Abstract
β-arrestin2 (β-arr2) identified as a scaffolding protein in GPCR desensitization, is a negative regulator of inflammation in polymicrobial sepsis. In this study we wanted to investigate the role of β-arr2 in intestinal inflammation, a site of persistent microbial stimulation. In the absence of β-arr2, mice exhibited greater extent of mucosal inflammation determined by cellular infiltration and expression of inflammatory mediators even under homeostatic conditions. Further, β-arr2 deficient mice were more susceptible to DSS induced colitis as demonstrated by greater body weight loss, higher disease activity index and shortened colon as compared to wild type (WT) mice. We also show that T cells from β-arr2 KO mice exhibit altered activation status under both basal and colitic conditions, implicating their involvement in disease induction. Further assessment of the role of β-arr2 in intrinsic T cell differentiation confirmed its importance in T cell polarization. Utilizing the T cell transfer model of colitis we demonstrate that T-cell specific-β-arr2 is important in limiting colitic inflammation; however it plays a paradoxical role in concurrent systemic wasting disease. Together, our study highlights a critical negative regulatory role of β-arr2 in intestinal inflammation and demonstrates a distinct role of T-cell-specific β-arr2 in systemic wasting disease.
Keywords: β-arrestin2, colitis, regulatory T cells, arrestin-2, arrestin-3
INTRODUCTION
IBD is a multifactorial disease perpetuated by a dysregulated immune response. Being the site of constant interaction between the immune system and foreign antigens, maintaining a healthy balance between inflammatory and regulatory responses is particularly essential for gut homeostasis. An imbalance by virtue of aberrant T cell activation or macrophage response contributes to pathogenesis of IBD (1). Genetic susceptibility and environmental triggers can disrupt this balance and lead to inflammation against microbial or “self” antigens (2). Current therapies against IBD include anti-inflammatory and immunosuppressive agents, but given the diverse etiology of the disease, a significant proportion of patients are unresponsive to these therapies. Further understanding of progression and factors affecting pathogenesis of IBD are therefore required to identify inflammatory nodes or pathways that could be targeted for novel therapies.
β-Arrestins (1 and 2) identified as scaffolding proteins required for GPCR desensitization have lately been found to be important in receptor endocytosis as well as for GPCR-dependent and independent signaling. The family of GPCRs includes various receptors including the ones involved in immune responses such as chemotaxis, proliferation and differentiation of leukocytes. β-arrestins are important players in inflammation and consequent pathogenesis of sepsis (3, 4), allergic asthma (5), EAE (6) and rheumatoid arthritis (7). Their involvement in innate responses to TLRs (8), adenovirus (9) and microbial stimulation (4) has also been demonstrated. Further in T cells, CD28 mediated PDE4 recruitment (10) and T cell differentiation into Tregs was found to be dependent on β-arrestin2 expression (6). Involvement of G proteins in T cell activation (11) and colitis (12) has also been previously demonstrated. Additionally, recent studies from our lab demonstrated that β-Arrestin1 deficiency is protective in DSS and TNBS induced colitis (13). These studies demonstrate critical role of arrestins in mediating immune responses during disease pathogenesis. The role of β-arrestin2 in intestinal inflammation though is not well known. Therefore, in this study we sought to determine the role of β-arrestin2 in intestinal mucosal inflammation.
MATERIALS AND METHODS
Animals
β-arrestin2 knockout mice on C57BL/6 background (kindly provided by Dr. Robert Lefkowitz, Duke University) have been described earlier (14). Wild type C57BL/6 mice were purchased from NCI and all mice were bred and housed at Michigan State University in rooms maintained at 22–24°C with 50% humidity and a 12-hr light-dark cycle. Mouse chow and water were provided ad libitum to all animals. All experiments were performed with age- and sex-matched mice between 8–12 weeks of age. Animal procedures were approved by Michigan state University institutional Animal Care and Use Committee (IACUC) and conformed to NIH guidelines. For co-housing experiments, wild type and knockout animals were co-housed at weaning stage (4 weeks) and used 8 weeks later.
DSS induced colitis model
Mice were provided 3.5% or 5% DSS (w/v) in drinking water for 6 days and water for an additional day and sacrificed. Over this period, mice were weighed everyday and observed for disease activity index indicated through stool consistency (1-loose); blood in stool (1-mild, 2-gross) ruffled hair coat (0 or 1); crusty eyes (0 or 1) and hunched posture (0 or 1). At the time of harvesting, splenic weight, colon length and thymic weight were also measured.
RAG T cell transfer model of colitis
RAG2 knockout mice mice, obtained from NCI were subjected to T cell transfer model of colitis as previously described (15). Briefly, RAG2−/− mice were injected with 0.5 million cells (CD4+CD45RBhi T cells), intraperitoneally. As follow up of colitis development, they were weighed once a week for first 3 weeks and thrice a week after that. Signs of disease development were observed and recorded in form of ruffled hair coat and hunched posture. At the time of harvesting colon length and weight and splenic weight were also measured.
Sample Processing
At pre-determined time of harvesting, mice were euthanized using CO2 asphyxiation. Plasma, spleen and MLN were collected and processed as previously described (4). Briefly, spleen and MLN was crushed, subjected to RBC lysis, filtered through 40 μm nylon mesh and counted for stimulation. Colon length was noted and 5 mm segments from distal end flash frozen for mRNA isolation; rest or part of the colon was processed as previously described (16). Briefly, colon was cut into 5 mm segments and incubated in epithelial dissociation buffer at 25°C with gentle shaking for 30 minutes. The segments were further cut into 1 mm segments and incubated for an hour in 0.5 mg/ml collagenase D. It was then strained through 100 μm filter and loaded onto 80:40 percoll gradients. Cells were collected form the interface and used as leukocyte fraction following a wash in PBS.
T cell sorting
Spleen was processed as described above and subjected to CD4+ T cell enrichment by negative selection using miltenyi beads as per manufacturer’s instructions. The enriched population was stained with CD4, CD25, CD44 and CD62L in RPMI media and washed with the same. Cells were sorted using Influx as naïve (CD4+CD25−CD44−CD62Lhi) and activated (CD4+CD25+CD44+CD62L−) under sterile conditions and used as outlined below. For RAG T cell transfers, the CD4+ T cell enriched population was stained with CD4 and CD45RB antibodies and CD4+CD45RBhi cells were sorted under sterile conditions for transfer.
TCR Stimulation
Single cell suspensions from spleen or MLN were counted and stimulated with plate bound CD3 (5 μg/ml) and CD28 (4 μg/ml) for 48 hours and supernatant collected for cytokine analysis. Naïve and activated T cells isolated from spleen as described above were stimulated with plate bound CD3 (5 μg/ml) and soluble CD28 in the presence of differentiating factors: IL-12 (10 ng/ml) for Th1; IL-4 (40 ng/ml) and anti-IFNγ (5 μg/ml) for Th2; IL-6 (10 ng/ml), TGFβ (1 ng/ml), anti-IL2 (10 μg/ml) and anti IFNγ (5 μg/ml) for Th17 and TGFβ (1 ng/ml) and anti IFNγ (5 μg/ml) for Treg. The stimulation condition without these factors was termed Th 0. Following 5 days of stimulation, the supernatant was collected for ELISA analysis while cells were further stimulated with phorbol ester and ionomycin in the presence of golgi stop brefeldinA for four hours and processed for flow cytometry.
