Abstract
Background & Aims
Little is known about the prevalence of nonalcoholic fatty liver disease (NAFLD) among severely obese adolescents or factors that determine its development. We investigated the prevalence of NAFLD in a multicenter cohort of adolescents undergoing bariatric surgery and factors associated with it.
Methods
We enrolled 242 adolescents undergoing bariatric surgery between March 2007 and February 2012 at 5 tertiary care centers into a multicenter, prospective observational cohort study. Intra-operative core liver biopsies were collected from 165 subjects; 17 were excluded because of insufficient liver tissue or use of hepatotoxic medications, so 148 remained in the study (mean age 16.8±1.6 y old; median body mass index [BMI], 52 kg/m2). Liver tissues were analyzed by histology using validated criteria. Hepatic gene expression was analyzed in 67 samples.
Results
NAFLD was present in 59% of this predominantly female (72%), white (68%), non-Hispanic (91%) cohort. Of subjects with NAFLD, 24% had borderline and 10% had definite nonalcoholic steatohepatitis (NASH). Mild fibrosis (≤ stage 2) was observed in 18% of liver biopsies and stage 3 in 0.7%, but cirrhosis was not detected. Dyslipidemia was present in 78% of subjects, hypertension in 44%, and diabetes in 14%. More severe NAFLD was associated with increasing levels of alanine aminotransferase (ALT), fasting glucose level, hypertension (each P<.01) and white blood cell count (P=.04). Only diabetes was associated with detection of fibrosis (odds ratio, 3.56; 95% confidence interval, 1.93–6.56). Microarray analysis associated presence of NASH with altered expression of genes that regulate macrophage chemotaxis, cholesterol absorption, and fatty acid binding.
Conclusions
More than half of adolescents undergoing bariatric surgery in this cohort had NAFLD, yet the prevalence of severe or fibrotic NASH was low. Increasing severity of NAFLD was associated with level of ALT and cardio-metabolic risk factors, but not BMI. Based on gene expression analysis, borderline and definite NASH were associated with abnormal immune function, intestinal cholesterol absorption, and lipid metabolism.
Keywords: pediatric, severe obesity, microarray, inflammation
INTRODUCTION
Severe obesity is highly prevalent among children and adolescents with NAFLD in the United States. In large cohort studies of children with biopsy-confirmed NAFLD, reported mean BMI ranges from 31 to 34 kg/m2 (standard deviations ~5) in children with mean age of approximately 13 years (range 4 to 17 years).1-6
Although consensus is lacking on whether bariatric surgery should be a specific treatment for NASH in severely obese patients, it is not contraindicated in patients with non-cirrhotic NASH.7 Current adolescent bariatric surgery guidelines include severe NASH as a criterion for surgery.8-10 Significantly, adolescents undergoing bariatric surgery are usually ≥ 13 years old with minimum BMI ≥ 35 kg/m2; mean BMI in surgical cohorts is often in the 50 kg/m2 range. They are therefore older and substantially more obese than other multicenter pediatric cohorts in whom NAFLD prevalence and determinants have been well-characterized.1-5
The prevalence and severity of NAFLD in severely obese adolescents undergoing bariatric surgery is unknown. A single center, retrospective study previously reported a 20% prevalence of biopsy-confirmed NASH in 41 adolescents at time of bariatric surgery, but was not adequately powered to identify determinants.11 The Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study, a prospective observational longitudinal cohort study of 242 adolescents undergoing bariatric surgery at five tertiary care centers in the United States, offers the opportunity to determine the biopsy-confirmed prevalence and determinants of NAFLD in a larger multicenter cohort of severely obese adolescents.
The primary aims of this prospective observational study were to determine the prevalence of biopsy-confirmed NAFLD, NASH and associated fibrosis and identify significant characteristics associated with histological severity of NAFLD in severely obese adolescents at time of bariatric surgery. Secondarily, we conducted gene expression analyses to elucidate biological pathways underlying NAFLD phenotypes in this unique cohort. We hypothesized that most participants would have NAFLD and the severity of liver disease would be predicted by BMI and cardio-metabolic features.
METHODS
Study population
The Teen-LABS study (NCT00474318) methodology has been previously described in detail.12 The observational cohort study enrolled 242 consecutive adolescents, age ≤ 19 years, undergoing bariatric surgery (March 2007 - February 2012) at five clinical centers in the United States: Cincinnati Children’s Hospital Medical Center (Cincinnati, Ohio), Nationwide Children’s Hospital (Columbus, Ohio), the University of Pittsburgh Medical Center (Pittsburgh, Ohio), Texas Children’s Hospital (Houston, Texas) and the Children’s Hospital of Alabama (Birmingham, Alabama). The study was approved by each center's Institutional Review Board. Written informed consent or assent, as appropriate for age, was obtained from all parents/guardians and adolescents.
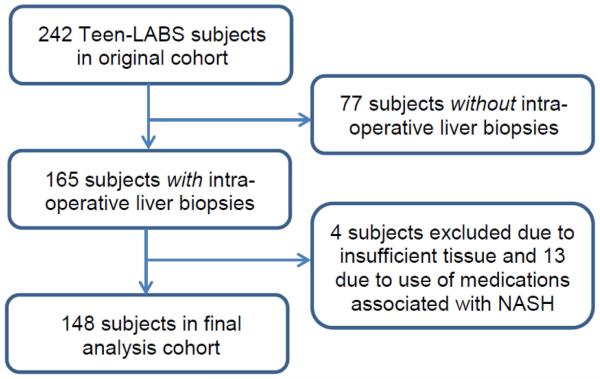
At time of surgery, 165 of the 242 participants had clinical intra-operative core liver biopsies performed. Due to the observational study design and lack of published consensus on whether intra-operative liver biopsies should be standard of care at time of bariatric surgery, the decision to perform a liver biopsy was deferred to the surgical teams at each site. Intra-operative liver biopsies were standard of care at three sites, but not at the remaining two sites. Accordingly, 99% of all biopsies were performed at the three sites where intraoperative biopsy was standard of care. Participants with insufficient liver tissue (n=4) or taking medications that may cause or treat NASH (n=13) were excluded from analysis. None reported alcohol intake >20 gm/day. After exclusions, 148 participants remained.
Preoperative liver testing was not required for participation in the Teen-LABS study. In the total cohort, 82.8% (n=199/242) had ALT measured, which did not differ among those with (n=136/165) or without liver biopsies (n=63/77), or those in the final analysis cohort (n=122/148).
