Abstract
This study investigated global gene expression in the small yellow follicles (6–8 mm diameter) of broiler-type B strain Taiwan country chickens (TCCs) in response to acute heat stress. Twelve 30-wk-old TCC hens were divided into four groups: control hens maintained at 25°C and hens subjected to 38°C acute heat stress for 2 h without recovery (H2R0), with 2-h recovery (H2R2), and with 6-h recovery (H2R6). Small yellow follicles were collected for RNA isolation and microarray analysis at the end of each time point. Results showed that 69, 51, and 76 genes were upregulated and 58, 15, 56 genes were downregulated after heat treatment of H2R0, H2R2, and H2R6, respectively, using a cutoff value of two-fold or higher. Gene ontology analysis revealed that these differentially expressed genes are associated with the biological processes of cell communication, developmental process, protein metabolic process, immune system process, and response to stimuli. Upregulation of heat shock protein 25, interleukin 6, metallopeptidase 1, and metalloproteinase 13, and downregulation of type II alpha 1 collagen, discoidin domain receptor tyrosine kinase 2, and Kruppel-like factor 2 suggested that acute heat stress induces proteolytic disintegration of the structural matrix and inflamed damage and adaptive responses of gene expression in the follicle cells. These suggestions were validated through gene expression, using quantitative real-time polymerase chain reaction. Functional annotation clarified that interleukin 6-related pathways play a critical role in regulating acute heat stress responses in the small yellow follicles of TCC hens.
Introduction
Global warming increases environmental temperatures and affects not only humans but also livestock [1,2,3]. Animal exposure to hot environments deleteriously affects their reproductive functions. In females, heat stress adversely affects oogenesis, oocyte maturation, fertilization, and embryo development and implantation rate [4,5]. In chickens, high ambient temperatures affect their endocrine systems and reproductive and egg-laying performance [6]. Thus, in tropical areas, such as Taiwan, high temperatures and humidity during summer induce stress in poultry. The average temperature in Taiwan has increased by 0.8°C in past decades, with summer temperature and humidity reaching 38°C and 80%, respectively (http://www.cwb.gov.tw/V7/index.htm).
Approximately 12,000 oocytes are present in the ovary of a mature hen. However, only a few hundred oocytes are selected for ovulation and subsequent egg formation. A functional hen ovary contains hundreds of white cortical follicles with a diameter of 1–5 mm, small yellow follicles (SYFs) with a diameter of 6–8 mm, and large yellow preovulatory hierarchy follicles with a diameter of 9–40 mm [7,8]. The SYFs are in a crucial prehierarchical stage related to the development of follicles and the laying performance [9]. A single follicle is selected from the SYF pool every day to join the group of preovulatory follicles destined for ovulation [10,11].
The normal body temperature of chicken is 40–41°C [12]. Panting is the primary mode of heat dissipation in birds. Heat insults exceeding the capacity of bodily thermoregulation detrimentally affect production performance. Taiwan country chickens (TCCs) are native, slow-growing breeds and exhibit higher thermotolerance than do nonnative breeds [13,14]. Broiler-type B strain TCCs have been bred for body weight and comb size for over 20 generations [15]. A few reports have investigated differential gene expression in chickens in response to heat stress [13,16,17,18]; however, the effect of acute heat stress on global gene expression in the ovary, particularly in native chickens of tropical regions, has not been explored. This study thus aimed to analyze the global mRNA expression of SYF in TCCs as a basis for delineating the mechanism of acute heat stress response in chicken hens.
Materials and Methods
Experimental animals and management
Twelve 30-wk-old broiler-type B strain TCC hens originally bred for meat production by National Chung Hsing University [19,20] were used in this study. The care and use of all animals in the study were complied with the guidelines and was approved by the Institutional Animal Care and Use Committee of National Chung Hsing University (Taichung, Taiwan; IACUC No. 102–06). The hens, housed in individual cages at 18 wk of age, peaked in egg production at 30 weeks [15]. The hens were placed in a climate chamber for over 2 weeks for adaptation under conditions of a light:dark photoperiod of 14:10 h at 25°C and 55% relative humidity (RH) before acute heat stress treatment. Feed and water were provided ad libitum, including the acute heat stress and recovery periods.
Conditions of acute heat stress and sample collection
After adaptation, hens were randomly allocated to four groups (three hens in each group). The control group was maintained at 25°C and 55% RH throughout the experiment. The hens in the other three groups were treated with an acute heat stress at 38°C for 2 h without recovery (H2R0), at 25°C with 2-h recovery (H2R2), and at 25°C with 6-h recovery (H2R6). The light: dark photoperiod and RH during the heat stress treatment and recovery remained the same as the adaptation period. Physiological parameters (respiratory rate and body temperature) were recorded during treatment and recovery. The respiratory rate was measured by counting the panting breaths of the chickens for 15 sec and the value was multiplied by 4 to give the number of breaths per min. The body temperature was obtained by introducing an alcohol thermometer into the cloaca of the chickens and recorded until the reading was stable. The hens were sacrificed by electric stunning and followed by bleeding from carotid artery at the end of each time point; their SYF were collected, placed overnight in cryogenic vials with 0.5 mL of RNAfter (GMbiolab Co, Ltd, Taichung, Taiwan) at 4°C, and stored at −80°C until RNA isolation. The time from sacrificing to the sample collection was limited to within 10 min.
