Abstract
Amyloid precursor protein (APP) has been modified by β and γ-secretase that cause amyloid deposits (plaques) in neuronal cells. Glyceraldhyde-derived AGEs has been identified as a major source of neurotoxicity in Alzheimer’s disease (AD). In a previous study, we demonstrated that glyceraldehyde-derived AGEs increase APP and Aβ via ROS. Furthermore, the combination of AGEs and Aβ has been shown to enhance neurotoxicity. In mice, APP expression is increased by tail vein injection of AGEs. This evidence suggests a correlation between AGEs and the development of AD. However, the role played by AGEs in the pathogenesis of AD remains unclear. In this report, we demonstrate that AGEs up-regulate APP processing protein (BACE and PS1) and Sirt1 expression via ROS, but do not affect the expression of downstream antioxidant genes HO-1 and NQO-1. Moreover, we found that AGEs increase GRP78 expression and enhance the cell death-related pathway p53, bcl-2/bax ratio, caspase 3. These results indicate that AGEs impair the neuroprotective effects of Sirt1 and lead to neuronal cell death via ER stress. Our findings suggest that AGEs increase ROS production, which stimulates downstream pathways related to APP processing, Aβ production, Sirt1, and GRP78, resulting in the up-regulation of cell death related pathway. This in-turn enhances neuronal cell death, which leads to the development of AD.
Introduction
Amyloid precursor protein (APP) is modified by β and γ-secretase [1] to produce amyloid β peptide (Aβ), which accumulates in neuronal cells as amyloid deposit [2, 3]. The deposit is a pathologic characteristic of Alzheimer’s disease (AD) [4–6]. In present studies have demonstrated that BACE (beta-site APP-cleaving enzyme) and PS1 (presenilin 1) have β- and γ-secretase activity which increase Aβ [7, 8]. Ample evidence has demonstrated that oxidative stress is closely related with AD. APP processing and Aβ production are regulated by oxidative stress [9]. Moreover, Aβ leads to neuronal cell death through reactive oxygen species (ROS) [10–12].
Advanced glycation end products (AGEs) are the production of Maillard reaction between carbohydrates and proteins. In clinical setting, AGEs and receptor of AGEs (RAGE) have been identified in neurons and hippocampus [13, 14]. Recent studies have suggested that AGEs and RAGE cause neurotoxicity via oxidative stress [15–24] [25, 26]. AGEs regulate Aβ aggregation and amyloid accumulation [27, 28]. Our findings in a previous study suggested that glyceraldehyde-derived AGEs increase the expression of APP and Aβ via ROS, and that this eventually leads to cell death [29]. However, the role played by AGEs in the development of Alzheimer’s disease (AD) remains unclear.
The NAD+-dependent deacetylase Sirtuin 1 (Sirt1) is correlated with aging and antioxidant function. Recent studies have suggested that Sirt1 may also play a neuroprotective role [30–32], whereby it helps prevent neuronal cell death by reducing oxidative stress [33, 34]. Furthermore, the antioxidant effects of ployphenolic compounds (-)-epigallocatechin-3-gallate (EGCG) and resveratrol are activated by Sirt1 [35, 36], and a previous study involving an AD mouse model showed that Sirt1 suppresses the production of Aβ by activating α-secretase [37–39]. A number of reports have suggested that Sirt1 is able to resist the effects of AGEs [40–42]; however, the precise relationship between AGEs and Sirt1 in AD has not been previously elucidated.
AGEs have been suggested to be closely related with AD, but a complete mechanism of AGEs remains unclear. In this study, we investigate a potential link between AGEs, APP processing, antioxidant pathway and neuronal cell death pathway in AD. We demonstrate that AGEs up-regulate the expression of Sirt1 via ROS but do not affect the expression of downstream antioxidant genes HO-1 and NQO-1. We also found that AGEs increase the expression of GRP78 and enhance the cell death related pathway p53, bax/bcl-2 ratio, caspase 3. Our results suggest that AGEs impair the protective functions of Sirt1 in neuronal cells and also cause neuronal cell death via ER stress. Taken together, these findings demonstrate the important role played by AGEs in the development of AD.
Materials and Methods
Reagents
Bovine serum albumin (BSA), 2',7’-Dichlorodihydrofluorescein diacetate (DCFH-DA), resveratrol, and DL-Glyceraldehyde were purchased from Sigma (St. Louis, MO, USA). DMEM/F12 media, fetal bovine serum (FBS), penicillin, streptomycin, and Hanks Balanced Salt Solution (HBSS) were purchased from Invitrogen (Carlsbad, CA, USA). APP 22C11 antibody was purchased from Chemicon (Temecula, CA, USA). Sirt1, NQO-1, and GRP78 antibodies were purchased from Epitomics (CA, USA). APP, Actin antibodies, and enhanced chemiluminescence (ECL) kit were purchased from Millipore (Billerica, MA, USA); and p53, Nrf-2, Ho-1, bcl-2, bax, caspase3, and β-amyloid antibodies were purchased from Santa Cruz biotechnology (Santa Cruz, CA, USA). Nitrocellulose membranes were purchased from PALL corp. (Ann Arbor, MI, USA).
Preparation of AGEs
AGEs were prepared by incubating BSA (pH = 7.4) in PBS with 20 mM DL-Glyceraldehyde at 37°C for 1 week. The product was dialyzed in PBS at 4°C for 2 hours, and this process was repeated five times. Using the Amicon Ultra protein concentration tube (Millipore), the solution was concentrated at 4°C before being centrifuged at 3,000 rpm for 30 min prior to storage at -80°C [29].
Cell culture and treatment
A neuroblastoma cell line SH-SY5Y (ATCC) was grown in DMEM/F12 (1: 1) media (Invitrogen) routinely supplemented with 10% FBS, 100 units/ml of penicillin, and 100 μg/ml of streptomycin. The culture was incubated at 37°C under an atmosphere of 5% CO2. Prior to treatment, cells were removed from the medium and incubated serum-free for 24 hours.
Western blot
Proteins (30 μg) were resolved using 10% SDS-PAGE gel and then transferred onto nitrocellulose membranes (PALL Corp.). The membranes were blocked using non-fat milk before being incubated with primary antibodies overnight at 4°C. Specifically, primary antibodies comprised mouse monoclonal clone 22C11 for APP (Chemicon) at a dilution of 1:1,000; rabbit monoclonal clones for Sirt1, HO-1, NQO-1, GRP78 p53, bcl-2, bax, and caspase 3 at dilutions of 1:1,000; and mouse monoclonal antibodies for Actin at a dilution of 1:10,000. Secondary antibodies included horseradish peroxidase conjugated anti-mouse, anti-rabbit, and anti-goat at a dilution of 1:1,000. Signals were detected using a Millipore enhanced chemiluminescence detection kit (Millipore). Antibody signals were quantified by normalization with the signal of Actin. Data are presented as mean values from experiments performed in triplicate.
