Abstract
Purpose
To assess the dosimetric accuracy of synthetic CT volumes generated from MRI data for focal brain radiotherapy.
Methods
A study was conducted on 12 patients with gliomas who underwent both MR and CT imaging as part of their simulation for external beam treatment planning. Synthetic CT (MRCT) volumes were generated from the MR images. The patients’ clinical treatment planning directives were used to create 12 individual Volumetric Modulated Arc Therapy (VMAT) plans, which were then optimized 10 times on each of their respective CT and MRCT-derived electron density maps. Dose metrics derived from optimization criteria, as well as monitor units and gamma analyses, were evaluated to quantify differences between the imaging modalities.
Results
Mean differences between Planning Target Volume (PTV) doses on MRCT and CT plans across all patients were 0.0% (range −0.1 to 0.2%) for D95%, 0.0% (−0.7 to 0.6%) for D5%, and −0.2% (−1.0 to 0.2%) for Dmax. MRCT plans showed no significant change in monitor units (−0.4%) compared to CT plans. Organs at risk (OARs) had an average Dmax difference of 0.0 Gy (−2.2 to 1.9 Gy) over 85 structures across all 12 patients, with no significant differences when calculated doses approached planning constraints.
Conclusions
Focal brain VMAT plans optimized on MRCT images show excellent dosimetric agreement with standard CT-optimized plans. PTVs show equivalent coverage, and OARs do not show any overdose. These results indicate that MRI-derived synthetic CT volumes can be used to support treatment planning of most patients treated for intracranial lesions.
Introduction
The current utilization of Magnetic Resonance Imaging (MRI) in Radiation Oncology typically involves alignment of MR image volumes with computed tomography (CT) scans. MR images provide the soft tissue contrast necessary for tumor identification and improved structure delineation, while CT images support convenient generation of the electron density maps necessary for dose calculation. An attractive alternative to this process is a solely MR-guided workflow. This has recently become feasible due to advances in field-gradient nonlinearity correction techniques that reduce MR geometric distortions [1–4], and new MR pulse sequences that improve distinction between bone and air [5–7]. An MR-only workflow would reduce both patient scan time and incurred costs, and eliminate alignment errors between MR and CT modalities [8,9] that have been shown to lead to systematic positioning errors of up to 2 mm [10,11]. Emerging MR-Linac technology [12,13] also motivates elimination of CT scans from the radiation treatment planning process.
To support MR-only treatment planning, several different synthetic CT (MRCT) creation methods have been explored, such as bulk density assignment [14], atlas-based electron density mapping [15–18], dual model HU conversion [19], voxel-based Gaussian mixture regression [20] and fuzzy tissue clustering [21]. In some initial treatment planning studies, comparisons of fluence maps calculated on MRCT versus CT-derived density grids showed mean differences ranging from 0.1–2% of doses to various reference points, such as the isocenter dose or Planning Target Volume (PTV) Dmax in focal brain [8,14–16,22,23] and prostate cases [18,19,23–25]. Recent work has turned toward assessing the impact of using MRCT density grids to drive optimization of treatment plans [23,26]. In this study, we investigate the accuracy and reproducibility of volumetric modulated arc therapy (VMAT) treatment planning for brain glioma cases based upon MRCT [21], as compared to our current CT-based treatment plan optimization paradigm.
Methods and Materials
Image Acquisition
As part of an internal review board approved prospective study, MR and CT images were acquired for 12 patients undergoing treatment for glioma. Tumor size (18 to 281 cm3) varied widely within the group, with locations ranging throughout the brain, from the most superior point, to the frontal lobes and the skull base (Figure e1). The prescribed target dose ranged from 40 to 60 Gy delivered in 15 to 30 fractions. Each patient underwent a standard simulation on our CT scanner (Brilliance, Phillips Medical Systems, Cleveland OH), with 3 mm slice thickness and in-plane pixel size ranging from 0.5×0.5 mm2 to 1×1 mm2. Volumetric brain MRI images were acquired of patients immobilized with a three-point mask (Posicast, CIVCO, Rotterdam NL) on a large-bore 3T MRI scanner (Skyra, Siemens Medical Systems, Malvern PA), using a combination of an 18-channel anterior surface coil and a 32-channel posterior coil.
A previously published MRCT generation method [21] defined the scans used for six patients in this study. This version used an ultrashort echo time sequence (UTE), with TE=0.06 ms, TR=7.7 ms, flip angle of 12° and voxel size ≈1.2×1.2×1.6 mm3. The UTE helps differentiate air from bone via an intensity threshold [27]. Turbo-spin echo (TSE) Dixon scans with in-phase and opposite-phase components (TE/TR=10/3700 ms, and voxel size ≈1.2×1.2×3.9 mm3) were acquired to calculate fat and water images [28]. A T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE, TE/TR/TI=2.4/1900/900 ms, and flip angle = 9°) as well as a T2-weighted scan (SPACE, TE/TR=4.1/3200 ms, and flip angle = 12°) were also acquired, both with voxel size ≈1.0×1.0×1.0 mm3. Finally a time-of-flight scan (TE/TR=4/8.6 ms, flip angle =20°) with voxel size ≈0.5×0.5×3.6 mm3 provided a map of large blood vessel locations.