Gene expression analysis in response to acute heat stress through microarray analysis
A chicken 44K oligo microarray (Agilent Technologies, Santa Clara, CA, USA) was used to determine differential gene expression between the control and acute-heat -stressed groups [13]. RNA isolated from the SYF of each hen was used for reverse transcription. The second strand complementary DNA (cDNA) was synthesized from 1 μg of the total RNA and amplified using a Quick-Amp Labeling Kit (Agilent Technologies). The cDNA served as the template for in vitro transcription for producing the target cRNA in the presence of Cy3-CTP (CyDye, Agilent Technologies). In total, 1.65 μg of Cy3-labled cRNA was fragmented to an average size of approximately 50–100 nucleotides through fragmentation buffer incubation at 60°C for 30 min. Subsequently, the corresponding fragment-labeled cRNA was hybridized to the microarray at 65°C for 17 h. After washing and drying, using a nitrogen gun, the microarrays were scanned using a microarray scanner (Agilent Technologies) at 535 nm for Cy3. The scanned images were analyzed using Feature Extraction 10.5.1.1 software (Agilent Technologies) and normalized for quantifying the signal and background intensities of each feature. Data was acquired using the following criteria: (1) p < 0.01 for gene expression difference using GeneSpring software (Agilent Technologies). (2) A distinct signal from the microarray image flagged by the software. (3) A false discovery rate of < 0.05. Results of the microarray analysis were filtered from the features when flags were present or marginal in at least one of the four groups (control, H2R0, H2R2, and H2R6). The dataset of microarray analysis were submitted to Gene Expression Omnibus in the National Center for Biotechnology Information under an accession number of GSE71091.
Gene annotation and gene network analysis of differentially expressed genes
The differentially expressed genes with known identities or with homologous sequences and functional definitions were categorized using the Gene Ontology (GO, http://www.geneontology.org/) and PANTHER (http://www.pantherdb.org/) databases according to their cellular components, biological processes, and molecular functions. Functional pathway analysis was performed using the STRING database (http://string-db.org/). Differentially expressed genes were input for generating biological networks by comparing the input list with a reference list from human databases.
Validation of gene expressions by using quantitative real-time polymerase chain reaction
Eight differentially expressed genes that played a critical role in the annotation analysis in response to acute heat stress—heat shock protein 25 (HSP25); interleukin 6 (IL6); vitellogenin 2 (VTG2); metalloproteinase 13 (MMP-13); polymerase I and transcript release factor (PTRF); collagen, type II, alpha 1 (COL2A1); discoidin domain receptor tyrosine kinase 2 (DDR2); and Kruppel-like factor 2 (KLF2) were validated using a quantitative real-time polymerase chain reaction (qRT-PCR) analysis [13]. The sample set used in the microarray analysis was used for validation. The qRT-PCR primers and their predicted product sizes are listed in Table 1. The qRT-PCR reactions were performed on the Roche Light-Cycler Instrument 1.5 using a Light-Cycler FastStart DNA MasterPLUS SYBR Green I kit (Roche Cat. 03 515 885 001, Castle Hill, Australia). For the PCR, 2 μL of master mix, 2 μL of 0.75 mM forward and reverse primer, and 6 μL of cDNA samples were used, with each sample tested three times. The RT-PCR program was run at 95°C for 10 min, 40 cycles each at 95°C for 10 s, 60°C for 15 s, and 72°C for 10 s; subsequently, a melt curve analysis was performed. At the end of each RT-PCR run, the data were automatically analyzed by the system and an amplification plot was generated for each cDNA sample. From each of these plots, the LightCycler3 data analysis software automatically calculated the crossing point value (Cp; the crossing point corresponds to the first maximum of the second derivative curve), which was interpreted as the beginning of exponential amplification. The fold expression or repression of the target gene relative to the internal control gene, GAPDH, in each sample was calculated [13]. For consistency with the microarray analysis, the cutoff value for the differentially expressed genes was set to two-fold or higher.
Table 1. Primers and product size of genes used for validation using quantitative real-time polymerase chain reaction.
| Gene symbol a | GenBank accession number | Forward (F) primers 5'-3' | Product size (base pairs) |
|---|---|---|---|
| Reverse (R) primers 5'-3' | |||
| HSP25 | NM_001010842 | F: CCGTCTTCTGCTGAGAGGAGTG | 117 |
| R: ACCGTTGTTCCGTCCCATCAC | |||
| IL6 | NM_204628 | F: AGCAAAACACCTGTTACATTTCT | 96 |
| R: AGTCTGGCTGCTGGACATTT | |||
| VTG2 | NM_001031276 | F: CAGCCTAACTGACAAACAGATGAAG | 100 |
| R: GCATTCCTCATTCTCACATGAACAC | |||
| MMP13 | AF070478 | F: TTGGTGCTAAGTATAGATGAATGCC | 131 |
| R: TGTAGGTAGTCAGTGCTTGTTCG | |||
| PTRF | NM_001001471 | F: CCCTGCCTGCTAGGACAAG | 149 |
| R: AGGTCTGGGCTCTGGAAGG | |||
| COL2A1 | NM_204426 | F: CACTGAACGGATGGCACGAC | 137 |
| R: CCTCCACCCGCCCTACG | |||
| DDR2 | CR387623 | F: TGCGGACGGGAGGAACTG | 103 |
| R: AGCAATAGGGTACTGCGAATGG | |||
| KLF2 | XM_418264 | F:CGCCGAGGATTGGACACAG | 139 |
| R: CACGGAGTTCACCCTTCACAG | |||
| GAPDH | NM_204305 | F: CATCACAGCCACACAGAAGA | 122 |
| R: TGACTTTCCCCACAGCCTTA |
a Abbreviations: HSP25, heat shock protein 25; IL6, interleukin 6; VTG2, vitellogenin 2; MMP13, metalloproteinase 13; PTRF, polymerase I and transcript release factor; COL2A1, type II alpha 1 collagen; DDR2, discoidin domain receptor tyrosine kinase 2; KLF2, Kruppel-like factor 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
The physiological parameters of the control and heat-stressed hens during acute heat stress and recovery were analyzed using a Student t test in Statistical Analysis System software [21]. Multiples of changes in the microarray and qRT-PCR analysis of each individual of each group are presented as the arithmetic mean of the three replicates.