ROS detection
ROS levels were determined according to fluctuations in fluorescence resulting from the oxidation of DCFH-DA (Sigma) [43]. To prepare for this experiment, cells were first incubated with 20 μM DCFH-DA at 37°C for 30 min before being washed with HBSS (Invitrogen) buffer 3 times and broken with a scraper. We then transferred 100 μl of the resulting product to a 96-well plate. A fluorescence microplate reader with an excitation wavelength of 480 nm and an emission wavelength of 540 nm was used to determine the intensity of fluorescence. Data are presented as mean values from experiments performed in triplicate.
Statistical analysis
Significant differences were analyzed using the student t-test and one-way ANOVA. Bars indicate the mean ± SEM obtained from experiments performed in triplicate.
Results
AGEs regulated APP processing via ROS
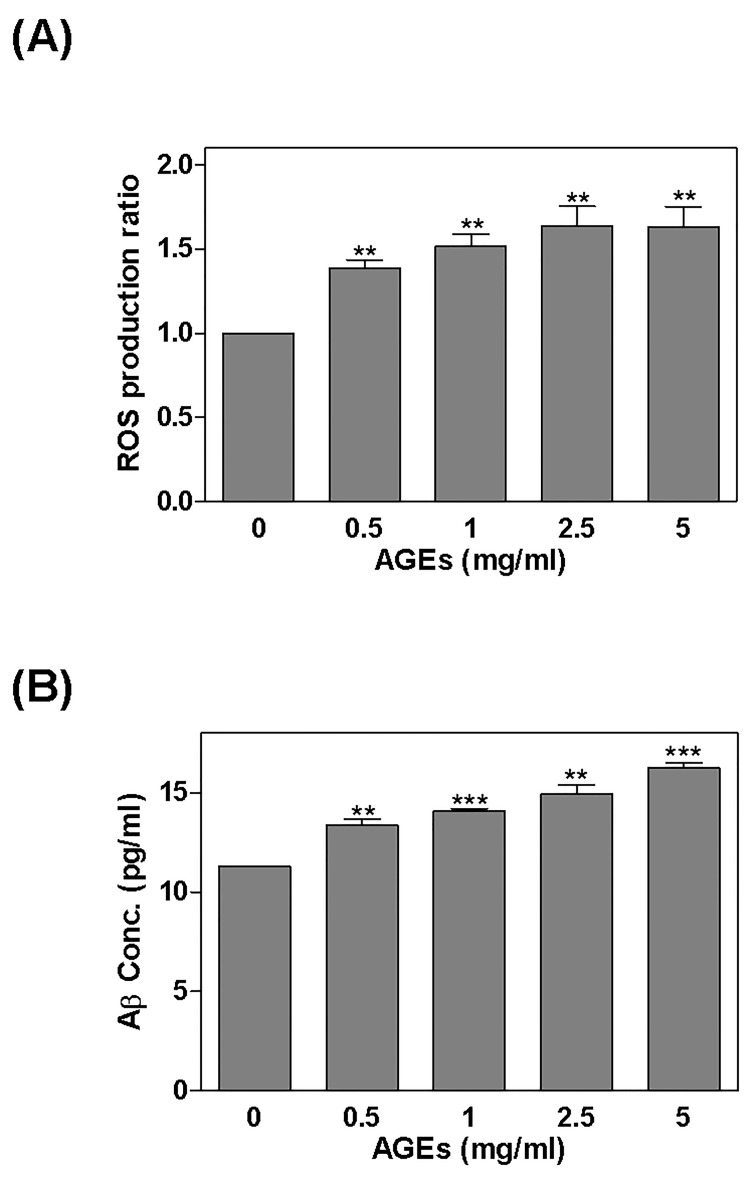
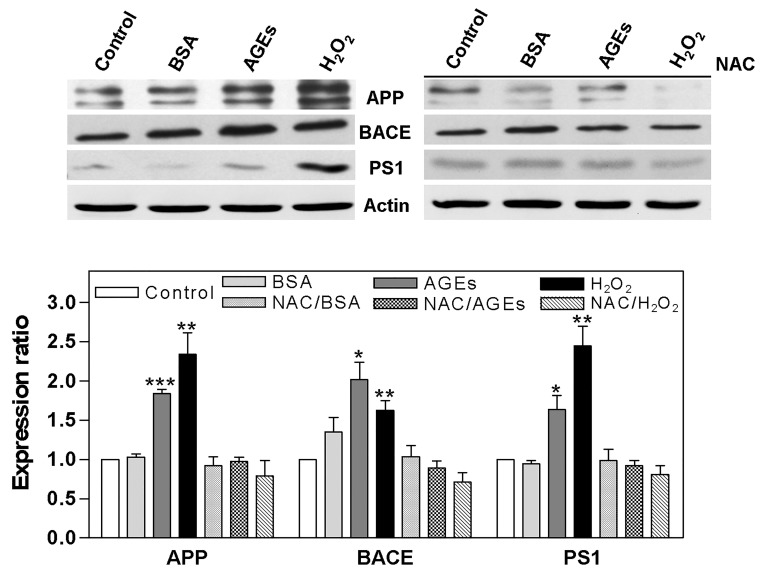
After treating cells with AGEs (0–5 mg/ml) for 24 hours, ROS was detected using DCFH-DA fluorescence (Fig 1A), and A1-42 was detected by ELISA (Fig 1B). ROS was found to have increased significantly to 1.39, 1.52, 1.63, and 1.63 times above normal levels. The level of A1-42 also increased significantly to 13.35 ± 0.32, 14.1 ± 0.1, 14.93 ± 0.48, and 16.25 ± 0.26 pg/ml (A1-42 was 11.27 ± 0.04 pg/ml in the control). After treating the cells with AGEs (0.5 mg/ml), BSA (0.5 mg/ml; as a negative control), or H2O2 (100 μM; as a ROS positive control) for 24 hours, we found that levels of APP (1.84 x), BACE (2.02 x), and PS1 (1.64 x) were increased by AGEs, and that H2O2 enhanced the expression of APP (2.34 x), BACE (1.62 x), and PS1 (2.45 x). Some cells were also pretreated with ROS scavenger NAC for 2 hours before being treated with AGEs, which reduced the effects of AGEs (Fig 2).
Fig 1. Regulation of ROS and Aβ production by AGEs.
After treating cells with AGEs (0–5 mg/ml) for 24 hours: (A) ROS was detected by DCFH-DA fluorescence; and (B) Aβ1-42 was detected by ELISA. Note that the levels of ROS and Aβ1-42 increased significantly. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. control.
Fig 2. Regulation of APP processing by AGEs via ROS.
Cells were treated with AGEs (0.5 mg/ml), BSA (0.5 mg/ml; as a negative control) or H2O2 (100 μM; as an ROS positive control) for 24 hours. AGEs increased the levels of APP, BACE, and PS1, and H2O2 enhanced the expression of APP, BACE, and PS1; some cells were also pretreated with ROS scavenger NAC for 2 hours before being treated with AGEs, which reduced the effects of AGEs. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. control.
