Abstract
Background
Patients with low-grade glioma (LGG) who are successfully treated with irradiation are at increased risk for cognitive and psychosocial late effects. Conformal radiation therapy (CRT) allows sparing of cognitive deficits, but how it affects emotional and behavioral functioning remains unclear. We performed a prospective longitudinal study of the emotional and behavioral functioning of pediatric patients with LGG in the first 5 years post-CRT.
Methods
Ninety-five pediatric patients with LGG treated on an institutional Phase II trial (August 1997– June 2009) underwent neuropsychological assessments pre-CRT and 6, 12, 24, 36, 48, and 60 months post-CRT. Parent-reported scores on the Child Behavior Checklist (CBCL) were analyzed. Three competence scales (School Competence, Social Competence, and Activities), 2 summary scales (Internalizing Problems and Externalizing Problems), and 2 subscales of theoretical interest (Attention Problems and Social Problems) from the CBCL were used.
Results
Among 80 eligible patients [44 female, 68 white], 51 had pilocytic astrocytoma and 13 had optic pathway glioma. Mean age at diagnosis was 6.8 years (SD=4.3 years) and at CRT initiation was 8.9 years (SD=3.4 years). Before CRT, deficits were demonstrated on the competence scales (mean scores below normative mean) and the Attention Problems and Social Problems subscales (mean scores above normative means). This trend continued at 5 years post-CRT. Longitudinal trajectories of emotional and behavioral functioning were stable over 5 years.
Conclusions
Emotional and behavioral deficits remain relatively stable over the 5 years post-CRT in patients with LGG, suggesting that CRT may not exacerbate pre-existing psychosocial difficulties in this population.
Keywords: low-grade glioma, conformal radiation therapy, emotional and behavioral functioning, pediatric brain tumors
Low-grade glioma (LGG; World Health Organization [1] Grade 1 and 2 tumors) is a heterogeneous group of pediatric brain tumors that account for 30%–50% of diagnoses [2]. Patients with cerebral hemispheric tumors and those involving the cerebellum are often treated successfully with gross-total resection and experience long-term, progression-free survival [3]. Those with unresectable tumors, most often involving the brainstem, diencephalon and optic pathways, may require adjuvant therapy or non-surgical approaches [2,3]. Chemotherapy is recommended for young children to delay irradiation to minimize risk for late effects [2]. With the advent of new methods that spare normal tissues from radiation exposure, it has been proposed that the role of irradiation be re-evaluated [4].
There is variability in development of cognitive, academic, and psychosocial late effects in patients with LGG. Studies on long-term functional outcomes are conflicting, with some suggesting patients perform well [5,6] whereas others report higher than expected rates of cognitive and psychosocial difficulties [7-10]. Variability in functioning occurs in patients treated with surgery [6,11,12] as well as those treated with chemotherapy [13,14], irradiation [13,15], or combination.
This report is part of a series describing a cohort of patients with LGG who completed serial neuropsychological assessments on an institutional Phase II trial of photon-based conformal radiation therapy (CRT) [4]. The trajectory of cognitive [14,16] and adaptive [17] functioning was previously reported, and suggested cognition is generally preserved in patients treated with CRT, though patients who are younger (<5 years) at CRT remain at risk for declines. Evidence from baseline assessments revealed pre-CRT factors such as tumor progression, surgical intervention, and treatment with chemotherapy may contribute to deficits [14,17].
We investigated the trajectory of emotional and behavioral functioning of these patients during the first 5 years after CRT. Previous studies have been predominantly cross-sectional and have not mapped functioning over time. The heterogeneous nature of this diagnosis makes it difficult to assess the influence of prognostic factors in the setting of multiple treatment modalities. We addressed this concern by limiting our sample to patients who received similar treatment doses and target volume margins and through the use of longitudinal follow-up. It was hypothesized there would be variability in functioning pre-CRT, but the trajectory of psychological functioning would be relatively stable over the 5 year study period.
Materials and Methods
Participants and Procedures
The current study included patients with LGG enrolled on a Phase II trial of photon-based CRT between August 1997 and June 2009 [4,18]. Total prescribed dose was 54 Gy and clinical target volume margin was 1.0–0.5 cm. Inclusion criteria included age 1-25 years at study entry, biopsy-proven or neuroimaging diagnosis of localized LGG, and no prior irradiation or current chemotherapy. The study was approved by the IRB and informed consent was obtained. Indications for CRT included age ≥8 years at diagnosis, progression after chemotherapy, and the discretion of the multidisciplinary team, acute loss of visual acuity or inability to tolerate chemotherapy.
