Abstract
Purpose of review
We review the most recent developments regarding the targeting of molecules involved in the traffic of leukocytes for the treatment of IBD.
Recent Findings
We discuss the most important findings of one published phase II trial that targeted the β7 integrin (Etrolizumab), two phase II trials that targeted the α4β7 integrin ligand: Mucosal Addressin Cell Adhesion Molecule-1 (MAdCAM-1, PF-00547659), a phase II targeting the chemokine IP-10 (CXCL10) in Crohn’s and a phase II trial that targeted the sphingosine-1-phosphate receptor-1 (S1P1): ozanimod in patients with ulcerative colitis (UC).
Summary
Targeting molecules involved in leukocyte traffic has recently become an effective and safe strategy for the treatment of IBD. Novel approaches now not only target the integrins on the lymphocyte surface, but also its endothelial ligand: MAdCAM-1. As with vedolizumab, antibodies against MAdCAM-1 appear most effective in ulcerative colitis rather than in Crohn’s. Targeting chemokines or their receptors does not appear to have the same efficacy as those that target the most stable integrin:immunoglobulin superfamily interactions between the lymphocyte and endothelium. Preliminary results also suggest that the sphingosine-1-phosphate pathway might also be targeted therapeutically in IBD, no longer with parenterally administered antibodies but with orally administered small molecules.
Keywords: α4β7 Integrin, MAdCAM-1, sphingosine-1-phosphate, Crohn’s, colitis
Introduction
The inflammatory bowel diseases (IBD) affect several million people, mostly in developed countries. Effective biologic therapies, such as anti-TNF antibodies are at best initially effective in 70 percent of patients, however they lose efficacy over time and increase the risk of infections and malignancy. Thus, there is a need for novel therapeutics that target other arms of the inflammatory cascade, beyond the proinflammatory cytokines.
T cells are key cellular mediators of chronic immune processes such as IBD. Since they respond to antigens through direct contact, they must leave the circulation and enter lymphoid compartments, where antigens are presented by antigen presenting cells. T cells then either become tissue residents (TRM)1 or recirculate, returning to areas with a similar micro-environment to that found where they first recognized their cognate antigen. To recirculate to effector sites, lymphocytes express a defined repertoire of adhesion molecules and chemokines/receptors on their cell surfaces that recognize counter-receptors on appropriate vascular endothelial beds 2.
Integrins are heterodimeric molecules formed by the noncovalent association of two subunits, namely a large (alpha, 120-170 kDa) and a small (beta, 90-100 kDa) subunit. Eighteen α and eight β subunits have been described, which combine to generate at least 24 different integrin heterodimers in vertebrates, fourteen of which are present in cells of the immune system 3-6. The high number of possible combinations ensures the wide functional diversity of these molecules. Each subunit is a type I transmembrane glycoprotein consisting of a large extracellular domain, a single pass transmembrane domain which induces intracellular signaling via binding to cytoskeleton7 and a short cytoplasmic tail (with the notable exception of β4 integrin)4. The particular β subunit that is present in each heterodimer defines discrete subtypes of integrins, with unique structures, tissue-specificities and function. According to this functional scheme, the β2 (CD18), α4 and β7 families of integrins play the most prominent roles during inflammatory conditions, as they are leukocyte-specific8, 9. Further diversity results from the fact that different leukocytes display unique patterns of integrin expression. Indeed, lymphocytes, macrophages, and polymorphonuclear cells all express distinct sets of β integrins which differ between subclasses and also between their resting and activated states10.
Combinatorial expression of adhesion molecules and chemokines, as well as their ligands and receptors serve as traffic signals for the differential migration of leukocytes to specific tissues. Gut-homing lymphocytes express the α4β7 integrin and gain access to the intestine and gastrointestinal-associated lymphoid tissues (GALT) through specialized high endothelial and postcapillary venules (HEV) that express its ligand Mucosal Addressin Cell Adhesion molecule-1 (MAdCAM-1).
