Abstract
The E1 helicase from BPV and HPV16 interacts with Ubc9 to facilitate viral genome replication. We report that HPV11 E1 also interacts with Ubc9 in vitro and in the yeast two-hybrid system. Residues in E1 involved in oligomerization (353–435) were sufficient for binding to Ubc9 in vitro, but the origin-binding and ATPase domains were additionally required in yeast. Nuclear accumulation of BPV E1 was shown previously to depend on its interaction with Ubc9 and sumoylation on lysine 514. In contrast, HPV11 and HPV16 E1 mutants defective for Ubc9 binding remained nuclear even when the SUMO pathway was inhibited. Furthermore, we found that K514 in BPV E1 and the analogous K559 in HPV11 E1 are not essential for nuclear accumulation of E1. These results suggest that the interaction of E1 with Ubc9 is not essential for its nuclear accumulation but, rather, depends on its oligomerization and binding to DNA and ATP.
Keywords: Papillomavirus, E1, Ubc9, Yeast two-hybrid, Sumoylation, DNA replication, Oligomerization, DNA-binding, ATPase, Gam1
Introduction
Human papillomaviruses (HPV) are small double-stranded DNA tumor viruses that cause benign and malignant lesions of the skin and mucosa, notably cervical cancer (reviewed in Chow and Broker, 1997; zur Hausen and de Villiers, 1994). Maintenance of the viral genome as a circular episome in the nucleus of infected basal epithelial cells is essential for the viral life cycle and the ensuing pathology (reviewed in Howley, 1996; Shah and Howley, 1996; Stubenrauch and Laimins, 1999). Replication of the HPV genome is performed by two viral proteins, E1 and E2, together with cellular DNA replication factors (reviewed in Chow and Broker, 1994; Hebner and Laimins, 2006). E1 is the replicative helicase of the virus that, with the help of E2, assembles into double hexamers at the origin of replication and unwinds the DNA ahead of the bidirectional replication fork (reviewed in Sverdrup and Myers, 1997; Wilson et al., 2002). Structure–function studies indicated that E1 comprised at least three functional domains. For HPV11 E1, these consist of an N-terminal region (amino acids 1–191) essential for replication in vivo but dispensable in vitro, an origin DNA-binding domain (OBD, amino acids 191–353), and a C-terminal helicase domain (amino acids 353–649) sufficient to form hexamers and to bind E2 (Amin et al., 2000; Sun et al., 1998; Titolo et al., 2000, 1999, 2003b; White et al., 2003). The helicase domain can be further subdivided into two regions. One comprised of residues 353–435 of HPV11 E1 mediates the oligomerization of the protein and constitutes the minimal E1–E1 interaction domain that can be identified in the yeast two-hybrid system (Titolo et al., 2000). The other, spanning amino acids 435–649, contains the ATPase motifs conserved among members of the SF3 family of helicases (Hickman and Dyda, 2005; James et al., 2003; White et al., 2001). E1 also interacts with several cellular replication factors to promote viral DNA replication, including polymerase α-primase, RPA, and topoisomerase I (Amin et al., 2000; Clower et al., 2006; Conger et al., 1999; Loo and Melendy, 2004; Masterson et al., 1998; Park et al., 1994).
E1 was also reported to interact with Ubc9 suggesting that it may be regulated by the Small Ubiquitin-like Modifier (SUMO) pathway (Rangasamy and Wilson, 2000; Yasugi and Howley, 1996; Yasugi et al., 1997). Sumoylation is a process biochemically akin to ubiquitination but involving the covalent attachment of SUMO to specific lysines of target proteins (reviewed in Hochstrasser, 2002; Kim et al., 2002; Schwartz and Hochstrasser, 2003). It has been shown to regulate a diverse array of processes including nuclear transport, chromosome segregation, cell cycle regulation, DNA replication, and regulation of the activity of several transcription factors (reviewed in Mascle et al., 2007; Matunis, 2002; Melchior and Hengst, 2002; Pichler and Melchior, 2002; Seeler and Dejean, 2003; Verger et al., 2003; Wilson and Rangasamy, 2001). The enzymology of sumoylation is similar to that of ubiquitination but is accomplished by a different set of activating (E1) and conjugating enzymes (E2) as well as E3 ligases (reviewed in Geiss-Friedlander and Melchior, 2007; Hilgarth et al., 2004). The human E1 activating enzyme is a heterodimer of the two proteins SAE1 and SAE2. Interestingly, the Gam1 protein of the avian adenovirus CELO (chicken embryo lethal orphan) has been shown to interfere with cellular sumoylation by abrogating the function of this heterodimer and inducing its ubiquitination and subsequent degradation by the proteasome (reviewed in Chiocca, 2007). Ubc9 is the only known SUMO-conjugating enzyme (E2) in yeast and human (Desterro et al., 1997; Johnson and Blobel, 1997; Schwarz et al., 1998). E3 ligases are dispensable in vitro where most of the substrate specificity comes from the consensus sumoylation site itself, as exemplified by the crystal structure of Ubc9 in complex with a portion of the target protein RanGAP1 (Bernier-Villamor et al., 2002; Sampson et al., 2001). The consensus sequence for sumoylation is ψ-K-x-D/E in which the lysine (K) subject to modification is preceded by a hydrophobic residue (ψ) and separated by any single amino acid (x) from an aspartic or glutamic acid (D or E).
The interaction of papillomavirus E1 with human Ubc9 was discovered in yeast two-hybrid screens, first for the HPV16 protein (Yasugi and Howley, 1996; Yasugi et al., 1997) and subsequently for that of BPV (Rangasamy and Wilson, 2000). Sumoylation of E1 from BPV, HPV1, and HPV18 has been demonstrated in vitro, in assays performed with Ubc9, SUMO-1 and in vitro translated E1 (Rangasamy and Wilson, 2000; Rangasamy et al., 2000). Sumoylation of BPV E1 was also shown to occur in vivo, in transfected cells overexpressing E1, Ubc9 and SUMO-1 (Rangasamy and Wilson, 2000). The site of SUMO attachment in BPV E1 was identified by mutagenesis as lysine 514, located in a highly conserved region of the C-terminal helicase domain (Rangasamy et al., 2000). Substitution of lysine 514 for alanine or arginine was shown to abrogate the ability of the protein to support transient DNA replication in vivo (Rangasamy et al., 2000). A similar result was obtained with a double amino acid substitution, L420P/K421A, that weakens binding to Ubc9 in vitro and in yeast and abrogates sumoylation in vitro and in vivo (Rangasamy and Wilson, 2000; Rangasamy et al., 2000). Strikingly, both the L420P/K421A and K514R BPV E1 mutant proteins were found to accumulate in the cytoplasm rather than in the nucleus of transfected COS-1 cells, when fused to GFP (Rangasamy et al., 2000). These studies led to the proposal that interaction with Ubc9 and conjugation with SUMO-1 are required for intranuclear accumulation of BPV E1.
Here we report that that HPV11 E1 also interacts with Ubc9 and provide evidence that the ability of E1 to oligomerize and to bind DNA and ATP is required for this interaction. We also demonstrate that the nuclear accumulation of E1 is not dependent on its interaction with Ubc9 and on a functional SUMO pathway in C33A cervical carcinoma cells.
