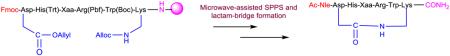
Abstract
Side-chain to side-chain lactam-bridged cyclic peptides have been utilized as therapeutic agents and biochemical tools. Previous synthetic methods of these peptides need special reaction conditions, form side products and take longer reaction times. Herein, an efficient microwaveassisted synthesis of side-chain to side-chain lactam-bridge cyclic peptides SHU9119 and MTII is reported. The synthesis time and efforts are significantly reduced in the present method, without side product formation. The analytical and pharmacological data of the synthesized cyclic peptides are in accordance with the commercially obtained compounds. This new method could be used to synthesize other side-chain to side-chain lactam-bridge peptides and amenable to automation and extensive SAR compound derivatization.
Keywords: Cyclic peptides, Microwave assisted synthesis, Lactam-bridge cyclic peptides, SHU9119, MTII
Graphical Abstract
Cyclic peptides offer great potential as therapeutic agents because they can exhibit membrane permeability,1 resistance to proteolytic degradation,2 and metabolic stability3. Cyclic peptides also serve as biochemical tools in studying protein-protein interactions and molecular probes.4-7 A wide variety of methodologies have been reported for syntheses of cyclic peptides using side-chain to side-chain bridging techniques, which include disulfide formation,8 lactamization,9 all-hydrocarbon linkage.10 Among these peptides, lactam-bridge peptides are finding an increasing number of applications in protein biology, which include protein folding, protein aggregation, peptide ligand–receptor recognition, and development of potent peptide therapeutics.11
In general, side-chain to side-chain lactam-bridged cyclic peptides can be synthesized by cyclizing the side-chain amino group of a lysine residue with the side-chain carboxyl group of a glutamic acid or aspartic acid residue of the peptide. Previous syntheses of these peptides used Nα-Boc strategy, which requires strong acid like trifluoroacetic acid (TFA) for repetitive removal of the Boc groups, while often relying on corrosive and toxic hydrofluoric acid (HF) for release of the assembled peptide from the support.12-15 Later approaches have used Nα-Fmoc strategy to synthesize this class of cyclic lactam-bridge peptides, where the Fmoc group can be removed under basic conditions.4,16-21 But, these methods require specialized reaction conditions such as inert atmosphere and closed reaction vessel without the presence of oxygen for selective deprotection of alloc, allyl protecting groups.16 These methods are also prone to form side products,19 which result in cumbersome purification steps and take longer time to achieve completion for peptide coupling.4,16,19,20 Hence, an efficient synthesis of side-chain to side-chain lactam-bridge cyclic peptides in shorter reaction time, without need for specialized reaction conditions and no side products formation is highly desirable.
The utilization of microwave heating can be advantageous in organic synthesis to synthesize diverse compounds, which increases reaction yields and shortens reaction times.22 The microwave-assisted solid-phase peptide synthesis have also made improvements to both the speed of the peptide coupling and Nα-Fmoc deprotection as well as increased the purity of the crude peptides.23 Here an efficient synthesis of side-chain to side-chain lactam peptides SHU9119, Ac-Nle-c[Asp-His-DNal(2’)-Arg-Trp-Lys]-NH2 and MTII, Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2 using microwave irradiation in an open vessel is reported. This method increases overall efficiency and significantly reduces the reaction time of each step including side-chain to side-chain lactam-bridge formation. No considerable side product formation was observed in this method.