Patients completed neuropsychological assessments pre-CRT (baseline) and 6, 12, 24, 36, 48, and 60 months post-CRT. For this study, patients were required to complete at least 2 assessments that included the Child Behavior Checklist (CBCL) [19]. As the CBCL is a parent-completed measure, the use of English as a primary language by parents/caregivers was also required. The median number of evaluations completed was 5 (range 2–7).
Of 95 patients enrolled, 80 (84.2%) met inclusion criteria for the current analysis. Of those not included, 11 completed no assessments and 4 completed one. There were no differences in demographic or clinical characteristics between patients included and not included.
Measures
The CBCL [19] is a widely-used parent-report questionnaire of emotional and behavioral functioning for children 4–18 years old. Open-ended and forced-choice questions combine to yield competence scales (e.g., grades, participation in clubs, frequency of peer interactions) designed to assess indicators of adaptive functioning, as well as scales more indicative of psychopathology such as withdrawn/depressed (“would rather be alone than with others,” “cries a lot,”) or aggressive behavior (“argues a lot,” “gets in many fights”). The measure has been used to differentiate between clinically-referred and typically-developing children in both clinical and research settings, and is ideally-suited to tracking functioning over time [19,20]. Raw scores are converted to age- and gender-normed T-scores with a mean of 50 and standard deviation (SD) of 10. Thresholds for clinical significance (borderline and clinical range; indicative of a possible need for intervention) vary by subscale, but typically result from T-scores that are 1-2 SDs above (problem scales) or below (competence scales) 50. The CBCL demonstrates solid content validity, as well as construct validity with other measures of psychological functioning [20]. The measure is frequently used clinically and in research investigations with pediatric oncology patients [see 10,21,22,23 for examples]. For this analysis, scales of interest included 3 competence scales (School Competence, Social Competence, and Activities), 2 summary scales (Internalizing Problems and Externalizing Problems), and 2 subscales of theoretical interest based on the literature (Attention Problems and Social Problems). The Attention and Social Problems subscales are not included in the overall summary scales.
Intellectual and adaptive functioning was also assessed. Baseline estimated intellectual function was assessed via the age-appropriate Wechsler scale: Preschool and Primary (ages 4-6; WPPSI-R [24]), Child (ages 6-16; WISC-III [25]) and Adult (age 16+; WAIS-III [26]). Three subtests (Block Design, Similarities, Information) combine to derive an estimate of IQ using a formula provided by Sattler [27]. Baseline adaptive functioning was assessed via semi-structured parent interview, the Vineland Adaptive Behavior Scales [28]. Four subscales and an overall score are derived; the overall Adaptive Behavior Composite was used for analyses. Both IQ and adaptive measures yield an age-normed standard score with a mean of 100 and a standard deviation of 15. Longitudinal trajectories of intellectual and adaptive functioning for this cohort have been previously described [16,17].
Analyses
Descriptive analyses were conducted to characterize demographic, diagnostic, and treatment-related features at baseline. One-sample t-tests were used to compare mean scores with normative means at specific time points of interest (e.g., pre-CRT and 60 months post-CRT). Pearson correlations were used to evaluate associations between CBCL subscales and intellectual and adaptive functioning scores at baseline. Linear mixed models were used to investigate the longitudinal change in CBCL indices over time. For significant findings only, relevant demographic and clinical variables were entered into the model individually to investigate the effects of that variable on the baseline and longitudinal trajectory of the CBCL subscale. Frequencies were calculated to determine the percentage of the sample that exceeded expected clinical cut-offs on the CBCL at each time point.
Results
Patient Characteristics
Table 1 provides demographic and treatment characteristics of patients. The majority of patients were female (55%) and white (85%). Average age at diagnosis was 6.8 years (SD=4.3), and 8.9 years (SD=3.4) at CRT. Thirty-two patients (40%) underwent a biopsy, 32 (40%) a sub-total resection, and 14 (17.5%) did not receive any surgery. A third of the sample received chemotherapy. The majority of patients were diagnosed with biopsy-proven pilocytic astrocytoma (63.75%) or neuro-imaging based optic pathway glioma (16.25%).
Table 1.