Measures
Preoperative baseline clinical data were collected within 30 days of operation at in-person visits by trained study personnel using standardized methodology, as previously described.12 Age and sex were recorded and race and ethnicity were self-identified by the participant and/or parent(s). Height, weight, waist circumference, systolic and diastolic blood pressure were measured by trained study personnel using standardized protocols. BMI was calculated as weight (kg)/height (m)2. Study personnel followed standard definitions (in Supplemental Materials) to determine presence or absence of co-morbid conditions (hypertension, diabetes mellitus, dyslipidemia) using medical records, physical exam, participant interview and laboratory values. Baseline laboratory measurements performed at a central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, Seattle, WA) included complete blood cell count, fasting glucose, insulin, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides. ALT, aspartate aminotransferase (AST) and alkaline phosphatase were abstracted for research use from the clinical chart at each site. Homeostatic model assessment-insulin resistance (HOMA-IR) was calculated as [glucose (mg/dL) × insulin (μU/mL)] / 405.
Liver histology
Liver biopsies were obtained by core needle technique after induction of anesthesia and before performing the bariatric surgery procedure. Location of the needle biopsy (left or right lobe) was at the discretion of the surgical team. Some surgical sites obtained biopsies from both lobes, submitting one specimen for histological evaluation and the second to research repositories. The liver biopsy location and any resulting complications were documented on the standard Teen-LABS operative evaluation form completed by the surgeon immediately post-operatively. Liver biopsy specimens were stained with hematoxylin-eosin and Masson’s trichrome stains, and reviewed and scored centrally by an experienced hepatopathologist (DEK) using the validated NASH Clinical Research Network scoring system.13 The NAFLD activity score (NAS, range 0-8) was calculated and fibrosis staged as detailed in the Supplemental Materials. Liver biopsies were also categorized as definite NASH, borderline NASH or NAFLD-not NASH (NAFL) based on the aggregate presence and degree of the individual histologic features of fatty liver disease.14 If there was no evidence of abnormal steatosis or injury consistent with NAFLD or NASH, the biopsy was designated as “Not-NAFLD.”
Statistical analysis
Categorical variables were presented using frequencies and percentages, while continuous variables were presented as means and standard deviations or medians and interquartile ranges (IQR). Chi-square, Fisher’s exact, Wilcoxon’s rank sum, and analysis of variance (ANOVA) tests were used to compare participant characteristics across liver phenotypes.
Multiple imputation (MI) by fully conditional specification was performed to address missing data (2.4% of data values) as outlined in the Supplemental Materials.
A cumulative logit model (SAS Proc Logistic) was used to evaluate predictors of increasing liver disease severity, ordinally defined as 1. Not-NAFLD, 2. NAFL (not NASH), 3. borderline NASH and 4. definite NASH. Since the outcome has four levels of ordinal severity, the model simultaneously evaluates three separate comparisons:
Definite NASH |vs| Borderline NASH or NAFL or Not-NAFLD.
Definite NASH or Borderline NASH |vs| NAFL or Not-NAFLD.
All NAFLD [Definite NASH or Borderline NASH or NAFL] |vs| Not-NAFLD.
The fundamental model assumption was that the odds ratio (OR) estimate for all three comparisons was the same; a non-significant p-value confirms no difference in estimates between the three comparisons. The final model met the proportional odds assumption (p = 0.74). Modified poisson regression with robust estimates (SAS Proc GENMOD) was used to evaluate predictors of fibrosis.15,16 A cumulative logit model was further used to evaluate predictors of increasing liver disease severity among only those with NAFLD, e.g. NAFL (not NASH), borderline NASH and definite NASH and a modified poisson regression with robust estimates was used to evaluate predictors of having any NAFLD (all subgroups combined) versus Not-NAFLD. SAS Proc MiAnalyze was used to generate all estimates from the multiply imputed data. All aforementioned descriptive and clinical variables were considered for inclusion in the final models. In the models, ALT elevation was defined as normal (<22 U/L for females, <26 U/L for males), mild (22-39 U/L for females, 26-39 U/L for males) and high (≥ 40 U/L), based on population-derived upper limits (≥26 U/L for adolescent males and ≥22 U/L for females) and a more typical clinical laboratory upper limit (≥ 40 U/L).17 Fasting plasma glucose levels were categorized as: <100 mg/dL (normal), 100-125 mg/dL (impaired), and ≥126 mg/dL (abnormal, consistent with diabetes).
Sensitivity and specificity values were calculated to evaluate the use of ALT values in identifying histologically-confirmed NAFLD (NAFL, borderline and definite NASH combined) or definite NASH alone. Calculations were done using the 122 participants with measured ALT values (no imputed values). Two ALT thresholds were analyzed: the population-derived upper limit (≥26 U/L for adolescent males and ≥22 U/L for females) and a typical clinical laboratory upper limit (≥ 40 U/L).17 Sensitivity was defined as the percentage of participants whose ALT values exceeded the above thresholds, among those with NAFLD or NASH. Specificity was defined as the percentage of participants whose ALT levels were below the thresholds described above, among those without NAFLD or NASH. Reported p-values are two-sided and considered statistically significant at ≤0.05.
Characterization of Hepatic Gene Expression
Relative hepatic gene expression was measured using the Human Exon 1.0 ST v1 Array in a subset of 67 participants from the analysis cohort with extra liver tissue available for microarray analyses (detailed in Supplementary Materials). The distribution of liver phenotype in this convenience sample was: 51% (n=34) Not-NAFLD, 39% (n=26) NAFL, 7% (n=5) borderline NASH and 3% (n=2) definite NASH. All analysis was performed in GeneSpring version 12.6.1.