Results
Effect of heat stress on physiological parameters in broiler-type B strain TCCs
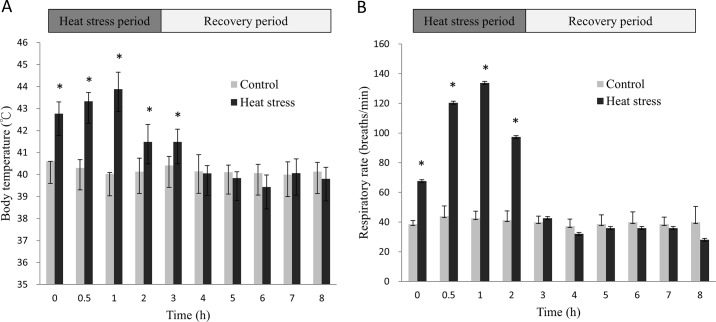
To evaluate the response of hens to acute heat stress, the hens were exposed to 38°C heat stress for 2 h. The acute heat stress increased the respiratory rate and body temperature immediately after the heat treatment began (p < 0.05; Fig 1). The hens started panting 30 min after heat stress, which continued until 1 h of recovery after the heat stress. The respiratory rate and body temperature normalized during the recovery period.
Fig 1. Body temperature (A) and respiratory rate (B) of acute-heat-stressed and control hens during stress and recovery periods.
Data are mean ± standard error (n = 12; n = 6 and n = 3 in groups with 2-h and 6-h recovery after heat stress, respectively). * Values differed between heat-stressed and control groups (p < 0.05).
Effects of heat stress on gene expressions in the SYFs of broiler-type B strain TCCs after acute heat stress
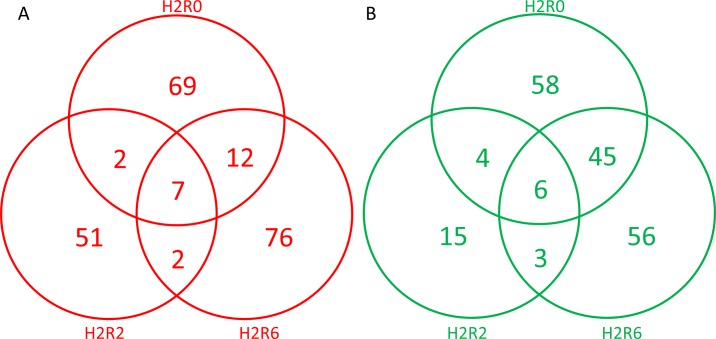
The mRNA profile of SYFs from control and heat-stressed hens were analyzed using a microarray. When using a cutoff value of a two-fold change, 406 genes showed differential expression on treatment (p < 0.05). The expression patterns of the 406 distinct genes are presented in Fig 2. Compared with the control group, the H2R0, H2R2, and H2R6 groups differed in 203, 90, and 147 genes, respectively; 69, 51, and 15 gene transcripts upregulated (S1 Table) and 58, 15, and 56 genes downregulated (S2 Table) specifically in the H2R0, H2R2, and H2R6 groups, respectively. After heat exposure, seven genes—HSP25, MYOC, PTRF, RGPD1, SOGA3, ChEST305c2 (Gallus gallus finished cDNA), and ChEST920a4 (Gallus gallus finished cDNA)—exhibited higher expression for all recovery times. The other six genes—ABI3, GAL2, GAL7, SERPINB10, alpha-2-macroglobulin-like 1 [ENSGALT00000023052], and ChEST478o11 (Gallus gallus finished cDNA)—exhibited downregulation for all recovery times.
Fig 2. Venn diagram analysis of 219 upregulated (A) and 187 downregulated (B) genes in the small yellow follicles of broiler-type Taiwan country chickens with 38°C acute heat stress for 2 h and recovery for 0, 2, and 6 h.
H2R0, recovery for 0 h after heat stress; H2R2, recovery for 2 h after heat stress; H2R6, recovery for 6 h after heat stress.