Results and discussion
HPV11 E1 binds to human Ubc9 in vitro through a region required for its oligomerization
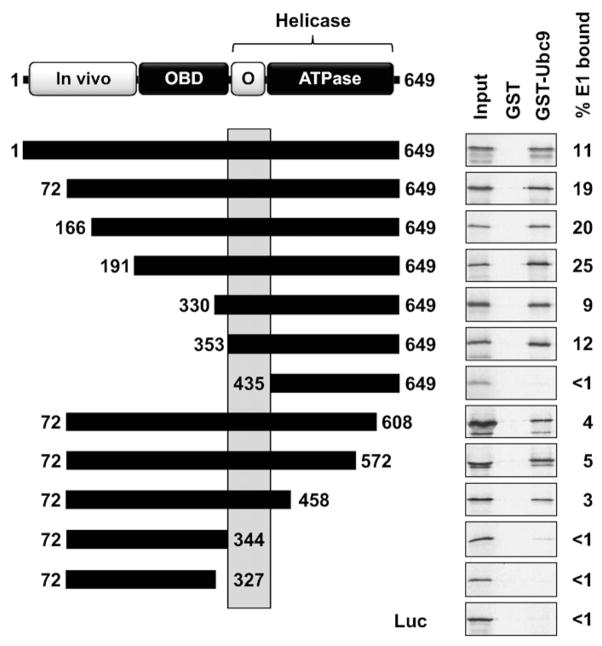
To determine if HPV11 E1 could interact with Ubc9 similarly to what was observed for BPV and HPV16 E1, we first performed an in vitro binding assay using GST-Ubc9 purified from bacteria and in vitro translated E1 produced in a rabbit reticulocyte lysate. Using this pulldown assay, a positive interaction was detected between both proteins (Fig. 1). The specificity of this interaction was demonstrated by showing that E1 did not bind to GST alone and that GST-Ubc9 could not pulldown an unrelated protein, firefly luciferase (Luc). We also showed that the E1-Ubc9 interaction was not sensitive to ethidium bromide in vitro (data not shown), indicating that it is not artefactually mediated through DNA present in the E1 lysate. We then used the set of deletions shown in Fig. 1 to map the domain of HPV11 E1 required for binding to Ubc9. E1 fragments were tested for their binding to purified GST-Ubc9, or to GST alone as a control. Among the N-terminally truncated E1 fragments, the one comprised of amino acids 353–649 was the shortest one that retained binding to Ubc9 (Fig. 1). For the C-terminally truncated fragments, we found that the one comprised of the first 458 amino acids of E1 could bind to Ubc9, whereas a shorter one ending at amino acid 344 could not (Fig. 1). Incidentally, the fact that an E1 fragment lacking the ATPase domain (amino acids 435–649), which is essential for single-stranded DNA binding (Enemark and Joshua-Tor, 2006), could still interact with Ubc9 ruled out the possibility that the interaction was artefactually mediated by single-stranded DNA present in the E1 lysate. Collectively, these studies indicate that amino acids 353–435 of HPV11 E1 are required for interaction with Ubc9. Interestingly, we previously reported that this region is also required for the oligomerization of E1 into hexamers (Titolo et al., 2000).
Fig. 1.
Interaction of HPV11 E1 with Ubc9 in vitro. A schematic representation of the HPV11 E1 protein, 649 amino acids in length, is shown at the top of the figure. The N-terminal domain essential for in vivo replication (in vivo, amino acids 1–191), the origin DNA-binding domain (OBD, amino acids 191–353) and the helicase domain encompassing a region required for oligomerization (O, amino acids 353–435) and ATPase activity (amino acids 435–649) are indicated. Truncated E1 proteins that were tested for interaction with Ubc9 in GST-pulldown assays are diagrammed as black boxes. The region of HPV11 E1 essential for its interaction with Ubc9 in vitro is highlighted by a grey box. The results of GST-pulldown assays performed with these truncations and the percentage of input E1 bound to GST-Ubc9 (% E1 bound) are shown on the right of the panel. In these experiments, in vitro translated and 35S-labeled E1 proteins were chromatographed over GST or GST-Ubc9 columns and the bound proteins eluted with high-salt were analyzed by SDS-PAGE and autoradiography. Firefly luciferase (Luc) was used as a negative control.
HPV11 E1 interacts with human Ubc9 in the yeast two-hybrid system
We determined that HPV11 E1 also interacts with Ubc9 in the yeast two-hybrid system, giving rise to high levels of β-galactosidase activity comparable to those obtained with BPV E1 (Fig. 2). As we previously reported, the complete HPV11 E1 protein (1–649) can activate transcription by itself in yeast, although not to an extent that prevents detection of its interaction with Ubc9 (Titolo et al., 1999). Fortunately, the interaction was conserved with a shorter HPV11 E1 fragment that lacks the first 71 amino acids and does not activate transcription in yeast. We also found that the interaction is independent of Ubc9 enzymatic activity since this E1 fragment could also interact with an inactive form of Ubc9 carrying the amino acid substitution C93S at the site of thioester linkage (Chakrabarti et al., 1999) (Fig. 2). The domain of HPV11 E1 required for interaction with Ubc9 was then mapped using a series of deletions shown in Fig. 2. These studies revealed that a fragment spanning amino acids 191–649 was the smallest region of E1 that gave rise to high levels of β-galactosidase activity in presence of Ubc9. This fragment of E1 encompasses the origin-binding domain (OBD; amino acids 191–353) and the C-terminal helicase domain (amino acids 353–649). Interestingly, the E1 (191–649) fragment and all of the larger ones that interacted strongly with Ubc9 were only weakly expressed in yeast (Supplementary Fig. 1). Truncations that removed part of the ATPase region (72–572) or the OBD (353–649) resulted in substantially lower levels of β-galactosidase activity, suggesting that both domains are necessary for interaction with Ubc9. Neither the OBD nor the C-terminal helicase domain interacted strongly with Ubc9 although low levels of β-galactosidase activity were measured for both domains, suggesting that they can each interact weakly with Ubc9. A fragment encompassing the OBD and minimal E1–E1 oligomerization domain (191–438) gave rise to higher levels of β-galactosidase activity in presence of Ubc9 than the OBD alone (191–353), despite the fact that it was expressed at lower levels than the OBD (Supplementary Fig. 1). This finding re-enforces the role of amino acids 353–438 for interaction with Ubc9. Collectively, these results indicate that an E1 fragment encompassing the OBD, minimal E1 oligomerization domain, and ATPase motifs interacts robustly with Ubc9 and that all three regions participate in this interaction.
Fig. 2.
Interaction of HPV11 E1 with Ubc9 in the yeast two-hybrid system. A schematic representation of the HPV11 E1 protein is shown at the top of the figure, as described in the legend of Fig. 1. Truncated E1 proteins tested for interaction with Ubc9 in the yeast two-hybrid system are diagrammed as black boxes. The level of expression of the different E1 fragments (Supplementary Fig. 1) are summarized on the left as low (+), intermediate (++), and high (+++) expression level. Yeast two-hybrid results are indicated on the right of each truncation as relative levels of β-galactosidase activity measured in cells expressing either human Ubc9 fused to the Gal4 activation domain (AD-Ubc9) or the Gal4 activation domain (AD) alone. The value for the interaction between E1 and a mutant Ubc9, carrying the C93S (Mut) substitution in the site of thioester linkage, is indicated in parentheses. All values are presented relative to that obtained for the combination of E1 (72–649) and Ubc9, which was set arbitrarily at 100. Negative and positive controls were the Gal4-DBD and BPV E1 (amino acids 1–605), respectively.
Effect of single amino acid substitutions in HPV11 E1 on its interaction with Ubc9 in vitro and in yeast
The results presented above identified amino acids 353–435 of E1 as important for Ubc9 binding in vitro and in yeast (Fig. 3A). Furthermore, they revealed that the OBD and ATPase region also contribute to the E1–Ubc9 interaction in yeast (Fig. 3A). To substantiate these findings, we tested the effect of single amino acid substitutions in E1 that impair its origin binding, oligomerization or ATPase activity on its interaction with Ubc9 in vitro and in yeast (Figs. 3B and C). For BPV E1, it was previously shown that the K183A substitution in the OBD abrogates origin binding (Gonzalez et al., 2000). We therefore created the analogous amino acid substitution (K228A) in HPV11 E1, confirmed that it abrogates origin binding (see below), and analyzed its effect on the E1–Ubc9 interaction. We found that this substitution reduced the binding of HPV11 E1 to Ubc9 both in pulldown assays (Fig. 3B) and in yeast (Fig. 3C), thereby confirming a role for the OBD in this interaction. To investigate the role of E1 oligomerization in its interaction with Ubc9, we used previously described amino acid substitutions, Y380A, N389A, and F393A that abolish the oligomerization of HPV11 E1 (Titolo et al., 2000; White et al., 2001). These three substitutions also affect the ATPase activity of HPV11 E1 (Titolo et al., 2000; White et al., 2001) as E1 needs to assemble into hexamers to be enzymatically active. We found that the three substitutions reduced interaction of E1 to Ubc9 in vitro and yeast, confirming a role for the oligomerization domain in Ubc9 binding. We previously demonstrated that these substitutions do not affect the interaction of E1 with E2 (Titolo et al., 2000), indicating that the folding of the helicase domain is not grossly disturb. Finally, we tested the effect of mutating the highly conserved K484 residue of the HPV11 E1 Walker A ATPase motif that is predicted to make critical hydrogen bounds with the triphosphate tails of ATP (Enemark and Joshua-Tor, 2006; James et al., 2003, 2004). Substitution of K484 for either alanine or arginine had no effect on the interaction of E1 with Ubc9 in vitro but completely abolished it in yeast. This result provided further support that the ATP-binding activity of E1 is needed for its interaction with Ubc9 in yeast. Together, the results described above support the notion that the minimal E1 oligomerization domain contains a binding site for Ubc9 that is sufficient for interaction in vitro. This domain of E1 is also required for interaction with Ubc9 in yeast but additional sequences in the origin-binding domain and C-terminal ATPase domain of E1 are also needed for maximal interaction (i.e., for high levels of β-galactosidase activity).
Fig. 3.
