Abstract
This study compared the seven treatment plan options in achieving the dose-volume criteria required by the RTOG 1005 protocol. Dosimetry plans were generated for 15 representative early stage breast cancer patients based on the protocol required dose-volume criteria for each of the following seven treatment options: 3D conformal radiotherapy (3DCRT) whole breast irradiation (WBI) plus 3DCRT lumpectomy boost, 3DCRT WBI plus electron boost, 3DCRT WBI plus intensity-modulated radiation therapy (IMRT) boost, IMRT WBI plus 3DCRT boost, IMRT WBI plus electron boost, IMRT WBI plus IMRT boost, and simultaneous-integrated boost with IMRT. A variety of dose-volume parameters, including target dose conformity and uniformity, and normal tissue sparing, for these plans were compared. For the patients studied, all plans met the required acceptable dose volume criteria, with the majority meeting the ideal criteria. When averaged over patients, the majority of dose-volume goals for all plan options can be achieved with a positive gap of at least a few tenths of standard deviations. The plans for all seven options are generally comparable. The dose-volume goals required by the protocol can in general be easily achieved. IMRT WBI provides better whole breast dose uniformity than 3DCRT WBI, but causes no significant difference for the dose conformity. All plan options are comparable when lumpectomy dose uniformity and conformity are concerned. Patient anatomy is always an important factor when whole breast dose uniformity and conformity, and lumpectomy dose conformity are considered.
Keywords: early stage breast cancer, radiation treatment planning, hypofractionated breast irradiation
Introduction
Early stage breast cancer (ESBC) patients can benefit from post lumpectomy radiation therapy (RT). Evidence have shown that post lumpectomy RT is associated with a promising long-term local control with equivalent survival outcomes as mastectomy,1,2 and the overall survival can be improved with post lumpectomy irradiation as compared to surgery alone.3 The current standard management for ESBC is to treat, after breast conserving surgery (BCS), the entire breast with photon external beam radiotherapy (EBRT) for 45-50 Gy in a fractional dose of 1.8-2 Gy. Frequently, electron or photon EBRT, or brachytherapy has been used to boost the tumor excision site with an additional 10-20 Gy dose, resulting in an overall treatment time of 5-7 weeks. Due to the prolonged treatment time, a substantial number of women potentially suitable for breast conserving therapy (BCT) were treated with breast-conserving surgery alone.4 Reducing the overall treatment time can bring great convenience for patients, lower treatment costs, and increase the utilization of the radiation therapy resources.
Accelerated whole breast irradiation (WBI) with hypofractionation plus concurrent boost is a method to reduce the overall treatment time for ESBC. However, normal and malignant tissues have different responses to fraction size. In linear-quadratic (LQ) model, the fractionation sensitivity is governed by the α/β ratio. Generally, lower α/β ratios support more hypofractionation. Qi's analysis5 with available clinical data from multiple institutions supported that breast cancer has a low α/β ratio, encouraging hypofractionated radiotherapy regimens for breast cancer. The UK Standardisation of Breast Radiotherapy (START) group had two trials to test the effects of radiotherapy schedules using fraction sizes larger than 2.0 Gy.6-7 Based on the LQ model with α/β ratio of 4.6 Gy (95% CI 1.1-8.1) derived by the START group, the biologically equivalent doses in ~2 Gy fractions delivered to the whole breast and the tumor bed can be estimated. Several prospective randomized clinical trials have shown promising results with hypofractionated schedules for WBI and confirmed that the validity of the LQ model effect estimates at least up to 3.3 Gy per fraction. 6-10 With 5-10 year follow-up of these studies, there has been similar in-breast local control between the hypofractionated and standard fractionated treatments. Despite these prior randomized trials, many questions still remain regarding the use of WBI hypofractionated schedules, such as the length of treatment, the breast size, cardiac toxicity etc. Recently, Radiation Therapy Oncology Group (RTOG) initiated a phase III randomized trial, RTOG 1005 protocol, aiming to determine whether an accelerated course of hypofractionated WBI including a concomitant boost to the tumor bed in 15 fractions following lumpectomy will prove to be non-inferior in local control to a regimen of standard WBI with a sequential boost following lumpectomy for early-stage breast cancer patients. The protocol allows seven irradiation options using 3D conformal radiotherapy (3DCRT), intensity-modulated radiation therapy (IMRT) or electron beams. There have been concerns whether these options are dosimetrically equivalent and whether the required dose-volume goals can be achieved by all of the options. The purpose of this work is to assess difficulties for achieving the dose-volume goals required by the protocol, and to compare plan quality for the seven allowed treatment options.
