Abstract
BACGORUND
PTEN/AKT signaling plays a key role in prostate cancer development and maintenance of prostate cancer stem cells. How other oncogenes or tumor suppressors interact with this pathway remain to be elucidated. SLUG is an zinc finger transcription factor of the Snail superfamily, and it promotes cancer metastasis and determines the mammary stem cell state.
METHODS
SLUG was overexpressed in cells by retroviral vector and knockdown of SLUG and PTEN was mediated by shRNAs-expressing lentiviruses. Expression level of SLUG and PTEN was examined by Western blot, RT-PCR, and qPCR analyses. PTEN promoter activity was measured by luciferase reporter assay. ChIP assay was used to measure the binding between SLUG and the PTEN promoter in vivo.
RESULT
We showed that overexpression of SLUG decreased expression of PTEN tumor repressor in prostate cancer cell lines 22RV1 and DU145; conversely, knockdown of SLUG expression elevated PTEN expresson at both protein and RNA level in these cells. We demonstrated that SLUG overexpression inhibits PTEN promoter activity through the proximal promoter region in prostate cancer cells. By ChIP assay, we confirmed that SLUG directly binds to the PTEN promoter region covering the E-box sites. We also showed that Slug deficiency leads to an increased expression of PTEN in mouse embryo fibroblasts and prostate tissues. Importantly, we found that overexpression of SLUG increases drug resistance of DU145 prostate cancer cell line and knockdown of SLUG by shRNA sensitizes DU145 cell line to chemotherapeutic drugs. We further demonstrated that PTEN knockdown converts drug sensitivity of DU145 cells expressing SLUG shRNA to anticancer drugs.
CONCLUSION
We provide compelling evidence showing that PTEN is a direct functional target of SLUG. Our findings offer new insight in the regulation of the PTEN/AKT pathway and provide a molecular basis for potential targeted therapies of prostate cancer
Keywords: Slug, Prostate cancer, Cell growth, PTEN, Tumor suppressor
INTRODUCTION
Prostate cancer is the most common cancer in men and is predominantly a disease of elderly men, with more than 75% of new prostate cancer cases diagnosed in men older than 65 years. Prostate cancer is a heterogeneous disease and its development proceeds through a progression of defined steps, including prostate intraepithelial neoplasia (PIN), prostate in situ, evasion and metastasis. Many genes are involved in prostate cancer development. BRCA1 and BRCA2, two important genes for ovarian and breast cancers, have also been implicated in prostate cancers. The common tumor suppressor, p53, is also mutated in some primary prostate cancers and frequently mutated in metastatic cases.
PTEN (phosphatase and tension homology deleted on chromosome 10) is one of the most frequently mutated or deleted tumor suppressor genes in prostate cancers (1). PTEN mutations and/or deletions have been found in 30% of primary prostate cancers and up to 70% of metastatic prostate cancers (2,3). Therefore, PTEN alteration is strongly implicated in early stages of prostate cancer development. The role of PTEN in prostate cancer development has been confirmed using mouse models. Mice with prostate-specific deletion of PTEN recapitulate the following progression of human prostate cancers: initiation of prostate cancer with PIN, progression to invasive adenocarcinoma, and subsequent metastasis (4). The PTEN tumor suppressor is a lipid and protein phosphatase, and it dephosphorylates PIP3, a PI3K product. Loss-of-function of PTEN results in accumulation of PIP3 that activates signaling pathways, including PI3K/AKT. PI3K/AKT downregulates cell cycle inhibitors such as p21 and p27, which leads to inhibition of proapoptotic factors, including Bax, Bim, Bad, and activation of antiapoptotic factors such as Bcl-2 and XIAP (5). PTEN is also implicated in regulation of mouse and human prostate cancer stem cells (5–7). Mice with Pten deletion show expansion of prostatic stem/progenitor cell populations (7). In human prostate cancer cell lines, PTEN knockdown increases prostate cancer stem-like cell populations that are accompanied by an increase in sphere formation as well as increased clonogenic and tumorigenic potential (6).
SLUG is a highly evolutionarily conserved zinc-finger transcription factor. It belongs to the Slug/Snail superfamily whose members share the same SNAG domain and a similar zinc finger domain, which has the same binding motif (AGAGGTG). SLUG is implicated in many signaling pathways, including the normal development process of cancer formation (8), and radiation-induced apoptosis in hematopoietic stem and progenitor cells (9). SLUG is a key regulator of the epithelial-mesenchymal transition (EMT) of mesodermal cells (10). EMT is a critical process during morphogenesis of multi-cellular organisms and is not only a normal developmental process but is also essential for tumor invasion and metastasis. We recently showed that SLUG promotes prostate cancer cell migration and invasion via CXCR4/CXCL12 axis in vitro, but inhibits growth of prostate cancer cells (11). Therefore, SLUG-mediated migration and invasion in cancer cells is not dependent on cell proliferation.
According to previous studies, either upregulation of SLUG or loss of PTEN contributes to resistance to apoptosis, bone metastasis, and maintenance of cancer stem cells (5–7,12–15). Thus far, how SLUG and PTEN regulate molecular pathways important for drug resistance in prostate cancers remains to be elucidated. In this study, we dissected molecular mechanisms by which SLUG negatively regulates PTEN expression in both human prostate cancer cell lines and mouse prostate tissues. We demonstrated that SLUG confers drug resistance of prostate cancer cells partially through inhibition of PTEN expression. Our findings offer new insight in the regulation of the SLUG-PTEN pathway and provide a molecular basis for potential targeted therapies of prostate cancers.
