Abstract
Pancreatic cancer is one of the most deadly types of cancer for both genders. Classified as a human carcinogen, cadmium has been related to diverse cancers. However, the association between cadmium exposure and the risk of pancreatic cancer is still unclear. We quantitatively reviewed the observational studies on the association of cadmium exposure with pancreatic cancer risk among individuals without occupational exposure history published through July 2014 in PubMed by using a fixed–effects model. Four prospective cohort studies (112,934 participants with 335 events) and two case-control studies (177 cases and 539 controls) were identified. The summarized relative risk (RR) with a 95% confidence interval (CI) was 2.05 (95% CI=1.58 – 2.66), comparing the highest to the lowest category of cadmium exposure. This positive association persisted in men (RR=1.78; 95% CI=1.04 – 3.05), but not in women (RR=1.02; 95% CI=0.63 – 1.65). Further research is needed to provide more solid evidence on the association of cadmium exposure with pancreatic cancer risk and to elucidate the underlying biological mechanism of the potential gender difference.
Keywords: Cadmium, Pancreatic cancer, Meta-analysis, Trace elements, General population, Cancer
Introduction
Pancreatic cancer is one of the most deadly cancers in the U.S. (American Cancer Society 2013) and worldwide (Hariharan et al. 2008). No specific symptoms appear until an advanced and incurable stage of pancreatic cancer, which contributes to its high mortality. Pancreatic cancer patients are likely to die within 6 months after diagnosis and the 5-year survival rate is less than 5% (American Cancer Society 2013). In the past decade, pancreatic cancer mortality gradually increased among U.S. men and women, while the death rates of other major cancers decreased mildly (American Cancer Society 2013).
Discovered in 1817 and classified as a human carcinogen in 1993 by the International Agency for Research on Cancer (Boffetta 1993), cadmium is currently one of the most extensive occupational and environmental pollutants. Working in cadmium-emitting industries and nonferrous metal mines significantly increases the level of cadmium exposure by inhaling dust and fumes, and by incidentally ingesting dust from contaminated hands, cigarettes or foods (U.S. Department of Health and Human Services 2011). Meanwhile, diet and tobacco smoking are the major sources of cadmium exposure in the “general” population (i.e. population without occupational exposure history) because of the ubiquitous cadmium pollution in air, soil and water released from industries to the atmosphere (U.S. Department of Health and Human Services 2011). Epidemiological studies have confirmed its toxic effects on lung (IARC 1993), kidney (Pollack et al. 2015) and bone functions (Thomas et al. 2011). Additionally, numerous studies found positive associations between cadmium exposure and breast cancer (Julin et al. 2012a; Julin et al. 2012b; McElroy et al. 2006; Nagata et al. 2013; Romanowicz-Makowska et al. 2011; Strumylaite et al. 2011), prostate cancer (Elghany et al. 1990; Julin et al. 2012b; Sharma-Wagner et al. 2000; Vinceti et al. 2007), lung cancer (Park et al. 2012; Sorahan and Lancashire 1997; Stayner et al. 1992; Zhang et al. 2012), and all cancer mortality (Adams et al. 2012; Elinder et al. 1985; Lin et al. 2013). However, little evidence has been revealed to link cadmium to pancreatic cancer.
The hypothesis that cadmium may be a risk factor of pancreatic cancer was first proposed in 2000 in a meta-analysis of three studies including individuals with occupational exposure (Schwartz and Reis 2000) and has been investigated in several occupational studies among workers in cadmium-emitting industries and smelters (Binks et al. 2005; Elinder et al. 1985; Jarup et al. 1998; Marsh et al. 2009; Sorahan et al. 1995). However, the patterns of pancreatic cancer mortality by cadmium exposure in the general population, in which a great majority do not have a job that substantially increases the possibility of cadmium exposure, have not been examined extensively. The association between cadmium exposure and the risk of pancreatic cancer in a general population with both genders and a relatively low dose of exposure (Chaumont et al. 2011) remains unclear. Thus, we conducted this study to quantitatively assess the overall association between cadmium exposure and the risk of pancreatic cancer in the general population by weighing evidence from observational studies.
Methods
Study selection
The meta-analysis was performed based on the PRISMA 2009 Checklist (Moher et al. 2009). The relevant observational studies published in English-language journals through July 2014, which investigated the association between cadmium exposure and the risk of pancreatic cancer, were identified by searching the PubMed database using the expression “((“pancreatic neoplasms”[MeSH Terms] OR (“pancreatic”[All Fields] AND “neoplasms”[All Fields]) OR “pancreatic neoplasms”[All Fields] OR (“pancreatic”[All Fields] AND “cancer”[All Fields]) OR “pancreatic cancer”[All Fields]) OR (“neoplasms”[MeSH Terms] OR “neoplasms”[All Fields] OR “cancer”[All Fields]) OR (“mortality”[Subheading] OR “mortality”[All Fields] OR “mortality”[MeSH Terms])) AND (“cadmium”[MeSH Terms] OR “cadmium”[All Fields])”. Additional information was retrieved by searching Google Scholar and the reference lists of the relevant articles. We did not search for randomized controlled trials because of the toxic nature of cadmium.
All relevant articles were independently reviewed by two of our coauthors (C.C. and P.X.). Disagreements were resolved by group discussion. The inclusion criteria included that 1) the study had either a cohort, case-control, or cross-sectional design; 2) the study reported hazards ratio (HR), relative risk (RR), or odds ratio (OR) with corresponding 95% confidence intervals (CIs) of pancreatic cancer relating to cadmium exposure or such information can be recalculated from the published results; and 3) the study was conducted in general population exposed non-occupationally to cadmium, in which the great majority of participants did not report occupational history related to cadmium exposure. We also included one study that provided us de novo results which were not previously reported (Li et al. 2011).