Methods and Materials
RTOG 1005 protocol
Patients registered for RTOG 1005 protocol are randomized to one of the two treatment arms: (1) ARM I: patients undergo standard WBI comprising IMRT or 3DCRT with 2 Gy/Fx for 25 fractions followed by a sequential boost to the lumpectomy site for an additional dose of 12-14 Gy in 6-7 fractions; and (2) ARM II: patients undergo accelerated hypofractionated WBI comprising 3DCRT or IMRT with 2.67 Gy/Fx for 15 fractions with a concurrent boost to the lumpectomy site to reach 3.2 Gy/Fx. seven treatment approaches are allowed: (1) 3DCRT WBI plus 3DCRT boost (3D+3D), (2) 3DCRT WBI plus electron boost (3D+e), (3) 3DCRT WBI plus IMRT boost (3D+IMRT), (4) IMRT WBI plus 3DCRT boost (IMRT+3D), (5) IMRT WBI plus electron boost (IMRT+e), (6) IMRT WBI plus IMRT boost (IMRT+IMRT), and (7) simultaneous-integrated boost (SIB) with IMRT. The SIB option is applied to ARM II only. For any of these options, the treatment plan must meet the dose-volume constraints for the two targets, including whole breast and lumpectomy site planning target volumes (PTV), and organs at risk (OARs), including contralateral breast, ipsilateral and contralateral lungs, and heart. The dose-volume criteria are specified, in two levels—ideal or acceptable, for a series of parameters including VD (percentage of volume covered by dose D), Dmax (maximum dose), and Dmean (mean dose) for the breast PTV for evaluation (Breast PTV_Eval), lumpectomy PTV for evaluation (Lump PTV_Eval) and OARs. The ideal and acceptable dose-volume criteria for these dose-volume parameters can be found in the RTOG 1005 protocol.
Dosimetric plan generation
CT data acquired with GE lightspeed CT scanner for 15 ESBC patients treated previously with post lumpectomy RT were used to generate dosimetric plans for the allowed seven irradiation options with a treatment planning system (Xio, Elekta). Among these 15 patients, 8 had left-sided breast cancer, 10 were positioned in supine (5 of these 10 were left-sided). For each patient, all required target contours, such as lumpectomy clinical target volume (CTV) and PTV, lumpectomy PTV for evaluation (Lump PTV_Eval), breast CTV and PTV, Breast PTV_Eval, and OAR contours were drawn according to the guidelines in the RTOG 1005 protocol. It is worth mentioning that, according to RTOG 1005 protocol, the breast PTV is a 7 mm 3D expansion of the breast CTV (excluding heart and do not cross midline), therefore, the breast PTV will most probably have a substantial part of it extends outside the patient or deeper than the anterior surface of the ribs. The Breast PTV_Eval is a copy of the breast PTV with editing. It is limited anteriorly to exclude the part outside the patient and the first 5 mm of tissue under the skin to remove most of the build up region, and it is limited posteriorly no deeper to the anterior surface of the ribs. The Breast PTV_Eval volumes are ranged from about 230 to over 2600 cc. Seven out of the 15 patients had Breast PTV_Eval volume over the average (~1000 cc), and 3 of these were in the prone setup. The patient positioning setups were initially determined by physician according to the breast size, lung function, and radiotherapy treatment history.
For each supine patient, one plan for each ARM II allowed seven treatment options were generated. However, due to the clearance needed, electron boost may be not deliverable for prone breast patients. Therefore, only 5 non-electron boost ARM II plans were generated for each prone breast patient. From dosimetric point of view, there is no difference between sequential and concurrent boosts for non-SIB plans. Non-SIB plans were generated separately for WBI and boost. The boost could be delivered concurrently (ARM II) or sequentially (ARM I) with appropriate dose scaling. Therefore, no plan was generated specifically for ARM I.
For WBI, parallel opposed tangential photon beams of 6 and/or 15 MV were utilized. For 3DCRT WBI, the MLC leaf positions, wedge, gantry angle, and collimator angle were adjusted to achieve the required dose-volume goals by either a trial and error method or an automated optimization method,11 and the field-in-field technique with static MLC is also an option. To include the whole breast PTV, an additional beam margin of 5 mm beyond the PTV was used in general, but this can be adjusted so that the inclusion of heart and lung can be minimized. For IMRT WBI, a beamlet-based inverse planning with simulate annealing algorithm 12-13 was adopted to generate a WBI plan based on the required dose-volume constraints. Only 6 MV photon beams were used for IMRT planning and beams were still tangential to avoid excessive dose to OARs. However, non-tangential IMRT beams are allowed in the protocol.
