SUMMARY
The Hippo pathway and its regulatory target, YAP, has recently emerged as an important biochemical signaling pathway that tightly governs epithelial tissue growth. Initially defined in Drosophilia, this pathway has shown remarkable conservation in vertebrate systems with many components of the Hippo/YAP pathway showing biochemical and functional conservation. The liver is particularly sensitive to changes in Hippo/YAP signaling with rapid increases in liver size becoming manifest on the order of days to weeks after perturbation. The first identified direct targets of Hippo/YAP signaling were pro-proliferative and anti-apoptotic gene programs, but recent work has now implicated this pathway in cell fate choice, stem cell maintenance/renewal, epithelial to mesenchymal transition, and oncogenesis. The mechanisms by which Hippo/YAP signaling is changed endogenously are beginning to come to light as well as how this pathway interacts with other signaling pathways, and important details for designing new therapeutic interventions. This review focuses on the known roles for Hippo/YAP signaling in the liver and promising avenues for future study.
The Core Hippo Pathway Members in Biology
A fundamental question in biology is defining the underpinnings of how organisms and their constituent parts “know” the size they are to grow and when to stop. What are these mechanisms that restrict tissue and organism growth, and if this tight regulatory control is lost, could this then lead to the development of cancer? The liver in particular has the ability to regrow to its original mass within a few weeks after partial hepatectomy, permitting life-saving procedures such as split-liver living donor liver transplantation to be performed. The molecular mechanisms governing this phenomenon are starting to become to clear, but sufficient detail to develop new therapeutics to stimulate liver regeneration or inhibit cancer growth remain in its infancy. To this end, many scientists have used various model systems to define the molecular mechanisms that initiate and restrict tissue growth. The Hippo pathway has emerged as an important biochemical signaling pathway that tightly governs tissue growth. This review will focus on the known roles for Hippo signaling in the liver, how it affects its primary regulatory target, YAP, and promising avenues for future study.
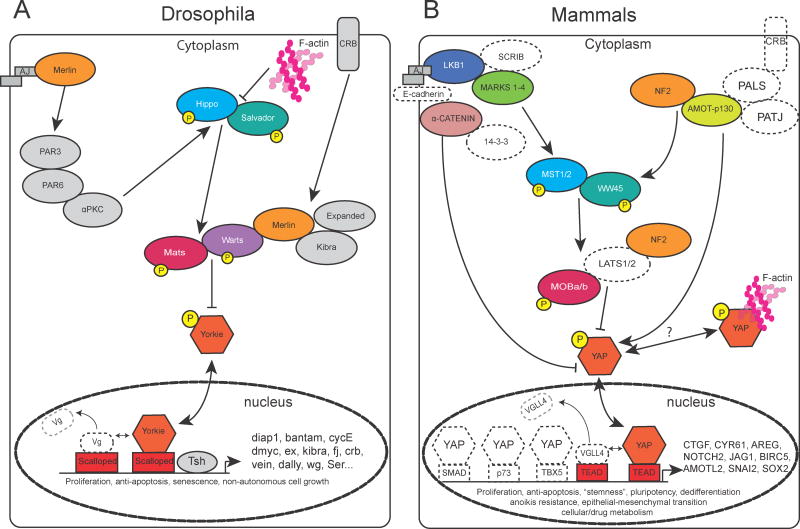
Initially, the Hippo pathway was defined through genetic screens in Drosophila for regulators of growth (Figure 1A). The pathway is aptly named for the serine/threonine kinase hippo (hpo), which when lost, results in tissue overgrowth due to excessive proliferation and decreased apoptosis [1]. Two other Drosophila mutants had been discovered several years prior to this publication, the warts (wts) kinase [2] and the scaffolding molecule, salvador (sav) [3]. The primary activity of the pathway is to restrict the cytoplasmic localization of yorkie (yki) through critical phosphorylation sites [4–6]. yki is a transcriptional co-activator that integrates Hippo signaling, acting as a “gatekeeper” regulating proliferation and growth. The hpo, sav and wts mutants exhibit a similar overgrowth phenotype as overexpression of yki, suggesting that these proteins interact to form a growth regulatory gene program. Through a number of biochemical and genetic epistasis experiments, these molecules form the core of the canonical Hippo pathway. Hippo signaling is commonly referred to as a tumor suppressor, as its “baseline” activity is to restrict yki to the cytoplasm. Hippo signaling loss results in accumulation of yki, its translocation into the nucleus to trigger a downstream gene program. Subsequent work has greatly expanded our biochemical understanding of the pathway (Figure 1A), but a major challenge is developing a clear understanding of the contexts within which Hippo regulates yki.
Figure 1. The Hippo pathway in Drosophila and Mammals.
Schematic of a subset of the Hippo pathway with known roles in the liver. The primary activity of the Hippo pathway is to restrict yorkie (YAP in mammals) to the cytoplasm. Reduced activity of the Hippo pathway results in translocation of yorkie to the nucleus where it binds to the TEAD family of transcription factors to upregulate gene expression. Known target genes of the pathway in Drosophila and Mammals are indicated after the arrowhead in the nucleus. Known roles for the pathway are indicated below the diagram of the transcriptional element.
A. The Hippo pathway in Drosophila. Colored shapes represent known effectors of the Hippo pathway. Selected effectors have homologous partners in mammals that are associated with liver proliferation/cancer. These are color/shape-matched for clarity.
B. The Hippo pathway in Mammals. Colored shapes represent known effectors of liver proliferation/cancer in the mammals. Open/dotted shapes represent potential/expected partners of the Hippo pathway that have yet to be functionally demonstrated in the liver, but have evidence from other tissues for their interaction.
Remarkably, many of the components of this pathway that were initially described in Drosophila, have vertebrate homologs, which share functional and biochemical activity (Figure 1B). For example, YAP, the vertebrate homolog of yorkie, when expressed in Drosophila can rapidly expand its tissues [7]. There are two homologs of yorkie in vertebrates, YAP and TAZ. Although, these homologs have different sizes, both molecules have strong co-transcriptional activity, have critical phosphorylation sites that control their nuclear localization which regulates their ability to activate downstream genes and contain WW motifs that modulate protein-protein binding [8–10]. A large body of work supports the Hippo pathway regulating YAP’s activity in the liver leading to stem cell renewal, regeneration and oncogenesis. Because of the high degree of evolutionary conservation, invertebrate members of the Hippo pathway serve as a resource to interrogate their potential roles in the liver.
With regards to TAZ, it may have similar or overlapping roles in the liver to YAP, but this has not been as extensively explored. Future studies will have to address what roles, if any TAZ may play in liver homeostasis and regeneration.
The Consequence of Manipulating Hippo/YAP Activity in the Liver
Two groups independently provided the first direct evidence that high levels of YAP in the liver rapidly leads to hepatomegaly and eventually hepatocellular carcinoma in the mouse [7, 11]. Within a week, the liver has doubled in size and by two weeks is typically 20% of the mouse’s body weight (Figure 2A, normal 3–5%). Persistent elevation of YAP levels results in tumor development (Figure 2A, 5 month). Restoring endogenous YAP levels after a period of overexpression leads to rapid reversal of the hepatomegaly, and normalization of the parenchymal architecture; strongly suggesting that Hippo signaling may act as an important regulator of overall liver size.