Amino acid substitutions affecting the interaction of HPV11 E1 with Ubc9. (A) Diagram of HPV11 E1 highlighting the portion of the protein required for interaction with Ubc9 in vitro (amino acids 353–435) and in the yeast two-hybrid system (amino acids 191–649). (B) GST-pulldown assays showing the binding of wild-type (WT) and the indicated mutant E1 proteins to GST-Ubc9 or GST as a control. The percentage of input E1 bound to GST-Ubc9 (% E1 bound) is shown on the right of the panel. (C) Yeast two-hybrid results showing the interaction of wild-type and mutant E1 (72–649) proteins with Gal4 AD-Ubc9 or the Gal4 AD alone as a control. β-Galactosidase levels are reported as a percentage of those measured in cell expressing wild-type E1 (72–649) and AD-Ubc9.
Nuclear accumulation of HPV11 E1 is not affected by substitutions that weaken Ubc9 binding and by inhibition of the SUMO pathway
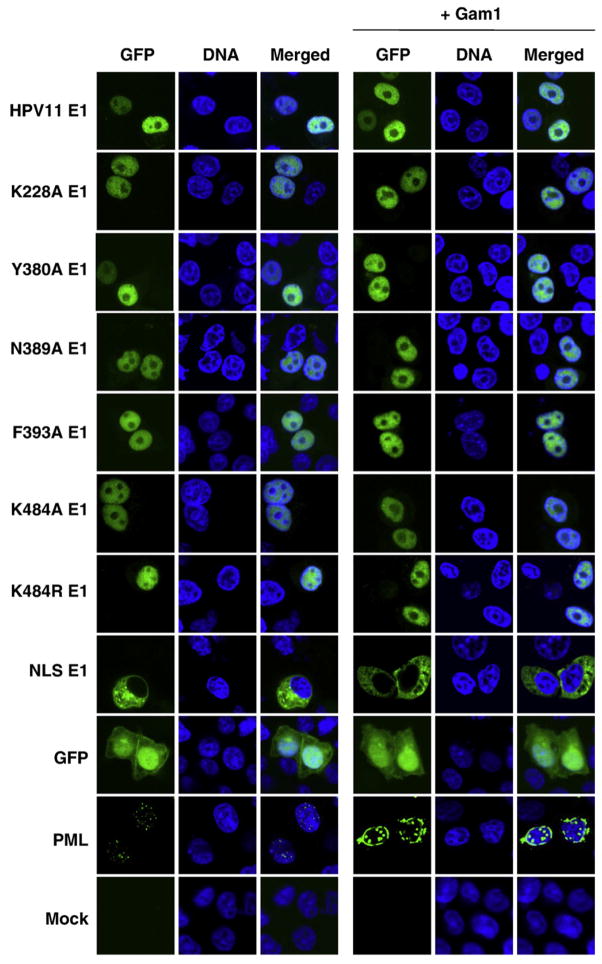
It was previously reported that intranuclear accumulation of BPV E1 is dependent on its interaction with Ubc9 (Rangasamy and Wilson, 2000; Rangasamy et al., 2000). We therefore examined the intracellular localization of the HPV11 E1 mutant proteins described above. As was done in the BPV study, we chose to visualize HPV11 E1 by fusing it to GFP. We were cautious to use a GFP-E1 construct in which a splicing donor site within the E1 ORF had been inactivated by silent mutations to prevent expression of a truncated fusion protein (Cote-Martin et al., 2008; Deng et al., 2003) and verified proper expression of each GFP-E1 protein by Western blotting (Supplementary Fig. 2A). In transfected C33A cells, this GFP-HPV11 E1 fusion protein was nuclear (Fig. 4), as previously reported (Deng et al., 2003). We also observed that the HPV11 E1 mutant proteins defective for Ubc9 binding were nuclear (Fig. 4), in contrast to what would have been expected from the BPV study (Rangasamy and Wilson, 2000; Rangasamy et al., 2000). These results suggested that nuclear accumulation of HPV11 E1 is independent of its interaction with Ubc9.
Fig. 4.
Intracellular localization of wild-type and mutant HPV11 E1. C33A cells transiently expressing the indicated GFP-HPV11 E1 proteins, or GFP-PML, with or without Gam1, were fixed, mounted, and visualized by fluorescence confocal microscopy. Nuclei (DNA) were stained with TO-PRO-3.
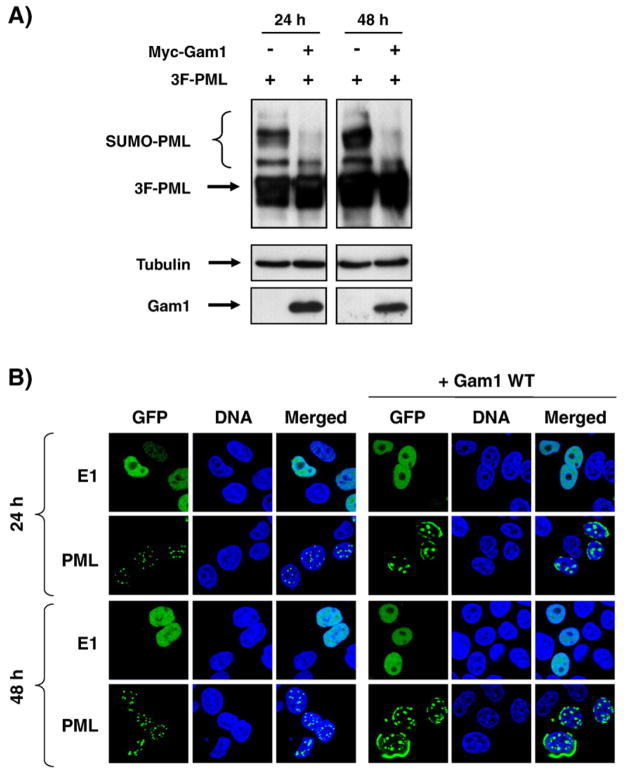
Given that the primary function of Ubc9 is to conjugate SUMO to its target proteins (Johnson, 2004) and that the nuclear accumulation of HPV11 E1 is independent of its interaction with Ubc9, we surmised that nuclear accumulation of E1 was independent of the SUMO pathway. To conclusively rule out the involvement of the SUMO pathway in HPV11 E1 nuclear accumulation, we initially used the Gam1 protein of the CELO adenovirus, a known inhibitor of the SUMO-activating enzyme (SAE1/SAE2 heterodimer) (reviewed in Chiocca, 2007). First, we confirmed that expression of Gam1 could inhibit the SUMO pathway in C33A cells by showing that it could prevent the sumoylation of the promyelocytic leukemia protein (PML), a prototypical substrate of the SUMO pathway, and also disrupt its characteristic punctuate intranuclear localization. Specifically, we showed by Western blotting that Gam1 could effectively inhibit the accumulation of the sumoylated forms of PML in C33A cells, 24 h post-transfection (Fig. 5A). PML is known to accumulate in discrete nuclear foci known as nuclear domains 10 (ND10) or PML oncogenic domains (PODs) (Zhong et al., 2000) whose formation depends on the sumoylation of PML (Shen et al., 2006). To further demonstrate that Gam1 was capable of inhibiting the SUMO pathway in C33A cells, we showed that its cotransfection resulted in the redistribution of GFP-PML from discrete nuclear foci to the nuclear periphery and in larger intranuclear domains (Figs. 4 and 5B), similar to what was reported by Shen et al. (2006). Together, these results demonstrated that Gam1 was indeed inhibiting the SUMO pathway under our assay conditions. When cotransfected with GFP-E1, Gam1 had no effect on the nuclear accumulation of wild-type E1 in C33A cells (Figs. 4 and 5B), suggesting that the SUMO pathway is not involved in this process. To exclude the possibility that residual activity of the SUMO pathway was responsible for the nuclear accumulation of HPV11 E1, we also verified that the effect of Gam1 on PML and HPV11 E1 was the same at 48 h post-transfection. At this time point, sumoylation of PML was still inhibited and its cellular localization was disturbed (Fig. 5). However, the nuclear accumulation of HPV11 E1 remained unchanged, further supporting the notion that HPV11 E1 nuclear accumulation is independent of the SUMO pathway.
Fig. 5.
Effect of the Gam1 protein on PML sumoylation and intracellular localization of PML and HPV11 E1. (A) Sumoylation of PML. C33A cells were transfected with a plasmid encoding a triple Flag-tagged PML (3F-PML) either alone or together with a plasmid encoding Myc-Gam1. Anti-Flag, anti-tubulin, and anti-Myc immunoblots were performed on whole cell extracts (WCE) 24 and 48 h post-transfection. (B) Cellular localization of PML and HPV11 E1. C33A cells transiently expressing GFP–E1 or GFP-PML, with or without Gam1, were fixed, mounted, and visualized by fluorescence confocal microscopy 24 and 48 h post-transfection. Nuclei (DNA) were stained with TO-PRO-3.
