Abstract
Broad-range 16S ribosomal RNA gene PCR coupled with Sanger sequencing was originally employed by soil scientists and was subsequently adapted for clinical applications. PCR coupled with electrospray ionization mass spectrometry has also progressed from initial applications in the detection of organisms from environmental samples into the clinical realm and has demonstrated promise in detection of pathogens in clinical specimens obtained from patients with suspected infection but negative cultures. We review studies of multiplex PCR, 16S ribosomal RNA gene PCR and sequencing and PCR coupled with electrospray ionization mass spectrometry for detection of bacteria in specimens that were obtained from patients during or after administration of antibiotic treatment, and examine the role of each for assisting in antimicrobial treatment and stewardship efforts. Following an exploration of the available data in this field we discuss the opportunities that the preliminary investigations reveal, as well as the challenges faced with implementation of these strategies in clinical practice.
Keywords: 16S rDNA PCR, PCR/ESI-MS, Multiplex PCR, Antimicrobial stewardship, culture negative infections, mass spectrometry
Introduction
In the treatment of infectious diseases, specific antimicrobial therapy can be administered when the identity of the pathogen is known. With the rising prevalence of antimicrobial resistance, the choice of the appropriate empiric antibiotic remains difficult when patients present with signs and symptoms of infection, yet cultures are negative.
Antibiotic pretreatment negatively impacts the likelihood of recovering viable organisms in culture. [1,2] When patients fail to respond to empiric antimicrobial treatment initiated without cultures for guidance, body fluids and tissue specimens subsequently obtained from suspected sites of infection and submitted for culture are less likely to identify the bacterial pathogen causing the infection. [3] Consequently, clinicians are commonly faced with the dilemma of continuing or changing antimicrobial therapy in the face of negative cultures. Finding ways to improve the detection of pathogens and discerning their potential response to therapy remains an important, but elusive goal.
“Salvage Microbiology” is a term that originated three years ago for the application of molecular diagnostic techniques in the detection of bacterial DNA directly from clinical specimens submitted for culture from patients who were treated with antimicrobial therapy. Although the term “salvage microbiology” was originally associated with the use of PCR combined with electron-spray ionization mass spectrometry (PCR/ESI-MS), this concept may apply to any similar application of molecular diagnostics to guide antimicrobial treatment when cultures are negative, or are expected to be negative due to prior empiric antimicrobial treatment. [3] Newer molecular methods that rely on nucleic acid amplification technology (NAAT) offer a unique advantage in the detection of pathogens collected after initiation of antimicrobial treatment. NAAT may also provide an opportunity to target antimicrobial therapy and “salvage” both individual treatment regimens as well as institutional antimicrobial stewardship efforts in settings with strong working collaborations between pharmacy, microbiology and infectious disease departments. A variety of techniques that employ NAAT offer potential applications in this setting: we review studies of real-time multiplex PCR, 16S ribosomal RNA gene PCR and sequencing, and PCR/ESI-MS for detection of bacteria in specimens that were obtained from patients during or after administration of antibiotic treatment and discuss the opportunities these platforms provide as well as the challenges facing successful implementation of these strategies moving forward. Interference of early empiric antimicrobial treatment with culture based diagnostics remains a common clinical conundrum, finding methods to improve the detection of pathogens in these cases and integrating salvage microbiology diagnostic algorithms into patient care, antimicrobial stewardship programs, and clinical microbiology laboratory practices remains a formidable challenge.
In addition to PCR/ESI-MS, other molecular diagnostic platforms that have potential for application in this setting include multiplex PCR as well as broad-range ribosomal RNA gene PCR and sequencing.
Opportunities
NAATs offer opportunities not only for detection of pathogens in specimens obtained from patients following initiation of antibiotic treatment, but also for detection of genes associated with specific antimicrobial resistance such as mecA in methicillin resistant Staphylococcus aureus (MRSA), vanA and vanB in vancomycin resistant enterococcus (VRE), ampC in plasmid mediated cephalosporin resistance in Enterobacteriaciae, as well as genetic mechanisms of extended spectrum beta-lactamase resistance (ESBL) and carbapenem resistance in Enterobacteriaciae (e.g., KPC, NDM, CTX-M). Detection of the presence of organisms harboring these genes is relevant not only in diagnostic testing related to treatment decisions for individual patients, but also screening for colonization for infection control purposes. In this review, we will restrict our discussion to diagnostic testing related to detection of bacterial pathogens in patients who have been treated with antibiotics prior to diagnostic testing.
Symptoms associated with infection from viral and bacterial respiratory pathogens are clinically indistinguishable. When patients present with suspected respiratory tract infections, antimicrobial treatment is often administered unnecessarily for disease resulting from viral infections, and overly broad antibiotics may be given for simple bacterial infections that should be expected to respond to a single antibiotic. Patients are commonly evaluated in prompt-care facilities or emergency departments that typically have a limited array of diagnostic testing options. When outpatient diagnostic testing is not performed or no pathogens are detected with the limited point of care diagnostic assays available, empiric antibiotic treatment is often recommended. Clinicians must realize that the respiratory tract is a non-sterile site. As a result, point of care (POC) diagnostics that use NAATs should distinguish colonization versus infection. To address this, POC testing ought to only include detection of the most common respiratory pathogens (e.g. influenza A and B, RSV, parainfluenza) and of those the ones that are treatable.
At most tertiary hospitals and academic medical centers, the antimicrobial stewardship team (AST) is the group charged with preventing unnecessary or excessive use of antibiotics. [4] It has been demonstrated that ASTs alone, or in combination with rapid diagnostics, improve inpatient outcomes compared to standard microbiology reporting. [5–7] However, since patients with viral upper respiratory tract infections typically present to an outpatient clinic and do not require hospitalization, most patient encounters with potential to benefit from for the combination of molecular diagnostics and AST actually occur outside of the scope of traditional hospital-based AST. Because of the cost of molecular testing, these cases are rarely afforded the advantage of molecular diagnostics for detection and diagnosis of the infectious etiology, and to date, the benefits of both antimicrobial stewardship and salvage microbiology have only been demonstrated in the inpatient setting. [5–7]
Multiplex PCR
Multidrug resistant organisms (MDROs) are not exclusively encountered in hospitals, and have become common in many communities, but outpatient clinics are typically not equipped for screening or detection of MDROs. In outpatient clinics and emergency departments, point-of-care testing is favored because rapid diagnostics facilitate faster determination of patient disposition. [8] Although rapid antigen detection assays for Group A streptococcus, pneumococcus and influenza (early in the flu season) are helpful when positive, they will fail to detect MDROs or viral pathogens other than influenza, and are much too limited to have a meaningful impact on antimicrobial use in a community facility. [6,8] Multiplex PCR, which is not yet available as a point-of-care assay, is the most likely platform to extend the salvage microbiology approach to the outpatient setting. Multiplex PCR employs multiple primer sets within a single PCR mixture. Each primer set is specific for DNA sequences that are unique to individual viral or bacterial species and is designed to produce multiple amplicons of varying lengths that are readily detected based on their differing sizes. For multiple distinct detections to occur reproducibly within a single PCR mixture, the amplicon base pair lengths must be sufficiently unique, requiring careful calibration to optimize the annealing temperatures for each of the primer sets in the PCR mixture. In clinical settings, real-time detection of each amplicon is employed as opposed to the more time and labor consuming traditional method of gel-electrophoresis.