Figure 2. The Effect of Liver YAP Expression.
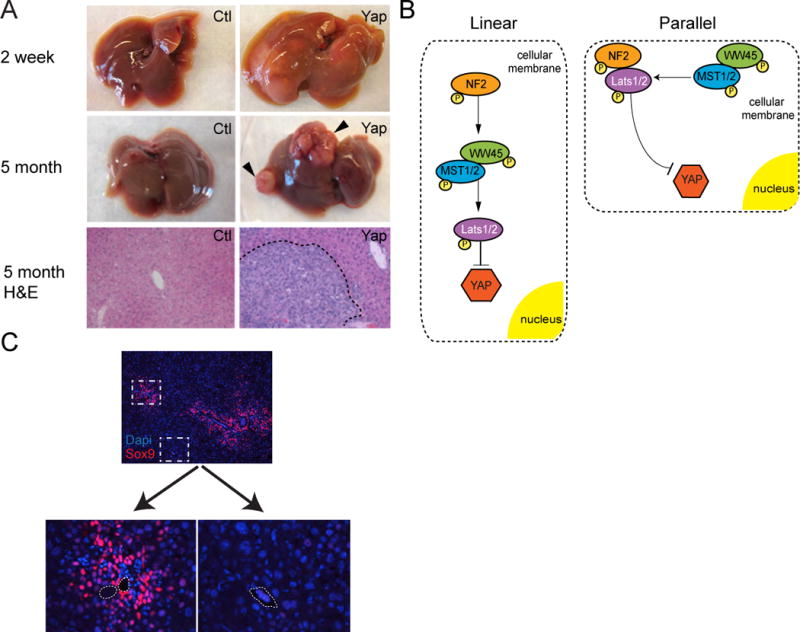
A. 2 weeks – Gross morphology of littermate control and YAP S127A overexpressing livers 2 weeks after high yield YAP induction in the adult. 5 month – Gross morphology of littermate control and a YAP S127A overexpressing liver with tumors (arrowheads) 5 months after low yield induction in the adult. H&E slides show histology of a highly undifferentiated HCC in the YAP overexpressing liver (dotted line).
B. Linear versus Parallel models of YAP modulation. Colored shapes represent known effectors of the Hippo Pathway that directly modulate YAP expression. They are arranged in the two proposed models of YAP regulation, linear and parallel [22].
C. Sox9 staining demonstrates differential hepatocyte response to YAP S127A expression in periportal (PP) as compared to central venous (CV) areas 2 weeks after induction. Dashed boxes are enlarged in pictures noted below. Dashed lines in the PP/CV pictures indicate the borders of the blood vessel [23].
When Hippo signaling is active, YAP is phosphorylated and restricted to the cytoskeleton through its binding to 14-3-3 [12]. Loss of phosphorylation whether by decreased kinase activity [13, 14] or through increased phosphatase activity is associated with nuclear localization of YAP [15] and the subsequent activation of downstream proliferative and anti-apoptotic gene programs. Several mouse liver knockouts of Hippo pathway regulation have been reported including MST1/2 [16–18], NF2 [19, 20], and WW45 [18, 21]. These models universally display increased levels of YAP, decreased YAP phosphorylation resulting in its nuclear relocalization. Each model developed liver overgrowth due to excessive cellular proliferation, but the primary cells composing these enlargements varied with the targeted gene. MST1/2 deletion affects hepatocyte proliferation, NF2 deletion leads to biliary hyperplasia and WW45 mutants demonstrated an abundance of hepatic progenitor cells. These results suggest that loss of various components of the Hippo pathway have differential effect on YAP activity, or that there is heterogeneity in how liver epithelial cells respond to changes in Hippo signaling. Accumulating evidence suggests that both of these hypotheses regarding Hippo signaling in the liver may be true.
Hippo Signaling Fine-tunes YAP Activity and Its Resulting Output
For many years, it had been presumed that a linear relationship between the core Hippo pathway components existed (Figure 2B, Linear). This model predicts that loss of one or more core members of the pathway lead to comparable levels of YAP activation and phenotypes that would be highly similar. As noted previously, this is not the case in the liver.
Duojia Pan and his group has refined this view; supporting a model that an actin-associated MST1/2-WW45 complex phosphorylates a plasma membrane-associated NF2/LATS1/2 complex (Figure 2B, Parallel). The parallel model predicts that these core components intimately cooperate to regulate YAP activity. Loss of a single regulator would yield a mild phenotype, while loss of multiple components leads to high levels of YAP activity and profound liver overgrowth. In the liver, loss of either NF2 or WW45 leads to a mild proliferative biliary phenotype with minimal liver hypertrophy. Concomitant loss of both molecules results in potent biliary hyperplasia, near complete loss of YAP phosphorylation-a sign of high YAP activity, and a profound enlargement of the liver [22].
Similarly, we have found that varying the level of YAP expression in the liver leads to different cellular phenotypes. Several years ago, using a hepatocyte specific driver to control a tetracycline-inducible form of YAP, we described that YAP’s primary function at high levels was to drive cell proliferation [11]. Although some progenitor markers began to appear in this context, the cells that drove YAP expression did not completely lose their phenotypic and biochemical hepatocytic markers. More recently, using a different system, we documented that high levels of YAP can drive hepatocytes to completely develop the properties of hepatic progenitor cells. These cells are easily expanded in tissue culture, can be transplanted to other animals for therapy, and revert to hepatocytes or assumed the characteristics of cholangiocytes when endogenous levels of YAP were restored [23]. The difference in driving a hepatocyte to proliferate versus dedifferentiating under the influence of YAP is likely accounted for by the level and persistence of YAP expression in these contexts. In our earlier publication, YAP expression was driven by a liver specific promoter, which limited YAP expression to those cells in the “hepatocyte-specific” context. The more recent publication utilized a driver of expression that is less affected by cell type, resulting in consistently high YAP expression that uncovered the dedifferentiation phenotype. Our results as well as those of the Pan group, suggest that varying levels of YAP activity may act as a rheostat for guiding cell behavior. Modest YAP levels may cause proliferation with higher levels being associated with changes in cell fate (Figure 3). Levels of TAZ activity have previously been suggested to guide cell fate choices as well [24], making it likely that tight regulation of the Hippo pathway transcriptional co-activators is a common mean by which cells guide these choices. Additional cellular choices involving varying degrees of Hippo activity will likely be uncovered, as we understand how cells and tissues tightly regulate the activity of these proteins.
Figure 3. Hippo/YAP Levels Mediate Cellular Behavior.