To rule out any potential pleiotropic effect of Gam1, we used two additional methods of interfering with the SUMO pathway. First, we made use of a dominant negative version of Ubc9 (Ubc9dn, C93S) that can still interact with its target proteins, including E1 (Fig. 2), but is unable to conjugate SUMO (Chakrabarti et al., 1999). As expected, Ubc9dn, but not Ubc9WT, significantly reduced the sumoylation of PML and led to a redistribution of GFP-PML at the nuclear periphery and in larger intranuclear domains 24 and 48 h post-transfection (Supplementary Fig. 3B). Importantly, it had no effect on the intranuclear accumulation of E1 (Supplementary Fig. 3B). We then tested the effect of depleting the levels of Ubc9 in C33A cells using a specific shRNA (shUbc9 11077, Supplementary Fig. 4A). As anticipated, the Ubc9 shRNA, but not the inactive control shRNA, led to a redistribution of PML 96 h post-transfection but had no effect on the intranuclear accumulation of E1 (Supplementary Fig. 4B). From these studies, we conclude that a functional SUMO pathway is not required for nuclear accumulation of wild-type HPV11 E1.
Next, we wondered if HPV11 E1 mutants that are defective for Ubc9 binding would be more sensitive to inhibition of the SUMO pathway. We therefore investigated the nuclear accumulation of these E1 mutants in the presence of Gam1, which we have found is a stronger inhibitor of the SUMO pathway than Ubc9dn or the Ubc9 shRNA. We found that all of the Ubc9-binding defective E1 mutants were still localized to the nucleus when the SUMO pathway was inhibited by Gam1 (Fig. 4). Collectively, these results indicate that the nuclear accumulation of HPV11 E1 is independent of both its interaction with Ubc9 and a functional SUMO pathway.
Mutant HPV16 E1 proteins that are defective for interaction with Ubc9 also localize to the nucleus
One of our laboratories has previously identified five amino acid substitutions in HPV16 E1 that abrogate its interaction with Ubc9 in the yeast two-hybrid system (Yasugi et al., 1997). Of these substitutions, four lie in the C-terminal helicase domain (Y412F, W439R, G482D, and G496R) whereas the fifth one is in the OBD (S330R). To analyze the nuclear accumulation of these mutant HPV16 E1, we constructed GFP fusion proteins in which the major splicing donor site within the E1 ORF was mutated to prevent expression of a truncated fusion protein, essentially as we did for HPV11 E1 (Cote-Martin et al., 2008), and verified their proper expression by Western blotting (Supplementary Fig. 2B). We then determined their intracellular localization in C33A cells. We found that the wild-type GFP-HPV16 E1 fusion protein localizes predominantly to the nucleus and that this phenotype was not altered by amino acid substitutions that affect Ubc9 binding (Fig. 6). Furthermore, expression of Gam1 did not affect the nuclear accumulation of either wild-type or mutant E1 (Fig. 6). The fact that none of the five amino acid substitutions prevented nuclear accumulation of GFP-HPV16 E1, even in presence of Gam1, provides strong evidence that this process can occur independently of an interaction with Ubc9 and a functional SUMO pathway, similarly to what we observed for HPV11 E1.
Fig. 6.
Intracellular localization of wild-type and mutant HPV16 E1. C33A cells transiently expressing the indicated GFP–HPV16 E1 proteins, or GFP-PML, with or without Gam1 were fixed, mounted, and visualized by fluorescence confocal microscopy. Nuclei (DNA) were stained with TO-PRO-3.
The S330R substitution in the HPV16 E1 OBD reduces its affinity for DNA
In this study, we found that mutations that affect the oligomerization of HPV11 E1 also impair Ubc9 binding. A similar observation was made by Yasugi et al. (1997), who found that four of their five substitutions in HPV16 E1 that affected interaction with Ubc9 also affected oligomerization of the protein, detected as an E1–E1 interaction in the yeast two-hybrid system. Accordingly, these four mutant proteins showed reduced ATPase activity and were unable to support transient DNA replication (Yasugi et al., 1997). In contrast the fifth substitution, S330R, impaired the interaction of E1 with Ubc9 with little effect on its ATPase activity and oligomerization (Yasugi et al., 1997). The S330R substitution was also unique in that it conferred a dominant negative phenotype in transient DNA replication assays, unlike the other mutants (Yasugi et al., 1997). Since S330R lies in the OBD, albeit in a region opposite to the DNA-binding surface (Fig. 7A), we wondered if it had any effect on its affinity for DNA. To test this possibility, we produced and purified the wild-type and S330R HPV16 E1 OBDs (residues 190–352) fused to GST. For comparison, we also produced the analogous wild-type and mutant (S331R) OBD from HPV11. An HPV11 OBD carrying the K228A substitution, which lies near the DNA-binding surface (Fig. 7A), was also produced as a DNA-binding defective control. The DNA-binding activities of these different OBDs were then measured using a quantitative assay based on fluorescence anisotropy, which we previously described (Titolo et al., 2003a). In this assay, binding of the purified GST-E1 OBD to a 21-bp duplex DNA labeled at one end with fluorescein results in a change in anisotropy that reaches a plateau when all of the binding sites are occupied. The fluorescent duplex DNA used in these experiments contained two inverted E1 binding sites separated by 3 bp, (5′-Fluo-GGATACTTAATAATAATGGGC-3; the two binding sites are underlined), an optimal substrate for the GST-OBD (Titolo et al., 2003a). As expected, we observed that the wild-type HPV16 E1 OBD could readily bind to the probe, with an estimated dissociation constant (KD) of 875±100 nM (Fig. 7B). In contrast, the S330R mutant OBD had a much lower affinity for the probe, too weak to be accurately measured (Fig. 7B). These results indicate that the S330R substitution reduces the affinity of the E1 OBD for DNA. Similar results were obtained for the HPV11 OBD (Fig. 7C). Specifically, the S331R mutant was also inactive similar to the control mutant K228A. Since S330R affects the binding of HPV16 E1 to Ubc9 in the yeast two-hybrid system, these results provide additional evidence that the DNA-binding activity of E1 is required for interaction with Ubc9 in yeast. In addition, the fact that the S330R substitution reduces DNA binding (this study), while having little effect on the oligomerization of E1 or its interaction with E2 (Yasugi et al., 1997), provides a likely mechanism for its dominant negative phenotype in transient DNA replication assays (Yasugi et al., 1997).
Fig. 7.
Effect of the S330R and S331R substitutions on the DNA-binding activity of the HPV16 and HPV11 E1 OBD, respectively. (A) Location of S330/S331 and K228 in the structure of the OBD. Crystal structure of the BPV E1 OBD bound to DNA (PDB ID: 1KSY (Enemark et al., 2002)) showing the location of threonine 286 (in yellow), which is analogous to serine 330 and 331 in HPV16 and HPV11 E1, respectively. The location of lysine 183, analogous to lysine 228 in HPV11 E1, is shown in red. (B) Fluorescence anisotropy DNA-binding assays. Binding isotherms were performed with 10 nM of fluorescent DNA probe containing two E1 binding sites and increasing concentrations of purified wild-type (squares) or S330R (triangles) GST-HPV16 OBD. (C) Same as panel B but using the wild-type GST-HPV11 E1 OBD (squares) or mutant derivatives carrying the K228A (circles) or S331R (triangles) amino acid substitution. Each binding isotherm was performed in triplicate.
Nuclear accumulation of BPV E1 is independent of the SUMO pathway and of lysine 514 in C33A cells
Our finding that nuclear accumulation of HPV11 and HPV16 E1 is independent of Ubc9 binding and of the SUMO pathway is in contrast to what has been reported for BPV E1 by Rangasamy and Wilson (2000) and Rangasamy et al. (2000). This prompted us to re-examine the intracellular localization of BPV E1 in C33A cells, under our assay conditions. As expected, we observed that BPV E1, when fused to GFP as was done by Rangasamy and Wilson (2000) and Rangasamy et al. (2000), accumulated in the nucleus of transfected C33A cells. Surprisingly, however, BPV E1 remained nuclear when the SUMO pathway was inhibited by Gam1. It was previously reported that BPV E1 is sumoylated on lysine 514 and that substitution of this residue for either alanine or arginine abrogates its nuclear accumulation (Rangasamy et al., 2000). When tested for their effect in C33A cells, we observed that neither of the two K514 substitutions had an effect on the nuclear accumulation of BPV E1, even when the SUMO pathway was inhibited by Gam1 (Fig. 8). We also investigated if similar substitutions had any effect in the context of HPV11 E1. As was observed for BPV E1 both HPV11 mutants (K559A and K559R) accumulated in the nucleus of transfected cells even in presence of Gam1 (Fig. 8). From these results, we conclude that nuclear accumulation of BPV and HPV11 E1 is not dependent on a functional SUMO pathway and on the integrity of K514/K559 in C33A cells, regardless of whether K514/K559 is a site of sumoylation in these cells.
