a vector control strategy to be carried out by the local authorities during the remainder of the study period. The baseline data demonstrated that mosquito production was very low inside houses and that the most productive breeding sites were the catch basins (see Section 3). Therefore, the vector control strategy targeted only the approximately 4800 catch basins in the entire urban area of Buga. Pyriproxyfen, was selected for two reasons: First, we observed susceptibility in local Ae. aegypti, and second, we previously demonstrated that its effect in catch basins lasts longer than insecticides (Mina et al., 2008). Each catch basin was treated with 2 g of pyriproxyfen (aprox 0.05 mg/mL). Treatment was carried out monthly from February to August 2009. Since pyriproxyfen was not provided by the Ministry of Health, political decisions limit the acquisition of additional supplies. Treatment stopped due to no other insecticide being available.
2.4.1. Evaluation of the intervention
The effect of the intervention was measured based on (1) the percentage of positive catch basins; (2) Stegomya indices from the entomological surveys in houses; and (3) dengue case incidence. For the latter, a neighboring town (Palmira) was selected as a control as it was found to have the most similar environmental conditions to those of Buga among all the other towns in the same department. The city of Palmira is located 66 km to the south of Buga at an altitude of 1000 m and with a population of 223,049 (DANE, 2005a). Like Buga, Palmira has high coverage of public services (electricity 99%, water supply 96,4%, and sewage system 92% (DANE, 2005b), presence of Ae. aegypti, and the circulation of all 4 DENV serotypes. In Palmira, the average dengue incidence from 2003 to 2008 was 234.7 per 100,000 inhabitants (Secretary of Health of Valle del Cauca personal communication 2008).
The positivity of catch basins was measured based on the presence of immature stages (larvae and pupae) of Aedes or Culex in a sample of 10% of the total catch basins in the town. The sampling was done systematically every 10 catch basins before the monthly treatment was applied. Counting of pupae was not done for evaluation purposes due to logistic reasons as technicians consider it time consuming. However, a sample of pupae collected from randomly selected catch basins were transported to the laboratory to observe the effect of the treatment on adult emergence.
For the Stegomya indices, besides the baseline during the pre-intervention survey, two more entomological surveys were carried out inside houses during the intervention (March and July, 2009) and two more after the intervention (November 2009 and March 2010).
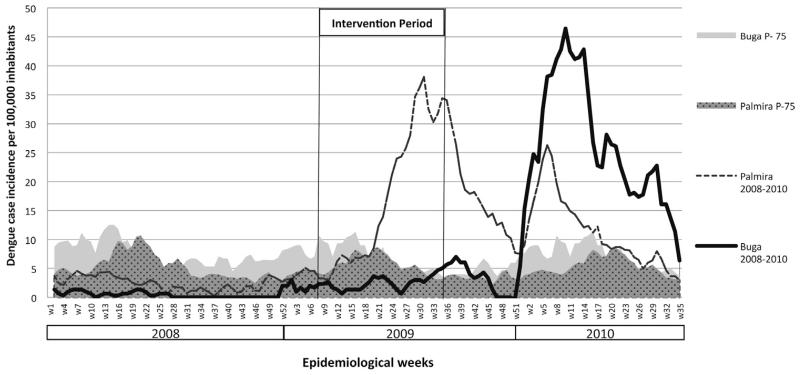
Incidence of dengue cases were reported weekly according to national guidelines during the entire duration of the study, including the post-intervention period (March 2008–March 2010). Endemic channels were draw for both Buga and Palmira with dengue cases reported from 2004 to 2008 using median and inter quartile ranges. The endemic channels are used to describe the expected number of cases and allows the early identification of epidemics when new cases are plotted against the expected (Bortman, 1999). The 75th percentile of the number of expected (historic) cases was used as a threshold to define the epidemic zone. The weekly number of dengue cases observed in 2008 and 2010 against the corresponding endemic channels in Buga and Palmira is shown in Fig. 3.
Fig. 3.
