SUMMARY
Prior studies have found that functional networks can rapidly add neurons as they build short-term memories, yet little is known about the principles underlying this process. Using voltage-sensitive dye imaging we found that short-term sensitization of Tritonia’s swim motor program involves rapid expansion of the number of participating neurons. Tracking neurons across trials revealed that this involves the conversion of recently discovered variably-participating neurons to reliable status. Further, we identify a candidate serotonergic cellular mechanism mediating this process. Our findings reveal a new mechanism for memory formation, involving recruitment of pre-positioned, variably-committed neurons into memory networks. This represents a shift from the field’s long-term focus on synaptic plasticity, toward a view that certain neurons have characteristics that predispose them to join networks with learning.
INTRODUCTION
The formation of both short-term [1–3] and long-term memories [4–7] can involve network expansion, with neurons recruited into existing functional networks. The mechanisms controlling such network reorganization lie at the heart of how our brains store information acquired through learning. For decades, research on the cellular mechanisms involved in memory formation has focused almost exclusively on the role played by synaptic plasticity in this process [8, 9]. Could memory formation involve additional principles? For example, are some neurons especially eligible for recruitment? How extensively do networks reorganize as memories form? Even less is known about forgetting - is it simply the reverse of learning, such that neurons added during memory formation leave with forgetting? Such issues are becoming more accessible due to recent improvements in our ability to track the activity of large numbers of neurons across trials as memories form and then dissipate.
Sensitization is a non-associative form of learning in which an unexpected, typically aversive stimulus potentiates responsiveness to the same or other stimuli. Prior studies have shown that sensitization of the marine mollusk Tritonia’s escape swim is accompanied by several behavioral modifications, including a faster onset latency and lowered threshold [10–12]. This non-associative learning can be recapitulated in isolated brain preparations, where for several minutes following a nerve-elicited swim motor program (SMP) subsequent stimuli elicit SMPs that begin more quickly [13]. The present study used voltage-sensitive dye (VSD) imaging to track the activity of several dozen neurons in the T. diomedea pedal ganglion as the memory for sensitization formed and then dissipated. Our findings identify a strategy for memory formation: neuronal networks may normally operate with mixtures of reliably- and variably-participating neurons, with memory formation occurring, in part, via neuromodulatory enhancement of the degree of commitment of neurons to the motor program.
RESULTS
The short-term memory for sensitization involves a rapid expansion of the escape swim network
In order to investigate how the swim network encodes the memory for sensitization, we performed VSD imaging with a photodiode array, allowing us to optically record the action potential activity of several dozen neurons in the dorsal pedal ganglion, where the majority of the known swim network is located [14]. We used a same-site sensitization protocol, in which an initial stimulus produces sensitization of SMP responses to subsequent stimuli [13].
Our first experiment involved two groups: experimental (N = 7) and control (N = 6). Each stimulus consisted of a 1 Hz train of seven 4 – 7 V, 2 ms pulses applied to pedal nerve 3, which contains the axons of sensory afferents. In the experimental group, three SMPs were elicited two minutes apart (Fig. 1A). The first of these served as the naïve SMP, while the second and third incorporated sensitization produced by the prior stimuli. In the control group, two SMPs were elicited 18 minutes apart (Fig. 2A), which preliminary experiments showed was sufficient for the sensitization evoked by an initial stimulus to dissipate. For both groups, the SMP files were concatenated and spike-sorted with independent component analysis (ICA, see supplemental experimental procedures), allowing us to examine the activity of all neurons across trials.
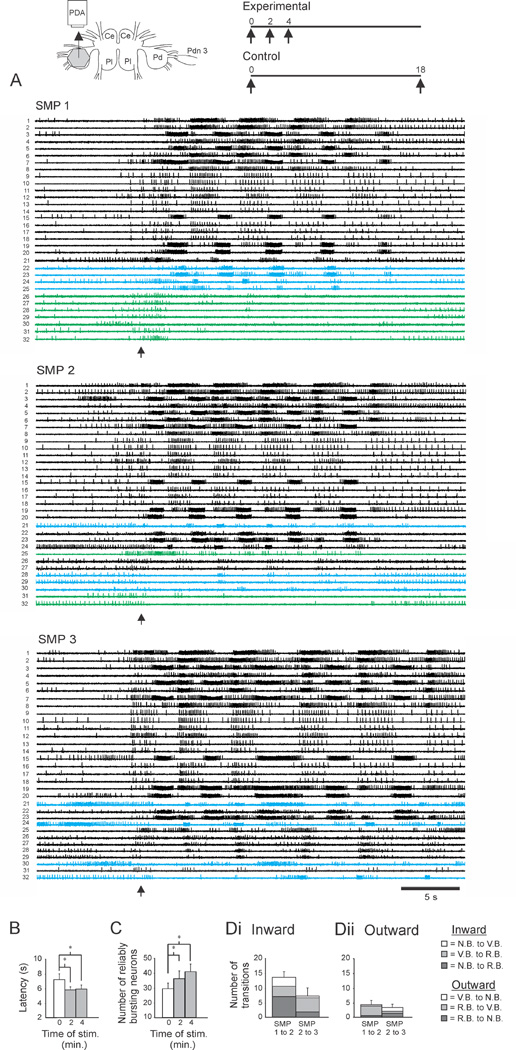
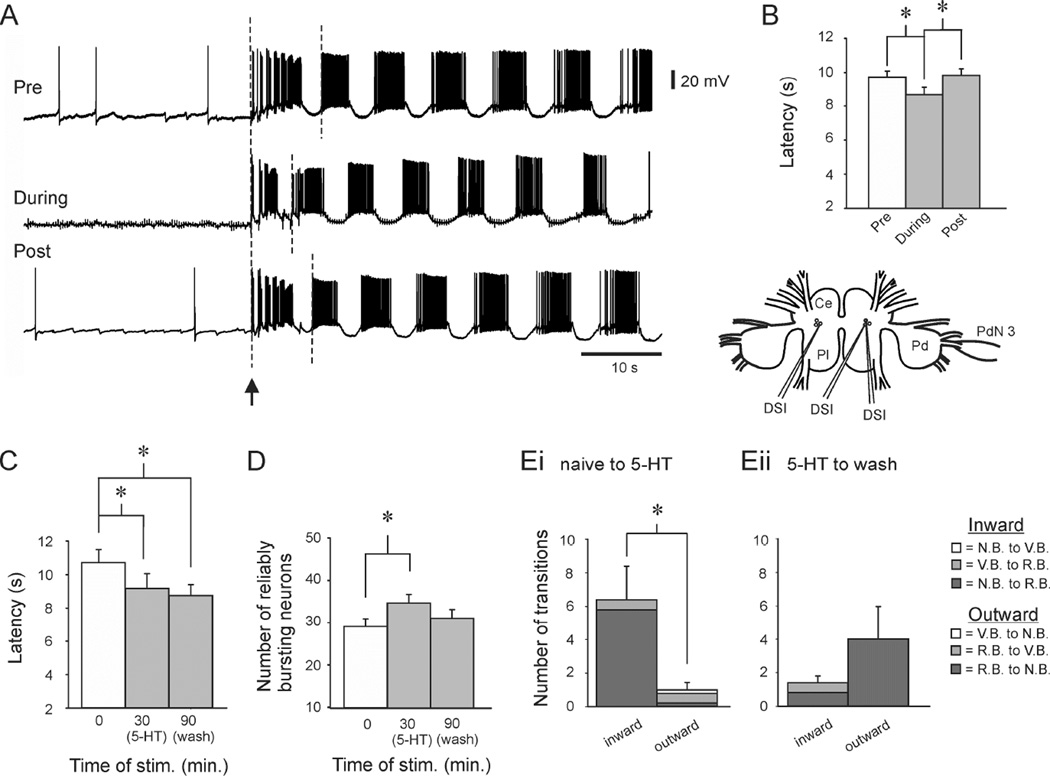
Figure 1.