Fig. 8.
K559 and K514 are not essential for the intracellular localization of HPV11 and BPV E1, respectively, in C33A cells. C33A cells transiently expressing the indicated GFP–E1 proteins, or GFP-PML, with or without Gam1, were fixed, mounted, and visualized by fluorescence confocal microscopy. Nuclei (DNA) were stained with TO-PRO-3.
The finding presented above promoted us to test more directly if BPV and HPV11 E1 were sumoylated in C33A cells. We found that both proteins, either in their wild-type or mutant forms (K514/K559), were not significantly sumoylated in C33A cells as compared to PML, which was used as a positive control in these experiments (Supplementary Fig. 5). We cannot rule out that a very small amount of E1 sumoylation occurs in C33A cells, below the detection limit of our assay. However, if this were the case, we would anticipate that this low level of sumoylation would be very efficiently inhibited by Gam1 as this adenoviral protein can inhibit the much more robust sumoylation of PML (Fig. 5A). Overall, these findings provide further support to the idea that nuclear accumulation of E1 is independent of sumoylation in C33A cells.
Lysine 559 in HPV11 E1 is not essential for transient HPV DNA replication
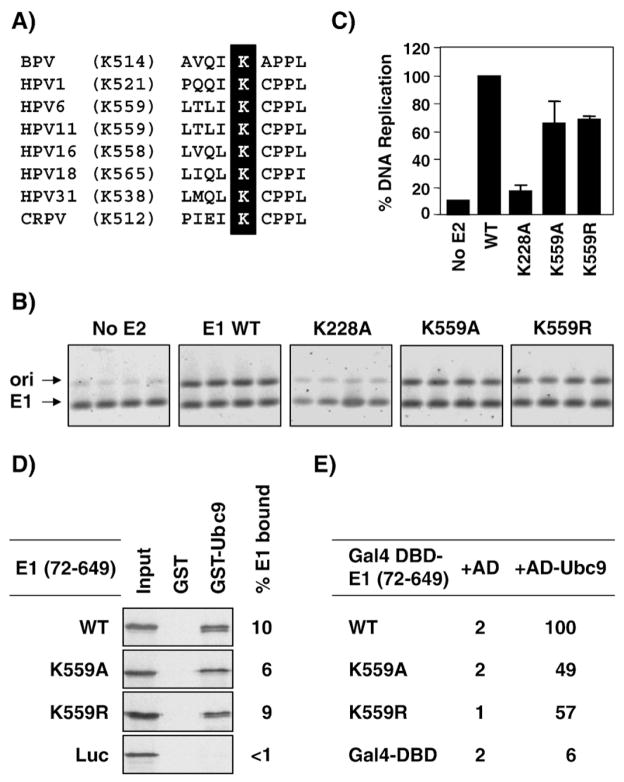
K514 in BPV E1 has been shown to be essential for viral DNA replication. Specifically, it was reported that BPV E1 mutants carrying the K514A and K514R substitutions were unable to support transient BPV DNA replication (Rangasamy et al., 2000). Because K514 is highly conserved (Fig. 9A), we tested if this residue was also essential for viral DNA replication in the context of HPV11 E1. Surprisingly, we found that the K559A and K559R substitutions had little effect in a transient HPV DNA replication assay performed with untagged HPV11 E1 and E2 (Figs. 9B and C). The fact that the K559A and K559R mutant E1 were nearly as active as their wild-type counterpart indicated that not only are they functional but also that they must localize to the nucleus where viral DNA replication takes place. Consistent with the fact that the two K559 mutant E1 are functional, they both retained the ability to interact with Ubc9 in vitro and in yeast (Figs. 9D and E). Thus, unlike what was found for BPV E1, K559 is not essential for HPV11 E1’s ability to support viral DNA replication.
Fig. 9.
K559 is not essential for the replication activity of HPV11 E1. (A) Sequence alignment showing the conservation of K559 in the E1 proteins from different papillomaviruses. (B) Transient HPV11 DNA replication. Cells were transfected with expression plasmids for the indicated wild-type or mutant HPV11 E1 together with an E2-expression plasmid and an origin-containing plasmid. The amount of replicated origin plasmid was measured by PCR from DpnI-digested genomic DNA. A portion of the E1-expressing plasmid devoid of DpnI site was amplified as an internal control to normalize for variation in transfection efficiencies. Each E1 mutant was tested in quadruplicates. The figures show the amounts of origin (ori) and E1 PCR products separated on 1% agarose gels and stained with the fluorescent dye SYBRGreen I (Molecular Probes). The HPV11 E1 K228A mutant defective in DNA binding was used as negative controls. (C) DNA replication activity of mutant E1 proteins. The intensities of the ori and E1 PCR fragments shown in panel B were quantified using a Storm PhosphorImager (Amersham Biosciences) and used to calculate the replication activity of each mutant protein relative to that of wild-type E1, which was set at 100%. Each value is the average of four replicates with the standard deviation indicated. (D) GST-pulldown assays showing the binding of wild-type (WT) and the indicated mutant E1 proteins to GST-Ubc9 or GST as a control. The percentage of input E1 bound to GST-Ubc9 (% E1 bound) is shown on the right of the panel. (E) Yeast two-hybrid results showing the interaction of wild-type and mutant E1 (72–649) proteins with Gal4 AD-Ubc9 or the Gal4 AD alone as a control. β-Galactosidase levels are reported as a percentage of those measured in cell expressing wild-type E1 (72–649) and AD-Ubc9.
Concluding remarks
In this report, we provide evidence that HPV11 E1 interacts with the human SUMO-conjugating enzyme Ubc9 in yeast and in vitro, similarly to what was reported for HPV16 and BPV E1 (Rangasamy and Wilson, 2000; Yasugi and Howley, 1996; Yasugi et al., 1997). We determined that the region of HPV11 E1 spanning amino acids 353–435 is important for interaction with Ubc9 both in yeast and in vitro, in agreement with the mapping data reported for BPV E1, which implicated residues 315 to 459 (corresponding to amino acids 360 to 504 of HPV11 E1). In addition to this region, we found that the OBD and the ATPase domain of E1 are also required for the interaction of E1 with Ubc9 in yeast, although both domains are dispensable in vitro. Based on our previous experience in using the yeast two-hybrid system and in vitro GST-pulldown assays to characterize protein–protein interactions, we would argue that the E1–Ubc9 interaction gives rise to a robust β-galactosidase signal in yeast but to weaker binding in pulldown assays. One reason for this difference may be the oligomerization status of E1. We previously determined that in vitro translated E1 is mostly monomeric (Titolo et al., 2000), and this may explain why it interacts only weakly with Ubc9 in pulldown assays. In yeast, interaction with Ubc9 may be enhanced by oligomerization of E1, which we have shown previously to occur in the two-hybrid system (Titolo et al., 2000). Interestingly, we also reported before that amino acids 353–435 of HPV11 E1, which we determined here to be necessary for interaction with Ubc9, mediate the oligomerization of E1 (Titolo et al., 2000). Furthermore, we have found in this study that substitutions in this region that abrogate the oligomerization of E1 (Y380A, N389A, and F393A) also prevent its interaction with Ubc9 (Titolo et al., 2000). Collectively, these findings suggest that the ability of E1 to oligomerize and bind to Ubc9 may be functionally related. We previously showed that the oligomerization of E1, as well as its ATPase activity, is abolished by substitution of the ATPase Walker A residue K484 to either alanine or arginine (Titolo et al., 2000; White et al., 2001). Here we found that these same substitutions also affect the interaction of E1 with Ubc9 in yeast. Similarly, four of the five substitutions in HPV16 E1 that reduce its interaction with Ubc9 in yeast also inhibit its oligomerization (E1–E1 interaction in yeast) and ATPase activity (Yasugi et al., 1997). Collectively, these results support the notion that oligomerization and ATP-binding/hydrolyzing activity of E1 are needed for its robust interaction with Ubc9 in yeast. Based on the two-hybrid and in vitro interaction data, we propose that residues 353–435 of E1 contain the primary binding site for Ubc9 and that this interaction can be further modulated by oligomerization of the protein.
