Abstract
Aims
In anesthetized rats, voiding is typically associated with phasic activation (bursting) of the external urethral sphincter (EUS). During spontaneous voiding in unanesthetized, unrestrained rats, EUS bursting is the most common form of EUS activity exhibited, but it is not necessary for productive voiding to occur. The aim of the present study was to determine which aspects of EUS activity contributed to void size during bursting and non-bursting voiding in conscious, freely moving rats.
Methods
Female rats were implanted with electrodes adjacent to the EUS for recording electromyographic activity (EMG). EUS EMG recordings were performed during 24-hr sessions in a metabolic cage while voided urine was continuously collected and weighed.
Results
Void size was positively correlated with the duration of the intra-burst silent and active periods and variables reflecting the overall intensity and duration of bursting, particularly at lower frequencies within the 3–10 Hz range of EUS bursting. In addition, void size was inversely related to the frequency of bursting and to the average EMG amplitude during voiding, both in voids with and without bursting.
Conclusions
EUS bursting contributes to productive voiding when bursting is present. Lower bursting frequencies elicit more productive voiding than do higher frequencies. In the absence of bursting, the association of increased void size with smaller average EUS EMG amplitude suggests that conscious rats can perform synergic voiding (i.e., bladder contraction with EUS relaxation) that is comparable to that seen in humans and other typically non-bursting species.
INTRODUCTION
The external urethral sphincter (EUS) plays a crucial role in continence and voiding1. It is active during urine retention and inactive during voiding, although the pattern of inactivity varies among species. In humans, the EUS is silent throughout voiding; in rats, the EUS exhibits a phasic pattern of short bursts of activity alternating with periods of silence (bursting). The ubiquity of EUS bursting during voiding in anesthetized animals and the reduced void size that follows impairment of EUS activity2–5 has led to the commonly accepted interpretation that EUS bursting is necessary for productive voiding in rats6.
We have recently reported in a study of EUS EMG during spontaneous voiding in unanesthetized, unrestrained rats that, while bursting is the most common form of EUS EMG activity during voiding, it is not essential for voiding to occur7. On average, 25% of voids were not associated with EUS bursting. During voids of comparable size, EUS EMG activity ranged from markedly phasic bursting to fully tonic ongoing activity. This finding in awake, unrestrained rats contradicts the prevailing view (largely based on studies in anesthetized animals) that EUS activity during voiding in rats is entirely phasic. The contribution of phasic and tonic EUS activity to voiding productivity has not been evaluated on a void-by-void basis. In this study, we evaluated the relationship of void size to EUS activity and other urodynamic properties. We used individual voids in conscious, freely moving rats to determine the aspects of EUS activity that accounted for variation in void size during spontaneous micturition.
MATERIALS AND METHODS
Animals
Animals were 23 8–12-week-old female Sprague-Dawley rats that were subjects in a previous study7. Rats were housed individually with a 12-hr light/12-hr dark cycle. All animal procedures were in accord with the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council (National Academy Press, Washington, DC, 2010), and had been reviewed and approved by the Wadsworth Center IACUC.
Implantation surgery
Implant construction and surgery have been reported in detail7 and are only described here briefly. Implants consisted of 3–4 Teflon-insulated stainless steel wires attached to a plastic pedestal. The free ends of the pedestal wires formed a pair of EUS EMG recording electrodes and 1–2 grounding electrodes.
All procedures were performed using sterile surgical technique under isoflurane anesthesia. The EUS EMG wires were routed subcutaneously by inserting the electrodes in a trocar into a skin incision over the skull and exiting through a skin incision over the abdomen. The EUS electrodes were inserted bilaterally through small holes drilled in the pubic bone five mm caudal to the rostral bone edge and just lateral to the pubic symphysis. The electrode tips were then sutured to holes drilled at the rostral edge of the pubic bone, which positioned the electrodes along the dorsal surface of the pubic bone parallel and adjacent to the EUS. One or two grounding wires were positioned either subcutaneously over the lower vertebral column or within the abdominal cavity. The abdominal wall and skin incisions were closed in layers. The implant pedestal was attached to the skull with screws and dental cement. Animals received antibiotics and an analgesic for 5–10 days after surgery.