For 3DCRT and IMRT boost plans, either tangential or non-tangential beams were used depending on the location of the lumpectomy PTV. The margins between PTV and beam shape edge in beam's eye view at isocenter used were generally 3-5 mm for 3DCRT photon beams and 1-5 mm for electron beams, the ultimate goal is to ensure adequate coverage of the lumpectomy PTV. The WBI dose was considered in the optimization for the IMRT boost plan. For an electron boost plan, enfaced electron beam(s) was used, with the beam energy determined by the depth of the lumpectomy PTV. Similar to IMRT WBI, the SIB plans were generated with tangential photon beams only. Unlike IMRT+IMRT plan, where two sequential optimizations were carried out to optimize two groups of beams, an SIB plan optimizes only one group of beams.
Special considerations were given to certain specific situations. For example, the gantry angle for one or more tangential beams might be rotated by a few degrees from their ideal tangential directions, or the jaws/MLCs might be moved to avoid the direct irradiation of the heart, or to reduce the dose for the contralateral breast.
Dose calculation for all plans was performed using a convolution/superposition dose calculation algorithm with tissue heterogeneity correction. The calculation grid used was 2.0 mm if IMRT is involved, and 2.5 mm otherwise.
Plan evaluation
The 3D dose distributions and dose-volume histograms (DVH) of all targets and OARs were compared between the seven plan options based on a series of dose-volume parameters, including the uniformity index (UI) and conformity index (CI). The UI was defined as: 14
| (1) |
where D5 and D95 are the doses covering 5% and 95% volume of the target, respectively. The value of UI is always greater than 1, with the UI value of 1 representing uniform dose in the target. The CI was calculated based on ICRU report 62 as: 15
| (2) |
where V95% and VPTV are the volume covered by the 95% of the prescription dose and the volume of PTV, respectively. In this work, we calculated UI and CI for the Breast PTV_Eval and Lump PTV_Eval for each plan generated. In addition, for each plan generated, all dose volume parameters specified in RTOG 1005 protocol were evaluated for both targets (e.g., Breast PTV_Eval, Lump PTV_Eval) and OARs (e.g., contralateral breast, ipsilateral and contralateral lungs, and heart). To find the influence of patient anatomy and plan option on UI and CI for the Breast PTV_Eval and Lump PTV_Eval, with statistics analysis software (GraphPad Prism 5.04), we performed a two-way analysis of variance (ANOVA) for these quantities using plan option and patient as independent variables.
Results and Discussion
All required acceptable dose volume criteria were met in all 95 plans generated, with most of plans (89 out of 95 plans) achieving the ideal criteria. Those plans failing to achieve the ideal criteria were from two patients, one of them (supine positioned) had only the IMRT+e plan failing to reach the ideal dose-volume criteria of the Lump PTV_Eval coverage, and the other patient (prone positioned) had no plan achieving all ideal dose-volume criteria at all (most of them failed to reach the Breast PTV_Eval coverage). The PTV_Eval dose coverage, and other dose-volume constraints for both PTV_Eval and OARs, is competing with each other. The patient specific anatomy is the most important factor for determining whether all ideal dose-volume criteria can be satisfied or not. In general, plans for various anatomic situations, such as deep-seated targets, targets close to OARs (e.g., heart), large lumpectomy PTV_Eval volume relative to the Breast PTV_Eval volume, are more difficult to meet the ideal criteria. The 3DCRT plan option has more beam energy choices (e.g. 15 MV can be used if the Lump PTV_Eval is deep), and can utilizes other treatment aids such as wedge and block etc. Bolus was not used in our plan generation as skin bolus is not allowed per protocol. The averages and standard deviations (SD) over the 15 patients, if applicable for the relevant plan option, for selected dose-volume parameters achieved for the seven treatment options are compared with the ideal dose volume criteria in Tables 1 through 5. The reported maximum doses are from dose calculation voxels as mentioned before. Some other parameters, such as V4Gy which is required to be less than 10% for the contralateral lung per protocol, are not tabulated because their achieved values are trivial (all zeros). The planning difficulty to achieve the required dose-volume goals can be represented by average gap to goal for each parameter. The average gap to goal for a dose-volume parameter is defined as a ratio of the difference between the patient average of the achieved parameter value and the protocol required dose-volume goal, and the corresponding SD of the achieved parameter values, positive if the goal was met and negative otherwise. The average gaps to goal were calculated for each individual dose-volume parameter and each individual plan option. For the ideal level dose-volume criteria, most of the average gaps to goal were 0.7 and larger, with an exception of V45.6Gy for Lump PTV_Eval, which has a value of 0.2 for IMRT+e plan option, indicating that, the plan with IMRT WBI plus electron boost sometimes may need extra effort to achieve the 95% volume coverage goal for Lump PTV_Eval, depending on its depth. On the other hand, for the acceptable level dose-volume criteria, all average gaps to goal were 1.3 at least. Therefore, in general, the dose-volume goals required by the protocol are not very hard to be achieved.
Table 1.