We also observed that the OBD can modulate the interaction of E1 with Ubc9 in the yeast two-hybrid system. This is most obvious when comparing an HPV11 E1 fragment containing both the OBD and helicase domain (aa 191–649) to one encompassing the helicase domain alone (aa 353–649). Although both E1 fragments bind to GST-Ubc9 with comparable affinities in vitro, the larger one gives rise to substantially higher levels of β-galactosidase activity in yeast, suggesting that it interacts more efficiently with Ubc9. The contribution of the OBD is also highlighted by the finding that the HPV11 K228A and HPV16 S330R substitution that abrogate the DNA-binding activity of the OBD also weakens the E1–Ubc9 interaction in yeast. Thus the origin-binding activity of both HPV11 and HPV16 E1 is essential for their interaction with Ubc9 in yeast. Given that we previously demonstrated that the OBD modulates the oligomerization of E1 (Titolo et al., 2000), it may not be surprising that it also affects its interaction with Ubc9 in yeast.
Previous studies on BPV E1 suggested that nuclear accumulation of the protein was dependent on its interaction with Ubc9 and sumoylation on lysine 514 (Rangasamy et al., 2000). In contrast to what was reported for BPV E1, we found that lysine 559 of HPV11 is neither required for the replication activity of the protein nor for its nuclear accumulation. More importantly, we found that HPV11 and HPV16 E1 proteins defective for Ubc9 binding could still accumulate in the nucleus, even when the SUMO pathway was inhibited by the Gam1 protein of the CELO adenovirus. The lack of effect of Gam1 is consistent with our observation that HPV11 E1 proteins are not significantly sumoylated in C33A cells even when the SUMO conjugation capacity of these cells is augmented by co-expression of Ubc9 and SUMO-1. Collectively, our findings indicate that nuclear accumulation of HPV11 and HPV16 E1 can occur independently of their sumoylation and interaction with Ubc9. Surprisingly, we also observed that the nuclear accumulation of BPV E1 was not changed by Gam1 or by mutation of lysine 514 in C33A cells, in contrast to what was reported previously in COS-1 cells (Rangasamy et al., 2000). This discrepancy between the two studies is unlikely due to our use of a GFP-E1 fusion protein, since a similar fusion was used in the BPV study. Furthermore, Deng et al., 2003 have shown that HPV11 E1 remains functional in transient DNA replication assays when fused to GFP. Our localization studies were performed in C33A cervical carcinoma cells, rather than in the COS-1 monkey kidney cells, because we believe that the C33A cell line is a more relevant system in which to study the function and regulation of HPV proteins. In support of our findings, we note that Yu et al. (2007) also observed that the K559A and K559R substitutions in HPV11 E1 have no effect on nuclear accumulation of the protein, in their case in COS-7 cells. We further note that the group that described the nuclear accumulation defect of the K514 BPV E1 protein has recently published that this mutant E1 is nuclear (Rosas-Acosta and Wilson, 2008). Finally, we stress that the nuclear accumulation of HPV11 and HPV16 E1 was unaffected by the combined effect of mutations that prevent Ubc9 binding and inhibition of the SUMO pathway by Gam1, thus providing strong evidence that sumoylation of E1 is not required for its nuclear accumulation.
In summary, the fact that all of the Ubc9-binding defective E1 mutants identified so far are unable to support viral DNA replication (Titolo et al., 2000; Yasugi et al., 1997), despite being in the nucleus, suggests that Ubc9 is required for some, yet unidentified, aspects of viral DNA replication. In addition, the results in this manuscript indicate that the interaction of E1 with Ubc9 depends on its oligomerization and binding to DNA and ATP (Titolo et al., 2000; Yasugi et al., 1997). Together, these findings support a model whereby Ubc9 facilitates viral DNA replication at a step following assembly of E1 into a double hexamer at the origin.
Materials and methods
Plasmid constructions and mutagenesis
Plasmids to express GFP–E1 fusion proteins were constructed by inserting PCR fragments encoding the HPV11 and BPV1 E1 ORFs into plasmid pQBI25-fc1 (Qbiogene) using BamHI and EcoRI. The HPV11 E1 ORF in these constructs carries silent mutations at codons 5–6 (nucleotides 844–849) that inactivate an internal splicing donor site, which, if not mutated, leads to expression of a truncated GFP–E1 protein (Deng et al., 2003). Plasmids to express GFP–HPV16 E1 fusion proteins were construct by inserting PCR fragment encoding the HPV16 E1 ORF into plasmid pGFP2-C2 (Perkin-Elmer) using XhoI and BamHI. The HPV16 E1 ORF in these constructs also carries silent mutations at codons 5–6 (nucleotides 877–882) to inactivate an internal splicing donor site and prevent expression of a truncated GFP–E1 protein (data not shown). Plasmids to express the HPV16 E1 OBD (amino acids 190–352) and HPV11 E1 OBD (amino acids 191–353) fused to GST have been described (Titolo et al., 2003a). Site-directed mutagenesis was performed with the QuickChange Site-Directed Mutagenesis kit (Stratagene) according to the manufacturer’s instructions. All DNA constructs were verified by sequencing. Plasmids expressing 21-nucleotide short hairpin RNAs (shRNA) against Ubc9 were expressed from the pLKO.1-Puro plasmid and obtained from Open Biosystems (Cat: RHS4533-NM_003345). The targeting sequences for the shRNA against Ubc9 are available online and the targeting sequence for the shRNA control is 5′-GCTATGAGAATAACGGTAACA-3′. Details on construction of these plasmids will be made available upon request.
Yeast two-hybrid analysis
Yeast two-hybrid analysis was performed using Saccharomyces cerevisiae strain Y153 (MATa leu2-3,112 ura3-52 trp1-901 his3-Δ200 ade2-101 gal4Δ gal80Δ URA3::GAL-lacZ LYS::GAL-HIS3) as described (Titolo et al., 1999). β-Galactosidase assays were performed with the substrate chlorophenol red-β-D-galactopyranoside (Roche Applied Science) as described (Titolo et al., 1999). HPV11 E1 plasmids have been described (Titolo et al., 1999, 2000). Plasmids encoding BPV E1 and human Ubc9 fused to the Gal4 DNA-binding domain and activation domain, respectively, were a gift from Dr. Van Wilson (Texas A&M) and have been described (Rangasamy and Wilson, 2000).
GST-pulldown assay
GST and GST-Ubc9 proteins were purified from Escherichia coli BL21 (DE3) (Novagen) as previously described (Titolo et al., 2000). GST-pulldown assays were performed as described in Titolo et al. (2000). The GST-Ubc9 plasmid was a kind gift from Dr. Van Wilson (Texas A&M) and has been described (Rangasamy and Wilson, 2000). Plasmids used for in vitro translation of E1 have been described (Titolo et al., 2000).
Cell culture and transfections
The human cervical carcinoma cell line C33A was grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 0.5 IU/ml of penicillin, 50 μg/ml streptomycin, and 2 mM L-glutamine. Transfections of C33A cells were performed using the Lipofectamine 2000 reagent (Invitrogen).
Confocal fluorescence microscopy
C33A cells (8×106) were transfected with 400 ng of GFP–E1 expression plasmid and either 400 ng of Gam1 expression plasmid or the same amount of empty vector as control, and grown on coverslips. Twenty-four hours post-transfection cells were fixed with 4% formaldehyde and permeabilized with 0.2% Triton X-100 when required. DNA was stained with TO-PRO-3 (Molecular Probes). Cells were mounted using Vectashield mounting medium (Vector Laboratories). Images were acquired using a LSM510 confocal laser coupled to an Axiovert 100M inverted scanning microscope (Zeiss, Toronto, CAN) and analyzed using LSM Image Browser version 3.2.0.70 (Zeiss, Toronto, Canada).
Antibodies and Western blotting
Gal4 DNA-binding domain fusion proteins were detected in total yeast extracts using a mouse monoclonal antibody against Gal4-DBD from Santa Cruz Biotechnology (Cat: sc-510) and β-actin was detected using a mouse monoclonal antibody from Abcam (Cat: ab8224). GFP fusion proteins were detected using a mixture of two mouse monoclonal antibodies purchased from Roche (Cat: 11814460001) and β-tubulin was detected using a mouse monoclonal antibody from Sigma-Aldrich (Cat: T0426). Myc-Gam1 and endogenous Ubc9 were detected using a c-Myc mouse monoclonal antibody (Cat: sc-40) and a Ubc9 goat polyclonal antibody (Cat: sc-5231) from Santa Cruz Biotechnology. For Western blot analysis, proteins were transferred onto polyvinylidene difluoride membranes and detected using horseradish peroxidase-conjugated sheep anti-mouse secondary antibody from GE healthcare (Cat: NA931) or a horseradish peroxidase-conjugated rabbit anti-goat secondary antibody from Santa Cruz Biotechnology (Cat: 2768) and an enhanced chemiluminescence detection kit (GE Healthcare).
