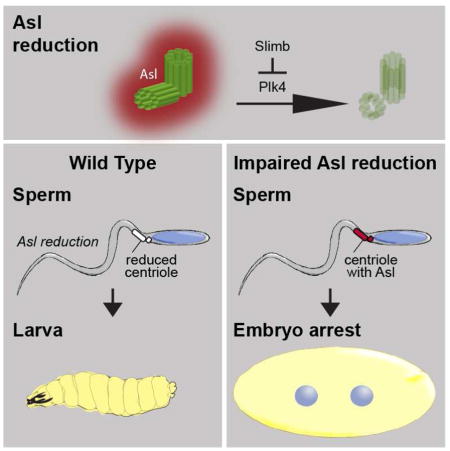
SUMMARY
Centrosome reduction is the decrease in centrosomal components during spermatid differentiation (spermiogenesis) [1, 2]. It is one of several dramatic subcellular reorganizations that leads to spermatozoa formation common to a wide range of animals [3]. However, the mechanism underlying centrosome reduction is unknown and its functions are unclear. Here, we show that in Drosophila melanogaster spermiogenesis, the quantity of centrosomal proteins is dramatically reduced [4, 5];for example, Asterless (Asl) is reduced ~500-fold and is barely detected in spermatozoa. Asl reduction is regulated through a subset of its domains by the master regulator of centriole duplication Plk4[6–8] and by the ubiquitin ligase that targets Plk4 for degradation: Slimb [9, 10]. When Asl reduction is attenuated by Asl overexpression, plk4 mutations, Plk4 RNAi, or Slimb overexpression, Asl levels are higher in spermatozoa, resulting in embryo’s with reduced viability. Significantly, overexpressing Plk4 and Asl simultaneously, or combining plk4 and slimb mutations, balances their opposing effects on Asl reduction, restoring seemingly normal fertility. This suggests that increased Asl levels cause the observed reduced fertility, and not other pleotropic effects. Attenuation of Asl reduction also causes delayed development and a failure to form astral microtubules in the zygote. Together, we provide the first insight into a molecular mechanism that regulates centrosome reduction and the first direct evidence that centrosome reduction is essential for postfertilization development.
Keywords: Asterless (Asl), Sak, Plk4, Slimb, Centrosome reduction, Spermatogenesis, Spermiogenesis, Fertilization, Development, Embryo, Zygote
Graphical Abstract
RESULTS
Asl Protein Levels are Dynamically Reduced During Spermiogenesis
During spermiogenesis, many centrosomal component proteins are diminished in a process known as centrosome reduction [1, 2]. It is unclear how and why centrosome reduction occurs even though it is ubiquitous throughout the animal kingdom and several indirect studies suggest it is necessary [11–13]. However, skeptics speculate that centrosome reduction is mediated by general sperm differentiation mechanisms and is inconsequential for male fertility, since centrosome reduction mechanism remains unknown. To gain insight into this universal phenomenon, we investigated centrosome reduction in Drosophila melanogaster, which serves as a manipulable model for human spermiogenesis. Drosophila exhibit dramatic diminishment of centrosomal proteins during spermiogenesis [14–17]; genetically-encoded fluorescent fusion tags show that Ana1, Ana2, Ana3, Sas-4, Sas6, Bld10, and Dplp’s Pact domain are reduced to nearly undetectable levels (Fig S1A). As in humans, centrosome reduction in Drosophila takes place in distinct and well-defined steps of sperm differentiation[1] and the centrosome is critical for the function of the zygote after fertilization[18, 19].
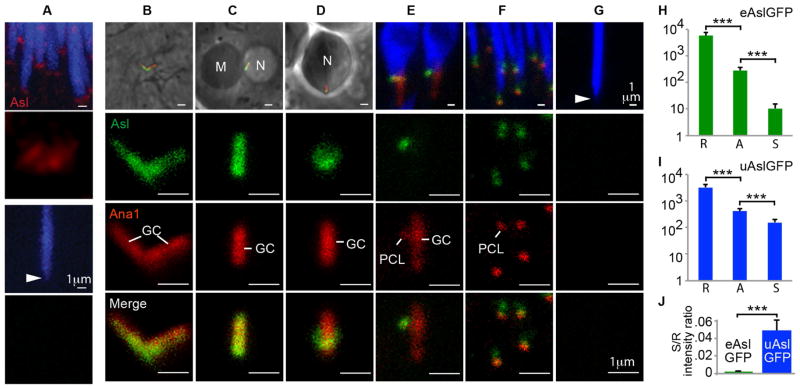
Like other centrosomal proteins, Asterless (Asl) is abundant in pre-reduced spermatid centrosomes [4, 5]; Asl levels decrease during spermiogenesis such that it is undetectable in mature sperm (spermatozoa) (Fig 1A). We named this phenomenon Asl reduction. To study Asl reduction, two GFP-tagged Asl’s were used: one expressed by the endogenous Asl promoter[4] (eAslGFP) and another strongly expressed by the exogenous ubiquitin promoter[20] (uAslGFP). Western blots showed that eAslGFP and uAslGFP, together with endogenously expressed Asl, results in a 1.6- and 2-fold greater than normal Asl content in testis (Figs S1B–C). Using eAslGFP as an indicator of Asl, before meiosis, in spermatocytes, Asl is normally found along the two very long centrioles (1.8μm long) that are named giant centrioles (GCs) (Fig 1B). After meiosis and centriole separation, in round spermatids, Asl’s presence is maintained along the GC (Fig 1C). As the spermatid differentiates, Asl shrinks gradually into a collar-like structure (Fig 1D). Later, Asl is only detectable in the second centriolar structure: the proximal centriole like (PCL) [21] (Fig 1E). In almost-needle spermatids, Asl is found in the PCL and in another location near the nucleus, presumably the GC’s proximal end (Fig 1F). Finally, in spermatozoa, eAslGFP is barely detectable (Fig 1G). Similar patterns of Asl reduction were observed with uAslGFP, yet with greater GFP-intensity, especially in almost-needle spermatids and spermatozoa (Fig. S1D). These data show that Asl is dramatically and dynamically reduced, initially from the GC and later from the PCL of spermatids.