Functional categories of the differentially expressed genes in the SYFs of broiler-type B strain TCCs after acute heat stress
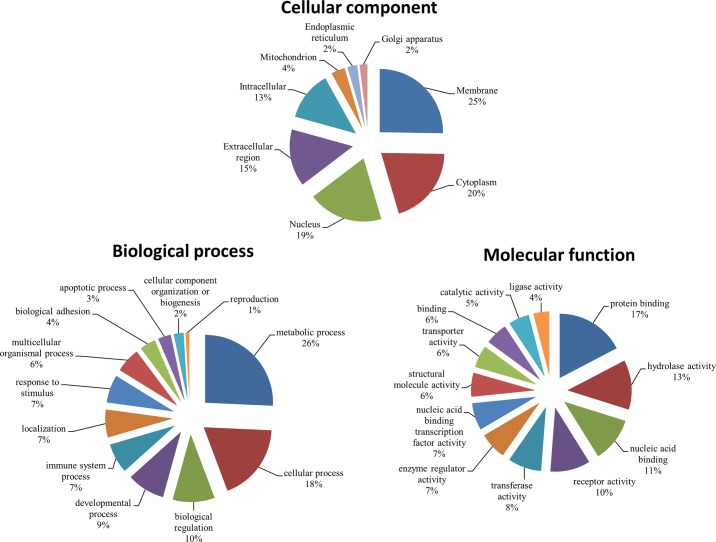
To characterize the functions of the differentially expressed genes, genes with known identities were subjected to GO annotation (Fig 3). The differentially expressed genes were primarily localized in the membrane, cytoplasm, nucleus, and extracellular regions. Most genes were associated with multiple biological processes and were involved in the metabolic process (26%), cellular process (18%), biological regulation (10%), developmental process (9%), immune system process (7%), localization (7%), response to stimulus (7%), and multicellular organismal process (6%). The majority of the differentially expressed genes were associated with multiple molecular functions, including protein binding (17%), hydrolase activity (13%), nucleic acid binding (11%), receptor activity (10%), transferase activity (8%), enzyme regulator activity (7%), and nucleic acid binding transcription factor activity (7%).
Fig 3. Classification of differentially expressed genes in small yellow follicles of broiler-type B strain Taiwan country chickens with 38°C acute heat stress for 2 h and recovery for 0, 2, and 6 h by cellular components (A), biological processes (B), and molecular functions (C).
Only the 212 genes with known functional definitions in the Gene Ontology and PANTHER databases were included.
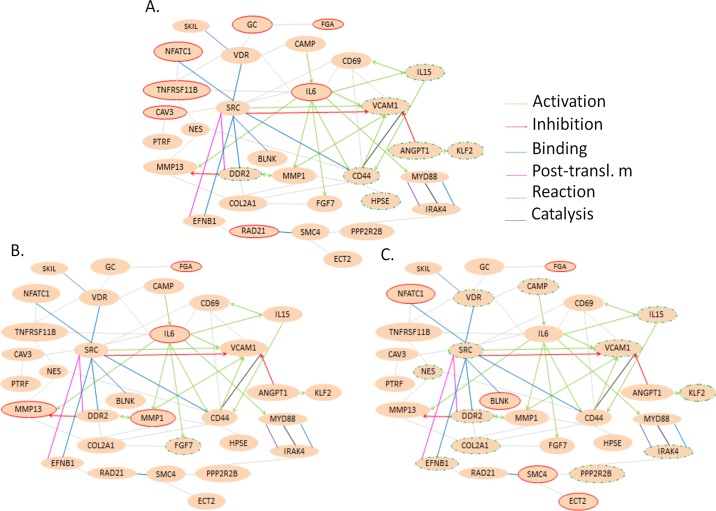
The functional annotation pathway analysis of the differentially expressed genes and their interrelationships are depicted in Fig 4. These networks were associated with the biological functions of reproduction, responses to stress, and regulation of such responses. The major upregulated genes in the network after heat stress and recovery for 0 h were IL6, GC, FGA, NFACT1, TNFRSF11B, CAV3, and RAD21; for 2 h were IL6, FGA, MMP1, and MMP13; and for 6 h were FGA, NFACT1, BLNK, SMC4, and ECT2 (S1 Table). The major downregulated genes in the network after heat stress and recovery for 0 h were CD44, IL15, DDR2, KLF2, VCAM1, and ANGPT1; for 2 h was FGF7 only; and for 6 h were SRC, VDR, NES, DDR2, IL15, CAMP, KLF2, VCAM1, EFNB1, IRAK4, COL2A1, and PPP2R2B (S2 Table).
Fig 4. Network analysis of the differentially expressed genes in small yellow follicles of heat-stressed broiler-type B strain Taiwan country chickens.
(A) H2R0, recovered for 0 h after heat stress; (B) H2R2, recovered for 2 h after heat stress; (C) H2R6, recovered for 6 h after heat stress.
Validation of representative differentially expressed genes in the SYFs of broiler-type B strain TCCs after acute heat stress
Through functional annotation pathway analysis, 8 significantly changed genes revealed through microarray analysis were further validated using qRT-PCR (Table 2). The coefficient of variation of Cp value of GAPDH in the 4 groups ranged from 1.2% to 2.3% and implied that the heat stress did not affect its expression. Consistent with the microarray analysis, HSP25, IL6, VTG2, and MMP13 were upregulated after heat stress. COL2A1 and KLF2 expressions were reduced by the acute heat stress in both the microarray and qRT-PCR analyses. PTRF and DDR2 expression of qRT-PCR differed from those of the microarray analysis, and DDR2 was upregulated after 2-h recovery in the qRT-PCR analysis. PTRF expression did not significantly differ after acute heat treatment in the qRT-PCR analysis.