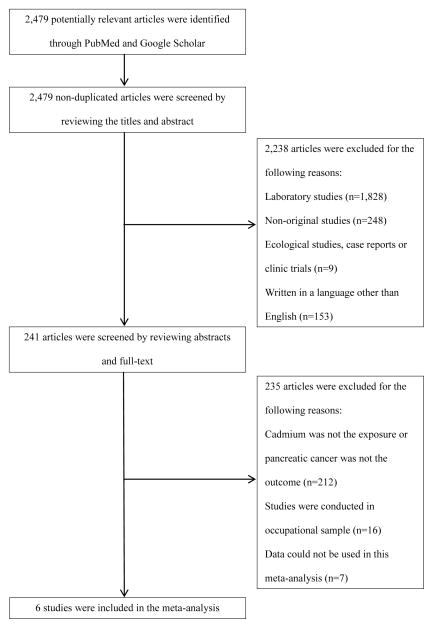
The detailed searching history is shown in Fig. 1. Of the 2,479 non-duplicated abstracts from PubMed and Google Scholar, 2,238 publications were excluded due to one of the following reasons: 1) laboratory studies (n=1,828); 2) non-original studies (reviews or letters to editor) (n=248); 3) ecological studies, case reports or clinic trials (n=9); or 4) not published in English (n=153). In addition, 235 articles were further excluded because 1) the exposure was not cadmium or the outcome was not pancreatic cancer (n=212); 2) studies were conducted in an occupational sample, but not the general population (n=16); or 3) the reported results were not able to be summarized and an attempt to request de novo results was not successful (n=7). In summary, six studies (4 prospective cohorts and 2 case-controls) met the criteria and were included in this meta-analysis.
Fig. 1.
Process of study selection
Quality assessment
Candidate studies were checked for methodological quality using a checklist (Margetts et al. 2007; Zaza et al. 2000) as follows: 1) “Did the study have a clear study aim with a clear hypothesis?”; 2) “Was the sample recruitment process clear (participant selection, sampling frame, and response rate)?”; 3) “Were the exposure and outcome measures valid and reliable?”; 4) “Did the authors conduct an appropriate analysis by conducting statistical testing, controlling for repeated measures, etc.?”; and 5) “Were the conclusions justified and appropriate to the results as presented?”. Each question was assigned one point and studies having ≥4 points were considered high quality. Among six included studies, five (Adams et al. 2012; Amaral et al. 2012; García-Esquinas et al. 2014; Luckett et al. 2012; Sawada et al. 2012) achieved full points and one study (Li et al. 2011) had four points.
Data extraction
For cohort studies, we collected data on the author’s name, year of publication, study name, country of origin, number of participants and events, age of participants (range or mean), proportion of men, follow-up year (mean), exposure assessment method, categories of cadmium exposure, outcome confirmation method, risk estimates for each exposure category versus the lowest one and the adjusted covariates in the final model. For case-control studies, the number of both cases and controls and the case confirmation method were collected instead of the number of both participants and events and the outcome confirmation method, while other data categories remained unchanged. HR, RR, or OR of pancreatic cancer for the highest versus the lowest exposure category were derived from primary studies and transformed to their natural logarithms (ln). Their corresponding 95% CI was used to calculate the standard error. Two of the coauthors (C.C. and P.X.) independently assessed each study and extracted data from included studies. Discrepancies were resolved by group discussion.
Statistical analysis
The summarized RR and OR were used as the measurement of the combined association between cadmium exposure and pancreatic cancer risk. HR was considered as RR. We estimated the summarized RRs and ORs and their corresponding 95% CIs by comparing the highest with the lowest category of cadmium exposure using a fixed-effects model. We also used the random-effects model to test the robustness of the findings. In addition, we performed sensitivity analyses among cohort studies to detect the influence of any single study on the overall estimate. Heterogeneity among studies was evaluated by calculating the I2 statistic along with Cochran’s test. Moreover, publication bias was assessed by using Egger’s regression asymmetry test (when the numbers of studies pooled was ≥3) or Begger’s rank correlation test (when the numbers of studies pooled was <3). A two-sided P value ≤0.05 was considered statistically significant. All analyses were performed by using STATA statistical software (Version 13.0, STATA Corporation LP, College Station, TX).
Results
Study characteristics
Six studies (Adams et al. 2012; Amaral et al. 2012; García-Esquinas et al. 2014; Li et al. 2011; Luckett et al. 2012; Sawada et al. 2012), including four independent prospective cohort studies and two independent case-control studies, were identified in this meta-analysis. All the studies had high methodological quality. The characteristics of the six studies are shown in Tables 1 and 2. The four prospective cohort studies contributed to a dataset comprised of 112,934 participants and 335 events with a mean follow-up of 15.5 years (Table 1). The two case-control studies included 177 cases and 539 controls (Table 2).
Table 1.