Images were interpolated onto a common 1.0×1.0×1.0 mm3 grid. An intensity threshold applied to the UTE images defined an air mask, excluding such labeled voxels from classification. Blood vessels were identified by applying an intensity threshold to the time-of-flight image volume and assigning a fixed attenuation to the voxels identified. A probabilistic classification using fuzzy c-means (FCM) clustering with spatial constraint [21] was applied to the set of T1-weighted, T2-weighted, fat and water images (Table e1). From this classification, each voxel in the head was assigned with a probability of belonging to each of five tissue classes (fat, water, white matter, grey matter and bone). The attenuation of each voxel was calculated from the weighted sum of these five components multiplied by assigned values.
A new MRI protocol with reduced acquisition time and improved spatial resolution of fat and water images was used for the remainder of the patient group. This version replaced the UTE scan with a pointwise time reduction with radial acquisition (PETRA) sequence, with TR=4.7 ms, TE=0.07 ms, flip angle = 6°, 20000 spokes and voxel size ≈1.2×1.2×1.2 mm3. The primary motivation was that the PETRA sequence is approved for clinical use. A T1-weighted volumetric interpolated breath-hold examination (VIBE) sequence (TE/TR=9/2.5 ms, flip angle = 9°) with voxel size ≈1.3×1.3×1.0 mm3 produced both T1-weighted as well as fat and water images in a single short (< 2 minute) acquisition. The T2-weighted and time-of-flight sequences were unchanged. Total scan time was reduced to 9 minutes. For comparative purposes, one patient was scanned with the full complement of sequences to support both MRCT generation methods.
For the patients scanned with the new imaging protocol, the MRCT generation procedure was slightly modified. In this updated version, post-processing was applied to images for bias field intensity correction [3] prior to normalization. The air mask was defined by applying an intensity threshold to the bias-corrected PETRA image volume, and was subsequently dilated by 1 mm [27]. A second set of intensity vectors (Table e2) was applied for the new imaging protocol. For both MRCT protocols all optimization occurred on separate data prior to this current investigation.
VMAT Planning and Dose Comparison
For each patient, the CT image was first interpolated onto a 1.0×1.0×1.0 mm3 grid, and then the MRCT rigidly aligned to the CT, so that both electron density maps had the same resolution. Both images were imported into the treatment planning system (Eclipse v11.0, Varian Medical Systems, Palo Alto CA). The patient’s clinically-defined structures were used; where physicians and dosimetrists delineate PTVs and OARs upon MR and CT images, then amalgamate the structure set upon the CT. Here each patient’s CT structure set was transferred onto the rigidly registered MRCT. The mean absolute error (MAE) in HU between registered images was calculated within two clinical contours; the brain and the entire superior portion of the head. A two-field full-rotation 6 MV VMAT plan was then optimized using density grids derived from each image set. Dose constraints for optimization were extracted from the patients’ treatment planning directives. Doses were calculated using the vendor’s analytical anisotropic algorithm (AAA).
The Progressive Resolution Optimization (PRO v11.0.31) algorithm used for VMAT treatment planning does not have identically reproducible results [29,30]. For identical input, repeating the optimization will generate slightly different fluence patterns as the optimizer settles onto different field weight and shape conditions [31]. To separate this variation from differences due to the MRCT versus CT density grids, the 12 individual patient VMAT plans were optimized 10 times on each of their respective CT and MRCT-derived density grids. For every patient, beam fluences from each of the MRCT-optimized VMAT plans were also transferred to the associated CT-derived density grids, and the dose subsequently recalculated. These transposed MRCT (tMRCT) plans were used to more directly evaluate the impact of density grid selection on dose calculation.
For each patient, measured dose values related to treatment planning objectives used for optimization were compared across CT and MRCT optimizations, as well as tMRCT calculations. All planning directives contained PTV (D95%, D5% and Dmax) as well as organ at risk (OAR) Dmax dose limits (structures included the brainstem, optic nerves, eyes, lenses, chiasm, and cochlea). The maximum doses to these OARs were compared, as well as the total energy deposited (by Monitor Units (MU) as well as average dose to the head external structure). Finally, a two-dimensional gamma analysis of dose distributions was performed at both 2% / 2 mm and 1% / 1 mm dose difference and distance levels, using planes passing through the isocenter.