Demographic and treatment characteristics of all patients (n = 80)
| Characteristic | N (%) | Mean ± SD | Range |
|---|---|---|---|
| Gender | |||
| Male | 36 (45.0) | ||
| Female | 44 (55.0) | ||
| Race | |||
| White | 68 (85.0) | ||
| Black | 7 (8.75) | ||
| Other | 5 (6.25) | ||
| Age at Diagnosis (years) | 6.80 ± 4.31 | 0.22–17.75 | |
| Age at CRT (years) | 8.94 ± 3.45 | 2.20–17.99 | |
| Time between Diagnosis and CRT | |||
| Less than 3 months | 36 (37.9) | 1.45 ± 0.81 | 0.23–3.00 |
| More than 3 months | 59 (62.1) | 41.53 ± 34.37 | 3.50–107.50 |
| Tumor Location | |||
| Infratentorial | 18 (22.5) | ||
| Supratentorial | 62 (77.5) | ||
| Tumor Locationa | |||
| Thalamus/Hypothalamus/Optic Pathways | 61 (76.25) | ||
| Cerebral Hemispheric | 3 (3.75) | ||
| Brainstem/Cerebellum | 15 (18.75) | ||
| Pre-CRT Extent of Resection | |||
| Biopsy | 32 (40.0) | ||
| Sub-total | 32 (40.0) | ||
| Near-total | 1 (1.25) | ||
| Gross-total | 1 (1.25) | ||
| Pre-CRT Surgeries (N) | 1.18 ± 0.81 | 0–3 | |
| 0 | 14 (17.5) | ||
| 1 | 44 (55.0) | ||
| 2 | 16 (20.0) | ||
| 3 | 6 (7.5) | ||
| Pre-CRT Chemotherapy | 29 (36.25) | ||
| VP Shunt | 28 (35.0) | ||
| Neurofibromatosis, Type 1 (NF1) | 12 (15.0) | ||
| History of Seizures | 9 (11.25) | ||
| Histologic Diagnosis | |||
| Pilocytic astrocytoma | 51 (63.75) | ||
| Optic pathway glioma | 13 (16.25) | ||
| Low-grade astrocytoma | 6 (7.50) | ||
| Pilomyxoid astrocytoma | 4 (5.00) | ||
| Ganglioglioma | 3 (3.75) | ||
| Neurocytoma | 1 (1.25) | ||
| Oligodendroglioma | 1 (1.25) | ||
| Pleomorphic xanthroastrocytoma | 1 (1.25) |
Abbreviation: CRT, conformal radiation therapy.
One of the supratentorial tumors could not be further categorized in this manner.
Baseline Functioning
Before CRT, mean parent-reported scores were lower on the Activities, Social Competence, and School Competence scales and higher on the Attention Problems and Social Problems subscales than normative means, suggestive of difficulties in these areas (Table 2). In contrast, parent-reported scores were lower on the Externalizing Problems scale, and scores on the Internalizing Problems scale were similar to the normative mean, suggestive of age-typical functioning.
Table 2.
Baseline and 60-month means of scores on the Child Behavior Checklist scales and longitudinal statistics
| Baseline+ | 60 months^ | Longitudinal Statistics† | ||||
|---|---|---|---|---|---|---|
| CBCL Scale | Mean ± SD | P-valuea | Mean ± SD | P-valuea | Annual Change | P-valueb |
| Competence Scalesc | ||||||
| Activities | 42.95 ± 9.36 | <.001 | 42.56 ± 10.25 | < .001 | 0.2363 | 0.38 |
| School Competence | 43.58 ± 9.91 | <.001 | 38.72 ± 9.54 | < .001 | –0.4281 | 0.12 |
| Social Competence | 44.18 ± 7.65 | <.001 | 42.95 ± 9.42 | < .001 | 0.0304 | 0.91 |
| Summary Scalesd | ||||||
| Internalizing Problems | 51.86 ± 9.71 | 0.18 | 49.26 ± 10.87 | 0.64 | –0.3869 | 0.16 |
| Externalizing Problems | 43.94 ± 10.2 | <.001 | 43.17 ± 9.98 | < .001 | 0.0311 | 0.91 |
| Individual Subscalese | ||||||
| Attention Problems | 53.98 ± 9.72 | <.003 | 55.48 ± 6.54 | < .001 | 0.4495 | 0.08 |
| Social Problems | 53.65 ± 12.51 | <.05 | 57.29 ± 7.96 | < .001 | 0.4149 | 0.09 |
Analyses completed with an n ranging from 38 to 58 patients (depending on subscale)
Analyses completed with an n ranging from 39 to 48 patients (depending on subscale)
Between 76 and 80 patients (depending on subscale) provided observations to the longitudinal analysis (at least 2 of a possible 7 completed evaluations were required for inclusion in analyses (median completed = 5)
P-values indicate univariate analysis against a normative mean of 50.
P-values indicate significance of change over time.