Characteristics of the 4 cohort studies included in the meta-analysis
| Authors (year), study |
Region | No. of participants (events) |
Agea, y |
Men, % |
Follow-upb, y |
Exposure assessment |
Exposure categories |
Outcome confirmation |
Risk estimates |
Adjusted covariates |
|---|---|---|---|---|---|---|---|---|---|---|
| García-Esquina (2014), SHS | U.S. | 3,792 (24) | 45–75 | 40.6 | 17.2 | Urinary Cd by inductively coupled plasma mass spectrometry (Agilent 7700x ICPMS; Agilent Technologies, Waldbronn Germany) | uCd (tertiles, μg/g CR): ≤0.70; 0.71–1.22; ≥1.23. |
Death certificates and autopsy records ICD-9 (code157) |
HR (95%CI): 1.00 (referent); --; 2.47 (1.01, 6.03). 80th vs 20th 2.40 (1.39, 4.17). |
Gender, age, smoking status, pack-years of smoking, BMI |
| Adams (2012), NHANES III | U.S. | 15,673 (37) | ≥17 | 47.6 | Men:13.4 Women:13.8 |
Urinary Cd by Perkin-Elmer Model 3030 atomic absorption spectrometry with Zeeman background correction Urinary CR by the Jaffe method with an ASTRA analyzer |
uCd (quartiles, μg/g CR): Men: ≤0.153; 0.154–0.297; 0.298–0.580; >0.580. Women: ≤0.210; 0.211–0.418; 0.419–0.819; >0.819. |
Death certificates and the National Death Index ICD-9 to ICD-10 |
HR (95%CI): Men: 4th quartile vs 1–3rd quartiles 7.25 (1.77, 29.80). Women: 4th quartile vs 1–3rd quartiles 1.24 (0.58, 2.63). |
Age, smoking history, BMI, education, race |
| Sawada (2012), JPHCBPS | Japan | 90,383 (236) | 45–74 | 46.5 | 9.2 | FFQ | Intake of cadmium by tertile (median intake, μg/day): Men: Low:19.7; Middle:26.7; High:35.4. Women: Low:19.2; Middle:24.9; High: 32.3. |
Hospital reports (64%), registry reports (26%), death certificates (10%) and responses to questionnaire and others (0.2%) | HR (95%CI): Men: 1.00 (referent); 1.36 (0.79, 2.34); 1.25 (0.66, 2.36). Women: 1.00 (referent); 1.12 (0.62, 2.02); 1.14 (0.56, 2.30). |
Age, area, BMI, smoking status, frequency of alcohol intake, leisure-time physical activity, intake of meat, soybean, vegetable and fruit (for men), additional covariates: menopausal status, and use of exogenous female hormones (for women) |
| Li (2011)c | Japan | 3,086 (38) | ≥50 | 45.0 | 22 | Urinary Cd by atomic absorption spectrometry Urinary CR by the Jaffe method | uCd (quartiles, μg/g CR): <3.0; 3.0–4.9; 5.0–9.9; ≥10.0. |
Death certificates, ICD-9 | RR (95%CI): Men: 1.00 (referent); 1.14 (0.31, 4.24); 0.87 (0.22, 3.46); 2.51 (0.62, 10.14). Women: 1.00 (referent); 0.32 (0.07, 1.42); 0.33 (0.10, 1.10); 0.41 (0.12, 1.46). |
Age, smoking |
Abbreviations: BMI=body mass index, ICD=International Classification of Diseases, -- = not applicable, CI=confidence interval, HR=hazard ratio, RR=relative risk, SHS=The Strong Heart Study, NHANES III=The Third National Health and Nutrition Examination Survey, JPHCBPS=The Japan Public Health Center-Based Prospective Study
The mean or range of age was reported
The mean or median years of follow-up were reported
The authors provided de novo results, which were not reported in their primary article, to this meta-analysis
Table 2.
Characteristics of the 2 case-control studies included in the meta-analysis
| Authors (year), study | Region | Cases, n | Controls, n | Age, y | Men, % | Exposure assessment | Exposure categories | Case confirmation | Risk estimates | Adjusted covariates |
|---|---|---|---|---|---|---|---|---|---|---|
| Amaral (2012), PANKRAS II & SBCES | Spain | 114 | 398b | Casea: 66.1 ±10.3 Controla: 63.8±12.5 |
81.4 | Toenail Cd by inductively coupled plasma mass spectrometry | Toenail Cd (quartiles, μg/g ): ≤0.0080; 0.0081–0.0134; 0.0135–0.0291; >0.0291. |
Hospital confirmation, reviewed by surgeons and gastroenterologists | OR (95%CI): 1.00 (referent); 0.87 (0.37.2.03); 2.04 (1.00, 4.17); 3.58 (1.86, 6.88). |
Age, gender, region, smoking status |
| Luckett (2012) | U.S. | 63 | 141c | >20 | 49.8 | Urinary Cd by Perkin Elmer atomic absorption spectrometry with a graphite furnace Urinary CR by a Beckman-Coul ter DXC 600 Pro | uCd (quartiles, μg/g CR): <0.5; 0.5-<1.0; 1.0-<1.5; ≥1.5. |
Registry records from Louisiana Tumor Registry and records of south Louisiana hospitals | OR (95%CI): 1.00 (referent); 3.34 (1.38, 8.07). 5.58 (2.03, 15.34). 7.70 (3.06, 19.34). |
Age, race, gender, pack years of smoking, current smoking status, alcoholic drinks per day, family history of pancreatic cancer, completed high school education |
Abbreviations: CI=confidence interval, OR=odds ratio, PANKRAS II & SBCES=PANKRAS II Study & The Spanish Bladder Cancer/EPICURO Study
The mean ± SD or range of age was reported
The control was hospital-based
The control was population-based
Of the four cohort studies, two (Adams et al. 2012; García-Esquinas et al. 2014) were conducted in the U.S. and the other two (Li et al. 2011; Sawada et al. 2012) were in Japan. The number of participants ranged from 3,086 (Li et al. 2011) to 90,383 (Sawada et al. 2012). All studies included both genders. The proportion of male participants did not differ considerably across studies. The mean follow-up year across studies ranged from 9.2 (Sawada et al. 2012) to 22 years (Li et al. 2011). Three out of the four cohort studies (Adams et al. 2012; García-Esquinas et al. 2014; Li et al. 2011) reported the adjusted HRs of pancreatic cancer mortality by tertiles or quartiles of urinary cadmium concentrations. The other one (Sawada et al. 2012) presented the adjusted HRs of pancreatic cancer incidence by tertiles of dietary cadmium intake.