Results
CT plan variation
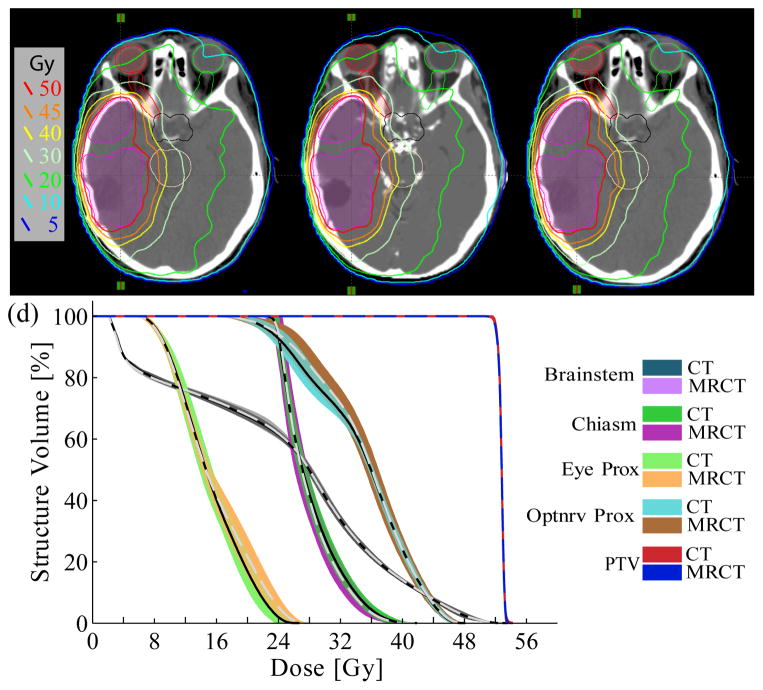
Figure 1 shows sample calculated dose distributions for a single CT plan and MRCT plan from the same patient. For the CT-optimized plans, the average standard deviation for observed PTV Dmax values, calculated by averaging across the standard deviations extracted from each of the 12 patient’s set of 10 optimizations, was 0.2 Gy (range 0.1–0.3 Gy). This corresponds to an average standard deviation of 0.4% (0.2 to 0.8%) of the plan maximum dose. The PTV D95% and D5% reference points both had average standard deviations of 0.1%. OAR Dmax values had an average standard deviation of 0.5 Gy (0.0 to 1.8 Gy) across all patients. The MU across CT optimizations also showed some variation, with standard deviations ranging from 1 to 4% (average σ = 2%) of the average total MU per case.
Figure 1.
Dose comparison of a single optimized VMAT plan calculated using (a) the CT, (b) the MRCT, and (c) the tMRCT (MRCT fluence on CT image) for patient #7. (c) The spread of DVH curves from ten optimizations on CT and MRCT. Solid lines represent mean DVH values from CT, dashed lines mean values from MRCT.
Variations between MRCT techniques
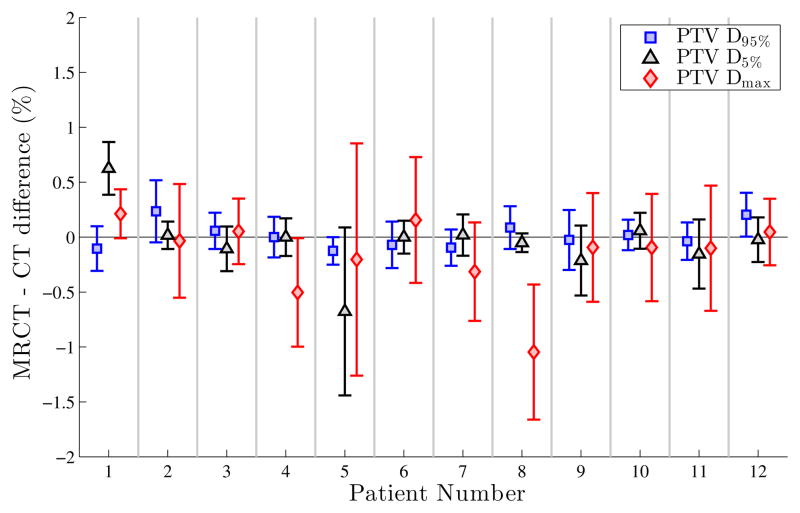
The 6-patient subgroup scanned under the initial MRCT protocol (UTE scans, T1 MPRAGE, TSE Dixon) was compared to the 6-patient subgroup with the new generation process (PETRA scans, T1 VIBE Dixon). Differences in dose metrics (PTV D95%, D5% and Dmax) between plans optimized using MRCT versus CT-derived density grids were comparable for both MRCT versions. Figure 2 illustrates this, with the results for patients #1–6 from the UTE-based MRCT generator, and #7–12 from the updated PETRA-based generation process. Additionally, patient #7 underwent both series of scans; differences in average D95%, D5% and Dmax values from 10 plans generated on each of the resulting MRCT volumes were 0.1%, 0.0%, and −0.1% respectively. As both MRCT generation methods yielded similar VMAT plans/doses the results from both MRCT methods are presented as a single ensemble.
