Abstract
Background
Dialysis patients have high cardiovascular mortality risk. This study aimed to investigate the association between SNPs of genes involved in vascular processes and mortality in dialysis patients.
Methods
Forty two SNPs in 25 genes involved in endothelial function, vascular remodeling, cell proliferation, inflammation, coagulation and calcium/phosphate metabolism were genotyped in 1330 incident dialysis patients. The effect of SNPs on 5-years cardiovascular and non-cardiovascular mortality was investigated.
Results
The mortality rate was 114/1000 person-years and 49.4% of total mortality was cardiovascular. After correction for multiple testing, VEGF rs699947 was associated with all-cause mortality (HR1.48, 95% CI 1.14–1.92). The other SNPs were not associated with mortality.
Conclusions
This study provides further evidence that a SNP in the VEGF gene may contribute to the comorbid conditions of dialysis patients. Future studies should unravel the underlying mechanisms responsible for the increase in mortality in these patients.
Introduction
Patients with end stage renal disease (ESRD) have a very high mortality risk as compared with the general population. Cardiovascular disease is a major cause of death in these patients, accounting for 40–50% of total mortality[1,2]. Recently, a large study showed that patients on chronic dialysis had an 8.8-times increased cardiovascular mortality risk as compared with the general population[3]. In addition to cardiovascular disease, declined kidney function and chronic kidney disease (CKD) are associated with increased hospitalization[4], infection[3,5,6], malignancies[7–9] and frailty[10] resulting in an 8.1-fold increased risk of non-cardiovascular mortality[3]. The latter illustrates the very high risk of both cardiovascular and non-cardiovascular death in these patients[3,3,6,11].
These increased mortality rates in ESRD patients are only in part explained by traditional risk factors, suggesting a role for CKD-related factors. CKD specific risk factors include chronic inflammatory state[12] and altered levels of circulating growth factors[13], the presence of uremic toxins[14], disturbed calcium/phosphate metabolism and coagulation[15] as well as endothelial dysfunction[16]. Alterations in the genetic profile of these processes in ESRD patients may further increase this dysbalance and enhance morbidity and mortality.
Interestingly, single nucleotide polymorphisms (SNPs) that influence the above mentioned processes have already been related to coronary restenosis[17–24] and vascular aneurysm formation[25,26] in the general population and to hemodialysis arteriovenous access failure[27–29] by changing vascular function through processes related to endothelial function and vascular remodeling, growth factors, inflammation, coagulation, and calcium/phosphate metabolism[20,24–31]. Thus far, the association between these SNPs and cardiovascular mortality has not been investigated in dialysis patients. Despite their strong cardiovascular link, these SNPs may not be exclusively related to cardiovascular morbidity and mortality. Indeed, the genes affected by these SNPs mediate a plethora of processes, and thus may also affect non-cardiovascular morbidity and mortality. Therefore, we hypothesized that SNPs involved in processes related to endothelial function, vascular remodeling, cell proliferation, inflammation, coagulation, and calcium/phosphate metabolism could influence cardiovascular and non-cardiovascular mortality in patients on dialysis. This study was performed in a large population of incident dialysis patients.
Subjects and Methods
Patients
The Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) is a prospective multicenter cohort study in which incident adult ESRD patients from 38 dialysis centers in the Netherlands were included[32]. The study was performed in accordance with the Declaration of Helsinki. The Medical Review Ethics Committee of the Leiden University Medical Center approved the study. All patients gave written informed consent. Adult patients that did not receive any prior renal replacement therapy were eligible. Patients were followed from January 1997 until death or censoring, i.e. transfer to a nonparticipating dialysis center, withdrawal from the study, transplantation or end of the follow-up period (June 2009). Data on dialysis modality, age, sex, and primary kidney disease were collected at the start of dialysis treatment. Primary kidney disease was classified according to the codes of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA)[33]. Patients were grouped into four classes of primary kidney disease: glomerulonephritis, diabetes mellitus, renal vascular disease and other kidney diseases. Other kidney diseases consisted of patients with interstitial nephritis, polycystic kidney diseases and kidney failure due to multisystem diseases. All-cause mortality was further subdivided in cardiovascular and non-cardiovascular mortality. Cardiovascular death was defined as death due to heart failure, myocardial infarction, ischemic or hemorrhagic stroke, sudden death without obvious non-cardiovascular cause, and death due to other cardiovascular causes. Non-cardiovascular death included all other causes of death.
SNP selection and genotyping
SNPs of interest were selected that could influence mortality risk by changing vascular processes in dialysis patients. Therefore, SNPs previously associated with vascular disease such as coronary restenosis, arteriovenous (AV) access failure and vascular aneurysm formation were selected after a systematic search of literature. Searching MEDLINE using keywords including ‘hemodialysis’, ‘single nucleotide polymorphism’, ‘arteriovenous access failure’, ‘coronary restenosis’, ‘percutaneous coronary intervention’ and ‘aortic aneurysm’ 42 SNPs in 25 candidate genes were identified[17–29,31,34–55]. Only SNPs with a minor allele frequency higher than 1% were included. The complete list of these candidate genes with associated outcomes is described elsewhere[32]. Two multiplex assays were designed using Assay designer software. When a SNP did not fit the multiplex, a proxy of that SNP was selected with the highest R2 value. The final set included 42 SNPs in 25 genes related to growth factors[18,24,26,34–36,38–42] (S1 Table), inflammation[20,21,23,28,43–46] (S2 Table), endothelial function and vascular remodeling[17,27,55] (S3 Table), calcium/phosphate metabolism[22,24,29,47,48] (S4 Table) and coagulation[24,31,50,51,53,54] (S5 Table). All SNPs were genotyped by MALDI-TOF mass spectrometry, using the MassARRAYtm methodology (Sequenom Inc., San Diego, CA, USA), following manufacturer's instructions. As quality control, 5% of the samples were genotyped in duplicate. No inconsistencies were observed. All the negative controls (2%) were negative.