Death related pathway was regulated by AGEs
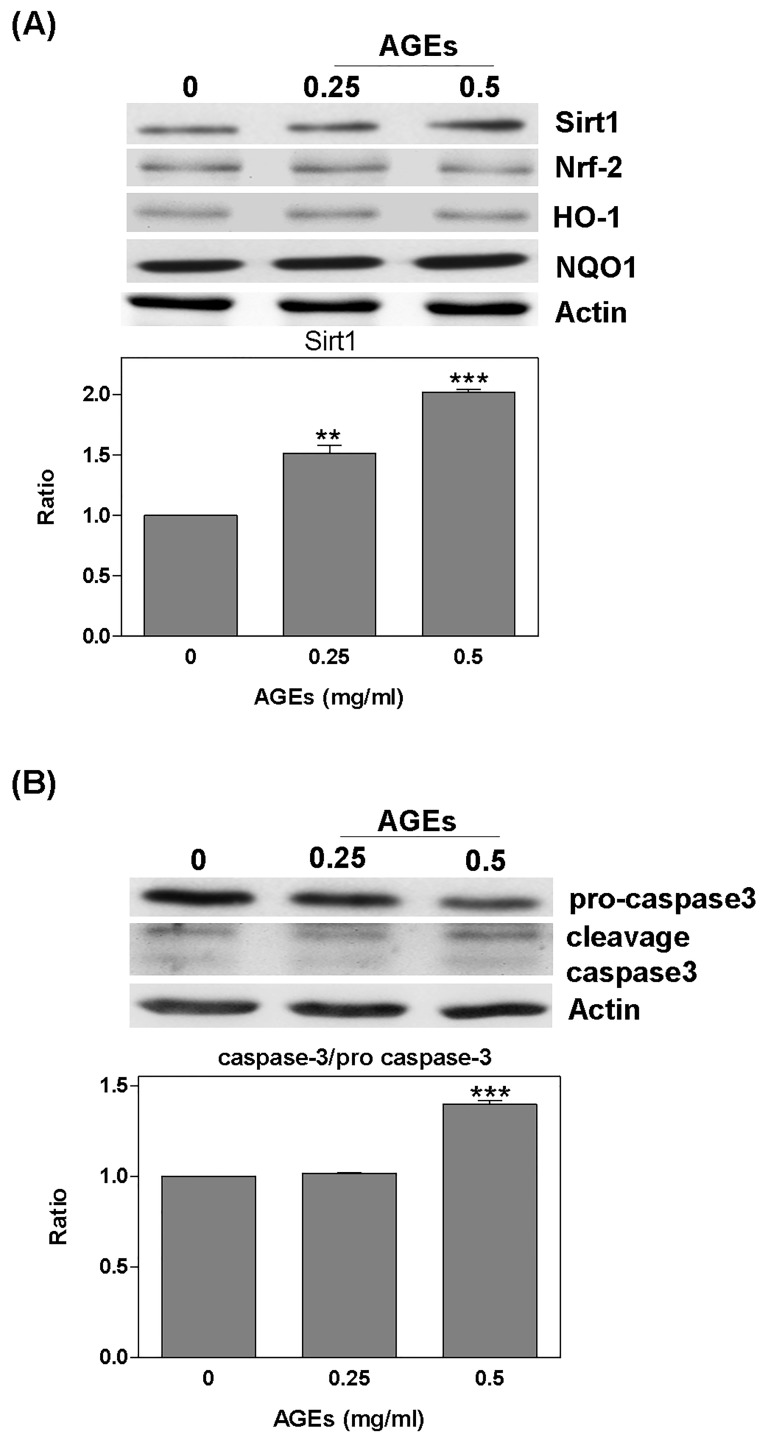
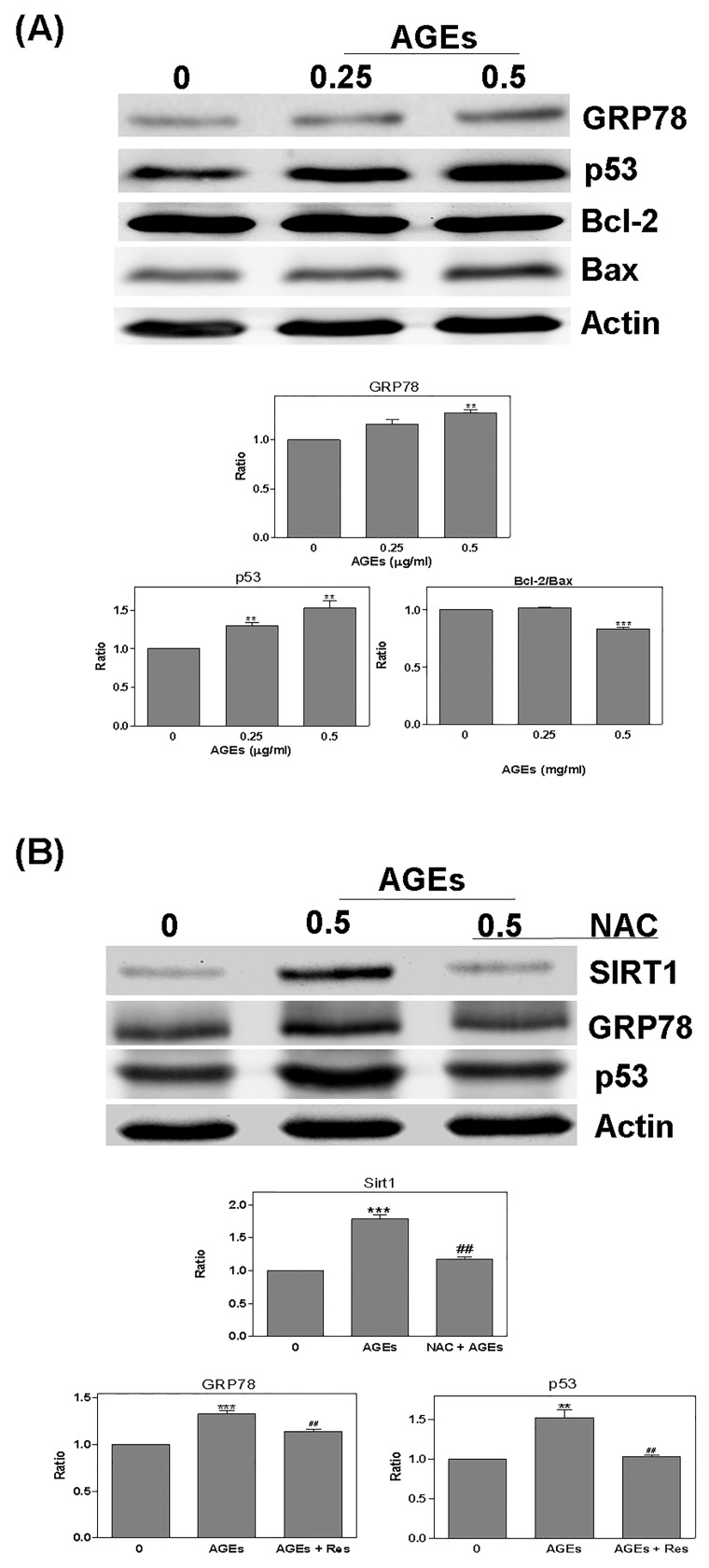
Cells were treated with AGEs (0–0.5 mg/ml) for 24 hours prior to the detection of the expression of the antioxidant protein Sirt1. The level of Sirt1 was increased by AGEs; specifically, 0.25 mg/ml AGEs led to an increase of 1.52 x; and 0.5 mg/ml AGEs led to an increase of 2.02 x. However, the expression of Nrf-2, HO-1, or NQO-1 was no difference change (Fig 3A). AGEs were also shown to increase the fragment of caspase 3 cleavage (AGEs 0.5: 1.4 x) (Fig 3B). Furthermore, the presence of AGEs significantly increased GRP78 (AGEs 0.5: 1.28 x), p53 (AGEs 0.25: 1.3; AGEs 0.5: 1.52 x); however, it decreased the bcl-2/bax ratio (AGEs 0.5: -1.2 x) (Fig 4A). Conversely, when we pretreated the cells with NAC for 2 hours, the effects of AGEs were less pronounced (Sirt1: AGEs: 1.79 x; NAC + AGEs: 1.18 x; GRP78: AGEs: 1.33 x; NAC + AGEs: 1.14 x; p53: AGEs: 1.52 x; NAC + AGEs: 1.03 x) (Fig 4B).