Rapid recognition of MRSA, VRE, and ESBL positive GNB are critical for successful antimicrobial stewardship in both inpatient and outpatient settings, but few multiplex platforms have the capacity to identify all these MDROs with a single assay, and few primary care physicians are trained to screen for colonization with these organisms. Multiplex PCR platforms have the capacity to identify pathogens such as pneumococcus, and detect the presence of antimicrobial resistance genes. [9] But recognizing the appropriate patient for screening or interrogation will require education. The platform that has achieved the most wide-spread acceptance in screening for MDROs is the Cepheid GenXpert™ real-time PCR platform that has assays capable of screening for MRSA, VRE, and CRE. [10] This assay requires minimal training, minimal sample preparation, and is capable of producing results in 90 minutes from receipt of specimen to result report.
Intracellular and anaerobic bacteria require special media and conditions to recover viable organisms in the laboratory. When patients are started on antibiotics before specimens are submitted to the microbiology laboratory, recovering viable bacteria becomes even more challenging. [1,2,11] Syndromically directed molecular diagnostic testing platforms intended to detect DNA from the most common respiratory pathogens are well-suited for salvage microbiology. Currently, four multiplex diagnostic platforms that employ DNA amplification have been approved in the U.S. by the FDA for molecular detection of respiratory pathogens: Luminex® xTAG RVPv1 and Luminex® xTAG RVP fast (Luminex Molecular Diagnostics, Austin, TX); FilmArray® respiratory panel (BioFire Diagnostics, Salt Lake City, UT); eSensor® respiratory viral panel (GenMark Dx, Carlsbad, CA). All four platforms are designed and approved for testing of nasopharyngeal (NP) swabs and have reported specificities of >99%; of course, there are unique advantages and disadvantages associated with each [12–14]. Among the four platforms, FilmArray could be the most readily integrated into an outpatient salvage microbiology/antimicrobial stewardship program due to its ease of use, minimal hands-on time, and less than 60 minutes from start of assay to results, but its poor sensitivity for adenovirus does compromise its appeal for this application. [15,16]
Popowitch, et al. [16] performed a direct comparison of all four platforms on 300 NP swabs from the 2011–2012 influenza season that had been stored at −4 °C. All four systems performed well; with sensivities and specificities > 95%. The specificities for all four assays were high, but the FilmArray was 100% specific for all targets (95% confidence interval = 96.2 – 100). The FilmArray, which targets 18 respiratory viral pathogens and three bacterial pathogens, had sensitivities > 92% for all targets except influenza virus A/2009:H1N1 (73.3%), influenza B virus (77.3%), RSV A (86.4%), rhinovirus/enterovirus (83.7%), and adenovirus (57.1%). Because of the cost associated with all of the multiplex assays, systematic incorporation of multiplex testing into a diagnostic and treatment algorithm for patients with signs and symptoms of respiratory tract infections would require commitment of time and resources of an institutional antimicrobial stewardship program committed to expanding its scope to the ambulatory setting.
16S ribosomal RNA Gene PCR and Sequencing
Bacterial ribosomes are composed of RNA and proteins. 16S ribosomal RNA (rRNA) is a component of the small subunit of prokaryotic ribosomes, and 23S rRNA is a component of the large subunit.[17] The genes that encode for 16S rRNA are referred to as 16S rDNA. The 16S ribosomal RNA gene is the most extensively employed gene for bacterial phylogenetic analysis, and 16S rDNA sequences are integral to constructing bacterial phylogenies.[17] Because of the close association between 16S rDNA sequences and phylogenetic classification of prokaryotic species, 16S rDNA PCR is widely accepted as an essential molecular tool for detection and identification of bacteria in clinical and environmental samples.
Broad-range 16S rDNA PCR has been applied in the clinical microbiology lab to facilitate the diagnosis of infectious diseases of bacterial origin by detecting 16S rDNA sequences in patient samples. Unlike the multiplex PCR assays discussed thus far, primers employed in 16S rDNA PCR do not target unique, species-specific DNA sequences.[18] Instead, highly-conserved regions of the 16S rDNA are targeted with a single primer set, averting the problem associated with conventional and multiplex PCR of correctly anticipating the potential pathogen(s) and optimizing multiple primers.[18,19] A significant advantage of 16S rDNA PCR over multiplex-PCR is that detection of unusual, unexpected or novel organisms is no more challenging than detection of expected or anticipated pathogens. 16S rDNA PCR is ideally suited to testing of sterile body fluids and tissues, but is poorly suited for analysis of respiratory specimens which typically compromise a complex mixture of bacteria and viruses.
Unlike multiplex PCR, interpretation of the results of broad-range 16S rDNA PCR is required. After the broad-range primers amplify a portion of the 16S rDNA genes from the bacteria present in the specimen, the PCR product is sequenced and the result is compared to 16S rDNA sequences in public databases such as the DNA Databank of Japan (DDBJ); European Nucleotide Archive (ENA); or GenBank (NCBI). Interpretation of the significance of the sequences matching unusual, unexpected or novel organisms requires experience, and should be done in collaboration between microbiologists and infectious disease clinicians. Nonetheless, post-amplification sequencing does have the advantage of facilitating identification of the infecting organism, and potentially identifying unexpected or novel organisms to the species level. Unfortunately, this enhanced detection of novel or unexpected pathogens comes at the expense of the assay’s sensitivity. Broad-range 16S rDNA PCR is less sensitive than a well-designed specific real-time PCR assay. [18]
The only study specifically designed to examine the salvage microbiology performance of 16S rDNA PCR was performed on heart valve tissue obtained in the operating room during valve surgery for patients with suspected infective endocarditis (IE). [20] Voldstedlund, et al. [20] examined the sensitivity and specificity of culture vs. 16S rDNA PCR in patients with suspected IE, all of whom had been treated, or were being treated with antibiotics. Broad-range 16S DNA PCR, culture, and histology were compared for heart valves from 74 IE patients. All patients were classified according to the Duke criteria for diagnosis of IE and blood culture results served as the gold standard. [21] The sensitivity and specificity of 16S rDNA PCR was 72% and 100%, respectively, as compared to sensitivity and specificity of 26% and 62%, respectively, for heart valve tissue culture. Examination of the dose response relationship between antibiotic treatment and recovery of organisms by culture vs. 16S rDNA PCR revealed that the sensitivity of culture and PCR were comparable for patients who had received less than 5 days of antibiotic treatment prior to surgery. In contrast, for patients who were treated for more than 5 days, the sensitivity of valve culture was significantly lower than the sensitivity of 16S rDNA PCR. [20]