Ideal dose-volume criteria for Breast PTV_Eval and corresponding achieved values (averages and standard deviations) from all 15 cases for all plan options, except for electron boost plans where only the 10 supine cases were included in calculation.
| Plan Options | V38Gy≥95% | V43.2Gy≤50% | V48Gy≤30% | Dmax/40Gy≤115% |
|---|---|---|---|---|
| 3D+3D | 96.3±1.3 | 42.9±8.4 | 17.5±7.7 | 112.1±1.7 |
| 3D+e | 96.4±1.2 | 39.4±8.6 | 18.0±6.3 | 112.3±1.4 |
| 3D+IMRT | 96.5±1.1 | 41.8±8.7 | 11.0±6.5 | 112.4±1.4 |
| IMRT+3D | 96.1±1.0 | 41.0±8.9 | 16.7±5.7 | 111.6±3.0 |
| IMRT+e | 95.9±0.8 | 34.0±9.1 | 15.7±5.1 | 110.8±2.6 |
| IMRT+IMRT | 96.1±0.9 | 41.8±9.5 | 12.7±6.3 | 111.6±3.0 |
| SIB | 96.2±1.1 | 42.5±8.8 | 12.7±5.3 |
Table 5.
Ideal dose-volume criteria for heart and corresponding achieved values (averages and standard deviations). Averages and standard deviations were calculated separately for left-sided and right-sided breasts for all plan options with data from applicable cases. V16Gy and V8Gy of heart for right-sided breast, which were all trivial for all plan options, are not listed.
| Plan Options | Left | Right | ||
|---|---|---|---|---|
| V16Gy≤5% | V8Gy≤30% | Dmean/40Gy≤8% | Dmean/40Gy≤8% | |
| 3D+3D | 2.0±1.8 | 3.8±2.5 | 4.9±2.1 | 2.0±1.5 |
| 3D+e | 1.8±1.8 | 3.5±2.5 | 4.4±2.1 | 2.3±2.0 |
| 3D+IMRT | 2.0±1.8 | 3.9±2.4 | 5.0±2.1 | 1.8±1.6 |
| IMRT+3D | 1.9±1.6 | 4.3±3.5 | 4.6±1.7 | 2.1±1.8 |
| IMRT+e | 1.7±1.7 | 4.0±4.1 | 4.4±1.7 | 1.9±1.2 |
| IMRT+IMRT | 1.9±1.6 | 4.3±3.5 | 4.6±1.7 | 1.6±1.1 |
| SIB | 2.2±1.6 | 4.9±3.8 | 4.8±1.8 | 1.5±1.4 |
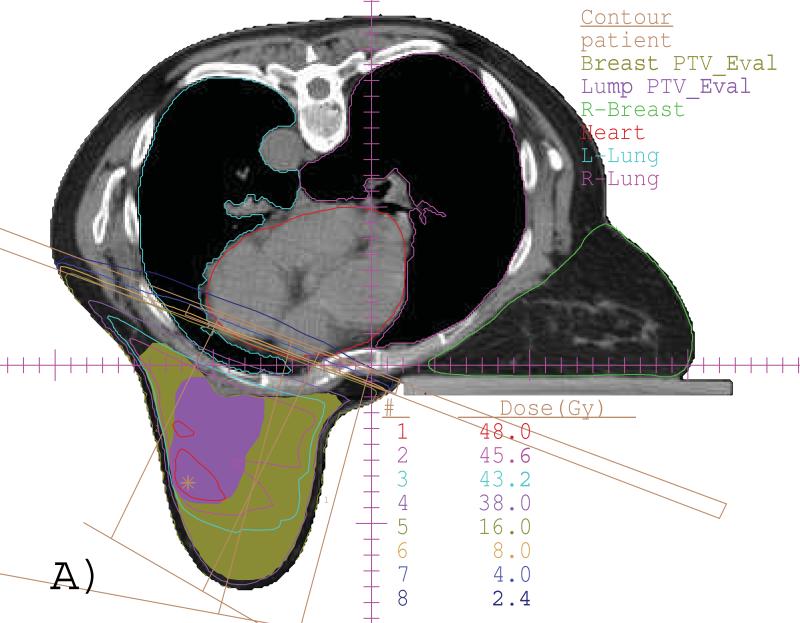
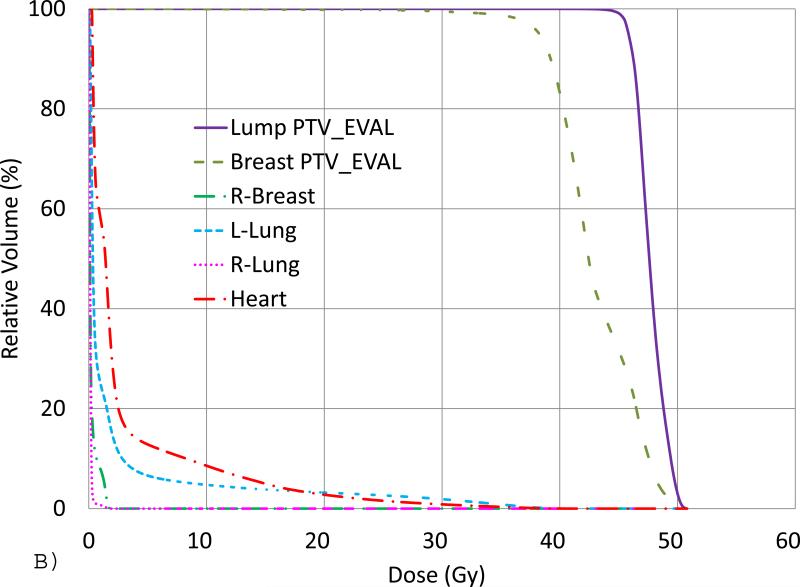
Fig. 1 shows, for an example of a SIB plan generated for a representative prone-positioned case, (A) the isodose lines in a transverse view and (B) the DVHs. For this SIB plan, only two 6 MV tangential beams were utilized, and all ideal dose-volume criteria were met as seen from the DVHs.