MATERIALS AND METHODS
Cell lines and culture
DU145, 22RV1, and 293T cells were obtained from American Type Culture Collection (ATCC, Manassas, VA) and were maintained in culture medium, according to the manufacturer’s instructions. Primary MEFs from Slug+/+, Slug+/−, and Slug−/− mice (9,16) were derived from day 13.5 embryos and cultured in DMEM with 10% FBS.
Plasmids
pLKO.1-SLUG shRNA1-5 were obtained as a set (RHS4533-NM_003068) from Open Biosystem (Huntsville, AL). pLKO.1-control shRNA (containing non-target scramble shRNA) were purchased from Addgene (Cambridge, MA). PTEN (WT)-LUC was constructed by cloning the human PTEN promoter region (1 kb) into pGL3-basic vector (Promega, Madison, WI).
Virus production and infection
293T cells were seeded at 3×105 cells/per well in a 6-well plate. The next day, a mixture of plasmid DNA containing viral vector and packaging plasmids was co-transfected into 293T cells using Superfect transfection reagent (Qiagen, CA). For generation of lentiviruses, the packaging plasmids (pCMV-VSVG and psPAX2) were co-transfected with pLKO.1-based shRNA vector or pLKO.1-control shRNA into 293T cells. For viral infection, the cells were seeded at 50% confluence in 6-well plates; the next day, virus-containing supernatants from 293T cultures were mixed with polybrene at a final concentration of 4 mg/ml. The plate was centrifuged at 2,000 rpm for 1 hr at 35°C, and subsequently was returned to cell culture incubator. Cells infected with pLKO.1-based lentiviruses were selected with puromycin (1 μg/ml) at 48 hr after infection.
RNA Isolation, reverse transcription, RT-PCR, and qPCR analysis
RNA was extracted with RNeasy Plus mini kit (Qiagen, Valencia, CA). cDNA was synthesized by random priming from 1 μg of total RNA with the SuperScript III First-Strand Synthesis Super Mix kit (Invitrogen, CA), according to the manufacturer’s protocols. Primers for RT-PCR and qPCR analysis were synthesized by Integrated DNA Technologies (Coralville, IA). RT-PCR was performed using Hotstar Taq DNA polymerase kit (McLab, San Francisco, CA), and qPCR was performed by using the perfecta SYBR Green FastMix (Quanta Bioscience, CA), according to the manufacturer’s protocol. Data were analyzed by the comparative CT method, as described previously (17).
Western-blot analysis
Cells were lysed in protein lysis buffer (0.5% Triton X-100, 20 mM Tris, 100 mM NaCl, 1 mM EDTA, 1mM beta glycerophosphate, 1mM sodium orthovanadate), supplemented with 1 ml protease inhibiter cocktail (Sigma, St. Louis, MO). Protein samples were analyzed by Western-blot analysis using an ECL kit (Pierce, Rockford, IL) with antibodies against the following antigens: SLUG (ANASPEC, Fremont, CA), PTEN (Sigma, Aldrich, St. Louis, MO), AKT (Cell signaling, Boston, MA), pAKT-Ser 473 (Cell signaling, Boston, MA), GAPDH (Bethyl Laboratories, TX).
Luciferase reporter assay
Cell were transfected with Fugene 6 (Roche, Indianapolis, IN), according to the manufacturer’s instructions, and luciferase activity was measured by the luciferase assay kit (Promega, Madison, WI). pCMV-LacZ was included in all transfections, and β-galactosidase activity was used to normalize luciferase activity in each sample.
ChIP assay
ChIP assay was performed using the ChIP-IT active express chromatin immunoprecipitation kit (Active motif, CA). 22RV1 and DU145 cells were transfected with infected with pMIG-Slug-Flag plasmid using Superfect transfection reagent (Qiagen, CA). Anti-Flag tag antibody (Thermoscientific, IL) was used for immunoprecipitation of DNA sequences associated with SLUG. An anti-HA antibody (Bethly, TX) was used as a negative control. DNA released from precipitated complexes was amplified by PCR using specific primers spanning the SLUG binding sites in the PTEN promoter region.
Drug resistance analysis
Adriamycin (doxorubicin) was dissolved in distilled H2O. Cells were treated with adriamycin (0.2 μM) for 5 days, and total number of viable cells was counted. Each experiment was repeated at least three times.
Immunochemistry staining
Following primary antibodies were used for immunostaining: anti-PTEN Ab (Clone PTN-18, diluted 1:500, Sigma Aldrich, St. Louis, MO), anti-SLUG Ab (diluted 1:500, ANASPEC, Fremont, CA). Paraffin-embedded tissue sections (4-μm thick) were deparaffinized by using heat; antigen retrieval was done with 1x EDTA; and sections were blocked with TNB blocking buffer which includes 1M Tris base pH 7.6, 5M NaCl, and blocking Reagent (TSA kit, Pelkin Elmer, MA). Sections were then incubated with primary monoclonal antibodies (mAbs) against PTEN, SLUG, or negative IgG at 4°C overnight. Sections were then incubated with biotinylated goat anti-rabbit or goat anti-mouse IgG (The Jackson Laboratory, ME) secondary antibodies for 30 min. Sections were visualized with the substrate diaminobenzidine (DAB; Merck, Darmstadt, Germany) and counterstained with haematoxylin. Digital images were obtained using an inverted microscope and camera system (IX71 and Pro600ES-D; Olympus, Tokyo, Japan).
