Abstract
Objective
Our objective in the present study was to conduct the first empirical study to examine regular physical activity habits and their relationship with brain volume and cortical thickness in patients in the early phase of schizophrenia. Relationships between larger brain volumes and higher physical activity levels have been reported in samples of healthy and aging populations, but have never been explored in first-episode schizophrenia patients.
Method
We collected MRI structural scans in fourteen first-episode schizophrenia patients with either self-reported low or high physical activity levels.
Results
We found a reduction in total grey matter volume, prefrontal cortex (PFC) and hippocampal grey matter volumes in the low physical activity group compared to the high activity group. Cortical thickness in the dorsolateral and orbitofrontal PFC were also significantly reduced in the low physical activity group compared to the high activity group. In the combined sample, greater overall physical activity levels showed a non-significant tendency with better performance on tests of verbal memory and social cognition.
Conclusions
Together these pilot study findings suggest that greater amounts of physical activity may have a positive influence on brain health and cognition in first-episode schizophrenia patients and support the development of physical exercise interventions in this patient population to improve brain plasticity and cognitive functioning.
Keywords: MRI, schizophrenia, exercise, cortical thickness, brain structure, cognition
INTRODUCTION
It is recognized that individuals suffering from schizophrenia have a greater prevalence of the metabolic syndrome (De Hert, Dekker et al. 2009, De Hert, Schreurs et al. 2009, Mitchell, Vancampfort et al. 2013), and premature mortality (Saha et al., 2007, McGrath et al., 2008) with a reduced life expectancy of about 20% compared with the general population (Newman and Bland 1991; Hausswolff-Juhlin et al., 2009). The leading natural cause of death in patients with schizophrenia is cardiovascular disease (Hennekens 2006; Brown et al., 2010). However, the cause-effect relationships in this context are not straightforward (Hausswolff-Juhlin, Bjartveit et al. 2009). Genetics, lifestyle factors (e.g., high fat diets, alcohol, smoking, and lack of regular exercise), disease-specific symptoms (e.g., anhedonia, avolition, apathy leading to obesity) and pharmacological treatments (e.g., antipsychotic medications) may each play a role (McCreadie 2003; Newcomer 2005, Joukamaa et al., 2006; Fritz-Wieacker et al., 2007; De Hert et al., 2009; De Hert et al., 2012).
Regarding modifiable lifestyle factors, 70–75% of patients with schizophrenia can be classified as physically inactive (Lindamer et al., 2008; Krogh et al., 2014) and 70-86% are overweight or obese (McCreadie, 2003; Fritz-Wieacker et al., 2007; Lee et al., 2013). Compared to healthy individuals, patients with schizophrenia have reduced maximal oxygen uptake (VO2 max); therefore, they are at higher risk for cardiovascular disease and lower quality of life (Heggelund, et al., 2011). VO2max is measured by respiratory gas analysis during subjection to gradually increasing workloads to the point of exhaustion (Maud et al, 2006) during incremental (i.e., progressively increasing work rate every n min) exercise as formally proposed by Taylor et al., 1955; Mitchell et al., 1958; and Åstrand & Saltin, 1961 (for review see Bassett & Howley, 2000). Peak oxygen uptake (VO2peak) is sometimes used interchangeably with VO2max for characterizing endurance during discontinuous exercise (where discrete square waves of different work rates are separated by periods of rest) (Day et al., 2003). A recent meta-analysis of the effects of physical activity in people with mental illness indicated reductions in symptoms of schizophrenia and depressive symptoms, and improvements in anthropometric measures, aerobic capacity, and quality of life (Rosenbaum et al., 2014). Schizophrenia outpatient case studies have reported improvements in social interest and reductions in anxiety and depression with exercise (Pelham et al., 1993; Adams 1994). There is also evidence that exercise therapy improves the cardiovascular fitness in patients with schizophrenia by increasing the VO2 max and peak work rate (Scheewe et al., 2012) while decreasing symptoms of schizophrenia, depression, and need of care when compared with occupational therapy (Scheewe et al., 2013; Krogh et al., 2014). High aerobic intensity training is reported to reduce distress and state anxiety, improve positive affect and well-being, enhance VO2max and contribute to lower risk of cardiovascular disease (Heggelund et al., 2014), although it had no effect on the psychiatric symptoms in patients with schizophrenia (Heggelund et al., 2011). Other studies were able to show reductions of positive and negative symptoms in schizophrenia patients after aerobic exercise (Beebe et al., 2005; Pajonk et al., 2010) though the differences did not reach statistical significance due to small sample sizes.
Recent neuroimaging research has highlighted the effect of physical (in)activity on brain morphology in patients with schizophrenia. Pajonk et al. (2010) showed a 12% hippocampal volume increase in the schizophrenia patients after aerobic exercise, in comparison with the non-exercising group, which was also related to improved short-term memory performance. Furthermore, the cognitive deficits in patients with schizophrenia, which affect attention, executive function, and memory (Nuechterlein et al. 2004; Malchow et al., 2013), have been correlated with physical inactivity (Lee et al., 2013). Studies in healthy subjects have previously showed improvement in cognitive functioning, particularly visuospatial learning and memory processes with regular exercise (Colcombe and Kramer 2003; Hillman et al., 2008) as a consequence of neurogenesis in the adult hippocampal dentate gyrus (Pereira et al., 2007). Exercise-induced structural changes can also be due to increased growth factors such as brain-derived neurotrophic factor (BDNF), which is highly expressed in the hippocampus, and essential in facilitating neurogenesis, synaptic plasticity, memory and learning (Poo 2001; Gomez - Pinilla et al., 2008). In chronic schizophrenia patients, improvements in physical fitness as a result of aerobic exercise training were related to increased serum BDNF levels (Kim et al., 2014; Kimhy et al., 2015). In a more recent study, Scheewe et al. (2013) reported an association of cardiorespiratory fitness improvement with cortical thickening in the left frontal, temporal and cingulate cortices, and a reduction in lateral and third ventricle volume in patients with schizophrenia. Falkai et al. (2013), however, found no difference in cortical thickness in schizophrenia patients after aerobic exercise, although he reported increased right frontal and occipital cortical gray matter density in healthy subjects, implying that any exercise effects on cortical gray matter are likely to be attenuated in chronic schizophrenia. Mittal et al. (2013) studied ultra high-risk individuals for psychosis, who had 65% higher rates of sedentary behavior than age-matched controls and found a decrease in medial temporal volumes of the patient group in comparison to the healthy controls. Furthermore, the total level of physical activity in the patient group was correlated with higher grey matter volumes in bilateral parahippocampal gyri (Mittal et al., 2013).