The catch basin intervention period (weeks 8–35, 2009) and the weekly dengue case incidence reported in Buga compared with the neighboring city of Palmira. The shaded areas represent the 75th percentile of historic weekly dengue incidence (light grey area, Buga and dark grey area, Palmira). The lines represent the weekly incidence of dengue cases reported in Buga and Palmira during 2008 and 2010.
2.5. Data management and statistical analysis
Entomological data was entered in MS Access 2007 and analyzed in Microsoft Excel. The following Stegomya indices were calculated at comuna level: House index (HI; the percentage of houses infested with larvae and/or pupae), Container Index (CI; the percentage of water-holding containers infested with immature mosquitoes), Breteau index (BI; the number of positive containers per 100 houses), and Pupae per person index (PI; mean number of pupae per person) (Focks, 2003). Despite the pupae counts categories agreed with technicians, the exact number of pupae in houses was obtained because it was always below 30. The pupae productivity of each type of domestic breeding site was calculated by dividing its total number of pupae by the number of sites of that type. The percentage of positive catch basins was calculated as the number of catch basins with immature stages (larvae and pupae) divided by the total number of catch basins surveyed times 100. Positivity of catch basins was calculated separately for Aedes and Culex. The effect of the intervention on the positivity of catch basins was estimated using χ2 test to compare each monthly survey conducted during and after the intervention against the baseline results.
Each reported dengue case was recorded in the Colombian national surveillance software (SIVIGILA) by health care personnel in charge of public health surveillance in each reporting institution. Quality control was done by the local health authorities on a weekly basis. The database was exported to MS Excel and then to R (version 3.0.1) for analysis. To explore the effect of the intervention on dengue incidence, the number of cases (whether severe or not) in Buga was modeled, using negative binomial regression, as a function of the Palmira incidence and the intervention status over time. Data for five years were included, up to and including week 36 of 2010. For this purpose, any effect of the intervention was assumed to act in weeks 11 to 38 of 2009 inclusive, this being a four-week shift of the implementation, allowing for lead-in and wash-out effects. The regression model, fitted using ‘glm.nb’ in R, therefore had two explanatory variables: intervention period or not (dichotomous), and the logarithm of incidence in Palmira. The latter was log transformed to analyze both cities’ incidence on the same scale, given the use of the default log link function. Ten of the 210 weeks with zero cases in Palmira could not be accommodated in this analysis and were excluded (there was no need to exclude weeks with zero cases in Buga). To take temporal autocorrelation into account, Newey–West standard errors (1987) were calculated from the regression output using the ‘newey.west.glm’ function. To parameterize this adjustment, the autocorrelation in the Buga incidence was found to be statistically significant up to a lag of three weeks, although the adjustment was made more conservative by including up to lag four, in accordance with the fourth-root of series length suggested by Baum (2006). A p-value of 0.05 was considered as statistically significant.
3. Results
3.1. Baseline (first phase)
3.1.1. Entomological survey in houses
The baseline entomological survey was conducted houses in September 2008. 603 houses from all 6 comunas were inspected. The expected sample size (185 per comuna) was not reached due to constraints on time and the number of field personnel Comuna 2 showed the lowest Stegomyia indices while comunas 5 and 6 showed the highest (Table 1). Breteau Index ranged from 3.5 to 15.3 and the pupae per person index varied from 0.01 to 0.18 (Table 1).
Table 1.
Entomological indices of larvae and pupae of Aedes aegypti calculated during the baseline assessment of house surveys in comunas of Buga.
| Comuna | Houses | People | Containers | CI | HI | BI | Pupae/person |
|---|---|---|---|---|---|---|---|
| 1 | 107 | 372 | 173 | 7.5 | 6.7 | 11.2 | 0.04 |
| 2 | 112 | 458 | 127 | 2.7 | 3.2 | 3.6 | 0.01 |
| 3 | 103 | 506 | 116 | 7.8 | 10.4 | 11.6 | 0.04 |
| 4 | 80 | 253 | 128 | 8.7 | 7.4 | 11.2 | 0.07 |
| 5 | 98 | 386 | 157 | 10.2 | 9.1 | 15.30 | 0.11 |
| 6 | 103 | 238 | 149 | 9.7 | 8.7 | 14.6 | 0.18 |
CI—container index; HI—house index; BI—Breteau index.