Of the two case-control studies, one was conducted in the U.S. (Luckett et al. 2012) and the other was from Spain (Amaral et al. 2012). The numbers of cases/controls were 114/398 and 63/141, respectively. Both genders were included in the two studies, but the proportion of male participants was substantially different. In addition, one measured cadmium in toenail (Amaral et al. 2012) and the other measured cadmium in urine (Luckett et al. 2012). Both of them reported multivariate-adjusted OR and the 95% CIs.
Meta-analysis
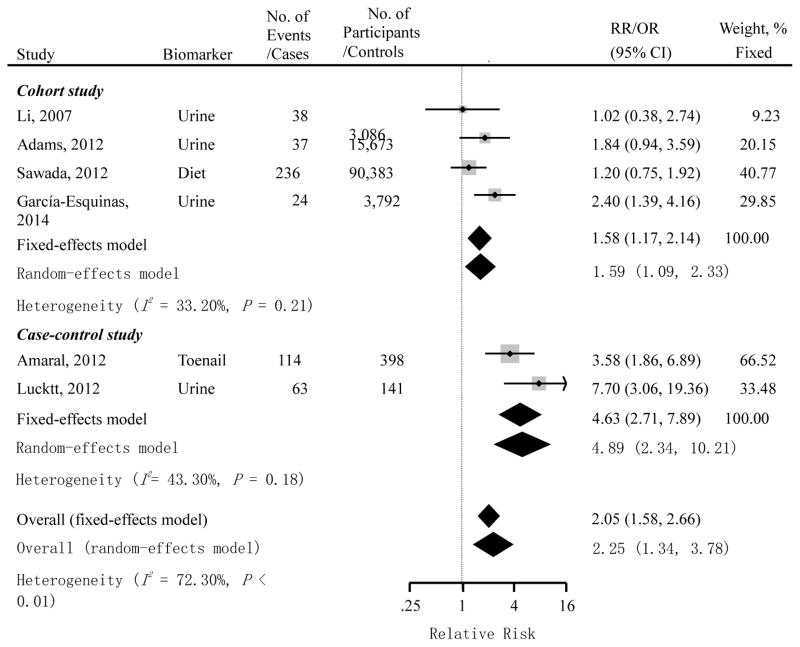
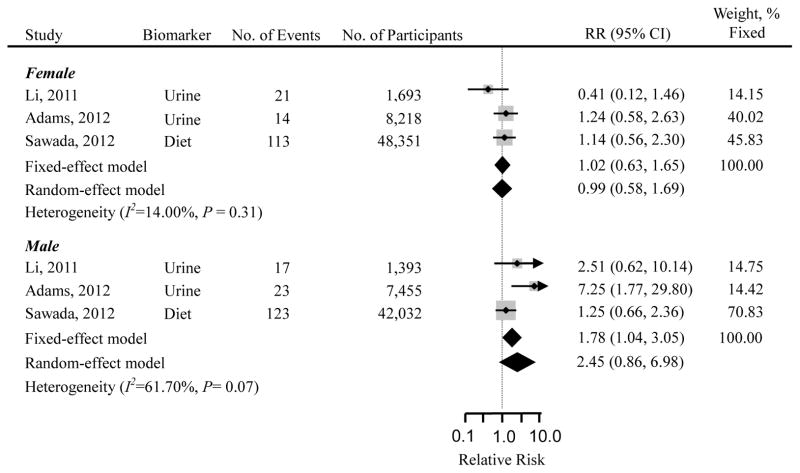
The summarized RR of cohort studies suggested a positive association between cadmium exposure and the risk of pancreatic cancer (RR=1.58; 95% CI=1.17 – 2.14, Fig. 2). The results did not considerably change when any study was excluded. Also, the conclusion remained when the random-effects model was used. There was no significant heterogeneity among studies (I2=33.20%, P=0.21) and Egger’s test showed no evidence of publication bias (P=0.89). Subgroup analysis was conducted among three cohort studies that reported gender-specific results (Adams et al. 2012; Li et al. 2011; Sawada et al. 2012) (Fig. 3). The observed positive association persisted in males (RR=1.78; 95% CI=1.04 – 3.05), but not in females (RR=1.02; 95% CI=0.63 – 1.65). However, when the random-effects model was used, the positive association found in males was attenuated and became statistically non-significant (RR=2.45; 95% CI=0.86 – 6.98), presumably due to reduced statistical power.
Fig. 2.
Multivariable adjusted relative risks (RRs) or odds ratios (ORs) and 95% confidence intervals (CIs) of pancreatic cancer risk by cadmium exposure from 4 prospective cohort studies and 2 case-control studies. The pooled estimates were obtained using fixed- and random-effects models. The dots indicate the adjusted RRs and ORs by comparing the highest to the lowest level of cadmium exposure. The size of the shaded square is proportional to the percent weight of each study using a fixed-effects model. The horizontal lines represent 95% CIs. The diamond data markers indicate the pooled RRs and ORs.
Fig. 3.
Gender-specific multivariable adjusted relative risks (RRs) and 95% confidence intervals (CIs) of pancreatic cancer risk by cadmium exposure from 3 prospective cohort studies. The pooled estimates were obtained using fixed- and random-effects models. The dots indicate the adjusted RRs by comparing the highest to the lowest level of cadmium exposure. The size of the shaded square is proportional to the percent weight of each study in a fixed-effects model. The horizontal lines represent 95% CIs. The diamond data markers indicate the pooled RRs.
