Abstract
Objectives
Calcium independent group VIA phospholipase A2 (iPLA2β) and Matrix Metalloproteinase-9 (MMP-9) are upregulated in many disease states; their involvement with cancer cell migration has been a recent subject for study. Further, the molecular mechanisms mediating nicotine-induced breast cancer cell progression have not been fully investigated. This study aims to investigate whether iPLA2β mediates nicotine-induced breast cancer cell proliferation and migration through both in-vitro and in-vivo techniques. Subsequently, the ability of Bromoenol Lactone (BEL) to attenuate the severity of nicotine-induced breast cancer was examined.
Method and Results
We found that BEL significantly attenuated both basal and nicotine-induced 4T1 breast cancer cell proliferation, via an MTT proliferation assay. Breast cancer cell migration was examined by both a scratch and transwell assay, in which, BEL was found to significantly decrease both basal and nicotine-induced migration. Additionally, nicotine-induced MMP-9 expression was found to be mediated in an iPLA2β dependent manner. These results suggest that iPLA2β plays a critical role in mediating both basal and nicotine-induced breast cancer cell proliferation and migration in-vitro. In an in-vivo mouse breast cancer model, BEL treatment was found to significantly reduce both basal (p<0.05) and nicotine-induced tumor growth (p<0.01). Immunohistochemical analysis showed BEL decreased nicotine-induced MMP-9, HIF-1alpha, and CD31 tumor tissue expression. Subsequently, BEL was observed to reduce nicotine-induced lung metastasis.
Conclusion
The present study indicates that nicotine-induced migration is mediated by MMP-9 production in an iPLA2β dependent manner. Our data suggests that BEL is a possible chemotherapeutic agent as it was found to reduce both nicotine-induced breast cancer tumor growth and lung metastasis.
Introduction
According to the American Cancer Society, Cancer Facts & Figures 2015, there are an estimated 1,658,370 new cancer cases diagnosed in the United States, of which 231,840 are attributed to breast cancer with 40,290 deaths. Breast carcinomas are at the forefront of cancer cases among women (41%) followed by uterine corpus (8%), and colon and rectum cancer (8%) [1]. Additionally, in the United States breast cancer afflicts 1 in 8 women during their lifetime; this high prevalence provides evidence for the need to foster therapeutic interventional research [2].
Of utmost concern is the malignant characteristics of breast cancer, in which, the major cause of mortality in breast cancer patients is metastasis to distant sites rather than the growth of the primary tumor [3]. 10–15% of breast cancer patients develop distant metastases within 3 years after the identification of a primary tumor, and remain at risk for metastatic disease during their entire lifespan [4]. Metastasis is a multi-step process involving local tumor cell invasion, intravasation, survival in the vasculature, extravasation, and colonization at a distant secondary site; all of which are consequently reliant on mechanisms of cell migration and movement [5].
A major risk factor for the development and exacerbation of breast cancer metastasis is cigarette smoke [6,7]. Smoke exposure has been found to increase anchorage-independent growth, motility, and invasiveness of breast cancer cells leading to higher rates of cell survival, colonization [8] and metastasis. Moreover, nicotine a known component in cigarette smoke has been shown to promote cancer development, cancer cell migration, and more recently apoptotic resistance in breast cancer cells [9,10,11]. Additionally, several epidemiological studies have indicated a strong link between smoke exposure and an increased risk of breast cancer [12,13,14]. Specifically, cigarette smoke was found to significantly increase breast cancer mortality by 60% and the probability of recurrence by 41% [15]. Supporting this association is the presence of tobacco carcinogens in the breast tissue of smokers [16]. In particular, nicotine is a known toxicant found in smoke that is linked to breast cancer apoptosis resistance, tumor growth, and migration, along with, facilitating angiogenic metastasis [9,17,18,19,20]. The exact cellular mechanisms promoting nicotine-induced breast cancer cell proliferation and migration remain undetermined.
In recent years, the enzyme iPLA2β (calcium-independent phospholipase A2 Beta) has emerged as an important cell membrane regulator and a contributor to various types of cancers including: survival signaling in prostate cancer, along with, proliferation, migration and tumorigenicity in ovarian cancer [21,22,23,24]. iPLA2β is a member of the PLA2 superfamily and is found both in the cytosol and bound to membranes; upon activation it translocates to the membrane in a calcium-independent process [25]. The other PLA2 family members are secretory PLA2 (sPLA2) and cytosolic (cPLA2), which are characteristically different based on their cellular locations and their calcium requirement for enzymatic activation. The primary catalytic function of iPLA2β is to cleave glycerophospholipids at the sn-2 position to release free fatty acids including arachidonic acid and 2-lysophospholipid [26]. Early on because of its catalytic activity, iPLA2β was classified as a housekeeping protein solely responsible for dynamically regulating membrane homeostasis/remodeling by altering the phospholipid composition of the membrane [27,28]. Currently, studies have now implicated iPLA2β as an important signaling molecule mediating various cellular functions and the progression of many disease states including hypertension, diabetes, alzheimer’s, and aneurysm formation [29,30,31,32]. Sequentially, iPLA2β has been found to regulate numerous membrane functions including vacuole formation, membrane transport, surface adhesion, cellular spreading, and proliferation [33,34].
Current research indicates Bromoenol Lactone (BEL), a suicide inhibitor of iPLA2β attenuates cigarette smoke induced-breast cancer cell mobility [7]. However, whether iPLA2β is involved in breast cancer tumor growth and metastasis remain unreported. BEL, irreversibly inhibits the enzymatic activity of iPLA2β by covalently binding to the lipase motif, rendering it inactive. The sPLA2s and cPLA2α isoforms, contain an enzymatic lipase domain similar to iPLA2; however, BEL has been shown to specifically inhibit iPLA2β [35]. Additionally, BEL has been indicated to have a 1000-fold more specificity towards iPLA2 than cPLA2 [36].
Our in-vitro and in-vivo studies introduce the novel idea that nicotine from cigarette smoke could enhance iPLA2β expression in breast cancer cells leading to enhanced tumor growth, along with, migration and metastatic ability. Here we present the effects of nicotine on cell proliferation and mobility in the 4T1 breast cancer cell line. The 4T1 is a mouse stage IV breast cancer cell line which is transplantable, highly tumorigenic and invasive, and has been characterized to spontaneously metastasize from the primary tumor in the mammary gland to multiple distant sites, imitating human clinical disease [37]. Thus far, the involvement of iPLA2β in mediating nicotine-induced breast cancer tumor growth and metastasis remains relatively unstudied and the exact signaling mechanisms regulated by iPLA2β in breast cancer could provide a critically needed new target for therapeutic intervention.
Methods
Cell Culture
The 4T1 mouse mammary tumor cell line (original commercially obtained from ATCC) was a gracious gift from Dr. Shu Liu and Dr. Kai Su from and stably transfected with GFP (Green Fluorescent Protein). The 4T1 cell line was cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% (vol/vol) Fetal Bovine Serum, 100U/ml penicillin, and 100μg/ml streptomycin. Cultures were maintained in a 37°C tissue culture incubator with a humidified atmosphere of 95% air and 5% CO2.
Animals
Female BALB/c mice, 10 weeks old, were purchased from Jackson Laboratory (BarHarbor, ME). The animals were allowed to acclimate for 1 week before experimentation and were maintained on a 12 hr light and dark cycle, and fed standard rodent chow (Prolab ISOPRO RMH 3000 Irradiated Lab Diet; Purina Mills International). Animal protocols were approved by the committee on animal research care and use at Eastern Kentucky University.
