Abstract
We examined the effect of knee osteoarthritis on the rate of torque development (RTD) of the knee extensors in older adults with advanced-stage knee osteoarthritis (OA; n=15) and recreationally-active controls (n=15) of similar age, sex and health status, as well as the relationship between RTD and the size and contractility of single muscle fibers. OA participants had lower RTD when expressed in absolute terms (Nm/ms). There were sex differences in peak RTD (P<0.05), with greater RTD in men, but no group by sex interaction effects for any variables. The lower RTD in OA versus controls was not explained by variation between groups in the fiber type admixture of the muscle, and was mitigated when RTD was normalized to peak torque (PT). In knee OA volunteers, we found strong correlations between the RTD expressed relative to PT and the velocity of contraction of single myosin heavy chain (MHC) I and IIA/X muscle fibers (r=0.652 and 0.862; both P<0.05) and power output of MHC I fibers (r=0.642; P<0.05). In controls, RTD relative to PT was related to fiber cross-sectional area of MHC IIA/X fibers (r=0.707; P<0.05), but not measures of single fiber contractile performance. To our knowledge, these results represent the first demonstration that variation in whole muscle contractile kinetics in patients with advanced-stage knee osteoarthritis and healthy older adults is related, in part, to the size and function of single muscle fibers.
Keywords: single muscle fiber, velocity, skinned fiber
1. INTRODUCTION
Knee osteoarthritis (OA) is the leading cause of disability in older adults (26) and its incidence is expected to increase several-fold in the coming decades with the expansion of the elderly population (18). As knee OA is the leading cause of functional disability in older adults (14), knowledge of the determinants of physical function has important implications for alleviating the burden of disability on these patients.
Functional disability is fundamentally related to an impaired capacity for physical work, with lower extremity skeletal muscle function being of central importance (28, 52). In individuals with knee OA, knee extensor strength is markedly reduced secondary to muscle atrophy (45), but also due to intrinsic deficits in the contractility of its constituent muscle fibers (11). Muscle force production, however, is likely not the only factor contributing to lower extremity dysfunction, as studies in mobility-limited older adults show that power output is more predictive of functionality than strength (4). Power output is the product of muscle force production and contractile velocity, suggesting that impairments in the velocity of muscle contraction may also contribute to disability (48, 52). Indeed, numerous daily functional tasks, including walking rapidly (55), descending stairs (44) and preventing falls (47), require rapid force development. In older adults with knee OA , work from our laboratories (61) and others (54) have found deficits in the rate of voluntary torque development (RTD) under isometric conditions in older adults with OA compared to controls that relate to functional impairments, such as reduced knee joint power output during walking (61). Moreover, RTD impairments in individuals with knee OA worsen acutely following total knee replacement (TKA; (61)) and are related to declines in functionality (40, 61). Thus, reduced RTD likely contributes to functional disability in older adults with knee OA (48, 52).
Several factors regulate the rate of joint torque development, including neural activation (22, 60), fiber type admixture (27, 29, 36), tendon/extracellular matrix stiffness (9, 51) and the contractile properties of individual muscle fibers (29). Variation in neural activation is generally assumed to be the most important determinant of the rate of torque development (22), and this could be particularly apparent in individuals with knee OA, who have deficits in neural activation (8, 45). However, variation in intrinsic muscle myofilament properties, such as fiber type admixture, atrophy of MHC II fibers (43, 50) and impaired single fiber contractile velocity (11) could also contribute. To our knowledge, however, no study has evaluated the relationship of these intrinsic muscle fiber size and myofilament properties to RTD in older adults with knee OA. Thus, our goal was to assess RTD during isometric contraction in older adults with knee OA and controls and evaluate potential relationships between RTD and muscle fiber type admixture and single muscle fiber size and contractility based on activity-dependent variation in these variables previously reported (11). Moreover, we investigated differences between men and women to determine whether sex-dependent variation in single muscle fiber contractile and structural characteristics described by our lab (10, 11) and others (37, 38) influence contractile properties at the whole muscle level.
2. METHODS
2.1 Participants
Fifteen (7 men, 8 women) older adults with symptomatic knee OA were recruited from the Adult Reconstruction Clinic of the Department of Orthopedics at the University of Vermont Medical Center and the surrounding community. All participants self-reported receiving a clinical diagnosis of knee osteoarthritis, with seven individuals recruited in close proximity to total knee arthroplasty surgery (bilateral or staged-bilateral in 3 volunteers and unilateral in 4). We confirmed that volunteers had symptomatic (5) and radiographic (Kellgren and Lawrence grade 3 or 4 (33)) evidence for advanced knee OA. Volunteers were excluded if they had/have had: a history or clinical signs or symptoms of diabetes, heart failure, pulmonary disease, thyroid disease, peripheral arterial disease, neurological or neuromuscular disease or autoimmune disease; a history (within 10 yrs) of smoking; a history (within 10 yrs) of malignancy, excluding non-melanoma skin cancer; or prior knee replacement in either knee. All volunteers had normal blood counts/chemistry and renal, liver and thyroid function, based on standard blood tests. No participants were taking sex steroid replacement therapy (estrogen or estrogen/progestin therapy in women or androgen replacement in men), oral or inhaled corticosteroids or any other medication that might affect muscle function. Four OA volunteers (2 women, 2 men) were on stable regimens of HMG CoA reductase inhibitors (statins). Plasma creatine kinase levels were within the normative range in these volunteers and none had symptoms or signs of statin-induced myopathy. Of note, we have recently found that chronic, stable statin therapy does not affect skeletal muscle fiber size, mitochondrial morphology or contractile function in patients without myalgia or elevated creatine kinase levels (Rengo et al. unpublished observations), suggesting that inclusion of these individuals would not likely influence results. Additionally, 6 OA participants (4 men, 2 women) had hypertension and were on stable anti-hypertensive therapy, consisting of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (33%), diuretics (33%) and adrenergic blocking agents (33%). Eight (4 women, 4 men) of the individuals were on non-steroidal anti-inflammatory medications for their OA. None had received an intra-articular injection (hyaluronan or corticosteroid) for 6 months prior to testing and none had participated in any prescribed rehabilitation program for the 6 months prior to testing.