Flow cytometry
Processed cells were surface stained with antibody cocktail made in 2.4G2 supernatant (fcγR blocking antibody) to block non-specific binding and washed with staining buffer (PBS with sodium azide and BCS). When intracellular staining was required, cells were fixed using fixation buffer (ebioscience) and permeabilised and washed with perm buffer (PBS with sodium azide and saponin). The antibodies against cell surface markers CD11b, F4/80, Gr-1, CD3, CD19, CD4 and CD8; intracellular cytokines, IFNγ, IL-17A and IL-4; and transcription factors RORγT and Foxp3 were obtained from eBiosciences and used as per manufacturer’s instructions. Cells were run on LSR II and data analyzed using Flowjo software. Total cell count was extrapolated from running a fixed fraction of the sample.
Cytokine/chemokine measurements
Cytokines were measured from plasma, splenic culture supernatant and peritoneal fluid using ELISA kits from eBiosciences Inc. as per manufacturer’s protocol.
Quantitative RT-PCR
To determine the relative levels of a specific RNA transcript, RNA was isolated from snap frozen tissue using Qiagen RNeasy mini kit using manufacturers’ protocol. Reverse transcription was carried out with 1 μg of RNA using promega cDNA synthesis kit. Q-RT-PCR was performed with ABI fast 7500 (Applied biosystems) and all genes were normalized to HPRT as previously described (4). Primer sequences are provided in supplementary Table 2.
Histopathology
Colon was harvested, swiss rolled and fixed overnight in 10% formalin followed by 70% ethanol. It was then embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E) and was read in a blinded fashion by board certified pathologist (Dr. P.C. Lucas).
Statistical Analysis
All experimental data in the figures is expressed as mean±SEM and analyzed using GraphPad Prism Software. Each “N” represents individual mouse. Student’s t-test (for comparing groups with equal variances) or Mann-Whitney (for comparing groups with unequal variances) was used to compare two experimental groups. P-values <0.05 were considered statistically significant.
Results
β-arrestin2 inhibits mucosal inflammation under homeostatic conditions
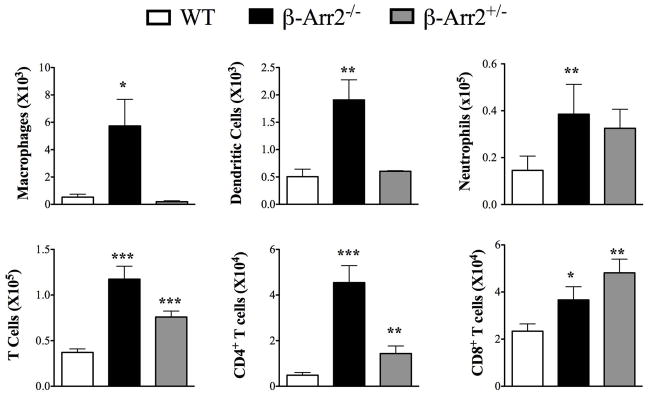
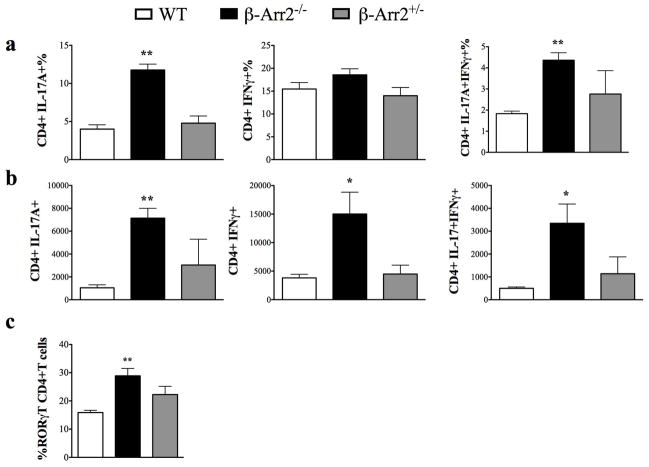
Given the negative regulatory role of β-Arrestin2 in polymicrobial sepsis, we hypothesized that β-arr2 could be a critical regulator of intestinal immune response. To ascertain its role under basal conditions, we determined cellular infiltration in colonic lamina propria (cLP) and expression of various inflammatory mediators in colon tissue of wild type (WT), β-arrestin2 knockout (β-arr2−/−) and β-arrestin2 heterozygous (β-arr2+/−) mice. We included mice heterozygous for β-arrestin2 in our initial studies to assess the effect of gene dosage of β-arr2 in intestinal inflammation. β-arr2−/− had higher number of cells of both innate (macrophages, dendritic cells and neutrophils) and adaptive (CD4+ and CD8+ T cells) leukocytes compared to the wild type mice (Fig 1). β-arr2+/− mice also displayed increased numbers of CD4+ and CD8+ T cells as compared to the WT, while the other cell types were similar (Fig 1). Production of inflammatory mediators including a vast array of innate cytokines, chemokines and T cell effectors were significantly higher in β-arr2−/− colon tissue even in the absence of any exogenous stimuli (Table 1). Particularly pronounced was the increase in type I T cell cytokine IFNγ, transcription factor T-bet, TNFα, GmCSF, IL-1β, IP-10, CXCL9, and CXCL11. Similar increase was observed in type 17 responses with higher expression of IL-17A and transcription factor RORγT. IL-22 expression, on the other hand was lower in β-arr2−/− colon tissue suggesting that β-arr2−/− colon might exhibit decreased anti-microbial defenses (17). Colon tissue from β-arr2+/− mice did not exhibit increases in inflammatory mediator expression; certain genes were in fact lower as compared to the WT. Together, these results suggest that β-arr2 is important in maintaining homeostatic immune cell composition and inflammatory gene expression in the colon and that gene dosage of β-arr2 differentially regulates these two responses.
Figure 1. β-arrestin2 negatively regulates mucosal inflammation under homeostatic conditions.
Total number of cells obtained from colonic lamina propria (cLP) of Wild type (WT), β-arrestin2 knockout (β-arr2−/−) and β-arrestin2 heterozygous (β-arr2+/−) mice under basal conditions. N=5–13 mice for each genotype. * p<0.05, **p<0.01, ***p<0.001 as compared to WT using student’s t-test.
Table 1. Inflammatory mediator expressions in basal colon.
RNA was extracted from distal colon segments of WT, β-arr2−/− and β-arr2+/− mice, subjected to cDNA synthesis and QPCR to quantify the expression of mentioned genes using primers noted in supplementary table 2. Data is presented as fold change over WT. Genes that are statistically significant in β-arr2−/− and β-arr2+/− colon compared to the WT are marked in bold.