Statistical analysis
Data from qPCR analysis and cell growth assays was analyzed by the Student’s t-test (one-tailed). P < 0.05 was used to define statistically significant differences.
RESULTS
SLUG regulates PTEN expression in prostate cancer cell lines
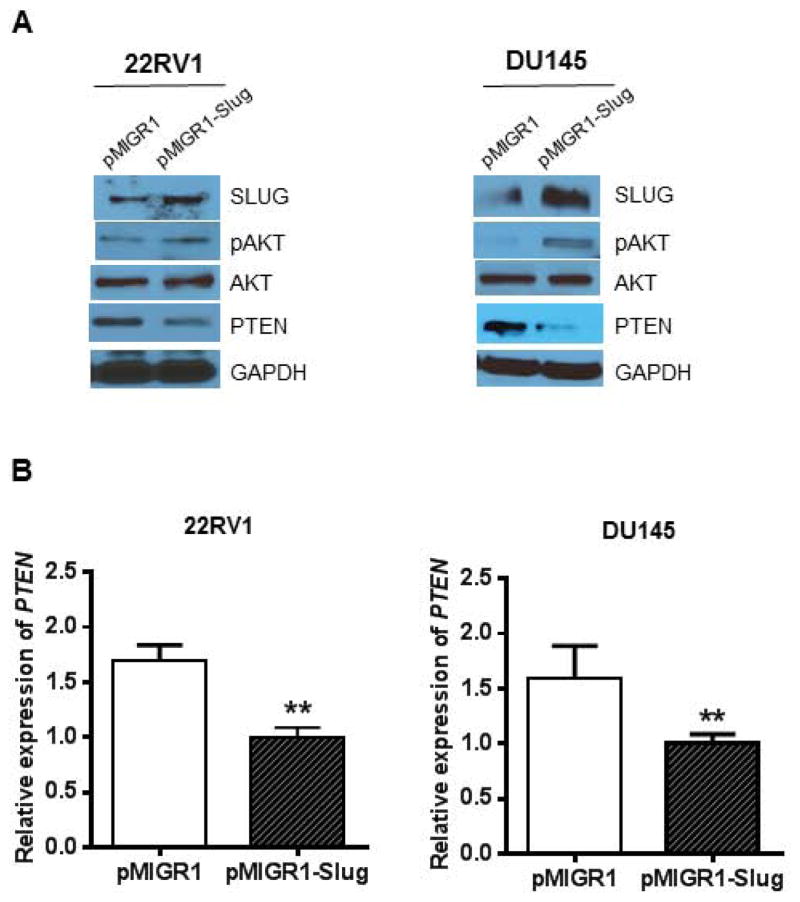
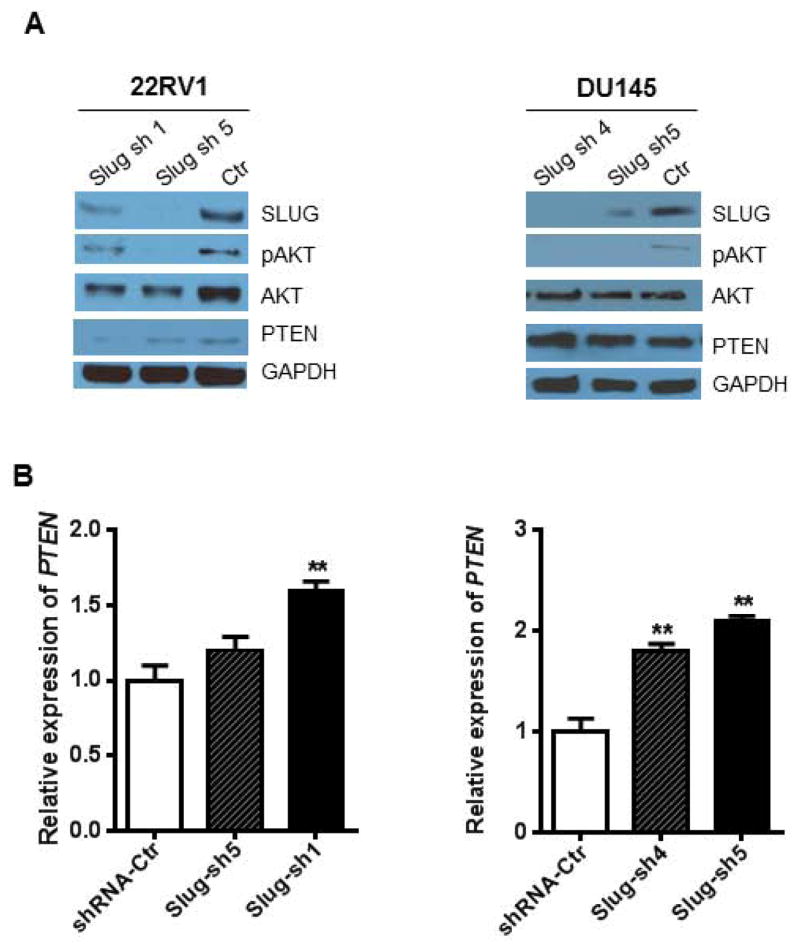
PTEN is a common tumor suppressor gene and has a very low expression in prostate cancer cell lines. In contrast, SLUG is an oncogene that is highly expressed in many tumors. Forced expression of SLUG (or Slug/Snail family members) or reduced expression of PTEN promotes tumorigenesis. Therefore, we are tempted to predict that the oncogenic function of SLUG is partially mediated through the inhibition of PTEN expression. To assess this possibility, we generated four stable cell lines by infecting 22RV1 and DU145 cells with retroviruses pMIGR1-Slug and pMIGR1, respectively. We examined expression of SLUG, PTEN, pAKT, AKT, and GAPDH in these stable cell lines. Our data showed that overexpression of SLUG downregulates PTEN expression and upregulates pAKT expression (Fig. 1A) in both 22RV1 and DU145 cells. By qPCR analysis we showed that SLUG overexpression also downregulates PTEN expression at RNA level (Fig. 1B) in these two cell lines. To assess effect of endogenous SLUG on expression of PTEN expression, we generated three stable cell lines by infecting 22RV1 and DU145 cells with lentiviruses expressing SLUG shRNA or control shRNA. Two of the stable lines expressed different shRNAs targeting SLUG and one was a control line carrying a control shRNA (Fig 2A). Western-blot analysis confirmed that SLUG expression was significantly reduced by corresponding shRNAs in 22RV1 (sh1 & sh5) and DU145 (sh4 & sh5) cell lines. Interestingly, PTEN expression was remarkably upregulated in 22RV1 and DU145 stable lines expressing SLUG-specific shRNAs. Considering SLUG is a zinc-finger transcription factor, we asked if SLUG could regulate PTEN at the transcriptional level. We quantified PTEN mRNA by qPCR analysis and found that SLUG knockdown increased expression level of PTEN transcripts in these stable cell lines (Fig. 2B). Together, these data indicate that SLUG negatively regulates PTEN expression in prostate cancer cell lines.
Figure 1. SLUG overexpression negatively regulates PTEN expression in human prostate cancer cell lines.
(A) Regulation of PTEN expression by SLUG in prostate cancer cells. 22RV1 (left panel) and DU145 (right panel) cells were infected with retroviruses expressing pMIGR1-Slug and pMIGR1 (control vector). Total protein was extracted from respective cell lines and analyzed by Western-blot analysis using anti-SLUG, anti-pAKT, anti-PTEN and anti-GAPDH (loading control) antibodies, respectively.