During the baseline a total of 850 potential breeding sites were inspected in houses. The most common type of potential breeding site was the ground level water storage tanks for laundry (56.7%), followed by plants in water (13%) and small miscellaneous (<500 mL) (8%). Plants in water were the most frequently found positive for immature stages (23.4% positive for larvae/pupae and 0.13% positive for pupae), followed by small miscellaneous (4.11% positive for larvae/pupae and 0.04% positive for pupae) and ground level water storage tanks for laundry (2.3% positive for larvae/pupae and 0.02% positive for pupae).
3.1.2. Entomological survey outside houses
A total of 108 catch basins were inspected of which, 58.3% were positive for immature stages of Aedes, 76% for Culex and 48% were positive for Aedes pupae with 2.8% of the total sample having an estimated range of 50 or more pupae (Fig. 1). No containers or natural breeding sites were found to be positive in parks and vacant lots visited during the survey.
Fig. 1.
Frequency of catch basins with different ranges of pupae productivity. 108 catch basins with water were surveyed for pupae productivity in six comunas of Buga. Water samples were collected and pupae count was averaged in the specific ranges.
3.1.3. Insecticide susceptibility
Adult Ae. aegypti showed 100% susceptibility to malathion and lambdacyhalothrin and decreased susceptibility to propoxur (52% mortality –30 min) (Fig. 2). In larvae, zero mortality after 24 h of exposure to temephos was observed. In contrast, a 100% inhibition of adult emergence was observed with pyriproxyfen (data not shown).
Fig. 2.
Insecticide susceptibility of Aedes aegypti adults to lambdacyhalothrin, malathion and propoxur determined by CDC bioassays (threshold at 30 min).
3.2. Intervention (second phase)
3.2.1. Entomological surveys during the second phase
During the second phase, four entomological surveys inside houses and in the catch basins were carried out. Overall, the Stegomyia indices were moderate (average of Breteau index ranged from 4.01 to 7.02 and Container Index ranged from 1.66 to 3.06), while pupae index were low (less than 0.02 pupae/person) indicating a low production of immature stages of Aedes in the houses during the study (Table 2).
Table 2.
Average of Stegomyia and pupae indices observed in houses during the six entomological surveys performed between December 2008 and March 2010.
| Comuna | Houses | People | Containers | Index average (min–max) | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Bretau | Container | Pupae/person | ||||
| 1 | 1053 | 13,936 | 2895 | 7.02 (3.2–21.4) | 2.55 (1.07–12.1) | 0.003 (0.0002–0.014) |
| 2 | 1222 | 15,560 | 3221 | 5.8 (3.6–8.6) | 2.23 (1.1–4.5) | 0.004 (0–0.008) |
| 3 | 1558 | 14,392 | 3134 | 6.16 (2.8–14.5) | 3.0 (1.7–7.7) | 0.008 (0–0.02) |
| 4 | 673 | 6814 | 1619 | 4.01 (0.9–7.9) | 1.66 (0.2–4.9) | 0.001 (0–0.003) |
| 5 | 1643 | 20,129 | 4119 | 6.0 (3.0–13.3) | 2.3 (1.2–9.0) | 0.001 (0–0.02) |
| 6 | 365 | 3777 | 896 | 7.1 (0.8–17.6) | 2.90 (0.3–10.7) | 0.0230 (0–0.12) |
A total of 15,872 potential breeding sites were inspected. The most common potential breeding site was the ground level water storage tanks for laundry (40%), followed by small miscellaneous (<500 mL) (26.9%) and “natural” breeding sites (10%). The potential breeding sites, showed generally low positivity of larvae and pupae, with bottles and cans, flower pots, drums, tires and plants in water most frequently found positive (6.0% < positivity < 8.9%) (Table 3). The pupae productivity observed in all domestic breeding sites was very low (<0.08) (Table 3).