Using an ANOVA, we generated a list of 8648 genes exhibiting differential regulation (ANOVA p<0.05) between Not-NAFLD, NAFL, borderline NASH and definite NASH. Among these genes, we identified those with > 1.5 fold change (FC) in the following comparisons:
NAFL vs. Not-NAFLD
Borderline NASH vs. Not-NAFLD
Definite NASH vs. Not-NAFLD
Borderline NASH vs. Definite NASH
Further, we intersected gene sets from the above comparisons to determine the following gene signatures: 1) genes shared between definite NASH and borderline NASH compared to Not-NAFLD; 2) genes specific to definite NASH compared to Not-NAFLD; and 3) genes specific to borderline NASH compared to Not-NAFLD. Each of these gene sets was submitted to an unbiased ontological analysis through ToppGene to identify meaningful enriched biological processes and pathways, using well established, previously described methods.18
RESULTS
Clinical and liver disease characteristics
A flow chart of the study cohort is shown in Figure 1. The 165 participants who had intra-operative liver biopsies were slightly younger than the 77 who did not undergo biopsies (p<0.01), had slightly higher BMI (p<0.01) and higher prevalence of dyslipidemia (p=0.02) [Supplemental Table 1]. Core needle liver biopsies were obtained from the right lobe in 27%, from the left lobe in 40%, and from both lobes in 33% of subjects. No complications occurred. There were no clinical differences between the final analysis cohort (n=148) and the 17 participants excluded. Table 1 presents the clinical characteristics of the analysis cohort. Mean age was 16.8 years and median BMI was 51.6 kg/m2. The majority of the cohort was female, white and non-Hispanic. Forty-four percent had hypertension, 14% diabetes and 78% dyslipidemia.
Figure 1.
Flow of Teen-LABS study participants excluded from and retained in final analysis cohort.
Table 1.
Demographic and Clinical Characteristics by Liver Disease Classification.
| Characteristics | Total (N=148) |
Not NAFLD (n=61) |
NAFL (not NASH) (n=57) |
Borderline NASH (n=21) |
Definite NASH (n=9) |
Overall p-value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Female, n (%) | 106 (71.6%) | 49 (80.3%) | 42 (73.7%) | 10 (47.6%) | 5 (55.6%) | 0.02 |
| Mean age at surgery, years ± SD | 16.8 ± 1.55 | 16.6 ± 1.62 | 17.0 ± 1.50 | 17.2 ± 1.54 | 16.4 ± 1.36 | 0.30 |
| White race, n (%) | 101 (68.2%) | 38 (62.3%) | 43 (75.4%) | 14 (66.7%) | 6 (66.7%) | 0.50 |
| Hispanic ethnicity, n (%) | 13 (8.8%) | 5 (8.2%) | 5 (8.8%) | 2 (9.5%) | 1 (11.1%) | 0.97 |
| BMI in kg/m2, median (IQR) | 51.6 (46.3, 60.5) | 49.8 (45.8, 56.1) |
53.5 (46.9, 62.9) |
55.3 (50.1, 63.0) |
52.9 (46.0, 55.4) |
0.10 |
| Pre-op weight loss >5%, n (%) | 17 (11.5%) | 10 (16.4%) | 5 (8.8%) | 2 (9.5%) | 0 (0.0%) | 0.47 |
| Histology | ||||||
| Fibrosis stage (score) | <0.01 | |||||
| None (0) | 120 (81.1%) | 57 (93.4%) | 50 (87.7%) | 12 (57.1%) | 1 (11.1%) | |
| Mild zone 3 only (1a) | 6 (4.1%) | 0 (0%) | 1 (1.8%) | 2 (9.5%) | 3 (33.3%) | |
| Moderate zone 3 only (1b) | 4 (2.7%) | 0 (0%) | 1 (1.8%) | 1 (4.8%) | 2 (22.2%) | |
| Periportal only (1c) | 13 (8.8%) | 4 (6.6%) | 4 (7.0%) | 5 (23.8%) | 0 (0.0%) | |
| Mild/moderate zone 3 and periportal (2) |
4 (2.7%) | 0 (0%) | 1 (1.8%) | 1 (4.8%) | 2 (22.2%) | |
| Bridging (3) | 1 (0.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (11.1%) | |
| Cirrhosis (4) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Fibrosis score, mean ± SD | 0.23 ± 0.52 | 0.07 ± 0.25 | 0.14 ± 0.40 | 0.48 ± 0.60 | 1.33 ± 0.87 | <0.01 |
| NAS score, mean ± SD | 1.8 ± 1.48 | 0.59 ± 0.53 | 2.1 ± 0.72 | 3.0 ± 1.20 | 5.1 ± 1.76 | <0.01 |
| Biopsy length (mm), median (IQR)* | 10.0 (7,13) | 9.0 (8,12) | 9.5 (7,14) | 13.0 (8,18) | 11.0 (9,12) | 0.14 |
| Lab Values, median (IQR) | ||||||
| ALT, U/L (n=122) | 27.0 (19, 38) | 22.5 (17, 31) | 27.0 (23, 38) | 30.0 (23, 38) | 63.5 (40.5, 106) |
<0.01 |
| AST, U/L (n=122) | 32.0 (24, 42) | 28.0 (22, 40) | 32.0 (23, 40) | 37.0 (27, 46) | 49.5 (33, 107.5) |
<0.01 |
| Alkaline phosphatase, U/L (n=120) | 92.5 (74, 113) | 99.5 (80, 114) | 97.0 (69, 113) |
86.0 (65, 102) |
91.5 (75.5, 138.5) |
0.56 |
| Fasting Glucose, mg/dL | 94.0 (87, 103) | 91.0 (85, 101) | 96.0 (87, 101) |
96.0 (93, 103) |
135 (122, 150) |
<0.01 |
| Fasting Insulin, mU/mL (n=144) | 25.2 (15.3, 37.5) | 24.0 (12.4, 31.9) |
25.8 (16.9, 42.5) |
26.8 (17.0, 32.3) |
41.9 (33, 73.6) |
0.11 |
| HOMA-IR (n=144) | 5.9 (3.7, 9.6) | 5.4 (2.8, 7.1) | 6.9 (4.2, 10.0) |
5.8 (4.0, 8.4) | 12.4 (9.6, 21.1) |
0.03 |
| Triglycerides, mg/dL | 113.0 (82, 163) | 103.0 (77, 134) | 121.0 (90, 173) |
139.0 (88, 215) |
142 (109, 197) |
0.01 |
| LDL cholesterol, mg/dL | 87.0 (72, 108) | 88.0 (71, 106) | 87.0 (74, 119) |
92.0 (74, 107) |
83.0 (71,92) | 0.66 |
| HDL cholesterol, mg/dL | 36.0 (32, 41) | 37.0 (32, 40) | 33.0 (30, 40) | 37.0 (33, 44) | 40.0 (38, 45) | 0.07 |
| Total cholesterol, mg/dL | 153.5 (134.5, 175.5) |
150.0 (130, 163) |
154.0 (136, 185) |
160.0 (142, 182) |
155.0 (153, 158) |
0.52 |
| Albumin (g/dl) (n=142) | 4.2 (4.0, 4.4) | 4.2 (4.0, 4.4) | 4.3 (4.2, 4.4) | 4.2 (4.0, 4.4) | 4.4 (4.3, 4.6) | 0.13 |
| White blood cell count (k/ul) (n=143) |
8.3 (6.9, 10.2) | 8.1 (6.5, 10.0) | 8.3 (6.9, 10.0) |
8.6 (6.4, 10.5) |
10.0 (9.2, 10.7) |
0.30 |
| Comorbidities | ||||||
| Diabetes, n (%) | 20 (13.5%) | 4 (6.6%) | 5 (8.8%) | 5 (23.8%) | 6 (66.7%) | <0.01 |
| Dyslipidemia, n (%) | 116 (78.4%) | 44 (72.1%) | 47 (82.5%) | 17 (81.0%) | 8 (88.9%) | 0.53 |
| Hypertension, n (%) | 65 (43.9%) | 19 (31.2%) | 26 (45.6%) | 13 (61.9%) | 7 (77.8%) | 0.01 |
n=1 missing.