Figure 2.
Differences between average MRCT and average CT PTV D95%, D5% and Dmax values for each patient, from the 10 plans optimized on each modality. Error bars represent the planning variation between paired CT and MRCT dose metrics.
Comparison of MRCT and CT-optimized plans
The average MAE between MRCT and CT was 142 HU and 24 HU for the skull and brain contours respectively, comparable to other synthetic CT efforts in the head [32,33]. Figure 2 illustrates dose coverage differences of the clinically-delineated PTVs between MRCT and CT plans for each case. Table 1 highlights the differences of mean D95%, D5% and Dmax values between MRCT and CT plans, as well as between tMRCT and CT plans; across all patient data sets there are no significant differences for these dose metrics. The largest observed difference between MRCT and CT plans across all 12 patient cases for any of the D95%, D5% and Dmax dose values was 1% in magnitude to a single Dmax reference point, corresponding to an MRCT-CT dose difference of 0.6 Gy.
Table 1.
Mean difference and range between the extracted dose metrics, across all 12 patients. MU values represent the sum per plan, while relative Dmean differences were calculated with respect to the body Dmax. The p-values are calculated from a paired sample t-test.
| MRCT vs. CT | p | tMRCT vs. CT | p | tMRCT vs. MRCT | p | ||
|---|---|---|---|---|---|---|---|
| PTV | D95% (%) | 0.0 (−0.1 to 0.2) | 0.67 | −0.3 (−1.4 to 0.8) | 0.05 | −0.3 (−1.2 to 0.9) | 0.06 |
| D5% (%) | 0.0 (−0.7 to 0.6) | 0.65 | −0.3 (−1.4 to 1.3) | 0.17 | −0.3 (−1.2 to 0.6) | 0.12 | |
| Dmax (%) | −0.2 (−1.0 to 0.2) | 0.13 | −0.2 (−1.4 to 1.7) | 0.44 | 0.0 (−1.1 to 1.7) | 0.88 | |
| OAR | Dmax (Gy) | 0.0 (−2.2 to 1.9) | 0.59 | 0.0 (−2.1 to 1.5) | 0.36 | 0.1 (−2.6 to 1.0) | 0.12 |
| Whole head | Dmax (%) | −0.2 (−1.0 to 0.2) | 0.08 | −0.3 (−1.3 to 1.7) | 0.24 | −0.1 (−1.3 to 1.5) | 0.63 |
| Dmean (%) | −0.1 (−0.2 to 0.0) | 0.03 | −0.1 (−0.2 to 0.0) | 0.04 | 0.0 (−0.1 to 0.2) | 0.70 | |
| Tot. MU (%) | −0.4 (−2.2 to 1.9) | 0.31 | −0.4 (−2.2 to 1.9) | 0.31 |
Organs at risk
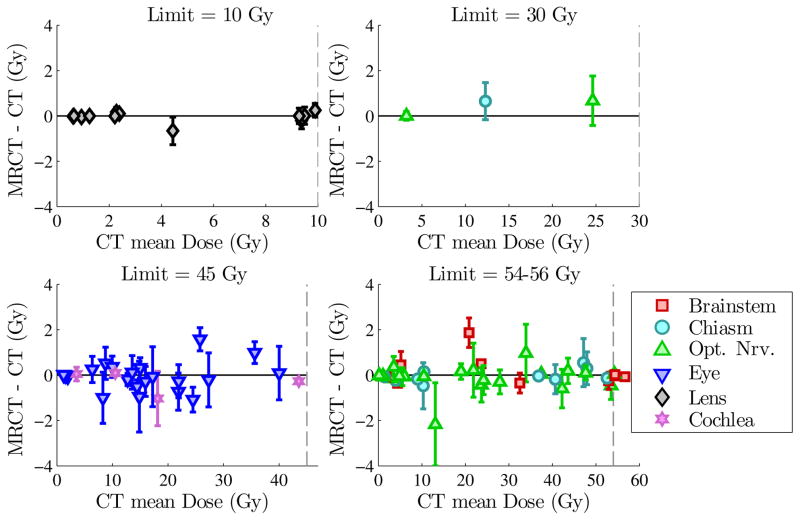
A total of 85 OARs were included in the VMAT optimization across the 12 patients. Table 1 shows the differences of mean Dmax values between MRCT and CT plans across all OARs. No significant difference was observed, with an average difference of 0.0 Gy (range −2.2 to 1.9 Gy, p = 0.59) across all OARs. Figure 3 shows all OAR Dmax differences, subdivided according to assigned structure Dmax limit. Although a few individual OARs show differences approaching 2 Gy, these are for doses below the planning constraints. In no case did the MRCT-based plans cause OAR Dmax values to exceed the assigned structure constraints.