Chronic recordings
Rats were housed individually in a metabolic cage for 24 hr 1–3x/week for up to 14 weeks. A flexible armored cable attached to the skull pedestal carried EUS EMG signals to recording equipment via a commutator; this allowed the rat to move freely about its cage. Voided urine was accumulated and continuously weighed by a force transducer mounted beneath the cage. The urine accumulation lagged behind the recorded EUS EMG due to the transit time of the urine drops along the inner surfaces of the metabolic cage (minimum transit time=0.6 s).
EMG data were bandpass filtered at 10–300 Hz, digitized at 600 Hz, full-wave rectified digitally, down-sampled to 200 Hz, and stored (except for two rats in which EMG data were bandpass filtered at 35–100 Hz, full-wave rectified, lowpass filtered again at 32 Hz, and digitized at 200 Hz). Urine weight recordings were low-pass filtered at 12 Hz.
Power spectra of EMG signals were calculated using the Fast Fourier Transform (FFT). Time-frequency spectrograms were calculated by repeating FFTs every 0.1 s on overlapping 1-s epochs of Hanning-windowed EMG data.
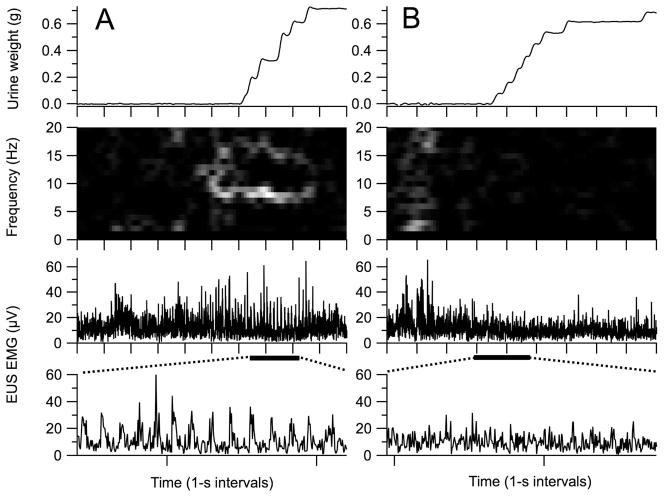
A urine weight increase of at least 0.04 g determined void onset. Typically, urine flowed as a series of closely spaced drops (top panel in Figure 1A), and void duration was calculated as the time between the first and last drop. For voids with drops delayed by ≥0.8 s from the preceding drop (top panel in Figure 1B), the void time was calculated as the time from the beginning of continuous urination to the end of voiding, minus pauses of ≥0.8 s.
Figure 1.
Voids recorded during one 24-hr session with comparable amounts of urine (top panels) but different EUS activity patterns (bottom three panels). (A) The most common EUS activity pattern during voiding is phasic (i.e., bursting). Bursting is evident in the expanded view of the EUS EMG (horizontal bar under upper EUS EMG trace denotes region of EUS EMG trace expansion shown in lower EMG trace) and in the time-frequency spectrogram of EUS EMG as a bright band at 7–9 Hz (second panel from top). Spectral power is calculated over 1-s bins at 0.1-s increments and represented from 0 to 384 μV2 by linear greyscale from black to white, respectively. (B) An EUS EMG pattern less commonly seen (~25% of voids) has only tonic activity (note lack of bursting in the expanded view of the EUS EMG (lower EUS EMG trace) and lack of a bright band between 3 and 10 Hz in the spectrogram (second panel from top), despite void size being similar to that of the bursting void shown in A. Tick marks on time axes show 1-s intervals.