Sensitization was accompanied by network expansion. Inset. Left. Isolated brain preparation. Ce: Cerebral ganglion, Pl: Pleural ganglion, Pd: Pedal ganglion, PDA: photodiode array, Pdn 3: Pedal nerve 3 with an attached suction electrode used to stimulate the swim motor program. Right. Stimulus protocols for the first imaging experiment. Data in Figs. 1 and 3 are from the experimental protocol. Data in Fig. 2 are from the control protocol. Numbers refer to minutes from the first SMP-inducing PdN3 stimulus. A. Three swim motor programs (SMPs) were elicited two minutes apart. In this and subsequent experiments, the data files were concatenated before spike sorting, allowing the firing of individual neurons to be tracked across trials (each numbered neuron is the same neuron in all trials shown). In all experiments, motor programs were elicited using a stimulus applied to pedal nerve 3, while spiking activity of neurons in the contralateral dorsal pedal ganglion was imaged with a 464-element photodiode array (inset). Neurons that fired bursts on all cycles of the SMP (reliably bursting (RB) neurons) are shown in black, neurons that skipped some cycles (variably bursting (VB) neurons) are shown in blue, and neurons that did not burst on any cycles (non-bursting (NB) neurons) are shown in green. B. The first stimulus produced sensitization, evident by the significant reduction in onset latency in the subsequent two SMPs. C. Sensitization was accompanied by a significant increase in the number of RB neurons. D. Network reorganization with learning. Di: Sensitization involved an “inward” movement of neurons toward/into the expanding RB neuron pool. This included neurons that transitioned directly to RB from the NB and VB pools, as well as neurons that moved toward RB by transitioning from NB to VB. Left: distribution of inward-transitioning neurons from SMP1 to SMP2. Right: distribution of same from SMP2 to SMP3. Dark grey: NB→RB transitions; Grey: VB→RB; White: NB→VB. Dii. While the active network grew with sensitization, on each trial some neurons simultaneously moved outward, becoming less involved in the SMP. Dark grey: RB→NB ; Grey: RB→VB; White: VB→NB. Error bars represent SEM.
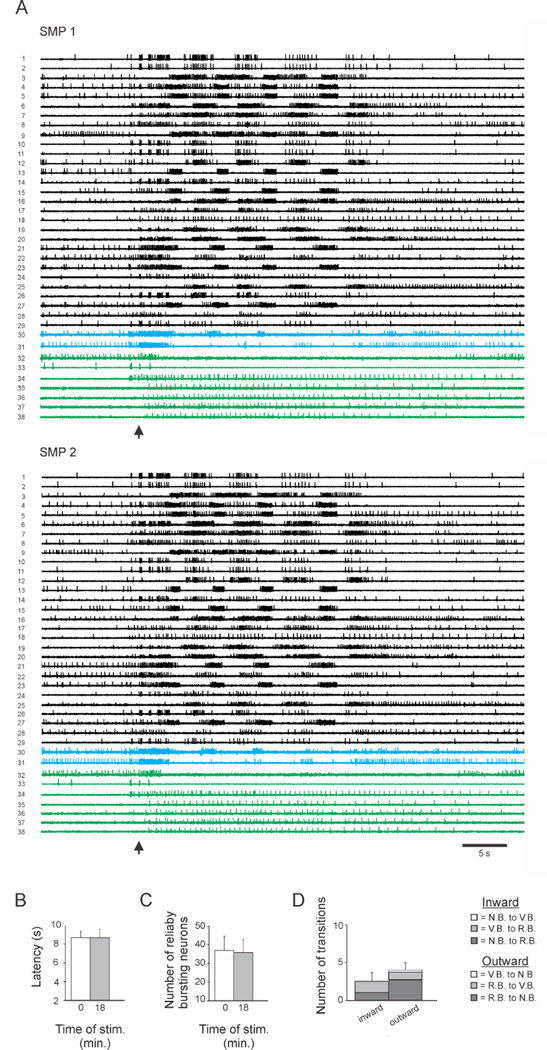
Figure 2.
Control group data. A. In the control paradigm two SMPs were elicited 18 minutes apart. The bursting patterns of the neurons were very similar in the two SMPs. Again VB neurons are shown in blue and NB neurons are shown in green. B. Both SMPs had long, naïve-type onset latencies, consistent with any sensitization produced from the first stimulus having dissipated by the time of the second stimulus. C. The number of RB neurons was not significantly different in the two SMPs. D. There was no significant difference between the number of inward vs. outward transitions between the two SMPs. Inward transitions: Dark Grey = NB→RB, Grey = VB→RB; outward transitions: Dark Grey = RB→NB, Grey = RB→VB, White = VB→NB. Error bars represent SEM.