Statistical analysis
Continuous variables are presented as median and interquartile range (IQR). Categorical variables are presented as number with percentages. Minor allele frequencies were calculated and a chi-squared test with 1 degree freedom was used to determine if observed and expected genotypes were in Hardy Weinberg equilibrium (HWE), using a p-value cut-off of <0.01, to reduce the likelihood of false positivity[32]. The hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated using Cox regression analysis for heterozygote genotypes and homozygous mutant genotypes as compared with wild-type genotypes for five-year mortality for the 42 SNPs. All analyses were performed using SPSS statistical software version 20.0 (SPSS, Chicago, Ill, USA). To adjust for multiple testing, the false discovery rate (FDR) was calculated using the method of Benjamini and Hochberg[56]. Although no universal FDR significance threshold has been defined, a cut-point of 0.20 has been suggested for candidate gene association studies, meaning that one should expect at most 20% of declared discoveries to be false[57]. Therefore a cutoff-point of 0.20 was chosen which resulted in a corrected level of significance of p = 0.0048 instead of p = 0.05.
Results
A total of 1330 dialysis patients were genotyped for the 42 SNPs. Baseline characteristics of the patients are shown in Table 1. The median age was 62.2 years, 39.0% was female, and 14.3% had diabetes mellitus as their primary kidney disease. In addition, approximately 8% of the patients had diabetes as co-morbidity. Of the 1330 patients, 474 (35.6%) died within five years of dialysis treatment. The overall mortality rate was 114 per 1000 person-years. Cardiovascular mortality accounted for 234 of these deaths (49.4%), whereas 50.6% of total mortality was due to non-cardiovascular causes (Table 2).
Table 1. Baseline characteristics.
| N = 1330 | ||
|---|---|---|
| Age (years, IQR) | 62.2 | 50.0–71.8 |
| Sex, female (n, %) | 515 | 39.0% |
| Race, white (n, %) | 1130 | 91.4% |
| Dialysis modality, hemodialysis (n, %) | 812 | 64.2% |
| Primary kidney disease (n, %) | ||
| Diabetes mellitus | 189 | 14.3% |
| Glomerulonephritis | 149 | 11.3% |
| Renal vascular disease | 206 | 15.6% |
| Others | 776 | 58.8% |
Table 2. Cardiovascular and non-cardiovascular mortality.
| N | % | ||
|---|---|---|---|
| Cardiovascular | Myocardial infarction | 41 | 8.7 |
| Heart failure | 29 | 6.1 | |
| Cerebrovascular accident | 28 | 3.8 | |
| Sudden death | 41 | 8.7 | |
| Other | 105 | 22.1 | |
| Total cardiovascular | 234 | 49.4 | |
| Non-cardiovascular | Infection | 56 | 11.8 |
| Withdrawal | 33 | 7.0 | |
| Suicide/refusal treatment | 59 | 12.4 | |
| Malignancy | 31 | 6.5 | |
| Other | 61 | 12.9 | |
| Total non-cardiovascular | 240 | 50.6 |
Minor allele frequencies and HWE p-values are summarized in S6 Table. Three SNPs were not in equilibrium: vitamin D receptor (VDR) rs4516035, interleukin-10 rs1800896 and TGF-β receptor1 rs1626340. Notably, none of these SNPs were significantly associated with mortality after correction for multiple testing.
SNPs and mortality
In total, 42 SNPs in 25 genes involved in vascular processes (endothelial function and vascular remodeling, growth factors, inflammation, coagulation, and calcium/phosphate metabolism) were genotyped. Without correction for multiple testing, three SNPs were associated with cardiovascular mortality. Vascular endothelial growth factor (VEGF) rs2010963 (HR0.62; 95% CI 0.38–1.00) and tumor necrosis factor rs1799964 (HR0.27; 95% CI 0.10–0.73) were associated with a decreased cardiovascular mortality, while VEGF rs699947 (HR1.52; 95% CI 1.07–2.17) resulted in an increased risk. In addition, without correction for multiple testing, matrix metalloproteinase-1 rs11292517 (HR0.67; 95% CI0.46–0.99) and VDR rs2238135 (HR0.33; 95% CI0.13–0.80) were associated with decreased risk of non-cardiovascular mortality, while rs9804922 (HR3.14; 95% CI1.17–8.46) in an intergenic region on 12q23.2, CD180 rs5744478 (HR3.25; 95% CI1.34–7.91) and interleukin-6 rs1800795 (HR1.52; 95% CI1.02–2.25) were associated with an increased non-cardiovascular mortality risk.