Void onset and offset were used as the basis for determining EUS EMG properties. EUS guarding (i.e., tonic pre-void EUS EMG) developed before almost every void (e.g., bottom panels in Figure 1), but subsequent phasic activity was variably expressed, ranging from robust bursting (bottom and middle panels in Figure 1A) to a completely tonic pattern (bottom and middle panels in Figure 1B). Thus, onset of EUS bursting could not reliably identify the transition from guarding to voiding. Instead, an empirical approach based on spectral analysis of voids with visually-identified bursting was devised that identified this transition point (see LaPallo et al.7 for presentation and justification of this approach). Void onset was defined as the time of transition from guarding to voiding as detected by the reduction in the combined EUS EMG power at 2, 11–12, and 21–30 Hz, regardless of the presence or absence of bursting. Void offset was calculated as the sum of the void EMG onset and urine accumulation duration.
The following void-associated EMG properties were calculated between void EMG onset and void EMG offset: EMG amplitude, defined as the average rectified EUS EMG calculated between void onset and void offset; bursting power, defined as the average signal power in the EUS bursting-frequency range (4–10 Hz in 22 rats and 3–9 Hz in 1 rat) expressed as a percent of the total average power; bursting time, defined as the cumulative time during which the bursting power was above a threshold value defined as the mean bursting power in amplitude-matched non-voiding epochs plus 2x its SD; and dominant frequency, defined as the average of the frequencies with the highest power from all spectra calculated during the void. In addition, the temporal structure of EUS bursting was further analyzed in a subset of voids (n=208 from 17 animals) that exhibited clear phasic activation with little tonic activity between the active phases by calculating: bursting silent period, defined as the time during a single cycle of bursting in which the EUS EMG was not active; bursting active period, defined as the time during a single cycle of bursting in which the EUS EMG was active; bursting period, defined as the duration of a single cycle of bursting (i.e., active period plus silent period); silent duty cycle, bursting silent period expressed as percent of the bursting period.
In preparation for regression analysis, data for each parameter were normalized by z-transformation for each individual animal by subtracting the mean value and then dividing by its standard deviation. Statistical significance of relationships between void size and other properties was determined by simple or multiple regression analysis.
RESULTS
Analyses were performed on 3629 voids recorded from 23 animals (median number of voids per animal=127, range=44–491). Five of the 23 animals were studied over ≥8 weeks, and thus contributed more voids to the analysis (median=291 and range=195–491 voids) than did the remaining 18 animals studied over 1–6 weeks (median=108 and range=44–210 voids). Mean values of the variables that characterize EUS EMG and voiding activity were calculated for each animal, and the means of these by-animal values are shown in Table I. Daily urine output varied significantly with daily water consumption (slope=0.39, r2=0.34, p<0.0001 for regression of urine weight/200 g/24 hr on water consumed/200 g/24 hr).
Table I.
EUS EMG, voiding, and other properties of chronically implanted rats
| Property | Mean ± SEM |
|---|---|
| Number of voids per 24 hr | 22.4 ± 1.4a |
| Intervoid interval (s) | 75.5 ± 4.1a |
| 24-hr urine output (g/200 g body weight) | 9.8 ± 0.6a |
| 24-hr water consumption (g/200 g body weight) | 19.1 ± 0.9 |
| Urine output/void (g/200 g body weight) | 0.49 ± 0.03a |
| Void duration (s) | 2.2 ± 0.1a |
| EMG amplitude (μV) | 28.6 ± 2.7a |
| Bursting power (% of total) | 14.8 ± 1.4a |
| Bursting time (s) | 0.93 ± 0.14a |
| Dominant frequency (Hz) | 6.7 ± 0.1a |
| Bursting active period (ms) | 70 ± 3 |
| Bursting silent period (ms) | 126 ± 12 |
| Bursting period (ms) | 196 ± 13 |
| Silent duty cycle (%) | 60.5 ± 1.8 |
Data from Table I of (7).