More research is needed to understand the effect and magnitude of the exercise and physical activity on brain morphology in patients with schizophrenia, especially in the early phase of illness, to devise better preventive measures for cardiovascular disease. The aim of this study was to investigate the relationship between naturalistic physical activity and brain morphology in first-episode psychosis patients who have been classified as having high or low levels of regular physical activity. Physical activity levels were assessed using the International Physical Activity Questionnaire (IPAQ) was used in individuals with schizophrenia in another study (Faulkner et al., 2006). In the present pilot study we were interested in examining the role of naturalistic physical activity levels on brain structure and cortical thickness in first-episode schizophrenia. Specifically, we were interested in investigating whether there is a global and/or regionally specific difference in prefrontal and hippocampal volumes between the first-episode schizophrenia patients with high versus low physical activity levels, and whether we could find any relationship between physical activity, neurocognition, and brain morphometry between these two groups of patients. Our a priori hypothesis was that first-episode schizophrenia patients with high physical activity levels would have higher cortical thickness and brain volumes than first-episode schizophrenia patients with low fitness activity levels. Since this was a novel, exploratory pilot investigation we used one-tailed statistical testing to investigate the effect of physical activity for the first time in first-episode schizophrenia patients.
METHOD
Participants
Baseline data collected from 14 recent-onset, first-episode schizophrenia (FE Sz) patients are included in this pilot investigation. Participants were all enrolled in the UCLA Aftercare Research Program, which is a longitudinal, NIMH-funded outpatient clinical research program for schizophrenia patients with a recent first psychotic episode. Patients are recruited from local inpatient and outpatient facilities in the Los Angeles area or are directly referred to the Program. Participants are provided with psychiatric medication management, psychoeducation, group skills training and individual case management for 18 months as participants in the program. Inclusion criteria were: 1) onset of a first psychotic episode within 24 months of program entry, 2) met criteria for schizophrenia, schizoaffective disorder, depressed type, or schizophreniform disorder (American Psychiatric Association 1994), 3) age of 18 to 45 years, and 4) sufficient fluency in English to allow for valid completion of the testing protocol. DSM-IV diagnoses were made using the Structured Clinical Interview for DSM-IV (SCID). Symptom ratings were made using the 18-item Brief Psychiatric Rating Scale (BPRS) to assess positive, negative and affective symptoms over the last two weeks. Additionally, all FE Sz patients were prescribed risperidone, and baseline testing occurred after medication stabilization, typically within three months of outpatient program entry. The university's institutional review board approved the protocol and all participants gave their written informed consent. Compliance with institutional research standards for animal or human research were completed in accordance with the Helsinki Declaration http://www.wma.net/e/policy/17-c_e.html).
Participant information, which was collected by the clinician who conducted the diagnostic interview, included demographic data (age, gender, handedness, ethnicity, education, parental education). Trained research staff members collected the weight and height data using a standard hospital scale to calculate BMI, and also administered and scored the neurocognitive battery (Nuechterlein et al., 2008), the Short-Form International Physical Activity Questionnaire (IPAQ; Craig et al., 2003) and the Starting the Conversation dietary assessment questionnaire (STC; Paxton, Strycker et al. 2011). The STC is an 8-item food frequency instrument validated for use in primary care and health promotion settings to assess regular dietary patterns. The item scores are added to create a summary score (range 0 –16), with lower summary scores reflecting a healthier diet and higher scores reflecting a lower healthy quality diet. For the purposes of this pilot investigation we were interested in the group differences in symptom severity (e.g., BPRS total scores) and dietary habits (e.g., STC total score) to examine the effects of more severe symptoms or poorer dietary habits, which could be driving the differences in brain morphometry.
Physical Activity Assessment
Participants completed the interviewer-administered IPAQ with the aid of a structured recall format that asked participants to recall activities for each of the last seven preceding days in morning, afternoon, and evening time periods. We used the seven-day reference period instead of asking what subjects ‘usually’ did in a week given the preference for this format in the initial validation of the IPAQ (Craig et al., 2003).
Data from the IPAQ were summarized according to walking, moderate (e.g., carrying light loads, bicycling at a regular pace, or easy swimming), and vigorous activities (e.g., heavy lifting, digging, aerobics, or fast bicycling) per week. On the basis of what activities subjects self-reported, the interviewer also clarified the perceived intensity of that specific activity. Responses were converted to Metabolic Equivalents in minutes per week (METS-min/wk) (Craig et al., 2003). According to the IPAQ scoring protocol: total minutes over last seven days spent on vigorous activity, moderate-intensity activity, and walking were multiplied by 8.0, 4.0, and 3.3, respectively, were assessed to create MET scores for each activity level. The low physical activity group met the following criterion, as indicated by the IPAQ scoring protocol: 1) No activity or 2) Less than: a) 3 days of vigorous-intensity activity of at least 20 min/day or b) 5 days of moderate-intensity activity and/or walking of at least 30 min/day or c) 5 days of any combination of walking, moderate-intensity or vigorous intensity activities with minimum total physical activity of at least 600 MET min/week. The high physical activity group met the following criterion: 1) vigorous-intensity activity on at least 3 days minimum total physical activity of at least 1500 MET min/week or 2) 7 or more days of any combination of walking, moderate-intensity or vigorous-intensity minimum total physical activity of at least 3000 MET min/week.
Neurocognitive Assessment
The Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) (Nuechterlein et al., 2008) includes seven cognitive domains: speed of processing (Tests: Category Fluency, BACS Symbol coding, Trail Making A), attention/vigilance (Continuous Performance-Identical Pairs Version), working memory (Letter Number Span, WMS-II Spatial Span), verbal learning (Hopkins Verbal Learning Test-R), visual learning (Brief Visuospatial Memory Test-R), reasoning and problem solving (NAB Mazes), and social cognition (MSCEIT Managing Emotions). In addition to the seven domain scores, the MCCB also provides an Overall Composite score, an index of cognitive functioning across domains. The Overall Composite score is derived through equal weighting of the seven MCCB domain scores (Nuechterlein, Green et al. 2008). All analyses of MCCB scores used age and gender correction based on the MCCB computer scoring program. Based on previous studies of the effects of physical activity on cognition in schizophrenia (Pajonk et al., 2010; Malchow et al., 2015) we looked at group differences from the sub-domain of verbal learning, in addition to the overall MCCB composite score. We also conducted an exploratory investigation of social cognition, due to the higher levels of social abilities seen in clinical samples of patients who regularly exercise (Taylor, Sallis et al. 1985).
Neurocognitive testing procedures for administration of the MCCB have been previously described (Nuechterlein, Green et al. 2008). Briefly, FE Sz patients completed the MCCB as part of their baseline assessment. Raw scores for each MCCB test were converted to age and gender corrected T-scores for the general population using the MCCB scoring program (Mean = 50, SD = 10). For all participants, testing was conducted by bachelor's level examiners who received extensive training in administration of the MCCB. All examiners participated in periodic checks on MCCB administration and scoring practices.