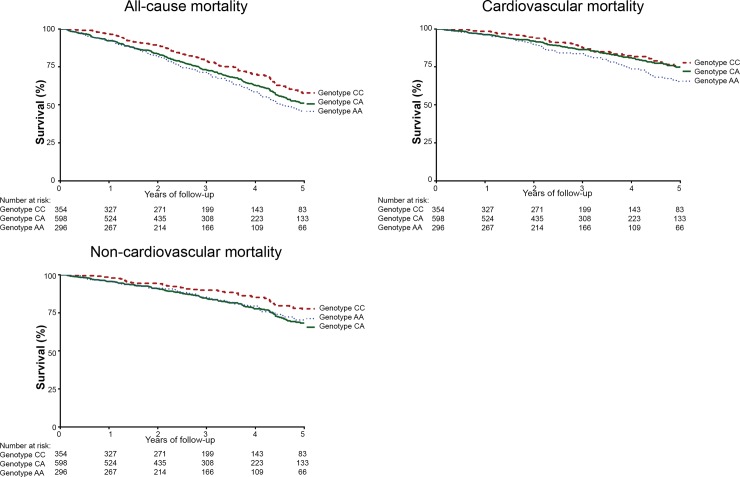
However, after correction for multiple testing, VEGF rs699947 only remained significantly associated with all-cause mortality (HR1.48, 95% CI 1.14–1.92, p = 0.003). Kaplan Meier curves for VEGF rs699947 are depicted in Fig 1. The results of all other SNPs are summarized in S1–S5 Tables.
Fig 1. Kaplan Meier survival curve for all-cause, cardiovascular and non-cardiovascular mortality for VEGF rs699947.
Discussion
Although it is widely recognized that patients on dialysis have substantially higher cardiovascular and non-cardiovascular mortality rates compared with the general population, little is known about the genetic predisposition to mortality of these vulnerable patients. In the present study, we investigated the association between mortality of chronic dialysis patients and 42 SNPs in 25 genes that have previously been linked to cardiovascular disease. We showed that, after correction for multiple testing, VEGF rs699947 was associated with an increased all-cause mortality risk. This emphasizes that this SNP is not exclusively associated with cardiovascular mortality, but also influences non-cardiovascular mortality. In concordance with previous studies[3,6,58], we observed that the burden of cardiovascular mortality was comparable with non-cardiovascular mortality in our cohort.
The VEGFA gene is located on chromosome 6 and is composed of a 14kb coding region with 8 exons and 7 introns[59]. VEGF rs699947 is situated in the promoter region and can thereby influence VEGF expression levels. Although we did not measure VEGF levels in our study, the effect of the rs699947 SNP in the VEGF gene on VEGF protein levels is reported in other studies. Indeed, carriers of the mutant A-allele of rs699947 on peritoneal dialysis have reduced serum VEGF levels[41]. Additional support for a detrimental effect of rs699947 SNP of the VEGF gene comes from in vitro studies which revealed that peripheral blood mononuclear cells isolated from healthy controls produced significantly more VEGF when compared to mononuclear cells from subjects with the AA genotype[60]. In our study the mutant AA genotype was associated with all-cause mortality, suggesting this can be attributed to both cardiovascular and non-cardiovascular causes. VEGF is involved in angiogenesis, arteriogenesis, vascular permeability, and endothelial cell migration and proliferation[61]. As such, VEGF plays a pivotal role in cardiovascular homeostasis and dysregulation can result in cardiovascular disease. VEGF mediated angiogenesis is important in hypoxic situations such as myocardial infarction since adequate vascular collaterals can preserve the myocardium during ischemia[62] and decrease cardiovascular events[63]. Indeed, carriers of the AA genotype of rs699947, associated with low levels of VEGF, were shown to have an increased risk of developing coronary artery atherosclerosis[64,65]. In addition to cardiovascular disease, the rs699947 SNP has also been associated to non-cardiovascular disease and mortality. AA carriers were shown to have an increased risk for thyroid cancer[66] and prostate cancer[67]. Moreover, patients with non-small cell lung cancer and AA genotype had poorer survival[68]. In addition to malignancies, VEGF rs699947 is also associated to other non-cardiovascular pathophysiology. Despite low systemic levels of VEGF, patients on peritoneal dialysis with the AA genotype expressed high mRNA VEGF levels in their peritoneal dialysis effluent as compared to the CC genotype, which was associated with progressive increase in peritoneal transport and even increased mortality[41].
Additional support for the detrimental effects of low VEGF levels comes from non-genetic studies, which revealed that reduced VEGF levels are associated with renal podocyte loss in diabetic nephropathy and progression of renal disease[69]. In addition, selective inhibition of VEGF with bevacuzimab, a monoclonal antibody against VEGF used in oncology, can induce hypertension and proteinuria[70]. Furthermore, females with were shown to have elevated levels of soluble VEGF receptor-1, an endogenous VEGF antagonist [71].
Next to reduced levels of VEGF, very high VEGF levels have been reported to be detrimental as well. Indeed, previous studies demonstrated that highly elevated VEGF levels increase all-cause mortality risk in ESRD patients[13,72]. Besides its pro-angiogenic actions, VEGF can exert pro-inflammatory effects[72] by enhancing vascular permeability and inducing leukocyte adhesion molecules[73]. These data suggest that a dysbalance in VEGF levels, either decreased or largely increased, may potentially be pathogenic.