Figure 3.
Dmax values for all OARs, divided by structure limit. Symbols representing structure types show differences between MRCT and CT mean Dmax values for each OAR. Error bars show the combined standard deviations of MRCT-optimized and CT-optimized plans. Dashed lines show the corresponding structure limits (10, 30, 45 and 54 Gy).
MU and body maximum dose
The mean difference in total MU from MRCT-based to CT-based plans was −0.4% (−2.2 to 1.9%, p = 0.31). The mean difference between MRCT and CT whole head maximum doses was −0.2% (−1.0 to 0.2%), a statistically insignificant difference. Mean dose to the head, relative to plan Dmax values, showed a difference of −0.1% (−0.2 to 0.0%, p = 0.03), corresponding to an absolute difference of −0.03 Gy.
Comparison of tMRCT and CT dose distributions
Dose distributions from the tMRCT, or MRCT-optimized fluence maps on the CT-derived density grids, showed increased variation in differences between dose metrics compared to CT-based optimizations, but did not lead to any systematic change in these differences. PTV D95%, D5% and Dmax points showed mean differences of −0.3%, −0.3% and −0.2% respectively between tMRCT and CT plans. The tMRCT OAR Dmax values showed a mean difference of 0.0 Gy (−2.1 to 1.5 Gy) as compared to the CT values (p = 0.36); no OAR overdose was caused by using the MRCT derived fluences and re-calculating doses upon the CT-based density grids.
Gamma analysis
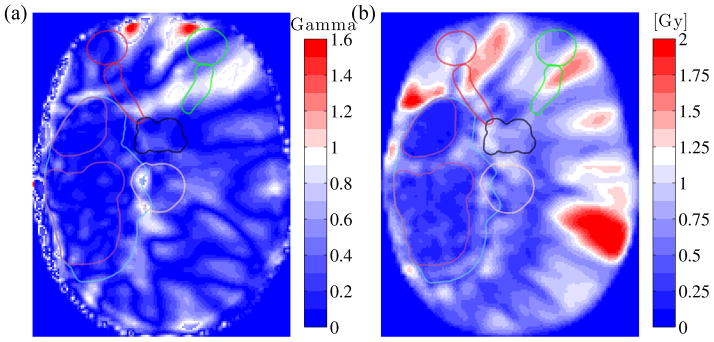
The two-dimensional gamma analysis comparing MRCT and CT plans using a 2% / 2 mm acceptance criterion revealed passing rates of 99.3%, 98.9% and 96.4% across the coronal, sagittal and transverse planes through isocenter respectively. Comparing tMRCT and MRCT plans, the mean passing rates were 99.9%, 99.7% and 99.8% in the three planes. Figure 4a shows a sample 1% / 1 mm gamma map in the axial plane from patient #7, comparing the mean tMRCT dose distribution against the mean MRCT dose distribution. Figure 4b shows the standard deviation of the dose from the 10 optimized MRCT plans for this plane.
Figure 4.
(a) Gamma analysis comparing mean tMRCT vs. mean MRCT dose in the transverse plane, for patient #7. A gamma value below 1 corresponds to acceptance under the 1% / 1 mm criterion. (b) Transverse standard deviation map from the 10 MRCT VMAT plans. Structure contours are overlaid for reference.
Table 2 summarizes the gamma analysis results in the axial plane, comparing individual CT versus mean CT dose distributions, as well as between mean CT, MRCT, and tMRCT dose distributions. The 2% / 2 mm passing rate when comparing MRCTmean and CTmean dose distributions (96.4%, range 89.1 to 100%) was higher than the rate when comparing individual CT plans (CTind) and CTmean (92.6%, range 81.0 to 98.6%), indicating that the dominant source of dose variation is VMAT optimization reproducibility instead of differences between the use of the MRCT-based versus CT-based density grid for optimization.
Table 2.
Average gamma analysis results over all 12 patients. For each patient, the two-dimensional gamma passing rate is calculated in the transverse plane through isocenter.