Active controls (8 men, 7 women) were selected to match OA participants for age and sex. Controls were healthy and free from disease or medications that could affect muscle size/function and were recruited using identical inclusion/exclusion criteria enumerated above for volunteers with knee OA, with notable exceptions. Controls did not have symptoms consistent with knee OA (5) or radiographic evidence of significant knee osteoarthritis (K&L grade > 2), and self-reported (via Stanford Brief Activity Survey) being recreationally-active, but were not actively training for athletic competition. Five individuals (3 men, 2 women) were on stable regimens of statins, although their plasma creatine kinase levels were within the normative range and none had symptoms or signs of statin-induced myopathy. Additionally, 6 controls (4 men, 2 women) had hypertension and were on stable anti-hypertensive therapy, consisting of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (67%), diuretics (33%) and adrenergic blocking agents (17%). Data on skeletal muscle size and contractile function at the cellular and molecular levels for individuals with OA and controls (n=31) have recently been published (11, 12). Physical characteristics are reproduced to provide necessary descriptive information and muscle fiber size and functional data for the purposes of correlations analyses to assess determinants of RTD. Written informed consent was obtained from each volunteer prior to their participation. The protocol was approved by the Committees on Human Research at the University of Vermont and conformed to the Declaration of Helsinki.
2.2 Knee extensor muscle function and size
Isometric knee extensor torque and torque-time parameters were assessed at 55°, as described previously (58), using a multi-joint dynamometer (HUMAC NORM; CSMi, Stoughton, MA). Briefly, volunteers were seated in the dynamometer with hips and shoulders firmly secured by padded straps and were instructed to perform maximum knee extension “as hard and as fast as you can” for 4-5 seconds, to best capture both peak torque and RTD, as has been shown during similar rapid isometric contractions (6). At least 2 minutes were provided to recover between subsequent attempts. Data recorded by the dynamometer corresponding to torque and position were analyzed after testing using custom-written MatLab software (MathWorks; Natick, MA). The highest torque value during each contraction was considered peak isometric torque for that trial. After smoothing the torque data by applying a 4th order Butterworth low-pass filter (10 Hz cutoff), the first derivative was taken to assess RTD. The peak RTD of the 5 trials was considered the peak RTD for that individual. The RTD was also calculated from the onset of torque development to 25% (RTD25) and 50% (RTD50) of peak isometric torque, as described (61) to facilitate comparison with previous studies and explore the potential that such measures may provide unique insights into muscle performance. The onset of torque development was defined as the point at which torque exceeded 2% of peak torque for a given contraction. Peak RTD was also normalized relative to peak torque of that contraction (RTDrel) to control for variation between subjects in muscle size and strength (21, 36, 49), as we observed that peak RTD was related to overall peak torque and muscle size (see Results, 3.2).
Whole muscle size was assessed using computerized tomography (CT) and dual energy xray absorptiometry (DEXA; GE Lunar Prodigy; Madison, WI) as described (12). Briefly, cross sectional area (CSA) of lean tissue (based on radiodensity) in the quadriceps muscle group were assessed by CT at mid-thigh. Muscle mass of the thigh region was assessed using DEXA. The thigh region was defined as tissue between the femoral condyles (distal) a proximal cut-point marking 60% of the distance between the femoral condyles and the greater trochanter.
2.3 Muscle biopsy processing
Biopsy of the vastus lateralis muscle was performed, as described (57). A portion of tissue was placed immediately into cold (4°C) dissecting solution (20 mM N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid (BES), 5 mM ethylene glycol-bis(2-aminoethylether)-N,N,N, N-tetraacetic acid (EGTA), 5 mM MgATP, 1 strength of 175 mEq, pH 7.0 and at pCa 8 (pCa = −log10 [Ca2+]), muscle fiber bundles were carefully dissected, tied to glass rods at slightly stretched lengths and chemically skinned, as described (42), with long-term storage at −20°C in storage solution with 50% glycerol (v/v) until measurements (performed within 3 weeks). Tissue not processed in this manner was frozen in liquid N2 and stored at −80°C until analysis.
2.4 Tissue MHC isoform expression
MHC isoform distribution was measured in tissue homogenates by gel electrophoresis, as described previously (42). MHC isoform expression was not assessed in one female control due to lack of sufficient tissue.