Fig 3. Sirt1 and caspase 3 were regulated by AGEs.
Detection of Sirt1 and antioxidant protein expression after treating cells with AGEs (0–0.5 mg/ml) for 24 hours: (A) The level of Sirt1 was increased by AGEs; however, no effect was observed for Nrf-2, HO-1, or NQO-1; (B) AGEs significantly increased cleavage caspase 3. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. control.
Fig 4. Cell death related pathway was regulated by AGEs.
Detection of the death related pathway, GRP78, p53, bcl-2, and bax: (A) AGEs increased GRP78 and p53 significantly, but decreased the bcl-2/bax ratio; (B) after pretreating cells with NAC for 2 hours, the effects of AGEs were inhibited. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. control. # P < 0.05, ## P < 0.01, ### P < 0.001 vs. AGEs.
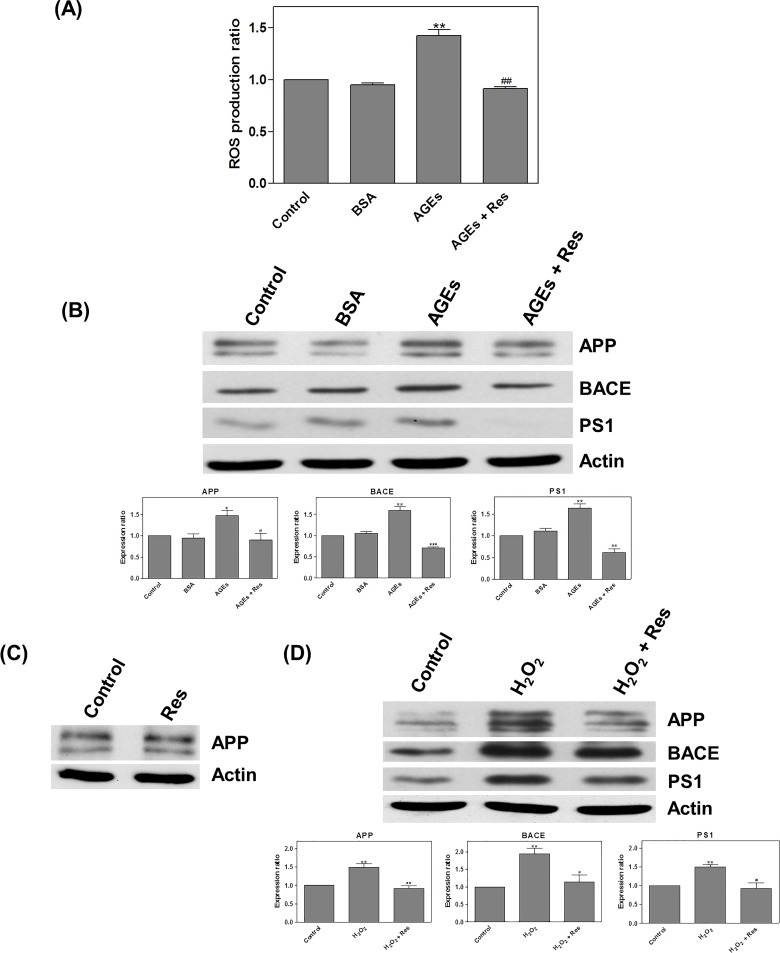
Resveratrol regulated the ROS-induced effects of AGEs
Following treatment with AGEs and resveratrol (Res; 20μM) for 24 hours, we observed a significant increase in the level of ROS (AGEs: 1.42 x; AGEs + Res: 0.91 x) (Fig 5A). AGEs were also found to increase the level of APP (1.47 x), BACE (1.6 x), and PS1 (1.64 x), and in cells treated with H2O2, we observed enhanced expression of APP (1.49 x), BACE (1.94 x), and PS1 (1.5 x) (Fig 5B). Treatment with resveratrol alone had no changes of APP expression (Fig 5C). We had postulated that H2O2 (as a ROS inducer) can induce expressions of APP and its related processing proteins. And then, resveratrol significantly reduced the effects of both AGEs and H2O2 (AGEs: APP: 0.9; BACE: 0.7; PS1: 0.62 x; H2O2 APP: 0.91; BACE: 1.13; PS1: 0.93 x) (Fig 5B and 5D).
Fig 5. Resveratrol regulated the effects of AGEs via ROS.
Detection of ROS by DCFH-DA fluorescence: (A) After treating cells with AGEs and resveratrol (Res; 20μM) for 24 hours, the level of ROS significantly increased; (B) After treating cells with AGEs the levels of APP, BACE, and PS1 were increased. (C) Treatment with resveratrol alone had no changes of APP expression. (D) H2O2 enhanced APP, BACE, and PS1 expression. (B and D) Resveratrol significantly inhibited the effects of AGEs and H2O2. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. control. # P < 0.05, ## P < 0.01, ### P < 0.001 vs. AGEs.