Natalizumab: First in the anti-integrin lineage
Proof of concept for the efficacy of targeting lymphocyte integrins in IBD appeared in 1998, when an antibody against α4 integrins (i.e. natalizumab), was FDA-approved for the treatment of multiple sclerosis (MS) and Crohn’s. By targeting the shared α4 subunit of two distinct integrin heterodimers, natalizumab blocks both α4β1 and α4β7 integrin interactions with fibronectin, VCAM-1, (expressed on inflamed endothelial and other cells) and MAdCAM-1 (expressed on intestinal endothelial cells and high endothelial venules) in intestine and gastrointestinal associated lymphoid tissues. Its use in IBD has been limited by its association with progressive multifocal leukoencephalopathy (PML), a demyelinating condition that results from reactivation of the John Cunningham (JC) polyoma virus. The pathogenesis of PML in patients receiving natalizumab is unknown, however it is primarily associated with the blockade of α4β1 integrin-VCAM-1 interactions by natalizumab. Therefore, significant resources have been dedicated to refining the anti-adhesion strategy in IBD, by targeting other lymphocyte trafficking molecules: CCR9 (Chemocentryx/GSK), IP-10 (Bristol-Myers Squibb), integrin-β7 (Genentech), integrin α4β7 (Millenium/Takeda, Amgen), MAdCAM-1 (Pfizer) and sphingosine-1-phosphate receptor (Receptos). The hope is that newer generation traffic-targeting strategies maintain the efficacy of natalizumab, while minimizing the risks of adverse events.
Vedolizumab: Specific Targeting of α4 β7 Integrin
Vedolizumab (Millennium Pharmaceuticals Inc.; Takeda) is a humanized IgG-1 monoclonal antibody directed against a combinatorial epitope on the integrin α4β7 heterodimer. Following promising results obtained in the cotton-top tamarin model of UC, human studies of this antibody were initiated, based on the concept that a more selective inhibition of gut-specific lymphocyte traffic would likely prove to be a safe and effective alternative to the less specific approach. Large phase 3 trials investigating vedolizumab as an induction and maintenance agent for both UC (GEMINI 1)11 and CD (GEMINI 2,3)12,13 were published in 2013 and have been extensively reviewed in the past year.
What is new with targeting traffic?
Etrolizumab: Targeting the β7 integrins: Is less specificity for α4β7 the same, better or worse than vedolizumab?
Etrolizumab (rhuMAb β7, Genentech) is a humanized IgG1 monoclonal antibody directed against the β7 integrin subunit. Therefore, the drug targets both αEβ7 and the α4β7 integrin heterodimers and blocks interactions to their respective ligands: MAdCAM-1 and E-cadherin. Pre-clinical studies showed that etrolizumab effectively inhibits migration of T cells to mucosal sites, without affecting traffic to non-mucosal tissues. The results from a double-blind, placebo-controlled randomized, phase II study on the use of etrolizumab were recently reported14. Study subjects were adults (age 18-75 years) with moderate to severe UC (Mayo Clinic Score (MCS) ≥ 6)), who had not responded to conventional therapy (immunomodulator, anti-TNF) and in whom disease extended 25 cm or more from anal verge. Patients were randomized (1:1:1) to one of two dose levels of subcutaneous etrolizumab (100 mg at weeks 0, 4, and 8, with placebo at week 2; or 420 mg loading dose at week 0 followed by 300 mg at weeks 2, 4, and 8 or placebo. The primary endpoint was clinical remission at week 10, defined as MCS of 2 or less (with no individual subscore of >1), analyzed in the modified intention-to-treat population (mITT; all randomly assigned patients who had received at least one dose of study drug, had at least one post-baseline disease-activity assessment, and had a centrally read screening endoscopic subscore of ≥2). Patients who had an endoscopic subscore of 0 or 1 were excluded from the mITT population. There were 39 patients in the 100 mg group, 39 in the 300 mg plus loading dose group, and 41 in the placebo group for the primary analyses. There were no patients in the placebo group who had clinical remission at week 10, compared with eight (21% [95% CI 7-36]) patients in the 100 mg group (p=0.004) and four (10% [0·2-24]) patients in the 300 mg plus loading dose group (p=0.048). Etrolizumab was more likely to lead to clinical remission than placebo at week 10. Safety and pharmacokinetics were examined at various time-points. Etrolizumab was safe and well tolerated. The most common side effect was exacerbation of colitis followed by nasopharyngitis. No serious opportunistic infections were reported. It is interesting that the higher dose was not any better than placebo. Although this could be a mere artifact of the sample size, we may speculate that higher drug tissue levels might potentially interfere with CD103:E-cadherin interactions. Further mechanism of action studies are needed.