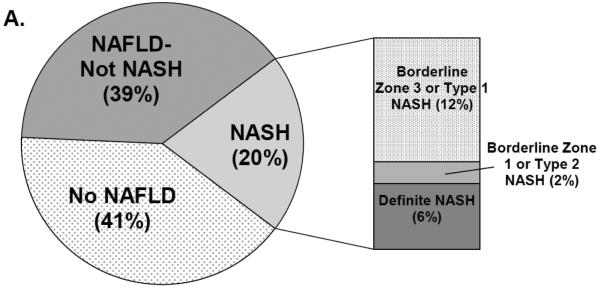
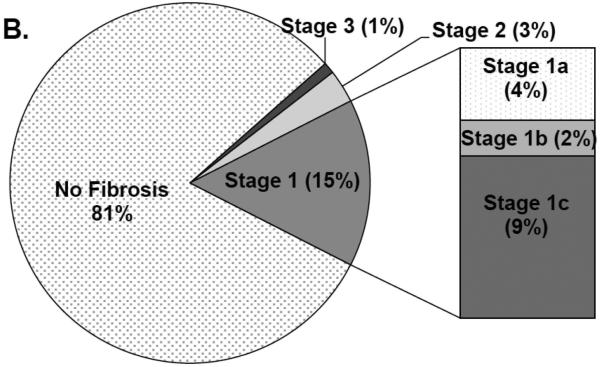
Figure 2 shows the prevalence and distribution of liver disease phenotypes. NAFLD was common among the cohort (58.8%). Of those with NAFLD, 34.5% had borderline or definite NASH (20% of the analysis cohort) [Panel 2A]. However, only 6% of the cohort had definite NASH. The borderline zone 1 pattern, most prevalent among pre-adolescent children, was rare in this cohort. Most participants with borderline NASH showed a zone 3 distribution of injury. Fibrosis was mild: 81% had none, while 18% had stage 1 or 2 fibrosis [Panel 2B]. Only 1 participant had stage 3 fibrosis and none had cirrhosis.
Figure 2.
Panel A: Distribution of NAFLD and NASH, including subtypes of borderline NASH. Panel B: Prevalence and distribution of fibrosis in the cohort. Stage 1 fibrosis was most frequent, predominantly stage 1c (periportal only), followed by stage 1a (mild zone 3 only) and stage 1b (moderate zone 3 only). Stage 2 (mild/moderate zone 3 and periportal fibrosis) and stage 3 (bridging) were rare. No participants had stage 4 (cirrhosis).
As shown in Table 1, ALT, AST, glucose, and triglycerides increased with ordinal severity of liver disease. The proportion of males also increased with increasing liver disease severity (p=0.02). Because candidates for bariatric surgery are often encouraged to lose weight pre-operatively and significant weight loss could influence NAFLD histology due to rapid reduction of steatosis, we analyzed pre-operative weight loss to determine if it differed by liver disease phenotype. Overall, 11.5% of the analysis cohort lost significant pre-operative weight (>5% of initial weight), but prevalence did not differ significantly among liver disease categories (p=0.47) [Table 1].
Independent risk factors for liver disease severity and fibrosis
After adjustment, increasing ALT, fasting glucose (both p<0.01), WBC (p=0.02), and hypertension (p=0.02) were associated with greater ordinal severity of liver disease [Table 2]. The only significant predictor of fibrosis (p=0.03) was presence of diabetes, as shown in Table 3.
Table 2.
Crude and Adjusted Odds Ratios of Categorical NAFLD by Characteristic
| Characteristic | Unadjusted | Adjusted | ||
|---|---|---|---|---|
|
|
||||
| (Groups) | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Sex | <.01 | |||
| Female | 1.00 | |||
| Male | 2.56 (1.31, 5.02) | |||
| Age Categories (yrs) | 0.48 | |||
| 13 – 15 | 1.00 | |||
| 16 – 17 | 1.41 (0.69, 2.89) | |||
| 18 + | 1.59 (0.72, 3.51) | |||
| Race | 0.38 | |||
| White | 1.00 | |||
| Black/Other | 0.75 (0.39, 1.43) | |||
| BMI (kg/m2) | 1.03 (1.00, 1.06) | 0.07 | ||
| ALT Elevation | <.01 | <.01 | ||
| Normal | 1.00 | 1.00 | ||
| Mild | 2.43 (1.11, 5.34) | 3.41 (1.43, 8.13) | ||
| High | 6.30 (2.42, 16.41) | 6.66 (2.50, 17.76) | ||
| ALK | Linear (p=0.09), Quadratic (p=0.08) |
|||
| Glucose (mg/dL) | <.01 | <.01 | ||
| < 100 | 1.00 | 1.00 | ||
| 100 – 125 | 1.43 (0.68, 2.99) | 1.48 (0.68, 3.23) | ||
| ≥ 126 | 7.76 (2.85, 21.11) | 8.10 (2.73, 23.88) | ||
| HOMA-IR | 1.08 (1.01, 1.15) | 0.03 | ||
| Triglycerides | 1.01 (1.00, 1.01) | <.01 | ||
| Albumin | 1.54 (0.55, 4.38) | 0.41 | ||
| White Blood Cell | 1.11 (0.99, 1.24) | 0.08 | 1.17 (1.02, 1.34) | 0.02 |
| Diabetes | <.01 | |||
| No | 1.00 | |||
| Yes | 6.77 (2.72, 16.89) | |||
| Hypertension | <.01 | 0.02 | ||
| No | 1.00 | 1.00 | ||
| Yes | 2.77 (1.48, 5.15) | 2.28 (1.16, 4.45) | ||
Table 3.