| WT | β-Arr2−/− | β-Arr2+/− | |
|---|---|---|---|
| Innate cytokines | |||
| IL-6 | 0.93±0.18 | 22.49±14.95 | 0.54±0.23 |
| TNFα | 1±0.20 | 4.61±1.59 | 0.96±0.14 |
| IL-1β | 0.98±0.22 | 11.84±6.66 | 0.63±0.21 |
| GmCSF | 0.98±0.15 | 7.30±2.40 | 0.77±0.27 |
| IL-18 | 1.13±0.27 | 0.91±1.3 | |
| TGFβ | 1±0.19 | 1.38±0.13 | |
| IL-12p40 | 1±0.28 | 2.36±1.06 | 0.39±0.17 |
| IL-15 | 1±0.10 | 1.9±0.44 | 2.39±0.72 |
| Chemokines | |||
| IP-10 | 1±0.44 | 3.36±0.86 | 0.74±0.26 |
| CXCL9 | 1.02±0.26 | 26.76±12.98 | 1.79±0.87 |
| CXCL11 | 1±0.15 | 5.65±1.26 | 1.9±0.39 |
| CCL20 | 1±0.22 | 0.84±0.19 | |
| CX3CL1 | 1±0.23 | 1.11±0.28 | 0.95±0.30 |
| KC | 1±0.40 | 0.73±0.18 | 0.25±0.06 |
| MIP2 | 1±0.42 | 2.28±0.71 | 0.30±.12 |
| T-Cell cytokines/Differentiation markers | |||
| IFNγ | 0.96±0.23 | 10.85±4.23 | 2.24±0.85 |
| IL-17A | 1.07±0.15 | 6.61±3.39 | 0.32±0.19 |
| IL-22 | 1.06±0.40 | 0.61±0.2 | 0.51±0.47 |
| Tbet | 1±0.15 | 3.0±0.60 | 0.74±0.22 |
| RORγT | 1±0.16 | 1.53±0.11 | 0.33±0.144 |
| GATA3 | 1±0.10 | 2.54±0.82 | 3.14±2.37 |
| Foxp3 | 1±0.26 | 1.40±0.53 | 0.50±0.14 |
Loss of β-arrestin2 alters T cell activation status under homeostatic conditions
As the β-arrestin2 deficient mice exhibited enhanced numbers of T cells in the colonic lamina propria, we further examined if the proportion of the effector, memory and naïve T-cells is differentially affected. T cells can be identified as effector, memory and naïve based on surface expression of activation marker CD44 and homing integrin CD62L (18). Colonic lamina propria (cLP) from β-arr2−/− mice had significantly higher proportion of effector T cells (CD4+ and CD8+) compared to the wild type mice (Fig S1). This was specific for the colon since associated lymphoid organs, spleen and MLN from the same mice had equivalent or lower memory T cell phenotype but higher proportion of naïve CD4+ T cells (Fig S1). These results demonstrate that β-arr2 deficiency leads to not only higher numbers but also greater proportion of activated T cells in the colonic lamina propria.
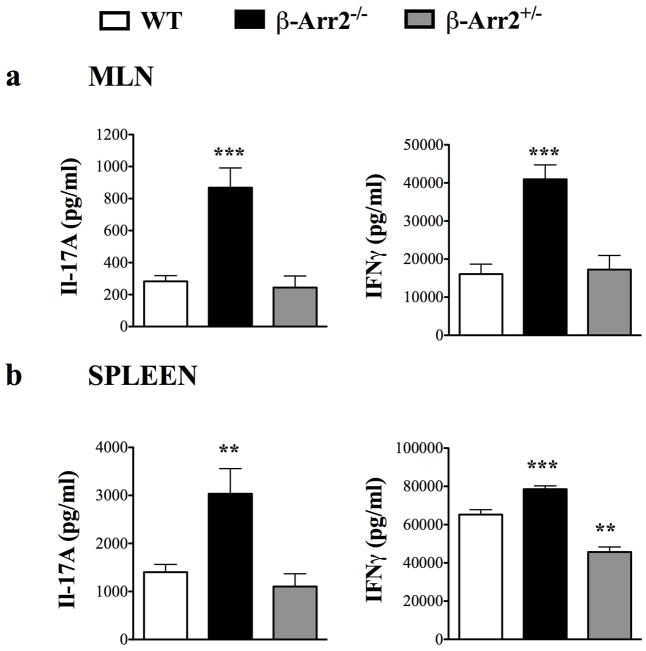
To further determine if T cells from spleen and MLN are functionally distinct between the WT and β-arr2−/− mice, we measured IFNγ and IL-17A in response to a polyclonal stimulation. Cells from β-arr2−/− mice produced significantly greater extent of IFNγ (Th1 cytokine) and IL-17A (Th17 cytokine) compared to the WT mice (Fig 2). In contrast to these data, cells from β-arr2+/− mice produced either equivalent or as in case of spleen lower IFNγ following T cell stimulation (Fig 2). Together, these results suggest that higher infiltration of T cells in the colon of β-arr2−/− mice is accompanied by higher functional responses to T cell stimulation from cells in the spleen and MLN. Importantly, these findings are from mice under naïve conditions with no exogenous stimuli.
Figure 2. Loss of β-arrestin2 affects T cell activation potential in MLN and spleen.
IL-17A and IFNγ production in response to CD3/28 stimulation of (a) MLN and (b) spleen cells, in WT, β-arr2−/− and β-arr2+/− mice. N=8–16 for WT and β-arr2−/− mice and N=4 for β-arr2+/− mice. **p<0.01, ***p<0.001 as compared to WT using student’s t-test.
β-arrestin2 protects mice from DSS induced colitis
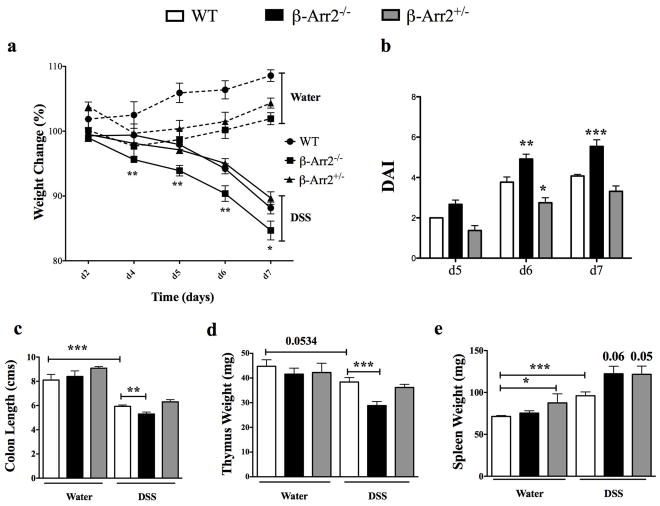
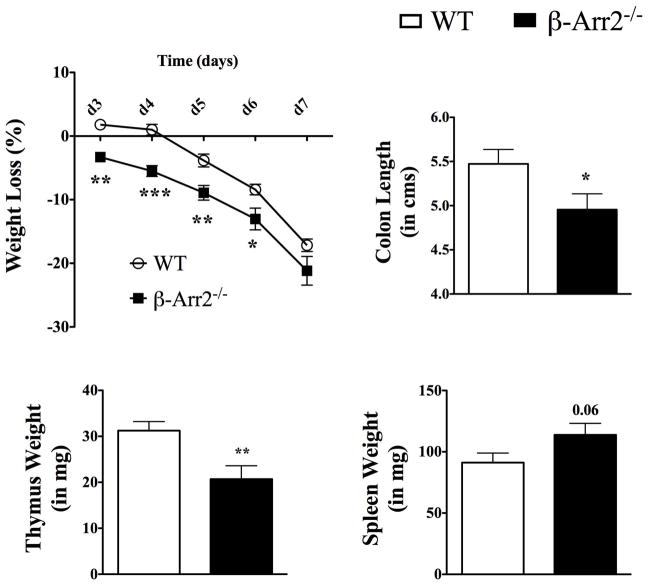
Inspite of increased immune cell infiltration, the colon tissue from β-arr2−/− mice did not display any gross or histopathological lesions or abnormalities under basal conditions (data not shown). We therefore posited that in response to a colonic insult, the knockout mice would be disposed to greater extent of injury. Mice were thus subjected to colitis through ingestion of 3.5% DSS in drinking water. Following DSS treatment, mice lost body weight starting day 5; exhibited signs of disease measured as disease activity score (DAI; indicated by loose and bloody stools, hunched posture, crusty eyes and ruffled hair coat) and had markedly shortened colons at the time of euthanasia and tissue collection (Fig 3). All these indices of colitis and associated wasting disease were significantly higher in mice lacking β-arrestin2 that lost significantly more weight as early as day 4, had higher DAI at days 6 and 7, and greater reduction in colon length in response to DSS induced colitis (Figure 3a–c). β-arr2+/− mice had colitis induction similar to WT as measured by the above-described parameters (Figure 3a–c), suggesting that one allele is able to compensate for and inhibit disease progression. Additionally, there was an inflammation-induced loss of thymic weight that was also significantly higher in the β-arr2−/− colitic mice (Figure 3d). On the other hand, splenic weight increased in response to colitis and this trended to be higher in the colitic β-arr2−/− animals (Figure 3e). β-arrestin2 therefore, is protective in DSS-induced colitis in mice, perhaps by virtue of inhibiting mucosal inflammation even under homeostatic conditions.