Nicotine Treatment In-Vivo and In-Vitro
An average cigarette contains 10-14mg of nicotine and approximately 1–1.5mg of nicotine is absorbed systemically during smoking [38]. Daily exposure to nicotine in cigarette smokers was found to range from 14.9 to 35.4 mg per day [39]. With rapid absorption and distribution in the body once the smoke reaches the alveolar lining, average human smokers have a peak plasma nicotine levels of 10–50 mg/ml (~60–310 nM). Thus, the nicotine levels of (5mg/kg/day) used in our study are well within the range of the plasma of human smokers. For comparison, mice treated with nicotine 13mg/kg/day were found to have plasma nicotine levels of ~49ng/ml or ~300nM [40]. Numerous studies have used a similar range of nicotine in-vivo treatment in mouse models [41,42,43,44]. Subsequently, human autopsy samples from human smokers indicated a high affinity for nicotine in body tissues in which skeletal muscle was found to have comparable nicotine levels to whole blood and distribution to the liver, kidney, spleen and lung where found to be high [38]. Additionally, nicotine is found to accumulate in breast milk of female smokers [12,38]. Moreover, for the in-vitro experiments 10μM concentration of nicotine was added to the cell cultures, similar to other studies [41,45].
In Vivo Xenograft Model of Breast Cancer and Drug Delivery
4T1-GFP cells (1X105) were suspended in 100μl of DMEM not supplemented with FBS and injected into the right second mammary fat pad of female BALB/c mice as previously described in literature [37]. Mini-osmotic pumps (Alzet model 2004, 28-day release, Alza Co., Palo Alto,CA) containing either Nicotine (Sigma-Aldrich [5mg/kg/day]) or Saline (50% DMSO) were subcutaneously implanted on the right flank via an incision in the scapular region. During the procedure the mice were anesthetized by inhalation of isoflurane mixed with O2 (3–5% isoflurane/97% O2) and maintained by inhalation of isoflurane missed with O2 (1–2% isoflurane/97% O2) throughout the procedure using a Drager 19.1 model isoflurane machine (Highland Medical Equipment). Daily administration of Bromoenol Lactone (BEL), [Cayman Chemical Company, lot 70700; (10ug/g/day)] or saline was delivered by intraperitoneal injection throughout the experiment. Treatment administration and scheduling during tumor growth was concurrent with previous studies [46,47,48].
Tumor growth was monitored daily and tumor volumes (mm3) were calculated using the formula: (width)2 × length/2, where width is the smaller of the two measurements. At 2 weeks the mice were sacrificed and tumor volume and weight were measured. Isolated tissues were either formalin fixed or mounted in Optimal Cutting Temperature compound (OCT; Tissue-Tek, Torrance, CA).
Histological and Immunohistochemical Staining
Additionally, paraffin-embedded sections (5μm thick) were deparaffinized using xylene, rehydrated, and immunohistochemistry conducted as previously described [49]. Slides were incubated with the following primary antibodies overnight at 4°C antibodies iPLA2β (1:100, gracious gift from Dr. Guo University of Kentucky, MMP-9 (1:500, Cell Signaling), MMP-2 (1:500, IHC World), GFP (1:500, Cell Signaling). Followed by counterstaining with hematoxylin and mounted on slides with paramount. Additionally, lung and liver sections were stained with Hematoxylin & Eosin (Surgipath). Representative images were taken under 20 to 40x magnification by Olympus IX70 microscope equipped with Olympus DP70 digital camera. Lung tumor area was analyzed using an Olympus digital camera with Olympus MicroSuit-B3 Software
Tissue mounted in Optimal Cutting Temperature compound (OCT; Tissue-Tek, Torrance, CA) was sectioned (5-μm), incubated in 4% paraformaldehyde, and blocked with normal goat serum (Vectastain ABC kit). Slides were incubated with CD31 antibody (1:200,Abcam) and counterstained with DAPI and mounted with glycerol gelatin (Sigma). Images were taken with an Olympus IX70 microscope equipped with Olympus DP70 digital camera.
Cell Proliferation
To determine the lowest nicotine concentration to significantly induce cell proliferation a cell count assay was conducted. Cells were seeded 1x104 in 6 well plates in media containing 10%FBS and treated with DMSO (control) or Nicotine (1uM, 5μM, or 10μM). Specifically, S-BEL (Cayman Chemical Company, 10006801) was used for all in-vitro experiments. Cells were allowed to expand for 24, 48, and 72 hours. To assess cell proliferation, the cells were trypsinized, suspended with typhan blue, and viable cells counted using a hemocytometer.
To assess the ability of BEL to attenuate nicotine-induced proliferation a MTT Assay was utilized. Cells were seeded 500 cells/100μl in a 96-well plate. The wells were treated with DMSO (control), 10μm Nicotine, 3μm BEL or both BEL and Nicotine; with retreatment after 24 hours. After 48 hours, cells were incubated at 37°C for 4 hours in 10μl MTT Solution obtained from Vbrant MTT Cell proliferation assay kit (Life Technologies). Cells were solubilized and mixed with SDS (sodium dodecyl sulfate). And the absorbance read at 595 nm on a Phenix Genios Tecon 96 well plate reader.
Cell Migration
Cell migration was examined using a scratch/wound healing assay and a transwell assay. For the scratch assay, cells were cultured in a 6-well plate until a confluent monolayer was formed. A 20μl pipet tip was used to scratch the wells. The wells were sequentially rinsed with PBS and cultured in DMEM supplemented with 10% FBS. The wells were treated with DMSO (control), 10μm Nicotine, 3μm BEL or both BEL and Nicotine. Four representative 10x images were taken at 0, 6, and 24 hours and the gap width was quantified using an average of three leading edge measurements for each image.
To further cell migration a transwell assay was conducted. For the transwell assay, 1X105 cells were treated with 3μm BEL (30 minutes pretreatment), 10μm Nicotine, both BEL and Nicotine, or DMSO (control). The cells were seeded in the upper insert (8-μm pore; corning) using DMEM containing 0.1% FBS and the fitted culture dishes contained 10% FBS. After 4 hours the chambers were removed from the plates, fixed, and the migrated cells were DAPI stained. For each well four representative 20x images were quantified.
Gel Zymography
To identify which MMP (matrix metalloproteinase) is the major player secreted by the Nicotine stimulation, a serial of parallel gel zymography experiments were performed. The gelatin zymography experiments were conducted without serum in the culture medium; all the treatments occurred 24 hours after starvation. There were no MMPs in the culture medium. The cells (1x106) were treated as described above for 24 hours and 10μl of culture medium was tested. The samples were ran with native Tris-Glycine containing 0.15% gelatin, incubated with reaction buffer, and stained with GelCode Blue Phalloidin (Life Technologies, Grand Island, NY). The bands were scanned and band density analyzed with ImageJ software.
In situ Zymography
To investigate nicotine-induced MMP-9 production 4T1 cells were seeded (1X104) on cover slides, serum starved for 24 hours then treated for 24 hours as mentioned above. The procedure was conducted as previously described [49] with cells incubated with MMP-9 (1:500, Cell Signaling) and counterstained by DAPI.
Statistics
Data were pooled prior to analyses. Data are illustrated as mean ± SEM and statistical analyses were carried out using GraphPad, Prism 6 (San Diego, CA). T-tests along with one- and two-way ANOVAs were used where appropriate. For study data used in the statistical analysis and figures refer to S1 Table.