Brain Imaging
MRI Image Acquisition
All scanning was performed on a Siemens Trio 3T scanner with a 12-channel head coil at the UCLA Staglin Center for Cognitive Neuroscience. Subject's head movement was minimized with the use of foam padding. A high-resolution T1-weighted anatomical scan was acquired using a magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence with a repetition time (TR) = 2300ms, echo time (TE) = 2.91ms, inversion time (TI) = 900ms, flip angle = 9 degrees, and field-of-view (FOV) of 256mm (anterior-to-posterior) × 240mm (superior-to-inferior) × 176mm (left-to-right), resulting in a voxel resolution of approximately 1mm × 1mm × 1.2mm.
Segmentation, volumetry cortical reconstruction, and cortical thickness calculation
Image processing and analyses of MR data including brain segmentation, cortical reconstruction and cortical thickness estimations were conducted in the UCLA Brain Tumor Imaging Laboratory (BTIL) within the Center for Computer Vision and Imaging Biomarkers (CVIB). MPRAGE scans were first assessed for quality to ensure the absence of artifacts such as aliasing. The MR data were then processed via FreeSurfer (version 4.3; http://surfer.nmr.mgh.harvard.edu) cortical reconstruction pipeline wherein each subjects cortical surface and thickness at each vertex were computed using a semi-automated approach previously described in detail (Dale and Sereno 1993; Dale et al., 1999, Fischl et al., 1999a, Fischl et al., 1999b; Fischl and Dale 2000; Salat et al., 2004). In short, automated serial manipulations of MR data for cortical rendering included: 1) transforming the 3D, T1-weighted MRI data into Talairach coordinates, 2) normalizing image signal intensity to correct for unwanted variations in intensity due to RF-field inhomogeneity, 2) the stripping of the skull and other extra-cerebral tissue using a watershed algorithm, 3) parcellating and labeling the white matter volume based upon normalized intensity, 4) correcting topological errors and smoothing the generated surfaces, and 5) the construction of cortical surface from the white/gray matter interface to the pial surface at the gray matter/CSF interface. The resulting segmentations were visually inspected on a slice-by-slice basis to ensure correct delineation of pial from dura surfaces and parcellation of subcortical white matter structures. Manual editing of the pial surface and white matter was done as needed to improve the accuracy of the segmentation. Cortical thickness measurements were then obtained from calculating the distance between pial surface and the gray/white matter boundary (Dale and Sereno 1993; Dale et al., 1999). The volume of gray matter and subcortical white matter structures including the hippocampus were measured automatically using FreeSurfer as described elsewhere (Fischl et al., 2002). Following this procedure, resulting three dimensional cortical surfaces were aligned to a standardized mesh surface with a mesh density linear depth of 141 for use in group comparisons, blurred using a Gaussian filter with a full width at half max (FWHM) of 8 mm, then labels of the region of interest comprising the prefrontal cortex (PFC) and hippocampus were examined in standard space and gray and white volumes were tabulated. Additionally, in lieu of a false discovery rate analysis, due to the limited sample size and degrees of freedom, we choose to use interactive clustering on the surface data to achieve the critical cluster size corresponding to a significance value of p ≤ 0.05. The application of the cluster size area 100 mm2 is therefore meant to reflect the corrected p-value of 0.05. Lastly, comparisons between patient groups were performed and cortical thickness comparisons were visualized in standard space.
Statistical Analysis
All analyses were performed in the statistical software SPSS 22.0. Prior to conducting group analyses we conducted Shapiro-Wilk's tests to test for normal distribution in this small sample. From the tests we found that brain volumes, cognition and physical data, were all not significant, indicating normality and we concluded that the parametric t-tests and Pearson correlations were the appropriate tests. All demographic, clinical, physical and neurocognitive data were compared across groups using Student's independent t-tests (two-tailed α=.05). Group comparisons of brain volumes and cortical thickness measurements from a priori regions of interest, including total cortical grey matter, prefrontal cortex (PFC) and hippocampus (Hillman et al. 2008; Pajonk et al., 2010; Scheewe et al., 2012; Mittal et al., 2013) were analyzed using Student's independent t tests (one-tailed α = .05), since we had a specific hypothesis on directionality in a sample of FE Sz patients. Also due to the small sample size and exploratory nature of this investigation Cohen's d effect size estimates were calculated for brain volume, cortical thickness and cognitive data.
As an exploratory investigation, we sought to test for associations between brain volume/cortical thickness and physical measurements (e.g. BMI) and also between physical activity levels (e.g. Total METS/week) and cognition (MCCB: Verbal learning, social cognition and overall composite MCCB scores). Pearson bivariate correlations were carried out across groups to explore potential relationships (two-tailed α = .05).
RESULTS
Demographic and Clinical Characteristics
Demographic, clinical, and physical information for all participants can be found in Table 1. There were no significant differences in age, sex, handedness, ethnicity, personal or parental education, or symptom ratings. All patients were on oral risperidone awaiting random assignment to treatment with either oral or injectable risperidone as part of the research protocol. Physical measurements showed that there was not a significant difference in BMI, even though the high physical activity group had a significantly higher amount of METS min/week (t = 21.99, p = .001). Additionally no group differences were found between dietary habits derived from the STC questionnaire.
Table 1.