Fig 1. Asl is reduced during spermiogenesis.
(A) An Asl antibody[4] identifies Asterless (Asl; in red) in spermatid centrioles (top two panels) but not in spermatozoan centrioles (bottom two panels; the centriole location is identified by arrowhead). Nuclei are shown in blue. (B–G) Asl reduction during spermiogenesis is a continuous process. Each top panel shows low magnification and each of the bottom three panels magnifies the centriole. The stages of spermiogenesis are: (B) spermatocyte, (C) round spermatid, (D) elongated spermatid, (E) leaf spermatid, (F) almost-needle spermatid, and (G) spermatozoon. The giant centriole (GC) and PCL are marked by Ana1-tdTomato [21], eAslGFP is shown in green, and nuclei are in blue or indicated by N; mitochondrion, M. In (G), the spermatozoan centrioles, identified by arrowhead in top panel, no longer have detectable levels of Ana1 or Asl. (H–J) Quantification of Asl reduction in Drosophila expressing eAslGFP or uAslGFP at three stages (R: round spermatid, A: almost-needle spermatid, or S: spermatozoa). Note that graphs in H and I have a logarithmic scaled (***, P<0.0001, N≥25, Mean±s.d.). See also Figure S1.
Photon counting of eAslGFP centrosomes was used to quantify Asl reduction during spermiogenesis. A~522-fold reduction in the GFP-intensity between the spermatid and spermatozoa centrioles was observed, with a spermatid / spermatozoa GFP-intensity ratio of 0.002 ± 0.001. Similarly, in uAslGFP, a ~22-fold reduction was observed (Figs H–J). Since Asl reduction occurs when Asl is expressed by the exogenous ubiquitin promoter, Asl reduction is unlikely due to transcriptional regulation. Altogether, these data suggest that the spermatid has sufficient capacity to reduce the amount of endogenous Asl and expressed eAslGFP, but it is unable to handle the excess Asl expressed as uAslGFP.
Asl Reduction is Attenuated in Asl with Domain Deletions
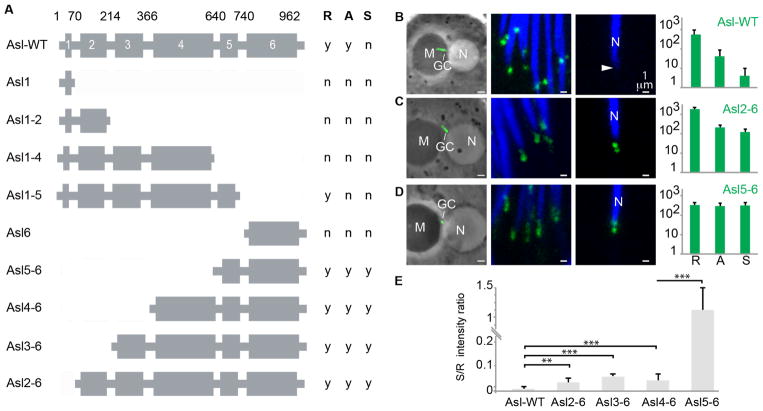
The mechanism of Asl reduction was then investigated. To determine which of Asl’s six coiled-coil domains[4]are essential for Asl reduction, transgenic flies were generated that expressed a GFP-fusion protein with a N-terminal or C-terminal deletion of Asl. Each transgene was built from Asl cDNA and expressed by the endogenous Asl promoter (Fig 2A). Fusion proteins lacking Asl’s coiled-coil domain 1 and having domains 2 to 6 (Asl2–6), 3 to 6 (Asl3–6), 4 to 6 (Asl4–6), or 5 to 6 (Asl5–6) showed GFP localized to the centrosome at all spermiogenesis stages. In centrosomes bearing the Asl2–6, Asl3–6, or Asl4–6 fusion proteins, Asl reduction is attenuated. Although there was some Asl reduction occurring early in spermiogenesis (between round and almost-needle spermatid stages), Asl reduction was greatly attenuated between the almost-needle spermatid and spermatozoa stages (Figs 2B–D; Fig S2). The Asl deletions showed variable GFP-intensity levels in the round spermatid centriole but all had lower intensity than eAslGFP, suggesting that attenuated Asl reduction is not due to Asl overexpression. Asl reduction was strongly attenuated in centrosomes bearing the Asl5–6 fusion protein, which showed similar GFP-intensity throughout spermiogenesis (Figs 2D–E). Together, Asl’s coiled-coil domains 1 and 4 appear to be critical for Asl’s reduction, with two mechanisms mediating Asl reduction: one dependent upon Asl’s domain 1, which relates to late Asl reduction, and another dependent upon Asl’s domain 4, which relates to early Asl reduction.