For the experimental group, the initial stimulus produced latency sensitization that was present at the time of the second trial, and was still in effect on the third trial (Fig. 1B; see supplemental experimental procedures and Fig. S1 for details of the intact animal electrophysiology experiments used to identify the SMP correlate of behavioral swim latency). A one-way repeated measures (RM) ANOVA indicated a significant effect of training history on SMP onset latency (F(2,6) = 5.81, p = 0.017). Student-Newman-Keuls (SNK) pairwise tests indicated that with respect to the onset latency on the first trial (7.3 ± 0.8 s), the latency on the second and third trials was significantly faster (SMP 2 = 5.9 ± 0.4 s, p = 0.026; SMP 3 = 6.0 ± 0.6s, p = 0.015). For the control group, a paired t-test found no difference in SMP onset latency for the two SMPs (Fig. 2B, SMP 1 = 8.66 ± 0.67 s, SMP 2 = 8.67 ± 0.89 s, p = 0.96), indicating that sensitization produced by the first stimulus was no longer present 18 minutes later. Consistent with this interpretation is the fact that both control group latencies were long compared to the mean latencies of all sensitized trials of this study, and thus had ample room to shorten if learning had occurred.
While numerous studies have examined plasticity at individual synapses in short-term memory, far fewer have addressed the effects of such plasticity on overall network structure and function. Several have reported that short-term memory formation can involve rapid network expansion (see discussion). To test whether sensitization of the Tritonia escape swim network employs this mechanism, we identified each neuron that fired on all cycles of the SMP, defined here as reliable bursters (RB neurons; see experimental procedures). For the experimental group, a one-way RM ANOVA found a significant effect of training history on the number of reliable bursters across the three trials (F(2,6) = 7.57, p = 0.007). SNK pairwise tests indicated that with respect to the number of reliable bursters in the first trial (29.7 ± 3.8), the number in the second (36.4 ± 5.1) and third (41.1 ± 5.0) trials significantly increased (p = 0.042 and 0.006 respectively; Fig. 1C). In contrast, the control group showed no difference in the number of reliable bursters in the two trials (SMP 1 = 37.0 ± 7.7, SMP 2 = 35.8 ± 7.1, paired t-test, p = 0.50; Fig. 2C). There was also no significant difference in the number of reliable bursters on the first trial of the experimental vs. control groups (unpaired t-test, p = 0.39), and no significant difference in the number of reliable bursters on the third trial of the experimental group vs. the second trial of the control group (unpaired t-test, p = 0.54).
These results indicate that the swim-eliciting stimulus used in this study produces rapid sensitization of SMP onset latency, accompanied by increases of 23% and 38% in the number of reliable bursters on the second and third stimulus trials. Network neurons also fired in a more correlated manner with learning (see supplemental results). These network changes seem likely to contribute to certain features of the enhanced escape swim behavior that occur in sensitization, such as the enhanced flexion powerstroke (see results below and [11]).
Network growth occurs by the addition of neurons from two sources
Our finding that sensitization is accompanied by an increase in the number of reliable bursters raised the question of where these neurons came from. One possibility was that they were recruited from outside the swim network. Another possibility was suggested by our recent discovery of variably bursting (VB) neurons, which burst on some but not all cycles of the Tritonia SMP [15]. Such neurons are marked blue in Figs. 1A and 2A. Their presence in the Tritonia swim network suggested a potentially novel mechanism for rapid network growth in learning – the recruitment of “pre-positioned” neurons that are variably-coupled to networks at rest.
To test this idea, neurons were objectively assigned to one of three categories: not bursting (NB), VB or RB. Neurons increasing their commitment across trials, referred to here as moving inward, could be of three types: NB→RB, NB→VB, and VB→RB. In the experimental group, 13.7 ± 1.9 neurons per preparation moved inward from the first to the second SMP (Fig. 1Di, left), whereas significantly fewer neurons (2.5 ± 1.1, Fig. 2D, left) did so across the two SMPs of the control group (unpaired t-test, t = 4.838, p < 0.001). Half of the inward transitioning experimental group neurons were non-bursters that directly transitioned to become reliable bursters in the second SMP (NB→RB: 7.1 ± 1.2 neurons). The other half involved VB neurons, including those that transitioned from NB to VB (3.1 ± 1.1 neurons), and another group that transitioned from VB to RB (3.4 ± 0.9 neurons) (Fig. 1Di, left). This inward transition of neurons was reinforced in the third SMP of the experimental group, where 7.4 ± 2.7 neurons transitioned to join the VB and RB pools, furthering the network expansion (Fig. 1Di, right). The majority of these, 5.0 ± 2.2, were VB neurons that transitioned to RB status. Of the remainder, 1.9 ± 0.8 were NB neurons that directly transitioned to RB, and 0.6 ± 0.3 were NB neurons that transitioned to VB.
These results demonstrate that as the memory for sensitization forms, many neurons either join or increase their participation in the network. Most of these transitions involved VB neurons, consistent with this category of neuron playing an important role in learning.
Some neurons depart as the network grows
Interestingly, with sensitization some neurons became less committed to the network. Such outward moving neurons, could be of three types, RB→VB, RB→NB, or VB→NB. In the experimental group, while a mean of 13.7 neurons moved inward from SMP 1 to SMP 2 (Fig. 1Di, left), a mean of 4.4 ± 1.5 simultaneously moved outward (Fig. 1Dii, left). From SMP 2 to SMP 3 this bidirectional reorganization continued, again with a majority of neurons transitioning in the inward vs. outward direction (7.4 ± 2.7 vs. 3.4 ± 1.2; Fig. 1Di, 1Dii, right) to mediate further expansion of the network (Fig. 1C).
The control group also showed a bidirectional reorganization across the two SMPs. However, there was no significant difference between the number of neurons transitioning inward vs. outward (2.5 ± 1.1 vs. 4.0 ± 1.0, paired t-test, p = 0.33; Fig. 2D), thus the RB network size was stable across trials (Fig. 2C). This result indicates that each nerve-evoked SMP, even without learning, engages a similar but non-identical population of bursting neurons, revealing an apparent stochastic feature of the swim network.
Since there was no difference in the number of outward transitioning neurons in the experimental (SMP 1 to 2) and control groups (4.4 ± 1.5 vs. 4.0 ± 1.0, unpaired t-test, p = 0.83), sensitization appears to reorganize the network by increasing the number of reliable bursters. Figure 3 shows an example of the states and transitions made by all neurons in one preparation across the three training trials.