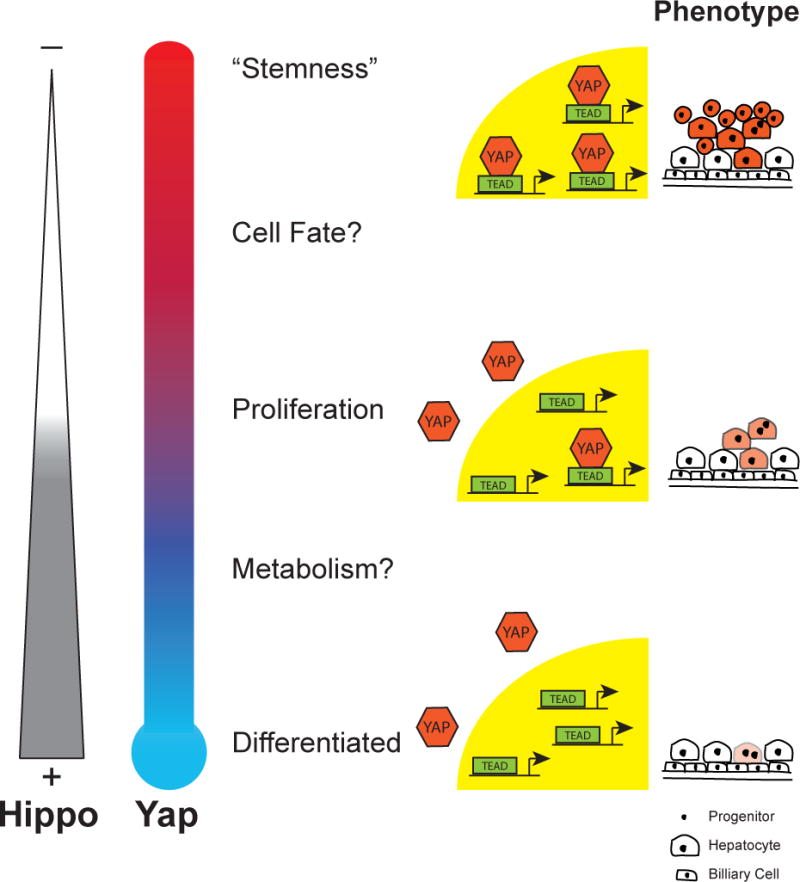
Hippo signaling and YAP activity is inversely related and determines particular cellular processes at various levels. High Hippo signaling activity is correlated with low levels of YAP protein and cytoplasmic YAP. Low Hippo activity is associated with high levels of YAP and a propensity for it to be localized to the nucleus. Proposed activities for varying levels of Hippo/YAP are depicted next to the YAP “thermometer”. At the far right, animations represent the proliferation and dedifferentiation of cells associated with the various levels of YAP expression. Darker shading suggests increased YAP expression.
The hepatocyte response to increased YAP activity throughout the liver is not uniform. Our recent studies have shown that there is a clear preferential expression of progenitor markers and proliferation that occurs in a periportal to central venous pattern (Figure 2C). These results suggest that Hippo signaling synergizes with other biochemical signaling pathways that predominate in the periportal areas. Preexisting factors such as hepatocyte growth factor, fibroblast growth factor, bone morphogenic factor 4, angiopoeitin-2, transforming growth factor β1, jagged1 and other ligands are found in these areas [25, 26]. Modulating these ligands or their cognate receptors in the context of Hippo signaling will provide useful insight on how we might improve our ability to generate liver stem cells.
The Requirement for YAP Activity During Liver Development, Homeostasis and Regeneration
Of the liver epithelial cells, biliary cells have the highest levels of YAP protein and activity [23, 27, 28]. YAP activity is critical for normal biliary development as liver-specific YAP knockout mice are born with hypoplastic biliary ducts, which are progressively lost as they age. YAP knockout hepatocytes are more sensitive to injury, due to a loss of survival factors. These mice gradually developed hepatitis and fibrosis, likely due to cholestatic liver injury from the immature biliary system and hepatocyte hypersensitivity to injury [19]. In the adult, YAP appears to be inconsequential, as liver YAP knockouts do not acutely develop biliary duct loss or hepatocyte necrosis. In contrast, YAP is required for recovery from liver injury as extensive hepatic necrosis and high mortality occurs after bile duct ligation [23, 29].
During chronic liver injury, ductal reactions often develop around the portal areas. They are likely responsible for pathologic liver remodeling and serve as a nidus for cancer stem cells [30]. Several recent reports support hepatocyte dedifferentiation as a significant contributor to the ductal reaction [31–33]. Since YAP activity is important to biliary development/survival [19] and induced YAP activity in the liver can generate a ductal reaction [23], human biliary diseases should also display evidence of Hippo Signaling loss. In the ductal reactions associated with human cases of primary sclerosing cholangitis, primary biliary cirrhosis and biliary atresia, high concentrations and evidence of nuclear YAP is present. This suggests that dysregulated Hippo signaling leads to the aberrant formation of a dysfunctional biliary system [29, 34]. In principle, inhibitors of YAP activity could ameliorate some of the pathology associated with these diseases.
Connecting Hippo/YAP Signaling to Organ Size
The liver is renowned for its ability to rapidly respond to partial hepatectomy by synchronously activating proliferation in the remaining hepatocytes. After hepatectomy, YAP protein levels rise, its phosphorylation status decreases and Hippo target genes are upregulated [35–37]. In particular, YAP localizes in the nucleus shortly (<4 hr) after partial hepatectomy suggesting that Hippo target genes are involved in liver regeneration [35]. What kinds of signals could facilitate these changes, particularly ones that may be rapidly transmitted throughout the liver?
One possible mechanism where partial hepatectomy could activate hepatocyte proliferation would be if cells could detect changes in shear stress from increased blood flow [38]. YAP is intimately associated with the actin cytoskeleton with the majority of it being cytoplasmic. It is clear that this association is to not simply act as a YAP reservoir, but directly facilitates its ability to sense overall changes in cell tension. Cytoskeletal changes can affect YAP localization in both a phosphorylation-dependent and -independent manner. The most direct evidence for cytoskeletal changes affecting YAP localization comes from work in fibroblasts where increased extracellular matrix stiffness leads to nuclear YAP localization in a Hippo pathway kinase independent manner [39, 40]. Mutations in YAP that uncouple its ability to sense cell tension lead to disorganized tissue and organ development, thereby providing a paradigm by which YAP activity can orchestrate liver size [41].
In addition, a number of cell surface molecules have been identified as YAP interactors, potentially transmitting changes in cell tension into the liver Hippo pathway. In the skin, the adherens junction (AJ) complex is an important sensor of tissue tension with loss of critical components resulting in hyperproliferation and cancer. α-catenin, an intracellular member of the AJ complex associates with YAP, anchoring it to the actin cytoskeleton with its loss being associated with increased YAP activity [42]. A similar situation exists in the adult liver, where α-catenin knockdown livers have disorganized liver sinusoids and are 60% larger in size after partial hepatectomy than controls. This disorganization is associated with elevated serum bile acids, presumably due to improper development of bile canaliculi. [43].
Another cell surface signaling complex that coordinates with YAP are the microtubule affinity-regulating kinase (MARK) family. A targeted siRNA screen of the known human kinome found several members of the MARK family to affect YAP phosphorylation and activity [44]. YAP is tightly associated with the MARK family as well as Scribble and LKB1, which have been previously described to regulate cell growth and proliferation [45, 46]. LKB1 knockdown in vitro affects YAP activity [47] and LKB1 liver knockouts show a 40% increase in liver to body weight that is attenuated by YAP knockout [44].
Finally, several groups have identified the cytoskeletal protein angiomotin (AMOT) as a strong binding partner of YAP [48–50]. While it had been predicted that loss of AMOT would result in excessive proliferation, genetic deletion of AMOT is indistinguishable from controls. Upon liver injury, AMOT knockout mice display less biliary proliferation than expected, suggesting that rather than restricting YAP’s activity, it potentiates downstream YAP gene activation by acting as a nuclear co-factor [51].