Fig 2. Specific coiled-coil domains in Asl are essential for normal Asl reduction.
(A) Asl coiled-coil domain organization and Asl truncations (fused to GFP) used in the present study. Coiled-coil domain numbers and their amino-acid positions are indicated. Asl truncations having GFP signal in round spermatid (R), almost-needle spermatid (A), or spermatozoa (S) are identified with “y” and those without GFP signal are identified with “n”. (B–D) Representative figures showing GFP signal for (B) a full-length AslGFP fusion having normal Asl reduction and two Asl truncation proteins,(C) an N-terminal Asl truncation lacking coiled-coil domain 1 (Asl2-6) and having abnormal Asl reduction, and (D) an N-terminal Asl truncation lacking coiled-coil domains 1 to 4 (Asl5-6) and having no Asl reduction. Shown is GFP signal in centrioles during three stages of spermiogenesis (round spermatid (left), almost-needle spermatid (middle), and spermatozoa (right)). GC, giant centriole; N, nucleus; M, mitochondria; an arrowhead points to the spermatozoan centriole. Right panels include graphs quantifying the GFP signal at each of the three stages (R: round spermatid, A: almost- needle spermatid, or S: spermatozoa). Scale bar, 5μm (round spermatids) and 1μm (almost-needle and spermatozoa). (E) The ratio of GFP-intensity in spermatozoan centrioles and spermatid centrioles shows significantly abnormal Asl reduction in the Asl truncations. Two-sided t-test, ***, P<0.001; N≥5; Mean±s.d. See also Figure S2.
Plk4 and Slimb Regulate Asl Reduction
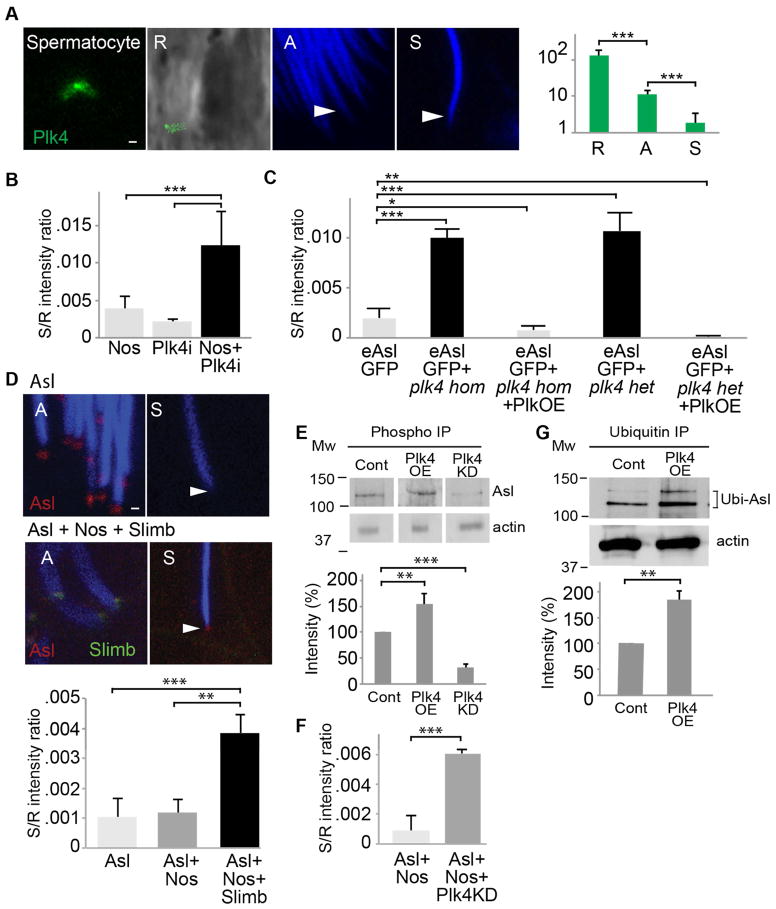
Asl’s domain 1 interact with Polo-like kinase 4 (Plk4)[6–8, 22], the master regulator of centriole formation. Like Asl, Plk4 is reduced during spermiogenesis (Fig. 3A). Plk4’s contribution to Asl reduction was then tested using a plk4 knockdown and a plk4 mutant. In late spermiogenesis, centrosomes having UAS-GAL4 knocked-down plk4 showed attenuated Asl reduction (Figs. 3B and S3A). Like the knockdown, homozygous or heterozygous partial loss of function P-element allele of plk4 (plk4c06612) [6] showed attenuated Asl reduction in late spermiogenesis (Figs.3C and S3B–D). When Asl reduction is attenuated, Asl is present in the spermatozoa as two foci that likely correspond to the PCL and a portion of the GC. This attenuation is reversed by overexpressing a GFP-tagged Plk4 (Plk4OE) driven by the ubiquitin promoter [23] or excising the P-element (Figs 3B and S3E–G), except in Asl2-6 spermatozoa, which has Asl levels that are not affected by Plk4OE (Fig. S3H). Together, these data suggest that Plk4 regulates Asl reduction via Asl domain 1. Furthermore, since Plk4OE enhances eAslGFP and uAslGFP reduction (Fig S3I–K) and Plk4 is reduced in parallel with Asl during spermiogenesis, it is likely that available amounts of Plk4 protein is a limiting factor in Asl reduction. In contrast, Asl does not appear to be essential for Plk4 reduction (Fig S3L).