Table 3.
Average of breeding site productivity in houses (n = 6514) according to types observed during six surveys performed between December 2008 and March 2010.
| Type of breeding site | N | Larvae/pupae positivity (%) |
Pupae productivity (no. of pupae/no. of tbs)* |
|---|---|---|---|
| Natural breeding sites | 1616 | 0.68 | 0 |
| Rooftop water storage tanks | 186 | 1.08 | 0.01 |
| Small miscellaneous (<500 mL) | 4273 | 1.24 | 0.01 |
| Large miscellaneous (>500 mL) | 614 | 1.3 | 0.01 |
| Ground level water storage tanks for laundry |
6357 | 1.9 | 0.02 |
| Bottles and cans | 516 | 6.01 | 0.05 |
| Flower pots | 1356 | 6.27 | 0.06 |
| steel water storage drums | 184 | 7.61 | 0.07 |
| Tires | 129 | 7.75 | 0.06 |
| Plant in water | 641 | 8.89 | 0.08 |
Pupae productivity calculated by dividing number of pupae per number of type of breeding site (tbs) observed.
A statistically significant reduction in the Aedes positivity of the catch basins was observed during the intervention compared to baseline. A reduction was observed in Culex in the first month post-intervention but not subsequently (Table 4). No emergence was observed in the treated catch basin-collected pupae brought to the laboratory (data not shown).
Table 4.
Percentage of positivity of catch basins for Aedes and Culex larvae and pupae observed in 10% of the catch basins treated with pyriproxyfen during the seven months intervention period.
| Month | Catch basins sampled n | Percentage of positivity in catch basins | ||
|---|---|---|---|---|
|
|
|
|||
| Aedes larvae | Culex larvae | Pupae◆ | ||
| February 28 | 360 | 60.5 | 39.7 | 26.9 |
| March 30 | 422 | 46.9** | 22.2** | 9.0** |
| April 30 | 387 | 42.9** | 47* | 17.2** |
| May 30 | 384 | 46** | 47* | 11** |
| June 30 | 383 | 42.3** | 39.4 | 13.1** |
| July 30 | 348 | 43.1** | 41.1 | 15.1** |
| August 30 | 389 | 40.1** | 35.4 | 13.3** |
Pupae positivity for both species compared to baseline (February).
p-value <0.05.
p-value <0.001.
3.2.2. Dengue incidence
Epidemic thresholds, defined as the 75th percentile of historic dengue cases (2004–2008), for Buga and Palmira, together with weekly incidence of dengue cases before, during and after the entomological intervention (2008–2010) are shown in Fig. 3. During the pre-intervention (2008–2010), Palmira reported relatively higher dengue incidence than Buga but both towns were below the 75th percentile of historic dengue cases. An epidemic from week 21 of 2009 to week 14 of 2010 was observed in Palmira. In contrast, before and during the intervention (weeks 8–35 of 2009), Buga was most of the time below the epidemic threshold except weeks 35 to 41 when a small increase in reported dengue cases was observed. When the intervention stopped (week 35 of 2009) an increase of dengue cases began to be observed in Buga reaching epidemic levels early in 2010. The rate ratio of dengue incidence in Buga relative to Palmira, adjusted for autocorrelation, was less in the intervention period, compared to the non-intervention period, by a factor of 0.19 (95% CI 0.12–0.30, p < 0.0001).The visual comparison of dengue cases against epidemic thresholds of both towns suggests that there was a delay in the onset of a dengue outbreak in Buga during the intervention period.
4. Discussion
This study reports the development and evaluation of a vector control strategy designed jointly by the research team and field staff (sanitation technicians) from a dengue hyperendemic area in Colombia. The vector strategy took into account the local entomological characterization of Aedes breeding sites inside and outside houses and aimed to control the main identified breeding site, the catch basins of kerbside storm drains.