Many studies have been done examining the utility of broad-range 16S rDNA PCR for clinical specimens other than heart valve tissue, but very few analyses examined the salvage microbiology performance of 16S rDNA PCR. Studies of 16S rDNA PCR for detection of bacterial pathogens in cerebrospinal fluid, abscess fluid and tissue, pleural fluid, synovial fluid and tissue, and ascitic fluid have been performed and published.[8,18,20,22,23] But unlike the study performed by Voldstedlund, et al. [20] these investigations do not exclusively target patients receiving antimicrobial treatment. Nonetheless, since each study includes a subset of patients who were treated with antibiotics, examination of the larger studies in this cohort does provide insight into potential opportunities for salvage microbiology. The largest study to date that included a subset of salvage microbiology surgical specimens was performed on bone and joint tissue, and synovial fluid samples. Fenollar, et al. [22] published a prospective, systematic evaluation of 16S rDNA PCR vs. culture in patients with suspected bone and joint infections. In this study, 525 bone and joint tissue samples were collected from 525 patients. 16S rDNA PCR followed by sequencing, as well as both aerobic and anaerobic cultures were each done twice for every specimen. When a potential pathogen was detected by 16S rDNA PCR from a culture negative specimen (suggesting a false negative culture), a third PCR assay was done that was a species-specific PCR to confirm the detection and identification made by 16S rDNA PCR and sequencing. Culture and 16S rDNA PCR results were identical in 475/525 (90%) cases. Not surprisingly, with such strong agreement between culture and 16S rDNA PCR, no statistically significant differences were observed between the two diagnostic approaches for sensitivity or specificity (P = 0.13 & 0.06, respectively). [22]
However, two important observations were noted. First, and perhaps most surprising, was the finding that contamination was a more common complication of conventional culture than molecular testing: 13 of the positive cultures were attributed to contamination, and only five positive 16S rDNA PCR tests were consistent with contamination. [22] Secondly, and most relevant to our current discussion, is that almost half of the antibiotic treated, culture-negative patients in the sample had detectable bacterial pathogens on 16S rDNA PCR. In this study, 104 of the 384 patients (49%) with negative cultures were receiving antibiotic therapy at the time of surgical removal or replacement of the orthopedic prosthesis. Interestingly, 47% (7/15) of patients with negative cultures who had two step sequential surgical extractions and were receiving antibiotics prior to both surgical procedures had the same bacterial pathogen detected by 16S rDNA PCR in the specimens collected from both the initial procedure and the follow-up procedure. In each case, these detections were validated by a confirmatory species-specific PCR. S. aureus was detected more than any other bacteria among the specimens with confirmed false negative cultures (3/7; 43%). Other pathogens detected included viridans streptococci, Streptococcus pneumoniae, and anaerobic gram negatives. Although S. aureus was the bacterium identified most frequently among the antibiotic treated patients with false negative cultures, there were also four cases of culture confirmed S. aureus infection that were not detected by 16S rDNA PCR, but were confirmed by S. aureus specific PCR. [22] None of these four patients had received antibiotic treatment prior to surgery.
Rampini, et al. [23] also used broad-range 16S rDNA PCR to demonstrate “missed opportunities” for diagnosis of infection by culture when specimens are submitted to the clinical microbiology lab for testing after initiation of antibiotics. Rampini, et al. compared results of culture and broad-range 16S rDNA PCR followed by amplicon sequencing for 536 clinical specimens from normally sterile body sites obtained from hospitalized patients. The two diagnostic methods were in agreement for 83% (445/536) of the samples tested. [23] Because of the overall strong agreement in this study between culture and 16S rDNA PCR, evidence of statistical significance between the two tests for the study population was not present. However, upon examination of the subset of patients in this study receiving antimicrobial treatment, impressive differences were observed: for salvage microbiology patients, 16S rDNA PCR detected bacterial DNA in 30.4 % (24/79) of patients receiving antimicrobial treatment compared with no detections by culture (Kappa = 0.334). [23]
Taken in total, our review of the experience comparing conventional culture to 16S rDNA PCR with particular attention to the subset of patients that had been treated with antibiotics prior to specimen sampling suggests that use of 16S rDNA PCR improves both sensitivity and specificity for recognition of infection and pathogen identification in this patient population. These studies demonstrate an important missed opportunity for antimicrobial stewardship programs, clinical microbiology labs, and treating healthcare providers. This opportunity is particularly relevant in the clinical practice of antimicrobial stewardship, where opportunities to avoid overly broad empiric antimicrobial treatment regimens are desperately needed. However, 16S rDNA PCR is time consuming and does require dedicated laboratory staff. This technology is not appropriate for a clinical lab with a small staff unless there is a dedicated technician with experience in molecular detection platforms and techniques.
PCR/ESI-MS
As an alternative to sequencing of PCR amplicons for identification of bacterial species and recognition of antimicrobial resistance genes, mass spectrometry techniques such as matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) have be employed for analysis of ionized proteins, and electrospray ionization mass spectrometry (ESI-MS) for rapid reliable analysis of PCR products.[24,25] Removal of PCR detergents, salts and buffers as well as deoxynucleotide triphosphates and metal ions which interfere with accurate mass measurement is essential prior to employing mass spectrometry for measurement of PCR products. But with development of reliable methods for obtaining pure, desalted PCR amplicons for mass spectrometry, this approach has gained traction because of the capacity to detect and accurately identify organisms to species level discrimination directly from clinical specimens, even with samples that may have a complex mixture of pathogens; and the ability to detect medically important genetic markers of antimicrobial resistance. [25]
The PCR/ESI-MS platform, which combines broad-range PCR with time-of-flight mass spectrometry, incorporates the advantages mentioned above and has been employed in most of the published investigations involving clinical specimens. [3,26–30] PCR/ESI-MS combines multiple broad-range primers to amplify all bacterial targets within a sample with several species-specific and resistance gene-specific PCR primers for detection of genes critical to pathogens that have acquired known mechanisms of antibiotic resistance (Table 1). [25] This assay is well suited for detection not only of common bacteria that may be suppressed by antimicrobial treatment, but is also well suited for detecting and deciphering uncommon pathogens and complex polymicrobial infections, recognition of antimicrobial resistance genes, and identification of viral and fungal pathogens.
Table 1.
PCR/ESI-MS primer target genes and their breadth of coverage.