2.5 Single fiber contractile function and cross-sectional area
Measures of single fiber contractile function were characterized by measuring maximally Ca2+-activated (pCa 4.5) isometric tension, shortening velocity and power output from isotonic load clamps performed at 15°C, as described (11). Muscle fiber CSA was assessed from the average of 5 top and side diameter measurement taken along the length of the fiber. Following completion of mechanical assessments, fibers were placed in gel loading buffer (2% sodium dodecyl sulfate, 62.5 mM Tris, 10% glycerol, 0.001% bromophenol blue, 5% β-mercaptoethanol, pH 6.8) and assessed for MHC isoform expression to determine fiber type, as described (42). Single fiber contractile measures were not conducted on two OA volunteers (1 man, 1 woman) because necessary equipment was unavailable at the time of biopsy. In addition, CSA data were omitted for one male control for MHC IIA and IIAX fibers because 10 out of 14 MHC IIA and IIAX fibers were statistical outliers (>3 SD from the mean), with the remaining 4 MHC II-type fibers all being greater than 2 SD from the mean.
2.6 Statistics
Differences in means between groups were determined using unpaired t-tests and by analysis of variance, with the latter including sex as an additional fixed effect to interrogate whether there were group by sex interactions. Associations between measures were determined using Pearson correlation coefficients for normally distributed data. For data that was not normally distributed (Shapiro-Wilk test), Spearman rank correlation coefficients were used (CSA in MHC I, IIA and IIA/X fibers and tension in MHC IIA fibers in the pooled sample and power in MHC I fibers in knee OA). In cases where multiple observations were available to characterize a parameter in each participant (eg, single fiber CSA, mechanical measures), data was collapsed to a single mean value for each individual for correlation analyses. All data are reported as mean ± SEM. Statistical analyses were performed using IBM SPSS Statistics (version 20.0, IVM, Armonk, NY).
3. RESULTS
3.1 Physical characteristics
Subject characteristics are listed in Table 1 by group assignment. There were no differences between groups in age or body mass or height, and no effect of sex to modify any of these parameters. As expected, physical activity level was significantly lower in volunteers with knee OA compared to controls (55.4%, P<0.01), while no group by sex interaction was noted.
Table 1.
Subject Characteristics
| Control | OA | |
|---|---|---|
| Sex (M/F) | 8/7 | 7/8 |
| Age (yrs) | 68 ± 1 | 71 ± 2 |
| Height (cm) | 168 ± 2 | 165 ± 3 |
| Physical Activity (kcal·d−1) | 494 ± 46 | 253 ± 26 ** |
Data are means ± SE.
, P<0.01 control versus OA.
3.2 RTD
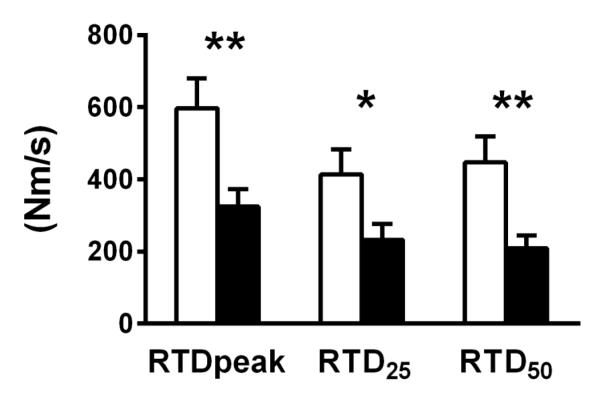
Parameters describing RTD are shown in Figure 1. Volunteers with knee OA had lower values for RTD (P<0.01), RTD25 (P<0.05) and RTD50 (P<0.01). There were no group by sex interaction effects for any of the RTD variables, although there were sex effects for RTD (P=0.05), with men having values than women. RTD was related to peak isometric torque, (r=0.743; P<0.001) and indices of whole muscle size (r=0.576 and 0.525; both P<0.01, from CT and DEXA, respectively), suggesting that the RTD measure may reflect the effects of knee OA on overall muscle size and/or force production. Thus, we chose to also express RTD relative to peak torque (RTDrel). This approach effectively accounted for variation in muscle size and strength, indicated by the fact that RTDrel was not related to either peak torque (r=0.120, P=0.54) or indices of whole muscle size (r=0.211, P=0.31 and r=0.191, P=0.32, for muscle CSA and mass, respectively). RTDrel did not differ between groups (P=0.753; not shown in figure).
Figure 1.
RTD variables in control (open bars) and knee OA (closed bars) groups. RTD, rate of isometric torque development; RTD25 and RTD50, rate of torque development per unit time from the onset of torque development to 25% and 50% of peak torque, respectively. Data are mean ± SE. *, P<0.05 and **, P<0.01 group effect.
3.3 Relationship between tissue MHC isoform expression and RTD parameters
No group differences, nor group X sex interaction effects were found in the relative expression of MHC I, IIA or IIX via gel electrophoresis, as recently reported (12). Neither RTD, nor RTDrel, were related to the fractional expression of MHC I, IIA or IIA/X in the pooled cohort (range of r-values: −0.002 to 0.160; range of P-values: 0.52 to 0.99), in OA (range of r-values: 0.021 to 0.254; range of P-values: 0.36 to 0.94) or in controls (range of r-values: 0.011 to - 0.269; range of P-values: 0.35 to 0.97).