Fig 3. Plk4 and Slimb regulate Asl reduction.
(A) Like Asl, Plk4 is subject to centrosome reduction during spermiogenesis as demonstrated by a Drosophila line having a ubiquitin promoted, GFP-tagged Plk4. (B) Plk4 knockdown by Nos-GAL4/UAS-Plk4 RNAi results in attenuated Asl reduction (Nos+Plk4i); normal Asl reduction is shown by data from Nos-GAL4flies (Nos) without a UAS activator and by data from UAS-Plk4 RNAi (Plk4i) flies without a GAL4 activator. (C) Similarly, spermatozoa homozygous or heterozygous forplk4c06612 (a P-element insertion, partial loss of function allele) have attenuated Asl reduction; normal Asl reduction is shown by flies bearing the eAslGFP transgene. Overexpression of Plk4 (Plk4OE) in plk4c06612 homozygotes (hom) or heterozygotes (het) rescues the plk4c06612–dependent attenuated Asl reduction. (D) Attenuation of Asl reduction is observed by overexpression of slimb using Nos-GAL4 + UAS-SlimbGFP (Asl + Nos + Slimb) in the background of Asl-td-Tomato, which labels Asl in red. Nuclei are stained in blue. Scale bar, 1μm; the spermatozoan centrioles are identified by arrowhead. In the right panel, normal Asl reduction is shown by data from Asl-td-Tomato files (Asl) or Asl-td-Tomato flies bearing Nos-GAL4 and without an UAS activator (Asl + Nos). (E) Immunoprecipitation with anti phosphothreonine antibody shows that Asl is phosphorylated. Asl phosphorylation is enhanced when Plk4 is overexpressed (Plk4OE) and is attenuated when Plk4KD is overexpressed. (F) Overexpression of Plk4KD attenuates Asl-td-Tomato reduction. (G) Immunoprecipitation with anti-Ubiquitin antibody found that Asl is Ubiquitinated. Asl ubiquitination is enhanced when Plk4 is overexpressed (Plk4OE). Two-sided t-test, ***P<0.001,**P<0.01, *P<0.05; N≥5; Mean ±s.d. See also Figure S3.
Plk4 is targeted for degradation by ubiquitin ligase SCF-Slimb/βTrCP-E3 via Slimb activity [9, 10]; therefore, we assessed whether Slimb regulates Asl reduction. For this, a GFP-tagged Slimb was overexpressed using the Nos-GAL4 driver in Asl-td-Tomato flies. Slimb overexpression attenuated Asl reduction, resulting in increased Asl levels in spermatozoa (Fig 3D), except in Asl2-6 spermatozoa, which lacks Asl’s domain 1 (Fig S3M). Furthermore, the slimb mutation counteracted the plk4c06612mutation’s impact on Asl reduction, resulting in undetectable eAslGFP levels in spermatozoa (Fig S3N). Together, these data suggest that Slimb controls Asl reduction by regulating Plk4.
To help clarify Plk4’s role in regulating centrosome reduction, we investigated Plk4-induced posttranslational modifications. Consistent with observations that Asl/Cep152 is phosphorylated by Plk4 in vitro [24], we found that Asl is phosphorylated in the testes (Figure 3E). Furthermore, Plk4 overexpression enhances Asl phosphorylation and Plk4kinase-dead (Plk4kd) overexpression attenuates Asl phosphorylation (Figure 3E). Since Plk4kd overexpression attenuated Asl-td-Tomato reduction (Figure 3F), it is likely that Plk4-induced Asl phosphorylation regulates Asl reduction. We also found that Asl is polyubiquitinated in testes and Plk4 overexpression enhanced Asl ubiquitination (Figure 3G), suggesting that ubiquitination also regulate Asl reduction.