| 2% / 2 mm | 1% / 1 mm | |
|---|---|---|
| CTind vs. CTmean | 92.6 (81.0 to 98.6) | 70.6 (56.2 to 87.2) |
| MRCTmean vs. CTmean | 96.4 (89.1 to 100.0) | 78.2 (53.7 to 96.8) |
| tMRCTmean vs. CTmean | 96.7 (90.1 to 100.0) | 80.1 (55.6 to 99.7) |
| tMRCTmean vs. MRCTmean | 99.8 (98.2 to 100.0) | 95.8 (81.4 to 99.9) |
Discussion
This study has shown that for treatment planning considerations, the images from our automated MRCT generation process are sufficiently similar to CT images to not impact clinical decisions related to treatment planning of gliomas using modulated arc therapy. The evaluation of PTV and OAR dose metrics showed sufficient similarity as to present a minimal risk from the use of MRCT in optimization. Further, the tMRCT dose maps, where the planned fluences optimized using the MRCT-derived electron density map were transferred onto the CT image for dose calculation, do not reveal any systematic shift to either PTV or total body dose metrics. This indicates that using the MRCT does not bias plans toward either any underdose or overdose of the target. Similarly, plan MUs were also equivalent when comparing VMAT plans optimized on MRCT and CT, indicating that no significant bias in total fluence and resulting body dose arises from using MRCT in place of CT.
Though closely matching, the MRCT and CT dose distributions do have some sources of disagreement. Differences were noted in regions of close proximity to air cavities. Figure 1 shows some mislabeled air as bone in the anterior sinus, and Figure 4a shows a larger dose difference in roughly the same region. While the absolute dose differences were not significant enough to have clinical impact, it is still appropriate to approach regions in which the target or highly involved critical organs are adjacent to larger air pockets with caution. Air-bone interfaces present a significant challenge for MRCT generation processes [20,23,25], although ultrashort echo sequences such as UTE and now PETRA have recently improved bone identification methods.
The PTV Dmax value for patient #8 (Figure 2) showed a large 1% difference between MRCT and CT. Here the tumor was located in the most superior portion of the brain, with the PTV encroaching on the cranium. Differences in HU within bony matter, where CT values can approach 2000 but MRCT values are set to 800 for a voxel bone probability of 1, may account in part for this unusual PTV discrepancy.
In this work, the CT-optimized plans were used as a gold standard. However, the CT modality contains its own sources of error. For example, structures are delineated in the clinic using MR images and then transferred over to the CT; in a practical sense, use of MRCT could avoid potential systematic errors related to image alignment for transferring structures from MRI to CT [10]. Furthermore, the CT scan itself may contain artifacts that impact the estimated electron density map. Such small discrepancies across small regions have little effect upon plan optimization and dose calculation, since high-energy photons are relatively insensitive to local electron density variations [34]. Regardless of whether CT-influenced electron density artifacts exist, this study determined that our MRCT-derived electron density maps do not lead to inferior decisions for focal brain treatment planning. While we did not observe visible MRCT distortion relative to matching CT images, we are aware that susceptibility issues may lead to local distortions and should be properly managed using high bandwidth scans and correction if needed [35,36].
As MRCT methods improve, recent work has shifted toward evaluating their efficacy in terms of clinical dosimetric accuracy, either by comparing dose calculations from the same fields [8,15,16,19,24,25] or by comparing the results of inverse planning optimizations [23,26]. Here, we have assessed the accuracy of our MRCT algorithm, requiring no user input, by applying patient-specific clinical treatment planning directives and studying metrics related to objective functions derived from such directives. Dose differences were evaluated with respect to the inherent variation of optimization-determined VMAT plans. The MRCT and CT modalities result in equivalent calculated dose distributions, validating the implementation of our MRCT generator for clinical use in focal brain cases. Future work will focus on expansion of the MRCT generation capabilities to include other sites such as head/neck and prostate, where additional complications, such as mobile air and motion of tumors and normal anatomy, exist.
Conclusions
The dosimetric accuracy of an in-house MRCT generation method was evaluated for VMAT plans on focal brain cases. Investigated metrics showed no statistically significant difference from CT-based optimization. This demonstration of functionally equivalent plans based on either image set validates a move toward MR-based treatment planning for focal intracranial targets.
Supplementary Material
Supplementary Figure e1: Representation of patient PTV size and location; as shown by transferring them onto a single CT image:
Supplementary Table e1: FCM centroid values for the initial MRI protocol (UTE image set):
Supplementary Table e2: FCM centroid values for the updated MRI protocol (PETRA image set):
Summary.
The dosimetric accuracy of generated synthetic computed tomography (MRCT) images was evaluated for focal brain tumor cases. Volumetric modulated arc therapy (VMAT) plans were generated for 12 patients on both MRCT and CT electron density maps, and assessed in terms of dose to target and avoidance references points, monitor units used, and via a gamma analysis. Dose discrepancies between modalities were insignificant, and smaller than the variation due to inverse planning.
Acknowledgments
This work was supported by NIH R01EB016079. Works-in-progress versions of the UTE and PETRA scanning sequences were provided under a research agreement with Siemens Medical Systems.
Footnotes
Conflict of interest: Dr. Balter and Dr. Cao report grants from Siemens Medical Systems, grants from National Institutes of Health, during the conduct of the study.