Our study has several potential limitations. The collective term cardiovascular disease comprises a plethora of disorders elicited by even more underlying processes. We investigated SNPs involved in endothelial function, vascular remodeling, cell proliferation, inflammation, coagulation and calcium/phosphate metabolism, as these processes play an important role in cardiovascular disease. Importantly, these processes are affected in CKD, and alterations in their genetic profile may further increase this dysbalance and enhance morbidity and mortality. Nonetheless, more mechanisms are involved in the broad scope of cardiovascular disease and the selection of SNPs in this article is not exhaustive. For example, polymorphisms in iron metabolism and vascular calcification are missing, while these could be relevant in a dialysis population. Future studies could elaborate on the current selection. In addition, we primarily selected the SNPs on their cardiovascular interactions. Because the genes affected by these SNPs also exert important non-cardiovascular effects and dialysis patients also suffer from a large burden of non-cardiovascular mortality[3], we did not want to neglect this and also investigated their influence on non-cardiovascular mortality. However, other SNPs that may contribute to non-cardiovascular mortality were not appraised in this study. Considering the increasing attention to non-cardiovascular mortality in ESRD patients[74], future studies should investigate the effects of other SNPs primarily influencing non-cardiovascular disease.
Secondly, despite the large size of our cohort, in some SNPs there was a very small sample size in especially the variant genotypes. These groups are likely insufficiently powered to detect an association and this may lead to underestimation of the actual effect of the SNPs.
Furthermore, we did not measure plasma VEGF levels in this study. However, previous studies convincingly demonstrated decreased VEGF levels in the rs699947 AA genotype in both healthy individuals[60] and dialysis patients[41], thereby providing support for our assumption that the increased mortality as observed in dialysis patients carrying the VEGF rs699947 SNP, might be explained by decreased serum VEGF levels.
In conclusion, this study provides evidence that VEGF rs699947 AA genotype is associated with all-cause mortality in a large cohort of dialysis patients, whereas there was no significant association with the other 41 SNPs. These results may help clarify the involved pathways in the increased mortality of these patients. Further studies should investigate the underlying mechanisms in order to develop new therapies aimed to reduce the dramatic mortality rates in dialysis patients. In addition, stratification of patients with genetic risk factors combined with clinical risk factors could be used to predict mortality for specific subgroups of dialysis patients and may facilitate tailored therapies.
Supporting Information
GT, genotype; SNP, single nucleotide polymorphism; N, number of subjects; HR, hazard ratio; CI confidence interval; NE, not estimable.
(DOC)
GT, genotype; SNP, single nucleotide polymorphism; N, number of subjects; HR, hazard ratio; CI confidence interval; NE, not estimable.
(DOC)
GT, genotype; SNP, single nucleotide polymorphism; N, number of subjects; HR, hazard ratio; CI confidence interval; NE, not estimable.
(DOC)
* rs397703 is a proxy for rs1207568 (R2 = 0.70). GT, genotype; SNP, single nucleotide polymorphism; N, number of subjects; HR, hazard ratio; CI confidence interval; NE, not estimable.
(DOC)
* rs1800787 was a proxy for rs1800790 (R2 = 1.0). † rs1718711 was a proxy for rs5918 (R2 = 0.93). GT, genotype; SNP, single nucleotide polymorphism; N, number of subjects; HR, hazard ratio; CI, confidence interval.
(DOC)
MAF, minor allele frequency, HWE p-value, Hardy Weinberg equilibrium χ2 test p-values. P-value <0.05 suggests a disequilibrium.
(DOC)
Acknowledgments
We thank the investigators and study nurses of the participating dialysis centers and the data managers of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) for collection and management of data. The members of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group include A.J. Apperloo, J.A. Bijlsma, M. Boekhout, W.H. Boer, P.J.M. van der Boog, H.R. Büller, M. van Buren, F.Th. de Charro, C.J. Doorenbos, M.A. van den Dorpel, A. van Es, W.J. Fagel, G.W. Feith, C.W.H. de Fijter, L.A.M. Frenken, W. Grave, J.A.C.A. van Geelen, P.G.G. Gerlag, J.P.M.C. Gorgels, R.M. Huisman, K.J. Jager, K. Jie, W.A.H. Koning-Mulder, M.I. Koolen, T.K. Kremer Hovinga, A.T.J. Lavrijssen, A.J. Luik, J. van der Meulen, K.J. Parlevliet, M.H.M. Raasveld, F.M. van der Sande, M.J.M. Schonck, M.M.J. Schuurmans, C.E.H. Siegert, C.A. Stegeman, P. Stevens, J.G.P. Thijssen, R.M. Valentijn, G.H. Vastenburg, C.A. Verburgh, H.H. Vincent, and P.F. Vos. We thank the nursing staff of the participating dialysis centers and the staff of the NECOSAD trial office for their invaluable assistance in the collection and management of data for this study. Furthermore, the authors would like to thank Dennis Kremer and Eka Suchiman from the Molecular Epidemiology Section of the Leiden University Medical Center, for their expert assistance with the Sequenom Massarray genotyping platform and Petra Noordijk from the Epidemiology Department for her practical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
JWJ was funded by grants from the Interuniversity Cardiology Institute of the Netherlands (ICIN), the European Community Framework KP7 Programme under grant agreement [n° HEALTH-F2-2009-223004], the Center for Medical Systems Biology (CMSB), a center of excellence approved by the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research (NWO) and the Netherlands Consortium for Healthy Ageing (NCHA). The funders had no role in study design, data collection and analysis, decision to publish or the preparation of the manuscript. TCR and JR were supported by the research program of the BioMedical Materials institute (P3.03 DialysisXS), co-funded by the Dutch Ministry of Economic Affairs, Agriculture and Innovation.