MAdCAM-1: the α4β7 integrin ligand
The endothelial ligands for integrins are members of the immunoglobulin superfamily. Proteins of this class contain at least one immunoglobulin domain, comprising of two β-pleated sheets held together by a disulfide bond (Figure 1). Among the several members of the immunoglobulin superfamily, the following have established pathogenetic roles in IBD: intercellular adhesion molecule-1 (ICAM-1) or CD54, Vascular Cell Adhesion Molecule-1 (VCAM-1) or CD106, and Mucosal Addressin Cell Adhesion Molecule-1 (MAdCAM-1). MAdCAM-1 is an endothelial cell adhesion molecule of the immunoglobulin superfamily (which also includes ICAM-1 and VCAM-1), expressed on specialized high endothelial venules (HEV) of gut associated lymphoid tissue (GALT) as well as on endothelial cells of the small and large intestine.
Figure 1. The leukocyte adhesion cascade.
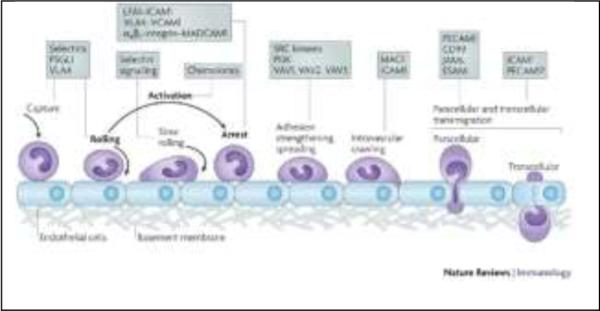
The original three steps are shown in bold: rolling, which is mediated by selectins, activation, which is mediated by chemokines, and arrest, which is mediated by integrins. Progress has been made in defining additional steps: capture (or tethering), slow rolling, adhesion strengthening and spreading, intravascular crawling, and paracellular and transcellular transmigration. Key molecules involved in each step are indicated in boxes. ESAM, endothelial cell-selective adhesion molecule; ICAM1, intercellular adhesion molecule 1; JAM, junctional adhesion molecule; LFA1, lymphocyte function-associated antigen 1 (also known as αLβ2-integrin); MAC1, macrophage antigen 1; MADCAM1, mucosal vascular addressin cell-adhesion molecule 1; PSGL1, P-selectin glycoprotein ligand 1; PECAM1, platelet/endothelial-cell adhesion molecule 1; PI3K, phosphoinositide 3-kinase; VCAM1, vascular cell-adhesion molecule 1; VLA4, very late antigen 4 (also known as α4β1-integrin).
There are increased numbers of intestinal mucosal vessels that stain positive for MAdCAM-1 in tissues from patients with IBD 15. In addition, MAdCAM-1 expression is increased in the colon of animal models of intestinal inflammation16. TNF-α and IL-1 are both abundant in areas of active CD or UC and have been shown to induce upregulation of MAdCAM-1 expression in the intestine, colon and MLN 16, 17. MAdCAM-1 expression is detected at extra-intestinal sites, such as the joints, eyes, skin and liver18. As these organs are frequently affected in patients with IBD, the aberrant expression of a gut-homing molecule in these tissues may attract pathogenic cells and induce extra-intestinal inflammation. In addition to IBD, MAdCAM-1 expression is upregulated on inflamed venules in several other chronic inflammatory conditions as those occurring in diabetes, primary sclerosing cholangitis, and cirrhosis 19.