Results
BEL Attenuates Nicotine-Induced Proliferation and Migration of Cultured Breast Cancer Cells
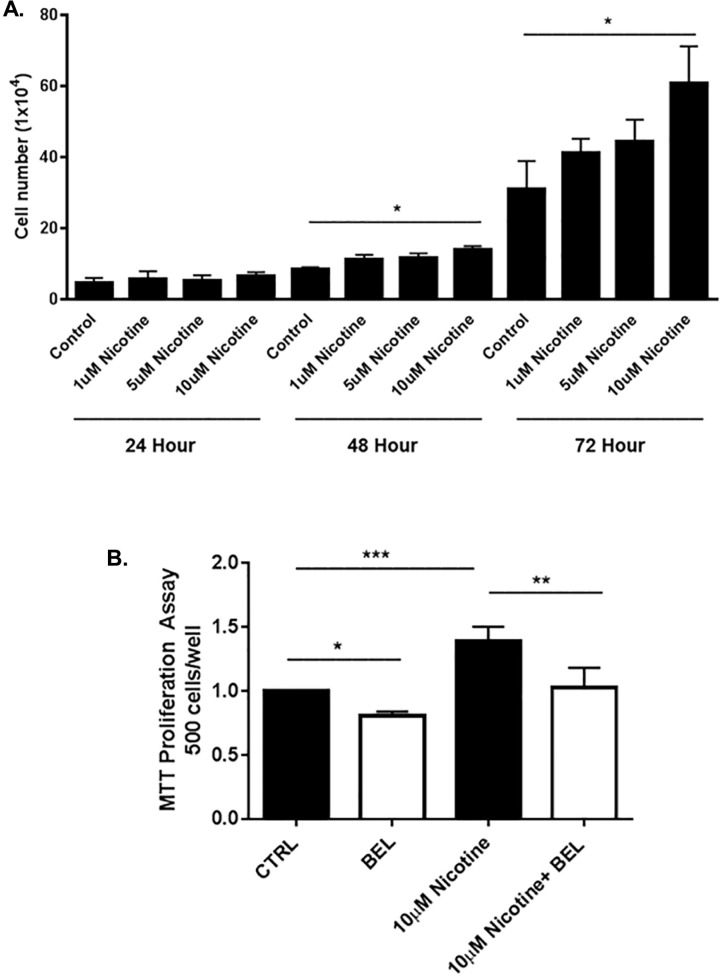
To examine the effects of nicotine on cell growth, 4T1 breast cancer cells were treated with varying concentrations of nicotine (1, 5, 10 μM). At both 48 and 24 hours nicotine 10μM was found to significantly exacerbate breast cancer cell proliferation (Fig 1A); the use of this dose in cell culture has been previously documented [9]. Additionally, an MTT assay found BEL significantly attenuated both basal and nicotine-induced breast cancer proliferation (Fig 1B).
Fig 1. Nicotine-induced Cell Proliferation is attenuated by BEL.
Promotion of 4T1 cell proliferation by nicotine dose dependently (1, 5, 10μM) was analyzed by cell count analysis for time intervals indicated above. Control wells were treated with DMSO (A). Cells were treated with nicotine (10μM) in the presence or absence of BEL (3μM) and proliferation measured by MTT assay (B). (n = 6–7). *, p<0.05 **, p<0.01 ***, p<0.00; two and one-way ANOVA.
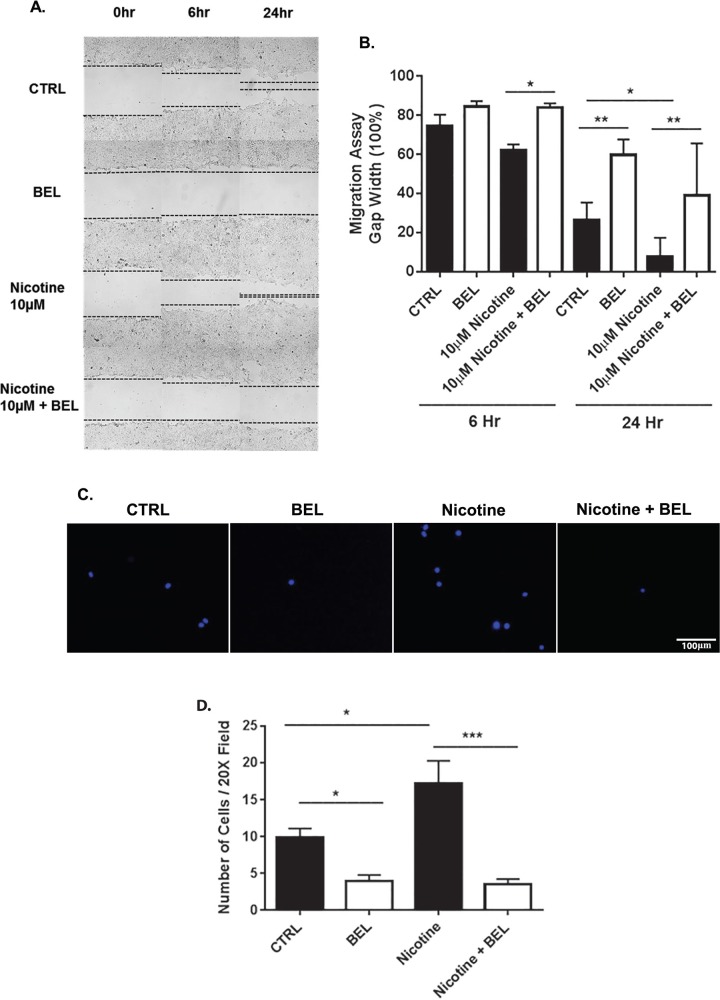
Furthermore, BEL was found to attenuate nicotine-induced breast cancer cell migration. Specifically BEL was found to attenuate gap closure promoted by nicotine at 6 and 24 hours (Fig 2A and 2B). The rate of gap closure can be facilitated by migration mechanisms and proliferation, hence, a short time point of 6 hours was investigated to show a reduction in cell migration ability. To further verify BEL attenuates nicotine-induced breast cancer migration a transwell assay was assessed at 4 hours (Fig 2C and 2D). Interestingly, for both migration experiments BEL was found to reduce basal breast cancer cell migration. Taken together this data suggests that iPLA2β is a main regulator of both basal and nicotine-induced breast cancer cell proliferation and migration.
Fig 2. Nicotine-induced Cell Migration is decreased by BEL.
Cultured 4T1 cells were scratched under normal conditions (DMSO) and nicotine (10μM) in the presence or absence of BEL (3μM). Representative 10x images of scratch migration assay, indicating BEL treatment attenuated nicotine induced migration (A). Cell migration was quantitatively evaluated by measuring the distance between the scratch edges after 6, 24, and 48 hr (B). Representative images of Boyden chamber migration assay, cells were treated with nicotine (10M) in the presence or absence of BEL (3μM, 30 minute pretreatment) for 4 hr (C). Cell migration was quantitatively evaluated by counting the number of cells migrated (D). (n = 3–5). *, p<0.05 **,p<0.01; one-way ANOVA.
BEL Decreases Nicotine-induced MMP-9 Expression and Secretion
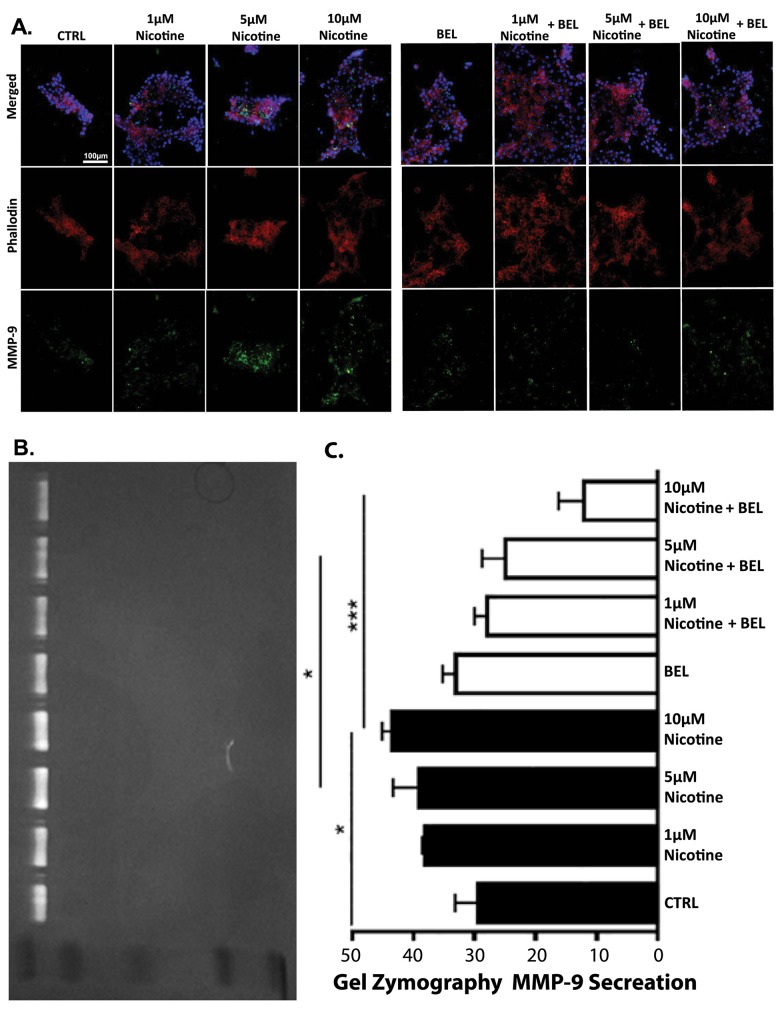
iPLA2β regulation of MMPs in breast cancer cells was investigated to determine if the role iPLA2β could lead to a substantial impact on overall metastasis progression. To identify which MMP is secreted by the Nicotine stimulation, a series of parallel gel zymography experiments were performed. Our findings suggests nicotine increases MMP-9 expression (Fig 3A) and secretion (Fig 3B and 3C), which is attenuated by BEL. Subsequently, MMP-2 was not found to be highly secreted by basal breast cancer cells or during nicotine stimulation which is consistent with others showing a major pro-MMP-9 band, the molecular weight around 92kDa [50]. Interestingly, BEL showed a significant attenuation of basal MMP-9 expression but not secretion (Fig 3A, 3B and 3C).