| Primer Pair | Target Clade | Primer Pair Target Gene |
|---|---|---|
| BCT346 | Broad Bacterial | 16S rDNA; Broad Bacterial Coverage |
| BCT348 | Broad Bacterial | 16S rDNA; Broad Bacterial Coverage |
| BCT361 | Broad Bacterial | 16S rDNA; Broad Bacterial Coverage |
| BCT349 | Broad Bacterial | 23S rDNA; Broad Bacterial Coverage |
| BCT3350 | GM + Coverage | rplB; 50S ribosomal subunit protein L2; Firmicutes |
| BCT2249 | Staphylococcus speciation | tufB Elongation factor EF-Tu; staphylococci |
| BCT358 | Gm - Enterobacteria | valS Valyl-tRNA synthetase; proteobacteria |
| BCT3346 | Gm - Gamma Proteobacteria | rpoB RNA polymerase; beta- and gamma-proteobacteria |
| BCT3921 | Gm - beta and gamma proteobacteria | rpoB; RNA polymerase; beta- and gamma-proteobacteria |
| BCT879 | mecA | mecA; Methicillin resistance |
| BCT4675 | kpc | blaKPC; Carbapenem resistance |
| BCT3767 | vanA | vanA/vanB; Vancomycin resistance |
| BCT3768 | vanB | vanA/vanB; Vancomycin resistance |
| FUN3030 | Broad Candida | 25S rDNA; Broad Fungal Coverage |
| FUN3031 | Broad Candida | 25S rDNA; Broad Fungal Coverage |
| FUN3766 | Broad Candida | 25S rDNA; Broad Fungal Coverage |
| FUN3865 | Broad Candida | Mitochondrial DNA; Candida |
| PLN4437 | Extraction Control | Pumpkin Extraction Control |
16S rDNA PCR and PCR/ESI-MS have the advantage compared to multiplex PCR that knowledge of a predetermined target is not necessary for pathogen detection. As with 16S rDNA PCR, broad-range PCR primers are employed in PCR/ESI-MS that target conserved regions of the bacterial genome. But instead of employing sequencing to identify the pathogens amplified by PCR, amplicons are detected and interpreted using mass spectrometry. Time-of-flight (TOF) mass spectrometry signals are translated into amplicon base composition signatures to provide distinct fingerprints of the organisms detected. [24,25] Unlike 16S rDNA PCR, PCR/ESI-MS does not rely on a single conserved sequence for broad-range PCR. PCR/ESI-MS employs a multiplex PCR process with four sets of broad-range primers that target conserved regions of both 16S rRNA and the 23S rRNA genes to amplify all bacterial species (Table 1). In addition, highly target-specific primers are used to detect bacterial species, and common antibiotic resistance genes (e.g., mecA, blaKPC, vanA & vanB). PCR/ESI-MS also provides an additional level of flexibility because fungal and viral primer sets can also be employed on the same platform. [24]
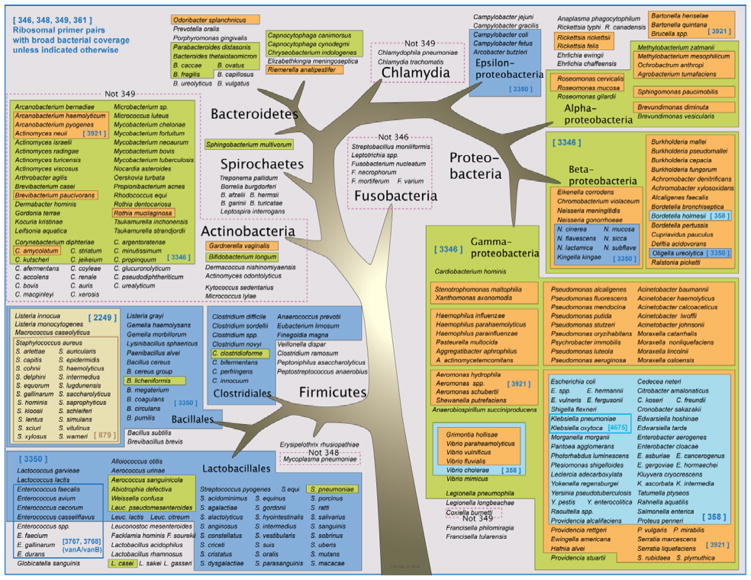
Just as with 16S rDNA PCR, PCR/ESI-MS requires routine nucleic acid extraction from the clinical specimen prior to PCR thermocycling. Desalting of the PCR product is not required for sequencing, but is a critical step prior to the sequential electrospray of PCR products into the mass spectrometer. Once the masses of the nucleic acids present in the sample have been measured, the analog signal of mass is converted to a digital signal of base composition based upon the mass measurement and the discrete masses associated with different combinations of the four nucleotide bases. [24] Mass spectrometry measures the mass and determines the base composition of all nucleic acids in the mixture, regardless of the complexity of the original sample (e.g., abscess fluid or purulent respiratory secretions). The amplicon base composition signals are then compared to signature signals of a database of all known species of bacteria (Figure 1). [22,23] Base composition is a useful metric for identification of infectious microbes. A base-composition signature can be thought of as a unique index of a specific gene from a specific organism. The composition signature is entered into a detection algorithm that searches a database that was developed by matching heterogeneous PCR amplicons from distinct species previously measured by ESI-MS to species-specific database signatures derived from both sequence data and analysis of representative strains of bacteria.[24,25] Ultimately, organism identification depends on demonstrating links between each signature for a particular organism and the presence of those signals in the sample. The ability to detect and determine the base composition of a large number of PCR amplicons in a mixed sample is crucial to accurate analysis and identification of multiple organisms in complex specimens; which is an extremely attractive characteristic for practicing clinicians and microbiologists, since this process involves less time and analysis than interpretation of GenBank Blast results following sequencing of 16S rDNA PCR amplicons. [23–25]
Figure 1.
Graphic representation of the breadth of coverage of the broad-range PCR primers employed by PCR/ESI-MS.
As with 16S rDNA PCR, studies of various clinical specimens for determination of the sensitivity and specificity of PCR/ESI-MS have also been performed. (Table 2) For example, analogous to the study of heart valve tissue by Voldstedlund, et al. [20] using 16S rDNA PCR, Brinkman, et al. [23] tested fixed heart valve tissue from patients who had received antibiotics for suspected IE with PCR/ESI-MS.[26] This was a retrospective study of conveniently available formalin-fixed, paraffin-embedded heart valves from subjects with endocarditis, and unfortunately antibiotic treatment data (if available) was not included in the report; limiting the application of this publication to the salvage microbiology literature. In this analysis, PCR/ESI-MS was performed on fixed heart valve tissue from 83 patients who had positive valve and/or blood cultures. Gram stains of heart valve tissue were positive for 63 of the specimens, suggesting non-viable bacteria were present for detection in the culture negative specimens. PCR/ESI-MS yielded 55% positivity with concordant microbiology at the genus/species or organism group level (e.g., viridans group streptococci), 11% positivity with discordant microbiology, and 34% with no detection. PCR/ESI-MS also identified a case of Tropheryma whipplei endocarditis that had not previously been recognized. Unfortunately, data is not available regarding length of antibiotic treatment before surgery for the patient population in this study.
Table 2.
Sensitivity and Specificity of multiplex PCR, 16S DNA PCR, and PCR/ESI MS in clinical applications
| Specimen | Multiplex PCR11,13–16,31 Sensitivity | 16S DNA PCR19,22,23,30,32,33 Sensitivity / Specificity | PCR/ESI-MS19,22,24–26,29,30,34 Sensitivity / Specificity |
|---|---|---|---|
| Fluids | 85% | 75% / 95% | 78–88% / 93–95% |
| Tissue | 25% | 70% / 95% | 88% / N/A |
| Fixed tissue | Case reports | Case reports only | 30% |
| Blood | 85% | 79% / 95% | 91% / 99% |
In a retrospective study of stored synovial fluid specimens that had been collected from patients with suspected prosthetic joint infection (PJI) who underwent removal of the prostheses between April, 2006 – May, 2011 (N=431), Greenwood-Quaintance et al. [29] demonstrated higher sensitivity for pathogen detection by PCR/ESI-MS as compared with culture: the sensitivities for detecting PJI were 77.6 and 69.7% for PCR-ESI/MS and culture, respectively (P = 0.01). In a related study of patients with suspicion of either prosthetic joint infection or non-infectious mechanical failure from the same group, Melendez et al. [30] compared culture of synovial fluid to PCR/ESI-MS (N=103), and found similar results between culture and PCR/ESI-MS for the patients with suspected infection (N=21), but detected potential pathogens in 4/82 of patients thought to have aseptic joint failure; versus 0/82 for culture (P = 0.045). [30]
In these studies only a minority of patients were receiving antibiotics at the time specimens were collected for testing, but in a prospective study exclusively of patients receiving antibiotics at the time samples were collected and submitted for clinical microbiologic testing, bacterial pathogens were detected directly from clinical specimens by PCR/ESI-MS in 60% of patients receiving antibiotic treatment (27/45) who had negative cultures. [3] Just as in the other studies discussed, the assay employed is designed to identify all known species of bacteria, as well as detect the presence of antibiotic resistance genes, but the patient population was unique in that only patients already receiving antibiotic treatment were enrolled. [3,29,30] In contrast, to the 16S rDNA PCR studies described, S. aureus was the most common organism detected by both culture and PCR/ESI-MS in the PJI studies and the study of antibiotic treated patients. (Table 3) But in the prospective study of patients receiving antibiotics, the most common organisms detected among the 60% of culture-negative patients were streptococci and anaerobes. [3] The 60% of culture-negative patients with positive PCR/ESI-MS detection also included cases of multidrug resistant bacteria that would have otherwise been missed, including methicillin resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococcus (VRE), and Klebsiella pneumoniae positive for the blaKPC carbapenemase gene.