Asl Reduction is Essential for Post-Fertilization Development
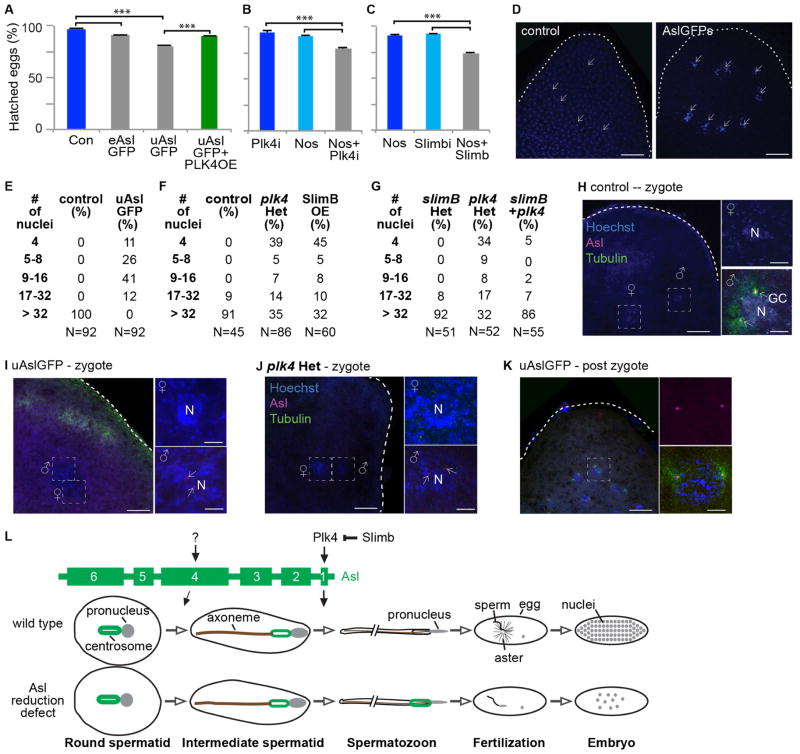
The role of Asl reduction in mature sperm function was studied using embryos fathered by males having increased Asl levels in their spermatozoa. In control experiments, 95% or more embryos hatched when fathered by flies with or without GFP-tagged sperm proteins (Fig S4A), whereas only 90% and 79% of embryos fathered by eAslGFP and uAslGFP flies hatched (Fig 4A), an inverse correlation with Asl levels in the testes (Fig S1A–B) and spermatozoa (Fig 1J). As expected from Plk4’s role in Asl reduction, Plk4 overexpression rescued uAslGFP’s embryo-hatching phenotype (Fig 4A). There was also decreased larval hatching of embryos fathered by Plk4-knockdown flies or by Slimb-overexpressing flies (Figs 4B–C). These observations suggest that increased Asl levels in spermatozoa interferes with embryonic development.
Fig 4. Attenuated Asl reduction reduces post-fertilization zygotic development.
(A–C) Embryos produced from spermatozoa with increased Asl have reduced larval hatching. (A) Compared to control flies (w1118), embryos fathered by eAslGFP or uAslGFP, which have attenuated Asl reduction, produced significantly fewer embryos that hatched. This phenomenon was rescued when Plk4 is overexpressed (PLK4OE). (B) Embryos fathered by Plk4 knockdown males (via Nos-GAL4/UAS-Plk4 RNAi; “Nos + Plk4i”) produced significantly fewer embryos that hatched when compared to control flies: Nos-GAL4 flies without an UAS activator (Nos) or UAS-Plk4 RNAi flies without a GAL4 activator (Plk4i). (C) Embryos fathered by males overexpressing Slimb, which bear Nos-GAL4/UAS-Slimb-GFP (SlimbOE) produced significantly fewer embryos that hatched when compared to control flies: Nos-GAL4 flies without a UAS activator (Nos) or UAS-Slimb-GFP flies without a GAL4 activator (Slimb). Two-sided t-test, ***P<0.001; N≥5; Mean±s.d. (D–F) One-hour-old embryos fathered by control flies (w1118) or uAslGFP flies; all embryos from control fathers and no embryo having an uAslGFP father had greater than 32 nuclei (D,E); nuclei stained with Hoechest blue. (F) Embryos fathered by Plk4 heterozygotes or fathers overexpressing Slimb had delayed embryo development compared to control males. (G) Embryos fathered by plk4 heterozygotes but not slimb or fathers having bothplk4 and slimb had delayed embryo development compared to control fathers. (H–K) Microtubule asters are observed in zygotes fathered by control males (w1118) (H), but asters are not seen in zygotes fathered by uAslGFP (I) or plk4(j)males. Microtubule asters are eventually present in embryos fathered by uAslGFP males once they have passed the zygote stage (K). Scale bar, 25μm (egg panel) and 10μm (nucleus, N, panel). (L) Model for Asl reduction. At least two mechanisms regulate Asl reduction: one in early spermatogenesis and another in late spermatogenesis. Asl reduction in late spermiogenesis is mediated by the interaction of Asl’s first coiled-coil domain with PLk4. Additional Asl reduction pathways depends on Asl’s domain 4. When centrosome reduction (including Asl reduction) is attenuated, spermatozoa can fertilize an ovum, but the embryo develops abnormally.
To help determine the mechanism underlying the embryo-hatching phenotype, one hour-old embryos were studied. As expected, all control embryos progressed beyond the 32-nuclei stage, whereas all uAslGFP-fathered embryos showed significant delays in cleavage cycle progression and embryo development, as evident by reduced numbers of nuclei and no embryo reaching the 32-nuclei stage (Figs 4D–E). Similarly, developmental delay was observed in embryos fathered by plk4c06612 heterozygous flies or by Slimb-overexpressing flies (Fig 4F). As expected from Slimb’s role in Asl reduction, the slimb00295 mutation rescued plk4c06612’s embryo-hatching phenotype (Fig 4G). These data suggest that increased paternal Asl does not interfere with fertilization and instead interferes with early steps of embryo development.
Next, functions of zygotic centrosomes in uAslGFP-fathered embryos were studied. All zygotic embryos from control fathers had robust microtubule asters surrounding their centrosomes (Fig 4H), whereas all embryos fathered by uAslGFP or plk4c0661 had undetectable or reduced microtubule asters immediately after fertilization (Fig 4I and J). At later stages, uAslGFP-fathered embryos had normal microtubule asters and bipolar spindles (Fig 4K and S4B); this explains why many of these embryos eventually developed into larvae. The fact that all uAslGFP-fathered embryos had the paternal giant centrioles indicates that the uAslGFP sperm can fertilize an ovum; therefore, the embryonic phenotypes are due to post-fertilization defects. Apparently, high amounts of paternal Asl interferes with zygotic centrosome function and embryo development. In other words, centrosome reduction (at least regarding Asl) is essential for normal embryonic development.