Fig 3. BEL decreases Nicotine-induced MMP-9 Expression and Secretion.
An In-situ zymography was conducted with cells treated dose dependently with nicotine (1, 5, 10μM) in the presence or absence of BEL (3μM) for 24 hr. Cells were stained with MMP-9, phalloidin, and DAPI (A). Cell culture media was isolated from cells treated as previously described and a gel zymography was performed; the gel molecular weight standard from top to bottom is 103kDa, 77kDa, 49kDa, 34kDa, and 28kDa. A representative image of gel zymography shows a band corresponding to the molecular weight of proMMP-9 (92kDa) (B) and the area of gelatin dissolved was quantified (C). n = 3, *,p<0.05. ***, p<0.001; one-way ANOVA.
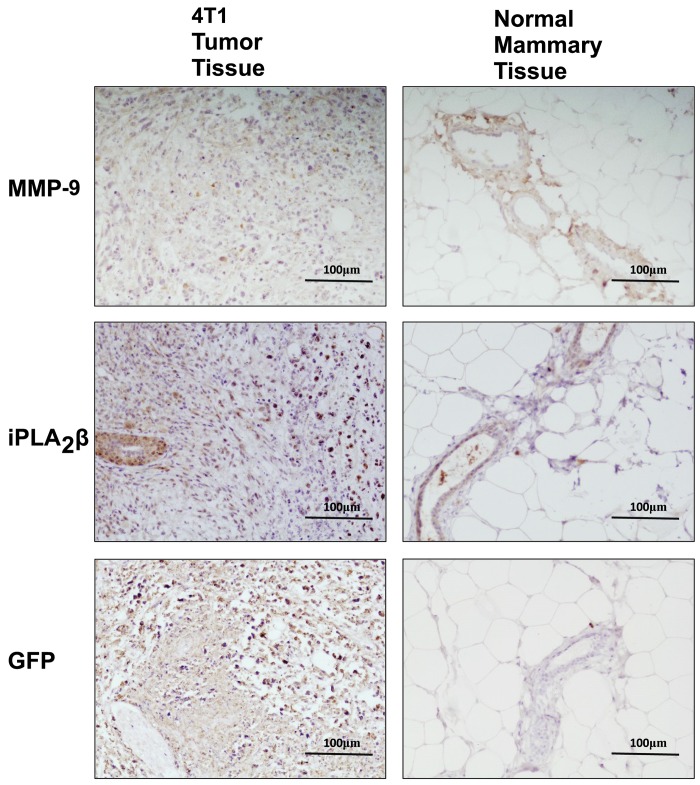
Furthermore, in breast tumor tissue iPLA2β was found overexpressed compared to normal mammary tissue (Fig 4). Subsequently, MMP-9 was found overexpressed in the tumor tissue (identified by GFP staining) and not MMP-2 (data not shown). This data provides strong evidence that iPLA2β may promote MMP-9 production and expression in breast cancer tumor tissue and suggests that iPLA2β may be a major mediator of metastasis in highly invasive stage IV breast cancer.
Fig 4. iPLA2β and MMP-9 Expression is upregulated in 4T1 tumor tissue.
Normal mammary or 4T1 tumor tissue grown for 2wks in 12wk old Female BALB/c mice were isolated and embedded in paraffin. Tissue sections were stained with anti-GFP (green-fluorescent protein), iPLA2β, or MMP-9 antibodies. Positive staining is indicated by dark brown coloration and representative images shown.
BEL Attenuates Nicotine-Induced Breast Cancer Tumor Growth
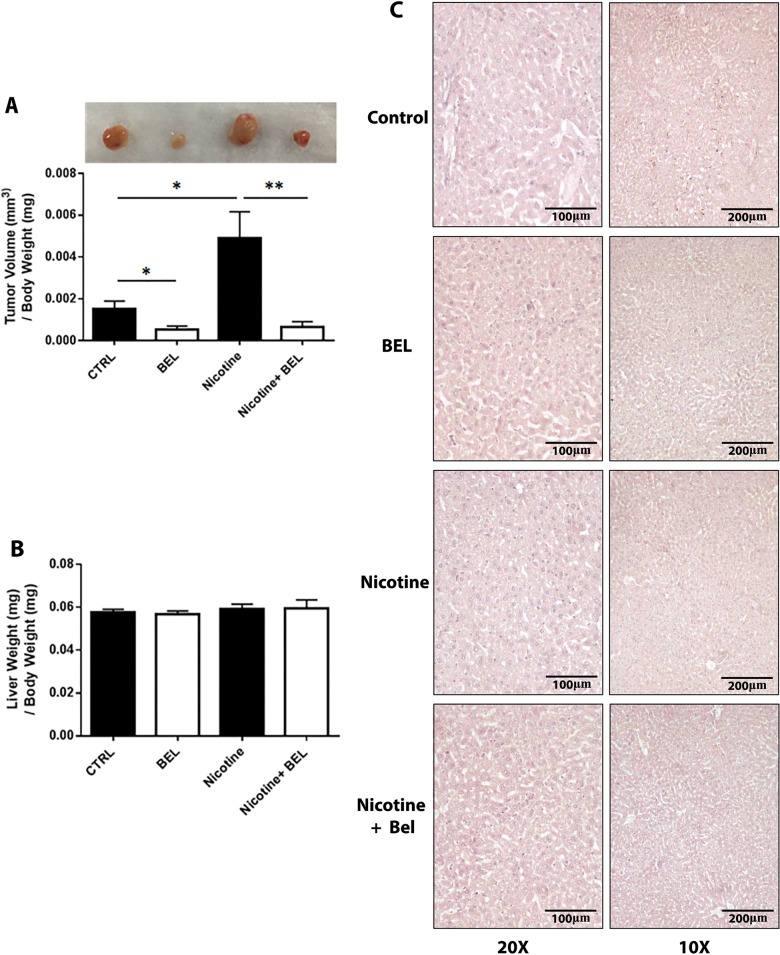
To examine the role of iPLA2β on nicotine-induced tumor growth, we administered BEL (10ug/g/day) to mice with tumors grown in the presence or absence of nicotine (5mg/kg/day). This dose of BEL is indicated to attenuate ovarian cancer development through reducing the adhesion, migration and invasion of epithelial ovarian cancer cells [51]. Subsequently, delivery of 5mg/kg/day of nicotine has been reported as an appropriate dose and is consistent with physiological range of blood nicotine levels in regular smokers [44,52]. Our data suggests that BEL significantly attenuated nicotine-induced tumor volume, along with decreasing normal tumor growth (Fig 5A). A decrease in basal tumor growth by BEL treatment was also found. Additionally, no physiological side effects from BEL were visible, subsequently, was no alteration in liver weight (Fig 5B) or liver cytotoxicity (Fig 5C).