Figure 3. β-arrestin2 is protective in DSS induced colitis.
Wild type (WT), β-arrestin2 knockout (β-arr2−/−) and β-arrestin2 heterozygous (β-arr2+/−) mice were fed 3.5% DSS in their drinking water for 6 days to induce colitis and euthanized on day-7. (a) Percentage body weight loss and (b) disease activity index observed over the course of the experiment. (c) Colon length, (d) thymus and (e) spleen weight recorded for control and colitic mice at day 7. N=14–28 mice per genotype. Data pooled from atleast three independent experiments. * p<0.05, **p<0.01, ***p<0.001 as compared to WT using 2-way ANOVA for weight loss and DAI and student’s t-test for colon length, spleen and thymus weight.
β-arrestin2 inhibits DSS induced T cell activation
To determine the cause of higher colitic response in β-arr2−/− mice, we examined the expression of various inflammatory mediators and cellular infiltration in colon tissue and colonic lamina propria respectively. In contrast to exacerbated clinical signs of colitis in β-arr2−/− mice, levels of TNFα, IL-17A and IL-10 were in fact lower in β-arr2−/− colitic tissue at day 7 following DSS treatment (Fig S2). Expression of inflammatory mediators as determined by QPCR analysis revealed increased expression of IL-1β and Th2 transcription factor GATA3 in the β-arr2−/− colon, while RORγT expression was significantly lower as compared to the WT mice (Suppl. Table 1) suggesting that by the end of colitis, β-arr2−/− colon tissue was no longer hyper-inflammatory (as opposed to under basal conditions).
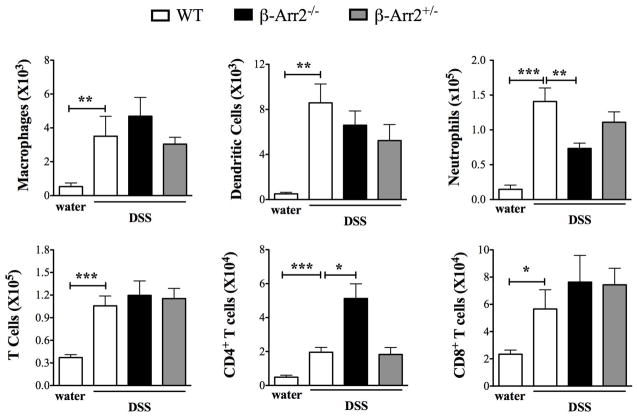
DSS induced colitis led to increased infiltration of various innate and adaptive immune cells in the cLP when compared to water fed mice (Fig 4). Comparison of cellular infiltration in colitic WT and β-arr2−/− mice showed increased numbers of CD4+ T cells but lower neutrophil numbers in β-arr2−/− colons as compared to the WT (Fig 4).
Figure 4. Cellular infiltration in colonic lamina propria of colitic mice.
Total number (and proportion) of inflammatory cells isolated from colonic lamina propria of colitic mice (WT, β-arr2−/− and β-arr2+/−) shown along with cell numbers in control WT mice (from figure 1). N=6–14 mice for each genotype. Data pooled from atleast 2 independent experiments. *p<0.05, **p<0.01, p<0.001 as compared to indicated group using student’s t test.
To directly determine the functional potential of T cells from colitic β-arr2−/− mice lamina propria cells isolated from colitic mice were subjected to T cell stimulation and analyzed for their activation pattern. Stimulation of cells from β-arr2−/− colitic tissue had significantly enhanced induction of Th1 and Th17 responses compared to WT colitic tissue demonstrated by higher numbers of IL-17A+, IFNγ+ and double positive (DP, IL-17A+IFNγ+) CD4+ T cells (Figure 5b). Additionally, T cells from β-arr2−/− colons also exhibited enhanced proportion (%) of IL-17A+ (Fig 5a) and RORγT+ CD4+ T cells (Fig 5c). This enhanced T cell differentiation and function was not observed in β-arr2+/− colitic mice, perhaps explaining why inspite of starting with higher T cell numbers in basal state, their colitic progression was comparable to the WT mice. Together, these data suggest that β-arrestin2 knockout mice exhibit enhanced T cell activation that is likely responsible for worsening of colitis, while the overall inflammatory mediator production and infiltration is similar or even lower with the notable exception of CD4+ T cells.
Figure 5. T cell differentiation in colitic mice.
Colonic lamina propria (cLP) cells from WT, β-arr2−/− and β-arr2+/− mice treated with DSS were stimulated ex vivo with PMA and ionomycine to determine a) proportion and b) total number of CD4+ T cells producing IL-17A, IFNγ or both cytokines. c) Cells were stained to determine proportion of RORγT+ CD4+ T cells in colons of colitic mice. N=4–7 mice for each genotype. *p<0.05, **p<0.01, p<0.001 as compared to WT using student’s t test.
β-arrestin2 protects against colitis independent of potential differences in microbiota
To demonstrate that the role of β-arrestin2 in mediating DSS induced colitis was independent of differences in microbiota, WT and β-arr2−/− mice were co-housed for 8 weeks before colitis induction. Similar to earlier results, KO mice had significantly higher weight loss and greater shortening of colon with DSS treatment (Figure 6). Further, the β-arr2−/− mice also showed significantly lower thymic and modestly increased splenic weights compared to the WT mice (Figure 6). Overall, mice lacking β-arrestin2 suffered from exacerbated colitis and associated wasting disease even when co-housed with wild type mice. Therefore, the aggravated colitic phenotype observed in the β-arr2−/− mice is likely independent of microbial diversity.
Figure 6. β-arrestin2 protects mice against DSS-induced colitis under co-housed conditions.
Wild type (WT) and β-arrestin2 knockout (β-arr2−/−) were co-housed at the time of weaning (4 weeks) for eight weeks and subjected to colitis. Colitic mice were observed for percentage body weight loss through course of the experiment and colon length, spleen and thymus weight at the end (day-7). N=9–11 mice per genotype. Data pooled from two independent experiments. * p<0.05, **p<0.01, ***p<0.001 as compared to WT using 2-way ANOVA for weight loss and student’s t-test for colon length, spleen and thymus weight.