Fig 1.
(A) An example of a SIB plan for prone case with isodose lines of 48 (red), 45.6 (pink), 43.2 (cyan), 38 (purple), 16 (dark green), 8 (brown), 4 (blue) and 2.4 (dark blue) Gy in a transverse slice. The Lump PTV_Eval (color wash, purple) and Breast PTV_Eval (color wash, dark green) are also shown. (B) DVHs from the same plan.
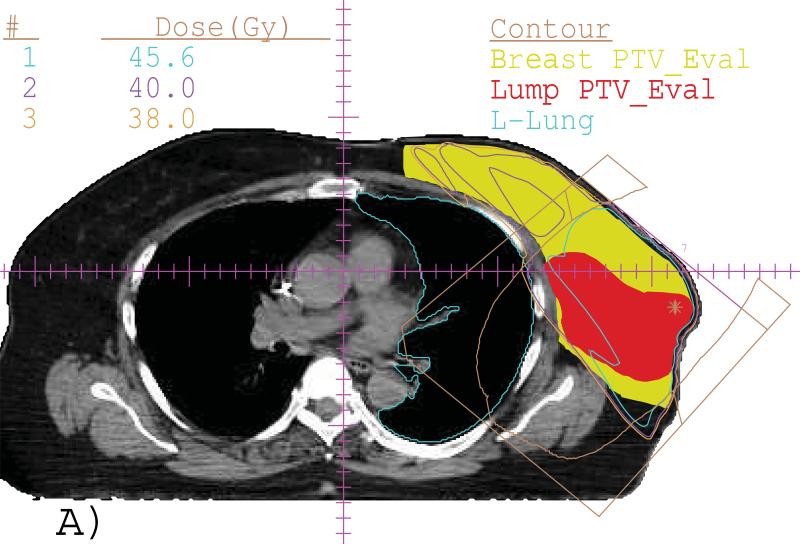
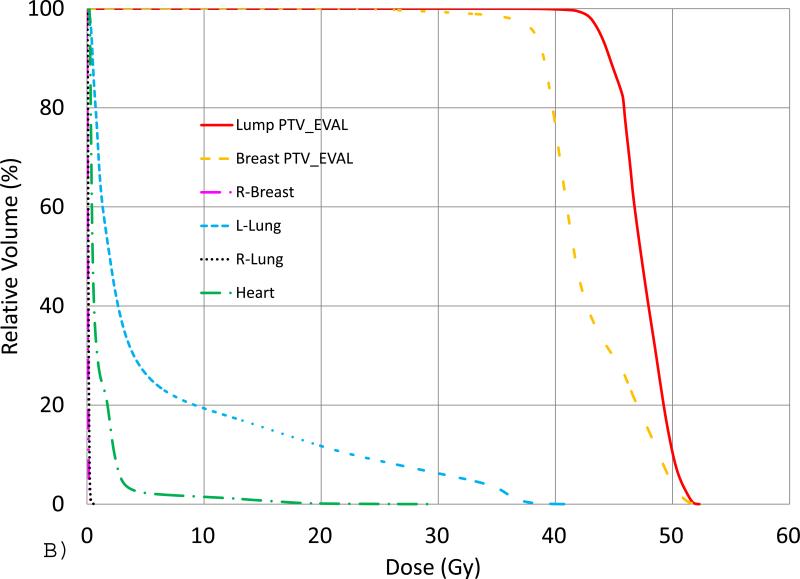
Fig. 2 shows an example of an IMRT+e plan generated for a supine case with lumpectomy in deep depth and breast in large size. The isodose lines in a transverse view (A) and the DVHs (B) were displayed. For this plan, only the ideal Lump PTV_Eval dose coverage criteria was not achieved because of the specific patient anatomy, even though the electron beam with highest energy available was selected.