(B) qPCR analysis of PTEN transcripts in stable cell lines expressing pMIGR1-Slug and control vector pMIGR1. PTEN mRNA transcripts in these cell lines were analyzed by qPCR using primers specific for PTEN gene. ** p < 0.01.
Figure 2. Knockdown of Slug upregulates PTEN expression.
(A) Western-blot analysis of PTEN expression in prostate cancer cell lines stably expressing Slug shRNA. 22RV1 (left panel) and DU145 (right panel) cells were infected with lentiviruses expressing SLUG-specific shRNAs and control shRNA, and then selected with puromycin and expanded. Total protein was extracted from respective cell lines and analyzed by Western-blot analysis using anti-SLUG, anti-pAKT, anti-PTEN and anti-GAPDH (loading control) antibodies, respectively.
(B) qPCR analysis of PTEN transcripts in stable cell lines expressing SLUG-specific shRNAs and control shRNA. ** p < 0.01.
SLUG is a direct transcription repressor of PTEN promoter
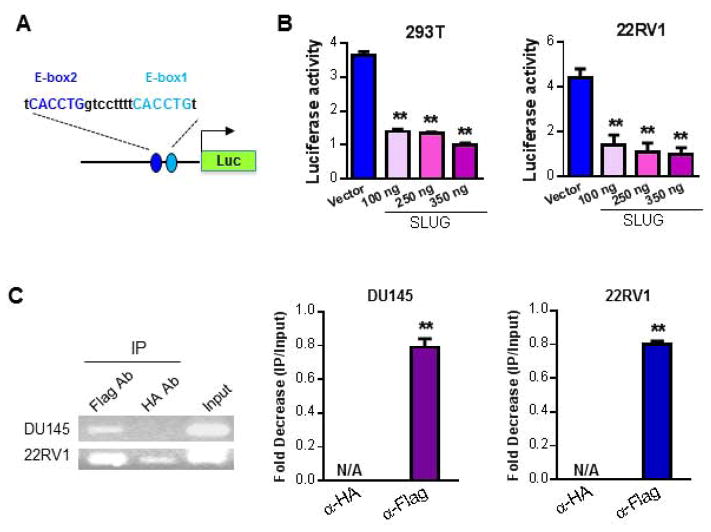
Our data show that SLUG negatively regulates PTEN expression at both protein and RNA level in 22RV1 and DU145 cell lines (Fig. 1 – 2). Therefore, we wonder whether SLUG acts as a transcriptional repressor of PTEN gene. By analyzing the DNA sequence of PTEN promoter, we identified two potential conserved SLUG binding sites (Fig. 3A). Thus, we cloned the DNA fragment covering the proximal promoter region of the PTEN gene into pGL3-basic vector to generate a luciferase reporter (Fig. 3A). Initially, we performed a luciferase reporter assay by transfecting PTEN-LUC into 293T and 22RV1 cells together with an increasing dose of SLUG expression plasmid. Our data showed that overexpression of SLUG inhibits PTEN promoter activity in a dose-dependent manner in 293T and 22RV1 cells (Fig. 3B). It has been demonstrated that SLUG acts as a transcriptional repressor when tethered to promoter (18). We previously also showed that SLUG directly downregulates Puma expression by binding to the E-box sites in the first intron of the Puma gene (9). According to our data (Fig. 1 – 2) showing that SLUG negatively regulated PTEN expression at protein and RNA level, we then hypothesized that SLUG regulates PTEN by direct binding to its promoter. To test this hypothesis, we performed ChIP assays in DU145 and 22RV1 cells that stably express SLUG with a Flag tag at its C-terminus. By PCR and qPCR analysis (Fig. 3C), we showed that anti-Flag antibody but not anti-HA antibody (negative control) immunoprecipitated the DNA sequences, containing the two E-box sites in PTEN promoter region in both 22RV1 and DU145 cells (Fig. 3C). Collectively, our data in Fig. 3 clearly demonstrated that PTEN is a direct transcriptional target of SLUG.