References
- 1. Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int 2004; 65: 2380–2389. [DOI] [PubMed] [Google Scholar]
- 2. Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 1998; 9: S16–S23. [PubMed] [Google Scholar]
- 3. de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009; 302: 1782–1789. 10.1001/jama.2009.1488 [DOI] [PubMed] [Google Scholar]
- 4. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 5. James MT, Quan H, Tonelli M, Manns BJ, Faris P, Laupland KB, et al. CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis 2009; 54: 24–32. 10.1053/j.ajkd.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 6. den Hoedt CH, Bots ML, Grooteman MP, Mazairac AH, Penne EL, van der Weerd NC, et al. Should we still focus that much on cardiovascular mortality in end stage renal disease patients? The CONvective TRAnsport STudy. PLoS One 2013; 8: e61155 10.1371/journal.pone.0061155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weng PH, Hung KY, Huang HL, Chen JH, Sung PK, Huang KC. Cancer-specific mortality in chronic kidney disease: longitudinal follow-up of a large cohort. Clin J Am Soc Nephrol 2001; 6: 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matas AJ, Simmons RL, Kjellstrand CM, Buselmeier TJ, Najarian JS. Increased incidence of malignancy during chronic renal failure. Lancet 1975; 1: 883–886. [DOI] [PubMed] [Google Scholar]
- 9. Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet 1999; 354: 93–99. [DOI] [PubMed] [Google Scholar]
- 10. Walker SR, Gill K, Macdonald K, Komenda P, Rigatto C, Sood MM, et al. Association of frailty and physical function in patients with non-dialysis CKD: a systematic review. BMC Nephrol 2013; 14: 228 10.1186/1471-2369-14-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol 2005; 16: 3728–3735. [DOI] [PubMed] [Google Scholar]
- 12. Carrero JJ, Stenvinkel P. Inflammation in end-stage renal disease—what have we learned in 10 years? Semin Dial 2010; 23: 498–509. 10.1111/j.1525-139X.2010.00784.x [DOI] [PubMed] [Google Scholar]
- 13. Thethi I, Bansal V, Khan H, Hoppensteadt D, Fareed J. Assessment of levels of vascular endothelial growth factor in patients with ESRD and its possible role in cardiovascular morbidity and mortality. Clin Appl Thromb Hemost 2012; 18: 534–537. 10.1177/1076029611435837 [DOI] [PubMed] [Google Scholar]
- 14. Aucella F, Maas R, Vigilante M, Tripepi G, Schwedhelm E, Margaglione M, et al. Methylarginines and mortality in patients with end stage renal disease: a prospective cohort study. Atherosclerosis 2009; 207: 541–545. 10.1016/j.atherosclerosis.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 15. Casserly LF, Dember LM. Thrombosis in end-stage renal disease. Semin Dial 2003; 16: 245–256. [DOI] [PubMed] [Google Scholar]
- 16. Brunet P, Gondouin B, Duval-Sabatier A, Dou L, Cerini C, Dignat-George F, et al. Does uremia cause vascular dysfunction? Kidney Blood Press Res 2011; 34: 284–290. 10.1159/000327131 [DOI] [PubMed] [Google Scholar]
- 17. Pons D, Monraats PS, Zwinderman AH, de Maat MP, Doevendans PA, de Winter RJ, et al. Metabolic background determines the importance of NOS3 polymorphisms in restenosis after percutaneous coronary intervention: A study in patients with and without the metabolic syndrome. Dis Markers 2009; 26: 75–83. 10.3233/DMA-2009-0609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Tiel CM, Bonta PI, Rittersma SZ, Beijk MA, Bradley EJ, Klous AM, et al. p27kip1-838C>A single nucleotide polymorphism is associated with restenosis risk after coronary stenting and modulates p27kip1 promoter activity. Circulation 2009; 120: 669–676. 10.1161/CIRCULATIONAHA.108.842179 [DOI] [PubMed] [Google Scholar]
- 19. Ewing MM, Karper JC, Sampietro ML, de Vries MR, Pettersson K, Jukema JW, et al. Annexin A5 prevents post-interventional accelerated atherosclerosis development in a dose-dependent fashion in mice. Atherosclerosis 2012; 221: 333–340. 10.1016/j.atherosclerosis.2012.01.037 [DOI] [PubMed] [Google Scholar]
- 20. Monraats PS, Kurreeman FA, Pons D, Sewgobind VD, de Vries FR, Zwinderman AH, et al. Interleukin 10: a new risk marker for the development of restenosis after percutaneous coronary intervention. Genes Immun 2007; 8: 44–50. [DOI] [PubMed] [Google Scholar]
- 21. Monraats PS, Pires NM, Schepers A, Agema WR, Boesten LS, de Vries MR, et al. Tumor necrosis factor-alpha plays an important role in restenosis development. FASEB J 2005; 19: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 22. Monraats PS, Fang Y, Pons D, Pires NM, Pols HA, Zwinderman AH, et al. Vitamin D receptor: a new risk marker for clinical restenosis after percutaneous coronary intervention. Expert Opin Ther Targets 2010; 14: 243–251. 10.1517/14728220903520929 [DOI] [PubMed] [Google Scholar]