Table 2. Multiples of changes of significantly differentially expressed genes in small yellow follicles of broiler-type B strain Taiwan country chickens after acute heat stress determined using microarray and quantitative reverse transcription polymerase chain reaction analyses.
| Fold change* | Gene a | |||||||
|---|---|---|---|---|---|---|---|---|
| HSP25 | IL6 | VTG2 | MMP13 | PTRF | COL2A1 | DDR2 | KLF2 | |
| H2R0/CTL | ||||||||
| M | 34.42 | 2.54 | 8.88 | 0.76 | 2.18 | 0.68 | 0.41 | 0.32 |
| Q | 54.40 | 1.93 | 3.37 | 0.96 | 0.91 | 0.81 | 1.09 | 0.27 |
| H2R2/CTL | ||||||||
| M | 38.20 | 2.11 | 1.21 | 3.21 | 2.08 | 0.98 | 0.82 | 0.95 |
| Q | 36.39 | 3.05 | 1.01 | 3.40 | 0.84 | 0.48 | 3.98 | 0.32 |
| H2R6/CTL | ||||||||
| M | 10.46 | 0.96 | 1.75 | 0.73 | 2.03 | 0.43 | 0.41 | 0.43 |
| Q | 8.40 | 1.82 | 1.65 | 0.82 | 0.74 | 0.53 | 0.94 | 0.34 |
* Multiples of changes of two-fold or higher increase or decrease were defined as different (p < 0.05). The fold expression or repression of the target gene were normalized using glyceraldehyde-3-phosphate dehydrogenase as an internal control gene.
a Abbreviations: HSP25, heat shock protein 25; IL6, interleukin 6; VTG2, vitellogenin 2; MMP13, metalloproteinase 13; PTRF, polymerase I and transcript release factor; COL2A1, type II alpha 1 collagen; DDR2, discoidin domain receptor tyrosine kinase 2; KLF2, Kruppel-like factor 2.
Discussions
Effect of acute heat stress on physiological parameters and gene expressions in the SYFs of broiler-type B strain TCCs
Numerous studies have shown that heat stress affects egg production, egg weight, egg quality, and shell quality in chickens [22,23,24,25]. Few studies, however, have explored the global changes of gene expressions in the ovarian follicles. The results of the current study showed that the respiratory rate and body temperatures of heat-stressed hens increased significantly during acute heat stress and normalized after recovery at 25°C, which is consistent with the a previous report on roosters [13]. Global gene expression changes in ovarian SYFs were associated with metabolic, developmental, immune system, multicellular organismal, apoptotic, and cellular processes, apoptosis, biological regulation, localization, response to stimulus, biological adhesion, cellular component organization (biogenesis), and reproduction changes in the SYFs after acute heat stress (Fig 3).
Heat shock protein family genes and other stress response related genes were induced in response to acute heat stress in the SYFs of broiler-type B strain TCCs
HSP25 expression was significantly upregulated after acute heat stress (S1 Table; Table 2). HSP25 is a small heat shock protein (sHSP) belonging to a family of conserved and ubiquitously expressed proteins [26]. HSP25 stabilizes the unfolding proteins and prevents them from precipitating in cells [27]. Moreover, HSP25 refolds numerous unfolding proteins and cooperates with other chaperones when organisms are recovered under optimal environmental conditions [28,29]. The elevated HSP25 expression in this study suggests that HSP25 facilitates protein refolding and chaperoning for preventing protein denaturation through acute heat insults in SYFs.
Acute phase response (APR) is a systemic and cellular reaction provoked by local or systemic disturbances in homeostasis caused by pathogen infection, tissue injury, trauma, stress, surgery, neoplasia, and immune disorders [30,31]. Numerous responses, including the production of proinflammatory cytokines (e.g., IL6, IL1β, and TNF-α) have been reported [32,33]. Furthermore, APR maintains physical homeostasis by activating the innate immune responses. IL6 production during APR suppresses the production of proinflammatory cytokines without hampering the other anti-inflammatory cytokines [34]. IL6 expression was significantly increased in the SYF (S1 Table; Table 2). Functional annotation analysis suggested that IL6 upregulates interleukin 15 (IL15), matrix metalloproteinase-1 (MMP-1), matrix metalloproteinase-13 (MMP-13), fibroblast growth factor 7 (FGF7), vascular cell adhesion molecule 1 (VCAM-1), myeloid differentiation primary response 88 (MYD88), and CD44 (Fig 4). However, the expression of FGF7 and VCAM-1 was downregulated, suggesting that epithelial cell injuries were exacerbated by acute stress [35,36]. Xing et al. [37] demonstrated that IL6 is critical in controlling the extent of local and systemic acute inflammatory responses, particularly the levels of proinflammatory cytokines. Because functional pathway analysis showed that the differentially expressed genes were primarily associated with the biological processes of reproduction, response to stress, and regulation of these responses (Fig 3), IL6 may initiate a protective mechanism against damage induced by heat stress in the SYF cells.
KLF2, a eukaryotic zinc finger transcription factor, has been reported to regulate various gene expressions in response to shear stress of vasculature endothelial cells for establishing and maintaining endothelial function [38,39]. KLF2 has 3 carboxy-terminal zinc fingers with high homology to KLF4, the expression of which was significantly upregulated after heat stress in several tissues [40]. Liu et al. [40] reported that the overexpression of KLF4 increased the mortality of C2C12 murine myogenic cells. Conversely, KLF4 deficiency reduced C2C12 cell injury after heat stress [40]. KLF2 expression was significantly downregulated (S2 Table; Table 2), implying that KFL2 play a role in preventing SYF damage in hens exposed to acute heat stress.