Targeting the endothelial α4β7 integrin ligand: anti-MAdCAM-1 (PF-00547659) in UC
PF-00547659 is a fully human IgG2K monoclonal antibody that binds specifically to human MAdCAM-1. Functional assays demonstrated that the drug blocked the adhesion of α4β7 integrin expressing cells to MAdCAM-120. The results of the phase II TURANDOT study were presented at the Digestive Diseases Week Meeting 2015 21. Three hundred fifty seven adults (ages 18-65 years) with ≥3 month history of UC extending >15 cm beyond the rectum and with a total Mayo Score ≥6/endoscopic subscore ≥2 and who had failed at least 1 prior therapy were enrolled. Anti-TNF therapy was discontinued ≥6 weeks prior to randomization. Stable doses of all other medications, including prednisone (<20mg/d) was required. Immunosuppressant agents were stopped by week 12. Patients were randomized to receive 7.5mg, 22.5mg, 75mg or 225mg of PF-00547659 or placebo every 4 weeks for 3 doses. The primary end point was remission at week 12, defined as total Mayo score ≤2 with no subscore >1. Secondary end points were clinical response at week 12 (Mayo score decrease ≥3 and ≥30% decrease from baseline) and mucosal healing (Mayo endoscopy subscore ≤1). Endoscopies were read centrally. The age and gender of the patients in the study arms were comparable (mean age 40.2 years, 58% male). Forty three percent of subjects had never received anti-TNF agents. The primary endpoint of clinical remission was significantly greater in the 7.5mg, 22.5mg and 75mg dose groups compared with placebo. Secondary endpoints included rates of clinical response (decrease in total Mayo score of at least 3 points or decrease in rectal bleeding subscore by greater than 30% or an absolute subscore of ≤1), mucosal healing (endoscopy subscore of ≤1), other levels of decrease in Mayo score, and change from baseline in fecal calprotectin. The secondary endpoint of mucosal healing was significantly greater in the 22.5 mg and 75 mg dose groups compared with placebo, while response was greater for 22.5 mg and 225 mg groups. Greater differences compared with placebo were observed in anti-TNF naïve patients. Overall and serious adverse event rates were similar between the placebo and all drug doses. There was no obvious dose-response relationship for any of the events monitored. There were no increases in mucosal tissue infections (gastrointestinal, nasal, spleen, bladder, uterus and lung) and no cases of PML were observed.
The 22.5 mg dose was the most effective dose for all endpoints, whereas the least effective dose for most outcomes was 225 mg, with the 7.5 mg dose usually the second-least effective. Major efficacy results with the 22.5 mg dose and placebo in a modified intent-to-treat analysis were clinical remission: 16.7% drug compared with 2.7% placebo. Clinical response was 54.2% drug compared with 28.8% placebo and mucosal healing of 27.8% drug vs. 8.2% placebo (allP<0.05). Fecal calprotectin levels decreased with all doses of the drug, whereas there was no change with placebo.
Anti-MAdCAM-1 Antibody (PF-00547659) for Crohn’s Disease: Why not as effective as in UC?
OPERA is a randomized, multicenter double-blind, placebo-controlled study of safety and efficacy of PF-00547659 in patients with Crohn’s disease (CD). The results were presented at the Digestive Diseases week 2015 22. Two hundred sixty seven adults (ages 18-75) with moderate to severe CD (CDAI 220-450), who had failed or did not tolerate anti-TNF and/or immunosuppressant drugs, had CRP >3.0 mg/L and ulcers on colonoscopy were enrolled. Study subjects were randomized to placebo, 22.5 mg, 75 mg or 225 mg PF-00547659. The primary end point was CDAI-70 response at week 8 or 12. Secondary end points included remission and CDAI-100 response. The frequency and level of β7 expression on peripheral CD4+ central memory T cells was studied via flow cytometry. CRP and soluble MAdCAM-1 levels we additionally monitored.