- 23. Agema WR, Monraats PS, Zwinderman AH, de Winter RJ, Tio RA, Doevendans PA, et al. Current PTCA practice and clinical outcomes in The Netherlands: the real world in the pre-drug-eluting stent era. Eur Heart J 2004; 25: 1163–1170. [DOI] [PubMed] [Google Scholar]
- 24. Sampietro ML, Trompet S, Verschuren JJ, Talens RP, Deelen J, Heijmans BT, et al. A genome-wide association study identifies a region at chromosome 12 as a potential susceptibility locus for restenosis after percutaneous coronary intervention. Hum Mol Genet 2011; 20: 4748–4757. 10.1093/hmg/ddr389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bown MJ, Jones GT, Harrison SC, Wright BJ, Bumpstead S, Baas AF, et al. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am J Hum Genet 2011; 89: 619–627. 10.1016/j.ajhg.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baas AF, Medic J, van 't Slot R, de Kovel CG, Zhernakova A, Geelkerken RH, et al. Association of the TGF-beta receptor genes with abdominal aortic aneurysm. Eur J Hum Genet 2010; 18: 240–244. 10.1038/ejhg.2009.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin CC, Yang WC, Chung MY, Lee PC. Functional polymorphisms in matrix metalloproteinases-1, -3, -9 are associated with arteriovenous fistula patency in hemodialysis patients. Clin J Am Soc Nephrol 2010; 5: 1805–1814. 10.2215/CJN.01500210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ram S, Bass K, Abreo K, Baier RJ, Kruger TE. Tumor necrosis factor-alpha -308 gene polymorphism is associated with synthetic hemodialysis graft failure. J Investig Med 2003; 51: 19–26. [DOI] [PubMed] [Google Scholar]
- 29. Kim Y, Jeong SJ, Lee HS, Kim EJ, Song YR, Kim SG, et al. Polymorphism in the promoter region of the klotho gene (G-395A) is associated with early dysfunction in vascular access in hemodialysis patients. Korean J Intern Med 2008; 23: 201–207. 10.3904/kjim.2008.23.4.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jukema JW, Verschuren JJ, Ahmed TA, Quax PH. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol 2012; 9: 53–62. [DOI] [PubMed] [Google Scholar]
- 31. Gungor Y, Kayatas M, Yildiz G, Ozdemir O, Candan F. The presence of PAI-1 4G/5G and ACE DD genotypes increases the risk of early-stage AVF thrombosis in hemodialysis patients. Ren Fail 2011; 33: 169–175. 10.3109/0886022X.2011.552151 [DOI] [PubMed] [Google Scholar]
- 32. Verschuren JJ, Ocak G, Dekker FW, Rabelink TJ, Jukema JW, Rotmans JI. Candidate gene analysis of arteriovenous fistula failure in hemodialysis patients. Clin J Am Soc Nephrol 2013; 8: 1358–1366. 10.2215/CJN.11091012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Dijk PC, Jager KJ, de Charro F, Collart F, Cornet R, Dekker FW, et al. Renal replacement therapy in Europe: the results of a collaborative effort by the ERA-EDTA registry and six national or regional registries. Nephrol Dial Transplant 2001; 16: 1120–1129. [DOI] [PubMed] [Google Scholar]
- 34. Cozzolino M, Biondi ML, Banfi E, Riser BL, Mehmeti F, Cusi D, et al. CCN2 (CTGF) gene polymorphism is a novel prognostic risk factor for cardiovascular outcomes in hemodialysis patients. Blood Purif 2010; 30: 272–276. 10.1159/000320706 [DOI] [PubMed] [Google Scholar]
- 35. Ma L, Zhang H, Han C, Tong D, Zhang M, Yao Y, et al. Fibroblast growth factor receptor 4 polymorphisms and susceptibility to coronary artery disease. DNA Cell Biol 2012; 31: 1064–1069. 1 10.1089/dna.2011.1552 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36. Oishi Y, Manabe I, Imai Y, Hara K, Horikoshi M, Fujiu K, et al. Regulatory polymorphism in transcription factor KLF5 at the MEF2 element alters the response to angiotensin II and is associated with human hypertension. FASEB J 2010; 24: 1780–1788. 10.1096/fj.09-146589 [DOI] [PubMed] [Google Scholar]
- 37. Coronary Artery Disease (C4D) Genetics Consortium. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet 2011; 43: 339–344. 10.1038/ng.782 [DOI] [PubMed] [Google Scholar]
- 38. Zhou J, Huang Y, Huang RS, Wang F, Xu L, Le Y, et al. A case-control study provides evidence of association for a common SNP rs974819 in PDGFD to coronary heart disease and suggests a sex-dependent effect. Thromb Res 2012; 130: 602–606. 10.1016/j.thromres.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 39. Pastuszczak M, Branicka A, Jakiela B, Stepien E, Jaworek AK, Wojas-Pelc A, et al. The +405 GG variant of vascular endothelial growth factor polymorphism is associated with poor prognosis in patients undergoing coronary artery bypass graft surgery. Pol Arch Med Wewn 2009; 119: 719–725. [PubMed] [Google Scholar]