Similarly, the pooled results of case-control studies suggested a significant association between cadmium exposure and the increased risk of pancreatic cancer (OR=4.63; 95% CI= 2.71 – 7.89, Fig. 2). The results did not appreciably change when using the random-effects model. Begger’s test showed no evidence on publication bias (P=1.00).
Because the rate of pancreatic cancer is very low (Siegel et al. 2014), OR can be used as an approximation of RR (Rigby 1999). Thus, we combined cohort and case-control studies to estimate the overall association. The summarized RR suggested a significant positive association between cadmium exposure and pancreatic cancer risk (RR=2.05; 95% CI=1.58 – 2.66, Fig. 2). This positive association remained when using the random-effects model. Also, the results did not change substantially when excluding any study in the summarized analysis.
Discussion
In this meta-analysis of prospective cohort and case-control studies based on available literature, we found that cadmium exposure was significantly associated with the increased risk of pancreatic cancer among individuals without occupational cadmium exposure. This positive association persisted in men, but not in women.
Our findings are consistent with the results from an early meta-analysis comprised of three occupational cohort studies (Schwartz and Reis 2000), but not in agreement with another meta-analysis combining one cohort study with one study reporting SMR/SIR in an occupationally exposed population (Ojajärvi et al. 2000). Of note, findings of studies conducted in industrial environments and assessing exposure using job history may or may not be parallel to results from the general population, because the levels of cadmium exposure, the male gender proportion, and the exposure assessment method may be substantially different. Studies of occupational cohorts have observed exposure-response curves with an increasing slope at low exposure levels. While the exposure level increased, the positive slope was attenuated or even turned negative (Stayner et al. 2003). Possible explanations include depletion of susceptible persons at high exposure levels and measurement error or misclassification of exposure (Stayner et al. 2003). Individuals in the two meta-analyses with occupational exposure are mostly males initially selected to be healthy enough to work, in comparison to participants from the general population comprised of both males and females, both apparently healthy and unhealthy persons. Additionally, exposure to more than one metal at a time makes it difficult to assess the hazard contributed by cadmium alone (Park et al. 2012). Results unadjusted for exposure to other substances in the primary studies included in the two meta-analyses might be biased.
Some potential mechanisms of cadmium carcinogenicity have been suggested. First, the replacement of zinc with cadmium may be a critical mechanism underlying the carcinogenicity of cadmium (Schwartz and Reis 2000). The pancreas contains high levels of zinc. Zinc is essential for DNA, RNA, and protein synthesis and thus for cell division (Prasad 1983). Cadmium may have influence on several biological systems by replacing zinc because they belong to the same column in the periodic table and share many physical and chemical properties (Schwartz and Reis 2000). Second, cadmium is known as one of the most potent agents to cause transdifferentiation of the pancreas (Waalkes et al. 1992). Cadmium may put cells at a higher risk of neoplasia since transdifferentiation involves cellular dedifferentiation, proliferation, and redifferentiation (Yuan et al. 1996). In addition to its direct effects on pancreatic cells, cadmium may influence carcinogenesis indirectly by inducing specific genes or by acting as a toxin and thereby disrupting cellular function (Koropatnick and Zalups 1997). Third, cadmium can induce or regulate the activation of several oncogenic proteins and tumor suppressor proteins that are overexpressed in human pancreatic cancers, such as ras proteins and the p53 protein (Ruggeri et al. 1992).
Our results showed that the risk of pancreatic cancer related to cadmium exposure was not statistically significant in women, while it was in men. The cause of this gender difference that was also reported in some primary studies was unclear (Rossouw 2002; Vahter et al. 2007). One possible explanation is that female steroid hormones are hypothesized to play a protective role in the development of pancreatic cancer (Lee et al. 2013), but menopausal status and the use of exogenous female hormones were not adjusted as potential confounders in most cohort studies (three out of four) (Adams et al. 2012; García-Esquinas et al. 2014; Li et al. 2011). This limitation may attenuate any possible association between cadmium exposure and the risk of pancreatic cancer in women. Additionally, women tend to have a higher consumption of fruits and vegetables (Blanck et al. 2008) which is a potential protective factor of pancreatic cancer due to increased antioxidant and dietary fiber intake (Pericleous et al. 2014). However, dietary intake was not adjusted in the primary studies, which may also partially explain the null association in women.
Relatively high heterogeneities (I2 =72.30%, P<0.01; I2 = 61.70%, P=0.07, respectively) were observed when combining cohort and case-control studies and in male subgroup analysis among cohort studies, which may be explained by the usage of different exposure assessments, i.e., dietary (1 study), urinary (4 studies) or toenail cadmium (1 study). The study measuring dietary cadmium had the largest number of participants and events, so might increase the heterogeneity. In addition, combining different types of studies might contribute to the high heterogeneity. Nevertheless, the pooled association remained significant in both fixed- and random-effects models when we excluded any one study.
Our study has several strengths. First, all included studies had high methodological quality, and the cohort studies had large sample sizes and long-term follow up periods. Second, our meta-analysis is the first one that examines the association between cadmium exposure and the risk of pancreatic cancer among individuals without occupational cadmium exposure history. Previous meta-analysis or systematic review only focused on occupational studies that investigated the mortality pattern of pancreatic cancer in cadmium-emitting industries (Ojajärvi et al. 2000; Schwartz and Reis 2000). Industrial workers are nearly 100% males who are concurrently exposed to multiple carcinogenic substances at high dose (Schwartz and Reis 2000), thus the conclusions from occupational studies may not be generalized to a general population that is comprised of both genders and has a relatively low intensity of cadmium exposure (Chaumont et al. 2011). Unfortunately, the general population has not received sufficient attention from health care as compared to occupational individuals, though pancreatic cancer incidence in the general population has increased in the past decade.