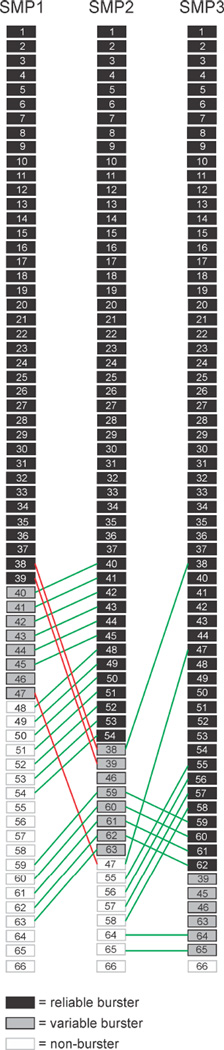
Figure 3.
Neuron transitions in a single preparation during sensitization, showing all 66 recorded neurons. Black: RB neurons, Grey: VB neurons, White: NB neurons. From the first to second SMP, the network of reliable bursters expanded by 11 neurons (green lines), drawn from both the VB and NB pools. Five NB neurons also transitioned to VB status. From SMP1 to SMP2 three neurons transitioned outward (red lines), joining pools having less commitment to the motor program. From the second to third SMP, the expansion of the RB pool continued again with neurons being drawn from both the VB and NB pools.
As the memory fades, the network returns to its original size, but with a different neuronal composition
In a second experiment we tracked network structure as the sensitization memory formed and then dissipated. Here three SMPs were elicited at 0, 5 and 75 minutes (N = 8 preparations). As before, SMP onset latency was used as the monitor of sensitization. A one-way RM ANOVA indicated a significant effect of training history for the three trials (Fig. 4A; F(2,7) = 8.74, p = .003). SNK pairwise tests indicated that with respect to the first SMP (6.3 ± 0.5 s), the onset latency was significantly faster on the second SMP (5.4 ± 0.3 s, p = 0.004), replicating our prior result. This effect was gone by the third SMP (75 min, 6.3 ± 0.4 s; versus SMP 1 p = 0.84, versus SMP 2 p = 0.006), indicating that the memory had dissipated by then.
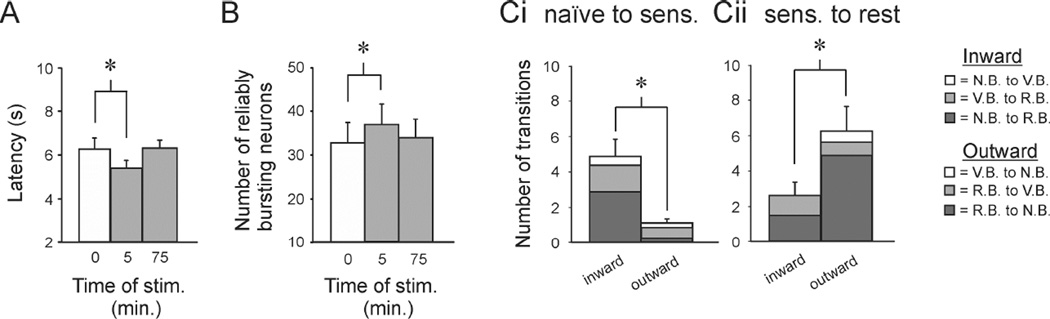
Figure 4.
Network reorganization as the memory forms and then dissipates. A. Latency sensitization: SMPs were elicited at 0, 5 and 75 minutes, with SMPs elicited at 5 minutes having significantly shorter onset latencies, which recovered after 70 minutes of rest. B. Latency sensitization was again accompanied by a significant increase in the number of RB neurons, which then reversed as the memory faded. C. Network transitions as the memory formed and then dissipated. Ci. As the memory formed, there were significantly more inward than outward transitions. Dark Grey: NB→RB transitions; Grey: VB→RB; White: NB→VB. Cii. As the memory faded, this reversed, with more outward than inward transitions. Dark Grey: RB→NB transitions; Grey: RB→VB; White: VB→NB. Error bars represent SEM.
Confirming our prior result, SMP sensitization was again accompanied by an increase in the number of reliable bursters (Fig. 4B). A one-way RM ANOVA on Ranks indicated a significant effect of training on the number of reliable bursters for the three trials (Chi-square = 6.26, p = 0.047). SNK pairwise comparisons indicated that with respect to the number of reliable bursters in the first trial (33.0 ± 6.7), the number in the second trial significantly increased (36.8 ± 6.4; p < 0.05), whereas by the third the network had returned to its initial size (33.5 ± 5.9; p > 0.05; SMP3 vs. SMP2, p < 0.05).
Again, we found that sensitization-related expansion of the network occurs on top of an underlying countercurrent of neurons leaving the network. With sensitization, significantly more neurons moved inward than moved outward (Fig. 4Ci; 4.88 ± 0.97 vs. 1.13 ± 0.23, paired t-test, p = 0.005). As the memory faded there were significantly more outward than inward transitions (Fig. 4Cii; 6.25 ± 1.4 outward vs. 2.63 ± 0.75 inward transitions; paired t-test, p = 0.046).
Did the same neurons that joined the network with learning leave as the memory dissipated? Across all preparations, 23 neurons transitioned from NB to RB status during sensitization. As the memory faded only 9 of these transitioned back to NB status, with one neuron transitioning from RB to VB status. 13 of the 23 remained as reliable bursters in the swim network after the memory had faded. Thus, a portion of the reliable bursters after the memory had faded were new with respect to those present in the naïve SMP. This could stem, in part, from trial-to-trial network variability or it could represent vestiges of the experience that remain embedded in the network after the overtly measurable signs of the memory have dissipated, perhaps representing a latent memory (see discussion).
The serotonergic DSI neurons are sufficient to mediate sensitization of SMP onset latency
We next turned to the cellular mechanisms that may mediate the learning-related network reorganization described above. Prior studies have identified the dorsal swim interneurons (DSIs) as members of the swim CPG [16] and as modulators of the excitability and synaptic efficacy of other members of the swim network [17, 18]. The DSIs fire rhythmically during the SMP and then tonically for several minutes afterward [19], during the time that animals are sensitized [12]. Thus we speculated that the DSIs might play a role in producing and actively maintaining the short-term memory for sensitization in Tritonia [17, 19].