Table 3.
Bacterial detection in specimens obtained from patients on antibiotic treatment by multiplex PCR, 16S DNA PCR, and PCR/ESI MS
PCR/ESI-MS is capable of identifying bacterial, viral, and fungal pathogens directly from clinical specimens, and the technology is clearly well-suited to complement conventional cultures in the clinical microbiology lab with detection of non-viable organisms or organisms not in growth phase from specimens obtained from patients on antimicrobial therapy. [3,27] Theoretically, broad-range amplification followed by detection of amplicon mass by time-of-flight mass spectrometry by PCR/ESI-MS should also have an advantage over 16S rDNA PCR in detection of unexpected pathogens without the price of lower sensitivity. [25] However, using PCR combined with electron-spray ionization mass spectrometry is not appropriate for all labs. While experience with time of flight mass spectrometry (eg, MALDI-TOF), is not necessary for a lab to consider PCR/ESI-MS, a small lab without experience in molecular detection platforms and techniques may not be an appropriate venue for PCR/ESI-MS.
Screening specimens for infection when the clinical suspicion is low, monitoring the response of patients receiving antimicrobial treatment for confirmed infection, and documenting resolution of infection are three additional potential opportunities for molecular testing.[29–31] Jacovides et al. [27] used PCR/ESI-MS to detect organisms in synovial fluid in 50/57 (88%) culture negative surgical tissue specimens from patients who had undergone extraction of the prosthesis for presumed noninfectious joint loosening or mechanical failure. And in a prospective study of prosthetic joint infections, Melendez et al. [30] demonstrated persistent detection of S. aureus in two cases following completion of antimicrobial treatment, which was also demonstrated with PCR/ESI-MS in a case study of serial CSF samples. [30]
Challenges
The premise developed with salvage microbiology is that species specific diagnostic information is valuable for management of individual cases of infection, and for institutional efforts to optimize antimicrobial prescribing practices. But skeptics will ask: What is the value of amplifying DNA associated with bacterial infections in culture-negative clinical specimens obtained from patients following initiation of antibiotic therapy if the DNA detected is from “dead” or non-viable organisms? While the reliability of amplification of non-viable bacterial DNA in tissues is undoubtedly variable between different tissues and fluids depending on the body site of origin, the duration of antibiotic treatment, and the infectious load, we would nonetheless argue that detection of DNA from non-viable organisms not only demonstrates a diagnostic advantage of PCR over culture when bacteria are not cultivated and isolated from the sample in the lab, but this also provides a potential clinical advantage when follow-up testing is employed for monitoring response to treatment. Absence of DNA amplification, or detection in lower quantities from subsequent clinical specimens, provides evidence of response to treatment when the diagnosis is in doubt. [28, 29]
It is important to stress that the intensity of signal and duration of detection of DNA by rDNA PCR and PCR/ESI-MS in specimens collected from patients following initiation of antibiotic treatment likely varies depending upon bacterial species and anatomic sites. PCR/ESI-MS has the advantage over 16S rDNA PCR in that it may have utility in evaluation of specimens from non-sterile sites, such as respiratory and GI tract specimens, but deciphering the mixed signals encountered with specimens from these sites and interpreting significance of various intensity detections presents a formidable challenge.
Frequent detection of environmental organisms of undetermined pathogenicity is another common conundrum associated with both 16S rDNA PCR and PCR/ESI-MS. Not infrequently, organisms of unknown pathogenicity are amplified by either rDNA PCR or PCR/ESI-MS from clinical specimens, which creates a challenge in the interpretation of microbiological relevance for the patient. Detection and subsequent identification of previously unrecognized bacterial species in human infection is one of the exciting possibilities anticipated with more routine clinical applications of 16S amplification and sequencing platforms in clinical microbiology laboratories. However, distinguishing infection from truly novel bacteria versus environmental contamination would present a considerable challenge for the clinical microbiology lab staff, and would require use of further costly resources for confirmation. [18]
Interpretation of 16S rRNA sequencing is reasonably straightforward for detection of bacterial pathogens in sterile sites; however, the results are more difficult to interpret when the sequence results indicate a mixed infection, such as may be expected in patients with lung abscess or empyema, an intra-abdominal infection, or a lower respiratory tract infection. 16S rDNA PCR results are usually uninterruptable for polymicrobic infections, and at best only the predominant organism will be identified. Comparing 16S rDNA PCR to culture in a prospective analysis of bone and joint tissue samples collected in the OR, Fenollar, et al. [22] documented polymicrobial infections in 6 patients, but they were unable to distinguish specific bacterial species in samples on the first pass sequencing of the 16S rDNA PCR amplicons. Species identification required bacterial cloning, which detected between 2 and 8 bacteria in the respective samples. Next-generation DNA sequencing can be employed to distinguish and identify the different organisms present in samples from polymicrobial infections, but this level of sophistication is beyond the current reach of diagnostic microbiology laboratories. [23–25]
PCR/ESI-MS, in contrast, is accurate and reliable for detection of single species or multiple species in mixed infections.[3,26,27,29] In their study of prosthetic joint synovial fluid, Greenwood-Quaintance et al. [29] used PCR/ESI-MS to detect organisms missed by conventional culture in 10/17 culture negative cases, including 4 cases of mixed infection in which culture was negative, and multiple organisms were detected by PCR/ESI-MS.[27] With this platform, no modifications in the extraction, amplification, or time of flight mass spectrometry processing are required when polymicrobic infection is suspected, and interpretation of results is straightforward.