Figure 3. SLUG regulates PTEN promoter activity through direct interaction.
(A) Diagram of PTEN promoter-driven luciferase reporter. The luciferase reporter (PTEN-Luc) driven by human PTEN promoter (left panel) contains two E-box sites (E-box1 and E-box2).
(B) PTEN Overexpression of SLUG suppresses PTEN promoter activity. PTEN-Luc and an increasing dose of Slug expression vector was transfected together into 293T (left panel) and 22RV1 (right panel) cells. pCMV-LacZ was included into the transfections as a control. Data were normalized by pCMV-LacZ activity, and graphed as fold change. ** p < 0.01.
(C) ChIP assay for SLUG binding to PTEN promoter. Retroviruses expressing Flag-tagged SLUG (Slug-Flag) were infected into DU145 and 22RV1 cells. DNA sequences that are associated with SLUG were precipitated with anti-Flag antibody. Anti-HA antibody was included as a negative control. Using specific primers, the precipitated DNAs containing the PTEN promoter were then detected by PCR (left panel) and qPCR (right panel). ** p < 0.01.
SLUG is a negative regulator of PTEN in vivo
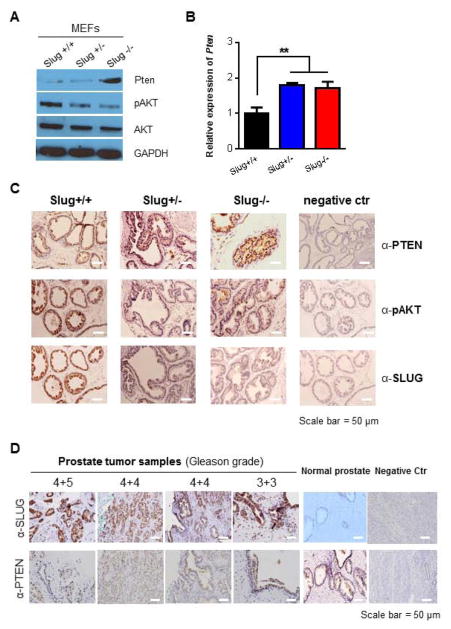
To further support the notion that SLUG is an essential regulator of PTEN and to determine if endogenous Slug regulates Pten expression, we initially examined the effects of endogenous Slug on PTEN expression in mouse embryo fibroblasts (MEFs) derived from Slug knockout mice (16). Our data showed that Pten expression was significantly higher in Slug−/− MEFs than Slug+/− and Slug+/+ MEFs (Fig. 4A). We also quantified Pten transcripts by qPCR and found that Pten expression at RNA level was higher in Slug−/− MEFs than in Slug+/− and Slug+/+ MEFs (Fig. 4B). Next, we asked if Slug regulates Pten expression in vivo. To address this question, we examined Pten in prostate tissue sections from Slug+/+, Slug+/−, and Slug−/− mice by immunohistochemistry staining (Fig. 4C). Our data showed that PTEN expression was more evident in prostate tissue from Slug−/− mouse than Slug+/− and Slug−/− mice. We also included negative control stains for each of the antibodies to exclude nonspecific staining. Based on these data, we then examined expression of SLUG and PTEN in human prostate tumor samples (Fig. 4D). Interestingly, we found that SLUG is highly expressed in different stages of these tumor samples, when compared with normal prostate tissues. In contrast, PTEN expression is lower in prostate tumor samples than normal prostate tissues. Collectively, these data strongly support the concept that SLUG is a key negative regulator of PTEN both in vitro and in vivo.
Figure 4. PTEN expression is regulated by endogenous SLUG in vivo.
(A) Western-blot analysis of AKT and PTEN expression in MEFs. Total protein was extracted from Slug+/+, Slug+/−, and Slug−/− MEFs (passage 1), and analyzed by Western-blot analysis using anti-pAKT, anti-PTEN, anti-AKT and anti-GAPDH (loading control) antibodies, respectively.
(B) qPCR analysis of PTEN transcripts in MEFs. Total RNA was extracted from the indicated MEFs and synthesized into cDNAs. Using specific primers, qPCR was used to analyze PTEN transcripts. ** p < 0.01.
(C) Expression of PTEN in prostate tissue in Slug-deficient mice. Expression of PTEN and SLUG in prostate tissues of Slug+/+, Slug+/−, and Slug−/− mice (2-month-old) was determined by immunohistochemistry using anti-PTEN, anti-pAKT, anti-SLUG antibodies, and counterstained with hematoxylin. Scale bar = 50 μm
(D) PTEN loss is related with endogenous SLUG expression in human prostate cancer tissue samples. Expression of SLUG and PTEN was detected in prostate cancer tissue samples by immunohistochemistry using anti-SLUG and anti-PTEN antibodies, and counterstained with hematoxylin. Scale bar = 50 μm.