The CDAI-70 response was not significantly different between any of PF-00547659 doses and placebo. However, in patients with baseline CRP level >18 (placebo 14% versus 37%, 24% and 39% with increasing doses) remission at week 12 was higher. Soluble MAdCAM-1 decreased significantly at week 2 compared with baseline, in a dose-dependent manner, and remained low during the study in patients who received drug. Circulating β7 CD4+ central memory T-lymphocytes increased at weeks 8 and 12, in patients treated with PF-00547659 in a dose-dependent manner, suggesting that the drug was effective at interfering with T cell recruitment. Baseline characteristics, age, disease duration and baseline CDAI were similar in all treatment arms. PF-00547659 was safe and well tolerated. Most common adverse events were related to the underlying disease, with no evidence of a dose response in any adverse event category. The primary endpoint was not met due to a high placebo response. However, PF-00547659 was pharmacologically active as shown by a dose-related increase in circulating β7+ T lymphocytes and a sustained dose-related decrease in soluble MAdCAM-1. Like in the ENACT trial (Natalizumab, designed nearly a decade ago), higher baseline CRP levels differentiated responders from placebo. This strongly argues for the need of additional objective readouts for Crohn’s trials beyond the CDAI and a revamped study design that allows enrollment of patients with objective evidence of inflammation.
Targeting chemokine receptors
Chemokines are chemoattractant cytokines that mediate several steps of leukocyte recruitment, such as chemoattraction and integrin activation. Trials of a small molecule antagonist that targeted chemokine receptor 9 (CCR9) in patients with Crohn’s showed no more efficacy than placebo23. Recently another chemokine: Interferon-γ-inducible protein-10 (IP-10, CXCL10) has been targeted. IP-10 is involved in inflammatory cell recruitment and the survival and migration of activated T cells, monocytes, eosinophils and natural killer cells. The receptor for IP-10 is chemokine (cys-x-cys motif) receptor 3 (CXCR3). IP-10 exerts chemotactic activity on T helper Th1 and Th17 cells and modulates epithelial and endothelial cell proliferation.
An Anti-IP-10 Antibody (BMS-936557, Eldelumab) in Patients with Crohn’s Disease
Eldelumab, is a fully human monoclonal antibody against IP-10 (CXCL10). A prior Phase IIb study of patients with ulcerative colitis showed modest efficacy.24 In this study25, patients (n=121) with Crohn’s Disease Activity Index (CDAI) ≥220 and ≤450 were randomly assigned 1:1:1 to placebo or eldelumab 10 or 20 mg/kg IV. Drug was administered on days 1 and 8 and every other week. Endoscopy videos were collected for all patients at baseline and patients with a score of 2–3 on the ulcerated surface subscore of the Simplified Endoscopic Score for Crohn’s Disease (SES-CD) in at least 1 of 5 segments (as judged by the investigator) underwent follow up endoscopy at 11 weeks. Endoscopies were read by a central reader in a blinded fashion. Trial endpoints included clinical response (reduction in CDAI ≥100 points from baseline or an absolute CDAI score <150) and clinical remission (CDAI <150), and endoscopic improvement at week 11.
Patients receiving eldelumab had numerically higher remission and response rate for the 10 mg/kg dose (22.5% and 47.5%, respectively) and 20 mg/kg (29.3% and 41.5%) compared with placebo (20% and 35%) at week 11. Clinical remission and response rates were higher in anti-tumor necrosis factor (TNF)-naïve patients versus anti-TNF failures. Greater reduction from baseline in mean endoscopy scores and a higher proportion of patients achieving 50% improvement on the SES-CD were observed for both eldelumab doses compared with placebo, in the cohort of patients with total SES-CD >2 at baseline. The level of endoscopic improvement was similar in the eldelumab-treated groups across the anti-TNF-naïve and anti-TNF failure subgroups. Serious adverse events were more common in the eldelumab groups (7.5% and 9.8%) compared with placebo (5%). Induction treatment with eldelumab demonstrated trends towards efficacy as assessed by both clinical and endoscopic endpoints. However, the study was not statistically powered to test efficacy endpoints.