As a step toward evaluating this hypothesis, we tested whether directly driving DSIs at an elevated tonic rate could reduce SMP onset latency. In each experiment, DSIs were impaled with electrodes before each of three SMPs (Fig. 5 inset). Each trial was preceded by a minimum 3 hour rest period. On the first and third trials a single DSI was impaled and recorded during an SMP. On the middle trial, three DSIs were impaled, and two were made to fire at 3–3.5 Hz, starting one minute before the SMP and continuing until the end of the SMP (Fig. 5A). A one-way RM ANOVA found a significant effect of treatment on the SMP latency across the 3 trials (F(2,10) = 7.49, p = 0.004). SNK pairwise tests indicated that the SMP onset latency on the second trial (8.7 ± 0.4 s) was significantly shorter than the latency on the first (9.7 ± 0.4) and third trials (9.8 ± 0.4 s, p < 0.01, N = 11 preparations; Fig. 5B). This ability to implant a false sensitization memory by driving individual DSI neurons supports the hypothesis that the DSIs produce and maintain the memory for sensitization.
Figure 5.
The serotonergic DSI neurons may drive the network reorganization occurring during sensitization. A. False memory experiment. Upper trace: A DSI neuron was penetrated and used to monitor the latency of the SMP elicited by the first of three PdN3 stimuli. Middle trace: After a 3 hour rest, two additional DSIs were penetrated (not shown) and driven with discrete currrent pulses at 3.5 Hz, starting one minute before and continuing through the PdN3-elicited SMP, which caused a sensitization-like reduction in the SMP onset latency in the unstimulated DSI. Bottom trace: After another 3 hour rest, a third motor program was elicited and displayed a longer latency. In all traces the SMP latency was measured from the start of the nerve stimulus to the start of the second burst in the monitored DSI (dashed lines). B. Group data showing the ability of DSI stimulation to drive the change in SMP latency seen in sensitization. Inset: experimental set-up. C. In optical imaging experiments, serotonin perfusion mimicked the onset latency reduction observed with sensitization. SMPs were elicited before, in the presence of serotonin (10−6 M or 10−7 M), and after a 60 min wash. The serotonin SMP had a significantly shorter onset latency than the naïve SMP, which persisted with washing. D. SMPs elicited in the presence of serotonin had significantly more RB neurons than did the naïve SMP, an effect that reversed with 60 minutes of washing. Ei. In the transition from the naïve SMP to the serotonin SMP, there were significantly more inward transitions than outward transitions. Eii. In the transition from the serotonin SMP to the washout SMP outward transitions were more numerous than inward, but not significantly so. Inward transitions: Dark Grey = NB→RB, Grey = VB→RB; outward transitions: Dark Grey = RB→NB, Grey = RB→VB, White = VB→NB. Error bars represent SEM.
We then used imaging to determine whether the DSI transmitter serotonin [17, 18] could induce the network modifications occurring during sensitization. Following a naïve SMP, the preparation was allowed to rest for 20 minutes, and then serotonin (10−6 to 10−7M) was perfused over the preparation for 10 minutes, at which time a second SMP was elicited (N = 7 preparations). The preparation was then perfused with serotonin-free saline for 60 minutes before a third SMP was elicited. We found that SMPs elicited in the presence of serotonin had a significantly reduced onset latency, which persisted even after washing (Fig. 5C; naïve: 10.24 ± 0.81s, serotonin: 8.82 ± 0.83, wash: 8.59 ± 0.59; One-way RM ANOVA, F(2,6) = 13.67, p < 0.001; SNK pairwise tests, pre vs. serotonin, p = 0.001, pre vs. wash, p = 0.001). The persistent latency reduction could stem from incomplete serotonin washout from the neuropil.
Serotonin also induced the network expansion observed with sensitization. A one-way RM ANOVA found a significant effect of serotonin on the number of reliable bursters across the three trials (F (2,6) = 6.03, p = 0.015). SNK pairwise tests indicated that with respect to the number of reliable bursters in the first trial (28.7 ± 2.8), the number in the serotonin trial (34.6 ± 2.9) was significantly increased (p = 0.013; Fig. 5D). This effect reversed with washing, such that the number of reliable bursters was no longer significantly different from the pre-serotonin level (31.0 ± 3.0; p = 0.20). These data suggest that serotonin may affect network size and onset latency independently. As with the nerve stimulus-induced network expansion, the serotonin-induced expansion occurred with a countercurrent of neurons leaving the network. In serotonin, inward transitions were significantly greater than outward (Fig. 5Ei; 6.4 ± 1.99 inward vs. 1.0 ± 0.45 outward transitions; t-test, p = 0.03), and with washing, as the network expansion dissipated, outward transitions were more numerous than inward, though not significantly so (4.0 ± 2.0 vs. 1.4 ± 0.4; t-test, p = 0.23; Fig. 5Eii). While serotonin application did elicit network expansion, most neurons transitioned directly from NB to RB, bypassing the VB state. This could stem from exogenously applied serotonin causing higher neuropil levels of the transmitter than occurs with nerve-evoked sensitization.
Potential behavioral relevance – sensitized animals have more forceful swim flexions
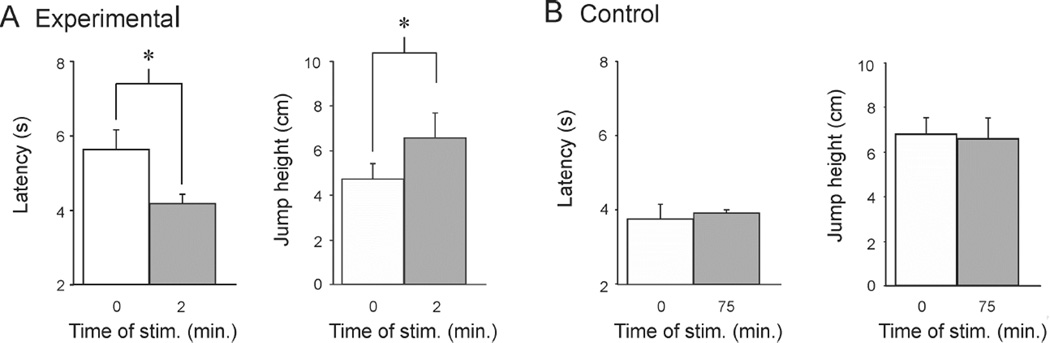
What are the behavioral consequences of the network expansion observed here with sensitization? Prior studies have shown that animals undergo several behavioral alterations with sensitization, including shortened swim onset latency and lowered swim threshold [11]. Since the pedal ganglion is the locus of swim flexion neurons [14, 20], we hypothesized that expansion of this motor network was well-suited to produce an increase in the vigor of the escape behavior. To test this hypothesis, 5M NaCl was applied to the tail to elicit swims two minutes apart. The swims were filmed, and we measured onset latency as well the height of the initial “jump”, when the first ventral flexion propels the animal off the substrate. Swim onset latency was significantly faster in the second swim, indicating a sensitized state (Fig. 6A, left, swim 1: 5.64 ± 0.52 s, swim 2: 4.18 ± 0.25 s; paired t-test, p = 0.008, n = 7). Sensitization was accompanied by a significant increase in the height of the animal’s jump off the substrate (Fig. 6A, right, swim 1: 4.71 ± 0.72 cm, swim 2: 6.57 ± 1.1 cm; paired t-test, p = 0.044, n = 7), consistent with our above finding of a larger and more correlated pedal ganglion neuron pool (Figs. 1, 4, and supplemental results).