Fig 5. Nicotine-induced Tumor Growth is decreased by BEL.
Female BALB/c mice were implanted with 4T1 cells (1X105) in the right second mammary fat pad. Tumors were grown for 2wks in mice infused either with nicotine (5mg/kg/day) or saline (50% DMSO) and in the presence or absence of BEL (10ug/g/day). Tumors were isolated and final tumor volume (A) and liver weight (B) measured. Liver cytotoxicity was analyzed by HE staining with representative 10x and 20x images shown (C) (n = 8). *, p<0.05 **, p<0.01; unpaired t-test.
Nicotine-Induced Metastatic Tumor Environment and Progression Is Decreased by BEL
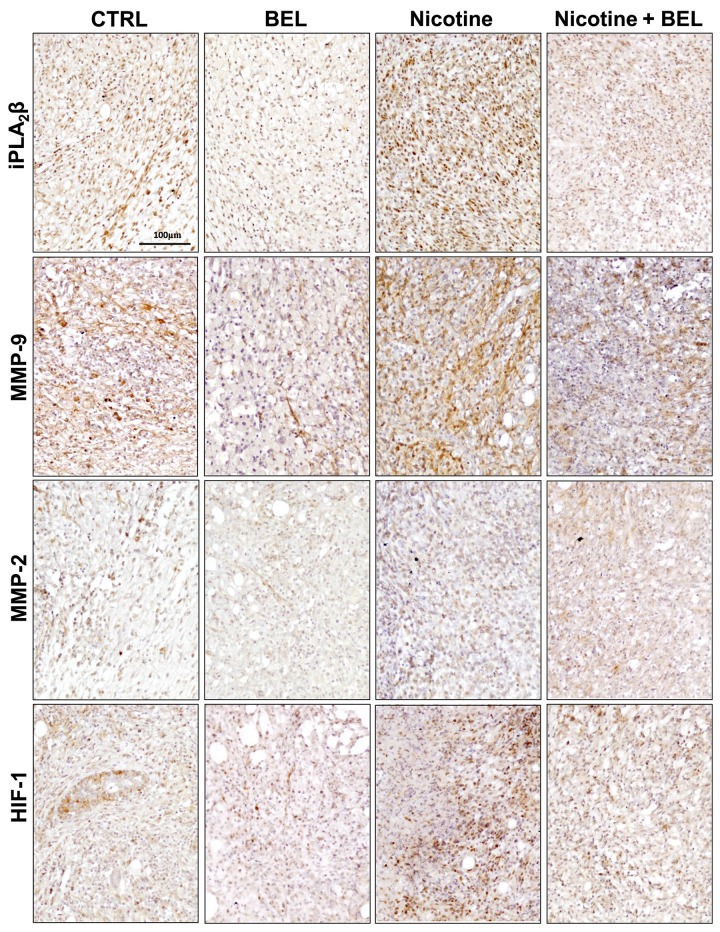
To determine the ability of nicotine to promote breast cancer metastasis, tissue immunohistochemistry was utilized. Nicotine was found to upregulate iPLA2β expression in the tumor tissue, which was attenuated by BEL along with basal iPLA2β expression (Fig 6). Subsequently, to elucidate the mechanism via which iPLA2β enhances nicotine-induced breast cancer metastasis we investigated MMP expression. We found that nicotine enhanced MMP-9 expression in the tumor tissue and both nicotine-induced and basal MMP-9 expression was decreased by BEL (Fig 6). Additionally, no difference was found in MMP-2 expression during nicotine or BEL treatment (Fig 6), representative images from four mice groups were imaged (S1 Fig). This data suggests that nicotine could promote metastasis through upregulating MMP-9 in an iPLA2β dependent manner.
Fig 6. Nicotine-induced iPLA2β and MMP-9 Expression in Tumor Tissue is decreased by BEL.
Control versus BEL and Nicotine versus Nicotine plus BE (n = 4): 4T1 tumor tissue grown for 2wks in 12wk old Female BALB/c mice were isolated and embedded in paraffin. Tissue sections were stained with anti-iPLA2β, MMP-9, MMP-2, and HIF-1alpha antibodies. Positive staining is indicated by dark brown coloration and representative images shown.
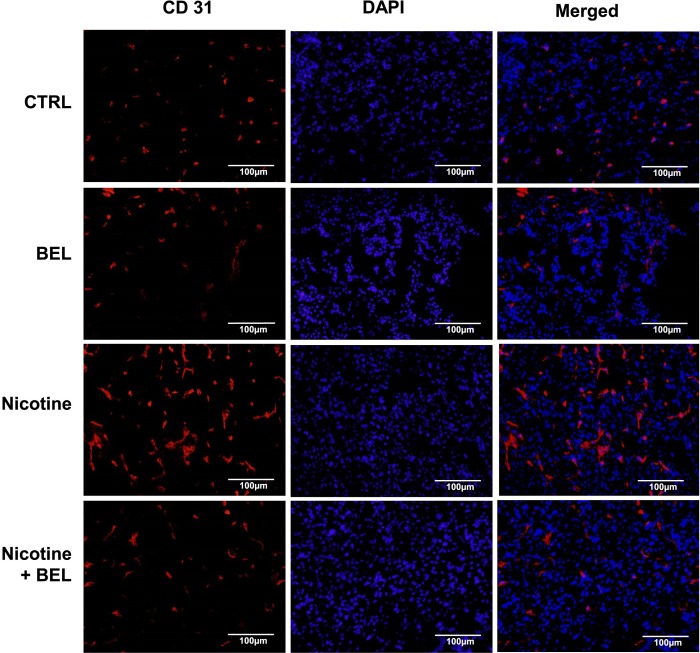
Furthermore, tumor angiogenesis plays a major role in tumor cell intravasation and metastasis. Our preliminary data suggests that nicotine increases both HIF-1alpha (Fig 6) and CD31 expression (Fig 7) in breast cancer tumor tissue, which is attenuated by BEL treatment. Hypoxic tumor tissue produces an environment which promotes angiolytic factor production and blood vessel formation. In Fig 6 we found that nicotine exacerbates tumor hypoxia conditions as an increase in HIF-1alpha was found. Subsequently, there was an increase in endothelial cells in the nicotine-induced tumor tissue, suggesting an increase in blood vessel presence and formation within the tumor tissue. This suggests that nicotine increase the accessibility of the breast cancer cells to blood vessels, hence, facilitating metastasis.
Fig 7. Nicotine-induced CD31 is decreased by BEL.
Isolated tumor tissue was embedded in OCT and stained with CD31 and counterstained with DAPI. Representative images are shown. n = 4.
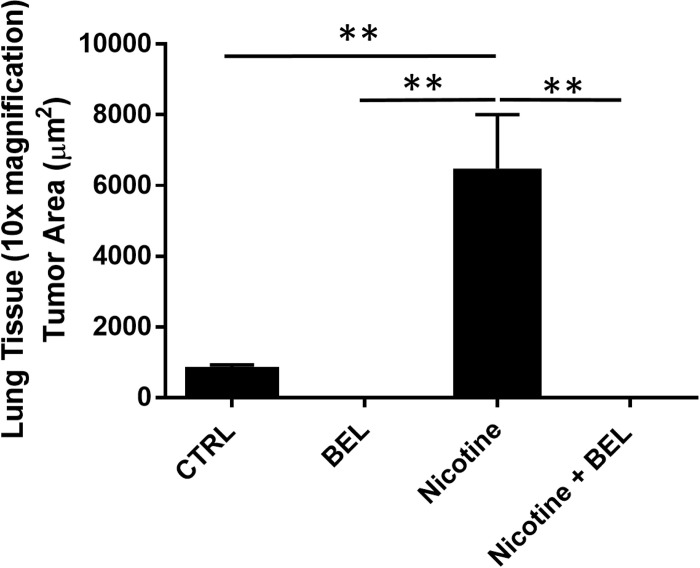
From our investigation of the tumor tissue we found that nicotine induces an environment highly suitable for promoting metastasis. To further examine metastasis we isolated the lung tissue and found a visible increase in breast cancer tumor colonization within the lungs from mice treated with nicotine (Fig 8). Moreover, BEL treatment decreased both nicotine-induced and basal lung metastasis. Subsequently, lung tissue sections stained with HE and GFP showed nicotine-induced tumor colonization was attenuated by BEL treatment (Fig 8). Area analysis conduction on the HE stained lung sections showed nicotine significantly enhanced tumor colonization in the lung tissue which was attenuated by BEL (Fig 9).