Fig. 2.
(A) Isodose lines of 45.6 (cyan), 40 (purple) and 38 (brown) Gy in a transverse slice for an IMRT+e plan for a supine patient with deep-seated Lump PTV_Eval (color wash, red) and Breast PTV_Eval (color wash, yellow) with large volume. The enface electron beam is indicated as brown color. (B) DVHs from the same plan.
The prone setup increases the distance between the breast and chestwall, improving the sparing of lung and heart.16-17 Formenti et al reported a prospective study of 400 (~60% eligible) patients underwent simulation in both supine and prone positions to test the hypothesis that prone positioning is superior to standard supine positioning,18 In their study, comparable coverage of the breast regardless of position was ensured by placing the posterior edge of the field on a plane connecting the midline to the anterior extent of the latissimus dorsi muscle. The in-field heart and lung volumes were measured as surrogates for doses to these organs. Their statistical results indicated that the prone positioning was associated with reduced lung volumes irradiated in all patients and reduced heart volumes irradiated in 85% of left-sided breast patients. Table 4 lists the comparisons for V16Gy, V8Gy, as well as V4Gy of ipsilateral lung for supine versus prone positions for the non-electron boost treatment options. Our results, even though with limited statistics, agree with, setting up patient in the prone position can spare more ipsilateral lung than setting up the patient in the supine position. Table 5 lists the averaged parameters V16Gy, V8Gy and Dmean/40Gy of heart for all the eight left-sided cases either in supine or prone positions for all the seven options (three prone cases were not included in electron boost plan options). There is no obvious difference in heart sparing between the seven options.
Table 4.
Ideal dose-volume criteria for ipsilateral lung and corresponding achieved values (averages and standard deviations). The averages and standard deviations were calculated separately for supine and prone for relevant plan options with data from applicable cases.
| Plan Options | V16Gy≤15% | V8Gy≤35% | V4Gy≤50% | |||
|---|---|---|---|---|---|---|
| Supine | Prone | Supine | Prone | Supine | Prone | |
| 3D+3D | 12.6±2.5 | 3.3±2.7 | 19.0±3.9 | 5.1±3.8 | 32.7±10.6 | 7.4±5.2 |
| 3D+e | 12.6±2.4 | 18.3±3.7 | 27.6±6.8 | |||
| 3D+IMRT | 12.2±2.2 | 3.5±2.7 | 17.7±2.8 | 5.2±3.7 | 30.0±9.4 | 7.7±4.6 |
| IMRT+3D | 12.7±1.6 | 3.3±2.5 | 19.2±3.0 | 4.7±3.3 | 33.7±10.7 | 6.7±4.5 |
| IMRT+e | 13.1±1.9 | 19.2±3.8 | 30.0±9.7 | |||
| IMRT+IMRT | 12.7±1.6 | 3.3±2.5 | 18.5±2.8 | 4.7±3.4 | 30.3±9.1 | 6.7±4.5 |
| SIB | 12.7±2.0 | 3.4±2.7 | 18.0±3.8 | 4.8±3.5 | 25.3±7.1 | 6.9±4.7 |
In planning, beams for the whole breast irradiation were placed to deliver dose to the whole breast as uniformly as possible. However, with dose contribution from the boost fields, the whole breast dose uniformity changes. To eliminate the uncertainty from the boost fields, the UI values for Breast PTV_Eval were calculated with dose contribution from WBI only (i.e., without boost fields). Therefore, SIB plan option is irrelevant for such calculation. For each patient, since the 3D/IMRT WBI part can be identical or very similar among the subsequent 3D, e, or IMRT boost plan options, the average of the UI values from these three plan options were calculated. To determine the effects of plan option and individual patient on plan quality, especially the dose uniformity and conformity of the Breast PTV_Eval and Lump PTV_Eval, two-way ANOVA was performed for these quantities using plan option and patient as independent variables. For Breast PTV_Eval, plan options are for 3D and IMRT WBI only. For Lump PTV_Eval, all plan options were included for supine patients and only non-electron boost plan options were considered for all patients (supine plus prone). The P values calculated from the two-way ANOVA are shown in Table 6. For Breast PTV_Eval UI, the variance caused by plan option and patient are both significant. When averaged over patient, the Breast PTV_Eval UI value is smaller with IMRT WBI than with 3D WBI. Kestin et al's study with ten supine patients concluded that the use of IMRT for tangential breast RT can achieve uniform dose throughout the breast and is dosimetrically superior to the treatment techniques that employ only wedges.19 Goodman et al reported a dosimetric analysis of a simplified IMRT technique to plan prone-position RT to the intact breast. They concluded that an IMRT planning approach for prone breast patients can improve dose homogeneity.20 Our results basically confirmed their conclusions. For Breast PTV_Eval CI, the variance caused by patient is significant but the variance caused by plan option is not. This is due to the tangential beam setup for both 3D and IMRT WBI fields. The P values for Lump PTV_Eval are calculated in two scenarios: all plan options with supine patients only and non-electron boost plan options with both supine and prone patients. For example, the P values of UI for Lump PTV_Eval with plan option as variable are 0.5043 and 0.3642 when all plan options with supine patients only and non-electron boost plan options with both supine and prone patients, respectively. For Lump PTV_Eval UI, the variance caused by either plan option or patient is not significant, no matter all plan options are considered for supine patient only, or only non-electron boost plan options are considered for all patients. For Lump PTV_Eval CI, the variance caused by patient is always significant, and the variance caused by plan option is always insignificant.