Transient HPV DNA replication assay
Transient HPV DNA replication was performed as described previously (Titolo et al., 2003a). Briefly, CHO-K1 cells were transfected with three plasmids encoding HPV11 E1, E2, and the minimal origin of DNA replication (pN9), respectively. Replication of the origin-containing plasmid was quantified 48 h post-transfection by PCR from Dpn1-digested genomic DNA. As a control, a fragment of the E1 expression plasmid devoid of Dpn1 restriction sites was amplified in the same PCR reaction. A low number of PCR cycles were used to ensure that amplification reactions remain in the linear range (data not shown). PCR products were separated on a 1% TBE agarose gel and visualized by staining with the intercalating dye SYBRGreen I (Molecular Probes). Amount of replicated ori-plasmid was quantified by exposure on a STORM 860 Phosphorimager (Molecular Dynamics) and normalized to the amplified E1 signal. Transfection and detection of replicated ori plasmid were performed in quadruplicates.
Fluorescence anisotropy DNA-binding assay
The HPV11 and HPV16 E1 OBDs were expressed as fusions to GST and purified from bacteria as described previously (Fradet-Turcotte et al., 2007). The duplex DNA probe encoding two E1 binding sites was described previously (Titolo et al., 2003a) and was prepared by annealing a fluorescein-labeled oligonucleotide to a complementary oligonucleotide as described (Titolo et al., 2003a). Binding reactions (150 μl) were assembled in 96-well HTRF plates (Packard) using 10 nM fluorescein-labeled probe and the indicated concentrations of protein in the following buffer: 20 mM Tris (pH 7.6), 50 mM NaCl, 0.01% NP-40, and 1 mM DTT. Fluorescence readings were recorded and KD values calculated as previously described (Fradet-Turcotte et al., 2007; Titolo et al., 2003a).
Supplementary Material
Acknowledgments
We thank Dr. Van G. Wilson (Texas A&M University) for providing the expression plasmid encoding GST-Ubc9 and the yeast two-hybrid plasmids encoding UBC9 and BPV E1 and Dr. Muriel Aubry (University of Montreal) for the gift of the GFP-SUMO-1 expression plasmid. We also thank Dr. Susanna Chiocca (Milan, Italy) and Dr. Eric Cohen (IRCM) for providing us with the Gam1 and eGFP-PML expression plasmids, respectively. This work was supported by a grant from the Canadian Institutes for Health Research to J.A. A.F.-T. hold a studentship from the Fonds de la Recherche en Santé du Québec (FRSQ). J.A. is a senior scholar from the FRSQ.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.virol.2009.09.020.
References
- Amin AA, Titolo S, Pelletier A, Fink D, Cordingley MG, Archambault J. Identification of domains of the HPV11 E1 protein required for DNA replication in vitro. Virology. 2000;272(1):137–150. doi: 10.1006/viro.2000.0328. [DOI] [PubMed] [Google Scholar]
- Bernier-Villamor V, Sampson DA, Matunis MJ, Lima CD. Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell. 2002;108(3):345–356. doi: 10.1016/s0092-8674(02)00630-x. [DOI] [PubMed] [Google Scholar]
- Chakrabarti SR, Sood R, Ganguly S, Bohlander S, Shen Z, Nucifora G. Modulation of TEL transcription activity by interaction with the ubiquitin-conjugating enzyme UBC9. Proc Natl Acad Sci U S A. 1999;96(13):7467–7472. doi: 10.1073/pnas.96.13.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocca S. Viral control of the SUMO pathway: Gam1, a model system. Biochem Soc Trans. 2007;35(Pt 6):1419–1421. doi: 10.1042/BST0351419. [DOI] [PubMed] [Google Scholar]
- Chow LT, Broker TR. Papillomavirus DNA replication. Intervirology. 1994;37(3–4):150–158. doi: 10.1159/000150373. [DOI] [PubMed] [Google Scholar]
- Chow LT, Broker R. Small DNA tumor viruses. In: Nathanson N, editor. Viral pathogenesis. Lippencott-Raven Publ; Philadelphia: 1997. pp. 267–301. [Google Scholar]
- Clower RV, Fisk JC, Melendy T. Papillomavirus E1 protein binds to and stimulates human topoisomerase I. J Virol. 2006;80(3):1584–1587. doi: 10.1128/JVI.80.3.1584-1587.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger KL, Liu JS, Kuo SR, Chow LT, Wang TS. Human papillomavirus DNA replication. Interactions between the viral E1 protein and two subunits of human DNA polymerase alpha/primase. J Biol Chem. 1999;274(5):2696–2705. doi: 10.1074/jbc.274.5.2696. [DOI] [PubMed] [Google Scholar]
- Cote-Martin A, Moody C, Fradet-Turcotte A, D’Abramo CM, Lehoux M, Joubert S, Poirier GG, Coulombe B, Laimins LA, Archambault J. Human papilloma-virus E1 helicase interacts with the WD repeat protein p80 to promote maintenance of the viral genome in keratinocytes. J Virol. 2008;82(3):1271–1283. doi: 10.1128/JVI.01405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Jin G, Lin BY, Van Tine BA, Broker TR, Chow LT. mRNA splicing regulates human papillomavirus type 11 E1 protein production and DNA replication. J Virol. 2003;77(19):10213–10226. doi: 10.1128/JVI.77.19.10213-10226.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desterro JM, Thomson J, Hay RT. Ubch9 conjugates SUMO but not ubiquitin. FEBS Lett. 1997;417(3):297–300. doi: 10.1016/s0014-5793(97)01305-7. [DOI] [PubMed] [Google Scholar]
- Enemark EJ, Joshua-Tor L. Mechanism of DNA translocation in a replicative hexameric helicase. Nature. 2006;442(7100):270–275. doi: 10.1038/nature04943. [DOI] [PubMed] [Google Scholar]
- Enemark EJ, Stenlund A, Joshua-Tor L. Crystal structures of two intermediates in the assembly of the papillomavirus replication initiation complex. EMBO J. 2002;21(6):1487–1496. doi: 10.1093/emboj/21.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradet-Turcotte A, Vincent C, Joubert S, Bullock PA, Archambault J. Quantitative analysis of the binding of simian virus 40 large T antigen to DNA. J Virol. 2007;81(17):9162–9174. doi: 10.1128/JVI.00384-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev, Mol Cell Biol. 2007;8(12):947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Bazaldua-Hernandez C, West M, Woytek K, Wilson VG. Identification of a short, hydrophilic amino acid sequence critical for origin recognition by the bovine papillomavirus E1 protein. J Virol. 2000;74(1):245–253. doi: 10.1128/jvi.74.1.245-253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebner CM, Laimins LA. Human papillomaviruses: basic mechanisms of pathogenesis and oncogenicity. Rev Med Virol. 2006;16(2):83–97. doi: 10.1002/rmv.488. [DOI] [PubMed] [Google Scholar]
- Hickman AB, Dyda F. Binding and unwinding: SF3 viral helicases. Curr Opin Struct Biol. 2005;15(1):77–85. doi: 10.1016/j.sbi.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Hilgarth RS, Murphy LA, Skaggs HS, Wilkerson DC, Xing H, Sarge KD. Regulation and function of SUMO modification. J Biol Chem. 2004;279(52):53899–53902. doi: 10.1074/jbc.R400021200. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. New structural clues to substrate specificity in the “ubiquitin system”. Mol Cell. 2002;9(3):453–454. doi: 10.1016/s1097-2765(02)00486-0. [DOI] [PubMed] [Google Scholar]
- Howley PM. Papillomaviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. 3. Vol. 2. Lippincott-Raven; Philadelphia: 1996. pp. 2045–2076. [Google Scholar]
- James JA, Escalante CR, Yoon-Robarts M, Edwards TA, Linden RM, Aggarwal AK. Crystal structure of the SF3 helicase from adeno-associated virus type 2. Structure. 2003;11(8):1025–1035. doi: 10.1016/s0969-2126(03)00152-7. [DOI] [PubMed] [Google Scholar]
- James JA, Aggarwal AK, Linden RM, Escalante CR. Structure of adeno-associated virus type 2 Rep40-ADP complex: insight into nucleotide recognition and catalysis by superfamily 3 helicases. Proc Natl Acad Sci U S A. 2004;101(34):12455–12460. doi: 10.1073/pnas.0403454101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- Johnson ES, Blobel G. Ubc9p is the conjugating enzyme for the ubiquitin-like protein Smt3p. J Biol Chem. 1997;272(43):26799–26802. doi: 10.1074/jbc.272.43.26799. [DOI] [PubMed] [Google Scholar]
- Kim KI, Baek SH, Chung CH. Versatile protein tag, SUMO: its enzymology and biological function. J Cell Physiol. 2002;191(3):257–268. doi: 10.1002/jcp.10100. [DOI] [PubMed] [Google Scholar]