Of the three cytoskeletal proteins/complexes cited above, AMOT, α-catenin, and LKB1, only loss of LKB1 in the adult liver causes changes in organ size. Genetic loss of the other noted proteins requires some form of injury to reveal the process it modulates. This suggests there may be some sort of hierarchy to liver Hippo pathway regulation, or that these cell surface complexes detect fundamentally different forms of input. An understanding of how these complexes cooperate to modulate YAP activity will significantly contribute to our understanding of liver disease.
Alternatively, partial hepatectomy increases blood flow to the remaining lobes, effectively upregulating the concentration of metabolic products and signaling molecules that may activate hepatocyte proliferation [52]. G protein coupled receptors (GPCRs) are an important link into the Hippo pathway that may sense increases in metabolites associated with hepatectomy. GPCRs were identified through small molecule screening for extracellular ligands that can modulate Hippo signaling. Small molecules such as epinephrine, estrogen, lyosphosphatidic acid, sphingosine 1-phosphophate and thrombin have been identified as GPCR ligands [53–55]. Moreover, this work described that the Hippo pathway could be either positively regulated through the Gα subset or negatively impacted by the G12/13 subset. The GPCRs and their ligands should be considered potential endogenous sensors and signals of liver size.
The mechanisms above preferentially affect hepatocytes, but biliary cells display high YAP activity. Could these cells be in direct contact with molecules that inactivate Hippo signaling? A primary physiologic activity of biliary cells is to efflux of bile salts from the liver and obstructive cholestasis often results in biliary proliferation. Bile salts can act as signaling molecules to stimulate regeneration [56] or as detergents, disrupting the cell membrane. Directly increasing bile salt concentration without mechanical obstruction increases YAP levels in the liver, resulting in liver hypertrophy and the development of hepatocellular carcinoma [57]. These results suggest bile acids may upon biliary cells through an unidentified receptor, or by directly changing the properties of the plasma membrane to inactivate the Hippo pathway.
Accumulating evidence indicates that the Hippo/YAP pathway integrates phosphorylation dependent and independent inputs (likely through changes in cytoskeletal tension) to make decisions to activate downstream transcription. In the liver and even at the cellular level, it is unlikely that all of these mechanisms, which are described above are simultaneously present and active in the same cell. Which cellular subsets utilize these mechanisms and can we identify the extracellular inputs that regulate downstream Hippo gene programs in the liver?
Transcriptional Partners (and Antagonists) of YAP and Signaling Cross Talk
As a transcriptional coactivator, YAP cannot bind to DNA, but it interacts with transcription factors to activate gene expression. The primary binding partners of YAP are the TEAD family of transcription factors [58–62]. They are highly associated with anti-apoptotic, pro-proliferative and “stemness” gene programs [23, 62–64]. Vertebrates have four TEAD family members that are expressed in a tissue-restricted pattern, but seem to have similar affinity for YAP. VGLL4 antagonizes the effects of nuclear YAP by binding to TEAD and interfering with its ability to activate downstream target genes [65, 66].
Prior to the identification of the TEAD family of proteins as major effectors of the Hippo transactivators, several other transcription factors were identified as binding partners of YAP including p73 [67], PEBP2α [9] and ErbB4 [68]. There is accumulating evidence that although, proliferative/anti-apoptotic/”stemness” programs may be transmitted through the TEAD family, YAP has critical interactions with other transcription factors. Some phenotypes include intestinal cell fate changes through Klf4 [69], enable pancreatic adenocarcinoma relapse utilizing E2F and TEAD2 [70], specifying the mouse trophectoderm in conjunction with RBP-J [71] as well as facilitating epithelial to mesenchymal transition in lung cancer through co-binding with KRAS/FOS [72]. While YAP-TEAD interactions appear to be the most prevalent, other potential YAP binding partners as described above, may facilitate critical activities or cell fate choices in the liver.
Notch signaling is well known to be important in the normal development and maintenance of the liver [73–75]. Subsequent work has shown that Notch and Hippo signaling synergize to potentiate liver cell growth and remodeling. The Notch pathway components, Jagged-1 and Notch2 are downstream targets of Hippo signaling leading to the dedifferentiation of hepatocytes into hepatic progenitors [23, 76]. Loss of Notch signaling in the context of increased YAP activity leads to poor cellular growth and blunting hepatocyte dedifferentiation, supporting the importance of Notch targets and its synergy with the Hippo pathway to support efficient clonal growth.
The WNT pathway interacts with Hippo signaling on multiple levels to modulate cellular output. WNT signaling is well known for its potent roles in stem cell maintenance, cell fate determination, cell migration, neural patterning and oncogenesis [77]. Our group has shown an antagonist relationship regarding YAP and WNT signaling occurs in the cytoplasm through DISHEVELLED inhibition [78]. In other contexts, YAP and β-catenin directly cooperate to activate gene targets [79] and the transcription factor TBX5 can direct the YAP/β-catenin complex to target genes [80]. More recently, another group has proposed that YAP and β-CATENIN may be primed in a quiescent cytoplasmic complex with AXIN. Upon WNT stimulation, there is a concomitant release of YAP and β-CATENIN, whereupon these molecules translocate into the nucleus and separately activate their respective pathways [81].
From studies in our laboratory as well as others, there is clear heterogeneity in the signaling complexes that contain YAP. Defining the composition and contexts that these YAP complexes operate in the liver is the next challenge to develop a clear understanding of how Hippo/YAP signaling contribute to liver disease.
Loss of Hippo signaling is an oncogenic driver of Liver Cancer
A role for Hippo signaling in cancer began to emerge approximately 10 years ago, when it was discovered that the chromosome region containing YAP is amplified in breast and liver cancer [82, 83]. Embryonic hepatoblasts with a p53 null, c-myc overexpressing background were used in these early screens, making it unclear how characteristic these tumors might compare to HCCs that arise in the adult. Subsequently, it was shown that Yap overexpression in the adult mouse liver, even for as little as 2 months results in hepatocellular carcinoma [7]. Of the HCC models that exist, Yap overexpression results in the most rapid means of generating HCC, which could bode well for in vivo chemical drug screening. YAP expression has since been demonstrated to be elevated in a number of human cancer samples including including colon [78], breast [84], lung [85, 86], ovarian [87, 88], medulloblastoma [89], cholangiocarcinoma (CC) and hepatocellular carcinoma [16, 27, 90–93]. It is estimated that 5–10% of human HCCs have genomic amplification of the genomic locus containing YAP [83].
Chemically-induced injury models of HCC suggest that increased YAP activity may be an early oncogenic event. Nuclear YAP was found in preneoplastic hepatic foci within 4 weeks of injury with the number and size of tumors being dramatically reduced when YAP inhibitors were administered [94]. In contrast, late induction of YAP expression can also be a common mechanism that tumors use to overcome targeted blocks to their growth. For example, in hepatocytes, loss of the Retinoblastoma (RB) protein is initially accompanied by rapid cellular proliferation, which slows due to cellular senescence. Genes initially activated by RB loss become silenced during this process. YAP expression restores hepatocyte proliferation partially by reactivating some of these silenced genes [95].