The results of the baseline entomological characterization showed pupa productivity was low in houses and high in of catch basins. Although, Stegomyia indices were moderate risk, the pupae per person index in houses (0.02) was below the 0.5 to 1.5 pupae/person transmission risk threshold proposed by Focks et al. (2000) and Focks and Chadee (1997). By contrast, high infestation of immature stages of Aedes was observed in catch basins (58.3% of 108) (Fig. 1). Taking in account the fraction sampled of each one, the positivity and average ranges observed in catch basins baseline showed a higher production of pupae than in houses. These results suggested the catch basins were the main Aedes breeding site in Buga. The low pupae per person index in houses could be the result of previous dengue control campaigns focused on promoting the elimination of water holding containers and the identification of mosquito larvae by the community. This, together with the high coverage of public services, potentially limits the production of immature mosquitoes in houses. The results of the house survey contrast with the findings in the catch basins. Although those responsible for vector control in Buga recognized that the catch basins were one potential breeding site, they did not identify them as the main breeding site since the routine entomological surveillance was focused on houses and on traditional Stegomyia indices. The analysis of pupae index and the surveillance of other potential breeding sites in this study, allowed us to identify the most productive breeding sites in the town. Although we found that the field technicians initially objected to counting pupae, once they were aware of the low productivity of mosquitoes in houses, they began to understand the importance of obtaining these data. Index has previously been validated and is a recommended component of entomological surveillance (Focks, 2003). Our study joins others that highlight the importance of catch basins as Aedes breeding sites (Anderson et al., 2011; Hitoshi et al., 2010; Suarez and Suarez, 2004). A review of their current design to prevent them from holding stagnant water and the development of new eco-friendly technology for vector control in these breeding sites is required.
The detection of resistance to propoxur and temephos in Buga is in line with previous studies reported in other localities in Colombia (Fonseca-Gonzalez et al., 2011; Ocampo et al., 2011; Santacoloma et al., 2010). Temephos is the most frequently used insecticide for the control of breeding sites in houses and has also been used intermittently to treat catch basins in Buga (approximately 2 to 3 times per year). However, the high resistance to temephos observed in this study (zero mortality after 24 h post treatment) suggests that this insecticide was not effective and should no longer be used. Pyroproxyfen was chosen for catch basin intervention since it was highly efficacious in preventing adult emergence in immature stages of Aedes collected from Buga, and no cross resistance was observed between temephos and pyriproxyfen has been previously reported (Ricardo Leyva et al., 2010). One advantage of pyriproxyfen is that it requires very low doses (≤1 ppm), to inhibit the emergence of A. aegypti mosquitoes. It interferes with the metamorphosis of juvenile stages (Hirano et al., 1998) and/or causes morphological and functional aberrations in emerging adults, such as decreased fertility (Iwanaga and Kanda, 1988). Although some studies have demonstrated 5 months, previous studies by our group showed that the effect in catch basins decreased after a month, potentially due to rain water turnover (Mina et al., 2008).
The monthly treatment of catch basins with pyriproxyfen showed a significant and sustained reduction of their positivity for immature stages of Aedes. A reduction in positivity of the catch basins was not expected since it acts mainly on late instars or pupae as an inhibitor, not an insecticide in the strict sense. The effectiveness of the treatment was confirmed by the observed 100% inhibition of adult emergence in the laboratory. Only Ae. aegypti was observed during the collections. The reduction in the catch basin positivity suggests an effect of pyriproxyfen on mosquito density and hence on larvae infestation. The maintenance of positive catch basins also suggests the presence of other breeding sites producing mosquitoes in Buga. However, the low variation of the Stegomya indices and pupae in the house surveys suggests that Aedes did not increase its productivity in the houses, supporting a potential effect of the mass control of the main breeding sites on mosquito density. However, the before/after nature of the comparisons limits the extent to which we can attribute the observed changes to the intervention. Confirming this in future work would require other types of sampling such as adult mosquito catches. Moreover, pupae counts will be required to objectively measure the effect of pyriproxyfen in catch basins productivity. Therefore, future studies should measure the effect of the pyriproxyfen treatment at different levels including larva indices, pupae productivity and adult mosquito density.