Borderline range: 30–35; Clinical range: <30
Borderline range: 60–63; Clinical range: >63
Borderline range: 65–69; Clinical range: ≥70
Baseline ratings were associated with several demographic and treatment factors. Children who underwent a biopsy had higher parent-reported scores on the Externalizing Problems scale than those who underwent subtotal resection (P=.004). However, children who underwent a biopsy were also engaged in more activities than those who underwent subtotal resection (P<.001). Race was associated with variability across the competence scales, with black children engaging in fewer activities (P=.04), spending less time with peers (P=.04), and having more difficulty in school (P=.002) than white children. Scores on the School Competence scale were also affected by tumor location: patients with infratentorial tumors had more difficulties with school performance than children with cerebral hemispheric tumors (P=.04) and tumors involving the thalamus, hypothalamus, and optic pathways (P=.04). At baseline, females had more attention difficulties than males (P<.05), as did those with a history of seizures (P<.02). Other diagnostic and treatment-related factors including pre-CRT chemotherapy, extended time before radiation (>3 months), age at diagnosis or CRT, history of NF1 and VP-shunt placement were unrelated to baseline functioning.
Correlations were computed between baseline CBCL scores, estimated IQ and adaptive functioning (Table 3). The Competence Scales were significantly positively associated with baseline estimated IQ, such that higher IQ was associated with greater engagement in activities and with peers and better school performance. In contrast, Attention and Social Problems were negatively associated with baseline IQ suggesting children with lower baseline IQs were more apt to have difficulty paying attention and more peer issues. There was no significant association between IQ and Internalizing or Externalizing Problems.
Table 3.
Associations between baseline CBCL scores and baseline estimated IQ and adaptive functioning
| CBCL Scales | Estimated IQa Pearson's r | Adaptive Behavior Compositeb Pearson's r |
|---|---|---|
| Competence Scales | ||
| Activities | .62*** | .50* |
| School Competence | .73*** | .54* |
| Social Competence | .43** | .55* |
| Summary Scales | ||
| Internalizing Problems | −.10 | .02 |
| Externalizing Problems | −.03 | −.12 |
| Individual Subscales | ||
| Attention Problems | −.55** | −.36 |
| Social Problems | −.45*** | −.18 |
p < .05
p < .01
p < .001
N = 49, Mean = 97.3, SD = 17.90, Range 52 – 136 (normative mean = 100, SD = 15)
N = 27, Mean = 92.0, SD = 20.29, Range 49 – 121 (normative mean = 100, SD = 15)
Competence Scales were significantly positively correlated with the Adaptive Behavior Composite at baseline (Table 3), such that higher parent-reported adaptive functioning was associated with better school performance and more engagement with peers and in activities. There was a trend (P=.06) for a negative relationship between parent-reported attention problems and adaptive functioning such that more attention problems were associated with poorer adaptive skills. There was no relationship between adaptive functioning and the Internalizing, Externalizing, or Social Problems scales.
Longitudinal Trends
Longitudinal analyses revealed relative stability across all scales and subscales (Table 2, Figure 1). Although there was a trend toward an increase in parent-reported scores on the Attention and Social Problems subscales, these did not reach significance.
Fig. 1.
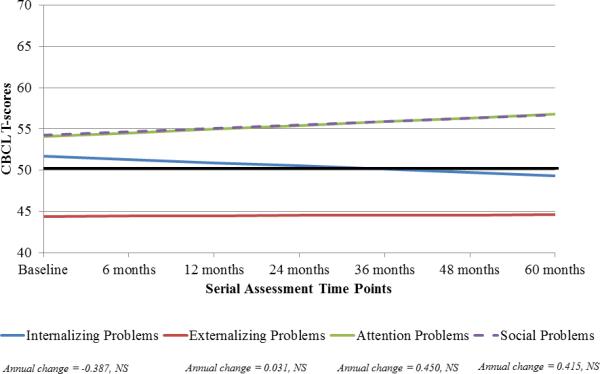
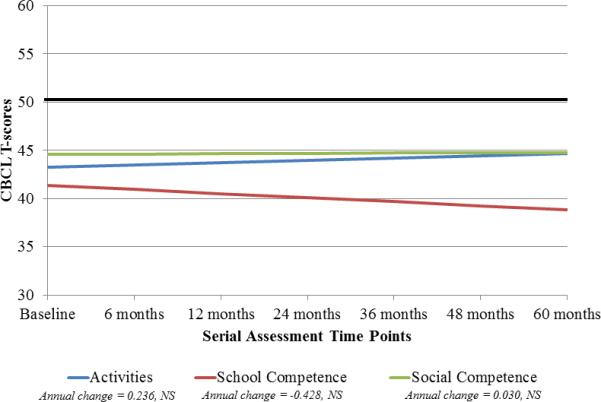
Longitudinal trajectory of emotional and behavioral functioning in patients with LGG at baseline and at different time points post-CRT. The solid black line at 50 indicates the normative mean (standard deviation 10). (A) For the Internalizing Problems and Externalizing Problems scales, higher scores are indicative of more problems. T-scores between 60 and 63 are in the borderline range and scores above 63 are in the clinical range. For the Attention Problems and Social Problems scales, T-scores between 65 and 69 are in the borderline range and scores 70 and above are in the clinical range. (B) For the competence scales, lower scores are indicative of more problems. T-scores between 30 and 35 are in the borderline range and scores below 30 are in the clinical range. NS, not significant.