What other mutations may lead to Hippo inactivation and liver cancer? In a small study of HCC patients, more than half had evidence of reduced MST1/2 activity, which leads to increased YAP activity. The mouse model of MST1/2 loss consistently developed HCC supporting its mechanistic role [16]. Hepatitis B infection is commonly is associated with HCC. One of the viral products, Hepatitis B Virus X protein directly interacts with the cyclic AMP responsive element binding protein to promote YAP transcription and increase its activity [90].
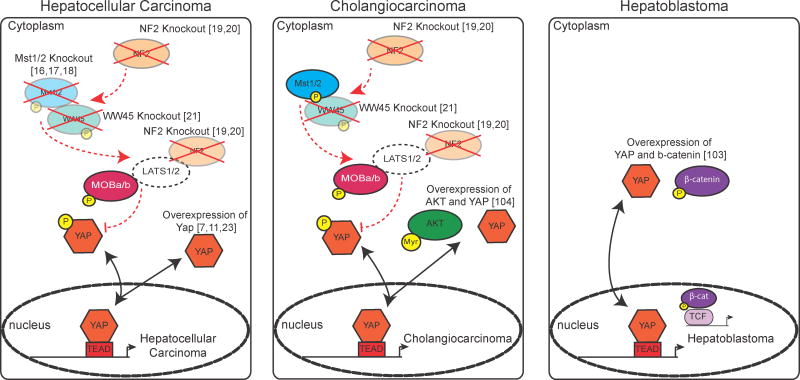
Mouse knockouts of WW45 [18, 21] and NF2 [19, 20] knockouts lead to the development of HCC and CC. It is interesting to note, that while loss of NF2 clearly leads to liver cancer in the mouse, there are no clinical reports of NF2-associated liver cancers in humans. Neurofibromatosis 2 patients are often reported to have spontaneous schwannomas and meningiomas. In addition, mutations in NF2 have been commonly documented in glioblastoma [96], mesothelioma [97], renal [98] and breast cancer [99] suggesting that in humans, the cells of the liver that lead to HCC or CC may not be susceptible to NF2 mutation or loss. Other unidentified genetic modifiers may make these kinds of cancer particularly rare due to monoallelic loss of NF2.
WNT signaling is commonly activated in a large number of cancers including HCC and hepatoblastoma [100, 101]. An important target of WNT signaling in liver cancer is TRIB2. This pseudokinase stabilizes YAP levels and downregulates C/EBPα expression, which antagonizes YAP-TEAD interactions [102]. In vivo, activating mutations of β-catenin, the WNT pathway co-activator does not lead to oncogenesis. Using similar techniques, in vivo hepatocyte transfection of YAP also does not lead to cancer, but co-transfection of activated YAP and β-catenin consistently led to the development of HB [103]. These two reports suggest that liver cancer, particularly HB develops in the context of simultaneous WNT and Hippo pathway activation.
In a similar fashion, transducing the biliary epithelium with activated forms of YAP and AKT results in the development of cholangiocarcinoma in mice. These tumors share a transcriptional profile similar to CC arising in human patients, suggesting that the PI3K and Hippo pathway interact in this oncogenic context [104]. Several prior reports suggested that increased YAP activity alone is sufficient for HCC or CC to develop [16, 18–21, 102, 105], although these models were not well characterized for mutations in other signaling pathways. It is likely that cells with Hippo pathway mutations rapidly proliferate and acquire additional mutations that lead to cancer.
The immunohistochemical localization of YAP has long been interpreted to be a sign of high YAP activity, but this may neglect modest levels of YAP activity that also have clinical significance. Uniform classification of cancer by molecular markers, such as by high throughput RNA sequencing is becoming commonplace [106, 107]. This should be done more uniformly for patients as well as our mouse models so that accurate comparisons particularly across studies can be made. This can then be used to help predict potentially effective treatments for a group of tumors that currently have limited available therapy.
Mutations in the Hippo pathway leading to elevated YAP levels/activity commonly lead to liver cancer in mice and humans (Figure 4). Targeting the inactivation of the Hippo/YAP pathway to hepatocytes versus cholangiocytes can partially explain how HCC, HB or CC arises. Why do MST1/2 mutants only develop HCC while NF2 and WW45 mutants can develop both HCC and CC remains a mystery. This suggests that there are subtle differences in the construction of the Hippo pathway in cells that generate HCC versus CC. The studies of how Hippo Signaling interacts with other important signaling programs is adding to our understanding of cancer evolution and should allow us to specifically targeted cancerous cells over normal cells.
Figure 4. Known Mutations in Hippo/YAP signaling in Liver Cancer.
Colored shapes represent the known effectors of the Hippo pathway that, when knocked out or overexpressed, are associated with the development of the indicated form of cancer. References to the papers are indicated.
Therapeutic avenues for Modulating Hippo Signaling
The mechanisms through which Hippo signaling can be therapeutically inhibited are so far limited, but it is a particularly active area of research. As noted previously, NF2 loss results in the liver cancer; interestingly, this can be rescued by hemizygous loss of YAP [19]. Dominant-negative TEAD2 (which binds to YAP, but not DNA) can also ameliorate the liver tumors that develop in the NF2 knockout model [108]. It is also reassuring that loss of YAP in quiescent adult hepatocytes does not immediately lead to their widespread loss [23]. These data suggest that partial inhibition of YAP activity is sufficient to suppress the development of oncogenesis. YAP siRNA lipid nanoparticles have been shown to effectively treat a mouse model of advanced HCC. Reduction in YAP using lipid-encapsulated siRNA converted highly aggressive HCC to undergo hepatocyte differentiation [109]. These data support the idea that reduced YAP activity can serve as the basis of treatment for HCC (and likely other forms of YAP-dependent cancers) with limited effect upon normal tissues.
Since much of the transcriptional activity of the Hippo pathway is directed through the TEAD family of transcription factors, a number of groups have focused on disrupting the YAP-TEAD interaction. Screening chemical libraries of commercially available compounds identified verteporfin, a porphyrin molecule currently in use as a photosensitizer for macular degeneration. Verteporfin binds to YAP interfering with its ability to bind TEAD. Importantly, verteporfin shows efficacy in vivo to limit YAP driven liver cancer [108]. Currently, the affinity of this drug is poor for clinical use, but serves as an important lead compound for future development.
Many of the proteins identified in the Hippo pathway are adaptor proteins. It is conceivable that development of a competitive inhibitor at critical junctions will have important therapeutic benefit. For several years, vestigial-like4 (VGLL4) has been reported to be an important competitive inhibitor of TEAD activation acting to partially mask the YAP-TEAD binding site [110, 111]. By identifying the critical peptides that mediate VGLL4-TEAD binding, this group generated a cell permeable peptide that out competes YAP for TEAD4 binding. It appears a promising candidate for the treatment of gastric cancer [65] and could be a useful adjunctive therapy for liver cancer particularly since TEAD4 is the highest expressed member of this family in the liver.