SLUG confers chemotherapeutic drug resistance of prostate cancer cells through PTEN tumor suppressor
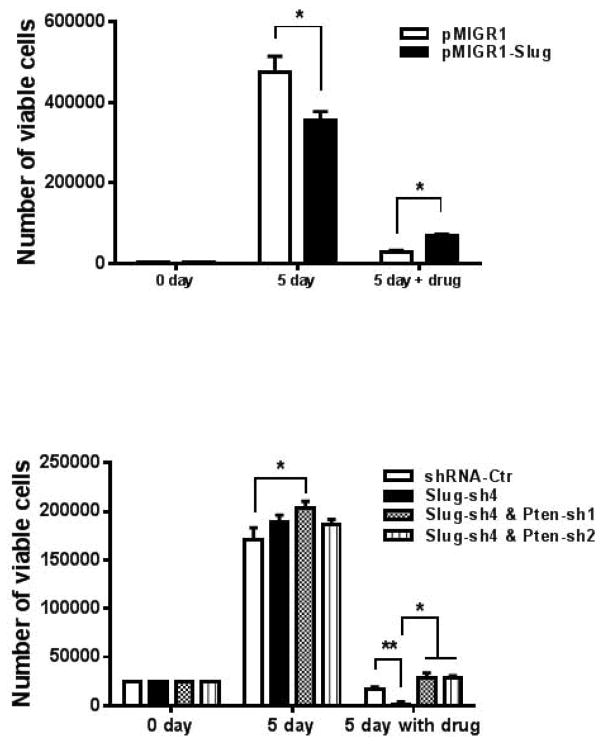
It has been shown that ectopic expression of SLUG increases drug resistance in cancers (19–21) and suppression of PTEN expression increases drug resistance in various cancers (22–25). According to our current data (Fig. 1 – 4), PTEN is a direct target gene of SLUG. Therefore, we decided to explore the possibility that SLUG regulates drug resistance of prostate cancers through PTEN. To this end, we generated DU145 cell line stably expressing SLUG by retroviral infection (Fig. 5A). We treated these two stable cell lines with or without Adriamycin (0.2 μM) for 5 days. Our data showed that the DU145 cell line-overexpressing SLUG grows slower then the control group, which is consistent with our previous finding that overexpression of SLUG inhibits cell growth of prostate cancer cells (11). Five days after treatment with Adriamycin, total number of viable cells in both of the two stable lines decreased significantly. But, DU145 cell line with stable expression of SLUG has a higher cell number than the control group (Fig. 5A). This data suggest that SLUG increases chemotherapeutic drug resistance of prostate cancer cells. Next, we examined effect of endogenous SLUG and PTEN on the resistance of prostate cancer cells to chemotherapeutic drugs. To do so, we established four DU145 stable cell lines expressing control shRNA, SLUG shRNA, or both SLUG shRNA and PTEN shRNA (Fig. 5B). We seeded the same number of cells from each of the four stable lines, and then treated these cells with or without Adriamycin (0.2 μM) for 5 days. We found that all the four stable cell lines expand seven times and have a comparable cell number after five days of cell culture, although DU145 cells expressing SLUG shRNA grow slightly faster. When cultured in medium with Adriamycin, cell number of the group expressing control shRNA remained almost the same. Notably, viable cell number of the cell group expressing SLUG shRNA (Slug-sh4) dramatically decreased after treatment with Adriamycin for 5 days. Interestingly, cell number of DU145 cells expressing both SLUG shRNA and PTEN shRNA is ten-times higher than those expressing SLUG shRNA and is also higher then the control shRNA-expressing DU145 cells (Fig. 5B). These data indicate that reduced expression of SLUG sensitizes DU145 cells to Adriamycin and knockdown of PTEN rescues drug sensitivity of the SLUG shRNA-expressing DU145 cells. Collectively, our data suggest that SLUG controls drug resistance of prostate cancer cells through its regulation of PTEN.
Figure 5. Opposing effect of SLUG and PTEN on drug resistance of prostate cancer cells.
(A) Cell growth assay of DU145 cells with SLUG overexpression after treatment with Adriamycin. DU145 cells expressing pMIGR1-Slug or pMIGR1 (vector control) were treated with or without 0.2 μM of Adriamycin for 5 days. Number of viable cells was determined by trypan blue exclusion cell viability assay. *p < 0.05.
(B) Cell growth assay of DU145 cells stably expressing compound SLUG and PTEN shRNA. DU145 cell lines stably expressing SLUG shRNA or control shRNA with or without PTEN shRNA were treated with 0.2 μM Adriamycin for 5 days, and then number of viable cells was determined. *p < 0.05, ** p < 0.01.
Discussion
The PTEN/AKT pathway is involved in initiation and development of many types of cancers, including prostate cancers (6,24,26–28). This pathway also plays important roles in maintenance of normal adult stem cells and prostate cancer stem cells (5,6,29,30). Many studies have focused on roles of PTEN/AKT pathway in different aspects of biological processes, but transcription factors that directly regulate PTEN expression remain to be elucidated.