Finally, false positives resulting from either persistent microbial DNA in fluid or tissue following successful treatment, or extraneous PCR amplicons present a common dilemma, particularly for labs without adequate experience with molecular techniques. Highly sensitive PCR primers targeting conserved 16S rDNA sequences will readily amplify extraneous DNA fragments from environmental contaminants.[31,32] And contamination of reagents or the clinical sample with minute bacterial DNA fragments at any point during collection and processing will ultimately generate false positive results with either 16S DNA PCR or PCR/ESI-MS.[25,27,32] Diagnostic labs that only have experience with commercially available real-time PCR platforms may not be appropriate settings to introduce broad-range PCR assays that require the use of DNA-free reagents; negative and positive controls, duplicate independently prepared template DNA samples, all performed in separate pre- and post-PCR areas to provide added protection against false positive results from contamination.[33]
Conclusions
Molecular methods have supplanted culture for the diagnosis of viral and intracellular bacterial infections in most clinical microbiology labs. But use of molecular techniques for complementing or augmenting conventional culture when specimens are submitted to the lab from patients following initiation of antimicrobial treatment is still considered experimental and has not truly gained traction in the diagnostic microbiology laboratory or antimicrobial stewardship community. There is a growing recognition among clinicians that broad-range 16S rDNA PCR may offer an alternative to culture if infection is strongly suspected despite cultures returning negative. Currently, 16S DNA PCR is offered for testing of clinical samples by a few select reference labs in the U.S.; while PCR/ESI-MS remains restricted to the research setting in the U.S. and is not currently offered for clinical applications on patient samples by any reference labs. At this time the only time-of-flight mass spectrometry tool that has gained wide-spread acceptance in diagnostic microbiology labs is MALDI-TOF which is designed to assist with rapid analysis of organisms that grow in culture, but would not have any potential application in the salvage microbiology setting. Like 16S rDNA PCR, PCR/ESI-MS testing offers a potential complement to culture for salvage microbiology applications, but it is currently not a viable alternative to culture due to cost and regulatory limitations. One PCR/ESI-MS platform has been approved for clinical testing in Europe, but there are no PCR/ESI-MS platforms currently approved for clinical testing in the U.S.
Much of the current focus of conventional antimicrobial stewardship efforts is on syndromic categorization of infections, and pairing patients with the briefest, narrowest antibiotic treatment based on the syndrome that fits the patient’s presentation. This approach ignores the value of pathogen recognition, and even for the most straightforward syndromes such as community acquired pneumonia, inevitably results in proposing overly broad empiric treatment recommendations. As multidrug resistant organisms perpetuate in our communities and healthcare facilities, and Clostridium difficile infections become a more and more costly burden on our healthcare systems in terms of both monetary and mortality losses, can we afford to enable a culture of perpetual empiric treatment?
The everyday practice of treating patients with empiric antibiotic regimes provides an enormous opportunity for novel approaches to identify pathogens in specimens submitted to the lab from patients after they have been started on antimicrobial therapy. As we have reviewed above, using broad-range 16S rDNA PCR, Rampini, et al. [23] demonstrated detection of unrecognized bacterial pathogens directly from clinical specimens in 30% of culture negative patients whom had been treated with antibiotics. Likewise, our group demonstrated direct detection of bacterial pathogens by PCR/ESI-MS directly from clinical specimens in 60% of patients on antimicrobial treatment who did not have any organisms recovered in culture. [3]
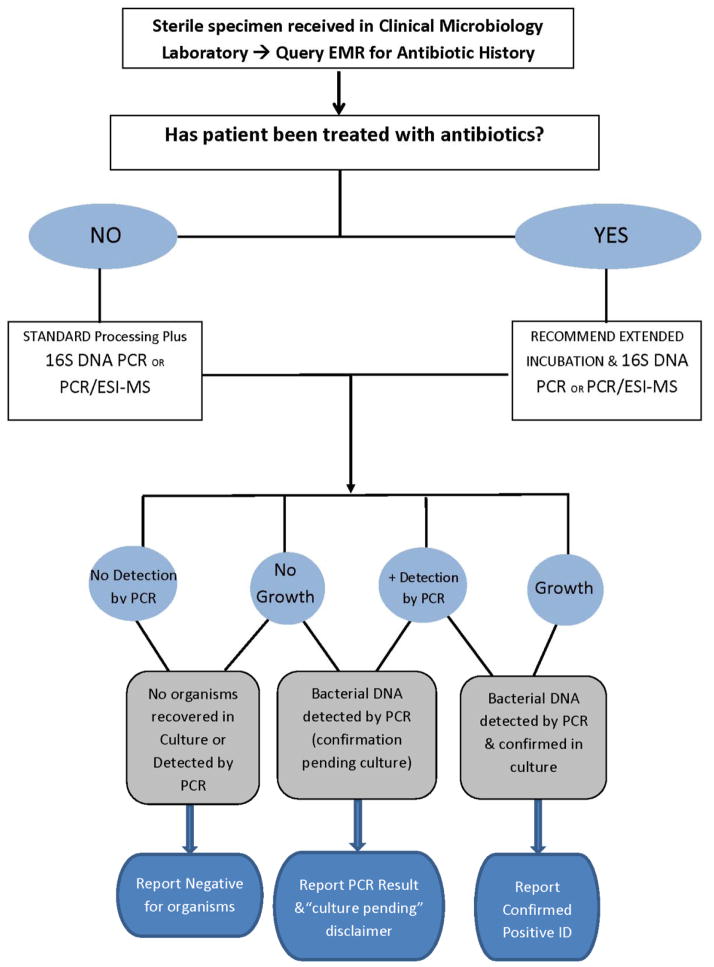
The evidence in the literature shows that both 16S rDNA PCR and PCR/ESI-MS provide opportunities to adjust antimicrobial treatment against clinically relevant pathogens detected from specimens obtained from 30–60% of patients who have cultures submitted following initiation of antimicrobial treatment, so the potential for minimizing empiric treatment and avoiding unnecessary antibiotic exposures is enormous. But incorporating these platforms into the standard diagnostic testing algorithms in the microbiology laboratory will require a commitment to development of local laboratory expertise, and paradigm shift in the thinking of clinicians, microbiologists and pharmacists alike to recognize that one size fits all testing is not an appropriate use of limited, valuable resources. Despite the obvious temptation to envision a culture free microbiology lab of the future, we are not suggesting that bacterial cultures are obsolete; and we are not recommending wide-spread adaptation of molecular diagnostics and elimination of culture based clinical microbiology (Figure 2). We do favor, and we are engaged in performing larger studies to determine the duration of antibiotic treatment that should prompt use of 16S rDNA PCR or PCR/ESI-MS testing of specimens collected from patients on empiric antimicrobial treatment for suspected infection with negative cultures. We need more clarity regarding when a negative culture is most likely to be due to the interference of early empiric antibiotic treatment and inhibiting the recovery of bacteria in culture.
Figure 2.
Suggested Diagnostic Microbiology Algorithm for Sterile Clinical Specimens
Expert Commentary
Molecular diagnostic platforms with either proven or potential application in the emerging field of salvage microbiology include multiplex PCR, broad-range rDNA PCR and sequencing, and PCR/ESI-MS. However, regardless of the platform employed, there remains a dearth of data demonstrating clinical utility, mortality benefit, cost savings, and impact on antibiotic utilization with this approach. Although the early work in the field shows promise, with capacity to detect clinically relevant pathogens by 16S rDNA PCR and PCR/ESI-MS in between 30–60% of patients who have negative cultures from specimens that are obtained following initiation of antimicrobial treatment, and detection of bacterial pathogens in 60–75% of sterile body fluids and 75–90% of surgical tissue specimens that have been collected form patients treated with antibiotics by 16S rDNA PCR and PCR/ESI-MS, respectively (Table 3). [22–33] But many questions remain to be explored such as the relationship between duration of antibiotic treatment and persistence of DNA from pathogenic bacteria; the role of NAAT in monitoring response to treatment, the optimal applications of (and most appropriate specimens for) 16S rDNA PCR vs. PCR/ESI-MS, the value and utility of salvage microbiology for common bacterial pathogens versus intra-cellular pathogens that are likely to have persistent molecular evidence in samples for weeks to months following antibiotic treatment, and finally, reliable methods for distinguishing DNA signals associated with active infection versus persistent molecular remnants following completion of successful antibiotic treatment. [34] Studies designed to test the hypothesis that salvage microbiology is a beneficial diagnostic approach which can prevent or decrease emergence of multidrug resistant pathogens and Clostridium difficile infections in specific targeted patient populations treated with empiric antibiotic treatment prior to collection of sterile specimens for culture need to be funded and performed to address these unanswered questions and further this emerging academic field of study which is lagging behind clinical practice.