Elevating YAP activity has been proposed to be a potentially effective way of expanding the liver in the transplantation setting, particularly where there may be some graft to host size mismatch and accelerating graft growth may prove advantageous. Utilizing RNAi technology to reduce expression of regulatory kinases of the Hippo pathway, small molecule activation of the Gα GPCR or even transient expression of YAP are possible means of momentarily accelerating cell proliferation. Recent reports evaluating hepatocyte ploidy in the context of YAP overexpression raises some concern over the long-term stability of cells after YAP induction or if induced YAP expression may accelerate the accumulation of potential oncogenic mutations. Typically mature hepatocytes are tetraploid. In the presence of YAP, the vast majority of hepatocyte ploidy shifts to 8N and higher [112]. Presumably, normalization of YAP levels results in either loss of the 8N hepatocytes, or subsequent cell division reducing the ploidy of these hepatocytes to the normal 4N number. As YAP expression is used to overcome a number of physiologic checkpoints during expansion, what will be the quality of these expanded cells and can techniques or strategies be used to minimize the potential deleterious effects? Additional work to understand cell health and quality after induced YAP expression is important for potential application in the transplantation setting.
Conclusions
The Hippo/YAP pathway has been recognized as important in regulating overall tissue proliferation and growth with loss of proper control associated with oncogensis. But, these ideas are likely an oversimplification due to the limitations of our current models. Roles for Hippo/YAP in cell fate determination, metabolism and epithelial to mesenchymal transition are beginning to emerge and it is likely that there are many important nuances left to be appreciated.
Many inputs into Hippo signaling have been identified, but the composition of the pathway is presumed to be context dependent. A number of Hippo Pathway members have been identified to be active in the liver (Table 1), but an even larger number are known in other contexts. Some of these mechanisms will apply to Hippo/YAP in the liver as well, such as in the case of α-catenin. The future challenge is in identifying the biologically and clinically relevant mechanisms to liver processes in order to develop new therapeutic paradigms.
Table 1.
Hippo pathway mutations cause liver specific phenotypic changes.
| Genetic Mutation | Mutation induction | Liver Phenotype | Reference |
|---|---|---|---|
| α-catenin knockdown | siRNA-LNP | Increased hepatocyte proliferation and liver size after hepatectomy | Herr, K et. al. 2014 |
| Amot-p130 knockout | Amotflox/flox/Alb-Cre | Reduced biliary hyperplasia after bile duct ligation | Yi, C et. al. 2013 |
| LKB1 knockout | Lkb1flox/flox/Ad-Cre | Increased hepatocyte proliferation and liver size | Mohseni, M et. al. 2014 |
| Mob1a/1b double deletion | Mob1aΔ/Δ1btr/+ | 19% of mice develop HCC | Nishio, Miki et. al 2012 |
| MST1/MST2 knockout | Mst1−/− Mstflox/−/Ad-Cre Mst1−/− Mstflox/−/Alb-Cre Mst1−/− Mstflox/flox/Alb-Cre |
Development of HCC | Zhou, D et. al. 2009; Song, H et. al. 2010; Lu, L et. al 2010 |
| Nf2 knockout | Nf2flox/flox/Alb-Cre Nf2flox/flox/Ad-Cre |
Increased/disorganized biliary cell proliferation, biliary hyperplasia and HCC, rare cholangiocarcinoma | Zhang, N et al. 2010; Benhamouche, S et. al. 2010 |
| Nf2/Salvador double knockout | Nf2flox/flox Sav1flox/flox/Alb-Cre | Biliary epithelial cell hyperplasia | Feng, Y et. al. 2013 |
| Salvador knockout | Sav1flox/flox/Alb-Cre | Increased proliferation and expansion of hepatic progenitor cells leading to HCC and CC phenotypes | Lee, K.P. et. al. 2010; |
| Yap knockout | Yapflox/flox : AAV-TBG-Cre Yapflox/flox : Alb-Cre |
Biliary hypoplasia, hepatocyte sensitivity to stress | Yimlamai, D et al. 2014; Zhang et. al. 2001 |
| Yap Overexpression | TetOYap-S127A-R26-rtTa LAP1/tTA-Yap(S127A) ApoE/rtTa-Yap |
Hepatocyte dedifferentiation, liver overgrowth, HCC development | Yimlamai, D et. al. 2014; Camargo, F et. al. 2007 Dong, J et. a;. 2007 |
Liver-specific gene mutations within the Hippo pathway leading to survival and proliferative changes of epithelial cells in the mouse liver. Adeno-associated thyroid binding globin virus Cre (AAV-TBG-Cre); Adenovirus Cre (Ad-Cre); albumin promoter Cre (Alb-Cre); villin-Cre); siRNA packaged in lipid nanoparticles (LNP).
Table 2.
Description of Hippo pathway Components
| Vertebrates | ||
|---|---|---|
| Name | Abbreviation | Role |
| 14-3-3 | YWHA | Regulatory binding proteins |
| α-catenin | CTNNA | Adherens junction structural proteins |
| Adherens junction | AJAP | Protein complex found in cell-cell junctions in epithelium and endothelium |
| Angiomotin | AMOT | Adaptor protein |
| E-cadherin | CDH | Transmembrane receptor |
| Large tumor suppressor homolog 1/2 | LATS1/2 | Serine/threonine kinase |
| Liver kinase B1 | LKB1 | Serine/threonine kinase |
| Microtubule affinity-regulating kinase | MARK | Serine/threonine kinase |
| Mob kinase activator 1a/1b | MOB1A/B | Co-factor |
| Mammalian STE20-like protein kinase | MST1/2 | Serine/threonine kinase |
| Neurofibromin 2 | NF2 | Adaptor protein |
| Salvador homolog 1 | SAV1 | Adaptor Protein |
| Scribble | SCRIB | Adaptor protein |
| Transcriptional co-activator with PDZ-binding motif | TAZ | Transcription co-activator |
| TEA domain-containing sequence-specific transcription factor | TEAD | Transcription factor |
| Vestigial-like protein 4 | VGL-4 | Co-factor |
| Yes-associated protein | YAP | Transcriptional Co-activator |
| Drosophila | ||
|---|---|---|
| Name | Abbreviation | Role |
| Crumbs | crb | Transmembrane receptor |
| Expanded | ex | Adaptor protein |
| Kibra | kibra | Adaptor protein |
| Mob as tumor suppressor | mats | Co-factor |
| Merlin | mer | Adaptor protein |
| Salvador | sav | Transcription factor |
| Scalloped | sd | Transcription factor |
| Hippo | hpo | Serine/threonine kinase |
| Warts | wts | Serine/threonine kinase |
| Yorkie | yki | Transcriptional co-activator |
KEY POINTS.
Hippo signaling is a recently described tumor suppressor pathway which tightly regulates overall liver size by controlling pro-proliferative and anti-apoptotic gene programs. Hippo pathway mutants commonly lead to liver overgrowth phenotypes and the development of cancer.
The transcriptional co-activator YAP is the primary regulatory target of Hippo signaling. Hippo signaling sequesters YAP to the cytoplasm through phospho-specific and cell-tension based mechanisms.
YAP primarily binds to the TEAD family of transcription factors to regulate genes associated with Hippo signaling. Small molecule and peptide-based inhibitors of the YAP-TEAD interaction can attenuate phenotypes associated with inactivation of Hippo signaling.
There is emerging evidence for Hippo/YAP signaling as important for stem cell maintenance/renewal, cell fate choice, overriding senescence, regulating cell metabolism and conferring epithelial to mesenchymal transition.
Increased YAP expression and activity is associated with the development of multiple types of liver cancer including hepatoblastoma, hepatocellular carcinoma and cholangiocarcinoma. Experimental models support YAP activity to be an early event and an important oncogenic driver of liver cancer development.