At 60 months post-CRT, patients had lower scores on the Externalizing Problems scale than the normative mean, suggesting fewer concerns than typically-developing children. Scores on the Internalizing Problems scale were similar to the normative mean. However, parents reported significantly lower rates of engagement for patients across the competence scales and higher scores on the Attention Problems and Social Problems subscales than the normative means (Table 2), indicating more difficulties than typical for each subscale.
Variables within the Clinical Range
While mean scores are different from normative means, averages are generally within the normal range. There was interest in determining the clinical significance of performance over time. The percentage of patients falling within the clinical range (e.g., scores indicative of clinically-impairing symptoms) was compared to the normative percentage (2.3%) for each subscale at each assessment.
More patients than expected were in the clinical range on the Internalizing Problems scale at 24 and 36 months post-CRT (9.52%, n=6, P<.001 and 6.45%, n=4, P<.03, respectively). In contrast, only 1 patient each at 24 and 48 months post-CRT was in the clinical range for Externalizing Problems. A significant percentage of patients were in the clinical range on the Social Problems subscale at 36 (7.58%, n=5, P=.004) and 48 months post-CRT (8.93%, n=5, P<.001) and on the Attention Problems scale at 48 months post-CRT (7.14%, n=4, P=.01).
A significant percentage of patients were within the clinical range across the competence scales and across time points (Table 4). For the Activities scale, a significant percentage of patients were in the clinical range at baseline, 12 months and 60 months post-CRT. Similarly, a significant percentage of patients were in the clinical range on the Social Competence scale at 36 and 60 months post-CRT and in the School Competence scale at all the time points except 6 months post-CRT. These findings suggest that although mean scores were generally within normal limits, a notable percentage of patients experienced clinically-significant difficulties related to engagement in activities and with peers as well as difficulties in school.
Table 4.
Number of patients in the clinical range (T < 30) on the competence scales at each assessment point.
| Activities N (%) | School Competence N (%) | Social Competence N (%) | |
|---|---|---|---|
| Baseline | 7 (17.1)*** | 5 (12.5)*** | 1 (2.6) |
| 6 months | 2 (4.9) | 2 (5.3) | 1 (2.4) |
| 12 months | 4 (7.7)** | 7 (15.2)*** | 2 (4.0) |
| 24 months | 3 (4.9) | 10 (17.2)*** | 1 (17) |
| 36 months | 0 (0.0) | 10 (18.2)*** | 4 (6.7)* |
| 48 months | 3 (5.7) | 7 (14.6)*** | 3 (5.8) |
| 60 months | 5 (11.6)*** | 7 (17.9)*** | 5 (11.4)*** |
P < .03
P < .01
P < .001
Note. P-values indicate the comparison between the found proportion of patients within the clinical range as compared to the expected proportion of 2.3% in the normative sample.
Discussion
Pediatric patients with LGG are at risk for emotional and behavioral problems. Previous studies have been inconsistent about factors associated with these concerns, with studies identifying diagnosis and treatment (e.g., surgical resection, chemotherapy, radiation therapy) as both risk and protective factors. In our prospective assessment, patients with LGG referred for CRT showed problems with emotional and behavioral functioning before receiving CRT, but deficits remained relatively stable through 5 years post-CRT. These findings suggest that CRT may not exacerbate emotional and behavioral problems in children with LGG.