Crude and Adjusted Prevalence Ratios for Presence of Fibrosis
| Characteristic | Unadjusted | Adjusted | ||
|---|---|---|---|---|
|
|
||||
| (Groups) | PR (95% CI) | P-Value | PR (95% CI) | P-Value |
| Sex | 0.06 | |||
| Female | 1.00 | |||
| Male | 1.89 (0.98, 3.65) | |||
| Age Categories (yrs) | 0.64 | |||
| 13-15 | 1.00 | |||
| 16-17 | 1.69 (0.71, 4.07) | |||
| 18+ | 1.46 (0.55, 3.86) | |||
| Race | 0.97 | |||
| White | 1.00 | |||
| Black/Other | 1.02 (0.46, 2.25) | |||
| BMI (kg/m2) | 1.01 (0.98, 1.05) | 0.36 | ||
| ALT Elevation | 0.02 | 0.08 | ||
| Low | 1.00 | 1.00 | ||
| Mild | 0.99 (0.33, 2.97) | 0.99 (0.33, 2.96) | ||
| High | 3.19 (1.16, 8.77) | 2.41 (0.84, 6.98) | ||
| ALK | 1.00 (0.99, 1.01) | 0.76 | ||
| Glucose (mg/dL) | 0.08 | |||
| <100 | 1.00 | |||
| 100-125 | 1.22 (0.46, 3.19) | |||
| > 126 | 3.21 (1.34, 7.70) | |||
| HOMA-IR | 1.03 (0.98, 1.08) | 0.26 | ||
| Triglycerides | 1.00 (1.00, 1.01) | 0.05 | ||
| Albumin | 0.63 (0.18, 2.15) | 0.46 | ||
| White Blood Cell | 1.03 (0.90, 1.19) | 0.65 | ||
| Diabetes | <.01 | 0.03 | ||
| No | 1.00 | 1.00 | ||
| Yes | 3.56 (1.93, 6.56) | 2.56 (1.10, 5.96) | ||
| Hypertension | 0.26 | |||
| No | 1.00 | |||
| Yes | 1.47 (0.76, 2.87) | |||
Among those with NAFLD (excluding Not-NAFLD from the model), only presence of diabetes (p<0.01) was associated with having more severe liver disease (Supplemental Table 2). Increasing ALT was the only predictor (p<0.01) of having any degree of NAFLD (combining NAFL, borderline and definite NASH together) vs. Not-NAFLD (Supplemental Table 3).
Utility of ALT in identifying NASH
Using population-derived norms (≥22 U/L for females,≥26 U/L for males), abnormal ALT was present in 71.8% of the population, with 82% sensitivity and 35% specificity for identifying definite NASH, and 81% sensitivity and 48% specificity for identifying NAFLD. Applying a more common clinical threshold of ≥ 40 U/L, 19.7% of the cohort had abnormal ALT with 75 % sensitivity and 84% specificity for identifying definite NASH, and 28% sensitivity and 92% specificity for identifying NAFLD in this cohort.
Hepatic Gene Expression Profiles
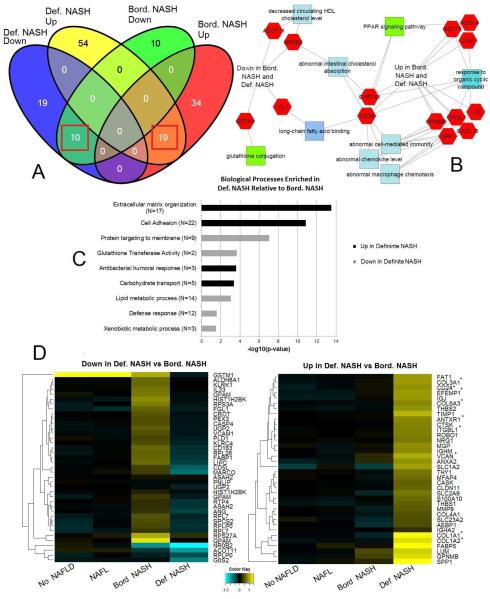
We found a single gene was differentially up-regulated (FC >1.5) when comparing NAFL to Not-NAFLD (CYP7A1; FC = 2.08, ANOVA p-value= 0.0003). This suggests that biological differences between these groups are minimal, or that a consistent genomic signature does not adhere to the histological groupings of bland steatosis vs. histologically normal-appearing liver in the setting of severe obesity. In contrast, 78 genes in the comparison of borderline NASH vs. Not-NAFLD and 105 genes in the comparison of definite NASH vs Not-NAFLD, had FC >1.5, as shown in Figure 3, Panel A. Of these genes, there were 19 up-regulated genes and 10 down-regulated genes in common between these two gene sets. We also observed gene signatures specific to borderline NASH (39 up- and 10 down-regulated genes) and definite NASH (57 up- and 19 down-regulated genes; Figure 3, Panel A) Comparing borderline NASH to definite NASH confirmed a unique genetic signature, generating 163 genes with FC >1.5 (73 up- and 90 down-regulated genes). Supplemental Table 4 lists the top differentially expressed genes identified by these comparisons (top 15 up- and 15 down-regulated genes in each comparison), including their attributed functions and relative fold changes.
Figure 3.
Panel A: Venn diagram of genes differentially regulated between definite NASH and Not-NAFLD, and borderline NASH and Not-NAFLD. Section numbers inside red squares indicate gene sets shared between definite and borderline NASH that were used to generate the ontological network in Panel B. Ontological enrichments associated with gene lists built through comparison of definite NASH to borderline NASH are presented in Panel C. Relative expression values of genes associated with the ontologies in Panel C underwent hierarchical clustering to generate heatmaps in Panel D. Asterisks denote genes previously reported to be associated with severe fibrotic NASH in adults.24
Unbiased ontological analysis of the shared genomic signatures of definite NASH and borderline NASH revealed relevant common pathways/processes (Figure 3, Panel B). In particular, we found enriched up-regulation of macrophage chemotaxis (p<0.0001) and elevated chemokine-related ontologies (p=0.04). Additionally, we saw dysregulation of genes involved in intestinal cholesterol absorption (p<0.0001), fatty acid binding (p=0.01) and high-density lipoproteins (p=0.04), as well as significantly depressed glutathione conjugation (p=0.01).