Figure 6.
Onset latency sensitization in intact animals was accompanied by an increase in the strength of the swim flexions, which reversed as the memory dissipated. A. Two trials at a two minute inter-trial interval. Sensitization was evident as a reduction in swim onset latency, which was accompanied by a newly documented feature of sensitization, an increase in swim flexion strength. This is evident as a higher jump height off the substrate powered by the first ventral flexion of the swim. B. Two trials at a 75 minute interval. This interval was deliberately set long enough for sensitization to dissipate between trials. There was no difference in swim onset latency at the 75 minute timepoint, and there was also no difference in the initial jump height. Error bars represent SEM.
A control experiment with a separate group of animals used a 75 minute interval between the two stimuli. Swims measured with this protocol had no difference in onset latency (Fig. 6B, left, Swim 1: 3.75 ± 0.39 s, Swim 2: 3.82 ± 0.18 s; paired t-test, p = 0.82, n = 5), and no significant difference in the height of the jump off the substrate (Fig. 6B, right; Swim 1: 6.8 ± 0.74 cm, Swim 2: 6.6 ± 0.93 cm; paired t-test, p = 0.82, n = 5).
DISCUSSION
While much has been learned about the types and mechanisms of synaptic plasticity believed to encode memory [9, 21], less attention has been paid to the network consequences of changes in synaptic strength, or to the properties of the neurons involved in this process. To investigate this, we monitored the firing of several dozen neurons as a short-term memory formed and then dissipated.
Rapid network expansion
Prior studies in both vertebrates and invertebrates have shown that learning can involve rapid expansion of network size, with neurons quickly joining functional networks [1–4, 7]. Here we demonstrated this phenomenon in the marine mollusk Tritonia. Across three SMPs elicited two minutes apart, the number of reliable bursters participating in the SMP increased by 23% and 38% on the second and third trials, and these increases correlated with decreases in onset latency. Given that many pedal ganglion neurons are efferents that fire in bursts correlated with swim contractions and produce flexion contractions when driven [20, 22], an expanded network of such neurons might be predicted to generate more forceful swim flexions, as was observed here.
A feature favoring the recruitment of certain types of neurons
Which neurons join networks with learning? Do some neurons have characteristics that make them especially eligible for selection? A recent study of amygdala neurons in fear conditioning found that elevated spontaneous firing at the time of training made neurons more likely to be incorporated into memory traces [23]. Our present study suggests a different mechanism, in which resting networks contain neurons that are effectively pre-positioned for the rapid construction of memories. In the first experiment, VB neurons made up 47% of the neurons transitioning to or toward RB status from SMP 1 to SMP 2; this increased to 76% from SMP 2 to SMP 3. It remains to be seen whether vertebrate networks employ this mechanism.
Due to focal plane and viewing area constraints, each of our recordings monitored a subset of neurons in the pedal ganglion, thus our method was well-suited for tracking neurons across trials and for revealing principles of network reconfiguration with learning. However, for the same reason it was not well-suited for addressing issues such as whether a given number of RB neurons are causally related to an absolute SMP onset latency.
Evidence for stochasticity in the swim network
Our control data revealed that in the absence of learning there was a moderate bidirectional flux in the composition of the network. Trial-to-trial stochasticity in the participation of neurons in repeated, similar behaviors has been observed in monkey motor cortex and mouse hippocampus [24, 25]. Our data support the possibility that each instance of Tritonia’s highly stereotypic SMP may engage a somewhat different set of neurons. The learning-related expansion of the swim network that we observed appears to be layered on top of this underlying network variability. Taken together, our findings support the notion that memories may not be encoded, as traditionally conceived, by consistently activated sets of neurons, but instead by “evanescent networks” that continually change their neuronal constituents [26].
A possible latent memory persisting after learning has dissipated
Many of the neurons that joined the network with sensitization remained after the memory faded, while some bursters that fired reliably in the naïve SMP left (see Fig. 7 for a schematic representation). Thus, the network is in an altered state following memory formation and dissipation. Consistent with the discussion above, the altered network composition present after the memory fades may stem, at least in part, from trial-to-trial network stochasticity and therefore be unrelated to learning. Alternatively, these enduring network changes may represent a latent memory that, while not behaviorally evident, can impact later learning. Latent memory has been well described in vertebrates, as well as in Aplysia [27, 28]. Further work is needed to distinguish between these possibilities.