The cyclic SHU9119 and MTII peptides were selected as examples of side-chain to side-chain lactam-bridge peptides, because of their tremendous applicability as biochemical tools for in vivo and in vitro characterization of the melanocortin receptor system.15,17,18,24-28 Melanocortin receptors (MC1R-MC5R) belong to the family of G-protein coupled receptors (GPCRs) and have been known to mediate numerous physiological processes including energy homeostasis,29 steroidogenesis,30 feeding behavior,28 sexual function,31 and skin pigmentation.32 The β-lactam containing SHU9119 peptide is an antagonist and partial agonist at the mMC3R and a complete antagonist at the mMC4R.13 The MTII peptide is a potent agonist at the MC1, MC3-5 receptors.12,14
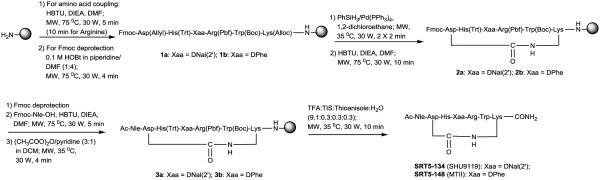
The SHU9119 and MTII peptides, which are C-terminal amidated lactam-bridge cyclic peptides were synthesized efficiently by microwave-assisted Fmoc solid phase peptide synthesis using Rink Amide MBHA resin (0.35 meq/g loading) in a manual microwave synthesizer (Discover SPS™, CEM Corp., supporting information). Initially, these peptides were elongated up to the Asp residue to afford linear peptide resins 1a and 1b respectively (Scheme 1) under microwave irradiation. Aspartimide formation is predominant in the deprotection of Fmoc group of the aspartic acid in the peptide, when an allyl ester is present as the side chain protection for aspartic acid.33 To avoid aspartimide formation, the alloc and allyl groups were selectively deprotected and the lactam cyclization was performed prior to the removal of the Fmoc of the Asp residue. Traditionally, allyl and alloc groups were selectively removed with Pd(PPh3)4/PhSiH3 at room temperature for 30 min (2 times).16 This reaction was done in a sealed reaction vessel under argon atmosphere with a complete absence of oxygen, which poisons the catalyst.16 Deprotection of the allyl and alloc functional groups under microwave-assisted conditions was presented at the American Peptide Symposium.34 However, the presently described method utilized modified microwave conditions to selectively deprotect alloc and allyl functional groups. While on resin, peptides 1a and 1b were treated with 20 equiv. of PhSiH3, and 0.25 equiv. of Pd(PPh3)4, in 1,2-dichloroethane under microwave conditions (30 W, 35 °C) for 2 min in an open reaction vessel and the above process was repeated. After the deprotection, a Kaiser test35 indicated the presence of free amino group.
Scheme 1.
Microwave-assisted syntheses of SRT5-134 (SHU9119) and SRT5-148 (MTII)
Traditionally, lactam-bridge formation was reported in the presence of HBTU/PyBOP/HATU, HOBt and DIEA for 2-24 h, at room temperature.4,16,19,20 This coupling was repeated until a negative Kaiser test resulted.16,19 In the present method, the peptides were successfully cyclized on the resin under microwave conditions in 10 min by treating with HBTU and DIEA in DMF to afford 2a and 2b (Scheme 1). Subsequent Nα-Fmoc deprotection, coupling of the final Fmoc-Nle-OH amino acid and final Nα-Fmoc removal were performed using same conditions described above.
The resulting free N-terminal peptide resins were acetylated under microwave conditions in 4 min to afford final peptide resins 3a and 3b. Cleavage of the final cyclic peptides from resin was done in 10 min under microwave irradiation using modified conditions of a previously published method.36 Treatment of final cyclic peptide resins 3a and 3b with a mixture of trifluoroacetic acid, thioanisole, triisopropylsilane and water (9.1:0.3:0.3:0.3) under microwave conditions in 10 min gave crude peptides SRT5-134 (SHU9119) and SRT5-148 (MTII). A complete comparison of synthetic conditions of these cyclic peptides under traditional Fmoc/t-Bu approach at room temperature and microwave-assisted solid-phase peptide synthetic conditions used in the current methodology are summarized in Table 1.