To assess the impact of vector control interventions on dengue incidence is more challenging as there are multiple and complex confounders. Community randomized trials are not always feasible on the necessary scale in this case, entire towns would need to be randomized. As an ideal control town for Buga does not exist, the use of Palmira, which we found to be the most comparable in terms of geography, public service coverage and dengue transmission, is useful to complement the before and after quasi-experimental design within Buga. Visual comparison of the weekly reported dengue cases in Buga and Palmira suggests that there was a delay in the onset of a dengue epidemic in Buga during the intervention period. Statistical analysis estimated an approximately five-fold reduction in dengue cases during the intervention period. However, this analysis cannot completely rule out causes other than our intervention for the observed patterns. Other vector control interventions were not implemented in Buga during the study but contamination from national and local mass media dengue campaigns for vector control inside houses cannot be excluded. The dengue campaigns increased in Colombia in 2010, when the government declared a dengue epidemic and all national vector control activities were strengthened. At this time, we were unable to continue the intervention due to lack of pyriproxyfen supply and changes in the personnel of the Secretary of Health from Buga. However, we were able to continue supporting the local dengue case surveillance and a local mass media campaign to promote community participation in dengue control in houses. The local mass media campaign did not show an effect in the presence of positive breeding sites inside the houses (Carabali et al., 2013).
Some of the lessons learned from this study include the importance of engaging field staff in designing and operationalizing entomological surveillance. At first, counting pupae and increasing the number of houses to be sampled was strongly highly opposed by technicians. During the training activities an agreement was achieved to classify visually the number of pupae but other methods with a perceived lower workload could be developed.
A better knowledge of the local characteristics and risk factors for dengue transmission is fundamental to the design of effective vector control strategies. In this present case, the capacity to identify the importance of catch basins and to prioritize them for vector control supports previous reports that massive control of the most productive breeding site can decrease urban dengue. This has been suggested to be cost-effective, which should be assessed in future studies (Tun-Lin et al., 2009).
Acknowledgments
We acknowledge the participation of the Secretary of Health of Buga, specifically the Vector Borne Diseases technicians from the Sanitation group, the Secretary of Health from the Department of Valle del Cauca, James Becerra at CIDEIM for data management, and the community in general. We also acknowledge the colleagues from “Caja de Compensación Familiar del Valle del Cauca—COMFANDI” for taken part in the design and implementation of the Educational Campaign.
This study is part of the project: “Mass dissemination of the results of monitoring to promote community participation in controlling the transmission of dengue in the municipality of Buga, Valle” funded by Colciencias, Contract No.: 2229-408-20422.