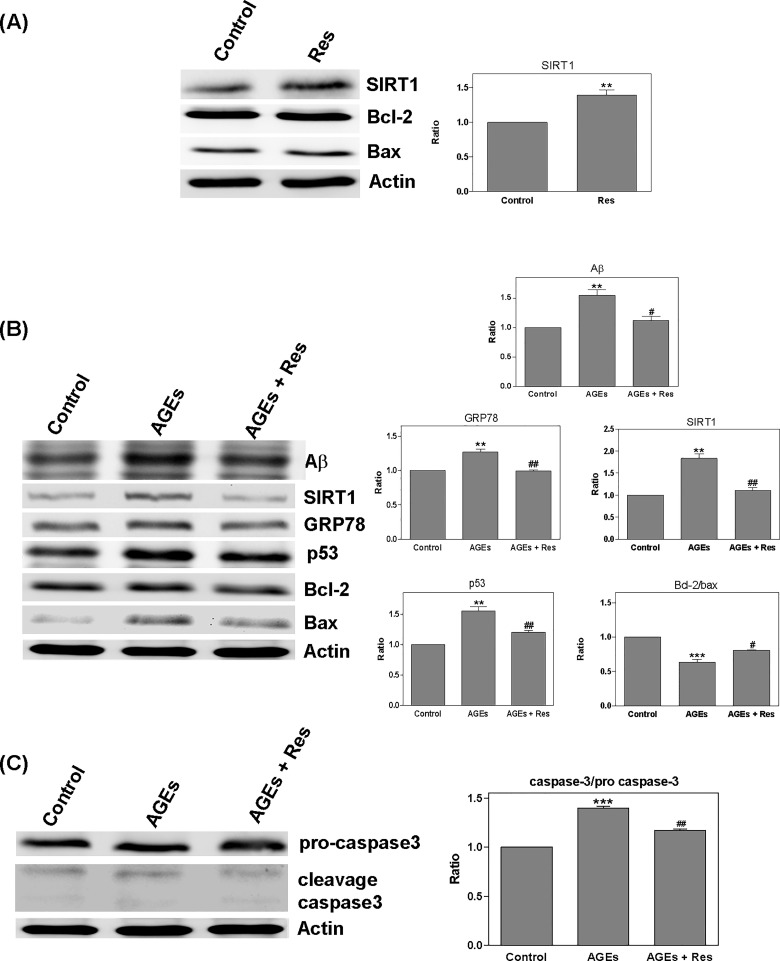
Interaction between resveratrol and AGEs
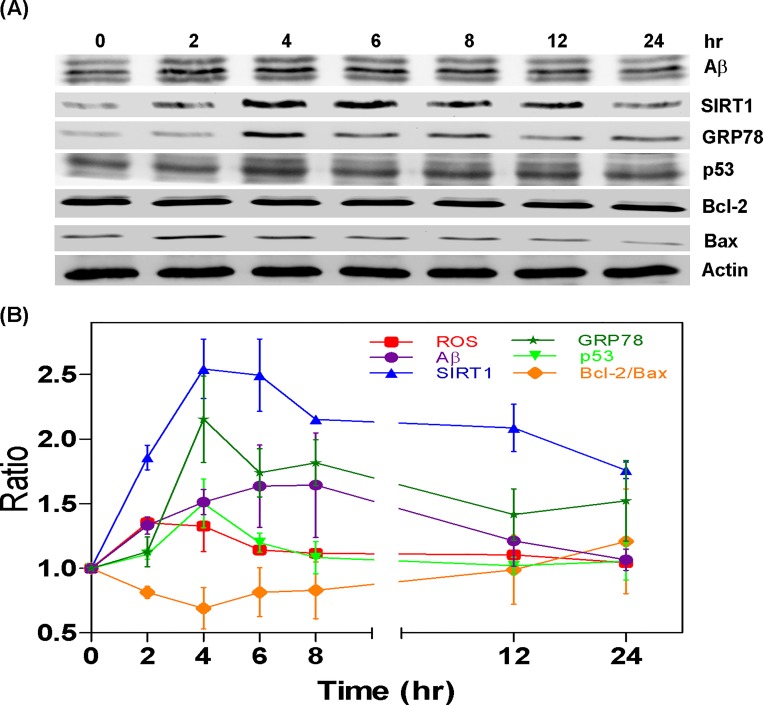
After treating cells with resveratrol (Res; 10μM) for 24 hours, we sought to detect the quantity of Sirt1 and determine the ratio of bcl-2/bax. Resveratrol treatment increased the level of Sirt1 (1.39 x); however, it did not appear to affect the bcl-2/bax ratio (Fig 6A). Conversely, treating cells with a combination of AGEs (0.5 mg/ml) and resveratrol for 24 hours increased in the bcl-2/bax ratio (AGEs: 0.63; AGEs + Res: 0.81 x); however, this led to a reduction in A (AGEs: 1.55; AGEs + Res: 1.12 x), Sirt1 (AGEs: 1.84; AGEs + Res: 1.11 x), GRP78 (AGEs: 1.27; AGEs + Res: 0.99 x), p53 (AGEs: 1.55; AGEs + Res: 1.2 x), and cleavage caspase 3 (AGEs: 1.4; AGEs + Res: 1.17 x) (Fig 6B and 6C). Interestingly, treating cells with a combination of AGEs and resveratrol for 24 hours led to a decrease in Sirt1 expression, but the bcl-2/bax ratio was still increased. We then treated cells with a combination of AGEs and resveratrol for a specific time course (0–24 hours) and investigated the effects of the treatment on ROS, A, Sirt1, GRP78, p53, and the bcl-2/bax ratio. At 2–6 hours, the quantity of ROS was shown to increase, whereas it took 2–12 hours for the expression of Sirt1 to increase. Between 6 and 24 hours, the bcl-2/bax ratio increased whereas the expressions of Aβ, p53, and GRP78 all presented a decrease. All analyses were conducted using one-way ANOVA with results deemed statistically significant at P < 0.0001 (Fig 7A and 7B).
Fig 6. Regulation of AGEs effects by resveratrol.
Detection of Sirt1, bcl-2/bax ratio after treating cells with resveratrol (Res; 10μM) for 24 hours: (A) The level of Sirt1 was increased; however, no effect was observed on the bcl-2/bax ratio; (B) Treating cells with a combination of AGEs (0.5 mg/ml) and resveratrol for 24 hours led to an increase in the bcl-2/bax ratio; (B and C) levels of Aβ, Sirt1, GRP78, p53, and cleavage caspase 3 decreased. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. control. # P < 0.05, ## P < 0.01, ### P < 0.001 vs. AGEs.
Fig 7. The interaction between Resveratrol and AGEs.
Detection of ROS, Aβ, Sirt1, GRP78, p53, and bcl-2/bax ratio after treating cells with a combination of AGEs and resveratrol for 0–24 hours: (A and B) The level of ROS increased at 2–6 hours; Sirt1 expression increased at 2–12 hours; at 6–24 hours, the bcl-2/bax ratio was enhanced and expressions of Aβ, p53, and GRP78 were decreased.