β-arrestin2 inhibits T cell differentiation in response to TCR stimulation
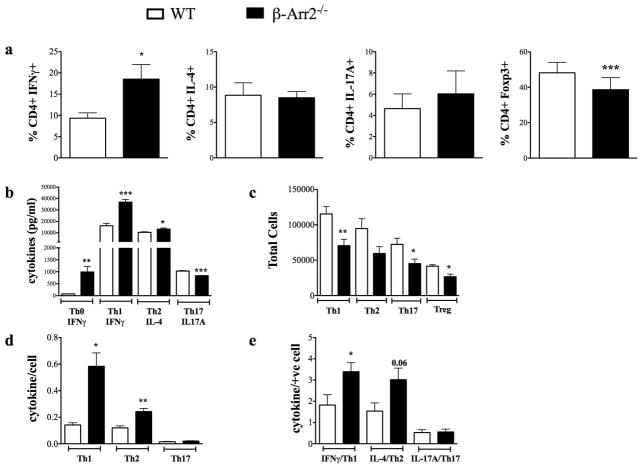
Activation potential of T cells could be regulated via both homeostatic and inflammatory processes in the local tissue or systemic environment. Further, the function of β-arrestin2 in T cells in the context of intestinal inflammation could be due to extrinsic or intrinsic factors. To address this question, naïve T cells (CD4+CD44−CD25−CD62Llo) were sorted from the spleen and activated in vitro under differentiation conditions favoring Th1, Th2, Th17 or Treg induction. In support of T cell intrinsic role of β-arrestin2, T cells lacking β-arrestin2 had significantly altered T cell differentiation: higher Th1 (%IFNγ+) but lower Treg (Foxp3+) compared to WT cells. Th2 (IL-4+) and Th17 (Il-17A+) however were similar between the two genotypes (Fig 7a). We also assessed cytokine production from these differentiated cells and found that IFNγ production under Th1 and IL-4 production under Th2 conditions were respectively higher in the β-arr2−/− cells compared to the WT. IL-17A under Th17 conditions however, was slightly albeit significantly lower from T cells lacking β-arrestin2 (Fig 7b). Strikingly, even under Th0 (neutral) conditions, IFNγ production was significantly higher from T cells lacking β-arrestin2 (Fig 7b). Compared to these findings, total numbers of CD4+ T cells at the end of the differentiation were significantly lower in the β-arr2−/− under all differentiation conditions including Tregs (Fig 7c). Therefore, when cytokine production was normalized for total cell number (Fig 7d) or cells positive for a particular cytokine as determined in 7a (Fig 7e), β-arr2−/− T cells have significantly higher differentiation potential towards Th1 and Th2. Since Treg induction was lower in the β-arr2−/− mice it is possible that in the absence of β-arrestin2, T cell differentiation in vivo is skewed more towards higher Th1 (and possibly Th2) responses.
Figure 7. Loss of β-arrestin2 alters T cell differentiation potential.
Sorted naïve (CD4+CD44−CD25−CD62Lhi) CD4+ T cells isolated from WT and β-arr2−/− mice were stimulated with CD3/28 under different conditions to induce Th0, Th1 (IL-12), Th2 (anti-IFNγ, IL-4), Th17 (anti-IFNγ, anti-IL-2, IL-6, TGFβ) and Treg (anti-IFNγ, TGFβ) differentiation for 5 days. a) Proportion of differentiated Th1, Th2, Th17 and Treg cells. b) Levels of indicated cytokine released in response to different differentiation protocol. c) Total number of cells recovered at the end of in vitro differentiation and re-stimulation. Cytokine production normalized to total number of cells is shown in d and total number of cells positive for the cytokine as determined in (a) is shown in e. N=6, with data pooled from 3 independent experiments. * p<0.05, **p<0.01, ***p<0.001 as compared to WT paired t-test.
T cells lacking β-arrestin2 have a higher colitogenic potential in T cell transfer model of colitis
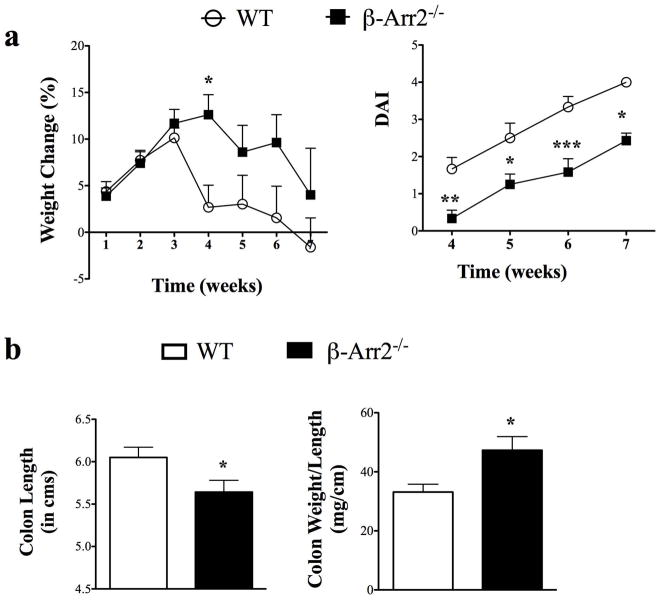
Our in vitro T cell differentiation studies clearly demonstrate that β-arr2 has an intrinsic role in T cell differentiation. To directly confirm if this role of β-arr2 is important in its ability to inhibit colitis development, we employed T cell transfer model of colitis. For this, CD4+CD45RBhi T cells from the spleen of WT and β-arr2−/− mice were sorted and injected into RAG2−/− mice. The recipients were then followed over course of time for signs of colitis and associated wasting disease. In contrast to our prediction based on DSS-induced colitis, weight loss and disease activity index were both higher in RAG2−/− mice injected with WT CD4+CD45RBhi cells compared to the RAG2−/− injected with β-arr2−/− T cells. This was observed as early as 4 weeks post transfer (Fig 8). While the weight loss became similar between the two sets of mice at the end of the experiment (7–8 weeks), disease activity index continued to be higher in mice that received WT cells (Fig 8). Even though the systemic wasting disease was lower in the β-arr2−/− mice, gross parameters of colitis including colon shortening and increase in colon weight (likely due to inflammation) were significantly higher for RAG2−/− injected with β-arr2−/− T cells as compared to WT T cells.
Figure 8. β-arrestin2 inhibits T cell colitogenic potential.
Sorted CD4+CD45RBhigh isolated from WT and β-arr2−/− mice were injected into RAG2−/− mice to induce T cell mediated colitis. a) Weight loss and disease activity index of T cell injected mice over the period of 7 weeks. (b) Colon length and colon weight normalized to colon length as a markers of gross inflammation at the end of the experiment (week 7–8). N=12 with data pooled from two independent experiments. * p<0.05, **p<0.01, ***p<0.001 as compared to WT using 2-way ANOVA for weight loss and disease activity index in (a) and student’s t-test for (b).