Acute heat stress may cause damage to the SYFs of broiler-type B strain TCCs
In chickens, vitellogenin, the major precursor protein of yolk, is synthesized in the liver [41]. Three vitellogenin genes exist, and the VTG2 transcript is the most abundant [42]. VTG2 expression in SYF was significantly increased after acute heat stress (Table 2). The role of upregulated VTG2 expression in response to acute heat stress in chickens SYFs remains unknown. In this study, the expression of MMP1 was upregulated after acute heat stress (Table 2). MMPs are zinc-dependent endopeptidases capable of degrading various extracellular matrix components [43,44]. Furthermore, MMPs play a critical role in follicular extracellular remodeling in mammalian ovaries [45]. Park et al. [46] reported that heat shock increased the MMP1 and MMP3 expression through an autocrine interleukin-6 loop. IL6 inhibition by a monoclonal antibody significantly reduced the MMP1 and MMP13 expression in response to heat shock. MMP1 expression was stimulated by a follicle-stimulating hormone, luteinizing hormone, progesterone, and estrogen, and remained low in the preovulatory follicles but increased in postovulatory follicles in chicken ovaries [45]. MMP1 upregulation after heat stress thus may be disturbed by disordered secretion of sex hormones and can induce matrix disintegration in the follicles. This suggestion was further confirmed by the COL2A1 downregulation and the transient upregulation of MMP13 because of heat stress. DDR2 induces MMP13 expression [47], and COL2A1 plays a critical role in collagen synthesis [48] and shares a majority of the total collagen genes in the ovary [49]. Liang et al. [49] reported that large amounts of misfolded procollagen were synthesized and retained in the dilated endoplasmic reticulum in COL2A1 knockout mice [48]. In addition, COL2A1 downregulation was observed in hypothyroid ovarian tissue, accompanied by the upregulation of MMP1, MMP8, and MMP13 [49]. Thus, the downregulation of COL2A1 and upregulation of MMP1, MMP13, and IL-6 after acute heat stress suggest the proteolytic disintegration of the structural matrix and inflamed damage of the follicle cells after acute heat insults. In this study, DDR2 was downregulated in H2R0 and H2R6 in the microarray analysis after acute heat stress.
PTRF, also known as cavin-1, participated in the dissociation of transcription complexes [50,51]. PTRF was recently reported to respond to mechanical stress by disassembling caveolae, [52] which, as a compact and rigid microdomain on the plasma membranes, has been implicated in several biological processes, including cell signaling, lipid regulation, and endocytosis [53]. Mechanical stress, such as osmotic swelling and unsymmetrical stretching, results in the rapid disappearance of caveolae [54]. The inner surface of caveolae is coated with a scaffolding protein formed by caveolin members [53]. CAV3 concentration is significantly increased in damaged chicken muscle [55]. PTRF expression was significantly upregulated after acute stress, and CAV3 expression was significantly upregulated at 0 h of recovery after heat stress (S2 Table; Table 2). These results indicate membrane permeability damaged by acute heat stress in SYF cells.
Conclusions
Heat stress affects SYF gene expression in broiler-type B strain TCCs. The differentially expressed genes participated in such biological processes as metabolic, cellular, and developmental processes and biological regulation. Functional pathway analysis showed that IL6 is a key regulator in the networks and connects the processes of reproduction, responses to stress, and regulation of such responses. The upregulation of heat shock protein 25, interleukin 6, metallopeptidase 1, and metalloproteinase 13, and downregulation of type II alpha 1 collagen, discoidin domain receptor tyrosine kinase 2, and Kruppel-like factor 2 suggest that acute heat stress induces proteolytic disintegration of the structural matrix and inflamed damage and adaptive responses of follicle cell gene expressions.
Supporting Information
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files. All results of microarray dataset files are available from the Gene Expression Omnibus in the National Center for Biotechnology Information database (accession number GSE71091).
Funding Statement
This study was supported in part by grants from Ministry of Science and Technology (NSC# 102-2321-B-005-013, MOST# 103-2321-B-005-010, and NSC# 101-2311-B-005-008-MY3), and the Ministry of Education (under the ATU plan), Executive Yuan, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Knight J, Harrison S. Evaluating the impacts of global warming on geomorphological systems. Ambio 2012; 41: 206–210. 10.1007/s13280-011-0178-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMichael AJ, Powles JW, Butler CD, Uauy R. Food, livestock production, energy, climate change, and health. Lancet 2007; 370: 1253–1263. [DOI] [PubMed] [Google Scholar]
- 3. New M, Liverman D, Schroeder H, Anderson K. Four degrees and beyond: the potential for a global temperature increase of four degrees and its implications. Philos Trans A Math Phys Eng Sci 2011; 369: 6–19. 10.1098/rsta.2010.0303 [DOI] [PubMed] [Google Scholar]