Discussion
APP is a precursor of Aβ, which is a pathogenesis of amyloid deposit in AD. In a previous study, we demonstrated that AGEs up-regulate APP via ROS and enhance the production of Aβ [29]. Nonetheless, the mechanism underlying the relationship between AGEs and AD has yet to be elucidated. This is the first study to investigate a potential link between AGEs, APP processing and neuronal cell death pathway in AD. Our data show that AGEs increase BACE and PS1 expression, and it is suppressed by NAC pretreatment. The same discuss has been demonstrated oxidation stress inference APP processing and Aβ production [44]. This suggests that AGEs regulate APP and APP processing pathway to increase Aβ through ROS.
Previous researchers have suggested that a relationship may exist between Sirt1 and ROS in neuronal cells [33–36]. Sirt1 plays a neuroprotective function by inhibiting the effects of ROS [30–34]. Sirt1 has also been shown to decrease the production of Aβ through the activation of α-secretase [37–39, 45]. Our results reveal that AGEs increase the expression of Sirt1; however, AGEs do not appear to affect the antioxidant proteins Nrf-2, HO-1, NQO-1. Furthermore, we observed a decrease in the bcl-2/bax ratio with an increase in the expression of p53 and cleavage caspase3. We also found that AGEs increased the expression of APP, Aβ, and ROS. Our findings differ from those of previous reports, which suggested that Sirt1 does not have a neuroprotective function. For example, Yuyun et al. claimed that the high expression of Sirt1 may compromise the mass and function of mitochondria through the overproduction of ROS [46], which suggests that AGEs increase the expression of Sirt1 but also promotes cell death pathway.
Recent studies have suggested that Aβ induces stress-mediated apoptosis in the endoplasmic reticulum (ER) during AD progression [47]. In this study, we observed that AGEs up-regulated GRP78, p53, and caspase 3 and a decrease in the bcl-2/bax ratio. Lin et al. suggested that ER-stress stimulates the expression of p53 [48], and enhances the expression of GRP78, thereby inducing the cell death pathway [49] and promoting neurotoxicity [50]. Moreover, Aβ has been shown to regulate the transcription of p53 [51]. These reports confirm that AGEs cause the production of ROS and Aβ as well as the downstream ER stress-mediated apoptosis pathway. In contrast, a number of reports have suggested that Sirt1 and ER stress are antagonistic [52, 53]. Our data indicates that AGEs increase the expression of Sirt1 and GRP78, which implies that they are closely related to the neuronal cell death pathway.
Resveratrol has a direct sirtuin activation function [54], and regulate suppress ROS production [55–57]. Moreover, resveratrol has been shown to suppress the neurotoxicity of ROS and Aβ [58] via β -secretase inhibition [59]. Our results show that APP, BACE, and PS1 are increased by AGEs or H2O2, however, it is decreased by resveratrol treatment. Our data conform to resveratrol inhibit ROS, and inhibit AGEs effecting through ROS. Moreover, Our results reveal that Sirt1 and the bcl-2/bax ratio are enhanced by resveratrol. Interestingly, treating cells with a combination of AGEs and resveratrol for 24 hours led to a decrease in Sirt1 expression, but the bcl-2/bax ratio was still increased. Furthermore, when cells were treated with AGEs and resveratrol for time course of 2 to 24 hours, the level of ROS, Aβ, Sirt1, GRP78, p53, and bcl-2/bax ratio are dynamic change the same. This suggests that 2 to 12 hours, resveratrol induces the suppression of ROS and the downstream pathway by Sirt1. Our findings also confirm that AGEs and resveratrol decrease Sirt1 at 24 hours (Fig 6B). More importantly, they demonstrate that the effect of AGEs on Sirt1 and GRP78 regulation is correct.
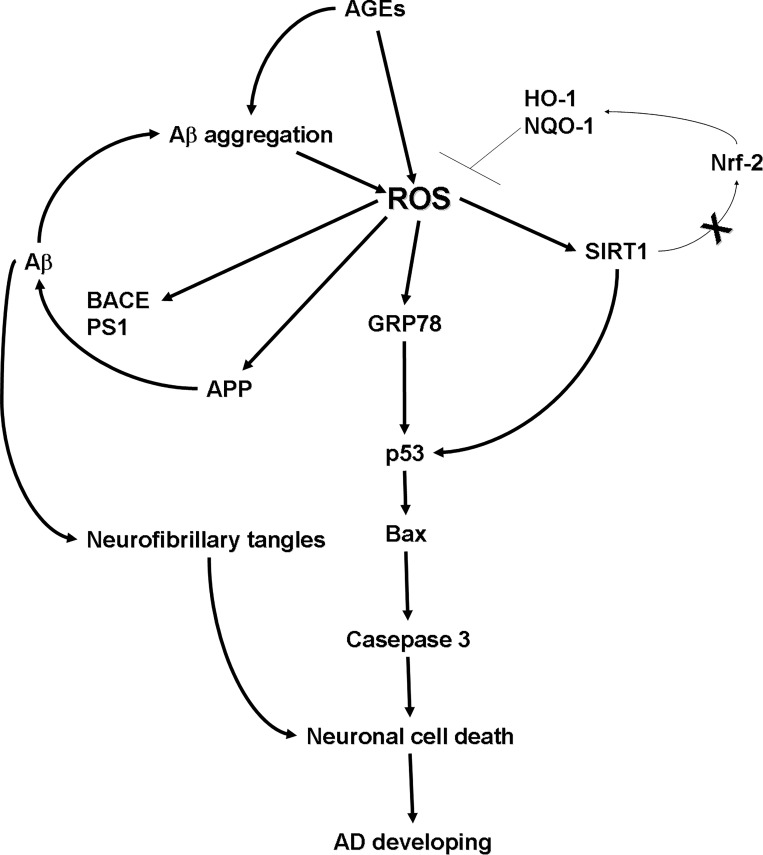
In this research, we demonstrated that AGEs increase the expression of Sirt1 and GRP78, while promoting cell death pathways. This is the first study to demonstrate that AGEs regulates the expression of Sirt1 and GRP78 via ROS. More importantly, we were able to elucidate the mechanism played by AGEs in the pathogenesis of AD through the regulation of ROS. The mechanism by which AGEs affect the development of AD is presented in Fig 8. As shown in the figure, AGEs increase the production of ROS, which stimulates the downstream pathways of Sirt1, GRP78, APP processing, and Aβ production. Fourthermore, the formation of Aβ aggregation and neurofibrillary tangles are enhanced. This in-turn ultimately results in the up-regulation of the cell death pathway, which enhances neuronal cell death, leading to the development of AD. The accumulation of AGEs is a natural process that increases slowly with aging; however, abnormal accumulation of AGEs can be induced by disease, dietary habits, and other factors. AGEs rapidly accumulate in the body, then through in the brain, and turn on the mechanism of AGEs effecting. Finally, in life span, the level of AGEs is an important key point for AD developing.
Fig 8. The mechanism by which AGEs promotes the development of AD.