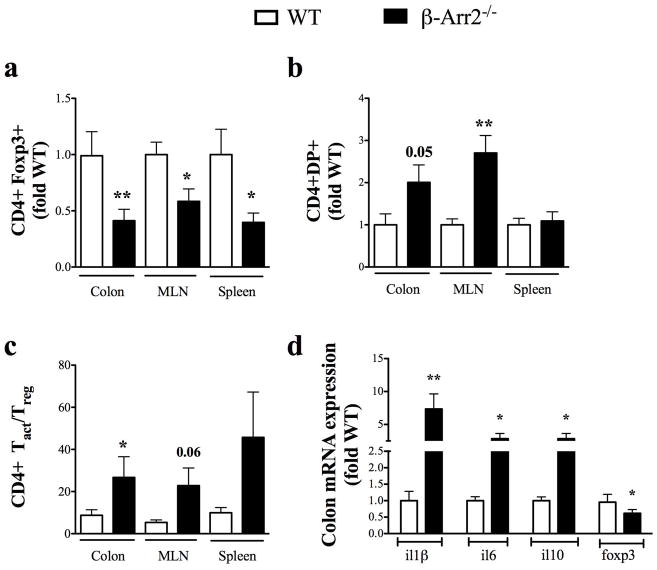
We then analyzed whether differential T cell homing or activation could account for the differences observed but found no difference in total number of T cells in cLP or spleen of RAG2−/− mice at the end point (data not shown). T cell differentiation pattern however was drastically different between the two genotypes. Proportion of Foxp3+CD4+ T cells (Tregs) isolated from cLP, spleen and MLN of RAG2−/− that received β-arr2−/− T cells was significantly lower when compared to RAG2−/− mice that received WT T cells (Fig 9a). Ex vivo restimulation of these T cells showed no major differences in the proportion of single positive IFNγ+ (Th1) and IL-17A+ (Th17 cells) (data not shown). Double positive (DB) T cells (IFNγ+IL17+), however, were significantly higher in the colon (p=0.05) and MLN of RAG2−/− mice injected with β-arr2−/− T cells compared to mice that received WT T cells (Fig 9b). In addition, the ratio of activated T cells (IFNγ+, IL-17A+ and DP) to regulatory T (Foxp3+) cells was significantly higher in colon from RAG2−/− that received β-arr2−/− T cells compared to the ones that received WT cells (Fig 9c). Since this ratio would be reflective of overall T-cell activation status, these results suggest that lack of β-arr2 in T cells potentially renders the mice more colitic. Further, expression of pro-inflammatory mediators IL-1β, IL-6 and IL-22 was increased while that of anti-inflammatory IL-10 and Foxp3 were significantly decreased (Fig. 9d); reinstating the paradigm of overt intestinal inflammatory response in this model of colitis. Together, these results demonstrate that T cells lacking β-arrestin2 are less able to differentiate into regulatory T cells while their ability to differentiate into activating T cell is increased and together this likely enhances their colitogenic potential.
Figure 9. β-arrestin2 inhibits Treg induction in vivo and alters T cell activation balance.
Cells from cLP were stained with or without ex vivo stimulation to determine proportion of Th1, Th17 or Treg cells. Proportion of (a) Treg cells, (b) double positive (DP, Th17+IFNγ+) cells and (c) ratio of activated T cells (Th1, Th17 and DP) to Foxp3+ T (Treg) cells in colon, mesenteric lymph node (MLN) and spleen. (d) mRNA expression of inflammatory mediators differentially regulated in RAG donors with either WT or β-arrestin2−/− naïve T cells. N=12 with data pooled from two independent experiments and shown as fold WT for each experiment. * p<0.05, **p<0.01, ***p<0.001 as compared to WT using paired t test.
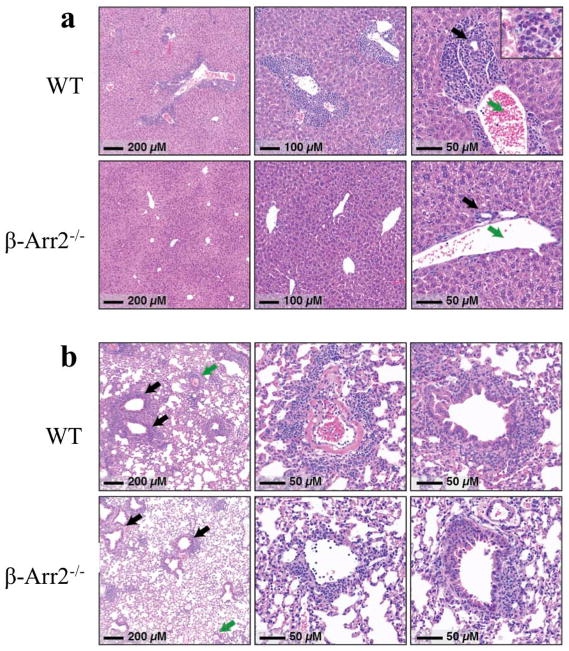
It was intriguing that even though β-arr2−/− T cells induced higher colitis in RAG2−/− mice, animals that received WT cells had poorer clinical scores and greater weight loss. Previous studies have implicated other organs including the liver to be important sites of inflammation in this T-cell transfer model of colitis (15). We therefore examined liver and lungs for any histological evidence of systemic inflammation. In contrast to our findings in the colon (supplementary figure 3), RAG2−/− mice that received β-arr2−/− T cells had almost no evidence of inflammation in the liver whereas the animals that received WT T cells showed marked liver inflammation (Fig. 10a). A similar, but less dramatic, trend towards reduced inflammation was seen in the lungs (Fig 10b). It is possible that this marked reduction in extra-colonic organ inflammation in RAG2−/− that received β-arr2−/− T cells protects the mice from systemic inflammation leading to diminished weight loss and clinical scores. Future studies in our lab will focus on the mechanisms that modulate these differential responses. Together, these results demonstrate the importance of T cell specific β-arrestin2 in colitis pathogenesis as well as underscore the differential effects of β-arrestin2 in the immunopathology of organ inflammation.
Figure 10. Loss of β-arrestin2 in T cells protects against liver and Lung damage in T cell transfer model of colitis.
Histopathology of liver and lung tissue from colitic mice 7–8 weeks post T cell transfer. (a) In the liver, inflammation was mostly confined to periportal regions (Zone 1), and consisted of a mixture of lymphocytes, eosinophils, and some neutrophils (inset, right panel). In most portal regions, inflammatory cells formed a thick rim encompassing both bile ducts (black arrow) and portal veins (green arrow). (b) Lungs from WT mice showed marked acute and chronic pneumonitis that was primarily peri-vascular (green arrows and middle panel) and peri-bronchiolar (black arrows and right-most panel) in distribution. Many of the involved parenchymal vessels showed evidence of fibrin deposition, as seen in the middle panel. Alveolar spaces were relatively spared, although significant inflammation was frequently seen extending away from vascular and airways to impinge on neighboring alveoli. Photomicrographs shown here were taken from mice with less severe, but notable inflammation that was of the same character and distribution as that seen in the WT mice.
DISCUSSION
In this study, we show that mice lacking β-arrestin2 had higher colonic inflammation under basal conditions and disruption of the intestinal barrier by DSS leads to exacerbated colitis in β-arr2−/− mice. Further in the absence of β-arrestin2, T cells have skewed differentiation and increased colitogenic potential. Even though the role of β-arrestin2 in mucosal inflammation could be modulated by innate response to LPS (microbial component) and polymicrobial stimulation as demonstrated in previous studies (4, 19, 20), our results clearly demonstrate that β-arr2 inhibits the colitogenic potential through a dominant role in T cell differentiation.
Under basal conditions, we observed higher T cell numbers in β-arrestin2 deficient colons and this correlated with higher T cell chemokine production and/or in situ T cell proliferation instigated by higher number of APCs. The latter could also provide stimulation for increased differentiation and activation as observed by proportion of effector T cells in cLP of β-arr2−/− animals. This effect of β-arr2 in T cells in the context of intestinal inflammation is in contrast to its role in allergic asthma model of lung inflammation (5), where T cell infiltration and consequent disease induction were abrogated in the absence of β-arrestin2. In the same study, LPS induced T cell infiltration was unaffected by loss of β-arrestin2, implicating that the role of β-arrestin2 at a particular site is stimuli dependent. Underscoring this is our observation in T cell transfer model of colitis, where transfer of β-arr2−/− T cells enhanced intestinal inflammation but suppressed hepatic and pulmonary inflammation.