3.4 Relationship between RTD and single muscle fiber CSA and function
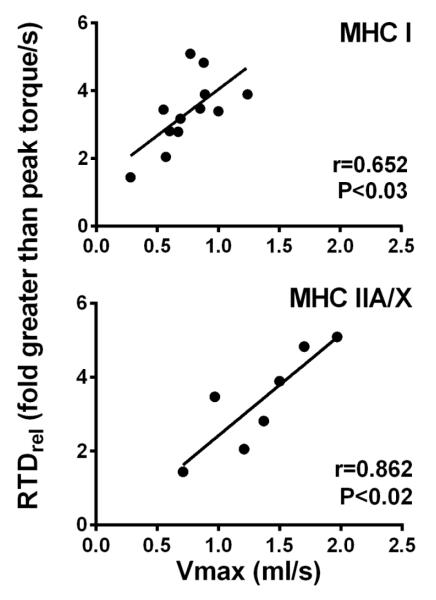
Single muscle fiber functional parameters have been recently described for this cohort (11). Briefly, group differences were apparent in tension in MHC IIA fibers, although no other variables differed between groups. Additionally, group by sex interactions were noted for MHC IIA power output, which was related to a trend towards a similar pattern of differences in shortening velocity. In the entire cohort, RTDrel was related to CSA of MHC IIA/X fibers (n=22; r=0.424; P<0.05). In knee OA, RTDrel was related to shortening velocity in MHC I (n= 12; r=0.652; P<0.03) and IIA/X (n=7; r=0.862; P<0.02; Figure 2) fibers and power output in MHC I fibers (n=11; r=0.642; P<0.04), but was not related to single muscle fiber CSA or tension. In controls, RTDrel was related to CSA of MHC IIA/X fibers (n=9; r=0.707; P<0.05), but was not related to single fiber functional parameters. RTD was related to MHC IIA CSA (r=0.519; P<0.01) and tension (r=0.592; P<0.01) and MHC I CSA (r=0.377; P<0.05) in the pooled cohort. Of note, our findings regarding RTDrel were consistent regardless of whether peak torque of the contraction producing greatest RTD, or that producing the greatest torque overall, were used to normalize RTD. In 11 of 29 cases, these occurred in the same contraction. Measures of relative RTD normalized to either peak torque value were themselves highly correlated (r=0.98, P<0.001). Furthermore, correlations with variables of interest were similar both qualitatively and in their statistical significance regardless of which torque value was used to calculate RTDrel.
Figure 2.
Relationship of RTDrel to shortening velocity (Vmax) in MHC I (n=12) and IIA/X (n=7) fibers in knee OA volunteers. Note that variation in sample size is related to the fact that not all volunteers had fibers of a specific fiber type analyzed due to simple random variation in the fibers that were selected for analysis (ie, fiber type is blinded until after analysis, when MHC isoform is determined) and because data from one male knee OA volunteer was removed because his average data were outliers for MHC IIA and IIA/X fibers (detailed in Methods).
In the knee OA group, RTD was related to shortening velocity in MHC I fibers (r=0.680; P<0.02) and showed a trend toward being related to MHC IIA/X shortening velocity (r=0.681; P=0.09). In controls, RTD was related to MHC IIA/X CSA (r=0.882; P<0.01).
4. DISCUSSION
Our investigation uncovered novel relationships between single fiber contractile performance and the rate of whole muscle force development in older adults with knee OA, suggesting that the mechanical properties of the constituent muscle fibers scale to tissue level function. Considering that RTD has been shown to predict whole body functionality in older volunteers with knee OA (61), these results suggest that their functional disability may relate, in part, to deficits in the fundamental contractile properties of muscle fibers.
An interesting aspect of our findings is the group-specific nature of the relationships between single fiber and whole muscle function. In older adults with knee OA, our results suggest the possible involvement of myofilament functional properties in explaining variability in RTD; whereas, in non-diseased controls, RTD was primarily correlated to MHC IIA/X fiber size, an association that held up when data were pooled. This relationship between the size of MHC IIA/X fibers, the most powerful fibers in older adults (11), and RTD in the pooled sample and controls is logical and generally in keeping with data from younger adults (27, 29, 31, 36). The absence of relationships of RTD to contractile parameters in controls (and the pooled sample) may relate to their greater variability in single MHC IIA/X fiber CSA compared to the OA group (coefficient of variation is >2-fold higher in controls vs. OA: 55 vs. 21%; (12)). This is because, mathematically, correlations are a function of the standard deviations of the dependent and independent variable. Accordingly, this may explain why single fiber function, rather than MHC II fiber size, was a better predictor of RTD in volunteers with knee OA. In this context, differences in relationships between groups may depend on the relative degree of variability between single muscle fiber size and functional measures within each group. Of course, we recognize that the RTD measure is also dependent on variability in other physiological regulators, such as tendon stiffness (9), motor unit discharge rate (35), the extent of voluntary neural activation (2, 54) and other factors. Thus, variation in the single fiber correlates of RTD may be explained by the fact that this measure is dependent on muscle fiber size and function differently in the two groups because of differences in these other determinants of RTD. Nonetheless, the fact that relationships were apparent between RTD and single fiber size and functional measures in our groups despite the influence of these other well-known regulators reinforces the notion that the amount and functionality of myofilament proteins determine whole muscle contractility in older adults.