- 40. Doi K, Noiri E, Nakao A, Fujita T, Kobayashi S, Tokunaga K. Functional polymorphisms in the vascular endothelial growth factor gene are associated with development of end-stage renal disease in males. J Am Soc Nephrol 2006; 17: 823–830. [DOI] [PubMed] [Google Scholar]
- 41. Szeto CC, Chow KM, Poon P, Szeto CY, Wong TY, Li PK. Genetic polymorphism of VEGF: Impact on longitudinal change of peritoneal transport and survival of peritoneal dialysis patients. Kidney Int 2004; 65: 1947–1955. [DOI] [PubMed] [Google Scholar]
- 42. Chen Y, Dawes PT, Packham JC, Mattey DL. Interaction between smoking and polymorphism in the promoter region of the VEGFA gene is associated with ischemic heart disease and myocardial infarction in rheumatoid arthritis. J Rheumatol 2011; 38: 802–809. 10.3899/jrheum.101095 [DOI] [PubMed] [Google Scholar]
- 43. Balakrishnan VS, Guo D, Rao M, Jaber BL, Tighiouart H, Freeman RL, et al. Cytokine gene polymorphisms in hemodialysis patients: association with comorbidity, functionality, and serum albumin. Kidney Int 2004; 65: 1449–1460. [DOI] [PubMed] [Google Scholar]
- 44. Girndt M, Kaul H, Sester U, Ulrich C, Sester M, Georg T, et al. Anti-inflammatory interleukin-10 genotype protects dialysis patients from cardiovascular events. Kidney Int 2002; 62: 949–955. [DOI] [PubMed] [Google Scholar]
- 45. Jaber BL, Rao M, Guo D, Balakrishnan VS, Perianayagam MC, Freeman RB, et al. Cytokine gene promoter polymorphisms and mortality in acute renal failure. Cytokine 2004; 25: 212–219. [DOI] [PubMed] [Google Scholar]
- 46. Hernesniemi JA, Raitakari OT, Kahonen M, Juonala M, Hutri-Kahonen N, Marniemi J, et al. Toll-like receptor 4 gene (Asp299Gly) polymorphism associates with carotid artery elasticity. The cardiovascular risk in young Finns study. Atherosclerosis 2008; 198: 152–159. [DOI] [PubMed] [Google Scholar]
- 47. Stenvinkel P, Wang K, Qureshi AR, Axelsson J, Pecoits-Filho R, Gao P, et al. Low fetuin-A levels are associated with cardiovascular death: Impact of variations in the gene encoding fetuin. Kidney Int 2005; 67: 2383–2392. [DOI] [PubMed] [Google Scholar]
- 48. Shimoyama Y, Taki K, Mitsuda Y, Tsuruta Y, Hamajima N, Niwa T. KLOTHO gene polymorphisms G-395A and C1818T are associated with low-density lipoprotein cholesterol and uric acid in Japanese hemodialysis patients. Am J Nephrol 2009; 30: 383–388. 10.1159/000235686 [DOI] [PubMed] [Google Scholar]
- 49. Friedman DJ, Afkarian M, Tamez H, Bhan I, Isakova T, Wolf M, et al. Klotho variants and chronic hemodialysis mortality. J Bone Miner Res 2009; 24: 1847–1855. 10.1359/jbmr.090516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oguri M, Kato K, Hibino T, Yokoi K, Segawa T, Matsuo H, et al. Identification of a polymorphism of UCP3 associated with recurrent in-stent restenosis of coronary arteries. Int J Mol Med 2007; 20: 533–538. [PubMed] [Google Scholar]
- 51. Monraats PS, Rana JS, Zwinderman AH, de Maat MP, Kastelein JP, Agema WR, et al. -455G/A polymorphism and preprocedural plasma levels of fibrinogen show no association with the risk of clinical restenosis in patients with coronary stent placement. Thromb Haemost 2005; 93: 564–569. [DOI] [PubMed] [Google Scholar]
- 52. Allon M, Zhang L, Maya ID, Bray MS, Fernandez JR. Association of factor V gene polymorphism with arteriovenous graft failure. Am J Kidney Dis 2012; 59: 682–688. 10.1053/j.ajkd.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weiss EJ, Bray PF, Tayback M, Schulman SP, Kickler TS, Becker LC, et al. A polymorphism of a platelet glycoprotein receptor as an inherited risk factor for coronary thrombosis. N Engl J Med 1996; 334: 1090–1094. [DOI] [PubMed] [Google Scholar]
- 54. Pastinen T, Perola M, Niini P, Terwilliger J, Salomaa V, Vartiainen E, et al. Array-based multiplex analysis of candidate genes reveals two independent and additive genetic risk factors for myocardial infarction in the Finnish population. Hum Mol Genet 1998; 7: 1453–1462. [DOI] [PubMed] [Google Scholar]
- 55. He D, ia M, horyshi M, Lemaire S, Milewicz D, Keeley F, et al. Identification and characterization of polymorphism in tropoelastin and their role in late onset cardiovascular disease. Canadian cardiovascular congress 2010. [Google Scholar]
- 56. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. 1995; 57: 289–300. [Google Scholar]
- 57. Smith NL, Hindorff LA, Heckbert SR, Lemaitre RN, Marciante KD, Rice K. Association of genetic variations with nonfatal venous thrombosis in postmenopausal women. JAMA 2007; 297: 489–498. [DOI] [PubMed] [Google Scholar]