Fig 8. Nicotine-induced breast cancer metastasis in lung tissue was attenuated by BEL.
Lungs were isolated and representative 10x images of tumor colonization shown by HE and GFP staining.
Fig 9. Nicotine-induced breast cancer metastasis in lung tissue was attenuated by BEL.
Tumor area (μm2) was quantified in the lung sections stained with HE. n = 4, **, p<0.01; one-way ANOVA.
Discussion
The exact cellular mechanisms regulating nicotine-induced breast cancer development remain unclear. However, cigarette smoke has been shown to enhance breast cancer cell mobility, along with, metastasis and adherence to lung endothelium in an iPLA2β dependent manner [7,53,54]. Recently, iPLA2β has emerged as a main regulator of cancer development and progression including prostate cancer cell growth, lung cancer cell invasion, and ovarian cancer cell proliferation [21,22] [23,55,56]. More importantly, iPLA2β has been shown to mediate cigarette smoke-induced cell proliferation and migration. Subsequently, our study provides the first research indicating that nicotine-induced breast cancer cell proliferation and migration occurs in an iPLA2β dependent manner.
The data presented in this study lays a preliminary foundation that targeting iPLA2β is a promising new strategy against nicotine-induced stage IV metastatic breast cancer. Inhibition of iPLA2β with BEL strongly attenuated nicotine-induced breast cancer proliferation and migration in-vitro and showed a decrease in both tumor growth and metastasis in -vivo. In women, adverse outcome from metastatic breast cancer is the leading cause of death among cancer cases, suggesting the critical need for novel pharmacological strategies to maintain and combat tumor progression and metastasis.
Previous work indicates that breast cancer tumors grown in genetically suppressed iPLA2β knockout mice showed no reduction in tumor size, however, an 11-fold attenuation in lung metastasis was found [53]. Importantly, our study shows that iPLA2β expression is upregulated in 4T1 tumors compared to normal mammary tissue, and furthermore, direct intraperitoneal administration of BEL attenuated both control and nicotine-induced tumor size and metastasis. This leads to an important observation that inhibition of iPLA2β in the tumors environment alone is not sufficient to influence tumor size and that direct suppression of iPLA2β in the cancer cells is needed to attenuate tumor development along with metastasis.
Additionally, our data suggests BEL is an effective treatment for early-stage breast cancer as it will interfere with the critical steps of cellular proliferation, migration, and metastasis. Furthermore, our study found a significant effect of BEL on short term exposure of nicotine on aggressive stage IV 4T1 cells. This provides evidence along with others that BEL will be effective during long term exposure in patients and potentially in less invasive breast cancer cells lines [7]. Moreover, studies using low doses of BEL in mice show toxicity tolerance and no liver toxicity as was similarly found in our study [51,57]. Additionally, supporting the limited toxicity and side effects that would occur in women from BEL treatments, is our results showing increase iPLA2β expression in the tumor tissue, while others have found that iPLA2β is not an essential gene in normal tissue in female mice [58]. Pharmacological drug design is progressing into tumor-specific treatments; in which BEL should be considered. To determine the mechanism promoting nicotine-induced breast cancer migration and metastasis, both MMP-2 and MMP-9 production and secretion were assessed. The production of MMP-2 and MMP-9 are considered by many publications as tumor markers, in which, they promote the development and progression of tumor cells, along with facilitating migration, invasion and metastasis [59]. High levels of MMP-9 are known to promote breast cancer invasion and migration to distant tissue sites [59] and downregulation of MMP-9 has been shown to be an effective mechanism to attenuate metastasis [60]. Furthermore, overexpression of MMP-9 in breast cancer has been shown to facilitate tumor cell invasiveness [61]. Our results supports the role that iPLA2β regulates 4T1 breast cancer cell production of MMP-9, which is amplified by nicotine. Moreover, BEL significantly reduced MMP-9 production, secretion and sequentially, lead to a decrease in both basal and nicotine-induced metastasis and colonization in lung tissue.
Future Directions
The nicotinic acetylcholine receptors (nAChR) form either heteropentamers, consisting of a combination of α (α1-α6) and β (β2-β4) subunits or homopentamers derived from α7-α10 subunits [41]. To date, analysis of nAchRs expressed on mouse 4T1 breast cancer cells has not been thoroughly conducted. However, the expression of nAchRs on various human breast cancer cell lines have been examined including α9-nAChR and α7-nAChR which are found to promote human breast cancer cell proliferation and invasion [41,45,62]. Further experiments investigating the expression levels of the nAChR subtypes on 4T1 cells should be conducted along with determining with the nAChR potentially involved with nicotine-induced 4T1 cell proliferation and migration. Subsequently, further research needs to be conducted to determine the exact cellular role of iPLA2β in mediating both basal and nicotine-induced MMP-9 expression and secretion in-vivo.
Conclusion
Taken together our research suggests that nicotine exacerbates the severity of breast cancer through facilitating metastasis by enhancing MMP-9 production in an iPLA2β dependent manner. Furthermore, our study indicates that BEL treatment could attenuate nicotine-induced and (non-nicotine induced) breast cancer growth and metastasis.
Supporting Information
Representative 20x images are shown from primary breast tumors stained with MMP-2. n = 4.
(EPS)
Observed experimental data analyzed in the results section and displayed in the manuscript’s figures.
(XLSX)
Acknowledgments
Recognition of Arnold Stromberg and Joshua Lambert from the University of Kentucky, Department of Statistics for their contribution to data analysis in the paper.
Data Availability
All relevant data are in the paper and its Supporting Information files.
Funding Statement
Funded by National Science Foundation, 1355438, Public Health Service Grant GM102776, and Hebei Providence Nature Science Foundation, H2015201137.