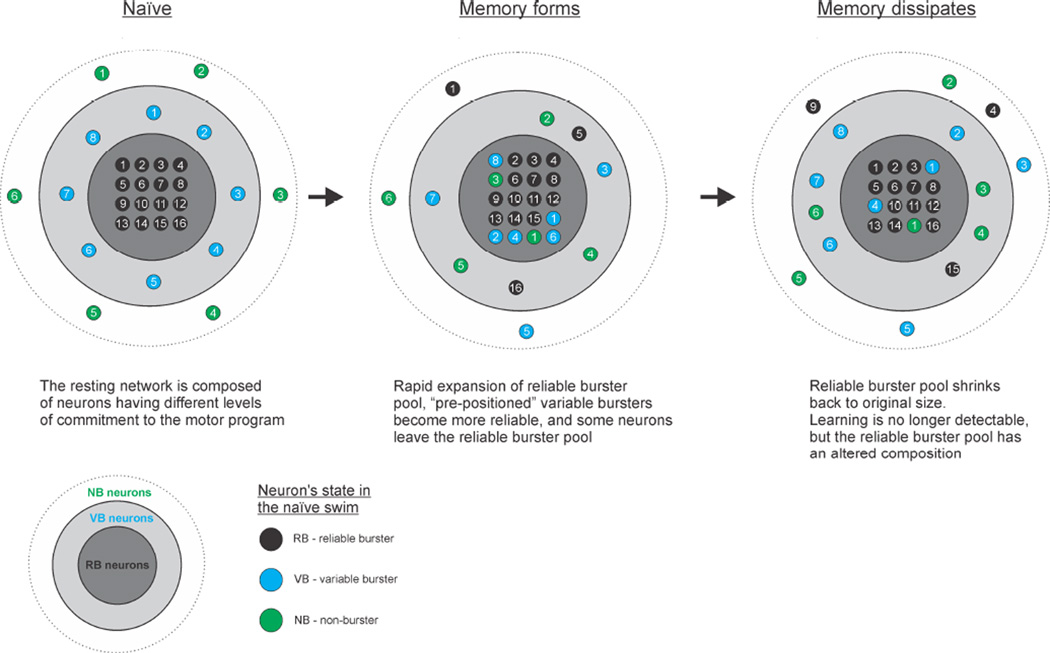
Figure 7.
Schematic representation of how the T. diomedea escape swim network reorganizes as the memory for sensitization forms and then dissipates. Colors of neurons refer to their position in the naïve network state (Green = non-burster, NB; Blue = variable burster, VB; Black = reliable burster, RB). Left. Before sensitization the naïve network consists of pools of RBs, VBs and NBs. VBs are in effect pre-positioned for rapid transition to RB status. Middle. In the sensitized state, the RB pool expands by recruiting neurons from the NB and VB pools, which occurs along with a simultaneous smaller outward transition of some neurons from the RB pool. Right. As sensitization dissipates, the expanded RB pool returns to its naïve size, but with an altered neuronal composition.
The DSIs may drive formation of the sensitization memory
The serotonergic DSIs fire rhythmically during the SMP and then continue to fire tonically for several minutes afterward [19], during the period that animals and isolated brain preparations display sensitization. Given this firing behavior, and the finding that the DSIs are potent modulators of other neurons in the swim network, we previously hypothesized that they may play a role mediating sensitization in Tritonia [17, 19]. The present study offers strong support for this with the finding that a false sensitization memory can be implanted by driving 2 DSIs at an elevated tonic firing rate similar to how they fire during sensitization, but in the absence of a training stimulus. Such false memory sufficiency tests have also been recently performed in vertebrates [29, 30].
Our findings raise the interesting possibility that while Tritonia’s sensitization memory may be mediated by cellular and synaptic modifications lasting several minutes, these modifications themselves may be maintained through tonic DSI firing. Such active maintenance of a memory by tonic firing has been demonstrated previously for Aplysia motor neurons in sensitization [31] and is consistent with current views of working memory in prefrontal cortex [32] and the role of pyramidal neurons in entorhinal cortex [33].
In support of the idea that the DSIs produce the learning-related network effects reported in this study, we found that bath applied serotonin produced both the quickening of SMP onset latency as well as the expansion of the reliable burster pool seen in sensitization. How might the DSIs and serotonin produce these effects? Both are known to enhance the excitability [34], and synaptic strength of CPG neurons [17, 35], which could act to draw neurons into the swim network. Additionally, serotonin can switch on inactive synapses in Aplysia’s withdrawal reflex circuitry [36]. A switching on and off of synapses cycle-by-cycle during the SMP could underlie the unusual firing behavior of the variably-participating neurons in the Tritonia swim network.
Perspective
Invertebrate preparations such as Tritonia offer advantages for the pursuit of memory mechanisms. Imaging can reveal the activity of a reasonable percentage of specific circuits, easing discovery of fundamental principles of network function. Next, the presence of individually identifiable neurons enables sufficiency and necessity tests of their roles in behavior. For example, since there are just six DSIs it may be possible to prevent their elevated firing during sensitization to determine whether the memory is actively maintained by their activity. Furthermore, the cellular mechanisms maintaining their tonic firing can be pursued, as well as whether and how they act to increase the commitment of variably-committed neurons in learning. Finally, it should be possible to determine the network roles of neurons of interest identified in optical recordings.
EXPERIMENTAL PROCEDURES
Optical recording
Isolated brain preparations were stained with the fast voltage-sensitive dye RH-155 (Anaspec), and imaged with a 464-element photodiode array (NeuroPDA-III, RedShirtImaging) sampled at 1600 Hz. For more details see supplemental experimental procedures.
Sharp electrode recordings
Intracellular recordings were obtained with 15–30 MΩ electrodes filled with 3M KCl or 3M K-acetate connected to a Dagan IX2–700 dual intracellular amplifier. The resulting signals were digitized at 2 KHz with a BioPac MP 150 data acquisition system.
Category assignment of recorded neurons
Custom MATLAB scripts were used to objectively determine the assignment of each recorded neuron to RB, VB or NB status. See supplemental experimental procedures and Figs. S1 and S2 for details of these algorithms.
Supplementary Material
Highlights.
Rapid expansion of a functional network as a short-term memory forms
Recruitment of a pre-positioned pool of variably-participating neurons
The network doesn’t return to its original state after the memory dissipates
Driving serotonergic neurons implants a false memory for sensitization
Acknowledgements
The authors thank Oscar Hernandez, Jody Buck, Lani Leong, Yusuf Ismail and Ryan Porter for assistance with behavioral experiments, and Lise Eliot for comments on the manuscript. The work was supported by NIH NS060921 and NSF 1257923 to W.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions – E.H., W.F. designed experiments; E.H., W.F., J.W. performed experiments; E.H., S.V., A.B., W.F,. analyzed data; E.H., W.F. wrote the paper.