References
- Alexander N, Lenhart AE, Romero-Vivas CM, Barbazan P, Morrison AC, Barrera R, Arredondo-Jiménez JI, Focks DA. Sample sizes for identifying the key types of container occupied by dengue-vector pupae: the use of entropy in analyses of compositional data. Annals of Tropical Medicine and Parasitology. 2006;100(Suppl 1):S5–S16. doi: 10.1179/136485906X105471. [DOI] [PubMed] [Google Scholar]
- Anderson J, Ferrandino F, Dingman D, Main A, Andreadis T, Becnel J. Control of mosquitoes in catch basins in Connecticut with Bacillus thuringiensis israelenis, Bacillus shpaericus, and spinosad. Journal of the American Mosquito Control Association. 2011;27:45–55. doi: 10.2987/10-6079.1. [DOI] [PubMed] [Google Scholar]
- Bath S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons C, Scott TW, Farrar J, Hay SI. The global distribution and burden of dengue. Nature. 2013;000:1–5. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum C. An Introduction to Modern Econometrics using Stata. Texas: 2006. [Google Scholar]
- Bortman M. Elaboración de corredores o canales endémicos mediante planillas de cálculo. Revista Panamericana de Salud Pública/Pan American Journal of Public Health. 1999;5:1–8. doi: 10.1590/s1020-49891999000100001. [DOI] [PubMed] [Google Scholar]
- Brogdon WG, McAllister JC. Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. Journal of the American Mosquito Control Association. 1998;14:159–164. [PubMed] [Google Scholar]
- Carabali M, Ocampo C, Toledo M, Osorio L. Difusión masiva de reportes situacionales sobre dengue: efectos de la intervención en Guadalajara de Buga, Colombia. Biomedica: Revista del Instituto Nacional de Salud. 2013;33(Suppl 1):130–141. [PubMed] [Google Scholar]
- DANE . Censo General 2005 Perfil Buga Valle del Cauca. DANE; Bogota, Colombia: 2005a. http://www.dane.gov.co. [Google Scholar]
- DANE . Censo General 2005 Perfil Palmira Valle del Cauca. DANE; Bogota, Colombia: 2005b. http://www.dane.gov.co/files/censo2005/perfiles/valle/palmira.pdf. [Google Scholar]
- Focks D. A review of entomological sampling methods and indicators for dengue vectors. World Health Organization; 2003. TDR/IDE/Den/03. [Google Scholar]
- Focks D, Alexander N. Multicontry study of Aedes aegypti pupal productivity survey methodology: Findings and recommendations. 2007. TDR/IRM/DEN/06.1. [Google Scholar]
- Focks DA, Brenner RJ, Hayes J, Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. American Journal of Tropical Medicine and Hygiene. 2000;62:11–18. [PubMed] [Google Scholar]
- Focks DA, Chadee D. Pupal survey: an epidemiological significant surveillance method for Aedes aegypti: an example using data from Trinidad. American Journal of Tropical Medicine and Hygiene. 1997;56:159–167. doi: 10.4269/ajtmh.1997.56.159. [DOI] [PubMed] [Google Scholar]
- Fonseca-Gonzalez I, Quinones ML, Lenhart A, Brogdon WG. Insecticide resistance status of Aedes aegypti (L.) from Colombia. Pest Management Science. 2011;67:430–437. doi: 10.1002/ps.2081. [DOI] [PubMed] [Google Scholar]
- Giraldo-Calderon GI, Perez M, Morales CA, Ocampo CB. Evaluation of the triflumuron and the mixture of Bacillus thuringiensis plus Bacillus sphaericus for control of the immature stages of Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) in catch basins. Biomedica: Revista del Instituto Nacional de Salud. 2008;28:224–233. [PubMed] [Google Scholar]
- Gubler DJ. Human arbovirus infections worldwide. Annals of the New York Academy of Sciences. 2001;951:13–24. doi: 10.1111/j.1749-6632.2001.tb02681.x. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Archives of Medical Research. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. The changing epidemiology of yellow fever and dengue, 1900 to 2003: full circle? Comparative Immunology, Microbiology & Infectious Diseases. 2004;27:319–330. doi: 10.1016/j.cimid.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Hirano M, Hatakoshi M, Kawada H, Takimoto Y. Pyriproxyfen and other juvenil hormone analogues. Reviews in Toxicology. 1998;2:357–394. [Google Scholar]