Our meta-analysis also has some limitations. First, the number of studies included in the meta-analysis was limited partially due to the low incidence rate of pancreatic cancer. In addition, cohort studies large enough to have appreciable numbers of pancreatic cancer cases might have not collected or analyzed biological specimens for cadmium. However, our study has combined the most comprehensive and updated findings in the literature. Second, the exposure assessments differ across studies. Four studies measured cadmium in urine, while one assessed dietary cadmium intake, and the other one quantified toenail cadmium levels. However, the cadmium levels from urine and toenails as well as diet should have a similar pattern and will enable us to compute the relative risk of pancreatic cancer by ranking the participants based on cadmium exposure levels. Third, the urinary cadmium level in the Li et al study (Li et al. 2011) was higher than that in others, but the conclusion did not change after we excluded the study. Forth, although all primary studies in this meta-analysis investigated the association of interest in noninstitutionalized general populations exposed non-occupationally to cadmium and studies of explicitly occupation-related cadmium exposure were excluded, the possibility of including a few or a small group of individuals who had occupational exposure cannot be completely excluded. Fifth, the possibility of residual confounding cannot be ruled out as in other observational studies. Sixth, a potential publication bias resulting from the exclusion of articles published in a language other than English and unpublished results that found a null association due to lack of power with low incidence of pancreatic cancer was possible, even though Egger’s regression asymmetry test (P=0.88) and Begger’s rank correlation test (P=1.00) did not suggest a publication bias. In addition, we reviewed all the English abstracts of the excluded articles written in other languages in the PubMed database. None of them met the inclusion criteria.
Cigarette smoking is considered one of the main sources of cadmium exposure in smokers. The risk of developing pancreatic cancer is approximately doubled among smokers as compared with non-smokers (American Cancer Society). Although smoking status was adjusted as a potential confounder in most included studies, none of these studies reported results based on smoking status. Future studies are warranted to investigate the modification effect of smoking.
In summary, our meta-analysis of prospective cohort and case-control studies indicates that cadmium exposure is significantly associated with an elevated risk of pancreatic cancer among individuals without occupational cadmium exposure history, particularly in men. Further research is needed to provide more solid evidence on the association of cadmium exposure with the risk of pancreatic cancer and to develop prevention and treatment programs aiming to prolong the survival of pancreatic cancer patients.
Supplementary Material
Acknowledgments
Source of funding Drs. Xun and He were partially supported by grants from the NIH (R01HL081572, R01ES021735 and R03CA139261). The funding sources had no involvement in the study design; the collection, analysis and interpretation of data; the writing of the report; and the decision to submit the article for publication.
Footnotes
Conflict of interest None declared.
Checklist S1 PRISMA 2009 Checklist. (DOC)
Contributor Information
Cheng Chen, Department of Epidemiology and Biostatistics, School of Public Health -- Bloomington, Indiana University, Bloomington, IN 47405, USA.
Pengcheng Xun, Department of Epidemiology and Biostatistics, School of Public Health -- Bloomington, Indiana University, Bloomington, IN 47405, USA.
Muneko Nishijo, Department of Epidemiology and Public Health, Kanazawa Medical University, Ishikawa, 920–0293, Japan.
Akira Sekikawa, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA 15261, USA.
Ka He, Email: kahe@indiana.edu, Department of Epidemiology and Biostatistics, School of Public Health -- Bloomington, Indiana University, Bloomington, IN 47405, USA.
References
- American Cancer Society. Cancer facts & figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- Adams SV, Passarelli MN, Newcomb PA. Cadmium exposure and cancer mortality in the Third National Health and Nutrition Examination Survey cohort. Occup Environ Med. 2012;69:153–156. doi: 10.1136/oemed-2011-100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral AF, Porta M, Silverman DT, Milne RL, Kogevinas M, Rothman N, Cantor KP, Jackson BP, Pumarega JA, López T. Pancreatic cancer risk and levels of trace elements. Gut. 2012;61:1583–1588. doi: 10.1136/gutjnl-2011-301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binks K, Doll R, Gillies M, Holroyd C, Jones SR, McGeoghegan D, Scott L, Wakeford R, Walker P. Mortality experience of male workers at a UK tin smelter. Occup Med (Lond) 2005;55:215–226. doi: 10.1093/occmed/kqi026. [DOI] [PubMed] [Google Scholar]
- Boffetta P. Carcinogenicity of trace elements with reference to evaluations made by the International Agency for Research on Cancer. Scand J Work Environ Health. 1993;19:67–70. [PubMed] [Google Scholar]