Acknowledgments
We appreciate the members of the Camargo laboratory for stimulating and insightful discussions. DY is a Gilead Sciences Scholar in Liver Disease and is supported by a Boston Children’s Hospital Career Development Award. This work was supported by awards from the NIH K08 DK105351 (DY), NIH R01 AR064036 (FDC) and NIH R01 DK099559-01 (FDC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 2.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 3.Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 4.Pan D. The Hippo Signaling Pathway in Development and Cancer. Developmental cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao B, Lei Q-Y, Guan K-L. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Current opinion in cell biology. 2008;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer cell. 2008;13:188–192. doi: 10.1016/j.ccr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yagi R, Chen LF, Shigesada K, Murakami Y, Ito Y. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 1999;18:2551–2562. doi: 10.1093/emboj/18.9.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui CB, Cooper LF, Yang X, Karsenty G, Aukhil I. Transcriptional coactivation of bone-specific transcription factor Cbfa1 by TAZ. Molecular and cellular biology. 2003;23:1004–1013. doi: 10.1128/MCB.23.3.1004-1013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Current Biology. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 12.Basu S, Totty N, Irwin M, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Molecular cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 13.Oka T, Mazack V, Sudol M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP) J Biol Chem. 2008;283:27534–27546. doi: 10.1074/jbc.M804380200. [DOI] [PubMed] [Google Scholar]
- 14.Zhao B, Li L, Tumaneng K, Wang C-Y, Guan K-L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF-TRCP. Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribeiro PS, Josué F, Wepf A, Wehr MC, Rinner O, Kelly G, et al. Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Molecular cell. 2010;39:521–534. doi: 10.1016/j.molcel.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Zhou D, Conrad C, Xia F, Park J-S, Payer B, Yin Y, et al. Mst1 and Mst2 Maintain Hepatocyte Quiescence and Suppress Hepatocellular Carcinoma Development through Inactivation of the Yap1 Oncogene. Cancer cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proceedings of the National Academy of Sciences. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proceedings of the National Academy of Sciences. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Developmental cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benhamouche S, Curto M, Saotome I, Gladden AB, Liu C-H, Giovannini M, et al. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010;24:1718–1730. doi: 10.1101/gad.1938710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee K-P, Lee J-H, Kim T-S, Kim T-H, Park H-D, Byun J-S, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proceedings of the National Academy of Sciences. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, et al. Hippo Pathway Activity Influences Liver Cell Fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 25.Boulter L, Lu WY, Forbes SJ. Differentiation of progenitors in the liver: a matter of local choice. The Journal of clinical investigation. 2013;123:1867–1873. doi: 10.1172/JCI66026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kordes C, Haussinger D. Hepatic stem cell niches. The Journal of clinical investigation. 2013;123:1874–1880. doi: 10.1172/JCI66027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Wolfe A, Septer S, Edwards G, Zhong X, Bashar Abdulkarim A, et al. Deregulation of Hippo kinase signalling in Human hepatic malignancies. Liver international : official journal of the International Association for the Study of the Liver. 2012;32:38–47. doi: 10.1111/j.1478-3231.2011.02646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai H, Gayyed MF, Lam-Himlin DM, Klein AP, Nayar SK, Xu Y, et al. Expression of Yes-associated protein modulates Survivin expression in primary liver malignancies. Human pathology. 2012;43:1376–1385. doi: 10.1016/j.humpath.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai H, Zhang N, Xu Y, Chen Q, Khan M, Potter JJ, et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012;56:1097–1107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oertel M, Shafritz D. Stem cells, cell transplantation and liver repopulation. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2008;1782:61–74. doi: 10.1016/j.bbadis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, et al. Cholangiocarcinomas can originate from hepatocytes in mice. The Journal of clinical investigation. 2012;122:2911–2915. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell stem cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurda GT, Zhu Q, Bai H, Pan D, Schwarz KB, Anders RA. The use of Yes-associated protein expression in the diagnosis of persistent neonatal cholestatic liver disease. Human pathology. 2014;45:1057–1064. doi: 10.1016/j.humpath.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Zhang L, He Q, Feng X, Zhu J, Xu Z, et al. Differences in Yes-associated protein and mRNA levels in regenerating liver and hepatocellular carcinoma. Molecular medicine reports. 2012;5:410–414. doi: 10.3892/mmr.2011.640. [DOI] [PubMed] [Google Scholar]
- 36.Wu H, Xiao Y, Zhang S, Ji S, Wei L, Fan F, et al. The Ets transcription factor GABP is a component of the hippo pathway essential for growth and antioxidant defense. Cell reports. 2013;3:1663–1677. doi: 10.1016/j.celrep.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grijalva JL, Huizenga M, Mueller K, Rodriguez S, Brazzo J, Camargo F, et al. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. American journal of physiology Gastrointestinal and liver physiology. 2014;307:G196–204. doi: 10.1152/ajpgi.00077.2014. [DOI] [PubMed] [Google Scholar]
- 38.Sato Y, Koyama S, Tsukada K, Hatakeyama K. Acute portal hypertension reflecting shear stress as a trigger of liver regeneration following partial hepatectomy. Surgery today. 1997;27:518–526. doi: 10.1007/BF02385805. [DOI] [PubMed] [Google Scholar]
- 39.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 40.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 41.Porazinski S, Wang H, Asaoka Y, Behrndt M, Miyamoto T, Morita H, et al. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature. 2015;521:217–221. doi: 10.1038/nature14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herr KJ, Tsang YH, Ong JW, Li Q, Yap LL, Yu W, et al. Loss of alpha-catenin elicits a cholestatic response and impairs liver regeneration. Scientific reports. 2014;4:1–11. doi: 10.1038/srep06835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, et al. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nature cell biology. 2014;16:108–117. doi: 10.1038/ncb2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baas AF, Smit L, Clevers H. LKB1 tumor suppressor protein: PARtaker in cell polarity. Trends in cell biology. 2004;14:312–319. doi: 10.1016/j.tcb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Yamanaka T, Ohno S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Frontiers in bioscience : a journal and virtual library. 2008;13:6693–6707. doi: 10.2741/3182. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen HB, Babcock JT, Wells CD, Quilliam LA. LKB1 tumor suppressor regulates AMP kinase/mTOR-independent cell growth and proliferation via the phosphorylation of Yap. Oncogene. 2013;32:4100–4109. doi: 10.1038/onc.2012.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. 2011;286:7018–7026. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, Huang J, Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem. 2011;286:4364–4370. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yi C, Shen Z, Stemmer-Rachamimov A, Dawany N, Troutman S, Showe LC, et al. The p130 isoform of angiomotin is required for Yap-mediated hepatic epithelial cell proliferation and tumorigenesis. Sci Signal. 2013;6:1–12. doi: 10.1126/scisignal.2004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hohmann N, Weiwei W, Dahmen U, Dirsch O, Deutsch A, Voss-Bohme A. How does a single cell know when the liver has reached its correct size? PloS one. 2014;9:1–15. doi: 10.1371/journal.pone.0093207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller E, Yang J, DeRan M, Wu C, Su AI, Bonamy GM, et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chemistry & biology. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 57.Anakk S, Bhosale M, Schmidt VA, Johnson RL, Finegold MJ, Moore DD. Bile acids activate YAP to promote liver carcinogenesis. Cell reports. 2013;5:1060–1069. doi: 10.1016/j.celrep.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135:4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- 60.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Developmental cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Developmental cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao B, Ye X, Yu J, Li L, Li W, Li S, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao B, Kim J, Ye X, Lai Z, Guan K. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res. 2009;69:1089–1098. doi: 10.1158/0008-5472.CAN-08-2997. [DOI] [PubMed] [Google Scholar]