Demographic, clinical, physical measurements and neurocognitive data of first-episode schizophrenia patients, grouped by physical activity levels
| Low PA (n = 7) | High PA (n = 7) | t-test/Chi-square/Effect sizea | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t/X2 | p | d | |
| Demographics | |||||||
| Age (years)b | 23.4 | 3.6 | 23.3 | 2.8 | 0.01 | 0.94 | |
| Sex (n, Female/Male) | 2 / 5 | 1 / 6 | 4.24 | 0.50 | |||
| Handedness (n, Left/Right) | 1 / 6 | 1 / 6 | 0.01 | 0.91 | |||
| Ethnicity (n) | 4 Latino, Mexican 2 African American 1 Latin, White |
1 Latino, Mexican 2 Latin, White 3 Non-Latin, White 1 Non-Latin, Mexican |
8.13 | 0.08 | |||
| Education (years) | 12.6 | 1.0 | 12.7 | 1.7 | 0.04 | 0.85 | |
| Parental Education | 15 | 2.0 | 15.1 | 2.0 | 0.17 | 0.90 | |
| Symptom ratings | |||||||
| BPRS Total Score | 58 | 12 | 49 | 15 | 1.20 | 0.25 | 0.65 |
| Health and Physical Measures | |||||||
| BMI | 27 | 5.0 | 29 | 6 | 0.46 | 0.51 | −0.35 |
| Total METS min/week (IPAQ) | 323 | 153 | 5510 | 2922 | 21.99 | 0.001 | −2.51 |
| Dietary Habits (STC) | 6.4 | 2.4 | 6.6 | 2.5 | 0.11 | 0.96 | −0.08 |
| Neurocognitive Assessment | |||||||
| MCCB: Composite | 26.7 | 7.3 | 29.0 | 16.9 | 0.11 | 0.75 | 0.32 |
| MCCB: Verbal Memory | 34.7 | 6.7 | 38.0 | 10.8 | 0.47 | 0.51 | 0.50 |
| MCCB: Social Cognition | 32.1 | 8.1 | 43.7 | 13.3 | 3.86 | 0.07 | 1.43 |
PA = Physical Activity; BPRS = Brief Psychiatric Rating Scale; BMI = Body Mass Index; METS = Metabolic equivalents; IPAQ = International Physical Activity Questionnaire; STC = Starting Conversation; MCCB = MATRICS Consensus Cognitive Battery
Significance tests were two-tailed
Age was defined as age at MRI scanning
Group Differences in Cognitive Performance
Baseline cognitive data are presented in Table 1. Although there was not a significant difference between groups on the neuropsychological measures, there was a non-significant tendency toward lower cognitive functioning on all three measures in the low physical activity group. The largest effect size observed was for social cognition (Cohen's d = 1.43), a moderate effect size for verbal memory (d = 0.50), and a small effect size for the MCCB overall composite (d = 0.32).
Group Differences in Brain Volumes
Comparisons of total cortical grey matter volume, total PFC grey matter volume, left PFC grey matter volume, right PFC grey matter volume all yield significant group differences revealing reduced volumes in the low physical activity group (p = 0.03, Cohen's d = 1.09; p = 0.04, d = 1.00; p = 0.04, d = 1.02; and p = 0.05, d = 0.98 respectively) (Table 2). When we examined the cortical parcel sub-regions comprisimising the PFC we found that although almost all sub-regions were reduced in the low physical activity group eight focal regions were potentially driving the differences in overall PFC and included the: left middle frontal gyrus (p = .05), left orbital gyrus (p = .02), left precentral gyrus (p = .04), left superior frontal sulcus (p = .05), left supeior part of the precentral sulcus (p = .05), right traverse frontopolar gyrus and sulcus (p = .04), right straight gyrus (p = .005), and right inferior part of the precentral sulcus (p = .01). Figure 1 displays a hippocampal statistical distribution for the two patient groups. There was also a significant reduction in total hippocampal volume (p = 0.05, d = 0.94) and left hippocampal volume (p = 0.05, d = 0.95) in the low physical activity group (Table 2), but not for the right hippocampal volume, although it was on average smaller in the low activity group (p = 0.07, d = 0.84).
Table 2.
Baseline volumetry and group differences for total cortical grey matter volume and regions of interest
| Low Physical Activity |
High Physical Activity |
t-testa/Effect size |
|||||
|---|---|---|---|---|---|---|---|
| Cortical Region | Mean | SD | Mean | SD | t | p | d |
| Total Grey Matter Volume (cm3) | 444.5 | 46.0 | 499.3 | 54.0 | t = 2.03 | p = 0.03 | d = 1.09 |
| Total PFC Volume | 137.2 | 15.0 | 151.5 | 17.0 | t = 1.88 | p = 0.04 | d = 1.00 |
| Left PFC Volume | 69.6 | 7.9 | 76.9 | 7.7 | t = 1.91 | p = 0.04 | d = 1.02 |
| Right PFC Volume | 67.6 | 7.9 | 74.5 | 9.4 | t = 1.83 | p = 0.05 | d = 0.98 |
| Total Hippocampal Volume | 8.1 | 0.5 | 8.9 | 1.0 | t = 1.75 | p = 0.05 | d = 0.94 |
| Left Hippocampal Volume | 4.0 | 0.3 | 4.4 | 0.5 | t = 1.78 | p = 0.05 | d = 0.95 |
| Right Hippocampal Volume | 4.1 | 0.3 | 4.5 | 0.6 | t = 1.56 | p = 0.07 | d = 0.84 |
PFC = Prefrontal Cortex
Significance tests were one-tailed
Figure 1.
Hippocampal probability distribution region of interest overlaid atop of MNI atlas brain. Patient brains and hippocampal ROI data were registered to MNI space and then averaged, resulting in (A) high physical activity patient population hippocampal probability distribution and (B) low physical activity patient population hippocampal probability distribution. (C) and (D) are magnified High PA (top) and Low PA (bottom) probablitiy distributions to underscore the difference in mean hippocampal volume between the two groups. PA= Physical Activity
Group Differences in Cortical Thickness
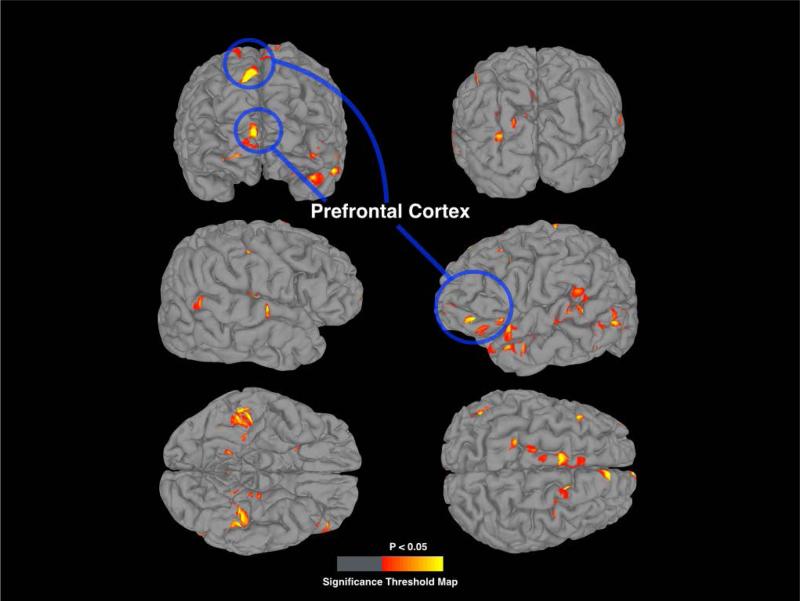
Figure 2 displays the average cortical thickness from each group. Figure 3 shows a corrected statistical map threshold of p = 0.05 for whole brain cortical thickness, showing regions that had reduced thickness in the low physical activity group compared to the high physical activity group, overlaid onto a standardized brain. Cortical thickness within sub-regions of the PFC was signficantly reduced in the low physical activity group in the dorsolateral and orbitofrontal cortex. Table 3 lists the location, anatomic labels, and comparison statistics for significant clusters.