Even though there were some differences in the outcome of β-arr2 deficiency in T cell differentiation in the two different colitis models versus in vitro polarization experiments (higher Th17 in DSS model while in vitro differentiation was similar; higher Th1 in vitro but no difference in transfer model colitis); they do not undermine the role of β-arrestin2 in regulating T cell differentiation. It instead highlights the involvement of different co-stimulatory molecules (in addition to CD28 used in vitro) and cytokine milieu in the context of ongoing inflammation guiding and dictating the final differentiation potential. Other studies have demonstrated contrasting role for β-Arrestin2 in T cell differentiation in allergic asthma model (5, 21, 22) and in human primary T cells (10). Use of siRNA to modulate β-arrestin2 expression and lack of data on T cell activation status in these studies makes them difficult to compare to ours. However, a recent study using a modified T cell differentiation protocol (magnetically enriched naïve T cells and different concentration of CD3/CD28) demonstrated a decrease in Treg induction in β-arr2−/− T cells but no difference in Th1/2/17 polarization (6). Consistent with the colitis model shown here, β-arrestin2 mediated Treg induction appeared to be important in protection against EAE and the β-arr2−/− mice consequently exhibited more severe disease (6). Lower Treg induction was also observed in splenic and MLN T cells even under basal conditions in our studies (data not shown). Given the importance of Foxp3+ T cells in abating colon inflammation (23), the role of β-arrestin2 in Treg induction could be a prominent reason for increased colitis induction. Even though β-arrestin2 has been shown to be important for TGFβRIII receptor endocytosis and downstream anti-proliferative potential (24), further work will be necessary to determine the role of altered TGFβ signaling in context of T cell differentiation.
Even though colitis induction in both models of colitis was higher in the absence of β-arrestin2, weight loss and disease activity index in the two models showed completely different modulation by β-arrestin2. It is quite possible that distinction stems from difference in pathogenesis of acute and chronic models of colitis as well as due to differential effects of T cell-specific β-arr2 in different tissues. It must also be noted that while DSS-induced colitis is considered an equivalent of ulcerative colitis, T cell transfer is a model for crohn’s disease (25). Nevertheless, the role of β-arrestin2 in differentially affecting weight loss and colitis induction in T cell transfer model of colitis was quite surprising. Similar distinction between weight loss and colitis was also observed in animals treated with an anti-TLR4 antibody (26). Also, in T cell transfer in SCID mice, antibiotics and TNFR-Fc preventive treatment were shown to affect only colitis or weight change respectively (27), suggesting differential involvement of microbial components and TNF signaling in these parameters. Given the positive regulatory role for β-arr2 in LPS mediated inflammation (8), it is possible that systemic effects of β-arr2 such as hepatic inflammation is differentially mediated by β-arr2 compared to intestinal inflammation. Also, the role and differential production of other inflammatory mediators at systemic or local sites cannot be ruled out. Future studies will examine the role of differential homing of donor T cells (β-arr2−/−) to the colon versus liver or lung, which might explain greater colitis but diminished liver inflammation.
While this manuscript was in preparation, another study was published using 5% DSS model of colitis in wild type and β-arr2 knockout mice. These authors found β-arr2 expression to be a susceptibility factor in colitis (28). They demonstrated in cell culture studies that, β-arr2 binds eIF2α and induces ER stress thereby affecting epithelial apoptosis through p53-upregulated modulator of apoptosis (PUMA). Given the role of epithelial apoptosis in colitis progression (29), these results suggested that β-arr2 in the presence of inflammatory stimuli would induce epithelial apoptosis thereby increasing colitic susceptibility. Consistent with that notion, they found that β-arr2 knockout mice were less susceptible to 5% DSS-induced colitis. Even though this is contrary to our findings, both studies have uncovered different mechanisms through which β-arr2 can affect colitis progression. While mucosal immune cell development and epithelial apoptosis could be factors affecting colitis progression in the absence of β-arr2, in our studies, we demonstrate that T cell polarization is distinctly perturbed and the inflammatory balance skewed, promoting a colitic response in mice deficient for β-arr2. Using T-cell transfer model, we also demonstrate that T-cell specific β-arr2 is inhibitory to colitis development (in contrast to systemic wasting disease). Thus the role of β-arr2 in T cells versus other cell types in the context of colitis could be distinct and the different phenotypes in these two studies could be due to factors that make the role of β-arr2 in one cell type dominant over the other. It should also be noted that the role of β-arr2 in apoptosis in response to different inflammatory and physiological stimuli has led to pro-(30–32) and anti-apoptotic (33, 34) roles being ascribed to this protein. Therefore, loss of β-arr2 could exhibit differential phenotypes depending on the extent of stimuli and differential pathways engaged. Microbiota could be another confounding factor especially in mucosal immunity. We tried to address a potential difference in microbiota using co-caged mice that equilibrates microbiota, allowing the presence or absence of β-arr2 to play a dominant role in the eventual outcome (35). It is nonetheless possible that microbial differences between the two colonies (in the two studies) led to discrepancies in the final outcome either directly or by modulating the dominant factor (epithelial apoptosis versus T cell differentiation) affecting outcome. Microbiota induced discrepancies in disease progression have been previously demonstrated in other studies, for example nlrp3 knockout mice in different studies had contrasting outcomes (36–38).
In the present study, we demonstrate that the role of β-arrestin2 is directly opposite to that of β-arr1, where we found that the loss of β-arr1 is protective in colitis development. Thus β-arr1 and 2 respectively mediate and inhibit the pathogenesis of colitis (13). These findings are similar to the distinct role of these two proteins in pathogenesis of other autoimmune diseases, EAE (6, 39) and arthritis (7, 40) where loss of β-arr1 led to attenuation of disease but deficiency of β-arr2 led to exacerbated disease pathogenesis. It is interesting that even though both proteins have similar roles in endotoxemia and polymicrobial sepsis, their roles in autoimmune diseases, some of which are modulated by TLR signaling, are quite distinct.
In summary, this study demonstrates an important role for β-arrestin2 in regulating mucosal inflammation under both homeostatic and colitic conditions. Its mode of action involves negative regulation of T cell activation and its requirement for induction of regulatory T cells. Further work would determine if either TLR4 or other G proteins or GPCRs have a role to play in this and provide insight into crosstalk between TCR, TLR and G protein pathways in regulation of inflammatory diseases.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support from NIH (grants HL095637, AR055726 and AI099404 (NP)). We thank the university lab animal resources for taking excellent care of our animals. We are grateful to Dr. Robert J. Lefkowitz for kindly providing us the β-arrestin2 knockout mice.