This work was presented in part at the 56th annual meeting of the American Society for Radiation Oncology (2014)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janke A, Zhao H, Cowin GJ, Galloway GJ, Doddrell DM. Use of spherical harmonic deconvolution methods to compensate for nonlinear gradient effects on mri images. Magnetic resonance in medicine. 2004;52:115–122. doi: 10.1002/mrm.20122. [DOI] [PubMed] [Google Scholar]
- 2.Karger CP, Höss A, Bendl R, Canda V, Schad L. Accuracy of device-specific 2d and 3d image distortion correction algorithms for magnetic resonance imaging of the head provided by a manufacturer. Physics in medicine and biology. 2006;51:N253. doi: 10.1088/0031-9155/51/12/N04. [DOI] [PubMed] [Google Scholar]
- 3.Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. N4itk: Improved n3 bias correction. Medical Imaging, IEEE Transactions on. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanescu T, Jans H, Pervez N, Stavrev P, Fallone B. A study on the magnetic resonance imaging (mri)-based radiation treatment planning of intracranial lesions. Physics in medicine and biology. 2008;53:3579. doi: 10.1088/0031-9155/53/13/013. [DOI] [PubMed] [Google Scholar]
- 5.Grodzki DM, Jakob PM, Heismann B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (petra) Magnetic Resonance in Medicine. 2012;67:510–518. doi: 10.1002/mrm.23017. [DOI] [PubMed] [Google Scholar]
- 6.Keereman V, Fierens Y, Broux T, De Deene Y, Lonneux M, Vandenberghe S. Mri-based attenuation correction for pet/mri using ultrashort echo time sequences. Journal of nuclear medicine. 2010;51:812–818. doi: 10.2967/jnumed.109.065425. [DOI] [PubMed] [Google Scholar]
- 7.Robson MD, Gatehouse PD, Bydder M, Bydder GM. Magnetic resonance: An introduction to ultrashort te (ute) imaging. Journal of computer assisted tomography. 2003;27:825–846. doi: 10.1097/00004728-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Jonsson JH, Karlsson MG, Karlsson M, Nyholm T. Treatment planning using mri data: An analysis of the dose calculation accuracy for different treatment regions. Radiat Oncol. 2010;5:62. doi: 10.1186/1748-717X-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Price RA, Wang L, Li J, Qin L, McNeeley S, Ma C-MC, Freedman GM, Pollack A. Mri-based treatment planning for radiotherapy: Dosimetric verification for prostate imrt. International Journal of Radiation Oncology* Biology* Physics. 2004;60:636–647. doi: 10.1016/j.ijrobp.2004.05.068. [DOI] [PubMed] [Google Scholar]
- 10.Ulin K, Urie MM, Cherlow JM. Results of a multi-institutional benchmark test for cranial ct/mr image registration. International Journal of Radiation Oncology* Biology* Physics. 2010;77:1584–1589. doi: 10.1016/j.ijrobp.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyholm T, Nyberg M, Karlsson MG, Karlsson M. Systematisation of spatial uncertainties for comparison between a mr and a ct-based radiotherapy workflow for prostate treatments. Radiat Oncol. 2009;4:54. doi: 10.1186/1748-717X-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagendijk JJ, Raaymakers BW, Raaijmakers AJ, Overweg J, Brown KJ, Kerkhof EM, van der Put RW, Hårdemark B, van Vulpen M, van der Heide UA. Mri/linac integration. Radiotherapy and Oncology. 2008;86:25–29. doi: 10.1016/j.radonc.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Fallone B, Murray B, Rathee S, Stanescu T, Steciw S, Vidakovic S, Blosser E, Tymofichuk D. First mr images obtained during megavoltage photon irradiation from a prototype integrated linac-mr system. Medical physics. 2009;36:2084–2088. doi: 10.1118/1.3125662. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen BH, Laursen FJ, Løgager V, Geertsen PF, Krarup-Hansen A. Dosimetric and geometric evaluation of an open low-field magnetic resonance simulator for radiotherapy treatment planning of brain tumours. Radiotherapy and Oncology. 2008;87:100–109. doi: 10.1016/j.radonc.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Andreasen D, Van Leemput K, Hansen RH, Andersen JAL, Edmund JM. Patch-based generation of a pseudo ct from conventional mri sequences for mri-only radiotherapy of the brain. Medical Physics. 2015;42:1596–1605. doi: 10.1118/1.4914158. [DOI] [PubMed] [Google Scholar]
- 16.Uh J, Merchant TE, Li Y, Li X, Hua C. Mri-based treatment planning with pseudo ct generated through atlas registration. Medical Physics. 2014;41:051711. doi: 10.1118/1.4873315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greer PB, Dowling JA, Lambert JA, Fripp J, Parker J, Denham JW, Wratten C, Capp A, Salvado O. A magnetic resonance imaging-based workflow for planning radiation therapy for prostate cancer. Medical Journal of Australia. 2011;194:S24. doi: 10.5694/j.1326-5377.2011.tb02939.x. [DOI] [PubMed] [Google Scholar]