As multidrug resistant organisms perpetuate in our communities and healthcare facilities, and Clostridium difficile infections become a more and more costly burden on our healthcare systems in terms of both monetary and mortality losses, alternatives must be sought to broad antibiotic treatment based primarily on categorizing patients into syndromes when cultures are negative. “Salvage Microbiology” refers to the application of molecular diagnostic techniques for detection of bacterial DNA directly from clinical specimens submitted for culture from patients who were started on antimicrobial therapy prior to obtaining clinical specimens from the suspected source of infection. Interference of early empiric antimicrobial treatment with culture based diagnostics remains a common clinical conundrum, and finding ways to improve the detection of pathogens in these cases and discerning the response to therapy remains an important but elusive goal.
5 year view
Salvage microbiology, and specifically use of NAAT in combination with sequencing or mass spectrometry to detect organisms and direct clinical treatment decisions is a long way from gaining wide-spread acceptance in either the clinical microbiology or antimicrobial stewardship communities. While various NAAT platforms and even broad-range PCR applications have been introduced into clinical microbiology practice over the past decade, coordinating knowledge of the clinical circumstances such as duration and breath of prior antimicrobial treatment with the appropriate diagnostic platform in the lab has many systematic obstacles that remain in place for most diagnostic laboratories and stewardship programs for the foreseeable future. The direction of research exploring the utility of these technologies must be cognizant of these limitations and shift the focus from examining simply sensitivity and specificity to studying and examining optimal utility, i.e., which specific patients, or patient populations provide the highest yield for a specific diagnostic testing platform, and targeted potential salvage microbiology applications accordingly. It remains to be seen whether clinical microbiologists, or physicians and pharmacists engaged in institutional antimicrobial stewardship programs will lead the way with future trials to evaluate the clinical performance of these broad-range diagnostic assays. Clearly, a collaborative effort would most beneficial for all concerned.
Key Issues.
16S rDNA PCR and PCR/ESI-MS have both evolved from environmental scientific research tools to novel clinical applications.
16S rDNA PCR and PCR/ESI-MS share the advantage that knowledge of a predetermined target is not necessary for pathogen detection.
Both 16S rDNA PCR and PCR/ESI-MS provide opportunities to adjust antimicrobial treatment, avoiding the pitfalls associated with wide-spread use of overly broad empiric antimicrobial treatment.
The premise of salvage microbiology is that species specific diagnostic information derived for patients who have cultures submitted following initiation of antimicrobial treatment is valuable for management of individual cases of infection, and for institutional efforts to optimize antimicrobial prescribing practices.
16S rDNA PCR and PCR/ESI-MS have shown the capacity to detect clinically relevant pathogens from specimens obtained from 30–60% of patients who have cultures submitted following initiation of antimicrobial treatment. Unlike 16S rDNA PCR, PCR/ESI-MS is capable of distinguishing all pathogens in a polymicrobic infection without additional testing.
Unlike 16S rDNA PCR, PCR/ESI-MS provides a single platform for diagnostic testing of clinical specimens for yeast; invasive fungi, and viral pathogens.
Head to head comparisons of 16S rDNA PCR vs. PCR/ESI-MS are lacking. [34]
Exploration of the most appropriate role for 16S rDNA PCR vs. PCR/ESI-MS with various clinical samples (sterile body fluids, surgical specimens, blood, non-sterile specimens such as respiratory samples) and subsequent optimization are needed.
Implementation of systematic institutional programs to capitalize on the potential outcome and cost-saving opportunities these technologies offer will require close collaboration across multiple disciplines.
More clinical studies of these new technologies designed to compare outcomes for specific patient populations are required before these platforms can be incorporated into “real-world” quality improvement initiatives.
Acknowledgments
The authors are supported by the National Institutes of Health and VISN 10 Veterans Affairs Research Initiative Program
Footnotes
Financial & competing interests disclosure
R Sampath is a salaried employee of Ibis Biosciences, an Abbott Company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Breitkopf C, Hammel D, Scheld HH, Peters G, Becker K. Impact of a molecular approach to improve the microbiological diagnosis of infective heart valve endocarditis. Circulation. 2005;111(11):1415–1421. doi: 10.1161/01.CIR.0000158481.07569.8D. [DOI] [PubMed] [Google Scholar]
- 2.Siddiqui BK, Tariq M, Jadoon A, Alam M, Murtaza G, Abid B, et al. Impact of prior antibiotic use in culture-negative endocarditis: review of 86 cases from southern Pakistan. Int J Infect Dis. 2009;135:606–612. doi: 10.1016/j.ijid.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Farrell JJ, Sampath R, Ecker DJ, Bonomo RA. ‘Salvage microbiology’: detection of bacteria directly from clinical specimens following initiation of antimicrobial treatment. PLoS One. 2013;8(6):e66349. doi: 10.1371/journal.pone.0066349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellit TH, Owens RC, McGowan JE, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 5.Forrest GN, Mehta S, Weekes E, Lincalis DP, Johnson JK, Venezia RA. Impact of rapid in situ hybridization testing on coagulase-negative staphylococci positive blood cultures. J Antimicrob Chemother. 2006;58(1):154–158. doi: 10.1093/jac/dkl146. [DOI] [PubMed] [Google Scholar]
- 6.Holtzman C, Whitney D, Barlam T, Miller NS. Assessment of impact of peptide nucleic acid fluorescence in situ hybridization for rapid identification of coagulase-negative Staphylococci in the absence of antimicrobial stewardship intervention. J Clin Microbiol. 2011;49(4):1581–1582. doi: 10.1128/JCM.02461-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;4:CD003543. doi: 10.1002/14651858.CD003543.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Weatherall C, Paoloni R, Gottlieb T. Point-of-care urinary pneumococcal antigen test in the emergency department for community acquired pneumonia. Emerg Med J. 2008;25(3):144–148. doi: 10.1136/emj.2007.050179. [DOI] [PubMed] [Google Scholar]