It is notable that, in volunteers with knee OA, associations between single fiber functional properties and whole muscle RTD were nearly as strong in MHC I fibers as they were in MHC IIA/X fibers. This was somewhat unexpected considering that muscle fibers with faster, more powerful contractile properties (eg, MHC IIA, MHC IIA/X) are assumed to have greater influence over whole muscle contractile kinetics (31, 36), but may be explained by the nature of the RTD measure and adaptations unique to volunteers with knee OA . Regarding the former, as RTD typically occurs during the early phase of contraction, it may be disproportionately dependent on MHC I contractile properties because low-threshold motor neurons innervating slower-contracting muscle fibers are recruited prior to higher-threshold motor units (30). Regarding the latter point, in volunteers with knee OA, where there are well-known deficits in neural activation (8, 45), this may yield an even greater recruitment of MHC I fibers in this early phase of contraction and, in turn, RTD may be more dependent on MHC I fiber contractility compared to controls. Such a relative shift in the proportional activation of MHC I vs. II fibers in volunteers with knee OA might contribute to reduced RTD similar to the in vitro behavior of mixtures of slower and faster contracting myosins, where slow-contracting myosins have a disproportionately greater effect to slow velocity when mixed with faster isoforms (19). That is, increased relative recruitment of MHC I fibers during the early phase of contraction may provide a disproportionately large “kinetic brake” on rapid force development in volunteers with knee OA.
Building on this last point, and in light of group-specific relationships of RTD to single fiber properties, we put forth a hypothetical framework for the reduction in RTD in older adults. In healthy older adults, age-related decline in the relative proportion of MHC II fibers secondary to atrophy (36) may be the primary determinant of reduced RTD, albeit one cannot discount contributions from concomitant reductions in MHC I contractility (20, 39) and neural activation (35). In volunteers with knee OA, where there is the additional effect of impaired neural activation superimposed upon age-related changes (8, 45), promoting greater reliance on MHC I fiber contractile properties, rapid force development is further reduced. In this situation, impaired MHC I contractility may further impair RTD because of the predominant effect of slow MHC I contractile properties over MHC II fibers (8, 19). In this context, our results, together with findings from other laboratories (1, 16, 17, 35), provide a model that integrates both neural and intrinsic muscle impairments into the etiology of reduced rapid force development with aging and the resultant performance declines and disability. We recognize that this model assumes that motor unit recruitment order is maintained during rapid contractions, a conclusion that is supported by a substantial body of literature (3, 23, 24, 41, 53, 62). However, our interpretation of relationships between fiber type-specific determinants of RTD in OA, suggesting that MHC I contractile characteristics exert a greater than expected influence on whole muscle performance, may be less robust if recruitment order is less reliable with age (15) or deep tissue pain (59). To this point, while some evidence suggests altered fractional recruitment of different fiber types with muscle pain (59), clear demonstration of reversal in recruitment order are lacking, and others suggest that recruitment order is maintained with nociceptive stimulation (53).
Volunteers with OA had slower RTD compared to controls, calculated at multiple points along the torque generating phase of voluntary isometric knee extension, in agreement with prior reports (61). Given the proposed contribution of reduced contractile velocity and power to mobility impairments in older adults (4, 25, 56), it is appealing to extrapolate RTD results to decreased dynamic contractile function. Indeed, our laboratories and others have shown that impaired RTD is associated with decreased whole body functionality (40, 61). Interestingly, however, we found that group differences were eliminated when expressed relative to peak torque (RTDrel) to account for variation in whole muscle size/strength (21, 49). Moreover, isokinetic knee extensor power was not correlated with RTDrel (P=0.417) in the pooled cohort or in either group separately (P>0.4 for both), leaving us to conclude that the relationship of RTD to functional parameters may be explained by its co-linearity with peak torque generating capacity. Parenthetically, we should note that both absolute RTD and RTDrel were related to both MHC I and II size and contractility (see Results, 3.4), arguing that these single fiber attributes are relevant to either measure. Practically speaking, absolute RTD is relevant to real world function, as rapid generation of torque is necessary to adjust for variation in center of pressure within the base of support (32), which is critical to maintaining balance and preventing falls (7).
Finally, we were surprised that RTD did not differ by sex. Considering sex-dependent differences in single fiber atrophy and contractile function in MHC II fiber types that we have recently reported (11, 12), we anticipated lower RTD in women with OA (ie, group X sex interaction). This could relate to our modest sample size. Alternatively, the absence of sex differences may be explained by sex-specific variation in other determinants of RTD. Reports suggest that women with knee OA who are candidates for total knee arthroplasty have greater neural activation deficits than men with OA, while healthy older women have less activation deficits (46). Considering that more than half of our OA cohort were not actively being considered for total knee arthroplasty, it is possible that neural activation was greater in women and this compensated for reduced single fiber size and function (11, 12). Additionally, there may be sex-specific adaptations in other determinants (eg, tendon stiffness) or unique sex interactions between these determinants and medication regimens (13) that compensate for decreased single fiber properties in women with knee OA.