Comparing definite NASH to borderline NASH revealed enriched up-regulation of genes involved in extracellular matrix organization and collagen binding (both p<0.0001), carbohydrate transport (p<0.001), and immune response (p=0.02). Additionally, we observed down-regulation of genes involved in protein targeting (p<0.0001), lipid and glutathione metabolism (both p<0.001), and immune function (p=0.03; Figure 3, Panels C and D). Specific genes identified in these biological processes/pathways are shown in Supplemental Table 5.
DISCUSSION
Our prospective multi-center study found that 59% of severely obese adolescents in our cohort had NAFLD, with 14% having borderline and 6% definite NASH at time of bariatric surgery. Among those with NASH, advanced fibrotic NASH was uncommon. Our multi-site findings confirm an initial single center report (N=41 adolescents) of a 20% prevalence of mild to moderate grade NASH11 but further advances the literature by elucidating the clinical predictors associated with NASH in this larger cohort of severely obese adolescents undergoing bariatric surgery. Specifically, the odds of having more severe NAFLD were associated with presence of hypertension, increasing ALT and glucose levels, and WBC. Diabetes was the only predictor of fibrosis. Further, an analysis of hepatic gene expression uncovered relevant common and distinct genomic signatures in borderline and definite NASH, but minimal difference between NAFL and normal-appearing liver.
Increasing ALT was associated with the increasing odds of having more severe NAFLD in this cohort: for ALT ≥ 40 U/L, the odds ratio was 6.7 (95% CI: 2.5, 17.8). However, many adolescents with NAFLD, including those with borderline NASH, had ALT levels within the normal or mildly elevated range, with only 28% sensitivity of ALT ≥40 U/L for detection of NAFLD. Our findings therefore support routine histological assessment with intraoperative liver biopsy, regardless of pre-operative ALT elevation. Because both short and long-term outcomes of NAFLD after bariatric surgery remain unknown in adolescents, it is important to identify and stage this condition at time of surgery, so that these patients can be followed long-term for persistence or worsening of liver disease. This is particularly important as new pharmacotherapy options for NASH are actively being investigated in clinical trials and additional treatment options may emerge for adolescents who exhibit persistent disease in adulthood. An intra-operative liver biopsy at time of surgery adds minimal additional operative time, allows for adequate tissue sampling under direct vision and minimizes procedural risk.19
Consistent with our other identified determinants of more severe NAFLD in this cohort, various measures of abnormal glucose homeostasis (impaired fasting glucose and diabetes) have been reported to be risk factors for more histologically advanced NASH in other pediatric and adult cohorts.1,2,20 Hypertension is also very common among children with biopsy-confirmed NAFLD and associated with more severe steatosis.4 Elevated WBC count, a marker of inflammation and atherosclerosis, has been associated with sonographic evidence of hepatic steatosis in adults but to our knowledge not previously reported to be associated with histological severity of pediatric NAFLD.21
In contrast, NAFLD presence and severity was not significantly associated with BMI in this cohort, suggesting that increasing weight is not a key driver for NAFLD risk in this range of severe obesity. The prevalence of definite NASH was also lower than we anticipated in this severely obese cohort, given the higher prevalence of cardiometabolic risk factors typically associated with NASH in less obese pediatric cohorts. Several factors may contribute to this observation. First, this is an adolescent cohort and more severe NASH is associated with advancing age.22 Thus, the adolescent with NASH may be presenting for bariatric surgery before progression to more active NASH or significant fibrosis has occurred. However, younger, less obese pediatric cohorts with biopsy-confirmed NAFLD do have greater proportions with stage 3 and 4 fibrosis (8%-14%). Therefore, young age alone cannot completely account for the lack of more advanced histology.2,23 Notably, biological pathways linked to fibrogenesis were already strongly associated with definite NASH in our analysis. Specifically, several genes recently identified and validated as predictive of severe fibrotic NAFLD in adults were significantly up-regulated in adolescent definite NASH (denoted by asterisk in Figure 3D).24
Selection and/or referral bias may also contribute to the low prevalence of severe NAFLD in this cohort. Uncertainty exists about the role of bariatric surgery in treating NASH, which could lead to low referral rates of adolescents with more severe fibrotic NASH. Also, the majority of severely obese adolescents who currently undergo bariatric surgery are female, white and non-Hispanic, whereas male and Hispanic patients predominate in other cohorts of children with biopsy-confirmed NAFLD and NASH.1,3,5 In the general pediatric population, estimated NAFLD prevalence is 10% and NASH 3% based on a large autopsy study.25 Fatty liver was present in 38% of the obese subjects in that study, compared with 59% in our severely obese cohort. Yet, significant fibrosis (bridging or cirrhosis) in our bariatric cohort was virtually absent (0.7%), compared with a 9% prevalence in the autopsy study. Type 2 diabetes is a known risk factor for fibrotic NASH, but prevalence was not reported in the autopsy series.20 Notably, the 13% prevalence of diabetes in our cohort, though lower than reported in adult cohorts with NAFLD, was higher than the 2.4% to 8% prevalence of diabetes in three other large pediatric cohorts who had much higher prevalence of stage 3 or 4 fibrosis (8% to 20%).2,5,23
Interestingly, fibrosis among adults undergoing bariatric surgery is also relatively mild, despite a reported 25-55% prevalence of NASH.26,27 In a recent analysis of 693 liver biopsies performed in the multicenter Longitudinal Assessment of Bariatric Surgery study in adults (also scored by our study pathologist), 233 subjects (34%) had borderline (17%) or definite NASH (16%).28 However, fibrosis was mild with the majority (>82-90%) having ≤ stage 2 fibrosis and a minority (~5%) having bridging fibrosis or cirrhosis.26-28 Given that advanced fibrotic NASH is not very prevalent among adults undergoing bariatric surgery, some have hypothesized that there may be biological factors unique to severe obesity that protect against developing fibrotic NASH. There is currently little empirical evidence to support this hypothesis. Some studies have suggested that increased subcutaneous fat, particularly in those with the most visceral adipose tissue, is associated with a lower risk of impaired glucose metabolism and dyslipidemia independent of abdominal fat.29,30 However, it is not clear if fat depots differ significantly in distribution or metabolic activity among the severely obese. Further, the genetics of severe obesity have not yet been adequately studied to determine whether there may be associated genetic polymorphisms that could influence severity of associated comorbidities.