Discussion
Centrosome reduction is a conserved process in Drosophila and mammalian sperm [1, 18]. Elegant work in mammals by Schatten, Simerly, Manandhar, and colleagues showed that centrosome lose many of their components in a stepwise fashion and identified some proteins that are reduced [25, 26]. Additional studies provided indirect evidence for an essential role of centrosome reduction in embryo development [11–13]. The data disclosed here provide the first insight in to the molecular mechanisms regulating centrosome reduction and the first direct evidence that centrosome reduction is essential for postfertilization development.
Using several independent approaches, we show that one aspect of centrosome reduction, Asl reduction, is essential for normal zygotic aster formation, post-fertilization development, and, ultimately, embryo viability:(i) Asl overexpression, (ii) RNAi knockdown of plk4, (iii) plk4 mutants, and (iv) Slimb overexpression. Furthermore, we show that reduced fertility, caused by increased spermatozoan Asl, is specific in two ways:(i) reduced larva hatching caused by Asl overexpression, which is rescued by Plk4 overexpression, and (ii) delayed embryo development caused by plk4 mutant, which is rescued by slimb mutation. Our independent approaches, strongly argue that the described embryonic phenotypes are due to increased Asl levels rather than to other problems stemming from overexpression or mutations. These observations are consistent with indirect observations that centrosome reduction is essential for embryonic development in mammals; for example, in vitro fertilization using human, rabbit, or cat early spermatids, having unreduced centrosomes, provide reduced rates of zygotic aster formation and elevated miscarriage rates [11–13].
We found that Asl reduction is a regulated process (Fig 4J). First, Asl reduction occurs in two distinct steps: the initial step taking place early in spermiogenesis, when Asl is stripped from the GC, and the subsequent step in late spermiogenesis, when Asl is stripped from the PCL. Second, Asl reduction depends on specific domains in Asl: Asl domain1 regulates late Asl reduction and Asl domain 4 regulates early Asl reduction. Third, Asl reduction is attenuated by plk4c06612, plk4kd, and Plk4-RNAi. It is likely that Plk4 regulates Asl reduction via Asl’s domain 1, since Plk4 is known to bind to Asl’s N-terminal domain [27] and reduction of Asl lacking domain 1 is not enhanced by Plk4; data shown here indicates that Slimb regulates Asl reduction, likely via Slimb’s regulation of Plk4 [9, 10]. Finally, regulation of Asl reduction is independent of other processes occurring during spermiogenesis. As examples, conditions affecting Asl reduction do not affect nucleus differentiation or ovum fertilization. Also, a plk4 mutant that attenuates Asl reduction does not attenuate other centrosomal proteins’ reduction (Figs S3O–P). Such specificity in Asl reduction regulation argues against a common belief that centrosome reduction is simply due to loss of sperm protein production machinery [28, 29]. Since Plk4 only mediates late Asl reduction and Asl’s domain 4 appears to be essential for a complete Asl reduction block, and Plk4 is nonessential for reduction of other centrosomal proteins, there exist other complementary mechanisms that together control centrosome reduction.
The role of Plk4’s interaction with Asl/Cep152 differs between centrosome reduction and centriole duplication. In dividing cells, Asl serves as a receptor recruiting Plk4 to the centriole for centriole duplication [24, 27, 30, 31]. However, Asl’s interaction with Plk4 is complex and mediated via two distinct domains (Asl C-terminus and N-terminus), which have distinct functions during centriole duplication [22]. Here, we show that Plk4’sN-terminus regulates Asl attachment to the centriole during spermiogenesis. This regulation may be specific to spermiogenesis, since Asl/Cep152 levels are constant in centrioles of dividing cells [24, 31]. Therefore, Plk4’s interaction with Asl seems to be modulated to achieve distinct outcomes depending on a cell’s developmental state.
It remains unknown why paternal Asl reduction is essential for timely assembly of microtubules around the zygotic centriole; indeed, why must the parental Asl be lost and replaced with maternal Asl in the zygote? Asl, in itself, is clearly not harmful to the zygote since an ovum contains large amounts of maternal Asl, which is sufficient to form ~1000 centrioles [32]. The embryonic arrest we observe may be because much of the maternal Asl is unavailable after fertilization. This is consistent with the idea that the loss of centrosomal proteins inactivates the paternal centrosome until it recruits maternal centrosomal proteins in the zygote and at the appropriate time [33, 34]. Nonetheless, excess paternal Asl interferes with zygotic aster formation. This raises the possibility that paternal Asl is “toxic” to the zygote, perhaps because paternal centrosomal proteins act differently from maternal ones. Normally, such a “toxic” effect must be rapid, since we cannot detect paternal Asl in zygotic centrioles, suggesting that any residual paternal Asl is soon striped from the centriole once in the zygote (data not shown). Another possibility is that the sperm centriole, like other sperm organelles, need to be remodeled, and Asl reduction is essential to complete this remodeling. For example, during spermiogenesis, remodeling of the nucleus involves eliminating histones and replacing them with protamines [3]. Possibly, the centriole, after striping its original proteins, may require a yet unknown sperm-specific centriolar protein. Regardless of the pathological mechanism induced by increased paternal Asl, it appears that the inhibitory effect on astral microtubules formation is temporary; as the paternal centrioles regain their ability to assemble microtubules at later stages.