- Hitoshi K, Yoshihide M, Mayumi A, Kazunori O, Shin-ya O, Masahiro T. Spatial distribution and pyrethroid susceptibility of mosquitoe larvae collected from catch basins in parks in Nagasaki City, Nagasaki Japan. Japanese Journal of Infectious Diseases. 2010;63:19–24. [PubMed] [Google Scholar]
- Iwanaga K, Kanda T. The effects of a juvenile hormone active oxime ether compound on the metamorphosis and reproduction of an Anopheline vector, Anopheles balabacensis (Diptera:Culicidae) Applied Entomology and Zoology. 1988;23:186–193. [Google Scholar]
- Krebs C. Ecological Methodology. Menlo Park, CA: 1999. [Google Scholar]
- Mina NJ, Caicedo PA, Morales CA, Ocampo C. In: SOCOLEN, editor. Evaluacion del Piryproxyfen para el Control de los Estadios Inmaduros de Aedes aegypti y Culex quinquefasciatus en Sumideros de Cali, Colombia; Resumenes XXXV Congreso Sociedad Colombiana de Entomologia; Cali, Colombia. 2008.p. 132. [Google Scholar]
- Ministerio de la Protección Social, C . Guía de Vigilancia Entomológica y Control de Dengue. Ministerio de la Protección Social; Bogota, Colombia: 2012. Gestión para la vigilancia entomológica y control de la transmisión de dengue; pp. 1–126. [Google Scholar]
- Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Medicine. 2008;5:e68. doi: 10.1371/journal.pmed.0050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey W, West K. A simple, positive definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica. 1987;55:703–708. [Google Scholar]
- Ocampo CB, Salazar-Terreros MJ, Mina NJ, McAllister J, Brogdon W. Insecticide resistance status of Aedes aegypti in 10 localities in Colombia. Acta Tropica. 2011;118:37–44. doi: 10.1016/j.actatropica.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Ricardo Leyva Y, Rodriguez Coto MM, Bisset Lazcano JA, Perez Insueta O, Sanchez Valdes L. Effect of pyriproxyfen for the Aedes (S) aegypti control (Diptera: Culicidae) in strains with various degrees of temephos resistance. Revista Cubana de Medicina Tropical. 2010;62:224–229. [PubMed] [Google Scholar]
- Santacoloma L, Chaves B, Brochero H. Suceptibilidad de Aedes aegypti a DDT, deltametrina y lambdacialotrina en Colombia. Revista Panamericana de Salud Pública. 2010;27:66–73. doi: 10.1590/s1020-49892010000100010. [DOI] [PubMed] [Google Scholar]
- Scott TW, Morrison AC. Vector dynamics and transmission of dengue virus: implications for dengue surveillance and prevention strategies: vector dynamics and dengue prevention. Current Topics in Microbiology and Immunology. 2010;338:115–128. doi: 10.1007/978-3-642-02215-9_9. [DOI] [PubMed] [Google Scholar]
- SIVIGILA Comportamiento Epidemiologico del Dengue en Colombia Año 2010. 2013 http://www.ins.gov.co/lineas-de-accion/Subdireccion-Vigilancia/Informe%20de%20Evento%20Epidemiolgico/Dengue%202010.pdf.
- Suarez M, Suarez ME. The use of the copepod Mesocyclops longisetus as a biological control agent for Aedes aegypti in Cali, Colombia. Journal of the American Mosquito Control Association. 2004;20:401–404. [PubMed] [Google Scholar]
- Tun-Lin W, Lenhart A, Nam VS, Rebollar-Tellez E, Morrison AC, Barbazan P, Cote M, Midega J, Sanchez F, Manrique-Saide P, Kroeger A, Nathan MB, Meheus F, Petzold M. Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: a multi-country non-inferiority cluster randomized trial. Tropical Medicine & International Health: TM & IH. 2009;14:1143–1153. doi: 10.1111/j.1365-3156.2009.02341.x. [DOI] [PubMed] [Google Scholar]
- Wan SW, Lin CF, Wang S, Chen YH, Yeh TM, Liu HS, Anderson R, Lin YS. Current progress in dengue vaccines. Journal of Biomedical Science. 2013;20:37. doi: 10.1186/1423-0127-20-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Guidelines for laboratory and field testing of mosquito larvicides. WHO; 2005. WHO/CDS/WHOPES/GCDPP/2005. [Google Scholar]
- WHO . Report of the scientific working group on dengue 2006. WHO; 2006. TDR/SWG/08. [Google Scholar]
- Zea D, Osorio L. The status of the dengue surveillance system in a Colombian municipality. Revista de Salud Publica. 2011;13:785–795. doi: 10.1590/s0124-00642011000500007. [DOI] [PubMed] [Google Scholar]