To date strategies that target chemokine receptors have not had the same level of efficacy than those that target the stable protein-protein bonds formed between integrins and their endothelial ligands.
Ozanimod: a sphingosine-1-phosphate receptor-1 selective agonist
Conceptulally, lymphocyte trafficking may be additionally targeted by the administration of sphingosine-1-phosphate receptor agonists. FTY720 (Fimgolimod, GilenyaTM), the prototype drug, is a small-molecule agonist of sphingosine-1-phosphate receptors 1,3,4,5 (S1P1,3,4,5). It is approved by the FDA as the first oral drug for the treatment of multiple sclerosis (MS)26. This small molecule agonist exerts its therapeutic effect through a poorly understood mechanism, independent of known leukocyte integrin pathways. Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid and its concentration gradient, (between tissues and blood), regulates lymphocyte recirculation. Ozanimod (RPC1063) is an oral, selective agonist for sphingosine 1-phosphate receptors 1 and 5.
The recently reported phase II study27 examined the safety and efficacy of 0.5 mg (low dose, LD, n=65) and 1 mg (high dose, HD, n=67) RPC1063 compared with placebo (n=65) in 197 patients with moderate to severe UC (Mayo score of 6-12 with an endoscopic sub-score ≥2). The 8 week induction trial had a continuing maintenance period for responders. The primary endpoint was the proportion of patients in remission (Mayo score ≤2, no subscore >1) at week 8. Secondary endpoints included the proportion of patients exhibiting clinical response (reduction in Mayo score of ≥3 and ≥30 % with a decrease in the rectal bleeding score of ≥1 or a rectal bleeding score ≤1), proportion of patients with mucosal improvement (endoscopy score ≤1), and a change in Mayo score.
Ninety five percent of patients completed the induction portion of the study. The proportion of patients achieving clinical remission was 16.4% for HD (p=0.048 vs. placebo), 13.8% for LD (p=0.14), and 6.2% for placebo (Fig. 2A). The proportion of patients with clinical response was 56.7% for HD (p=0.01), 53.8% for LD (p=0.06), and 36.9% for placebo (Fig. 2B). The proportion of patients with mucosal improvement was 34.3% for HD (p=0.002), 27.7% for LD (p=0.03), and 12.3% for placebo (Fig. 2C). The improvement in Mayo score from baseline was 3.3 points for HD (p=0.003), 2.6 points for LD (p=0.098), and 1.9 for placebo. Safety assessments included ECG, Holter monitoring, pulmonary function testing, optical coherence tomography and adverse events. The adverse event profiles were comparable between groups, with approximately 31% of patients experiencing a treatment emergent event across all groups. The most common in the treatment event was worsening of ulcerative colitis (HD 1 [1.5%], LD 2 [3.1%], placebo 3 [4.6%]) and anemia/decreased Hgb (HD 0, LD 3 [4.6%], placebo 3 [4.6%]). Modest effects on heart rate were seen with no notable cardiac, pulmonary, ophthalmologic or malignancy observed. Transient transaminase (ALT ≥3x) occurred in 3 patients (HD 1 [1.5%], LD 2 [3.1%]) and decreased with continued treatment. Thus at the 1 mg dose, ozanimod induced clinical remission, clinical response, and mucosal improvement. The drug was safe and well tolerated. A phase 3 trial in UC and a phase II trial in Crohn’s are being planned.
Figure 2. The immunoglobulin superfamily of integrin ligands.