REFERENCES
- 1.Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012;73:1195–1203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 3.Zecevic D, Wu JY, Cohen LB, London JA, Hopp HP, Falk CX. Hundreds of neurons in the Aplysia abdominal ganglion are active during the gill-withdrawal reflex. J Neurosci. 1989;9:3681–3689. doi: 10.1523/JNEUROSCI.09-10-03681.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annual review of neuroscience. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 5.Taubert M, Lohmann G, Margulies DS, Villringer A, Ragert P. Long-term effects of motor training on resting-state networks and underlying brain structure. NeuroImage. 2011;57:1492–1498. doi: 10.1016/j.neuroimage.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 6.Nomura H, Nonaka A, Imamura N, Hashikawa K, Matsuki N. Memory coding in plastic neuronal subpopulations within the amygdala. NeuroImage. 2012;60:153–161. doi: 10.1016/j.neuroimage.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Aoki T, Kinoshita M, Aoki R, Agetsuma M, Aizawa H, Yamazaki M, Takahoko M, Amo R, Arata A, Higashijima S, et al. Imaging of neural ensemble for the retrieval of a learned behavioral program. Neuron. 2013;78:881–894. doi: 10.1016/j.neuron.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi T, Duszkiewicz AJ, Morris RG. The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Philosophical transactions of the Royal Society of London. 2014;369:20130288. doi: 10.1098/rstb.2013.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown GD, Frost WN, Getting PA. Habituation and iterative enhancement of multiple components of the Tritonia swim response. Behav Neurosci. 1996;110:478–485. doi: 10.1037//0735-7044.110.3.478. [DOI] [PubMed] [Google Scholar]
- 11.Frost WN, Brandon CL, Mongeluzi DL. Sensitization of the Tritonia escape swim. Neurobiol Learn Mem. 1998;69:126–135. doi: 10.1006/nlme.1997.3816. [DOI] [PubMed] [Google Scholar]
- 12.Mongeluzi DL, Frost WN. Learning & memory. Vol. 7. N.Y: Cold Spring Harbor; 2000. Dishabituation of the Tritonia escape swim; pp. 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown GD. Isolated-brain parallels to simple types of learning and memory in Tritonia. Physiol Behav. 1997;62:509–518. doi: 10.1016/s0031-9384(97)00008-5. [DOI] [PubMed] [Google Scholar]
- 14.Getting PA. Neural control of swimming in Tritonia. Symp Soc Exp Biol. 1983;37:89–128. [PubMed] [Google Scholar]
- 15.Hill ES, Vasireddi SK, Bruno AM, Wang J, Frost WN. Variable neuronal participation in stereotypic motor programs. PLoS One. 2012;7:e40579. doi: 10.1371/journal.pone.0040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Getting PA, Lennard PR, Hume RI. Central pattern generator mediating swimming in Tritonia. I. Identification and synaptic interactions. Journal of neurophysiology. 1980;44:151–164. doi: 10.1152/jn.1980.44.1.151. [DOI] [PubMed] [Google Scholar]
- 17.Katz PS, Getting PA, Frost WN. Dynamic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. Nature. 1994;367:729–731. doi: 10.1038/367729a0. [DOI] [PubMed] [Google Scholar]
- 18.Katz PS, Frost WN. Intrinsic neuromodulation in the Tritonia swim CPG: the serotonergic dorsal swim interneurons act presynaptically to enhance transmitter release from interneuron C2. J Neurosci. 1995;15:6035–6045. doi: 10.1523/JNEUROSCI.15-09-06035.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popescu IR, Frost WN. Highly dissimilar behaviors mediated by a multifunctional network in the marine mollusk Tritonia diomedea. J Neurosci. 2002;22:1985–1993. doi: 10.1523/JNEUROSCI.22-05-01985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hume RI, Getting PA, Del Beccaro MA. Motor organization of Tritonia swimming. I. Quantitative analysis of swim behavior and flexion neuron firing patterns. Journal of neurophysiology. 1982;47:60–74. doi: 10.1152/jn.1982.47.1.60. [DOI] [PubMed] [Google Scholar]
- 21.Mayford M, Siegelbaum SA, Kandel ER. Synapses and memory storage. Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willows AO, Dorsett DA, Hoyle G. The neuronal basis of behavior in Tritonia. 3. Neuronal mechanism of a fixed action pattern. J Neurobiol. 1973;4:255–285. doi: 10.1002/neu.480040308. [DOI] [PubMed] [Google Scholar]
- 23.Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang HL, Pressey J, Mahadevan V, Tran MM, Kushner SA, et al. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83:722–735. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nature neuroscience. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmena JM, Lebedev MA, Henriquez CS, Nicolelis MA. Stable ensemble performance with single-neuron variability during reaching movements in primates. J Neurosci. 2005;25:10712–10716. doi: 10.1523/JNEUROSCI.2772-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Routtenberg A. Lifetime memories from persistently supple synapses. Hippocampus. 2013;23:202–206. doi: 10.1002/hipo.22088. [DOI] [PubMed] [Google Scholar]
- 27.Antzoulatos EG, Wainwright ML, Cleary LJ, Byrne JH. Learning & memory. Vol. 13. N.Y: Cold Spring Harbor; 2006. Long-term sensitization training primes Aplysia for further learning; pp. 422–425. [DOI] [PubMed] [Google Scholar]
- 28.Philips GT, Tzvetkova EI, Marinesco S, Carew TJ. Learning & memory. Vol. 13. N.Y: Cold Spring Harbor; 2006. Latent memory for sensitization in Aplysia; pp. 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. Science. Vol. 341. New York, N.Y: 2013. Creating a false memory in the hippocampus; pp. 387–391. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez S, Tonegawa S, Liu X. Identification and optogenetic manipulation of memory engrams in the hippocampus. Frontiers in behavioral neuroscience. 2014;7:226. doi: 10.3389/fnbeh.2013.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frost WN, Clark GA, Kandel ER. Parallel processing of short-term memory for sensitization in Aplysia. J Neurobiol. 1988;19:297–334. doi: 10.1002/neu.480190402. [DOI] [PubMed] [Google Scholar]
- 32.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 33.Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- 34.Katz PS, Frost WN. Removal of spike frequency adaptation via neuromodulation intrinsic to the Tritonia escape swim central pattern generator. J Neurosci. 1997;17:7703–7713. doi: 10.1523/JNEUROSCI.17-20-07703.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakurai A, Katz PS. Spike timing-dependent serotonergic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. J Neurosci. 2003;23:10745–10755. doi: 10.1523/JNEUROSCI.23-34-10745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Royer S, Coulson RL, Klein M. Switching off and on of synaptic sites at aplysia sensorimotor synapses. J Neurosci. 2000;20:626–638. doi: 10.1523/JNEUROSCI.20-02-00626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.