Figure 2.
Standardized 3D cortical renders (left panel) and inflated cortex (right panel) overlaid with the average cortical thickness between the (A) high physical activity group and (B) low physical activity group. PA= Physical Activity
Figure 3.
Cortical thickness t-test comparison of high and low physical activity groups overlaid on standardize surface mesh displaying brain regions of greater cortical thickness. The t-statistical map is threholded to significance value of p = 0.05 and a cluster area threshold of 100 mm2. Regions highlighted in the blue circle signify brain regions in the dorsolateral and orbitofrontal prefrontal cortex which had reduced cortical thickness in the low physical activity group compared to the high physical activity group.
Table 3.
Location, anatomic labels, and comparison statistics for significant clusters from the cortical thickness analysis
| Low PA |
High PA |
||||
|---|---|---|---|---|---|
| Cortical Region | MNI Coordinates | Mean (mm) | Mean (mm) | T-stat (mm) | Effect size (mm) |
| Right Hemisphere | |||||
| Superior frontal gyrus | 10.3, 66.9, 29.4 | 2.399 | 3.169 | 5.651 | 0.77 |
| Transverse frontopolar gyri and sulci | 10.0, 89.3, −13.0 | 2.285 | 2.889 | 2.889 | 0.604 |
| Angular gyrus | 65.0, −29.0, 18.8 | 2.2 | 2.884 | 3.08 | 0.684 |
| Lateral occipito-temporal gyrus | 46.4, −28.7, −46.0 | 2.568 | 3.393 | 4.105 | 0.825 |
| Left Hemisphere | |||||
| Superior frontal gyrus | −6.9, 44.7, 44.6 | 3.074 | 3.433 | 4.583 | 0.359 |
| Lateral aspect of superior temporal gyrus | −43.5, 41.1, −47.9 | 3.191 | 3.883 | 3.44 | 0.692 |
| Inferior temporal gyrus | −60.1, −47.1, −24.9 | 2.681 | 3.496 | 3.804 | 0.815 |
| Supramarginal gyrus | −67.4, −12.1, 2.0 | 2.727 | 3.068 | 2.804 | 0.341 |
| Lateral occipito-temporal gyrus | −39.3, −22.6, −46.7 | 2.877 | 3.477 | 3.891 | 0.6 |
| Middle occipital gyrus | −31.8, −72.7, 13.1 | 3.011 | 3.467 | 3.164 | 0.456 |
PA = Physical Activity
Correlations of Brain Volumes, Cortical Thickness, and Cognition
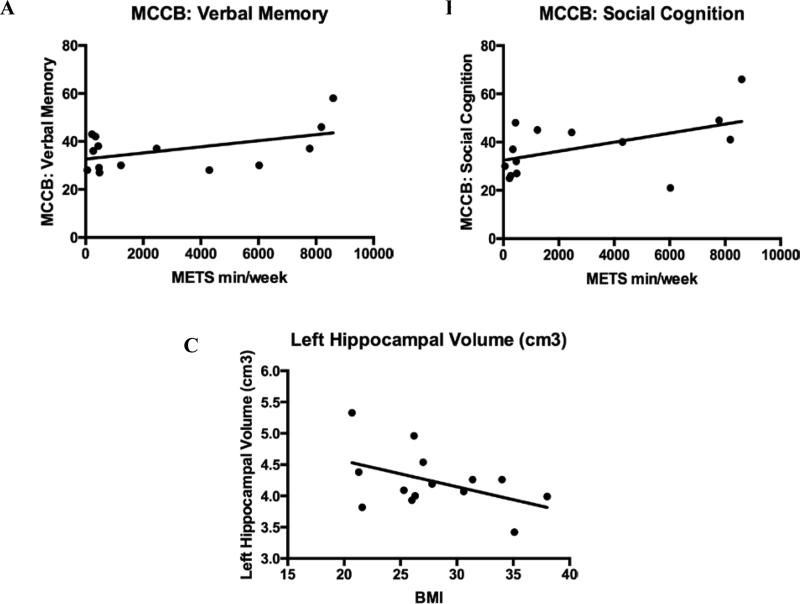
In an exploratory analysis we examined the relationship between brain volume/cortical thickness and physical measurements (e.g. BMI) and also between physical activity levels (e.g., Total METS/week) and neurocognition (MCCB: Verbal memory, social cognition and overall composite scores). Although none of the correlations were significant, there was a non-significant tendency toward relationships between the amount of total METS min/week (e.g. total physical activity) and scores on verbal learning (r = .49, p = .08) and social cognition (r = .52, p = .06) (Figure 4). There was also a non-significant tendency between lower BMI and larger left hippocampal volume (r = −.50, p = .09) (Figure 4).
Figure 4.
Correlations in the combined sample of first-episode schizophrenia patients (n = 14). Scatterplots showing the near significant relationship between METS min/week (e.g. Total physical activity per week) and neurocognitive performance and hippocampal volume and BMI. Trend level correlations between the amount of total METS min/week (e.g. total physical activity) and performance scores in (A) verbal learning and memory (r = .49, p = .08) and (B) social cognition (r = .52, p = .06). (C) Larger left hippocampal volume also showed a trend for a correlation with lower BMI (r = −.50, p = .09). METS = Metabolic equivalents; BMI = Body Mass Index
DISCUSSION
To our knowledge, this is the first study to investigate the role of naturalistic physical activity levels on brain structure and cortical thickness in first-episode schizophrenia. This investigation is an important starting point in a burgeoning field of stuying the role of exercise as a novel intervention to increase brain plasticity and improve cognition in schizophrenia. We found that FE Sz patients with self-reported low levels of physical activity had reductions in total grey matter volume, specifically within the PFC and hippocampus. We also found a reduction in cortical thickness in the PFC regions of the dorsolateral and obitofrontal cortex in patients with lower physical activity levels. In an exploratory analysis, we found non-significant tendencies toward relationships in FE Sz patients between greater self-reported physical activity and cognitive performance on tests of verbal learning and social cognition. Lastly, we found a trend level correlation between lower BMI and larger left hippocampal volume. Together these findings highlight the possibility that physical activity has an potential impact on brain morphometry in FE Sz patients and supports the need to initiate interventions promoting physical exercise in this patient population not only to stave off deletrious physical health but also to improve cognitive functioning and brain health. This study also highlights that prior to starting an exercise intervention study, researchers need to examine the role of baseline levels of physical activity and their relationships to brain structures at study entry, as this may have an effect on the magnitude of the treatment response.