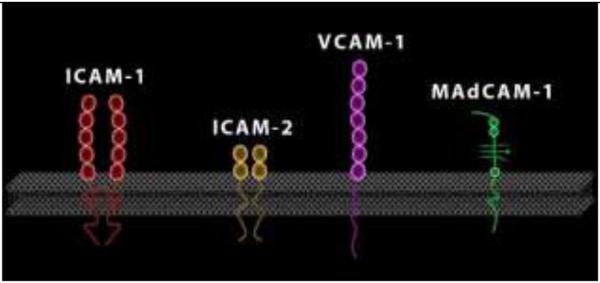
Of these ICAM-1 and MAdCAM-1 have been targeted for the treatment of IBD.
Conclusions
Of the several families of molecules that mediate the recruitment of leukocytes to the intestine (selectins, chemokine receptors, integrins and its endothelial ligands)2 targeting the last steps of the process (firm adhesion and transmigration) mediated by integrins and its ligands has provided the most viable strategy. Targeting chemokine receptors has yet to provide a viable prototype drug, while for the first time the targeting of endothelial integrin ligands (MAdCAM-1) appears promising, particularly in UC. Targeting the sphingosine-1-phosphate (S1P) pathway through the administration of oral S1P agonists is the newest strategy that likely acts via the modulation of lymphocyte traffic. With the approval of a second anti-integrin antibody (Vedollizumab) for the treatment of UC and CD and several other anti-traffic drugs undergoing clinical trials, the targeting of lymphocyte migration has become the next therapeutic frontier for IBD.
Keypoints.
Of the major families of molecules involved in leukocyte traffic (selectins, chemokine/receptors, integrin/immunoglobulins) it is the integrins and their immunoglobulin-superfamily of ligands that continue to show the most promise as viable therapeutic targets for IBD.
Blockade of specific “gut-selective” integrin-endothelial ligand interactions is to date a safe and effective alternative to anti-TNF antibodies.
All of the drugs in the family tested to date appear most efficacious in ulcerative colitis than in Crohn’s, however Crohn’s trials remain riddled by high placebo response rates.
Targeting the sphingosine-1-phosphate pathway with orally administered small molecules might represent a viable alternative to the therapeutic targeting of lymphocyte traffic via monoclonal antibodies.
Figure 3.
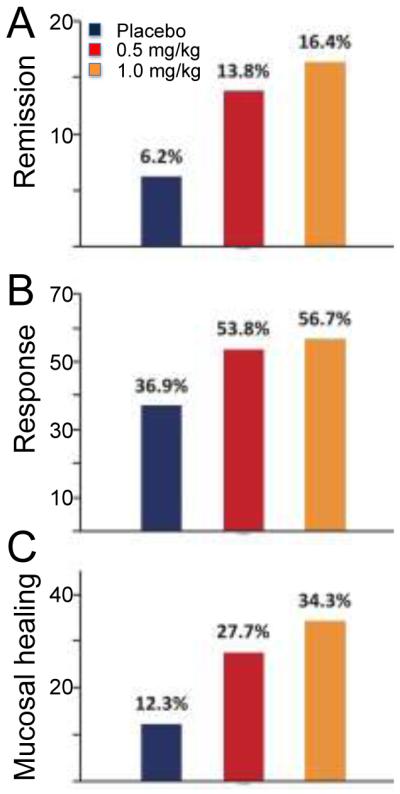
Endpoints of TOUCHSTONE trial testing safety and efficacy of low (LD, 0.5 mg/kg) and high (HD, 1 mg/kg) dose ozanimod in patients with moderate to severe UC. (Figure provided by Dr. Allan Olson (Receptos, Inc)
Acknowledgements
None
Financial Support and Sponsorship
This work was supported by NIDDK (USPHS DK080212) and by BLRD VA Merit Review award (1I01BX001051) and by a contract from Receptos Inc.
Footnotes
Conflicts of Interest
Jesus Rivera-Nieves received a research contract from Receptos Inc.