References
- 1. DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. (2014) Cancer treatment and survivorship statistics, 2014. CA: a cancer journal for clinicians 64: 252–271. [DOI] [PubMed] [Google Scholar]
- 2. Desantis C, Ma J, Bryan L, Jemal A (2013) Breast cancer statistics, 2013. CA: a cancer journal for clinicians. [DOI] [PubMed] [Google Scholar]
- 3. Heyder C, Gloria-Maercker E, Hatzmann W, Niggemann B, Zanker KS, Dittmar T. (2005) Role of the beta1-integrin subunit in the adhesion, extravasation and migration of T24 human bladder carcinoma cells. Clinical & experimental metastasis 22: 99–106. [DOI] [PubMed] [Google Scholar]
- 4. Weigelt B, Peterse JL, van 't Veer LJ (2005) Breast cancer metastasis: markers and models. Nature reviews Cancer 5: 591–602. [DOI] [PubMed] [Google Scholar]
- 5. van Zijl F, Krupitza G, Mikulits W (2011) Initial steps of metastasis: cell invasion and endothelial transmigration. Mutation research 728: 23–34. 10.1016/j.mrrev.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murin S, Pinkerton KE, Hubbard NE, Erickson K (2004) The effect of cigarette smoke exposure on pulmonary metastatic disease in a murine model of metastatic breast cancer. Chest 125: 1467–1471. [DOI] [PubMed] [Google Scholar]
- 7. Kispert S, Marentette J, McHowat J (2015) Cigarette smoke induces cell motility via platelet-activating factor accumulation in breast cancer cells: a potential mechanism for metastatic disease. Physiological reports 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Cello F, Flowers VL, Li H, Vecchio-Pagan B, Gordon B, Harbom K, et al. (2013) Cigarette smoke induces epithelial to mesenchymal transition and increases the metastatic ability of breast cancer cells. Molecular cancer 12: 90 10.1186/1476-4598-12-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guha P, Bandyopadhyaya G, Polumuri SK, Chumsri S, Gade P,Kalvakolanu DV, et al. (2014) Nicotine promotes apoptosis resistance of breast cancer cells and enrichment of side population cells with cancer stem cell-like properties via a signaling cascade involving galectin-3, alpha9 nicotinic acetylcholine receptor and STAT3. Breast cancer research and treatment 145: 5–22. 10.1007/s10549-014-2912-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bavarva JH, Tae H, Settlage RE, Garner HR (2013) Characterizing the Genetic Basis for Nicotine Induced Cancer Development: A Transcriptome Sequencing Study. PloS one 8: e67252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jayakumar R, Kanthimathi MS (2012) Dietary spices protect against hydrogen peroxide-induced DNA damage and inhibit nicotine-induced cancer cell migration. Food chemistry 134: 1580–1584. 10.1016/j.foodchem.2012.03.101 [DOI] [PubMed] [Google Scholar]
- 12. Johnson KC, Miller AB, Collishaw NE, Palmer JR, Hammond SK, Salmon AG, et al. (2011) Active smoking and secondhand smoke increase breast cancer risk: the report of the Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk (2009). Tobacco control 20: e2 10.1136/tc.2010.035931 [DOI] [PubMed] [Google Scholar]
- 13. Luo J, Margolis KL, Wactawski-Wende J, Horn K, Messina C, Stefanick ML, et al. (2011) Association of active and passive smoking with risk of breast cancer among postmenopausal women: a prospective cohort study. BMJ 342: d1016 10.1136/bmj.d1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nyante SJ, Gierach GL, Dallal CM, Freedman ND, Park Y, Danforth KN, et al. (2014) Cigarette smoking and postmenopausal breast cancer risk in a prospective cohort. British journal of cancer 110: 2339–2347. 10.1038/bjc.2014.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pierce JP, Patterson RE, Senger CM, Flatt SW, Caan BJ, Natarajan L, et al. (2014) Lifetime cigarette smoking and breast cancer prognosis in the After Breast Cancer Pooling Project. Journal of the National Cancer Institute 106: djt359 10.1093/jnci/djt359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Faraglia B, Chen SY, Gammon MD, Zhang Y, Teitelbaum SL, Neugut AI, et al. (2003) Evaluation of 4-aminobiphenyl-DNA adducts in human breast cancer: the influence of tobacco smoke. Carcinogenesis 24: 719–725. [DOI] [PubMed] [Google Scholar]
- 17. Grando SA (2014) Connections of nicotine to cancer. Nature reviews Cancer 14: 419–429. 10.1038/nrc3725 [DOI] [PubMed] [Google Scholar]
- 18. Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. (2011) Hazardous compounds in tobacco smoke. International journal of environmental research and public health 8: 613–628. 10.3390/ijerph8020613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishioka T, Kim HS, Luo LY, Huang Y, Guo J, Chen CY. (2011) Sensitization of epithelial growth factor receptors by nicotine exposure to promote breast cancer cell growth. Breast cancer research: BCR 13: R113 10.1186/bcr3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Momi N, Ponnusamy MP, Kaur S, Rachagani S, Kunigal SS, Chellappan S, et al. (2013) Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through alpha7nAChR-mediated MUC4 upregulation. Oncogene 32: 1384–1395. 10.1038/onc.2012.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nicotera TM, Schuster DP, Bourhim M, Chadha K, Klaich G, Corral DA. (2009) Regulation of PSA secretion and survival signaling by calcium-independent phopholipase A(2)beta in prostate cancer cells. The Prostate 69: 1270–1280. 10.1002/pros.20968 [DOI] [PubMed] [Google Scholar]
- 22. Sun B, Zhang X, Yonz C, Cummings BS (2010) Inhibition of calcium-independent phospholipase A2 activates p38 MAPK signaling pathways during cytostasis in prostate cancer cells. Biochemical Pharmacology 79: 1727–1735. 10.1016/j.bcp.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 23. Song Y, Wilkins P, Hu W, Murthy KS, Chen J, Lee Z, et al. (2007) Inhibition of calcium-independent phospholipase A2 suppresses proliferation and tumorigenicity of ovarian carcinoma cells. The Biochemical journal 406: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao X, Wang D, Zhao Z, Xiao Y, Sengupta S, Xiao Y, et al. (2006) Caspase-3-dependent activation of calcium-independent phospholipase A2 enhances cell migration in non-apoptotic ovarian cancer cells. The Journal of biological chemistry 281: 29357–29368. [DOI] [PubMed] [Google Scholar]
- 25. Akiba S, Sato T (2004) Cellular function of calcium-independent phospholipase A2. Biological & pharmaceutical bulletin 27: 1174–1178. [DOI] [PubMed] [Google Scholar]
- 26. Balsinde J, Winstead MV, Dennis EA (2002) Phospholipase A(2) regulation of arachidonic acid mobilization. FEBS Lett 531: 2–6. [DOI] [PubMed] [Google Scholar]
- 27. Balsinde J, Balboa MA (2005) Cellular regulation and proposed biological functions of group VIA calcium-independent phospholipase A2 in activated cells. Cell Signal 17: 1052–1062. [DOI] [PubMed] [Google Scholar]
- 28. Ma Z, Turk J (2001) The molecular biology of the group VIA Ca2+-independent phospholipase A2. Prog Nucleic Acid Res Mol Biol 67: 1–33. [DOI] [PubMed] [Google Scholar]
- 29. Calderon LE, Liu S, Su W, Xie Z, Guo Z, Eberhard W, et al. (2012) iPLA2beta overexpression in smooth muscle exacerbates angiotensin II-induced hypertension and vascular remodeling. PloS one 7: e31850 10.1371/journal.pone.0031850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xie Z, Gong MC, Su W, Xie D, Turk J, Guo Z. (2010) Role of calcium-independent phospholipase A2beta in high glucose-induced activation of RhoA, Rho kinase, and CPI-17 in cultured vascular smooth muscle cells and vascular smooth muscle hypercontractility in diabetic animals. The Journal of biological chemistry 285: 8628–8638. 10.1074/jbc.M109.057711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ali T, Kokotos G, Magrioti V, Bone RN, Mobley JA, Hancock W, et al. (2013) Characterization of FKGK18 as inhibitor of group VIA Ca2+-independent phospholipase A2 (iPLA2beta): candidate drug for preventing beta-cell apoptosis and diabetes. PloS one 8: e71748 10.1371/journal.pone.0071748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu S, Xie Z, Zhao Q, Pang H, Turk J, Calderon L, et al. (2012) Smooth muscle-specific expression of calcium-independent phospholipase A2beta (iPLA2beta) participates in the initiation and early progression of vascular inflammation and neointima formation. The Journal of biological chemistry 287: 24739–24753. 10.1074/jbc.M112.340216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vadali S, Post SR (2014) Lipid rafts couple class A scavenger receptors to phospholipase A2 activation during macrophage adhesion. Journal of leukocyte biology 96: 873–881. 10.1189/jlb.2A0414-214R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nikolic DM, Gong MC, Turk J, Post SR (2007) Class A scavenger receptor-mediated macrophage adhesion requires coupling of calcium-independent phospholipase A(2) and 12/15-lipoxygenase to Rac and Cdc42 activation. The Journal of biological chemistry 282: 33405–33411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ackermann EJ, Conde-Frieboes K, Dennis EA (1995) Inhibition of macrophage Ca(2+)-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem 270: 445–450. [DOI] [PubMed] [Google Scholar]