Table 1.
| Step | Traditional SPPS (room temperature) | Microwave-assisted SPPS |
|---|---|---|
| Fmoc deprotection | 20-25% Piperidine/DMF; 5 min and 30 min | 0.1 M HOBt in piperidine /DMF (1:4) solution; 2 min at rt; MW, 75 °C, 30 W, 4 min |
| Coupling | Fmoc-amino acid (3 equiv.), HBTU, HOBt, DIEA, 2 h |
Fmoc-amino acid (3 equiv.), HBTU, DIEA; MW, 75 °C, 30 W, 5 min (10 min for Arginine) |
| Alloc and Allyl group deprotection |
PhSiH3 (24 equiv.) or DMBA (10 equiv.), Pd(PPh3)4 (0.1-0.25 equiv.); Argon atm.; 2 X 30 min |
PhSiH3 (20 equiv.), Pd(PPh3)4 (0.25 equiv.); MW, 35 °C, 30 W, 2 X 2 min |
| Lactam-bridge Formation |
HOBt (6 equiv.), HBTU/PyBOP/HATU (1-6 equiv.), DIEA (3-12 equiv.) in NMP/THF for 2- 24 h (repeat the process until a negative Kaiser test resulted) |
HBTU, (6 equiv.) DIEA (12 equiv.) in DMF; MW, 75 °C, 30 W, 10 min |
| N-Acetylation | Acetic anhydride: pyridine (3:1), 30 min |
4 mL of acetic anhydride: pyridine (3:1) mixture in 4 mL DCM; MW, 35 °C; 30 W, 4 min |
| Cleavage from Resin |
TFA:TES:H2O (9:0.5:0.5), 3 h |
TFA:TIS:Thioanisole:H2O (9.1:0.3:0.3:0.3); MW, 35 °C, 30 W, 10 min |
HPLC profiles of these crude peptides SRT5-134 (SHU9119) and SRT5-148 (MTII) showed 91% and 85% crude peptide purity respectively (supporting information). In the traditional room temperature method, the reported crude peptides purity were in the range of 65-72%.33 After purification of the crude peptides using semi-preparative HPLC and lyophilization, mass analysis found that major peak for each peptide corresponded to the expected product (supporting information). Resulted peptides were found to be ≥ 99% pure (RP-HPLC) and the yields of the peptides were comparable to that of room temeperature method.16 Microwave-assisted peptide synthesis and resin loading could be the factors in obtaining lactam-bridge cyclic peptides with high crude peptide purity. Co-injection of synthesized SHU9119 (SRT5-134) and commercially obtained SHU9119 (Bachem) into analytical RP-HPLC resulted in elution of both compounds as one peak, matching the chromatograms of both synthesized SHU9119 (SRT5-134) and commercially obtained SHU9119 (supporting information). The methodology presented here allows the formation of the cyclic lactam peptides on solid support in an efficient manner with shorter reaction times compared to that of room temperature methods,4,16,19,20 without requiring any specialized reaction conditions and no considerable side product formation.
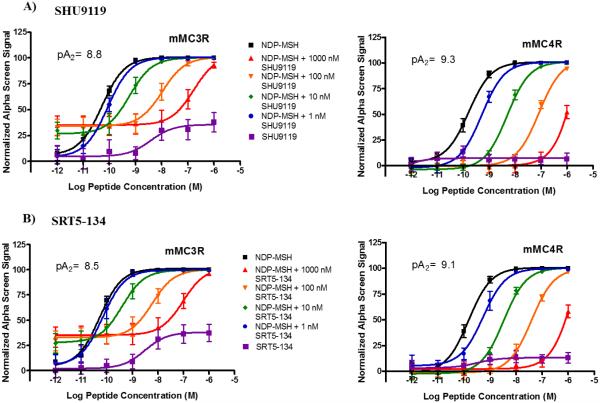
The synthesized SHU9119 and MTII peptides were tested for functional activity at the mouse MCRs using the cAMP-based AlphaScreen® assay37 (PerkinElmer) according to the manufacturer’s instructions. The results obtained are illustrated for the synthesized and commercially obtained SHU9119 and MTII peptides in Table 2. Since, the AlphaScreen® assay is a competition assay with loss of signal at higher concentrations, the concentration-activity curves were normalized for illustration purposes similar to Elster et al (Figure 1).38 These results support the hypothesis that the synthetic route do not alter functional potency or efficacy as anticipated.