- Loo YM, Melendy T. Recruitment of replication protein A by the papillomavirus E1 protein and modulation by single-stranded DNA. J Virol. 2004;78(4):1605–1615. doi: 10.1128/JVI.78.4.1605-1615.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascle XH, Germain-Desprez D, Huynh P, Estephan P, Aubry M. Sumoylation of the transcriptional intermediary factor 1beta (TIF1beta), the co-repressor of the KRAB Multifinger proteins, is required for its transcriptional activity and is modulated by the KRAB domain. J Biol Chem. 2007;282(14):10190–10202. doi: 10.1074/jbc.M611429200. [DOI] [PubMed] [Google Scholar]
- Masterson PJ, Stanley MA, Lewis AP, Romanos MA. A C-terminal helicase domain of the human papillomavirus E1 protein binds E2 and the DNA polymerase alpha-primase p68 subunit. J Virol. 1998;72(9):7407–7419. doi: 10.1128/jvi.72.9.7407-7419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis MJ. On the road to repair: PCNA encounters SUMO and ubiquitin modifications. Mol Cell. 2002;10(3):441–442. doi: 10.1016/s1097-2765(02)00653-6. [DOI] [PubMed] [Google Scholar]
- Melchior F, Hengst L. SUMO-1 and p53. Cell Cycle. 2002;1(4):245–249. [PubMed] [Google Scholar]
- Park P, Copeland W, Yang L, Wang T, Botchan MR, Mohr IJ. The cellular DNA polymerase alpha-primase is required for papillomavirus DNA replication and associates with the viral E1 helicase. Proc Natl Acad Sci U S A. 1994;91(18):8700–8704. doi: 10.1073/pnas.91.18.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler A, Melchior F. Ubiquitin-related modifier SUMO1 and nucleocytoplasmic transport. Traffic. 2002;3(6):381–387. doi: 10.1034/j.1600-0854.2002.30601.x. [DOI] [PubMed] [Google Scholar]
- Rangasamy D, Wilson VG. Bovine papillomavirus E1 protein is sumoylated by the host cell Ubc9 protein. J Biol Chem. 2000;275(39):30487–30495. doi: 10.1074/jbc.M003898200. [DOI] [PubMed] [Google Scholar]
- Rangasamy D, Woytek K, Khan SA, Wilson VG. SUMO-1 modification of bovine papillomavirus E1 protein is required for intranuclear accumulation. J Biol Chem. 2000;275(48):37999–38004. doi: 10.1074/jbc.M007777200. [DOI] [PubMed] [Google Scholar]
- Rosas-Acosta G, Wilson VG. Identification of a nuclear export signal sequence for bovine papillomavirus E1 protein. Virology. 2008;373(1):149–162. doi: 10.1016/j.virol.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson DA, Wang M, Matunis MJ. The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J Biol Chem. 2001;276(24):21664–21669. doi: 10.1074/jbc.M100006200. [DOI] [PubMed] [Google Scholar]
- Schwartz DC, Hochstrasser M. A superfamily of protein tags: ubiquitin, SUMO and related modifiers. Trends Biochem Sci. 2003;28(6):321–328. doi: 10.1016/S0968-0004(03)00113-0. [DOI] [PubMed] [Google Scholar]
- Schwarz SE, Matuschewski K, Liakopoulos D, Scheffner M, Jentsch S. The ubiquitin-like proteins SMT3 and SUMO-1 are conjugated by the UBC9 E2 enzyme. Proc Natl Acad Sci U S A. 1998;95(2):560–564. doi: 10.1073/pnas.95.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeler JS, Dejean A. Nuclear and unclear functions of SUMO. Nat Rev, Mol Cell Biol. 2003;4(9):690–699. doi: 10.1038/nrm1200. [DOI] [PubMed] [Google Scholar]
- Shah KV, Howley PM. Papillomaviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. 3. Vol. 2. Lippincott-Raven; Philadelphia: 1996. pp. 2077–2109. [Google Scholar]
- Shen TH, Lin HK, Scaglioni PP, Yung TM, Pandolfi PP. The mechanisms of PML-nuclear body formation. Mol Cell. 2006;24(3):331–339. doi: 10.1016/j.molcel.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubenrauch F, Laimins LA. Human papillomavirus life cycle: active and latent phases. Semin Cancer Biol. 1999;9(6):379–386. doi: 10.1006/scbi.1999.0141. [DOI] [PubMed] [Google Scholar]
- Sun Y, Han H, McCance DJ. Active domains of human papillomavirus type 11 E1 protein for origin replication. J Gen Virol. 1998;79(Pt 7):1651–1658. doi: 10.1099/0022-1317-79-7-1651. [DOI] [PubMed] [Google Scholar]
- Sverdrup F, Myers G. The E1 proteins. In: Myers G, Baker C, Münger K, Sverdruo F, McBride A, Bernard H-U, editors. Human Papillomavirus 1997. Theoretical Biology and Biophysics. Chapter III Los Alamos National Laboratory; Los Alamos, New Mexico: 1997. pp. 37–53. [Google Scholar]
- Titolo S, Pelletier A, Sauve F, Brault K, Wardrop E, White PW, Amin A, Cordingley MG, Archambault J. Role of the ATP-binding domain of the human papillomavirus type 11 E1 helicase in E2-dependent binding to the origin. J Virol. 1999;73(7):5282–5293. doi: 10.1128/jvi.73.7.5282-5293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titolo S, Pelletier A, Pulichino AM, Brault K, Wardrop E, White PW, Cordingley MG, Archambault J. Identification of domains of the human papillomavirus type 11 E1 helicase involved in oligomerization and binding to the viral origin. J Virol. 2000;74(16):7349–7361. doi: 10.1128/jvi.74.16.7349-7361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titolo S, Brault K, Majewski J, White PW, Archambault J. Characterization of the minimal DNA binding domain of the human papillomavirus e1 helicase: fluorescence anisotropy studies and characterization of a dimerization-defective mutant protein. J Virol. 2003a;77(9):5178–5191. doi: 10.1128/JVI.77.9.5178-5191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titolo S, Welchner E, White PW, Archambault J. Characterization of the DNA-binding properties of the origin-binding domain of simian virus 40 large T antigen by fluorescence anisotropy. J Virol. 2003b;77(9):5512–5518. doi: 10.1128/JVI.77.9.5512-5518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger A, Perdomo J, Crossley M. Modification with SUMO. A role in transcriptional regulation. EMBO Rep. 2003;4(2):137–142. doi: 10.1038/sj.embor.embor738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PW, Pelletier A, Brault K, Titolo S, Welchner E, Thauvette L, Fazekas M, Cordingley MG, Archambault J. Characterization of recombinant HPV6 and 11 E1 helicases: effect of ATP on the interaction of E1 with E2 and mapping of a minimal helicase domain. J Biol Chem. 2001;276(25):22426–22438. doi: 10.1074/jbc.M101932200. [DOI] [PubMed] [Google Scholar]
- White PW, Titolo S, Brault K, Thauvette L, Pelletier A, Welchner E, Bourgon L, Doyon L, Ogilvie WW, Yoakim C, Cordingley MG, Archambault J. Inhibition of human papillomavirus DNA replication by small molecule antagonists of the E1-E2 protein interaction. J Biol Chem. 2003;278(29):26765–26772. doi: 10.1074/jbc.M303608200. [DOI] [PubMed] [Google Scholar]
- Wilson VG, Rangasamy D. Intracellular targeting of proteins by sumoylation. Exp Cell Res. 2001;271(1):57–65. doi: 10.1006/excr.2001.5366. [DOI] [PubMed] [Google Scholar]
- Wilson VG, West M, Woytek K, Rangasamy D. Papillomavirus E1 proteins: form, function, and features. Virus Genes. 2002;24(3):275–290. doi: 10.1023/a:1015336817836. [DOI] [PubMed] [Google Scholar]
- Yasugi T, Howley PM. Identification of the structural and functional human homolog of the yeast ubiquitin conjugating enzyme UBC9. Nucleic Acids Res. 1996;24(11):2005–2010. doi: 10.1093/nar/24.11.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasugi T, Vidal M, Sakai H, Howley PM, Benson JD. Two classes of human papillomavirus type 16 E1 mutants suggest pleiotropic conformational constraints affecting E1 multimerization, E2 interaction, and interaction with cellular proteins. J Virol. 1997;71(8):5942–5951. doi: 10.1128/jvi.71.8.5942-5951.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JH, Lin BY, Deng W, Broker TR, Chow LT. Mitogen-activated protein kinases activate the nuclear localization sequence of human papillomavirus type 11 E1 DNA helicase to promote efficient nuclear import. J Virol. 2007;81(10):5066–5078. doi: 10.1128/JVI.02480-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Salomoni P, Pandolfi PP. The transcriptional role of PML and the nuclear body. Nat Cell Biol. 2000;2(5):E85–90. doi: 10.1038/35010583. [DOI] [PubMed] [Google Scholar]
- zur Hausen H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.