Table 6.
P values calculated from ANOVA with plan option and patient as independent variables. The Breast PTV_Eval UI and CI are for 3D and IMRT WBI only. The P values for Lump PTV_Eval are calculated for all plan options with supine patients only and non-electron boost plan options with all patients.
| Contour | Variables | Plan | Patient |
|---|---|---|---|
| Breast PTV_Eval | UI | 0.0377 | 0.0467 |
| CI | 0.2172 | < 0.0001 | |
| Lump PTV_Eval | UI | 0.5043/0.3642 | 0.3655/0.2842 |
| CI | 0.5151/0.3265 | <0.0001/<0.0001 |
In general, for non-SIB plans, the most time consuming part in generating a plan is the planning for the whole breast irradiation, because the doses to lung, heart and contralateral breast often need extra efforts to make them meet the required criteria, gantry angles need to be adjusted in addition to MLC leaf positions. Beam placement for lumpectomy PTV is usually straight forward and therefore, less time consuming. The time for treatment planning with different plan options is more or less the same, about 3-8 hours if contouring is included, depending again, on the patient anatomy.
Conclusion
All seven treatment options allowed in RTOG 1005 protocol can meet the required acceptable dose-volume criteria by the protocol, with most of plans achieving the ideal criteria for the patient cases studied. Even at the ideal level, when averaged over patients, the majority of dose-volume goals for all plan options can be achieved with a positive gap of at least a few tenths of standard deviations. The plan qualities in terms of target coverage and normal tissue sparing as measured by the dose-volume parameters are generally comparable for all the seven treatment options allowed in the protocol. For Breast PTV_Eval, the results of our study agree with other studies that, the dose uniformity with IMRT WBI is better than that with 3D WBI. For WBI with tangents, the dose conformity of Breast PTV_Eval only depends on patient anatomy, variance caused by 3DCRT or IMRT is not significant. For Lump PTV_Eval, the dose uniformity depends on neither patient anatomy nor plan option, the dose comformity depends on patient anatomy but there is no preference for the plan options available for the specific patient setup.
Table 2.
Ideal dose-volume criteria for Lump PTV_Eval and corresponding achieved values (averages and standard deviations) from all 15 cases for all plan options, except for electron boost plans where only the 10 supine cases were included in calculation.
| Plan Options | V45.6Gy≥95% | V52.8Gy≤5% | Dmax/48Gy≤115% |
|---|---|---|---|
| 3D+3D | 97.1±1.6 | 0.1±0.5 | 106.6±4.5 |
| 3D+e | 97.0±1.8 | 0.0±0.0 | 107.7±2.1 |
| 3D+IMRT | 96.0±1.1 | 0.5±0.9 | 107.0±5.9 |
| IMRT+3D | 96.7±1.3 | 0.0±0.0 | 106.2±4.0 |
| IMRT+e | 95.9±4.5 | 0.0±0.0 | 107.5±1.9 |
| IMRT+IMRT | 96.1±1.1 | 0.2±0.6 | 106.6±5.2 |
| SIB | 96.1±1.5 | 0.5±0.9 | 108.8±5.1 |
Table 3.