Table 2.
Pharmacology of commercial and synthesized SHU9119 and MTII at the mouse melanocortin receptors.a
| Peptide | mMC1R | mMC3R | mMC4R | mMC5R |
|---|---|---|---|---|
|
|
||||
| EC50 (nM) | ||||
| NDP-MSH | 0.04±0.01 | 0.23±0.03 | 0.47±0.21 | 0.18±0.07 |
| SHU9119 (Commercial) |
0.98±0.17 | Partial agonist PA2 = 8.8, Ki = 1.6 nM |
Antagonist PA2 = 9.3, Ki = 0.5 nM |
Partial agonist |
| SHU9119 (SRT5-134) | 1.43±0.33 | Partial agonist PA2 = 8.5, Ki = 3.2 nM |
Antagonist PA2 = 9.1, Ki = 0.79 nM |
Partial agonist |
| MTII (Commercial) |
0.06±0.02 | 0.18±0.04 | 0.15±0.06 | 0.14±0.05 |
| MTII (SRT5-148) |
0.05±0.01 | 0.15±0.04 | 0.14±0.02 | 0.12±0.02 |
The indicated errors represents the standard error of the mean determined from at least three independent experiments.
* The pA2 values were calculated by a Schild analysis. 39
Figure 1.
Antagonist Pharmacology of SHU9119 synthesized (SRT5-134) and commercial using Schild analysis at the mouse MC3R and MC4R.
In conclusion, an efficient microwave-assisted synthetic strategy for the syntheses of side-chain to side-chain lactam-bridged cyclic peptides on a solid support without aspartimide formation is reported. This synthetic route makes the purification of these peptides efficient and the synthesis amenable to rapid SAR compound generation and purification. Utilization of microwave irradiation in each step of the peptide synthesis, which include i) deprotection of Fmoc group, ii) coupling of amino acid, iii) selective deprotection of alloc and allyl protecting groups, iv) formation of lactam cyclic-bridge, v) N-terminal acetylation, and vi) cleavage of peptide from resin, reduces the reaction times at each step. The current method also permits the syntheses of these peptides efficiently in an open reaction vessel without need for complete absence of oxygen. The methodology presented here could be automated and applied to the synthesis of other side-chain to side-chain lactam-bridge cyclic peptides, decreasing the synthetic times and improving the synthetic efficiency.
Supplementary Material
Acknowledgments
This work has been supported by NIH Grants R01DK091906 (C. H.-L.).
Abbreviations
- MWA-SPPS
microwave-assisted solid phase peptide synthesis
- MBHA
methylbenzhydryl
- MCR
melanocortin receptors
- NDP
[Nle4, DPhe7]-αMSH
- MTII
melanotan-II
- RP-HPLC
reverse phase high performance liquid chromatography
- TFA
trifluoroacetic acid
- TIS
triisopropylsilane
- H2O
water
- HBTU
O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluoro-phosphate
- DIEA
N,N-diisopropylethylamine
- DMF
N,N-dimethylformamide
- DCM
dichloromethane
- HATU
O-(7-azabenzotrizol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluoro-phosphate
- PyBOP
(benzotriazol-1-yloxy)tripyrrolidinophospho-nium hexafluoro-phosphate
- HOBt
N-hydoxybenzotriazole
- TES
triethylsilane
- NMP
N-methyl-2-pyrrolidone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could afiect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Detailed procedure for the syntheses of peptides, analytical HPLC chromatograms, analytical data of peptides and AlphaScreen® cAMP assay are given in the supporting information.