Previous research on the benefits of physical activity on brain plasticity in older sedentary adults found that increasing physical exercise increased cerebral gray matter volume (Colcombe et al., 2006), and more specifically within the PFC (Erickson et al., 2012) suggeting the role of increasing physical activity on overall brain health and strucural integrity. In line with our PFC findings, a recent study with chronic schizophrenia patients, Scheewe et al. (2013) reported an association of cardiorespiratory fitness improvement with cortical thickening in the left frontal, temporal and cingulate cortices, and a reduction in lateral and third ventricle volume in patients with schizophrenia. Falkai et al. (2013), however, found no difference in cortical thickness in schizophrenia patients after aerobic exercise, although they did find increased right frontal and occipital cortical gray matter density in healthy subjects implying that any exercise effects on cortical gray matter may be attenuated in chronic schizophrenia.
The hippocampus is a brain region most commonly associated with the greatest sensitivity to volume increases as a result of greater physical activity levels. Much of this work valiating this relationship between increased exercise, hippocampal size, and memory functioning has been carried out in mice (van Praag et al., 1999) and aging humans (Erickson et al., 2012), but also in chronic schizophrenia patients (Pajonk et al., 2010) and ultra-high risk for psychosis youth (Mittal et al., 2013). Research from Mittal et al. (2013) in youth at high-risk for developing psychosis revealed that these patients at an imminent risk for developing schizophrenia have lower objectively recorded physical activity levels than matched healthy controls. Furthermore, they found that those at high-risk who also have a more sedentary lifestyle had smaller medial temporal structure volumes. Although they were not able to provide data on conversion to psychosis in the high-risk sample, this study highlights that lower physical activity levels and related smaller medial temproal lobe volumes might be potential treatment targets to delay or prevent conversion to a full-blown psychotic illness. These exercise-induced structural changes in the hippocampus have been found to be related to increased growth factors such as brain-derived neurotrophic factor (BDNF), which are highly expressed in the hippocampus, and essential for facilitating neurogenesis, synaptic plasticity, memory and learning (Poo 2001; Gomez - Pinilla et al., 2008).
Our findings suggest that higher amounts of physical activity levels are associated with larger brain volumes, overall and in the PFC and hippocampus, along with cortical thickness in the PFC in FE Sz patients. Although the underlying mechanisms of brain volume increases as a result of increased physical fitness is still relatively speculative in humans, increased production of neurotrophins, (e.g. BDNF, IGF-1, VEGF), increased neurogenesis, syntaptogenesis, vascularization and improved cell energy metabolosim all seem to play a critical role in animal studies (Neeper et al., 1995; Cotman et al., 2007; van Praag 2008). In schizophrenia patients there have been two published studies that have explored the role of serum-levels of BDNF before and after an exercise intervention (Kim et al. 2014; Kimhy et al., 2015). Both studies found elevated serum levels change in BDNF from baseline to 12-week follow-up, and one study also found a relationship between improved physical fitness and cognition, measured by the MCCB composite score (Kimhy et al., 2015)
Although the present correlations of physical activity levels and cognitive functioning were not significant, there was a large effect size with social cognition and a medium effect size with verbal learning. These findings were further supported by the non-significant tendency toward a correlation between total amounts of physical activity completed per week and higher scores on these neurocognitive measures. The finding of greater physical activity and increased verbal memory abilities is in line with an intervention study of exercise in chronic schizophrenia patients (Pajonk et al., 2010; Malchow et al., 2015). The finding of a tendency for higher social cognitive functioning to be associated with greater physical activity is a novel finding. Previous research has suggested that exercise can improve symptoms of depression, social, anhedonia and low self-esteem in patients with schizophrenia, which may provide he link between greater amounts of physical activity and social cognitive functioning (Gorczynski and Faulkner 2010). Additionally, one study in schizophrenia spectrum disorder patients found a relationship between greater amounts of sedentary behavior and lower performance on a test of metacongition (Snethen et al., 2014). We would posit that the increase in higher order social cognition is supported by the underlying increased PFC volume in the higher activity patients, which subserves these processes.
Although there is a growing literature to support the need to develop effective interventions to promote a physically active lifestyle in chronic schizophrenia patients, to date, there has been only one published exercise intervention study in FE Sz patients (Abdel-Baki et al., 2013). Although it was not a randomized controlled trial, it did show the feasibility of a 14-week aerobic exercise intervention and the ability of the intervention to improve physical fitness in a FE Sz sample. There have yet to be any published studies of the effects of exercise on cognition and brain structure in FE Sz. Published exercise interventions in schizophrenia samples are therefore limited to chronic patient samples (illness duration/chronicity ~10 years). These aerobic exercise interventions range in duration from 6-24 weeks, requiring ~90 minutes of exercise per week, have high adherence to supervised group exercise training (79%) and show significant improvements in physical health and symptomotology (for review see Firth et al. 2015). Together these studies show the feasibility of intensive exercise interventions in schizophrenia patients and the ability to reduce deletrious health outcomes and improve functioning. The next step is to develop small randomized controlled exercise trials to test feasibility and target behavioral outcomes in FE Sz. Then larger treatment trials can be implemented to explore the neural mechanisms underlying improvements in brain health and cognitive functioning in FE Sz.
Our study results must be evaluated in the context of some limitations. The sample size of this pilot investigation is very small. Further research of physical activity levels in larger samples is warranted to determine the relationship between brain morphometry and physical acitivty levels in FE Sz patients. Also due to the small sample size for the volumetric imaging analysis, we reported one-tailed significance tests and effect sizes, which proved to be a useful method for our pilot and exploratory analysis but need to be replicated with greater statistical rigor in a larger sample. We did seek to explore potential confounds that would affect brain structures that were not related to activity levels, such as anti-psychotic medication type (which was the same for all patients in our sample), age, gender, education, BMI, symptom severity and dietary habits, and we did not find any group differences. Future studies should also take into account these potentially confounding factors. Additionally, our study groupings depended on a self-report tool for the assessment of physical activity habits, which may influence the validity and reliability of recalled data, even though the IPAQ questionnaire used in our study had been previously used in another study with schizophnreia patients (Faulkner et al., 2006). Concern regarding the inconsistency between self-reported and directly measured physical activity were recently described by Prince et al. (2008), who systematically reviewed the literature to determine the extent of discrepancy between subjectively (self-report e.g. questionnaire, diary) and objectively (directly measured; e.g. accelerometry) assessed adult physical activity. Their findings suggest that self-report measures were both higher and lower than directly measured levels of physical activity, although no clear trends emerged in the over- or underreporting of physical activity by self-report compared to direct methods (Prince et al., 2008). Because of the potential overestimation of the physical activity values by subjective measures (Sebastiao et al., 2012), in future studies we would also seek to collect objective longitudinal measures of current physical activity habits, using actigraphs or pedometers. However, these measurements also have their limitations since they could interfere with naturalistic data collection and cause a change in the patients typical physical activty behaviors. Objective measures of physical fitness, such as VO2max, would help to assess cardiovascular fitness to determine group classification (e.g., low vs. high fitness levels). It is also likely that the correlation between the physical activity and social cognition and/or verbal learning might be due to the contribution of several mechanisms rather than a single mechanism acting in isolation (Faulkner & Carless, 2006). Therefore, we would also like to collect more historical data on lifetime physical activity habits, as current activity levels after having been diagnosed with psychosis may not be reflective of activity levels prior to illness onset. We currently have an RCT underway to examine the effects of a physical activity intervention on brain strucure, neurotrophin levels and cognitive functioning in FE Sz patients.