Ideal dose-volume criteria for contralateral breast and corresponding achieved values (averages and standard deviations) from all 15 cases for all plan options, except for electron boost plans where only the 10 supine cases were included in calculation.
| Plan Options | Dmax/40Gy≤6% |
|---|---|
| 3D+3D | 4.1±1.9 |
| 3D+e | 3.3±1.8 |
| 3D+IMRT | 4.2±1.8 |
| IMRT+3D | 3.7±1.6 |
| IMRT+e | 3.0±1.4 |
| IMRT+IMRT | 3.7±1.6 |
| SIB | 3.4±1.5 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E. Twenty-year follow-up of a randomized study comparing breastconserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002;347(16):1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative Group Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. The Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 4.Morrow M, White J, Moughan J, Owen J, Pajack T, Sylvester J, Wilson JF, Winchester D. Factors predicting the use of breast-conserving therapy in stage I and II breast carcinoma. J. Clin. Oncol. 2001;19(8):2254–2262. doi: 10.1200/JCO.2001.19.8.2254. [DOI] [PubMed] [Google Scholar]
- 5.Qi XS, White J, Li XA. Is α/β for breast cancer really low? Radiother. Oncol. 2011;100(2):282–288. doi: 10.1016/j.radonc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 6.The START Trialists' Group The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The START Trialists' Group The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. The Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarnold J, Ashton A, Bliss J, Homewood J, Harper C, Hanson J, Haviland J, Bentzen S, Owen R. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother. Oncol. 2005;75:9–17. doi: 10.1016/j.radonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Owen JR, Ashton A, Bliss JM, Homewood J, Harper C, Hanson J, Haviland J, Bentzen SM, Yarnold JR. Effect of radiotherapy fraction size on tumor control in patients with earlystage breast cancer after local tumor excision: long-term results of a randomised trial. Lancet Oncol. 2006;7(6):467–471. doi: 10.1016/S1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- 10.Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzi R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H, Perera F, Fyles A, Schneider K, Gulavita S, Freeman C. Long-Term Results of Hypofractionated Radiation Therapy for Breast Cancer. N. Engl. J. Med. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 11.Chen GP, Ahunbay E, Li XA. Auotmated computer optimization for 3D treatment planning of breast irradiation. Med. Phys. 2008;35(6):2253–2258. doi: 10.1118/1.2911869. [DOI] [PubMed] [Google Scholar]
- 12.Mihai A, Rakovitch E, Sixel K, Woo T, Cardoso M, Bell C, Ruschin M, Pignol JP. Inverse vs. forward breast IMRT planning. Med. Dosim. 2005;30(3):149–154. doi: 10.1016/j.meddos.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Hong L, Hunt M, Chui C, Spirou S, Forster K, Lee H, Yahalom J, Kutcher GJ, McCormick B. Intensity-modulated tangential beam irradiation of the intact breast. Int. J. Radiat. Oncol. Biol. Phys. 1999;44(5):1155–1164. doi: 10.1016/s0360-3016(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhang F, Zheng M. Dosimetric evaluation of conventional radiotherapy, 3-D conformal radiotherapy and direct machine parameter optimization intensity-modulated radiotherapy for breast cancer after conservative surgery. J. Med. Imaging. and Rad. Onc. 2011;55(6):595–602. doi: 10.1111/j.1754-9485.2011.02313.x. [DOI] [PubMed] [Google Scholar]
- 15.International Commission on Radiation Units and Measurements . Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU Report 50), ICRU Report 62. Bethesda, MD: 1999. [Google Scholar]
- 16.Formenti SC, Gidea-Addeo D, Goldberg JD, Roses DF, Guth A, Rosenstein BS, DeWyngaert KJ. Phase I-II trial of prone accelerated intensity modulated radiation therapy to the breast to optimally spare normal tissue. J. Clin. Oncol. 2007;25(16):2236–2242. doi: 10.1200/JCO.2006.09.1041. [DOI] [PubMed] [Google Scholar]
- 17.Lymberis SC, Dewyngaert JK, Parhar P, Chhabra AM, Fenton-Kerimian M, Chang J, Hochman T, Guth A, Roses D, Goldberg JD, Formenti SC. Prospective assessment of optimal individual position (prone versus supine) for breast radiotherapy: volumetric and dosimetric correlations in 100 patients. Int. J. Radiat. Oncol. Biol. Phys. 2012;84(4):902–909. doi: 10.1016/j.ijrobp.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Formenti SC, DeWyngaert JK, Jozsef G, Goldberg JD. Prone vs supine positioning for breast cancer radiotherapy. JAMA. 2012;308(9):861–863. doi: 10.1001/2012.jama.10759. [DOI] [PubMed] [Google Scholar]
- 19.Kestin LL, Sharpe MB, Frazier RC, Vicini FA, Yan D, Matter RC, Martinez AA, Wong JW. Intensity modulation to improve dose uniformity with tangential breast radiotherapy: initial clinical experience. Int. J. Radiat. Oncol. Biol. Phys. 2000;48(5):1559–1568. doi: 10.1016/s0360-3016(00)01396-1. [DOI] [PubMed] [Google Scholar]
- 20.Goodman KA, Hong L, Wagman R, Hunt MA, McCormick B. Dosimetric analysis of a simplified intensity modulation technique for prone breast radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004;60(1):95–102. doi: 10.1016/j.ijrobp.2004.02.016. [DOI] [PubMed] [Google Scholar]