References
- 1.Rezai T, Yu B, Millhauser GL, Jacobson MP, Lokey RS. J. Am. Chem. Soc. 2006;128:2510–1. doi: 10.1021/ja0563455. [DOI] [PubMed] [Google Scholar]
- 2.March DR, Abbenante G, Bergman DA, Brinkworth RI, Wickramasinghe W, Begun J, Martin JL, Fairlie DP. J. Am. Chem. Soc. 1996;118:3375–3379. [Google Scholar]
- 3.Gentilucci L, De Marco R, Cerisoli L. Curr. Pharm. Des. 2010;16:3185–3203. doi: 10.2174/138161210793292555. [DOI] [PubMed] [Google Scholar]
- 4.Murage EN, Gao GZ, Bisello A, Ahn JM. J. Med. Chem. 2010;53:6412–6420. doi: 10.1021/jm100602m. [DOI] [PubMed] [Google Scholar]
- 5.Park BW, Zhang HT, Wu CJ, Berezov A, Zhang X, Dua R, Wang Q, Kao G, O'Rourke DM, Greene MI, Murali R. Nat. Biotechnol. 2000;18:194–198. doi: 10.1038/72651. [DOI] [PubMed] [Google Scholar]
- 6.Colgrave ML, Korsinczky MJL, Clark RJ, Foley F, Craik DJ. Biopolymers. 2010;94:665–672. doi: 10.1002/bip.21415. [DOI] [PubMed] [Google Scholar]
- 7.Athanassiou Z, Dias RLA, Moehle K, Dobson N, Varani G, Robinson JA. J. Am. Chem. Soc. 2004;126:6906–6913. doi: 10.1021/ja0497680. [DOI] [PubMed] [Google Scholar]
- 8.Jackson DY, King DS, Chmielewski J, Singh S, Schultz PG. J. Am. Chem. Soc. 1991;113:9391–9392. [Google Scholar]
- 9.Felix AM, Heimer EP, Wang CT, Lambros TJ, Fournier A, Mowles TF, Maines S, Campbell RM, Wegrzynski BB, Toome V, Fry D, Madison VS. Int. J. Pept. Protein Res. 1988;32:441–454. doi: 10.1111/j.1399-3011.1988.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 10.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor JW. Biopolymers. 2002;66:49–75. doi: 10.1002/bip.10203. [DOI] [PubMed] [Google Scholar]
- 12.Al-Obeidi F, Castrucci AM, Hadley ME, Hruby VJ. J. Med. Chem. 1989;32:2555–61. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- 13.Hruby VJ, Lu D, Sharma SD, Castrucci AL, Kesterson RA, Al-Obeidi FA, Hadley ME, Cone RD. J. Med. Chem. 1995;38:3454–61. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- 14.Al-Obeidi F, Hadley ME, Pettitt MB, Hruby VJ. J. Am. Chem. Soc. 1989;111:3413–3416. [Google Scholar]
- 15.Haskell-Luevano C, Lim S, Yuan W, Cone RD, Hruby VJ. Peptides. 2000;21:49–57. doi: 10.1016/s0196-9781(99)00167-9. [DOI] [PubMed] [Google Scholar]
- 16.Grieco P, Gitu PM, Hruby VJ. J. Pept. Res. 2001;57:250–6. doi: 10.1111/j.1399-3011.2001.00816.x. [DOI] [PubMed] [Google Scholar]
- 17.Grieco P, Han G, Weinberg D, Van der Ploeg LH, Hruby VJ. Biochem. Biophys. Res. Commun. 2002;292:1075–80. doi: 10.1006/bbrc.2002.6739. [DOI] [PubMed] [Google Scholar]
- 18.Grieco P, Balse-Srinivasan P, Han G, Weinberg D, MacNeil T, Van der Ploeg LH, Hruby VJ. J. Pept. Res. 2003;62:199–206. doi: 10.1034/j.1399-3011.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 19.Vaz E, Dames SA, Geyer M, Brunsveld L. Org. Biomol. Chem. 2012;10:1365–73. doi: 10.1039/c1ob06422c. [DOI] [PubMed] [Google Scholar]