References
- 1.Gebhardt T, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–9. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 2.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 3.Lacy-Hulbert A, et al. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–8. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo BH, Carman CV. Springer, T.A. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pribila JT, Quale AC, Mueller KL, Shimizu Y. Integrins and T cell-mediated immunity. Annu Rev Immunol. 2004;22:157–80. doi: 10.1146/annurev.immunol.22.012703.104649. [DOI] [PubMed] [Google Scholar]
- 6.Travis MA, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–5. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–59. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 8.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 10.Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. The leukocyte integrins. J Biol Chem. 2000;275:23409–12. doi: 10.1074/jbc.R000004200. [DOI] [PubMed] [Google Scholar]
- 11.Feagan BG, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 12.Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711–21. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 13.Sands BE, et al. Effects of vedolizumab induction therapy for patients with Crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618–627. doi: 10.1053/j.gastro.2014.05.008. e3. [DOI] [PubMed] [Google Scholar]
- 14.Vermeire S, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384:309–18. doi: 10.1016/S0140-6736(14)60661-9. (**) Describes the latest findings of a third anti-integrin drug that targets the beta 7 integrin subunit shared by the α4β7 and αEβ7 heterodimers. [DOI] [PubMed] [Google Scholar]
- 15.Briskin M, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 16.Connor EM, Eppihimer MJ, Morise Z, Granger DN, Grisham MB. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leukoc Biol. 1999;65:349–55. doi: 10.1002/jlb.65.3.349. [DOI] [PubMed] [Google Scholar]
- 17.Sikorski EE, Hallmann R, Berg EL, Butcher EC. The Peyer's patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-alpha and IL-1. J Immunol. 1993;151:5239–50. [PubMed] [Google Scholar]
- 18.Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6:244–51. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- 19.Salmi M, Andrew DP, Butcher EC, Jalkanen S. Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J Exp Med. 1995;181:137–49. doi: 10.1084/jem.181.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pullen N, et al. Pharmacological characterization of PF-00547659, an anti-human MAdCAM monoclonal antibody. Br J Pharmacol. 2009;157:281–93. doi: 10.1111/j.1476-5381.2009.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinisch W, et al. 901a A Randomized, Multicenter Double-Blind, Placebo-Controlled Study of the Safety and Efficacy of Anti-MAdCAM Antibody PF-00547659 (PF) in Patients With Moderate to Severe Ulcerative Colitis: Results of the TURANDOT Study. Gastroenterology. 148:S-1193. (**) Describes the results of a clinical trial testing safety and efficacy of a novel drug that targets the endothelial α4β7 ligand MAdCAM-1 in patients with UC. [Google Scholar]
- 22.Sandborn W, et al. 825 Anti-MAdCAM-1 Antibody (PF-00547659) for Active Refractory Crohn's Disease: Results of the OPERA Study. Gastroenterology. 148:S-162. (*) Describes the results of a clinical trial testing safety and efficacy of a novel drug that targets the endothelial α4β7 ligand MAdCAM-1 in patients with Crohn's. [Google Scholar]
- 23.Wendt E, Keshav S. CCR9 antagonism: potential in the treatment of Inflammatory Bowel Disease. Clin Exp Gastroenterol. 2015;8:119–30. doi: 10.2147/CEG.S48305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer L, et al. Anti-IP-10 antibody (BMS-936557) for ulcerative colitis: a phase II randomised study. Gut. 2014;63:442–50. doi: 10.1136/gutjnl-2012-303424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandborn WJ, et al. 827 Phase IIA, Randomized, Placebo-Controlled Evaluation of the Efficacy and Safety of Induction Therapy With Eldelumab (Anti-IP-10 Antibody; BMS-936557) in Patients With Active Crohn's Disease. Gastroenterology. 148:S-162–S-163. (*) [Google Scholar]
- 26.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–64. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandborn W, et al. 445 The TOUCHSTONE Study: A Randomized, Double-Blind, Placebo-Controlled Induction Trial of an Oral S1P Receptor Modulator (RPC1063) in Moderate to Severe Ulcerative Colitis. Gastroenterology. 148:S-93. (**) Describes the result of a trial testing the safety and efficacy of a novel ORAL small molecule drug that targets shingosine-1-phosphate receptor-1. [Google Scholar]