- 9.Rudolph K, Bulkow L, Bruce M, Zulz T, Reasonover A, Harker-Jones M, et al. Molecular resistance mechanisms of macrolide-resistant invasive Streptococcus pneumoniae isolates from Alaska, 1986 to 2010. Antimicrob Agents Chemother. 2013;57(11):5415–5422. doi: 10.1128/AAC.00319-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenover FC, Canton R, Kop J, Chan R, Ryan J, Weir F, et al. Detection of colonization by carbapenemase-producing Gram-negative Bacilli in patients by use of the Xpert MDRO assay. J Clin Microbiol. 2013;51(11):3780–3787. doi: 10.1128/JCM.01092-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsalik EL, Jones D, Nicholson B, Waring L, Liesenfeld O, Park LP, et al. Multiplex PCR to diagnose bloodstream infections in patients admitted from the emergency department with sepsis. J Clin Microbiol. 2010;48(1):26–33. doi: 10.1128/JCM.01447-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gadsby NJ, Hardie A, Claas EC, Templeton KE. Comparison of the Luminex respiratory virus panel fast assay with in-house real-time PCR for respiratory viral infection diagnosis. J Clin Microbiol. 2010;48(6):2213–2216. doi: 10.1128/JCM.02446-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couturier MR, Barney T, Alger G, Hymas WC, Stevenson JB, Hillyard D, et al. Evaluation of the FilmArray® Respiratory Panel for clinical use in a large children’s hospital. J Clin Lab Anal. 2013;27(2):148–54. doi: 10.1002/jcla.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierce VM, Hodinka RL. Comparison of the GenMark Diagnostics eSensor respiratory viral panel to real-time PCR for detection of respiratory viruses in children. J Clin Microbiol. 2012;50(11):3458–3465. doi: 10.1128/JCM.01384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond SP, Gagne LS, Stock SR, Marty FM, Gelman RS, Marasco WA, et al. Respiratory virus detection in immunocompromised patients with FilmArray respiratory panel compared to conventional methods. J Clin Microbiol. 2012;50(10):3216–3221. doi: 10.1128/JCM.00538-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popowitch EB, O’Neill SS, Miller MB. Comparison of the Biofire FilmArray RP, Genmark eSensor RVP, Luminex xTAG RVPv1, and Luminex xTAG RVP fast multiplex assays for detection of respiratory viruses. J Clin Microbiol. 2013;51(5):1528–1533. doi: 10.1128/JCM.03368-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenner L, Demeshkina N, Yusupova G, Yusupov M. Structural rearrangements of the ribosome at the tRNA proofreading step. Nat Struct Mol Biol. 2010;17(9):1072–1078. doi: 10.1038/nsmb.1880. [DOI] [PubMed] [Google Scholar]
- 18.Harris KA, Hartley JC. Development of broad-range 16S rDNA PCR for use in the routine diagnostic clinical microbiology service. J Med Microbiol. 2003;52(8):685–91. doi: 10.1099/jmm.0.05213-0. [DOI] [PubMed] [Google Scholar]
- 19.Clifford RJ, Milillo M, Prestwood J, Quintero R, Zurawski DV, Kwak YI, et al. Detection of Bacterial 16S rRNA and Identification of Four Clinically Important Bacteria by Real-Time PCR. PLoS One. 2012;7(11):e48558. doi: 10.1371/journal.pone.0048558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voldstedlund M, Nørum Pedersen L, Baandrup U, Klaaborg KE, Fuursted K. Broad-range PCR and sequencing in routine diagnosis of infective endocarditis. APMIS. 2008;116(3):190–198. doi: 10.1111/j.1600-0463.2008.00942.x. [DOI] [PubMed] [Google Scholar]
- 21.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 22.Fenollar F, Roux V, Stein A, Drancourt M, Raoult D. Analysis of 525 samples to determine the usefulness of PCR amplification and sequencing of the 16S rRNA gene for diagnosis of bone and joint infections. J Clin Microbiol. 2006;44(3):1018–1028. doi: 10.1128/JCM.44.3.1018-1028.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rampini SK, Bloemberg GV, Keller PM, Büchler AC, Dollenmaier G, Speck RF, et al. Broad-range 16S rRNA gene polymerase chain reaction for diagnosis of culture-negative bacterial infections. Clin Infect Dis. 2011;53(12):1245–1251. doi: 10.1093/cid/cir692. [DOI] [PubMed] [Google Scholar]
- 24.Baldwin CD, Howe GB, Sampath R, Blyn LB, Matthews H, Harpin V, et al. Usefulness of multilocus polymerase chain reaction followed by electrospray ionization mass spectrometry to identify a diverse panel of bacterial isolates. Diagn Microbiol Infect Dis. 2009;63(4):403–408. doi: 10.1016/j.diagmicrobio.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Kaleta EJ, Clark AE, Johnson DR, Gamage DC, Wysocki VH, Cherkaoui A, et al. Use of PCR coupled with electrospray ionization mass spectrometry for rapid identification of bacterial and yeast bloodstream pathogens from blood culture bottles. J Clin Microbiol. 2011;49(1):345–353. doi: 10.1128/JCM.00936-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkman CL, Vergidis P, Uhl JR, Pritt BS, Cockerill FR, Steckelberg JM, et al. PCR-electrospray ionization mass spectrometry for direct detection of pathogens and antimicrobial resistance from heart valves in patients with infective endocarditis. J Clin Microbiol. 2013;51(7):2040–2046. doi: 10.1128/JCM.00304-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247–2254. doi: 10.2106/JBJS.L.00210. [DOI] [PubMed] [Google Scholar]
- 28.Farrell JJ, Tsung AJ, Flier L, Martinez DL, Beam SB, Chen C, et al. PCR and electrospray ionization mass spectrometry for detection of persistent Enterococcus faecalis in cerebrospinal fluid following treatment of postoperative ventriculitis. J Clin Microbiol. 2013;51(10):3464–3466. doi: 10.1128/JCM.01343-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenwood-Quaintance KE, Uhl JR, Hanssen AD, Sampath R, Mandrekar JN, Patel R. Diagnosis of prosthetic joint infection by use of PCR-electrospray ionization mass spectrometry. J Clin Microbiol. 2014;52(2):642–649. doi: 10.1128/JCM.03217-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melendez DP, Uhl JR, Greenwood-Quaintance KE, Hanssen AD, Sampath R, Patel R. Detection of prosthetic joint infection by use of PCR-electrospray ionization mass spectrometry applied to synovial fluid. J Clin Microbiol. 2014;52(6):2202–2205. doi: 10.1128/JCM.00570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucignano B, Ranno S, Liesenfeld O, Pizzorno B, Putignani L, Bernaschi P, et al. Multiplex PCR allows rapid and accurate diagnosis of bloodstream infections in newborns and children with suspected sepsis. J Clin Microbiol. 2011;49(6):2252–2258. doi: 10.1128/JCM.02460-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vollmer T, Piper C, Horstkotte D, Körfer R, Kleesiek K, Dreier J. 23S rDNA real-time polymerase chain reaction of heart valves: a decisive tool in the diagnosis of infective endocarditis. Eur Heart J. 2010;31(9):1105–1113. doi: 10.1093/eurheartj/ehp600. [DOI] [PubMed] [Google Scholar]
- 33.Harris KA, Yam T, Jalili S, Williams OM, Alshafi K, Gouliouris T, et al. Service evaluation to establish the sensitivity, specificity and additional value of broad-range 16S rDNA PCR for the diagnosis of infective endocarditis from resected endocardial material in patients from eight UK and Ireland hospitals. Eur J Clin Microbiol Infect Dis. 2014;33(11):2061–2066. doi: 10.1007/s10096-014-2145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacconi A, Richmond GS, Baroldi MA, Laffler TG, Blyn LB, Carolan HE, et al. Improved Sensitivity for Molecular Detection of Bacteria and Candida in Blood. J Clin Microbiol. 2014;52(9):3164–3174. doi: 10.1128/JCM.00801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]