- 58. Marks A, Macleod C, McAteer A, Murchie P, Fluck N, Smith WC, et al. Chronic kidney disease, a useful trigger for proactive primary care? Mortality results from a large U.K. cohort. Fam Pract 2013; 30: 282–289. 10.1093/fampra/cms079 [DOI] [PubMed] [Google Scholar]
- 59. Vincenti V, Cassano C, Rocchi M, Persico G. Assignment of the vascular endothelial growth factor gene to human chromosome 6p21.3. Circulation 1996; 93: 1493–1495. [DOI] [PubMed] [Google Scholar]
- 60. Shahbazi M, Fryer AA, Pravica V, Brogan IJ, Ramsay HM, Hutchinson IV, et al. Vascular endothelial growth factor gene polymorphisms are associated with acute renal allograft rejection. J Am Soc Nephrol 2002; 13: 260–264. [DOI] [PubMed] [Google Scholar]
- 61. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997; 18: 4–25. [DOI] [PubMed] [Google Scholar]
- 62. Cohen M, Rentrop KP. Limitation of myocardial ischemia by collateral circulation during sudden controlled coronary artery occlusion in human subjects: a prospective study. Circulation 1986; 74: 469–476. [DOI] [PubMed] [Google Scholar]
- 63. Regieli JJ, Jukema JW, Nathoe HM, Zwinderman AH, Ng S, Grobbee DE, et al. Coronary collaterals improve prognosis in patients with ischemic heart disease. Int J Cardiol 2009; 132: 257–262. 10.1016/j.ijcard.2007.11.100 [DOI] [PubMed] [Google Scholar]
- 64. Biselli PM, Guerzoni AR, de Godoy MF, Pavarino-Bertelli EC, Goloni-Bertollo EM. Vascular endothelial growth factor genetic variability and coronary artery disease in Brazilian population. Heart Vessels 2008; 23: 371–375. 10.1007/s00380-008-1057-6 [DOI] [PubMed] [Google Scholar]
- 65. Howell WM, Ali S, Rose-Zerilli MJ, Ye S. VEGF polymorphisms and severity of atherosclerosis. J Med Genet 2005; 42: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hsiao PJ, Lu MY, Chiang FY, Shin SJ, Tai YD, Juo SH. Vascular endothelial growth factor gene polymorphisms in thyroid cancer. J Endocrinol 2007; 195: 265–270. [DOI] [PubMed] [Google Scholar]
- 67. Ianni M, Porcellini E, Carbone I, Potenzoni M, Pieri AM, Pastizzaro CD, et al. Genetic factors regulating inflammation and DNA methylation associated with prostate cancer. Prostate Cancer Prostatic Dis 2013; 16: 56–61. 10.1038/pcan.2012.30 [DOI] [PubMed] [Google Scholar]
- 68. Masago K, Fujita S, Kim YH, Hatachi Y, Fukuhara A, Nagai H, et al. Effect of vascular endothelial growth factor polymorphisms on survival in advanced-stage non-small-cell lung cancer. Cancer Sci 2009; 100: 1917–1922. 10.1111/j.1349-7006.2009.01253.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Baelde HJ, Eikmans M, Lappin DW, Doran PP, Hohenadel D, Brinkkoetter PT, et al. Reduction of VEGF-A and CTGF expression in diabetic nephropathy is associated with podocyte loss. Kidney Int 2007; 71: 637–645. [DOI] [PubMed] [Google Scholar]
- 70. Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 2008; 358: 1129–1136. 10.1056/NEJMoa0707330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 2003; 88: 2348–2351. [DOI] [PubMed] [Google Scholar]
- 72. Yuan J, Guo Q, Qureshi AR, Anderstam B, Eriksson M, Heimburger O, et al. Circulating vascular endothelial growth factor (VEGF) and its soluble receptor 1 (sVEGFR-1) are associated with inflammation and mortality in incident dialysis patients. Nephrol Dial Transplant 2013; 28: 2356–2363. 10.1093/ndt/gft256 [DOI] [PubMed] [Google Scholar]
- 73. Melder RJ, Koenig GC, Witwer BP, Safabakhsh N, Munn LL, Jain RK. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat Med 1996; 2: 992–997. [DOI] [PubMed] [Google Scholar]
- 74. de Jager DJ, Vervloet MG, Dekker FW. Noncardiovascular mortality in CKD: an epidemiological perspective. Nat Rev Nephrol. 2014; 4: 208–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GT, genotype; SNP, single nucleotide polymorphism; N, number of subjects; HR, hazard ratio; CI confidence interval; NE, not estimable.
(DOC)
GT, genotype; SNP, single nucleotide polymorphism; N, number of subjects; HR, hazard ratio; CI confidence interval; NE, not estimable.
(DOC)
GT, genotype; SNP, single nucleotide polymorphism; N, number of subjects; HR, hazard ratio; CI confidence interval; NE, not estimable.
(DOC)
* rs397703 is a proxy for rs1207568 (R2 = 0.70). GT, genotype; SNP, single nucleotide polymorphism; N, number of subjects; HR, hazard ratio; CI confidence interval; NE, not estimable.
(DOC)
* rs1800787 was a proxy for rs1800790 (R2 = 1.0). † rs1718711 was a proxy for rs5918 (R2 = 0.93). GT, genotype; SNP, single nucleotide polymorphism; N, number of subjects; HR, hazard ratio; CI, confidence interval.
(DOC)
MAF, minor allele frequency, HWE p-value, Hardy Weinberg equilibrium χ2 test p-values. P-value <0.05 suggests a disequilibrium.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.