- 18.Dowling JA, Lambert J, Parker J, Salvado O, Fripp J, Capp A, Wratten C, Denham JW, Greer PB. An atlas-based electron density mapping method for magnetic resonance imaging (mri)-alone treatment planning and adaptive mri-based prostate radiation therapy. International Journal of Radiation Oncology* Biology* Physics. 2012;83:e5–e11. doi: 10.1016/j.ijrobp.2011.11.056. [DOI] [PubMed] [Google Scholar]
- 19.Korhonen J, Kapanen M, Keyriläinen J, Seppälä T, Tenhunen M. A dual model hu conversion from mri intensity values within and outside of bone segment for mri-based radiotherapy treatment planning of prostate cancer. Medical Physics. 2014;41:011704. doi: 10.1118/1.4842575. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson JH, Johansson A, Söderström K, Asklund T, Nyholm T. Treatment planning of intracranial targets on mri derived substitute ct data. Radiotherapy and Oncology. 2013;108:118–122. doi: 10.1016/j.radonc.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 21.XXX
- 22.Karlsson M, Karlsson MG, Nyholm T, Amies C, Zackrisson B. Dedicated magnetic resonance imaging in the radiotherapy clinic. International Journal of Radiation Oncology* Biology* Physics. 2009;74:644–651. doi: 10.1016/j.ijrobp.2009.01.065. [DOI] [PubMed] [Google Scholar]
- 23.Korsholm ME, Waring LW, Edmund JM. A criterion for the reliable use of mri-only radiotherapy. Radiation Oncology. 2014;9:16. doi: 10.1186/1748-717X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert J, Greer PB, Menk F, Patterson J, Parker J, Dahl K, Gupta S, Capp A, Wratten C, Tang C. Mri-guided prostate radiation therapy planning: Investigation of dosimetric accuracy of mri-based dose planning. Radiotherapy and Oncology. 2011;98:330–334. doi: 10.1016/j.radonc.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Glide-Hurst C, Doemer A, Wen N, Movsas B, Chetty IJ. Implementation of a novel algorithm for generating synthetic ct images from magnetic resonance imaging data sets for prostate cancer radiation therapy. International Journal of Radiation Oncology* Biology* Physics. 2015;91:39–47. doi: 10.1016/j.ijrobp.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Jonsson JH, Akhtari MM, Karlsson MG, Johansson A, Asklund T, Nyholm T. Accuracy of inverse treatment planning on substitute ct images derived from mr data for brain lesions. Radiation Oncology. 2015;10:1. doi: 10.1186/s13014-014-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.XXX
- 28.Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 29.Otto K. Volumetric modulated arc therapy: Imrt in a single gantry arc. Medical Physics. 2008;35:310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 30.Vanetti E, Nicolini G, Nord J, Peltola J, Clivio A, Fogliata A, Cozzi L. On the role of the optimization algorithm of rapidarc® volumetric modulated arc therapy on plan quality and efficiency. Medical Physics. 2011;38:5844–5856. doi: 10.1118/1.3641866. [DOI] [PubMed] [Google Scholar]
- 31.Jeraj R, Keall P. The effect of statistical uncertainty on inverse treatment planning based on monte carlo dose calculation. Physics in medicine and biology. 2000;45:3601. doi: 10.1088/0031-9155/45/12/307. [DOI] [PubMed] [Google Scholar]
- 32.Johansson A, Garpebring A, Karlsson M, Asklund T, Nyholm T. Improved quality of computed tomography substitute derived from magnetic resonance (mr) data by incorporation of spatial information-potential application for mr-only radiotherapy and attenuation correction in positron emission tomography. Acta Oncologica. 2013;52:1369–1373. doi: 10.3109/0284186X.2013.819119. [DOI] [PubMed] [Google Scholar]
- 33.Zheng W, Kim JP, Kadbi M, Movsas B, Chetty IJ, Glide-Hurst CK. Mr-based automatic air segmentation for generation of synthetic cts in the head region. International Journal of Radiation Oncology*Biology*Physics. doi: 10.1016/j.ijrobp.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Seco J, Evans P. Assessing the effect of electron density in photon dose calculations. Medical physics. 2006;33:540–552. doi: 10.1118/1.2161407. [DOI] [PubMed] [Google Scholar]
- 35.XXX
- 36.XXX
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure e1: Representation of patient PTV size and location; as shown by transferring them onto a single CT image:
Supplementary Table e1: FCM centroid values for the initial MRI protocol (UTE image set):
Supplementary Table e2: FCM centroid values for the updated MRI protocol (PETRA image set):