Footnotes
Disclosure of Funding:
National Institutes of Health
References
- 1.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World journal of gastroenterology: WJG. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham C, Cho JH. Inflammatory Bowel Disease. New England Journal of Medicine. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan H, Bitto A, Zingarelli B, et al. Beta-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology. 2010;130:344–351. doi: 10.1111/j.1365-2567.2009.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma D, Malik A, Lee E, et al. Gene Dosage-Dependent Negative Regulatory Role of β-Arrestin-2 in Polymicrobial Infection-Induced Inflammation. Infection and Immunity. 2013;81:3035–3044. doi: 10.1128/IAI.00653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker JKL, Fong AM, Lawson BL, et al. β-Arrestin-2 regulates the development of allergic asthma. The Journal of Clinical Investigation. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Liu C, Wei B, et al. Loss of beta-arrestin 2 exacerbates experimental autoimmune encephalomyelitis with reduced number of Foxp3+ CD4+ regulatory T cells. Immunology. 2013;140:430–440. doi: 10.1111/imm.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li P, Cook JA, Gilkeson GS, et al. Increased expression of beta-arrestin 1 and 2 in murine models of rheumatoid arthritis: isoform specific regulation of inflammation. Mol Immunol. 2011;49:64–74. doi: 10.1016/j.molimm.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter KJ, Gonipeta B, Parvataneni S, et al. Regulation of lipopolysaccharide-induced inflammatory response and endotoxemia by beta-arrestins. Journal of cellular physiology. 2010;225:406–416. doi: 10.1002/jcp.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seregin SS, Appledorn DM, Patial S, et al. beta-Arrestins modulate Adenovirus-vector-induced innate immune responses: differential regulation by beta-arrestin-1 and beta-arrestin-2. Virus research. 2010;147:123–134. doi: 10.1016/j.virusres.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjorgo E, Solheim SA, Abrahamsen H, et al. Cross talk between phosphatidylinositol 3-kinase and cyclic AMP (cAMP)-protein kinase a signaling pathways at the level of a protein kinase B/beta-arrestin/cAMP phosphodiesterase 4 complex. Molecular and cellular biology. 2010;30:1660–1672. doi: 10.1128/MCB.00696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Murray F, Koide N, et al. Divergent requirement for Gαs and cAMP in the differentiation and inflammatory profile of distinct mouse Th subsets. The Journal of Clinical Investigation. 2012;122:963–973. doi: 10.1172/JCI59097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hörnquist CE, Lu X, Rogers-Fani PM, et al. G(alpha)i2-deficient mice with colitis exhibit a local increase in memory CD4+ T cells and proinflammatory Th1-type cytokines. The Journal of Immunology. 1997;158:1068–1077. [PubMed] [Google Scholar]
- 13.Lee T, Lee E, Irwin R, et al. beta-Arrestin-1 deficiency protects mice from experimental colitis. Am J Pathol. 2013;182:1114–1123. doi: 10.1016/j.ajpath.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohn LM, Lefkowitz RJ, Gainetdinov RR, et al. Enhanced Morphine Analgesia in Mice Lacking β-Arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 15.Ostanin DV, Bao J, Koboziev I, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. American journal of physiology Gastrointestinal and liver physiology. 2009;296:G135–146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik A, Sharma D, St Charles J, et al. Contrasting immune responses mediate Campylobacter jejuni-induced colitis and autoimmunity. Mucosal Immunol. 2014;7:802–817. doi: 10.1038/mi.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. The Journal of experimental medicine. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J, August A. Naive and innate memory phenotype CD4+ T cells have different requirements for active Itk for their development. Journal of immunology (Baltimore, Md: 1950) 2008;180:6544–6552. doi: 10.4049/jimmunol.180.10.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Tang Y, Teng L, et al. Association of [beta]-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- 20.Fan H, Bitto A, Zingarelli B, et al. β-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology. 2010;130:344–351. doi: 10.1111/j.1365-2567.2009.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G, Liu Y, Yang M, et al. Effects of beta-arrestin 2 on cytokine production of CD4+ T lymphocytes of mice with allergic asthma. Indian journal of experimental biology. 2011;49:585–593. [PubMed] [Google Scholar]
- 22.Liu Y, Wang GY, Liu SK, et al. beta-arrestin2 stimulates interleukin-17 production and expression of CD4+ T lymphocytes in a murine asthma model. Iranian journal of allergy, asthma, and immunology. 2011;10:171–182. [PubMed] [Google Scholar]
- 23.Boehm F, Martin M, Kesselring R, et al. Deletion of Foxp3+ regulatory T cells in genetically targeted mice supports development of intestinal inflammation. BMC gastroenterology. 2012;12:97. doi: 10.1186/1471-230X-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Kirkbride KC, How T, et al. β-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 25.Wirtz S, Neufert C, Weigmann B, et al. Chemically induced mouse models of intestinal inflammation. Nature protocols. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 26.Ungaro R, Fukata M, Hsu D, et al. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. American journal of physiology Gastrointestinal and liver physiology. 2009;296:G1167–1179. doi: 10.1152/ajpgi.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindebo Holm T, Poulsen SS, Markholst H, et al. Pharmacological Evaluation of the SCID T Cell Transfer Model of Colitis: As a Model of Crohn’s Disease. International journal of inflammation. 2012;2012:412178. doi: 10.1155/2012/412178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng LX, Tao J, Liu HL, et al. [beta]-Arrestin2 encourages inflammation-induced epithelial apoptosis through ER stress/PUMA in colitis. Mucosal Immunol. 2014 doi: 10.1038/mi.2014.104. [DOI] [PubMed] [Google Scholar]
- 29.Edelblum KL, Yan F, Yamaoka T, et al. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflammatory bowel diseases. 2006;12:413–424. doi: 10.1097/01.MIB.0000217334.30689.3e. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Chen L, Zhang Y, et al. Chronic stress promotes lymphocyte reduction through TLR2 mediated PI3K signaling in a beta-arrestin 2 dependent manner. Journal of neuroimmunology. 2011;233:73–79. doi: 10.1016/j.jneuroim.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Sun X, LeSage G, et al. beta-arrestin 2 regulates Toll-like receptor 4-mediated apoptotic signalling through glycogen synthase kinase-3beta. Immunology. 2010;130:556–563. doi: 10.1111/j.1365-2567.2010.03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang P, Gao H, Ni Y, et al. Beta-arrestin 2 functions as a G-protein-coupled receptor-activated regulator of oncoprotein Mdm2. The Journal of biological chemistry. 2003;278:6363–6370. doi: 10.1074/jbc.M210350200. [DOI] [PubMed] [Google Scholar]
- 33.Qi S, Xin Y, Qi Z, et al. HSP27 phosphorylation modulates TRAIL-induced activation of Src-Akt/ERK signaling through interaction with beta-arrestin2. Cellular signalling. 2014;26:594–602. doi: 10.1016/j.cellsig.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 34.Revankar CM, Vines CM, Cimino DF, et al. Arrestins block G protein-coupled receptor-mediated apoptosis. The Journal of biological chemistry. 2004;279:24578–24584. doi: 10.1074/jbc.M402121200. [DOI] [PubMed] [Google Scholar]
- 35.Brinkman BM, Becker A, Ayiseh RB, et al. Gut microbiota affects sensitivity to acute DSS-induced colitis independently of host genotype. Inflammatory bowel diseases. 2013;19:2560–2567. doi: 10.1097/MIB.0b013e3182a8759a. [DOI] [PubMed] [Google Scholar]
- 36.Bauer C, Duewell P, Lehr HA, et al. Protective and aggravating effects of Nlrp3 inflammasome activation in IBD models: influence of genetic and environmental factors. Digestive diseases (Basel, Switzerland) 2012;30 (Suppl 1):82–90. doi: 10.1159/000341681. [DOI] [PubMed] [Google Scholar]
- 37.Bauer C, Duewell P, Mayer C, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 38.Zaki MH, Boyd KL, Vogel P, et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, Feng Y, Kang J, et al. Critical regulation of CD4+ T cell survival and autoimmunity by beta-arrestin 1. Nat Immunol. 2007;8:817–824. doi: 10.1038/ni1489. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Wei B, Guo A, et al. Deficiency of beta-arrestin1 ameliorates collagen-induced arthritis with impaired TH17 cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7395–7400. doi: 10.1073/pnas.1221608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.