An increase in ROS production stimulated downstream pathways related to APP processing, Aβ production, Sirt1, and GRP78. This resulted in the up-regulation of the cell death related pathway and enhanced neuronal cell death, which can lead to the development of AD.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Lendon CL, Ashall F, Goate AM (1997) Exploring the etiology of Alzheimer disease using molecular genetics. Jama 277:825–831. [PubMed] [Google Scholar]
- 2. Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A 82:4245–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Naslund J, Schierhorn A, Hellman U, Lannfelt L, Roses AD, Tjernberg LO, et al. (1994) Relative abundance of Alzheimer A beta amyloid peptide variants in Alzheimer disease and normal aging. Proc Natl Acad Sci U S A 91:8378–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Selkoe DJ (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81:741–766. [DOI] [PubMed] [Google Scholar]
- 5. Apelt J, Schliebs R (2001) Beta-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res 894:21–30. [DOI] [PubMed] [Google Scholar]
- 6. Dickson DW (1997) The pathogenesis of senile plaques. J Neuropathol Exp Neurol 56:321–339. [DOI] [PubMed] [Google Scholar]
- 7. Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. (1999) Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286:735–741. [DOI] [PubMed] [Google Scholar]
- 8. Nunan J, Small DH (2000) Regulation of APP cleavage by alpha-, beta- and gamma-secretases. FEBS Lett 483:6–10. [DOI] [PubMed] [Google Scholar]
- 9. Olivieri G, Brack C, Muller-Spahn F, Stahelin HB, Herrmann M, Renard P, et al. (2000) Mercury induces cell cytotoxicity and oxidative stress and increases beta-amyloid secretion and tau phosphorylation in SHSY5Y neuroblastoma cells. J Neurochem 74: 231–236. [DOI] [PubMed] [Google Scholar]
- 10. Olivieri G, Baysang G, Meier F, Muller-Spahn F, Stahelin HB, Brockhaus M, et al. (2001) N-acetyl-L-cysteine protects SHSY5Y neuroblastoma cells from oxidative stress and cell cytotoxicity: effects on beta-amyloid secretion and tau phosphorylation. J Neurochem 76: 224–233. [DOI] [PubMed] [Google Scholar]
- 11. Butterfield DA, Drake J, Pocernich C, Castegna A (2001) Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol Med 7: 548–554. [DOI] [PubMed] [Google Scholar]
- 12. Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262: 689–695. [DOI] [PubMed] [Google Scholar]
- 13. Wang MY, Ross-Cisneros FN, Aggarwal D, Liang CY, Sadun AA (2009) Receptor for advanced glycation end products is upregulated in optic neuropathy of Alzheimer's disease. Acta Neuropathol 118: 381–389. 10.1007/s00401-009-0513-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donahue JE, Flaherty SL, Johanson CE, Duncan JA, Silverberg GD 3rd, Miller MC, et al. (2006) RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta Neuropathol 112: 405–415. [DOI] [PubMed] [Google Scholar]
- 15. Mruthinti S, Sood A, Humphrey CL, Swamy-Mruthinti S, Buccafusco JJ (2006) The induction of surface beta-amyloid binding proteins and enhanced cytotoxicity in cultured PC-12 and IMR-32 cells by advanced glycation end products. Neuroscience 142:463–473. [DOI] [PubMed] [Google Scholar]
- 16. Woltjer RL, Maezawa I, Ou JJ, Montine KS, Montine TJ (2003) Advanced glycation endproduct precursor alters intracellular amyloid-beta/A beta PP carboxy-terminal fragment aggregation and cytotoxicity. J Alzheimers Dis 5:467–476. [DOI] [PubMed] [Google Scholar]
- 17. Loske C, Neumann A, Cunningham AM, Nichol K, Schinzel R, Riederer P, et al. (1998) Cytotoxicity of advanced glycation endproducts is mediated by oxidative stress. J Neural Transm 105:1005–1015. [DOI] [PubMed] [Google Scholar]
- 18. Gasic-Milenkovic J, Loske C, Deuther-Conrad W, Munch G (2001) Protein "AGEing"—cytotoxicity of a glycated protein increases with its degree of AGE-modification. Z Gerontol Geriatr 34:457–460. [DOI] [PubMed] [Google Scholar]
- 19. Deuther-Conrad W, Loske C, Schinzel R, Dringen R, Riederer P, Munch G (2001) Advanced glycation endproducts change glutathione redox status in SH-SY5Y human neuroblastoma cells by a hydrogen peroxide dependent mechanism. Neurosci Lett 312:29–32. [DOI] [PubMed] [Google Scholar]
- 20. Wei Y, Chen L, Chen J, Ge L, He RQ (2009) Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biol 10:10 10.1186/1471-2121-10-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choei H, Sasaki N, Takeuchi M, Yoshida T, Ukai W, Yamagishi S, et al. (2004) Glyceraldehyde-derived advanced glycation end products in Alzheimer's disease. Acta Neuropathol 108:189–193. [DOI] [PubMed] [Google Scholar]
- 22. Sato T, Shimogaito N, Wu X, Kikuchi S, Yamagishi S, Takeuchi M (2006) Toxic advanced glycation end products (TAGE) theory in Alzheimer's disease. Am J Alzheimers Dis Other Demen 21:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takeuchi M, Bucala R, Suzuki T, Ohkubo T, Yamazaki M, Koike T, et al. (2000) Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J Neuropathol Exp Neurol 59:1094–1105. [DOI] [PubMed] [Google Scholar]
- 24. Takeuchi M, Kikuchi S, Sasaki N, Suzuki T, Watai T, Iwaki M, et al. (2004) Involvement of advanced glycation end-products (AGEs) in Alzheimer's disease. Curr Alzheimer Res 1:39–46. [DOI] [PubMed] [Google Scholar]
- 25. Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, et al. (1996) RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature 382: 685–691. [DOI] [PubMed] [Google Scholar]
- 26. Mruthinti S, Capito N, Sood A, Buccafusc JJ (2007) Cytotoxicity of Abeta1-42, RAGE23-54, and an Abeta-RAGE complex in PC-12 cells. Curr Alzheimer Res 4: 581–586. [DOI] [PubMed] [Google Scholar]
- 27. Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, et al. (1994) Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci U S A 91: 4766–4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Munch G, Mayer S, Michaelis J, Hipkiss AR, Riederer P, Muller R, et al. (1997) Influence of advanced glycation end-products and AGE-inhibitors on nucleation-dependent polymerization of beta-amyloid peptide. Biochim Biophys Acta 1360: 17–29. [DOI] [PubMed] [Google Scholar]