- 36. Hazen SL, Zupan LA, Weiss RH, Getman DP, Gross RW (1991) Suicide inhibition of canine myocardial cytosolic calcium-independent phospholipase A2. Mechanism-based discrimination between calcium-dependent and -independent phospholipases A2. J Biol Chem 266: 7227–7232. [PubMed] [Google Scholar]
- 37. Pulaski BA, Ostrand-Rosenberg S (2001) Mouse 4T1 breast tumor model. Current protocols in immunology / edited by Coligan John E [et al. ] Chapter 20: Unit 20 22. [DOI] [PubMed] [Google Scholar]
- 38. Benowitz NL, Hukkanen J, Jacob P 3rd (2009) Nicotine chemistry, metabolism, kinetics and biomarkers. Handbook of experimental pharmacology: 29–60. 10.1007/978-3-540-69248-5_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andersson G, Vala EK, Curvall M (1997) The influence of cigarette consumption and smoking machine yields of tar and nicotine on the nicotine uptake and oral mucosal lesions in smokers. Journal of oral pathology & medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology 26: 117–123. [DOI] [PubMed] [Google Scholar]
- 40. Hao J, Shi FD, Abdelwahab M, Shi SX, Simard A, Whiteaker P, et al. (2013) Nicotinic receptor beta2 determines NK cell-dependent metastasis in a murine model of metastatic lung cancer. PloS one 8: e57495 10.1371/journal.pone.0057495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen CS, Lee CH, Hsieh CD, Ho CT, Pan MH, Huang CS, et al. (2011) Nicotine-induced human breast cancer cell proliferation attenuated by garcinol through down-regulation of the nicotinic receptor and cyclin D3 proteins. Breast cancer research and treatment 125: 73–87. 10.1007/s10549-010-0821-3 [DOI] [PubMed] [Google Scholar]
- 42. Wilkinson DS, Turner JR, Blendy JA, Gould TJ (2013) Genetic background influences the effects of withdrawal from chronic nicotine on learning and high-affinity nicotinic acetylcholine receptor binding in the dorsal and ventral hippocampus. Psychopharmacology 225: 201–208. 10.1007/s00213-012-2808-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang S, Zhang C, Zhang M, Liang B, Zhu H, Lee J, et al. (2012) Activation of AMP-activated protein kinase alpha2 by nicotine instigates formation of abdominal aortic aneurysms in mice in vivo. Nature medicine 18: 902–910. 10.1038/nm.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Davis JA, James JR, Siegel SJ, Gould TJ (2005) Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. The Journal of neuroscience: the official journal of the Society for Neuroscience 25: 8708–8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee CH, Chang YC, Chen CS, Tu SH, Wang YJ, Chen LC, et al. (2011) Crosstalk between nicotine and estrogen-induced estrogen receptor activation induces alpha9-nicotinic acetylcholine receptor expression in human breast cancer cells. Breast cancer research and treatment 129: 331–345. 10.1007/s10549-010-1209-0 [DOI] [PubMed] [Google Scholar]
- 46. Liu Z, Zhang B, Liu K, Ding Z, Hu X (2012) Schisandrin B attenuates cancer invasion and metastasis via inhibiting epithelial-mesenchymal transition. PloS one 7: e40480 10.1371/journal.pone.0040480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kanaya N, Adams L, Takasaki A, Chen S (2014) Whole blueberry powder inhibits metastasis of triple negative breast cancer in a xenograft mouse model through modulation of inflammatory cytokines. Nutrition and cancer 66: 242–248. 10.1080/01635581.2014.863366 [DOI] [PubMed] [Google Scholar]
- 48. Mandal CC, Ghosh-Choudhury T, Yoneda T, Choudhury GG, Ghosh-Choudhury N (2010) Fish oil prevents breast cancer cell metastasis to bone. Biochemical and biophysical research communications 402: 602–607. 10.1016/j.bbrc.2010.10.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu S, Xie Z, Daugherty A, Cassis LA, Pearson KJ, Gong MC, et al. (2013) Mineralocorticoid receptor agonists induce mouse aortic aneurysm formation and rupture in the presence of high salt. Arteriosclerosis, thrombosis, and vascular biology 33: 1568–1579. 10.1161/ATVBAHA.112.300820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. (1999) Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. The Journal of biological chemistry 274: 13066–13076. [DOI] [PubMed] [Google Scholar]
- 51. Li H, Zhao Z, Antalis C, Zhao Z, Emerson R, Wei G, et al. (2011) Combination therapy of an inhibitor of group VIA phospholipase A2 with paclitaxel is highly effective in blocking ovarian cancer development. The American journal of pathology 179: 452–461. 10.1016/j.ajpath.2011.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Q, Zhang M, Liang B, Shirwany N, Zhu Y, Zou MH. (2011) Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: the role of uncoupling protein 2. PloS one 6: e25436 10.1371/journal.pone.0025436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McHowat J, Gullickson G, Hoover RG, Sharma J, Turk J, Kornbluth J. (2011) Platelet-activating factor and metastasis: calcium-independent phospholipase A2beta deficiency protects against breast cancer metastasis to the lung. American journal of physiology Cell physiology 300: C825–832. 10.1152/ajpcell.00502.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kispert SE, Marentette JO, McHowat J (2014) Enhanced breast cancer cell adherence to the lung endothelium via PAF acetylhydrolase inhibition: a potential mechanism for enhanced metastasis in smokers. American journal of physiology Cell physiology 307: C951–956. 10.1152/ajpcell.00218.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ho JN, Lee SB, Lee SS, Yoon SH, Kang GY, Hwang SG, et al. (2010) Phospholipase A2 activity of peroxiredoxin 6 promotes invasion and metastasis of lung cancer cells. Molecular cancer therapeutics 9: 825–832. 10.1158/1535-7163.MCT-09-0904 [DOI] [PubMed] [Google Scholar]
- 56. Jo M, Yun HM, Park KR, Hee Park M, Myoung Kim T, Ho Pak J, et al. (2013) Lung tumor growth-promoting function of peroxiredoxin 6. Free radical biology & medicine 61C: 453–463. [DOI] [PubMed] [Google Scholar]
- 57. Saab-Aoude S, Bron AM, Creuzot-Garcher CP, Bretillon L, Acar N (2013) A mouse model of in vivo chemical inhibition of retinal calcium-independent phospholipase A2 (iPLA2). Biochimie 95: 903–911. 10.1016/j.biochi.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 58. Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, et al. (2004) Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. The Journal of biological chemistry 279: 38194–38200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Radenkovic S, Konjevic G, Jurisic V, Karadzic K, Nikitovic M, Gopcevic K. (2013) Values of MMP-2 and MMP-9 in Tumor Tissue of Basal-Like Breast Cancer Patients. Cell biochemistry and biophysics. [DOI] [PubMed] [Google Scholar]
- 60. Lee SJ, Hong S, Yoo SH, Kim GW (2013) Cyanidin-3-O-Sambubioside from Acanthopanax sessiliflorus Fruit Inhibits Metastasis by Downregulating MMP-9 in Breast Cancer Cells MDA-MB-231. Planta medica. [DOI] [PubMed] [Google Scholar]
- 61. Choi JY, Jang YS, Min SY, Song JY (2011) Overexpression of MMP-9 and HIF-1alpha in Breast Cancer Cells under Hypoxic Conditions. Journal of breast cancer 14: 88–95. 10.4048/jbc.2011.14.2.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dasgupta P, Rizwani W, Pillai S, Kinkade R, Kovacs M, Rastogi S, et al. (2009) Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. International journal of cancer Journal international du cancer 124: 36–45. 10.1002/ijc.23894 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative 20x images are shown from primary breast tumors stained with MMP-2. n = 4.
(EPS)
Observed experimental data analyzed in the results section and displayed in the manuscript’s figures.
(XLSX)
Data Availability Statement
All relevant data are in the paper and its Supporting Information files.