- International Agency for Research on Cancer, World Health Organization, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. 1993. p. 58. [PMC free article] [PubMed] [Google Scholar]
- Chaumont A, De Winter F, Dumont X, Haufroid V, Bernard A. The threshold level of urinary cadmium associated with increased urinary excretion of retinol-binding protein and beta 2-microglobulin: a re-assessment in a large cohort of nickel-cadmium battery workers. Occup Environ Med. 2011;68:257–264. doi: 10.1136/oem.2009.054122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elghany NA, Schumacher MC, Slattery ML, West DW, Lee JS. Occupation, cadmium exposure, and prostate cancer. Epidemiology. 1990;1:107–115. doi: 10.1097/00001648-199003000-00005. [DOI] [PubMed] [Google Scholar]
- Elinder C, Kjellström T, Hogstedt C, Andersson K, Spång G. Cancer mortality of cadmium workers. Br J Ind Med. 1985;42:651–655. doi: 10.1136/oem.42.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Esquinas E, Pollan M, Tellez-Plaza M, Francesconi KA, Goessler W, Guallar E, Umans JG, Yeh J, Best LG, Navas-Acien A. Cadmium exposure and cancer mortality in a prospective cohort: the Strong Heart Study. Environ Health Perspect. 2014;122:363. doi: 10.1289/ehp.1306587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB: The Official Journal of the International Hepato Pacreato Biliary Association. 2008;10:58–62. doi: 10.1080/13651820701883148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarup L, Bellander T, Hogstedt C, Spang G. Mortality and cancer incidence in Swedish battery workers exposed to cadmium and nickel. Occup Environ Med. 1998;55:755–759. doi: 10.1136/oem.55.11.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julin B, Wolk A, Bergkvist L, Bottai M, Åkesson A. Dietary cadmium exposure and risk of postmenopausal breast cancer: a population-based prospective cohort study. Cancer Res. 2012a;72:1459–1466. doi: 10.1158/0008-5472.CAN-11-0735. [DOI] [PubMed] [Google Scholar]
- Julin B, Wolk A, Johansson J-E, Andersson S-O, Andrén O, Åkesson A. Dietary cadmium exposure and prostate cancer incidence: a population-based prospective cohort study. Br J Cancer. 2012b;107:895–900. doi: 10.1038/bjc.2012.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatnick J, Zalups R. Effect of non-toxic mercury, zinc or cadmium pretreatment on the capacity of human monocytes to undergo lipopolysaccharide-induced activation. Br J Pharmacol. 1997;120:797–806. doi: 10.1038/sj.bjp.0700975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Horn-Ross PL, Rull RP, Neuhausen SL, Anton-Culver H, Ursin G, Henderson KD, Bernstein L. Reproductive factors, exogenous hormones, and pancreatic cancer risk in the CTS. Am J Epidemiol. 2013;178:1403–1413. doi: 10.1093/aje/kwt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Nishijo M, Nakagawa H, Morikawa Y, Sakurai M, Nakamura K, Kido T, Nogawa K, Min D. Relationship between urinary cadmium and mortality in habitants of a cadmium-polluted area: a 22-year follow-up study in Japan. Chin Med J. 2011;124:3504–3509. [PubMed] [Google Scholar]
- Lin Y-S, Caffrey JL, Lin J-W, Bayliss D, Faramawi MF, Bateson TF, Sonawane B. Increased risk of cancer mortality associated with cadmium exposures in older americans with low zinc intake. Journal of Toxicology and Environmental Health, Part A. 2013;76:1–15. doi: 10.1080/15287394.2012.722185. [DOI] [PubMed] [Google Scholar]
- Luckett BG, Su LJ, Rood JC, Fontham ET. Cadmium exposure and pancreatic cancer in south Louisiana. J Environ Public Health. 2012;2012:180186. doi: 10.1155/2012/180186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margetts B, Vorster H, Venter C. Evidence-based nutrition-review of nutritional epidemiological studies. South Afr J Clin Nutr 2007 [Google Scholar]
- Marsh GM, Esmen NA, Buchanich JM, Youk AO. Mortality patterns among workers exposed to arsenic, cadmium, and other substances in a copper smelter. Am J Ind Med. 2009;52:633–644. doi: 10.1002/ajim.20714. [DOI] [PubMed] [Google Scholar]
- McElroy JA, Shafer MM, Trentham-Dietz A, Hampton JM, Newcomb PA. Cadmium exposure and breast cancer risk. J Natl Cancer Inst. 2006;98:869–873. doi: 10.1093/jnci/djj233. [DOI] [PubMed] [Google Scholar]
- Blanck HM, Gillespie C, Kimmons JE, Seymour JD, Serdula MK. Trends in Fruit and Vegetable Consumption Among US Men and Women, 1994–2005. Prev Chronic Dis. 2008;5(2):A35. [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Nagata C, Nagao Y, Nakamura K, Wada K, Tamai Y, Tsuji M, Yamamoto S, Kashiki Y. Cadmium exposure and the risk of breast cancer in Japanese women. Breast Cancer Res Treat. 2013;138:235–239. doi: 10.1007/s10549-013-2414-4. [DOI] [PubMed] [Google Scholar]
- Ojajärvi IA, Partanen TJ, Ahlbom A, Boffetta P, Hakulinen T, Jourenkova N, Kauppinen TP, Kogevinas M, Porta M, Vainio HU. Occupational exposures and pancreatic cancer: a meta-analysis. Occup Environ Med. 2000;57:316–324. doi: 10.1136/oem.57.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park RM, Stayner LT, Petersen MR, Finley-Couch M, Hornung R, Rice C. Cadmium and lung cancer mortality accounting for simultaneous arsenic exposure. Occup Environ Med. 2012;69:303–309. doi: 10.1136/oemed-2011-100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericleous M, Rossi RE, Mandair D, Whyand T, Caplin ME. Nutrition and pancreatic cancer. Anticancer Res. 2014;34:9–21. [PubMed] [Google Scholar]