Together, we show that during spermiogenesis, Asl is gradually reduced from centrosomes and Plk4 and Slimb regulate its reduction. Additionally, we find that abnormally high amounts of Asl in spermatozoa are deleterious to embryonic development by interfering with the ability of the paternal centriole to form astral microtubules. This phenotype demonstrates that Asl reduction is an essential aspect of centrosome reduction and abnormalities in Asl reduction cause paternal effects.
Supplementary Material
Highlights.
Asl is a model protein for centrosome reduction during spermiogenesis
Asl reduction is an active process.
Asl reduction is regulated by Plk4 and Slimb.
Asl reduction is essential for embryo development and for fertility.
Acknowledgments
We thank Andrew Ha and Allen Church for technical assistance; Dr. Edmund Koundakjian, Sarah E. Hynek, and Emily Fishman for editing this manuscript; Dr. Richard Komuniecki and, Dr. Timothy Megraw for commenting on the manuscript; Dr. Deborah Chadee for advice. We also thank Dr. Jordan Raff for the uAslGFP, and Plk4OE flies, Dr. Daniel St Johnston for SlimbGFP, Dr. Gregory Rogers for the PlkKD plasmid, and Bloomington Stock Center for the plk4 mutants, nos-GAL4 lines and Piggybac-transposase line. This work was supported by grant 1121176 (MCB) from the National Science Foundation and R01GM098394 from the National Institute of General Medical Sciences. The authors declare no competing financial interests.
Footnotes
The authors declare no competing financial interests
AUTHOR CONTRIBUTION
T.A.R. conceived and supervised the project. T.A.R. and A.K. wrote the manuscript. A.K. performed most of the experiments described. A.A.V. performed Figures 1B–G, Figures 2B–E and related supplementary data. E.D. assisted in performing Figure 4A–C.
Methods, along with any additional Extended Data display items and Source Data, are available in the online version of the paper; references unique to these sections appear only in the online paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manandhar G, Schatten H, Sutovsky P. Centrosome reduction during gametogenesis and its significance. Biology of reproduction. 2005;72:2–13. doi: 10.1095/biolreprod.104.031245. [DOI] [PubMed] [Google Scholar]
- 2.Schatten G. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev Biol. 1994;165:299–335. doi: 10.1006/dbio.1994.1256. [DOI] [PubMed] [Google Scholar]
- 3.Fabian L, Brill JA. Drosophila spermiogenesis: Big things come from little packages. Spermatogenesis. 2012;2:197–212. doi: 10.4161/spmg.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blachon S, Gopalakrishnan J, Omori Y, Polyanovsky A, Church A, Nicastro D, Malicki J, Avidor-Reiss T. Drosophila asterless and vertebrate Cep152 Are orthologs essential for centriole duplication. Genetics. 2008;180:2081–2094. doi: 10.1534/genetics.108.095141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varmark H, Llamazares S, Rebollo E, Lange B, Reina J, Schwarz H, Gonzalez C. Asterless is a centriolar protein required for centrosome function and embryo development in Drosophila. Curr Biol. 2007;17:1735–1745. doi: 10.1016/j.cub.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol. 2005;15:2199–2207. doi: 10.1016/j.cub.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 7.Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. The Polo kinase Plk4 functions in centriole duplication. Nat Cell Biol. 2005;7:1140–1146. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- 8.Ko MA, Rosario CO, Hudson JW, Kulkarni S, Pollett A, Dennis JW, Swallow CJ. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nat Genet. 2005;37:883–888. doi: 10.1038/ng1605. [DOI] [PubMed] [Google Scholar]
- 9.Rogers GC, Rusan NM, Roberts DM, Peifer M, Rogers SL. The SCF Slimb ubiquitin ligase regulates Plk4/Sak levels to block centriole reduplication. J Cell Biol. 2009;184:225–239. doi: 10.1083/jcb.200808049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunha-Ferreira I, Rodrigues-Martins A, Bento I, Riparbelli M, Zhang W, Laue E, Callaini G, Glover DM, Bettencourt-Dias M. The SCF/Slimb ubiquitin ligase limits centrosome amplification through degradation of SAK/PLK4. Curr Biol. 2009;19:43–49. doi: 10.1016/j.cub.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 11.Borges E, Jr, Rossi-Ferragut LM, Pasqualotto FF, dos Santos DR, Rocha CC, Iaconelli A., Jr Testicular sperm results in elevated miscarriage rates compared to epididymal sperm in azoospermic patients. Sao Paulo Med J. 2002;120:122–126. doi: 10.1590/S1516-31802002000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tachibana M, Terada Y, Ogonuki N, Ugajin T, Ogura A, Murakami T, Yaegashi N, Okamura K. Functional assessment of centrosomes of spermatozoa and spermatids microinjected into rabbit oocytes. Molecular reproduction and development. 2009;76:270–277. doi: 10.1002/mrd.20951. [DOI] [PubMed] [Google Scholar]