- 4. Hansen PJ. Effects of heat stress on mammalian reproduction. Philos Trans R Soc Lond B Biol Sci 2009; 364: 3341–3350. 10.1098/rstb.2009.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sakatani M, Alvarez NV, Takahashi M, Hansen PJ. Consequences of physiological heat shock beginning at the zygote stage on embryonic development and expression of stress response genes in cattle. J Dairy Sci 2012; 95: 3080–3091. 10.3168/jds.2011-4986 [DOI] [PubMed] [Google Scholar]
- 6. Rozenboim I, Tako E, Gal-Garber O, Proudman JA, Uni Z. The effect of heat stress on ovarian function of laying hens. Poult Sci 2007; 86: 1760–1765. [DOI] [PubMed] [Google Scholar]
- 7. Onagbesan O, Bruggeman V, Decuypere E. Intra-ovarian growth factors regulating ovarian function in avian species: a review. Anim Reprod Sci 2009; 111: 121–140. 10.1016/j.anireprosci.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 8. Kang L, Cui X, Zhang Y, Yang C, Jiang Y. Identification of miRNAs associated with sexual maturity in chicken ovary by Illumina small RNA deep sequencing. BMC Genomics 2013; 14: 352 10.1186/1471-2164-14-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan TQ, Ge C, Mi Y, Jin Y, Zhang C. Ginsenosides promote proliferation of granulosa cells from chicken prehierarchical follicles through PKC activation and up-regulated cyclin gene expression. Cell Biol Int 2010; 34: 769–775. 10.1042/CBI20090244 [DOI] [PubMed] [Google Scholar]
- 10. Hernandez AG, Bahr JM. Role of FSH and epidermal growth factor (EGF) in the initiation of steroidogenesis in granulosa cells associated with follicular selection in chicken ovaries. Reproduction 2003; 125: 683–691. [PubMed] [Google Scholar]
- 11. Johnson AL, Woods DC. Dynamics of avian ovarian follicle development: cellular mechanisms of granulosa cell differentiation. Gen Comp Endocrinol 2009; 163: 12–17. 10.1016/j.ygcen.2008.11.012 [DOI] [PubMed] [Google Scholar]
- 12. Beaupré CE, Tressler CJ, Beaupré SJ, Morgan JL, Bottje WG, Kirby JD. Determination of testis temperature rhythms and effects of constant light on testicular function in the domestic fowl (Gallus domesticus). Biol Reprod 1997; 56: 1570–1575. [DOI] [PubMed] [Google Scholar]
- 13. Wang SH, Cheng CY, Tang PC, Chen CF, Chen HH, Lee YP, et al. Differential gene expressions in testes of L2 strain Taiwan country chicken in response to acute heat stress. Theriogenology 2013; 79: 374–382. 10.1016/j.theriogenology.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 14. Lee YP, Chen TL. Daytime behavioural patterns of slow-growing chickens in deep-litter pens with perches. Br Poult Sci 2007; 48: 113–120. [DOI] [PubMed] [Google Scholar]
- 15. Chen CF, Shiue YL, Yen CJ, Tang PC, Chang HC, Lee YP. Laying traits and underlying transcripts, expressed in the hypothalamus and pituitary gland, that were associated with egg production variability in chickens. Theriogenology 2007; 68: 1305–1315. [DOI] [PubMed] [Google Scholar]
- 16. Beckham JT, Wilmink GJ, Opalenik SR, Mackanos MA, Abraham AA, Takahashi K, et al. Microarray analysis of cellular thermotolerance. Lasers Surg Med 2010; 42: 752–765. 10.1002/lsm.20983 [DOI] [PubMed] [Google Scholar]
- 17. Kim HJ, Joo HJ, Kim YH, Ahn S, Chang J, Hwang KB, et al. Systemic analysis of heat shock response induced by heat shock and a proteasome inhibitor MG132. PLoS One 2010; 6: e20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li C, Wang X, Wang G, Li N, Wu C. Expression analysis of global gene response to chronic heat exposure in broiler chickens (Gallus gallus) reveals new reactive genes. Poult Sci 2011; 90: 1028–1036. 10.3382/ps.2010-01144 [DOI] [PubMed] [Google Scholar]
- 19. Chao CH, Lee YP. Relationship between reproductive performance and immunity in Taiwan country chickens. Poult Sci 2001; 80: 535–540. [DOI] [PubMed] [Google Scholar]
- 20. Kuo YM, Shiue YL, Chen CF, Tang PC, Lee YP. Proteomic analysis of hypothalamic proteins of high and low egg production strains of chickens. Theriogenology 2005; 64: 1490–1502. [DOI] [PubMed] [Google Scholar]
- 21. SAS. 2010. SAS/STAT User's Guide: Version 9.2 ed. SAS Institute Inc., Cary, NC, USA. [Google Scholar]
- 22. Emery DA, Vohra P, Ernst RA, Morrison SR. The effect of cyclic and constant ambient temperatures on feed consumption, egg production, egg weight, and shell thickness of hens. Poult Sci 1984; 63: 2027–2035. [DOI] [PubMed] [Google Scholar]
- 23. Mashaly MM, Hendricks GL 3rd, Kalama MA, Gehad AE, Abbas AO, Patterson PH. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult Sci 2004; 83: 889–894. [DOI] [PubMed] [Google Scholar]
- 24. Star L, Kemp B, van den Anker I, Parmentier HK. Effect of single or combined climatic and hygienic stress in four layer lines: 1. Performance. Poult Sci 2008; 87: 1022–1030. 10.3382/ps.2007-00142 [DOI] [PubMed] [Google Scholar]
- 25. Ajakaiye JJ, Perez-Bello A, Mollineda TA. Impact of heat stress on egg quality in layer hens supplemented with l-ascorbic acid and dl-tocopherol acetate. Vet Arhiv 2011; 81: 119–132. [Google Scholar]
- 26. Rogers RS, Beaudoin MS, Wheatley JL, Wright DC, GeigerPC. Heat shock proteins: in vivo heat treatments reveal adipose tissue depot-specific effects. J Appl Physiol 1985; 118: 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katoh Y, Fujimoto M, Nakamura K, Inouye S, Sugahara K, Izu H, et al. Hsp25, a member of the Hsp30 family, promotes inclusion formation in response to stress. FEBS Lett 2004; 565: 28–32. [DOI] [PubMed] [Google Scholar]