It was shown that p53 upregulates expression of PTEN through direct or indirect mechanisms (31). Egr-1 is another zinc finger transcription factor that directly upregulates PTEN expression through its binding site in the PTEN promoter (32). Egr-1 is required for induction of PTEN expression following irradiation (33). Unlike p53 and Egr-1, two positive regulators of PTEN, SLUG negatively regulates PTEN expression by binding directly to the two E-boxes in its promoter region. Because p53 and Egr-1 are DNA damage responsive transcription factors, it will be interesting to study how Slug, p53, and Egr-1 co-regulate DNA response pathways in prostate or other cancer cells. Recently, Maria at al. reported that Snail1 but not Slug represses the PTEN promoter during γ-irradiation in Madin-Darby canine kidney (MDCK) cells (34), which is contradictory with our findings in this study. The discrepancy between the two studies may be due to different cell lines used in these studies.
In addition to prostate cancer cell lines, we showed that SLUG also functioned as a transcription repressor of PTEN gene in primary MEFs and suppresses PTEN expression in prostate tissues. We showed that PTEN expression was elevated in MEFs and prostate tissues in Slug-deficient mice. Because SLUG can directly bind to the PTEN promoter and represses its transcriptional activity, we thus concluded that PTEN is a direct target of SLUG both in vitro and in vivo. It is conceivable that negative regulation of PTEN play an important role in prostate cancer development, at least for those cancers without PTEN mutation and/or deletion.
In agreement with this notion, our data showed that SLUG knockdown increases drug sensitivity of prostate cancer cells, and the addition of PTEN knockdown converts cells from “drug sensitive” to “drug resistant”. Although we did not examine the effects of AKT on drug sensitivity of prostate cancer cells with SLUG knockdown, we predict that overexpression of constitutively active AKT also have similar effects with PTEN knockdown on prostate cancer cells with SLUG knockdown. Thus, our data suggest that treatment with combination of AKT inhibitors and SLUG inhibitor (currently not available) might provide an effective therapeutic approach. Although we provided strong evidence to show that SLUG is a direct transcriptional repressor of PTEN tumor suppressor gene, it will be tempting in the future to explore (i) whether SLUG not only negatively regulates PTEN expression, but also is reciprocally regulated by PTEN, and (ii) whether SLUG promotes prostate tumor metastasis, at least partially, through the inhibition of PTEN expression in vivo.
Conclusion
We demonstrated that SLUG acts a transcriptional repressor of PTEN tumor suppressor both in vitro and in vivo. We further showed that SLUG and PTEN affect sensitivity of prostate cancer cells to chemotherapeutic drugs in an opposing manner. Our findings suggest that PTEN is a direct functional target of SLUG.
Acknowledgments
Financial Support: This study was supported in part by NIH/NIA and NIDDK grants (R01AG040182 and R01DK090478). J.L. was supported by a Scholarship (2008610038) from the China Scholarship Council. BU was supported by a MMCRI PhD student fund.
We thank members of the Wu laboratory for their contributions to valuable discussion. This work was supported in part by NIH/NIA and NIDDK grants (R01AG040182 and R01DK090478). BU was supported by a MMCRI PhD student fund.
Footnotes
Disclosure of Potential Conflicts of Interest: There are no potential conflicts of interest.
References
- 1.Deocampo ND, Huang H, Tindall DJ. The role of PTEN in the progression and survival of prostate cancer. Minerva Endocrinol. 2003;28(2):145–153. [PubMed] [Google Scholar]
- 2.Dahia PL. PTEN, a unique tumor suppressor gene. Endocr Relat Cancer. 2000;7(2):115–129. doi: 10.1677/erc.0.0070115. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, Isaacs WB, Bova GS. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998;58(2):204–209. [PubMed] [Google Scholar]
- 4.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4(3):209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 5.Hill R, Wu H. PTEN, stem cells, and cancer stem cells. J Biol Chem. 2009;284(18):11755–11759. doi: 10.1074/jbc.R800071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubrovska A, Kim S, Salamone RJ, Walker JR, Maira SM, Garcia-Echeverria C, Schultz PG, Reddy VA. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A. 2009;106(1):268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S, Garcia AJ, Wu M, Lawson DA, Witte ON, Wu H. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci U S A. 2006;103(5):1480–1485. doi: 10.1073/pnas.0510652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemavathy K, Ashraf SI, Ip YT. Snail/slug family of repressors: slowly going into the fast lane of development and cancer. Gene. 2000;257(1):1–12. doi: 10.1016/s0378-1119(00)00371-1. [DOI] [PubMed] [Google Scholar]
- 9.Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123(4):641–653. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 10.Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137(6):1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uygur B, Wu WS. SLUG promotes prostate cancer cell migration and invasion via CXCR4/CXCL12 axis. Mol Cancer. 10:139. doi: 10.1186/1476-4598-10-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camp ER, Findlay VJ, Vaena SG, Walsh J, Lewin DN, Turner DP, Watson DK. Slug expression enhances tumor formation in a noninvasive rectal cancer model. J Surg Res. 170(1):56–63. doi: 10.1016/j.jss.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Mancera PA, Gonzalez-Herrero I, Perez-Caro M, Gutierrez-Cianca N, Flores T, Gutierrez-Adan A, Pintado B, Sanchez-Martin M, Sanchez-Garcia I. SLUG in cancer development. Oncogene. 2005;24(19):3073–3082. doi: 10.1038/sj.onc.1208505. [DOI] [PubMed] [Google Scholar]