- 13.Comizzoli P, Wildt DE, Pukazhenthi BS. Poor centrosomal function of cat testicular spermatozoa impairs embryo development in vitro after intracytoplasmic sperm injection. Biology of reproduction. 2006;75:252–260. doi: 10.1095/biolreprod.106.051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blachon S, Khire A, Avidor-Reiss T. The origin of the second centriole in the zygote of Drosophila melanogaster. Genetics. 2014;197:199–205. doi: 10.1534/genetics.113.160523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mottier-Pavie V, Megraw TL. Drosophila bld10 is a centriolar protein that regulates centriole, basal body, and motile cilium assembly. Mol Biol Cell. 2009;20:2605–2614. doi: 10.1091/mbc.E08-11-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson PG, Zheng Y, Oakley CE, Oakley BR, Borisy GG, Fuller MT. Differential expression of two gamma-tubulin isoforms during gametogenesis and development in Drosophila. Dev Biol. 1997;184:207–221. doi: 10.1006/dbio.1997.8545. [DOI] [PubMed] [Google Scholar]
- 17.Li K, Xu EY, Cecil JK, Turner FR, Megraw TL, Kaufman TC. Drosophila centrosomin protein is required for male meiosis and assembly of the flagellar axoneme. J Cell Biol. 1998;141:455–467. doi: 10.1083/jcb.141.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avidor-Reiss T, Khire A, Fishman EL, Jo KH. Atypical Centrioles During Sexual Reproduction. Frontiers in Cell and Developmental Biology. 2015;3 doi: 10.3389/fcell.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simerly C, Wu GJ, Zoran S, Ord T, Rawlins R, Jones J, Navara C, Gerrity M, Rinehart J, Binor Z, et al. The paternal inheritance of the centrosome, the cell’s microtubule-organizing center, in humans, and the implications for infertility. Nat Med. 1995;1:47–52. 18. doi: 10.1038/nm0195-47. [DOI] [PubMed] [Google Scholar]
- 20.Stevens NR, Dobbelaere J, Brunk K, Franz A, Raff JW. Drosophila Ana2 is a conserved centriole duplication factor. J Cell Biol. 2010;188:313–323. doi: 10.1083/jcb.200910016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blachon S, Cai X, Roberts KA, Yang K, Polyanovsky A, Church A, Avidor-Reiss T. A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication. Genetics. 2009;182:133–144. doi: 10.1534/genetics.109.101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klebba JE, Galletta BJ, Nye J, Plevock KM, Buster DW, Hollingsworth NA, Slep KC, Rusan NM, Rogers GC. Two Polo-like kinase 4 binding domains in Asterless perform distinct roles in regulating kinase stability. J Cell Biol. 2015;208:401–414. doi: 10.1083/jcb.201410105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basto R, Brunk K, Vinadogrova T, Peel N, Franz A, Khodjakov A, Raff JW. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133:1032–1042. doi: 10.1016/j.cell.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatch EM, Kulukian A, Holland AJ, Cleveland DW, Stearns T. Cep152 interacts with Plk4 and is required for centriole duplication. J Cell Biol. 2010;191:721–729. doi: 10.1083/jcb.201006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manandhar G, Sutovsky P, Joshi HC, Stearns T, Schatten G. Centrosome reduction during mouse spermiogenesis. Dev Biol. 1998;203:424–434. doi: 10.1006/dbio.1998.8947. [DOI] [PubMed] [Google Scholar]
- 26.Manandhar G, Schatten G. Centrosome reduction during Rhesus spermiogenesis: gamma-tubulin, centrin, and centriole degeneration. Molecular reproduction and development. 2000;56:502–511. doi: 10.1002/1098-2795(200008)56:4<502::AID-MRD8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 27.Dzhindzhev NS, Yu QD, Weiskopf K, Tzolovsky G, Cunha-Ferreira I, Riparbelli M, Rodrigues-Martins A, Bettencourt-Dias M, Callaini G, Glover DM. Asterless is a scaffold for the onset of centriole assembly. Nature. 2010;467:714–718. doi: 10.1038/nature09445. [DOI] [PubMed] [Google Scholar]
- 28.Chenoweth PJ, Lorton S. Animal Andrology: Theories and Applications, (Cabi) 2014. [Google Scholar]
- 29.Gur Y, Breitbart H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006;20:411–416. doi: 10.1101/gad.367606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SY, Park JE, Kim TS, Kim JH, Kwak MJ, Ku B, Tian L, Murugan RN, Ahn M, Komiya S, et al. Molecular basis for unidirectional scaffold switching of human Plk4 in centriole biogenesis. Nature structural & molecular biology. 2014;21:696–703. doi: 10.1038/nsmb.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cizmecioglu O, Arnold M, Bahtz R, Settele F, Ehret L, Haselmann-Weiss U, Antony C, Hoffmann I. Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome. J Cell Biol. 2010;191:731–739. doi: 10.1083/jcb.201007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigues-Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt-Dias M. Revisiting the role of the mother centriole in centriole biogenesis. Science. 2007;316:1046–1050. doi: 10.1126/science.1142950. [DOI] [PubMed] [Google Scholar]
- 33.Mikeladze-Dvali T, von Tobel L, Strnad P, Knott G, Leonhardt H, Schermelleh L, Gonczy P. Analysis of centriole elimination during C. elegans oogenesis. Development. 2012;139:1670–1679. doi: 10.1242/dev.075440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schatten H, Sun QY. New insights into the role of centrosomes in mammalian fertilization and implications for ART. Reproduction. 2011;142:793–801. doi: 10.1530/REP-11-0261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.