ACKNOWLEDGEMENTS
We thank the participants for giving their time to this study. We gratefully acknowledge the assistance of the UCLA Aftercare Research Program research assistants Jacqueline Hayata, B.A., and Livon Ghermezi, B.A., and clinic staff Laurie Casaus, M.D., John Luo, M.D., Yurika Sturdevant, Psy.D., and Luana Turner, Psy.D. None of the study sponsors had any role in the data collection, analysis, or write up of the current paper. The authors have declared that there are no conflicts of interest in relation to the subject of this study. This work was supported by the National Institute of Mental Health (S.M., K01 MH099431), (K.N., R34 MH102529), (K.N., P50 MH066286).
REFERENCES
- Abdel-Baki A, Brazzini-Poisson V, Marois F, Letendre E, Karelis AD. Effects of aerobic interval training on metabolic complications and cardiorespiratory fitness in young adults with psychotic disorders: a pilot study. Schizophr Res. 2013;149(1-3):112–115. doi: 10.1016/j.schres.2013.06.040. [DOI] [PubMed] [Google Scholar]
- Adams L. How exercise can help people with mental health problems. Nursing times. 1994;91(36):37–39. [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Astrand P, Saltin B. Oxygen uptake during the first minutes of heavy muscular exercise. Journal of Applied Physiology. 1961;16(6):971–976. doi: 10.1152/jappl.1961.16.6.971. [DOI] [PubMed] [Google Scholar]
- Bassett DR, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Medicine and science in sports and exercise. 2000;32(1):70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- Beebe LH, Tian L, Morris N, Goodwin A, Allen SS, Kuldau J. Effects of exercise on mental and physical health parameters of persons with schizophrenia. Issues in Mental Health Nursing. 2005;26(6):661–676. doi: 10.1080/01612840590959551. [DOI] [PubMed] [Google Scholar]
- Brown S, Kim M, Mitchell C, Inskip H. Twenty-five year mortality of a community cohort with schizophrenia. The British journal of psychiatry. 2010;196(2):116–121. doi: 10.1192/bjp.bp.109.067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults a meta-analytic study. Psychological science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J Cogn Neurosci. 1993;5(2):162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Day JR, et al. The maximally attainable VO2 during exercise in humans: the peak vs. maximum issue. Journal of applied physiology. 2003;95(5):1901–1907. doi: 10.1152/japplphysiol.00024.2003. [DOI] [PubMed] [Google Scholar]
- De Hert M, Dekker J, Wood D, Kahl K, Holt R, Möller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). European Psychiatry. 2009;24(6):412–424. doi: 10.1016/j.eurpsy.2009.01.005. [DOI] [PubMed] [Google Scholar]
- De Hert M, Detraux J, Van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nature Reviews Endocrinology. 2012;8(2):114–126. doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- De Hert M, Schreurs V, Vancampfort D, Winkel R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8(1):15–22. doi: 10.1002/j.2051-5545.2009.tb00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18(1):82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkai P, Malchow B, Wobrock T, Gruber O, Schmitt A, Honer WG, Cannon TD. The effect of aerobic exercise on cortical architecture in patients with chronic schizophrenia: a randomized controlled MRI study. European archives of psychiatry and clinical neuroscience. 2013;263(6):469–473. doi: 10.1007/s00406-012-0383-y. [DOI] [PubMed] [Google Scholar]
- Faulkner G, Carless D. Physical activity in the process of psychiatric rehabilitation: Theoretical and methodological issues. Psychiatric Rehabilitation Journal. 2006;29(4):258. doi: 10.2975/29.2006.258.266. [DOI] [PubMed] [Google Scholar]
- Faulkner G, Cohn T, Remington G. Validation of a physical activity assessment tool for individuals with schizophrenia. Schizophr Res. 2006;82(2-3):225–231. doi: 10.1016/j.schres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Firth J, Cotter J, Elliott R, French P, Yung AR. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med. 2015;45(7):1343–1361. doi: 10.1017/S0033291714003110. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz-Wieacker A, Matschinger H, Heider D, Schindler J, Riedel-Heller S, Angermeyer MC. Health habits of patients with schizophrenia. Social psychiatry and psychiatric epidemiology. 2007;42(4):268–276. doi: 10.1007/s00127-007-0164-5. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. European Journal of Neuroscience. 2008;28(11):2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski P, Faulkner G. Exercise therapy for schizophrenia. Cochrane Database Syst Rev. 2010;(5):CD004412. doi: 10.1002/14651858.CD004412.pub2. doi:10.1002/14651858.CD004412.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausswolff-Juhlin V, Bjartveit M, Lindström E, Jones P. Schizophrenia and physical health problems. Acta Psychiatrica Scandinavica. 2009;119(s438):15–21. doi: 10.1111/j.1600-0447.2008.01309.x. [DOI] [PubMed] [Google Scholar]
- Heggelund J, Hoff J, Helgerud J, Nilsberg GE, Morken G. Reduced peak oxygen uptake and implications for cardiovascular health and quality of life in patients with schizophrenia. BMC psychiatry. 2011;11(188):1–8. doi: 10.1186/1471-244X-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggelund J, Kleppe KD, Morken G, Vedul-Kjelsås E. High aerobic intensity training and psychological states in patients with depression or schizophrenia. Frontiers in psychiatry. 2014;5(148):1–8. doi: 10.3389/fpsyt.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggelund J, Nilsberg GE, Hoff J, Morken G, Helgerud J. Effects of high aerobic intensity training in patients with schizophrenia-A controlled trial. Nordic journal of psychiatry. 2011;65(4):269–275. doi: 10.3109/08039488.2011.560278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekens CH. Increasing global burden of cardiovascular disease in general populations and patients with schizophrenia. The Journal of clinical psychiatry. 2006;68:4–7. [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature reviews neuroscience. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Joukamaa MM, Heliövaara M, Knekt P, Aromaa A, Raitasalo R, Lehtinen V. Schizophrenia, neuroleptic medication and mortality. The British Journal of Psychiatry. 2006;188(2):122–127. doi: 10.1192/bjp.188.2.122. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Song BK, So B, Lee O, Song W, Kim Y. Increase of circulating BDNF levels and its relation to improvement of physical fitness following 12 weeks of combined exercise in chronic patients with schizophrenia: A pilot study. Psychiatry research. 2014;220(3):792–796. doi: 10.1016/j.psychres.2014.09.020. [DOI] [PubMed] [Google Scholar]