5. CONCLUSION
In summary, our results demonstrate the potential relevance of muscle fiber size and contractility to whole muscle performance in healthy older adults and those with advanced-stage knee OA. The fact that these relationships were evident despite neural (34, 45) and biomechanical (9) limitations that accompany knee OA further underscores their robustness and potential relevance to functional outcomes. Considering that one of the defining characteristics of the knee OA population is reduced physical activity secondary to joint pain, these data suggest the need for rehabilitation strategies to improve muscle function and, more specifically, modalities that are designed to enhance the inherent contractile velocity of skeletal muscle, such as power training, to mitigate the development of disability.
Highlights.
Knee osteoarthritis (OA) reduces muscle size and strength.
Absolute knee extensor rate of torque development is reduced with OA.
When normalized to peak torque, RTD is not different with OA.
Contractile characteristics of single muscle fibers predict normalized RTD in OA.
Muscle fiber size of fast-twitch fibers predicts normalized RTD in controls.
ACKNOWLEDGEMENTS
We thank all the volunteers who dedicated their valuable time to these studies.
FUNDING
This study was funded by grants from the NIH (AG-033547 to MJT) and an Institutional National Research Service Award (HL-007647 to DMC) and Glenn/American Federation for Aging Research Postdoctoral Fellowship for Translational Research on Aging (DMC).
Footnotes
COMPETING INTERESTS
No competing interest or conflicts of interest are present.
AUTHOR CONTRIBUTIONS
BDB, PAA and MJT were involved in the conception and design of the experiments. DMC, TWT, JRS and MJT were involved in the collection and analysis of data. DMC and MJT were involved in the interpretation of the data. All of the authors were involved in drafting or critically revising the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93:1318–1326. doi: 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- 2.Barry B, Warman G, Carson R. Age-related differences in rapid muscle activation after rate of force development training of the elbow flexors. Exp Brain Res. 2005;162:122–132. doi: 10.1007/s00221-004-2127-3. [DOI] [PubMed] [Google Scholar]
- 3.Bawa PN, Jones KE, Stein RB. Assessment of size ordered recruitment. Front Hum Neurosci. 2014;8:532. doi: 10.3389/fnhum.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol. 2003;58A:728–733. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measureing clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoartritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 6.Bemben MG, Clasey JL, Massey BH. The effect of the rate of muscle contraction on the force-time curve parameters of male and female subjects. Res Q Exerc Sport. 1990;61:96–99. doi: 10.1080/02701367.1990.10607484. [DOI] [PubMed] [Google Scholar]
- 7.Bento PCB, Pereira G, Ugrinowitsch C, Rodacki ALF. Peak torque and rate of torque development in elderly with and without fall history. Clin Biomech. 2010;25:450–454. doi: 10.1016/j.clinbiomech.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Berth A, Urbach D, Awiszus F. Improvement of voluntary quadriceps muscle activation after total knee arthroplasty. Arch Phys Med Rehabil. 2002;83:1432–1436. doi: 10.1053/apmr.2002.34829. [DOI] [PubMed] [Google Scholar]
- 9.Bojsen-Møller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J Appl Physiol. 2005;99:986–994. doi: 10.1152/japplphysiol.01305.2004. [DOI] [PubMed] [Google Scholar]
- 10.Callahan DM, Bedrin NG, Subramanian M, Berking J, Ades PA, Toth MJ, Miller MS. Age-related structural alterations in human skeletal muscle fibers and mitochondria are sex specific: relationship to single-fiber function. J Appl Physiol. 2014;116:1582–1592. doi: 10.1152/japplphysiol.01362.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callahan DM, Miller MS, Sweeny AP, Tourville TW, Slauterbeck JR, Savage PD, Maugan DW, Ades PA, Beynnon BD, Toth MJ. Muscle disuse alters skeletal muscle contractile function at the molecular and cellular levels in older adult humans in a sex-specific manner. J Physiol. 2014;592:4555–4573. doi: 10.1113/jphysiol.2014.279034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callahan DM, Tourville TW, Miller MS, Hackett SB, Sharma H, Cruickshank NC, Slauterbeck JR, Savage PD, Ades PA, Maughan DW, Beynnon BD, Toth MJ. Chronic disuse and skeletal muscle structure in older adults: sex-specific differences and relationships to contractile function. Am J Physiol Cell Physiol. 2015;308:C932–943. doi: 10.1152/ajpcell.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Weinheimer EM, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of acetaminophen and ibuprofen on in vivo patellar tendon adaptations to knee extensor resistance exercise in older adults. J Appl Physiol. 2011;111:508–515. doi: 10.1152/japplphysiol.01348.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Prevalence and most common causes of disability among adults--United States, 2005. 2009.