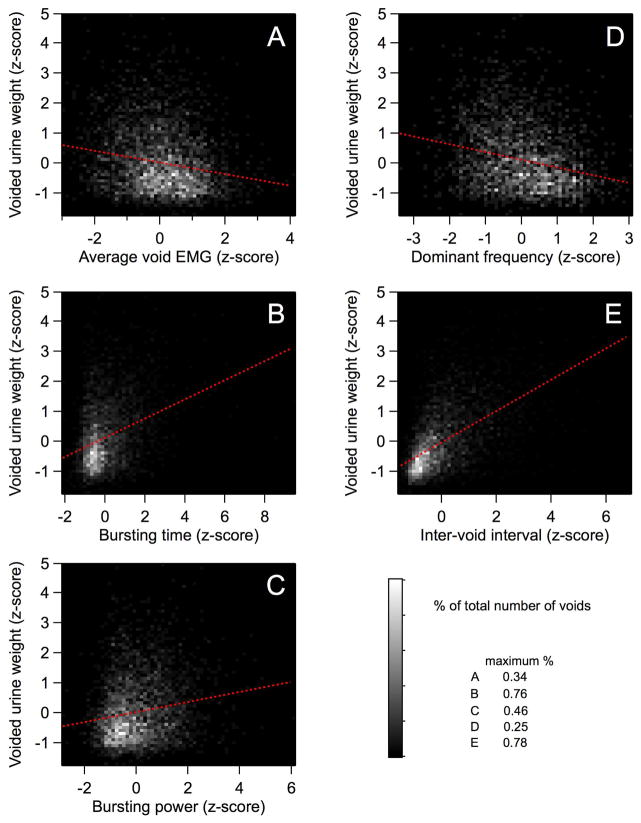
Figure 2 shows the relationships between urine weight per void and EUS or other urodynamic properties. Table II reports the slopes and coefficients of determination (r2) for simple regressions of void size on EUS and urodynamic variables. Significant relationships were found between void size and all EUS and urodynamic variables. Both bursting time and bursting power varied directly with void size, which is consistent with EUS bursting being an important contributor to urine expulsion in rats. Dominant frequency was inversely related to void size, which is consistent with previous reports that low-frequency bursting is associated with higher voiding efficiency8–10. The strength of the relationship between void size and low-frequency bursting is confirmed by regression analysis of void size on spectral power calculated at individual frequencies in the bursting range (3–10 Hz; Table II). Significant direct relationships were found at all frequencies between 3 and 7 Hz, with 4 Hz accounting for the largest amount of variation in void size of all the individual frequencies. It is noteworthy that void size was not significantly related to the amount of power expressed at 8–10 Hz, despite the fact that the largest peak in the entire spectrum of EUS EMG during voiding in conscious rats was observed at 8 Hz, with a smaller peak at 4 Hz (see Figure 7 in 7).
Figure 2.
Relationships between EMG parameters, inter-void interval, and void size. Contour plots show relationships between z-transformed values of void weight and average intra-void EMG (A), bursting time (B), bursting power (C), dominant frequency (D), and inter-void interval (E). Contour shading is a linear greyscale that indicates the number of voids represented by each pixel as a percent of total (maxima in lower right indicate the % of voids represented by pure white pixels in the respective panels). Linear regressions (dashed red lines) of void size on bursting power, bursting time, and inter-void interval show significant positive relationships; regressions of void size on average void EMG and dominant frequency exhibit significant inverse relationships.
Table II.