- Kimhy D, Vakhrusheva J, Bartels MN, Armstrong HF, Ballon JS, Khan S, Sloan RP. The Impact of Aerobic Exercise on Brain-Derived Neurotrophic Factor and Neurocognition in Individuals With Schizophrenia: A Single-Blind, Randomized Clinical Trial. Schizophr Bull. 2015 doi: 10.1093/schbul/sbv022. doi: 10.1093/schbul/sbv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh J, Speyer H, Nørgaard HCB, Moltke A, Nordentoft M. Can exercise increase fitness and reduce weight in patients with schizophrenia and depression? Frontiers in psychiatry. 2014;5(89):1–6. doi: 10.3389/fpsyt.2014.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EH, Hui CL, Chang WC, Chan SK, Li Y, Lee JT, Chen EY. Impact of physical activity on functioning of patients with first-episode psychosis—A 6months prospective longitudinal study. Schizophrenia research. 2013;150(2):538–541. doi: 10.1016/j.schres.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Lindamer LA, McKibbin C, Norman GJ, Jordan L, Harrison K, Abeyesinhe S, Patrick K. Assessment of physical activity in middle-aged and older adults with schizophrenia. Schizophrenia research. 2008;104(1):294–301. doi: 10.1016/j.schres.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow B, Keller K, Hasan A, Dörfler S, Schneider-Axmann T, Hillmer-Vogel U, Falkai P. Effects of Endurance Training Combined With Cognitive Remediation on Everyday Functioning, Symptoms, and Cognition in Multiepisode Schizophrenia Patients. Schizophr Bull. 2015 doi: 10.1093/schbul/sbv020. doi: 10.1093/schbul/sbv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow B, Reich-Erkelenz D, Oertel-Knöchel V, Keller K, Hasan A, Schmitt A, Falkai P. The effects of physical exercise in schizophrenia and affective disorders. European archives of psychiatry and clinical neuroscience. 2013;263(6):451–467. doi: 10.1007/s00406-013-0423-2. [DOI] [PubMed] [Google Scholar]
- Maud PJ, Foster C. Physiological assessment of human fitness. Human Kinetics. 2006 [Google Scholar]
- McCreadie RG. Diet, smoking and cardiovascular risk in people with schizophrenia Descriptive study. The British Journal of Psychiatry. 2003;183(6):534–539. doi: 10.1192/bjp.183.6.534. [DOI] [PubMed] [Google Scholar]
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiologic reviews. 2008;30(1):67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Vancampfort D, Sweers K, Van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophrenia bulletin. 2013;39(2):306–318. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Sproule BJ, Chapman CB. The physiological meaning of the maximal oxygen intake test. Journal of Clinical Investigation. 1958;37(4):538. doi: 10.1172/JCI103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Gupta T, Orr JM, Pelletier-Baldelli A, Dean DJ, Lunsford-Avery, Millman ZB. Physical activity level and medial temporal health in youth at ultra high-risk for psychosis. J Abnorm Psychol. 2013;122(4):1101–1110. doi: 10.1037/a0034085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper S, Gómez-Pinilla AF, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects. CNS drugs. 2005;19(1):1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. The Canadian Journal of Psychiatry/La Revue canadienne de psychiatrie. 1991;36(4):239–245. doi: 10.1177/070674379103600401. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophrenia Research. 2004;72(1):29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Meyer T. Hippocampal plasticity in response to exercise in schizophrenia. Archives of general psychiatry. 2010;67(2):133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Paxton AE, Strycker LA, Toobert DJ, Ammerman AS, Glasgow RE. Starting the conversation performance of a brief dietary assessment and intervention tool for health professionals. Am J Prev Med. 2011;40(1):67–71. doi: 10.1016/j.amepre.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Pelham TW, Campagna PD, Ritvo PG, Birnie WA. The effects of exercise therapy on clients in a psychiatric rehabilitation program. Psychosocial Rehabilitation Journal. 1993;16(4):75–84. [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nature Reviews Neuroscience. 2001;2(1):24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum S, Tiedemann A, Sherrington C, Curtis J, Ward PB. Physical activity interventions for people with mental illness: a systematic review and meta-analysis. The Journal of clinical psychiatry. 2014;75(9):964–974. doi: 10.4088/JCP.13r08765. [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Archives of general psychiatry. 2007;64(10):1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Scheewe T, Backx F, Takken T, Jörg F, Strater AV, Kroes A, Cahn W. Exercise therapy improves mental and physical health in schizophrenia: a randomised controlled trial. Acta Psychiatrica Scandinavica. 2013a;127(6):464–473. doi: 10.1111/acps.12029. [DOI] [PubMed] [Google Scholar]
- Scheewe TW, Takken T, Kahn RS, Cahn W, Backx F. Effects of exercise therapy on cardiorespiratory fitness in patients with schizophrenia. Med Sci Sports Exerc. 2012;44(10):1834–42. doi: 10.1249/MSS.0b013e318258e120. [DOI] [PubMed] [Google Scholar]
- Scheewe TW, Van Haren NE, Sarkisyan G, Schnack HG, Brouwer RM, De Glint M, Cahn W. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: A randomised controlled trial in patients with schizophrenia and healthy controls. Eur Neuropsychopharmacology. 2013b;23(7):675–685. doi: 10.1016/j.euroneuro.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Sebastião E, Gobbi S, Chodzko-Zajko W, Schwingel A, Papini CB, Nakamura PM, Netto AV, Kokubun E. The International Physical Activity Questionnaire-long form overestimates self-reported physical activity of Brazilian adults. Public Health. 2012;126(11):967–975. doi: 10.1016/j.puhe.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Snethen GA, McCormick BP, Lysaker PH. Physical activity and psychiatric symptoms in adults with schizophrenia spectrum disorders. J Nerv Ment Dis. 2014;202(12):845–852. doi: 10.1097/NMD.0000000000000216. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Sallis JF, Needle R. The relation of physical activity and exercise to mental health. Public Health Rep. 1985;100(2):195–202. [PMC free article] [PubMed] [Google Scholar]
- Taylor HL, Buskirk E, Henschel A. Maximal oxygen intake as an objective measure of cardio-respiratory performance. Journal of applied physiology. 1955;8.1:73–80. doi: 10.1152/jappl.1955.8.1.73. [DOI] [PubMed] [Google Scholar]
- Van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10(2):128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]