Prior hepatic gene expression studies in two non-bariatric pediatric cohorts with NAFLD/NASH (n=11 to 17 patients) have highlighted enrichment of pathways involved in lipid metabolism as well as alcohol metabolism, presumably endogenously produced by intestinal microbiota.31,32 Larger studies in adults with fibrotic NASH have identified differentially expressed genes that are linked to fibrogenesis, mitochondrial function, oxidative stress, lipid and glucose metabolism, cell adhesion and immune function.24,33-35 We likewise found enrichment of pathways and processes involved in fibrogenesis, lipid and carbohydrate metabolism, chemotaxis, cell adhesion and immune function. We also noted a striking down-regulation of glutathione metabolism as NAFLD severity increased; cysteamine bitartrate which increases endogenous glutathione is currently being tested as a treatment for pediatric NAFLD (NCT01529268). Other notable findings in our cohort included the enrichment of pathways linked to abnormal intestinal cholesterol absorption and bile acid physiology. Although only one gene, CYP7A1, was differentially expressed in bland steatosis (NAFL) versus histologically normal-appearing liver in severely obese adolescents, this gene encodes a P450 cytochrome enzyme that catalyzes the first reaction and rate-limiting step in the conversion of cholesterol to bile acids and is the major site of regulation of bile acid synthesis. Altered bile acid physiology has been implicated in the development of NASH and bile acid analogues have recently been proven to improve histology of NASH in clinical trials.36 Further, bariatric surgery favorably changes bile acid profiles in both animal models and human cohorts.37,38 Recently, the bile acid receptor FXR was found to be critical for the weight and metabolic improvement seen after bariatric surgery.39 Therefore, this may be a key mechanism by which bariatric surgery leads to the significant improvements in NASH reported in adults.
Importantly, due to the smaller than anticipated number of subjects with borderline and definite NASH in our bariatric cohort, our genomic signature results must be viewed as pilot in nature and hypothesis generating. Nevertheless, these findings underscore the need for further validation in severely obese cohorts with greater representation of definite NASH and more significant fibrosis. Further, because our normal liver controls were derived from severely obese adolescents, they are unlikely to reflect hepatic gene expression in normal non-obese adolescents. Future studies should ideally include controls from less obese (BMI 85th to 97th percentile) and normal weight (BMI < 85th percentile) adolescents in order to elucidate whether there are potential protective biological factors contributing to milder NAFLD among the severely obese.
Our study has several key strengths including a large multicenter cohort, rigorous standardized, prospective data collection of well-characterized clinical characteristics, and histological confirmation of liver disease by a central pathologist with extensive expertise in evaluating NAFLD. Participants taking medications that can cause or treat NASH were excluded and no participants reported excess alcohol intake. Finally, additional liver tissue was available in a subset of participants enabling pilot gene expression analyses to elucidate biological pathways underlying borderline and definite NASH in this unique cohort.
Because our study was observational, liver biopsies and ALT were not obtained in every participant in Teen-LABS. However, the only statistically significant differences in those not biopsied were a slightly lower rate of dyslipidemia, marginally older age, and minimally lower BMI, which are unlikely to be clinically significant. Further, the biopsy site varied between right and left lobe. NAFLD histological severity can be non-uniform, raising the potential for misclassification of liver disease status and severity within individuals.40 However, minimal variability has been reported between right and left lobe liver biopsies for NAS ≥ 5 and fibrosis in Potential sampling error of liver biopsy affects any study which relies on this current gold standard for diagnosis and staging of NAFLD.40 The variable biopsy practices among the surgical sites in our study do however underscore the current lack of consensus about whether and how to assess for NAFLD in patients undergoing bariatric surgery. Finally, our observational study was not able to systematically rule out more rare causes of steatohepatitis including hepatitis C and Wilson disease. However, histological analysis did not reveal overt signs of viral hepatitis. Further, only 24 participants had a baseline ALT ≥ 40 U/L. We will follow those participants prospectively to determine if ALT normalizes post-operatively.
In summary, we found that while NAFLD was common, advanced NASH was rare in this multicenter cohort of severely obese adolescents undergoing bariatric surgery. BMI did not predict NAFLD severity, highlighting that severe obesity is not the key driver in this population. ALT and cardiometabolic risk factors were associated with NAFLD severity, but the prevalence of NASH was lower than anticipated given the high rate of cardiometabolic risk factors. Selection and/or referral bias may have played a role, due in part to the current demographics of adolescents undergoing surgery and in part to uncertainty regarding outcomes of NASH after bariatric surgery. Whether there are potential protective biological factors unique to severe obesity requires further study. We are following a subset of adolescent participants in Teen-LABS longitudinally to determine long-term ALT change and histologic outcome of NASH, but it will also be critical to conduct prospective controlled studies in severely obese adolescents with more advanced NASH to guide practitioners and patients on optimal treatment for NASH in the most severely obese.
Supplementary Material
Acknowledgements
We acknowledge and are grateful for the important work performed by the research coordinators involved in Teen-LABS and this ancillary study.
Grant support: This study was conducted as a cooperative agreement and funded by the National Institute of Diabetes and Digestive and Kidney Diseases with grants to Cincinnati Children's Hospital Medical Center (K23 DK080888, R01 DK100429) PI: Stavra Xanthakos, MD, MS and (U01 DK072493/ UM1 DK072493) PI: Thomas Inge, MD, PhD and the University of Cincinnati (UM1 DK095710) PI: C. Ralph Buncher, ScD and Todd Jenkins, PhD, MPH. This project was also supported in part by PHS Grant P30 DK078392 Bioinformatics Core of the Digestive Disease Research Core Center in Cincinnati. We gratefully acknowledge the significant contributions made by the Teen-LABS Consortium as well as our parent study LABS Consortium (U01 DK066557). Research support for Dr. Kleiner was provided by the Intramural Research Program of the NIH, National Cancer Institute.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- HDL
high density lipoprotein
- LDL
low-density lipoprotein
- Teen-LABS
Teen Longitudinal Assessment of Bariatric Surgery
- IQR
interquartile range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Stavra Xanthakos: Nothing to disclose
Tawny W. Boyce: Nothing to disclose
David E. Kleiner: Nothing to disclose
Todd M. Jenkins: Nothing to disclose
Reena Mourya: Nothing to disclose
Rebekah Karns: Nothing to disclose
Mary L. Brandt: Nothing to disclose
Carroll M. Harmon: Nothing to disclose
Michael A. Helmrath: Nothing to disclose
MaMrc P. Michalsky: Nothing to disclose
Anita P. Courcoulas: Dr. Courcoulas has received research grants from Covidien, EndoGastric Solutions, Nutrisystem and is on the Scientific Advisory Board of Ethicon J & J Healthcare System
Margaret H. Zeller: Nothing to disclose
Thomas H. Inge: Dr. Inge has received research funding for an investigator-initiated study from Ethicon Endosurgery not related to this current study. Dr. Inge has also served as a consultant to NPS Pharma and Sanofi Pharmaceuticals not related to this current study.