Importantly, our data reveal that indicators of potential psychopathology, both internalizing and externalizing behaviors, were within normal limits both pre-CRT and at all points post-CRT. Specifically, mean scores on the Internalizing Problems scale were similar to the normative mean and those on the Externalizing Problems scale were below the normative mean both at baseline and at 5 years post-CRT, with no longitudinal change over time, suggesting no evidence of clinically-significant psychopathology in the population as a whole. Very few patients were in the clinical range at any time point before or after CRT. In contrast, indicators of competence and engagement in normal social and school activities were well below the normative means both at baseline and at 5 years post-CRT, and a significant proportion of patients were in the clinical range across the study period. This suggests that these patients were not engaging in school and social activities at the same rates as typically-developing peers. Relatedly, parents also reported difficulties with attention and social functioning.
The relative stability in emotional and behavioral functioning over time, coupled with the difficulties observed pre-CRT, begs the question of how a delay in CRT (by means of observation, multiple surgeries, and/or chemotherapy) could affect psychosocial outcomes. Notably, performance at baseline was primarily affected by non-modifiable factors such as gender, race, and tumor location, and was also associated with intellectual and adaptive functioning. However, our previous analysis with these patients showed those who received chemotherapy before CRT had greater cognitive difficulties than those who did not [14]. While pre-CRT chemotherapy, extended time prior to CRT, and time since diagnosis were not associated with baseline ratings on the CBCL, we are not able to definitively state that there were no changes in functioning from diagnosis to our “baseline” assessment (an average of 2 years post-diagnosis). Although randomized trials of CRT versus chemotherapy might not be ethically prudent, it could be beneficial to prospectively follow groups of patients to determine the cognitive outcomes of patients undergoing surgery only versus those receiving chemotherapy and/or CRT. Importantly, long-term follow-up data (<5 years post-CRT) are not yet available. A recent review suggested the frequency of many adverse health and cognitive outcomes increases after 5 years post-diagnosis [8]. Thus, follow-up studies are essential to confirm the long-term outcomes of our patients and to ensure that CRT does not increase emotional and behavioral difficulties in long-term survivors, particularly when compared to other treatment modalities.
When compared to normative expectations [19], our patients had higher than anticipated difficulties in school competence in the years after receiving CRT. The School Competence scale assesses performance across academic subjects as well as history of grade retention or receipt of special education services [19]. Difficulties in school competence have also been found in patients with LGG treated with surgery only [10,12]. These studies, coupled with our findings, suggest that patients with LGG need to be closely followed with regard to academic achievement and the need for special education services, regardless of treatment modality. We recommend all patients with LGG be referred for a neuropsychological evaluation after surgical resection, with the timing of continued follow-up determined based on clinical need.
Tumor location is highly associated with risk of functional deficits (e.g., visual impairment, endocrine issues, physical limitations) [2], which may have an impact on academic outcomes as well as activity and/or social participation. As such, difficulties with school competence at the pre-CRT assessment and its significant association with tumor location and baseline IQ indicate the need for early assessment and intervention for all patients with LGG and particularly for those who undergo extensive surgical resections and/or treatment to delay radiotherapy. Although many interventions for children with brain tumors primarily focus on the off-treatment period, patients with LGG may be particularly amenable to earlier remediation services [29]. More specifically, providers may consider offering intervention with stimulant medication [30] or cognitive remediation [31,32] shortly after diagnosis and/or initial treatment. Such strategies may be particularly beneficial for those patients who require adjuvant therapies or those who have demographic or treatment factors (infratentorial tumor location, biopsy or subtotal resection, female, black) that were associated with more difficulties.
The current study has some limitations. First, findings relied primarily on a single measure of emotional and behavioral functioning, and may be strengthened by the inclusion of self-report or additional proxy report (e.g., teacher). The corresponding self- and teacher-report versions of the CBCL are available [19,20], but were not collected for this study. Parent and teacher ratings on the CBCL are moderately correlated [20], suggesting adequate agreement between the two raters. The use of more symptom-specific measures (e.g., attention or academic functioning) may have also strengthened our findings, though correlations with baseline intellectual and adaptive functioning provide support for our conclusions. Third, this study in part relied on comparing scores with normative means. Although a comparison group of typically-developing children might not be ideal, future studies could consider alternative comparison groups such as patients with LGG treated with surgery alone. This would also serve to increase the generalizability of our findings to all patients with LGG, and not just those who required CRT. Fourth, though some patients were assessed shortly after diagnosis, a sizable proportion were either monitored and/or received additional treatment, resulting in a mean interval of approximately 2 years between diagnosis and CRT. Therefore, our “baseline” assessment likely does not accurately reflect the pre-diagnosis functioning of our sample. Future studies should be designed that assess patients at diagnosis and then follow longitudinally, regardless of the treatment modalities ultimately used.