- Pollack AZ, Mumford SL, Mendola P, Perkins NJ, Rotman Y, Wactawski-Wende J, Schisterman EF. Kidney biomarkers associated with blood lead, mercury, and cadmium in premenopausal women: a prospective cohort study. J Toxicol Environ Health A. 2015;78:119–131. doi: 10.1080/15287394.2014.944680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A. The role of zinc in gastrointestinal and liver disease. Clin Gastroenterol. 1983;12:713–741. [PubMed] [Google Scholar]
- Rigby AS. Statistical methods in epidemiology. III. The odds ratio as an approximation to the relative risk. Disabil Rehabil. 1999;21:145–151. doi: 10.1080/096382899297756. [DOI] [PubMed] [Google Scholar]
- Romanowicz-Makowska H, Forma E, Bry M, Krajewska WM, Smolarz B. Concentration of cadmium, nickel and aluminium in female breast cancer. Pol J Pathol. 2011;62:257–261. [PubMed] [Google Scholar]
- Rossouw JE. Hormones, genetic factors, and gender differences in cardiovascular disease. Cardiovasc Res. 2002;53:550–557. doi: 10.1016/s0008-6363(01)00478-3. [DOI] [PubMed] [Google Scholar]
- Ruggeri B, Zhang S, Caamano J, DiRado M, Flynn S, Klein-Szanto A. Human pancreatic carcinomas and cell lines reveal frequent and multiple alterations in the p53 and Rb-1 tumor-suppressor genes. Oncogene. 1992;7:1503–1511. [PubMed] [Google Scholar]
- Sawada N, Iwasaki M, Inoue M, Takachi R, Sasazuki S, Yamaji T, Shimazu T, Endo Y, Tsugane S. Long-term dietary cadmium intake and cancer incidence. Epidemiology. 2012;23:368–376. doi: 10.1097/EDE.0b013e31824d063c. [DOI] [PubMed] [Google Scholar]
- Schwartz GG, Reis IM. Is cadmium a cause of human pancreatic cancer? Cancer Epidemiology Biomarkers & Prevention. 2000;9:139–145. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Report on Carcinogens. 12. 2011. Cadmium and Cadmium Compounds; p. 80. [Google Scholar]
- Sharma-Wagner S, Chokkalingam AP, Malker HS, Stone B, McLaughlin JK, Hsing AW. Occupation and prostate cancer risk in Sweden. J Occup Environ Med. 2000;42:517–525. doi: 10.1097/00043764-200005000-00010. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Sorahan T, Lister A, Gilthorpe MS, Harrington JM. Mortality of copper cadmium alloy workers with special reference to lung cancer and non-malignant diseases of the respiratory system, 1946–92. Occup Environ Med. 1995;52:804–812. doi: 10.1136/oem.52.12.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorahan T, Lancashire RJ. Lung cancer mortality in a cohort of workers employed at a cadmium recovery plant in the United States: an analysis with detailed job histories. Occup Environ Med. 1997;54:194–201. doi: 10.1136/oem.54.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stayner L, Smith R, Thun M, Schnorr T, Lemen R. A dose-response analysis and quantitative assessment of lung cancer risk and occupational cadmium exposure. Ann Epidemiol. 1992;2:177–194. doi: 10.1016/1047-2797(92)90052-r. [DOI] [PubMed] [Google Scholar]
- Stayner L, Steenland K, Dosemeci M, Hertz-Picciotto I. Attenuation of exposure-response curves in occupational cohort studies at high exposure levels. Scand J Work Environ Health. 2003;29:317–324. doi: 10.5271/sjweh.737. [DOI] [PubMed] [Google Scholar]
- Strumylaite L, Bogusevicius A, Abdrachmanovas O, Baranauskiene D, Kregzdyte R, Pranys D, Poskiene L. Cadmium concentration in biological media of breast cancer patients. Breast Cancer Res Treat. 2011;125:511–517. doi: 10.1007/s10549-010-1007-8. [DOI] [PubMed] [Google Scholar]
- Thomas LD, Michaelsson K, Julin B, Wolk A, Akesson A. Dietary cadmium exposure and fracture incidence among men: a population-based prospective cohort study. J Bone Miner Res. 2011;26:1601–1608. doi: 10.1002/jbmr.386. [DOI] [PubMed] [Google Scholar]
- Vahter M, Åkesson A, Lidén C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;104:85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vinceti M, Venturelli M, Sighinolfi C, Trerotoli P, Bonvicini F, Ferrari A, Bianchi G, Serio G, Bergomi M, Vivoli G. Case-control study of toenail cadmium and prostate cancer risk in Italy. Sci Total Environ. 2007;373:77–81. doi: 10.1016/j.scitotenv.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Cherian MG, Ward JM, Goyer RA. Immunohistochemical evidence of high concentrations of metallothionein in pancreatic hepatocytes induced by cadmium in rats. Toxicol Pathol. 1992;20:323–326. doi: 10.1177/019262339202000302. [DOI] [PubMed] [Google Scholar]
- Yuan S, Rosenberg L, Paraskevas S, Agapitos D, Duguid WP. Transdifferentiation of human islets to pancreatic ductal cells in collagen matrix culture. Differentiation. 1996;61:67–75. doi: 10.1046/j.1432-0436.1996.6110067.x. [DOI] [PubMed] [Google Scholar]
- Zaza S, Wright-De Agüero LK, Briss PA, Truman BI, Hopkins DP, Hennessy MH, Sosin DM, Anderson L, Carande-Kulis VG, Teutsch SM, Pappaioanou M. Data collection instrument and procedure for systematic reviews in the guide to community preventive services1. Am J Prev Med. 2000;18:44–74. doi: 10.1016/s0749-3797(99)00122-1. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lv J, Liao C. Dietary exposure estimates of 14 trace elements in Xuanwei and Fuyuan, two high lung cancer incidence areas in China. Biol Trace Elem Res. 2012;146:287–292. doi: 10.1007/s12011-011-9252-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.