- 15.Chan KM, Doherty TJ, Brown WF. Contractile properties of human motor units in health, aging, and disease. Muscle Nerve. 2001;24:1113–1133. doi: 10.1002/mus.1123. [DOI] [PubMed] [Google Scholar]
- 16.Clark DJ, Manini TM, Fielding RA, Patten C. Neuromuscular determinants of maximum walking speed in well-functioning older adults. Exp Gertonol. 2013;48:358–363. doi: 10.1016/j.exger.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Muscle performance and physical function are associated with voluntary rate of neuromuscular activation in older adults. J Gerontol A Biol Sci Med Sci. 2011;66A:115–121. doi: 10.1093/gerona/glq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among medicare beneficiaries, 1991-2010. JAMA. 2012;308:1227–1236. doi: 10.1001/2012.jama.11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuda G, Pate E, Cooke R, Sellers JR. In vitro actin filament sliding velocities produced by mixtures of different types of myosin. Biophys J. 1997;72:1767–1779. doi: 10.1016/S0006-3495(97)78823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552:499–511. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Ruiter CJ, Jones DA, Sargeant AJ, de Haan A. Temperature effect on the rates of isometric force development and relaxation in the fresh and fatigued human adductor pollicis muscle. Exp Physiol. 1999;84:1137–1150. doi: 10.1017/s0958067099018953. [DOI] [PubMed] [Google Scholar]
- 22.de Ruiter CJ, Kooistra RD, Paalman MI, de Haan A. Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. J Appl Physiol. 2004;97:1693–1701. doi: 10.1152/japplphysiol.00230.2004. [DOI] [PubMed] [Google Scholar]
- 23.Desmedt JE, Godaux E. Ballistic contractions in fast or slow human muscles: discharge patterns of single motor units. J Physiol. 1978;285:185–196. doi: 10.1113/jphysiol.1978.sp012566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desmedt JE, Godaux E. Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiol. 1977;264:673–693. doi: 10.1113/jphysiol.1977.sp011689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, Pu CT, Hausdorff JM, Fielding RA, Fiatarone-Singh MA. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol. 2000;55:M192–M199. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 26.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, Kelly-Hayes M, Wolf PA, Kreger BE, Kannel WB. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Pub Health. 1994;84:351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gür H, Gransberg L, vanDyke D, Knutsson E, Larsson L. Relationship between in vivo muscle force at different speeds of isokinetic movements and myosin isoform expression in men and women. Eur J Appl Physiol Occup Physiol. 2003;88:487–496. doi: 10.1007/s00421-002-0760-8. [DOI] [PubMed] [Google Scholar]
- 28.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harridge SDR, Bottinelli R, Canepari M, Pellegrino MA, Reggiani C, Esbjornsson M, Saltin B. Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch-Eur J Physiol. 1996;432:913–920. doi: 10.1007/s004240050215. [DOI] [PubMed] [Google Scholar]
- 30.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motorneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- 31.Ivy JL, Withers RT, Brose G, Maxwell BD, Costill DL. Isokinetic contractile properties of the quadriceps with relation to fiber type. Eur J Appl Physiol Occup Physiol. 1981;47:247–255. doi: 10.1007/BF00422470. [DOI] [PubMed] [Google Scholar]
- 32.Izquierdo M, Aquodao X, Gonzalez R, Lopez JL, Hakkinen H. Maximal and explosive force production capacity and balance performance in men of different ages. Eur J Appl Physiol Occup Physiol. 1999;79:260–267. doi: 10.1007/s004210050504. [DOI] [PubMed] [Google Scholar]
- 33.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kittelson AJ, Stackhouse SK, Stevens-Lapsley JE. Neuromuscular electrical stimulation after total joint arthroplasty: a critical review of recent controlled studies. Eur J Phys Rehabil Med. 2013;49:909–920. [PubMed] [Google Scholar]
- 35.Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol. 2008;104:739–746. doi: 10.1152/japplphysiol.00550.2007. [DOI] [PubMed] [Google Scholar]
- 36.Korhonen MT, Cristea A, Alén M, Häkkinen K, Sipilä S, Mero A, Viitasalo JT, Larsson L, Suominen H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol. 2006;101:906–917. doi: 10.1152/japplphysiol.00299.2006. [DOI] [PubMed] [Google Scholar]
- 37.Krivickas LS, Fielding RA, Murray A, Callahan D, Johansson A, Dorer DJ, Frontera WR. Sex differences in single muscle fiber power in older adults. Med Sci Sports Exerc. 2006;38:57–63. doi: 10.1249/01.mss.0000180357.58329.b1. [DOI] [PubMed] [Google Scholar]
- 38.Krivickas LS, Suh D, Wilkins J, Hughes VA, Roubenoff R, Frontera WR. Age- and gender-related differences in maximum shortening velocity of skeletal muscle fibers. Am J Phys Med Rehabil. 2001;80:447–455. doi: 10.1097/00002060-200106000-00012. quiz 456-447. [DOI] [PubMed] [Google Scholar]
- 39.Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol. 1997;272:C638–C649. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- 40.Maffiuletti NA, Bizzini M, Widler K, Munzinger U. Asymmetry in quadriceps rate of force development as a functional outcome measure in TKA. Clin Orthoop Relat Res. 2010;468:191–198. doi: 10.1007/s11999-009-0978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masakado Y, Akaboshi K, Nagata M, Kimura A, Chino N. Motor unit firing behavior in slow and fast contractions of the first dorsal interosseous muscle of healthy men. Electroencephalogr Clin Neurophysiol. 1995;97:290–295. doi: 10.1016/0924-980x(95)00188-q. [DOI] [PubMed] [Google Scholar]