Our findings support consideration of photon-based CRT for patients with LGG who require adjuvant therapy. Indicators of emotional and behavioral functioning remained stable across the 5-year study period. Although mean scores were significantly higher than normative means, they were not clinically significant. Additional long-term follow-up studies are required to confirm stability in emotional and behavioral functioning post-CRT.
Acknowledgments
Funding: Supported in part by American Lebanese Syrian and Associated Charities (ALSAC)
Footnotes
Portions of this manuscript were presented at the 42nd Annual Meeting of the International Neuropsychological Society, Seattle, WA.
Conflict of Interest: None declared
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathology. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. doi:10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24:1397–1408. doi: 10.1177/0883073809342005. doi:10.1177/0883073809342005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher PG, Tihan T, Goldthwaite PT, Wharam MD, Carson BS, Weingart JD, Repka MX, Cohen KJ, Burger PC. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;41:245–250. doi: 10.1002/pbc.21563. doi:10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 4.Merchant TE, Kun LE, Wu S, Xiong X, Sanford RA, Boop FA. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27:3598–3604. doi: 10.1200/JCO.2008.20.9494. doi:10.1200/JCO.2008.20.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musial-Bright L, Panteli L, Driever PH. Pediatric low-grade glioma survivors experience high quality of life. Childs Nerv Syst. 2011;27:1895–1902. doi: 10.1007/s00381-011-1467-0. doi:10.1007/s00381-011-1467-0. [DOI] [PubMed] [Google Scholar]
- 6.Zuzak TJ, Poretti A, Drexel B, Zehnder D, Boltshauser E, Grotzer MA. Outcome of children with low-grade cerebellar astrocytoma: Long-term complications and quality of life. Childs Nerv Syst. 2008;24:1447–1455. doi: 10.1007/s00381-008-0692-7. doi:10.1007/s00381-008-0692-7. [DOI] [PubMed] [Google Scholar]
- 7.Aarsen FK, Paquier PF, Reddingius RE, Streng IC, Arts WM, Evera-Preesman M, Catsman-Berrevoets CE. Functional outcome after low-grade astrocytoma treatment in childhood. Cancer. 2005;106:396–402. doi: 10.1002/cncr.21612. doi:10.1002/cncr.21612. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong GT, Conklin HM, Huang S, Srivastava D, Sanford R, Ellison DW, Merchant TE, Hudson MM, Hoehn ME, Robison LL, Gajjar A, Morris EB. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13:223–234. doi: 10.1093/neuonc/noq178. doi:10.1093/neuonc/noq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beebe DW, Ris MD, Armstrong FD, Fontanesi J, Mulhern R, Holmes E, Wisoff JH. Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: Evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies (CCG 9891/POG 9130). J Clin Oncol. 2005;23:5198–51204. doi: 10.1200/JCO.2005.06.117. [DOI] [PubMed] [Google Scholar]
- 10.Ris MD, Beebe DW, Armstrong FD, Fontanesi J, Holmes E, Sanford RA, Wisoff JH. Cognitive and adaptive outcome in extracerebellar low-grade brain tumors in children: A report from the Children's Oncology Group. J Clin Oncol. 2008;28:4765–4770. doi: 10.1200/JCO.2008.17.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pompili A, Caperle M, Pace A, Ramazzotti V, Raus L, Jandolo B, Occhipinti E. Quality-of-life assessment in patients who had been surgically treated for cerebellar pilocytic astrocytoma in childhood. J Neurosurg. 2002;96:229–234. doi: 10.3171/jns.2002.96.2.0229. doi:10.3171/jns.2002.96.2.0229. [DOI] [PubMed] [Google Scholar]
- 12.Turner CD, Chordas CA, Liptak CC, Rey-Casserly C, Delaney BL, Ullrich NJ, Goumnerova LC, Scott RM, Begley HC, Fletcher WJ, Yao X, Chi S, Kieran MW. Medical, psychological, cognitive and educational late-effects in pediatric low-grade glioma survivors treated with surgery. Pediatr Blood Cancer. 2009;53:417–423. doi: 10.1002/pbc.22081. doi:10.1002/pbc.22081. [DOI] [PubMed] [Google Scholar]
- 13.Aarsen FK, Paquier PF, Arts WF, Van Veelen ML, Michiels E, Lequin M, Catsman-Berrevoets CE. Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytoma in childhood. J Clin Oncol. 2009;27:3526–3532. doi: 10.1200/JCO.2008.19.6303. doi:10.1200/JCO.2008.19.6303. [DOI] [PubMed] [Google Scholar]