- 29. Ko SY, Lin YP, Lin YS, Chang SS (2010) Advanced glycation end products enhance amyloid precursor protein expression by inducing reactive oxygen species. Free Radic Biol Med 49:474–480. 10.1016/j.freeradbiomed.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 30. Jiang M, Wang J, Fu J, Du L, Jeong H, West T, et al. (2012) Neuroprotective role of Sirt1 in mammalian models of Huntington's disease through activation of multiple Sirt1 targets. Nat Med 18:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, et al. (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J 26:3169–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao Y, Luo P, Guo Q, Li S, Zhang L, Zhao M, et al. (2012) Interactions between SIRT1 and MAPK/ERK regulate neuronal apoptosis induced by traumatic brain injury in vitro and in vivo. Exp Neurol 237:489–498. 10.1016/j.expneurol.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 33. Khan RS, Dine K, Das Sarma J, Shindler KS (2014) SIRT1 activating compounds reduce oxidative stress mediated neuronal loss in viral induced CNS demyelinating disease. Acta Neuropathol Commun 2:3 10.1186/2051-5960-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khan RS, Fonseca-Kelly Z, Callinan C, Zuo L, Sachdeva MM, Shindler KS (2012) SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front Cell Neurosci 6:63 10.3389/fncel.2012.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li J, Feng L, Xing Y, Wang Y, Du L, Xu C, et al. (2014) Radioprotective and antioxidant effect of resveratrol in hippocampus by activating Sirt1. Int J Mol Sci 15:5928–5939. 10.3390/ijms15045928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ye Q, Ye L, Xu X, Huang B, Zhang X, Zhu Y, et al. (2012) Epigallocatechin-3-gallate suppresses 1-methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1alpha signaling pathway. BMC Complement Altern Med 12:82 10.1186/1472-6882-12-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aso E, Semakova J, Joda L, Semak V, Halbaut L, Calpena A, et al. (2013) Triheptanoin supplementation to ketogenic diet curbs cognitive impairment in APP/PS1 mice used as a model of familial Alzheimer's disease. Curr Alzheimer Res 10:290–297. [DOI] [PubMed] [Google Scholar]
- 38. Donmez G, Wang D, Cohen DE, Guarente L (2010) SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell 142:320–332. 10.1016/j.cell.2010.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39. Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, et al. (2006) Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem 281:21745–21754. [DOI] [PubMed] [Google Scholar]
- 40. Huang K, Chen C, Hao J, Huang J, Wang S, Liu P, et al. (2014) Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-beta1 in rat glomerular messangial cells. Mol Cell Endocrinol 399:178–189. 10.1016/j.mce.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 41. Huang K, Huang J, Xie X, Wang S, Chen C, Shen X, et al. (2013) Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-beta1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med 65:528–540. 10.1016/j.freeradbiomed.2013.07.029 [DOI] [PubMed] [Google Scholar]
- 42. Uribarri J, Cai W, Pyzik R, Goodman S, Chen X, Zhu L, et al. (2013) Suppression of native defense mechanisms, SIRT1 and PPARgamma, by dietary glycoxidants precedes disease in adult humans; relevance to lifestyle-engendered chronic diseases. Amino Acids 46:301–309. 10.1007/s00726-013-1502-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2',7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231. [DOI] [PubMed] [Google Scholar]
- 44. Olivieri G, Brack C, Muller-Spahn F, Stähelin HB, Herrmann M, Renard P, et al. (2000) Mercury induces cell cytotoxicity and oxidative stress and increases beta-amyloid secretion and tau phosphorylation in SHSY5Y neuroblastoma cells. J Neurochem 74:231–236. [DOI] [PubMed] [Google Scholar]
- 45. Sun Q, Hu H, Wang W, Jin H, Feng G, Jia N (2014) Taurine attenuates amyloid beta 1-42-induced mitochondrial dysfunction by activating of SIRT1 in SK-N-SH cells. Biochem Biophys Res Commun 447:485–489. 10.1016/j.bbrc.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 46. Yuyun X, Jinjun Q, Minfang X, Jing Q, Juan X, Rui M, et al. (2013) Effects of Low Concentrations of Rotenone upon Mitohormesis in SH-SY5Y Cells. Dose Response 11:270–280. 10.2203/dose-response.12-005.Gao [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Costa RO, Ferreiro E, Martins I, Santana I, Cardoso SM, Oliveira CR, et al. (2012) Amyloid beta-induced ER stress is enhanced under mitochondrial dysfunction conditions. Neurobiol Aging 33:824 e825-816. 10.1016/j.neurobiolaging.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 48. Lin WC, Chuang YC, Chang YS, Lai MD, Teng YN, Su IJ, et al. (2012) Endoplasmic reticulum stress stimulates p53 expression through NF-kappaB activation. PLoS One 7:e39120 10.1371/journal.pone.0039120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou S, Yin X, Zheng Y, Miao X, Feng W, Cai J, et al. (2014) Metallothionein prevents intermittent hypoxia-induced cardiac endoplasmic reticulum stress and cell death likely via activation of Akt signaling pathway in mice. Toxicol Lett 227:113–123. 10.1016/j.toxlet.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 50. Yuan D, Wan JZ, Deng LL, Zhang CC, Dun YY, Dai YW, et al. (2014) Chikusetsu saponin V attenuates MPP+-induced neurotoxicity in SH-SY5Y cells via regulation of Sirt1/Mn-SOD and GRP78/caspase-12 pathways. Int J Mol Sci 15:13209–13222. 10.3390/ijms150813209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu T, Ren D, Zhu X, Yin Z, Jin G, Zhao Z, et al. (2013) Transcriptional signaling pathways inversely regulated in Alzheimer's disease and glioblastoma multiform. Sci Rep 3:3467 10.1038/srep03467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ghemrawi R, Pooya S, Lorentz S, Gauchotte G, Arnold C, Gueant JL, et al. (2013) Decreased vitamin B12 availability induces ER stress through impaired SIRT1-deacetylation of HSF1. Cell Death Dis 4:e553 10.1038/cddis.2013.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X, et al. (2011) Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J 25:1664–1679. 10.1096/fj.10-173492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gertz M, Nguyen GT, Fischer F, Suenkel B, Schlicker C, Fränzel B, et al. (2012) A molecular mechanism for direct sirtuin activation by resveratrol. PLoS One 7:e49761 10.1371/journal.pone.0049761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kao CL, Chen LK, Chang YL, Yung MC, Hsu CC, Chen YC, et al. (2010) Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J Atheroscler Thromb 17:970–979. [DOI] [PubMed] [Google Scholar]
- 56. Lee S, Van Bergen NJ, Kong GY, Chrysostomou V, Waugh HS, O'Neill EC, et al. (2011) Mitochondrial dysfunction in glaucoma and emerging bioenergetic therapies. Exp Eye Res 93:204–212. 10.1016/j.exer.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 57. Li YG, Zhu W, Tao JP, Xin P, Liu MY, Li JB, et al. (2013) Resveratrol protects cardiomyocytes from oxidative stress through SIRT1 and mitochondrial biogenesis signaling pathways. Biochem Biophys Res Commun 438:270–276. 10.1016/j.bbrc.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 58. Marambaud P, Zhao H, Davies P (2005) Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem 280:37377–37382. [DOI] [PubMed] [Google Scholar]
- 59. Choi CW, Choi YH, Cha MR, Kim YS, Yon GH, Hong KS, et al. (2011) In vitro BACE-1 inhibitory activity of resveratrol oligomers from the seed extract of Paeonia lactiflora. Planta Med 77:374–376. 10.1055/s-0030-1250370 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.