- 42.Miller MS, VanBuren P, LeWinter MM, Lecker SH, Selby DE, Palmer BM, Maughan DW, Ades PA, Toth MJ. Mechanisms underlying skeletal muscle weakness in human heart failure: alterations in single fiber myosin protein content and function. Circ: Heart Fail. 2009;2:700–706. doi: 10.1161/CIRCHEARTFAILURE.109.876433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura T, Kurosawa H, Kawahara H, Watarai K, Miyashita H. Muscle fiber atrophy in the quadriceps in knee-joint disorders. Histochemical studies on 112 cases. Acta Orthop Trauma Surg. 1986;105:163–169. doi: 10.1007/BF00433935. [DOI] [PubMed] [Google Scholar]
- 44.Nyland J, Frost K, Quesada P, Angeli C, Swank A, Topp R, Malkani AL. Self-reported chair rise ability relates to stair climbing readiness of total knee arthroplasty patints: a pilot study. J Rehabil Res Dev. 2007;44:751–759. doi: 10.1682/jrrd.2006.11.0146. [DOI] [PubMed] [Google Scholar]
- 45.Petterson SC, Barrance P, Buchanan T, Binder-Macleod S, Snyder-Mackler L. Mechanisms underlying quadriceps weakness in knee osteoarthritis. Med Sci Sports Exerc. 2008;40:422–427. doi: 10.1249/MSS.0b013e31815ef285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petterson SC, Raisis L, Bodenstab A, Snyder-Mackler L. Disease-specific gender differences among total knee arthroplasty candidates. J Bone Joint Surg Am. 2007;89:2327–2333. doi: 10.2106/JBJS.F.01144. [DOI] [PubMed] [Google Scholar]
- 47.Pijnappels M, Bobbert MF, Dieën JHv. Push-off reactions in recovery after tripping discriminate young subjects, older non-fallers and older fallers. Gait Posture. 2005;21:388–394. doi: 10.1016/j.gaitpost.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Pojednic RM, Clark DJ, Patten C, Reid K, Phillips EM, Fielding RA. The specific contributions of force and velocity to muscle power in older adults. Exp Gertonol. 2012;47:608–613. doi: 10.1016/j.exger.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ranatunga KW. Temperature-dependence of shortening velocity and rate of isometric tension development in rat skeletal muscle. J Physiol. 1982;239:465–483. doi: 10.1113/jphysiol.1982.sp014314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reardon K, Galea M, Dennett X, Choong P, Byrne E. Quadriceps muscle wasting persists 5 months after total hip arthroplasty for osteoarthritis of the hip: a pilot study. Intern Med J. 2001;31:7–14. doi: 10.1046/j.1445-5994.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 51.Reeves ND, Narici MV, Maganaris CN. Strength training alters the viscoelastic properties of tendons in elderly humans. Muscle Nerve. 2003;28:74–81. doi: 10.1002/mus.10392. [DOI] [PubMed] [Google Scholar]
- 52.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40:4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sohn MK, Graven-Nielsen T, Arendt-Nielsen L, Svensson P. Inhibition of motor unit firing during experimental muscle pain in humans. Muscle Nerve. 2000;23:1219–1226. doi: 10.1002/1097-4598(200008)23:8<1219::aid-mus10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 54.Suetta C, Aagaard P, Magnusson SP, Andersen LL, Sipilä S, Rosted A, Jakobsen AK, Duus B, Kjaer M. Muscle size, neuromuscular activation, and rapid force characteristics in elderly men and women: effects of unilateral long-term disuse due to hiposteoarthritis. J Appl Physiol. 2007;102:942–948. doi: 10.1152/japplphysiol.00067.2006. [DOI] [PubMed] [Google Scholar]
- 55.Suetta C, Aagaard P, Rosted A, Jakobsen AK, Duus B, Kjaer M, Magnusson SP. Training-induced changes in muscle CSA, muscle strength, EMG, and rate of force development in elderly subjects after long-term unilateral disuse. J Appl Physiol. 2004;97:1954–1961. doi: 10.1152/japplphysiol.01307.2003. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki T, Bean JF, Fielding RA. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J Am Geriatr Soc. 2001;49:1161–1167. doi: 10.1046/j.1532-5415.2001.49232.x. [DOI] [PubMed] [Google Scholar]
- 57.Toth MJ, Miller MS, VanBuren P, Bedrin NG, LeWinter MM, Ades PA, Palmer BM. Resistance training alters skeletal muscle structure and function in human heart failure: effects at the tissue, cellular and molecular levels. J Physiol. 2012;590:1243–1259. doi: 10.1113/jphysiol.2011.219659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toth MJ, Shaw AO, Miller MS, VanBuren P, LeWinter MM, Maughan D, Ades PA. Reduced knee extensor function in heart failure is not explained by inactivity. Int J Cardiol. 2010;143:276–282. doi: 10.1016/j.ijcard.2009.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tucker K, Butler J, Graven-Nielsen T, Riek S, Hodges P. Motor unit recruitment strategies are altered during deep-tissue pain. J Neurosci. 2009;29:10820–10826. doi: 10.1523/JNEUROSCI.5211-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Cutsem M, Duchateau J, Hainaut K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol. 1998;513:295–305. doi: 10.1111/j.1469-7793.1998.295by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winters JD, Christiansen CL, Stevens-Lapsley JE. Preliminary investigation of rate of torque development deficits following total knee arthroplasty. Knee. 2014;21:382–386. doi: 10.1016/j.knee.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoneda T, Oishi K, Fujikura S, Ishida A. Recruitment threshold force and its changing type of motor units during voluntary contraction at various speeds in man. Brain Res. 1986;372:89–94. doi: 10.1016/0006-8993(86)91461-7. [DOI] [PubMed] [Google Scholar]