- 14.Di Pinto M, Conklin HM, Li C, Merchant TE. Learning and memory following conformal radiation therapy for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys. 2012;84:e363–e369. doi: 10.1016/j.ijrobp.2012.03.066. doi:10.1016/j.ijrobp.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jalali R, Dutta D, Kamble R, Gupta T, Munshi A, Sarin R, Dinshaw K. Prospective assessment of activities of daily living using Modified Barthel's Index in children and young adults with low-grade gliomas treated with stereotactic conformal radiotherapy. J Neurooncol. 2008;90:321–328. doi: 10.1007/s11060-008-9666-6. doi:10.1007/s11060-008-9666-6. [DOI] [PubMed] [Google Scholar]
- 16.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. doi:10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. Longitudinal investigation of adaptive functioning following conformal irradiation for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys. 2013;85:1301–1306. doi: 10.1016/j.ijrobp.2012.10.031. doi:10.1016/j.ijrobp.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merchant TE, Zhu Y, Thompson SJ, Sontag MR, Heideman RL, Kun LE. Preliminary results from a Phase II trail of conformal radiation therapy for pediatric patients with localised low-grade astrocytoma and ependymoma. Int J Radiat Oncol Biol Phys. 2002;52:325–332. doi: 10.1016/s0360-3016(01)01807-7. doi:10.1016/S0360-3016(01)01807-7. [DOI] [PubMed] [Google Scholar]
- 19.Achenbach TM. Manual for the CBCL. University of Vermont; Burlington, VT.: 1991. [Google Scholar]
- 20.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth & Families; Burlington, VT.: 2001. [Google Scholar]
- 21.Willard VW, Conklin HM, Boop FA, Wu S, Merchant TE. Emotional and behavioral functioning after conformal radiation therapy for pediatric ependymoma. Int J Radiat Oncol Biol Phys. 2014;88:814–821. doi: 10.1016/j.ijrobp.2013.12.006. doi:10.1016/j.ijrobp.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrera M, Atenafu E. Cognitive, educational, psychosocial adjustment and quality of life of children who survive hematopoietic SCT and their siblings. Bone Marrow Transplant. 2008;42:15–21. doi: 10.1038/bmt.2008.84. doi:10.1038/bmt.2008.84. [DOI] [PubMed] [Google Scholar]
- 23.Dolson EP, Conklin HM, Li C, Xiong X, Merchant TE. Predicting behavioral problems in craniopharyngioma survivors after conformal radiation therapy. Pediatr Blood Cancer. 2009;52:860–864. doi: 10.1002/pbc.21947. doi:10.1002/pbc.21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence, Revised. The Psychological Corporation; San Antonio, TX.: 1989. [Google Scholar]
- 25.Wechsler D. Wechsler Intelligence Scale for Children - 3rd Edition. The Psychological Corporation; San Antonio, TX.: 1991. [Google Scholar]
- 26.Wechsler D. Wechsler Adult Intelligence Scale - 3rd edition. The Psychological Corporation; San Antonio, TX.: 1997. [Google Scholar]
- 27.Sattler JM. In: Assessment of Children. 3rd edn. Sattler Jerome M., editor. Publisher Inc.; San Diego: 1992. [Google Scholar]
- 28.Sparrow SS, Balla D, Cicchetti D. Vineland Adpative Behavior Scales. American Guidance Service; Circle Pines, MN.: 1984. [Google Scholar]
- 29.Askins MA, Moore BDI. Preventing neurocognitive late effects in childhood cancer survivors. J Child Neurol. 2008;23:1160–1171. doi: 10.1177/0883073808321065. doi:10.1177/0883073808321065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conklin HM, Reddick WE, Ashford J, Ogg S, Howard SC, Morris EB, Brown R, Bonner M, Christensen R, Wu S, Xiong X, Khan RB. Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. J Clin Oncol. 2010;28:4465–4472. doi: 10.1200/JCO.2010.28.4026. doi:10.1200/JCO.2010.28.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardy KK, Willard VW, Allen TM, Bonner MJ. Working memory training in survivors of pediatric cancer: A randomized pilot study. Psychooncology. 2013;22(8):1856–1865. doi: 10.1002/pon.3222. doi:10.1002/pon.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler RW, Copeland DR, Fairclough DL, Mulhern RK, Katz ER, Kazak AE, Noll RB, Patel SK, Sahler OJZ. A multicenter, randomized clinical trial of a cognitive remediation program for childhood survivors of a pediatric malignancy. J Consult Clin Psychol. 2008;76:367–378. doi: 10.1037/0022-006X.76.3.367. doi:10.1037/0022-006X.76.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]