- 14.Emadi Baygi M, Soheili ZS, Essmann F, Deezagi A, Engers R, Goering W, Schulz WA. Slug/SNAI2 regulates cell proliferation and invasiveness of metastatic prostate cancer cell lines. Tumour Biol. 31(4):297–307. doi: 10.1007/s13277-010-0037-5. [DOI] [PubMed] [Google Scholar]
- 15.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27(9):2059–2068. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 16.Inoue A, Seidel MG, Wu W, Kamizono S, Ferrando AA, Bronson RT, Iwasaki H, Akashi K, Morimoto A, Hitzler JK, Pestina TI, Jackson CW, Tanaka R, Chong MJ, McKinnon PJ, Inukai T, Grosveld GC, Look AT. Slug, a highly conserved zinc finger transcriptional repressor, protects hematopoietic progenitor cells from radiation-induced apoptosis in vivo. Cancer Cell. 2002;2(4):279–288. doi: 10.1016/s1535-6108(02)00155-1. [DOI] [PubMed] [Google Scholar]
- 17.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 18.Kajita M, McClinic KN, Wade PA. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol. 2004;24(17):7559–7566. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haslehurst AM, Koti M, Dharsee M, Nuin P, Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, Davey S, Squire J, Park PC, Feilotter H. EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer. 12:91. doi: 10.1186/1471-2407-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang TH, Tsai MF, Su KY, Wu SG, Huang CP, Yu SL, Yu YL, Lan CC, Yang CH, Lin SB, Wu CP, Shih JY, Yang PC. Slug confers resistance to the epidermal growth factor receptor tyrosine kinase inhibitor. Am J Respir Crit Care Med. 2011;183(8):1071–1079. doi: 10.1164/rccm.201009-1440OC. [DOI] [PubMed] [Google Scholar]
- 21.Mancini M, Petta S, Iacobucci I, Salvestrini V, Barbieri E, Santucci MA. Zinc-finger transcription factor slug contributes to the survival advantage of chronic myeloid leukemia cells. Cell Signal. 2010;22(8):1247–1253. doi: 10.1016/j.cellsig.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Wu HJ, Wu HT, Weng DH, Xing H, Lu YP, Ma D. Reversal of drug resistance in human ovarian cancer cells by wild-type PTEN gene and its mechanisms. Zhonghua Fu Chan Ke Za Zhi. 2007;42(9):612–616. [PubMed] [Google Scholar]
- 23.Steelman LS, Navolanic PM, Sokolosky ML, Taylor JR, Lehmann BD, Chappell WH, Abrams SL, Wong EW, Stadelman KM, Terrian DM, Leslie NR, Martelli AM, Stivala F, Libra M, Franklin RA, McCubrey JA. Suppression of PTEN function increases breast cancer chemotherapeutic drug resistance while conferring sensitivity to mTOR inhibitors. Oncogene. 2008;27(29):4086–4095. doi: 10.1038/onc.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D’Assoro AB, Salisbury JL, Mazzarino MC, Stivala F, Libra M. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Lake F. Outsmarting glioblastoma: phosphorylation of PTEN may be a marker of drug resistance. Biomark Med. 2012;6(5):638. [PubMed] [Google Scholar]
- 26.Bouali S, Chretien AS, Ramacci C, Rouyer M, Becuwe P, Merlin JL. PTEN expression controls cellular response to cetuximab by mediating PI3K/AKT and RAS/RAF/MAPK downstream signaling in KRAS wild-type, hormone refractory prostate cancer cells. Oncol Rep. 2009;21(3):731–735. [PubMed] [Google Scholar]
- 27.Ohigashi T, Mizuno R, Nakashima J, Marumo K, Murai M. Inhibition of Wnt signaling downregulates Akt activity and induces chemosensitivity in PTEN-mutated prostate cancer cells. Prostate. 2005;62(1):61–68. doi: 10.1002/pros.20117. [DOI] [PubMed] [Google Scholar]
- 28.Van de Sande T, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Role of the phosphatidylinositol 3’-kinase/PTEN/Akt kinase pathway in the overexpression of fatty acid synthase in LNCaP prostate cancer cells. Cancer Res. 2002;62(3):642–646. [PubMed] [Google Scholar]
- 29.Korsten H, Ziel-van der Made A, Ma X, van der Kwast T, Trapman J. Accumulating progenitor cells in the luminal epithelial cell layer are candidate tumor initiating cells in a Pten knockout mouse prostate cancer model. PLoS One. 2009;4(5):e5662. doi: 10.1371/journal.pone.0005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawson DA, Xin L, Lukacs R, Xu Q, Cheng D, Witte ON. Prostate stem cells and prostate cancer. Cold Spring Harb Symp Quant Biol. 2005;70:187–196. doi: 10.1101/sqb.2005.70.003. [DOI] [PubMed] [Google Scholar]
- 31.Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW. Regulation of PTEN transcription by p53. Mol Cell. 2001;8(2):317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 32.Baron V, Adamson ED, Calogero A, Ragona G, Mercola D. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther. 2006;13(2):115–124. doi: 10.1038/sj.cgt.7700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virolle T, Adamson ED, Baron V, Birle D, Mercola D, Mustelin T, de Belle I. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat Cell Biol. 2001;3(12):1124–1128. doi: 10.1038/ncb1201-1124. [DOI] [PubMed] [Google Scholar]
- 34.Escriva M, Peiro S, Herranz N, Villagrasa P, Dave N, Montserrat-Sentis B, Murray SA, Franci C, Gridley T, Virtanen I, Garcia de Herreros A. Repression of PTEN phosphatase by Snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol Cell Biol. 2008;28(5):1528–1540. doi: 10.1128/MCB.02061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]