Writing assistance: none
REFERENCES
- 1.Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008 Dec;135(6):1961–1971 e1962. doi: 10.1053/j.gastro.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patton HM, Yates K, Unalp-Arida A, et al. Association between metabolic syndrome and liver histology among children with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2010 Sep;105(9):2093–2102. doi: 10.1038/ajg.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011 Apr 27;305(16):1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwimmer JB, Zepeda A, Newton KP, et al. Longitudinal assessment of high blood pressure in children with nonalcoholic fatty liver disease. PLoS One. 2014;9(11):e112569. doi: 10.1371/journal.pone.0112569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter-Kent C, Yerian LM, Brunt EM, et al. Nonalcoholic steatohepatitis in children: a multicenter clinicopathological study. Hepatology. 2009 Oct;50(4):1113–1120. doi: 10.1002/hep.23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly AS, Barlow SE, Rao G, et al. Circulation. 2013 Sep 9; [Google Scholar]
- 7.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012 Jun;142(7):1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Michalsky M, Reichard K, Inge T, Pratt J, Lenders C. ASMBS pediatric committee best practice guidelines. Surg Obes Relat Dis. 2012 Jan-Feb;8(1):1–7. doi: 10.1016/j.soard.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Pratt JS, Lenders CM, Dionne EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity (Silver Spring) 2009 May;17(5):901–910. doi: 10.1038/oby.2008.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nobili V, Vajro P, Dezsofi A, et al. Indications and Limitations of Bariatric Intervention in Severely Obese Children and Adolescents With and Without Non-alcoholic Steatohepatitis: the ESPGHAN Hepatology Committee Position Statement. J Pediatr Gastroenterol Nutr. 2015 Feb 2; doi: 10.1097/MPG.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 11.Xanthakos S, Miles L, Bucuvalas J, Daniels S, Garcia V, Inge T. Histologic spectrum of nonalcoholic fatty liver disease in morbidly obese adolescents. Clin Gastroenterol Hepatol. 2006 Feb;4(2):226–232. doi: 10.1016/s1542-3565(05)00978-x. [DOI] [PubMed] [Google Scholar]
- 12.Inge TH, Zeller MH, Jenkins TM, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr. 2014 Jan;168(1):47–53. doi: 10.1001/jamapediatrics.2013.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005 Jun;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 14.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011 Mar;53(3):810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004 Apr 1;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 16.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. American journal of epidemiology. 2005 Aug 1;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 17.Schwimmer JB, Dunn W, Norman GJ, et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010 Apr;138(4):1357–1364. doi: 10.1053/j.gastro.2009.12.052. 1364 e1351-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009 Jul;37:W305–311. doi: 10.1093/nar/gkp427. Web Server issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009 Mar;49(3):1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 20.Loomba R, Abraham M, Unalp A, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012 Sep;56(3):943–951. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YJ, Lee HR, Shim JY, Moon BS, Lee JH, Kim JK. Relationship between white blood cell count and nonalcoholic fatty liver disease. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2010 Dec;42(12):888–894. doi: 10.1016/j.dld.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30(6):1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 23.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005 Aug 22;42(3):641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 24.Moylan CA, Pang H, Dellinger A, et al. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology. 2014 Feb;59(2):471–482. doi: 10.1002/hep.26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006 Oct;118(4):1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 26.Abrams GA, Kunde SS, Lazenby AJ, Clements RH. Portal fibrosis and hepatic steatosis in morbidly obese subjects: A spectrum of nonalcoholic fatty liver disease. Hepatology. 2004 Aug;40(2):475–483. doi: 10.1002/hep.20323. [DOI] [PubMed] [Google Scholar]
- 27.Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007 Feb;102(2):399–408. doi: 10.1111/j.1572-0241.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 28.Kleiner DE, Berk PD, Hsu JY, et al. Hepatic pathology among patients without known liver disease undergoing bariatric surgery: observations and a perspective from the longitudinal assessment of bariatric surgery (LABS) study. Semin Liver Dis. 2014 Feb;34(1):98–107. doi: 10.1055/s-0034-1371083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot. Diabetes Care. 2009 Jun;32(6):1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell metabolism. 2008 May;7(5):410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu L, Baker SS, Liu W, et al. Lipid in the livers of adolescents with nonalcoholic steatohepatitis: combined effects of pathways on steatosis. Metabolism. 2011 Jul;60(7):1001–1011. doi: 10.1016/j.metabol.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Baker SS, Baker RD, Zhu R, Zhu L. Systematic analysis of the gene expression in the livers of nonalcoholic steatohepatitis: implications on potential biomarkers and molecular pathological mechanism. PLoS One. 2012;7(12):e51131. doi: 10.1371/journal.pone.0051131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sreekumar R, Rosado B, Rasmussen D, Charlton M. Hepatic gene expression in histologically progressive nonalcoholic steatohepatitis. Hepatology. 2003 Jul;38(1):244–251. doi: 10.1053/jhep.2003.50290. [DOI] [PubMed] [Google Scholar]
- 34.Younossi ZM, Baranova A, Ziegler K, et al. A genomic and proteomic study of the spectrum of nonalcoholic fatty liver disease. Hepatology. 2005 Sep;42(3):665–674. doi: 10.1002/hep.20838. [DOI] [PubMed] [Google Scholar]
- 35.Bertola A, Bonnafous S, Anty R, et al. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS One. 2010;5(10):e13577. doi: 10.1371/journal.pone.0013577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2014 Nov 7; doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring) 2014 Feb;22(2):390–400. doi: 10.1002/oby.20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013 Apr;98(4):E708–712. doi: 10.1210/jc.2012-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014 May 8;509(7499):183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nalbantoglu IL, Brunt EM. Role of liver biopsy in nonalcoholic fatty liver disease. World J Gastroenterol. 2014 Jul 21;20(27):9026–9037. doi: 10.3748/wjg.v20.i27.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larson SP, Bowers SP, Palekar NA, Ward JA, Pulcini JP, Harrison SA. Histopathologic variability between the right and left lobes of the liver in morbidly obese patients undergoing Roux-en-Y bypass. Clin Gastroenterol Hepatol. 2007 Nov;5(11):1329–1332. doi: 10.1016/j.cgh.2007.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.