- 20.Guo H, Gallazzi F, Miao Y. Bioconjugate Chem. 2012;23:1341–8. doi: 10.1021/bc300191z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang B, Li X, Zhang C, Yan S, Wei W, Wang X, Deng X, Qian H, Lin H, Huang W. Org. Biomol. Chem. 2015;13:4551–61. doi: 10.1039/c5ob00333d. [DOI] [PubMed] [Google Scholar]
- 22.Lidstrom P, Tierney J, Wathey B, Westman J. Tetrahedron. 2001;57:9225–9283. [Google Scholar]
- 23.Pedersen SL, Tofteng AP, Malik L, Jensen KJ. Chem. Soc. Rev. 2012;41:1826–44. doi: 10.1039/c1cs15214a. [DOI] [PubMed] [Google Scholar]
- 24.Doering SR, Todorovic A, Haskell-Luevano C. ACS Med. Chem. Lett. 2015;6:123–7. doi: 10.1021/ml500340z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ericson MD, Wilczynski A, Sorensen NB, Xiang Z, Haskell-Luevano C. J. Med. Chem. 2015;58:4638–47. doi: 10.1021/acs.jmedchem.5b00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Testa C, Scrima M, Grimaldi M, D'Ursi AM, Dirain ML, Lubin-Germain N, Singh A, Haskell-Luevano C, Chorev M, Rovero P, Papini AM. J. Med. Chem. 2014;57:9424–9434. doi: 10.1021/jm501027w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irani BG, Xiang Z, Yarandi HN, Holder JR, Moore MC, Bauzo RM, Proneth B, Shaw AM, Millard WJ, Chambers JB, Benoit SC, Clegg DJ, Haskell-Luevano C. Eur. J. Pharmacol. 2011;660:80–7. doi: 10.1016/j.ejphar.2010.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 29.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 30.Haynes RC, Jr., Berthet L. J. Biol. Chem. 1957;225:115–24. [PubMed] [Google Scholar]
- 31.Wessells H, Hruby VJ, Hackett J, Han G, Balse-Srinivasan P, Vanderah TW. Neuroscience. 2003;118:755–62. doi: 10.1016/s0306-4522(02)00866-7. [DOI] [PubMed] [Google Scholar]
- 32.Smith PE. Science. 1916;44:280–2. doi: 10.1126/science.44.1130.280. [DOI] [PubMed] [Google Scholar]
- 33.Flora D, Mo H, Mayer JP, Khan MA, Yan LZ. Bioorg. Med. Chem. Lett. 2005;15:1065–8. doi: 10.1016/j.bmcl.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Wilson KR, Sedberry S, Pescatore R, Ballard S, Love B, Vinton D, Williamson E. American Peptide Symposium; Orlando, FL. 2015. p. P417. [Google Scholar]
- 35.Kaiser E, Colescott RL, Bossinger CD, Cook PI. Anal. Biochem. 1970;34:595–8. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- 36.Collins JM, Porter KA, Singh SK, Vanier GS. Org. Lett. 2014;16:940–3. doi: 10.1021/ol4036825. [DOI] [PubMed] [Google Scholar]
- 37.Singh A, Tala SR, Flores V, Freeman K, Haskell-Luevano C. ACS. Med. Chem. Lett. 2015;6:568–72. doi: 10.1021/acsmedchemlett.5b00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elster L, Elling C, Heding A. J. Biomol. Screen. 2007;12:41–9. doi: 10.1177/1087057106295895. [DOI] [PubMed] [Google Scholar]
- 39.Schild HO. Brit. J. Pharmcol. Chemother. 1947;2:251–8. doi: 10.1111/j.1476-5381.1947.tb00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.