- 64.Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Developmental cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 65.Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 66.Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F, et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell research. 2014;24:331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Strano S, Munarriz E, Rossi M, Castagnoli L. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. Journal of Biological …. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- 68.Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003:33334–33341. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- 69.Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nature cell biology. 2015;17:7–19. doi: 10.1038/ncb3084. [DOI] [PubMed] [Google Scholar]
- 70.Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, et al. Yap1 activation enables bypass of oncogenic kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rayon T, Menchero S, Nieto A, Xenopoulos P, Crespo M, Cockburn K, et al. Notch and hippo converge on Cdx2 to specify the trophectoderm lineage in the mouse blastocyst. Developmental cell. 2014;30:410–422. doi: 10.1016/j.devcel.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, et al. KRAS and YAP1 Converge to Regulate EMT and Tumor Survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hofmann JJ, Zovein AC, Koh H, Radtke F, Weinmaster G, Iruela-Arispe ML. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137:4061–4072. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geisler F, Strazzabosco M. Emerging roles of Notch signaling in liver disease. Hepatology. 2015;61:382–392. doi: 10.1002/hep.27268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530–1542 e1512. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 78.Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 82.Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Overholtzer M, Zhang J, Smolen G. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proceedings of the …. 2006 doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim JM, Kang DW, Long LZ, Huang SM, Yeo MK, Yi ES, et al. Differential expression of Yes-associated protein is correlated with expression of cell cycle markers and pathologic TNM staging in non-small-cell lung carcinoma. Hum Pathol. 2011;42:315–323. doi: 10.1016/j.humpath.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 87.Zhang X, George J, Deb S, Degoutin JL, Takano EA, Fox SB, et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30:2810–2822. doi: 10.1038/onc.2011.8. [DOI] [PubMed] [Google Scholar]
- 88.Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T, et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010;70:8517–8525. doi: 10.1158/0008-5472.CAN-10-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fernandez-L A, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009:2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang T, Zhang J, You X, Liu Q, Du Y, Gao Y, et al. Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology. 2012;56:2051–2059. doi: 10.1002/hep.25899. [DOI] [PubMed] [Google Scholar]
- 91.Xu MZ, Yao T-J, Lee NPY, Ng IOL, Chan Y-T, Zender L, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, et al. Expression of Yes-associated protein in common solid tumors. Human pathology. 2008:1–8. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pei T, Li Y, Wang J, Wang H, Liang Y, Shi H, et al. YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget. 2015:1–15. doi: 10.18632/oncotarget.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perra A, Kowalik MA, Ghiso E, Ledda-Columbano GM, Di Tommaso L, Angioni MM, et al. YAP activation is an early event and a potential therapeutic target in liver cancer development. J Hepatol. 2014;61:1088–1096. doi: 10.1016/j.jhep.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 95.Ehmer U, Zmoos AF, Auerbach RK, Vaka D, Butte AJ, Kay MA, et al. Organ Size Control Is Dominant over Rb Family Inactivation to Restrict Proliferation In Vivo. Cell reports. 2014;8:371–381. doi: 10.1016/j.celrep.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen H, Mei L, Zhou L, Zhang X, Guo C, Li J, et al. Moesin-ezrin-radixin-like protein (merlin) mediates protein interacting with the carboxyl terminus-1 (PICT-1)-induced growth inhibition of glioblastoma cells in the nucleus. The international journal of biochemistry & cell biology. 2011;43:545–555. doi: 10.1016/j.biocel.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 97.Guo G, Chmielecki J, Goparaju C, Heguy A, Dolgalev I, Carbone M, et al. Whole-Exome Sequencing Reveals Frequent Genetic Alterations in BAP1, NF2, CDKN2A, and CUL1 in Malignant Pleural Mesothelioma. Cancer Res. 2015;75:264–269. doi: 10.1158/0008-5472.CAN-14-1008. [DOI] [PubMed] [Google Scholar]
- 98.Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360–363. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morrow KA, Das S, Metge BJ, Ye K, Mulekar MS, Tucker JA, et al. Loss of tumor suppressor Merlin in advanced breast cancer is due to post-translational regulation. J Biol Chem. 2011;286:40376–40385. doi: 10.1074/jbc.M111.250035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Armengol C, Cairo S, Fabre M, Buendia MA. Wnt signaling and hepatocarcinogenesis: the hepatoblastoma model. The international journal of biochemistry & cell biology. 2011;43:265–270. doi: 10.1016/j.biocel.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 101.Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Seminars in cancer biology. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang J, Park JS, Wei Y, Rajurkar M, Cotton JL, Fan Q, et al. TRIB2 acts downstream of Wnt/TCF in liver cancer cells to regulate YAP and C/EBPalpha function. Molecular cell. 2013;51:211–225. doi: 10.1016/j.molcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tao J, Calvisi DF, Ranganathan S, Cigliano A, Zhou L, Singh S, et al. Activation of beta-catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology. 2014;147:690–701. doi: 10.1053/j.gastro.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamada D, Rizvi S, Razumilava N, Bronk SF, Davila JI, Champion MD, et al. IL-33 facilitates oncogene-induced cholangiocarcinoma in mice by an interleukin-6-sensitive mechanism. Hepatology. 2015;61:1627–1642. doi: 10.1002/hep.27687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nishio M, Hamada K, Kawahara K, Sasaki M, Noguchi F, Chiba S, et al. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. The Journal of clinical investigation. 2012;122:4505–4518. doi: 10.1172/JCI63735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li S, Mao M. Next generation sequencing reveals genetic landscape of hepatocellular carcinomas. Cancer letters. 2013;340:247–253. doi: 10.1016/j.canlet.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 107.Shibata T, Aburatani H. Exploration of liver cancer genomes. Nature reviews Gastroenterology & hepatology. 2014;11:340–349. doi: 10.1038/nrgastro.2014.6. [DOI] [PubMed] [Google Scholar]
- 108.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fitamant J, Kottakis F, Benhamouche S, Tian HS, Chuvin N, Parachoniak CA, et al. YAP Inhibition Restores Hepatocyte Differentiation in Advanced HCC, Leading to Tumor Regression. Cell reports. 2015;10:1692–1707. doi: 10.1016/j.celrep.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo T, Lu Y, Li P, Yin MX, Lv D, Zhang W, et al. A novel partner of Scalloped regulates Hippo signaling via antagonizing Scalloped-Yorkie activity. Cell research. 2013;23:1201–1214. doi: 10.1038/cr.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koontz LM, Liu-Chittenden Y, Yin F, Zheng Y, Yu J, Huang B, et al. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Developmental cell. 2013;25:388–401. doi: 10.1016/j.devcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ganem NJ, Cornils H, Chiu SY, O’Rourke KP, Arnaud J, Yimlamai D, et al. Cytokinesis failure triggers hippo tumor suppressor pathway activation. Cell. 2014;158:833–848. doi: 10.1016/j.cell.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]