- 28. Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J 1997; 16: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lindner RA, Carver JA, Ehrnsperger M, Buchner J, Esposito G, Behlke J, et al. Mouse Hsp25, a small shock protein. The role of its C-terminal extension in oligomerization and chaperone action. Eur J Biochem 2000; 267: 1923–1932. [DOI] [PubMed] [Google Scholar]
- 30. Gruys E, Toussaint MJ, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B 2005; 6: 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cray C, Zaias J, Altman NH. Acute phase response in animals: a review. Comp Med 2009; 59: 517–526. [PMC free article] [PubMed] [Google Scholar]
- 32. van Miert AS. Pro-inflammatory cytokines in a ruminant model: pathophysiological, pharmacological, and therapeutic aspects. Vet Q 1995; 17: 41–50. [DOI] [PubMed] [Google Scholar]
- 33. Kushner I. Regulation of the acute phase response by cytokines. Perspect Biol Med 1993; 36: 611–622. [DOI] [PubMed] [Google Scholar]
- 34. Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol 2003; 24: 25–29. [DOI] [PubMed] [Google Scholar]
- 35. Yen TT, Thao D T, Thuoc TL. An overview on keratinocyte growth factor: from the molecular properties to clinical applications. Protein Pept Lett 2014; 21: 306–317. [DOI] [PubMed] [Google Scholar]
- 36. Ross EA, Douglas MR, Wong SH, Ross EJ, Curnow SJ, Nash GB, et al. Interaction between integrin alpha9beta1 and vascular cell adhesion molecule-1 (VCAM-1) inhibits neutrophil apoptosis. Blood 2006; 107: 1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 1998; 101: 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dekker RJ, Boon RA Rondaij MG, Kragt A, Volger OL, Elderkamp YW, et al. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood 2006; 107: 4354–4363. [DOI] [PubMed] [Google Scholar]
- 39. Boon RA, Horrevoets AJ. Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie 2009; 29: 39–40, 41–43. [PubMed] [Google Scholar]
- 40. Liu Y, Wang J, Yi Y, Zhang H, Liu J, Liu M, et al. Induction of KLF4 in response to heat stress. Cell Stress Chaperones 2006; 11: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Byrne BM, Gruber M, Ab G. The evolution of egg yolk proteins. Prog Biophys Mol Biol 1989; 53: 33–69. [DOI] [PubMed] [Google Scholar]
- 42. Evans MI, Silva R, Burch JBE. Isolation of chicken vitellogenin I and III cDNAs and the developmental regulation of five estrogen-responsive genes in the embryonic liver. Genes Dev 1988; 2: 116–124. [DOI] [PubMed] [Google Scholar]
- 43. Kahari VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol 1997; 6: 199–213. [DOI] [PubMed] [Google Scholar]
- 44. Woessner JF Jr. Role of matrix proteases in processing enamel proteins. Connect Tissue Res 1998; 39: 69–73; discussion 141–149. [DOI] [PubMed] [Google Scholar]
- 45. Zhu G, Kang L, Wei Q, Cui X, Wang S, Chen Y, et al. Expression and regulation of MMP1, MMP3, and MMP9 in the chicken ovary in response to gonadotropins, sexhormones, and TGFB1. Biol Reprod 2014; 90: 1–11. [DOI] [PubMed] [Google Scholar]
- 46. Park CH, Lee MJ, Ahn J, Kim S, Kim HH, Kim KH, et al. Heat shock-induced matrix metalloproteinase MMP-1 and MMP-3 are mediated through ERK and JNK activation and via an autocrine interleukin-6 loop. J Invest Dermatol 2004; 123: 1012–1019. [DOI] [PubMed] [Google Scholar]
- 47. Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, et al. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem 2005; 280: 548–555. [DOI] [PubMed] [Google Scholar]
- 48. Liang G, Lian C, Huang D, Gao W, Liang A, Peng Y, et al. Endoplasmic reticulum stress-unfolding protein response-apoptosis cascade causes chondrodysplasia in a col2a1 p.Gly1170Ser mutated mouse model. PLoS One 2014; 9: e86894 10.1371/journal.pone.0086894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saha S, Ghosh P, Mitra D, Mukherjee S, Bhattacharya S, Roy SS. Localization and thyroid hormone influenced expression of collagen II in ovarian tissue. Cell Physiol Biochem 2007; 19: 67–76. [DOI] [PubMed] [Google Scholar]
- 50. Mason SW, Sander EE, Grummt I. Identification of a transcript release activity acting on ternary transcription complexes containing murine RNA polymerase I. EMBO J 1997; 16: 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jansa P, Mason SW, Hoffmann-Rohrer U, Grummt I. Cloning and functional characterization of PTRF, a novel protein which induces dissociation of paused ternary transcription complexes. EMBO J 1998; 17: 2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nassoy P, Lamaze C. Stressing caveolae new role in cell mechanics. Trends Cell Biol 2012; 22: 381–389. 10.1016/j.tcb.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 53. Thomas CM, Smart EJ. Caveolae structure and function. J Cell Mol Med 2008; 12: 796–809. 10.1111/j.1582-4934.2008.00295.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sinha B, Koster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 2011; 144: 402–413. 10.1016/j.cell.2010.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matsumoto H, Sasazaki S, Fujiwara A, Ichihara N, Kikuchi T, Mannen H. Accumulation of caveolin-3 protein is limited in damaged muscle in chicken muscular dystrophy. Comp Biochem Physiol A Mol Integr Physiol 2010; 157: 68–72. 10.1016/j.cbpa.2010.04.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. All results of microarray dataset files are available from the Gene Expression Omnibus in the National Center for Biotechnology Information database (accession number GSE71091).