Simple regression analysis of voided urine weight on urodynamic and EUS EMG parameters
| Independent variable | Slope | r2 | % of animals with significant slope
|
|
|---|---|---|---|---|
| + slope | − slope | |||
| Inter-void interval | 0.52 | 0.284** | 100 | 0 |
| EMG amplitude | −0.19 | 0.037** | 13 | 61 |
| Bursting power | 0.17 | 0.028** | 52 | 9 |
| Dominant frequency | −0.26 | 0.063** | 0 | 65 |
| Bursting time | 0.32 | 0.101** | 74 | 4 |
| 3-Hz power | 0.15 | 0.023** | 35 | 4 |
| 4-Hz power | 0.28 | 0.079** | 61 | 0 |
| 5-Hz power | 0.21 | 0.045** | 57 | 0 |
| 6-Hz power | 0.13 | 0.018** | 35 | 0 |
| 7-Hz power | 0.10 | 0.009** | 43 | 4 |
| 8-Hz power | 0.03 | 0.001 | 26 | 22 |
| 9-Hz power | −0.03 | 0.001 | 17 | 17 |
| 10-Hz power | −0.04 | 0.001* | 9 | 17 |
| Bursting active period | 0.32 | 0.103** | 6 | 0 |
| Bursting silent period | 0.52 | 0.267** | 35 | 0 |
| Bursting period | 0.55 | 0.300** | 59 | 0 |
Data for all variables converted to z-scores (in units of SD with a mean=0 for individual animals). Regression statistics (Slope and r2) for data from all animals shown in second and third columns (*P<0.05, **P<0.0001). Percent of animals with significant (P<0.05) direct (+ slope) or inverse (− slope) regressions of urine weight on EUS EMG and urodynamic variables are shown in the fourth and fifth columns. n-Hz power, power at individual frequency n expressed as a percentage of the total power.
EUS bursting-related variables (i.e., bursting time, bursting duration, and dominant frequency), while significantly related to void size, individually accounted for only about 3–10% of the total variation in void size. Inter-void interval accounted for a large proportion of variation in void size (~28%; see Table II). The time between successive voids represents a complex urodynamic parameter that reflects the influences of multiple factors, including the rate of urine production, bladder and urethral tissue biomechanics, and autonomic status. These factors jointly determine the bladder filling threshold and the rate at which this occurs. The diurnal variation in void size and inter-void interval11 further complicate the relationships among these variables. We have recently reported that intra-void EUS activity also exhibits diurnal variation, in that bursting time and power are higher and void EMG amplitude and dominant frequency are lower in the light than in the dark (see Figure 6 and Table I in 7). To dissect the contributions of these various elements to void size, we have performed multiple regression analyses of void size on inter-void interval, light-dark status, and each of the EUS-related variables described above (Table III). Inter-void interval uniquely accounts for more variation in void size than any other factors. Nevertheless, each of the EUS-related variables accounts for a significant fraction of the total variation in void size independent of inter-void interval and light-dark status. The slopes of the relationships between void size and EUS EMG amplitude, dominant frequency, bursting power and time, and individual bursting frequencies are similar to those of the simple regression analyses, and the preferential dependence of void size on bursting in the low-frequency range persists.
Table III.
Multiple regression analyses of voided urine weight on urodynamic and EUS EMG parameters
| Independent variable | Multiple regression (with inter-void interval and light vs. dark)
|
|||||
|---|---|---|---|---|---|---|
| Slope indep | r2 indep | r2 inter-void interval | r2 light vs. dark | r2 common | r2 total | |
| EMG amplitude | −0.07 | 0.038* | 0.125* | 0.003** | 0.157 | 0.327* |
| Bursting power | 0.10 | 0.036* | 0.134* | 0.006* | 0.147 | 0.330* |
| Dominant frequency | −0.17 | 0.031* | 0.128* | 0.018* | 0.149 | 0.338* |
| Bursting time | 0.22 | 0.028* | 0.115* | 0.034* | 0.163 | 0.350* |
| 3-Hz power | 0.08 | 0.004* | 0.139* | 0.034* | 0.144 | 0.329* |
| 4-Hz power | 0.17 | 0.022* | 0.130* | 0.029* | 0.133 | 0.323* |
| 5-Hz power | 0.15 | 0.015* | 0.134* | 0.034* | 0.156 | 0.351* |
| 6-Hz power | 0.09 | 0.006* | 0.143* | 0.036* | 0.136 | 0.328* |
| 7-Hz power | 0.03 | 0.001 | 0.148* | 0.036* | 0.132 | 0.324* |
| 8-Hz power | 0.00 | 0.000 | 0.149* | 0.038* | 0.129 | 0.322* |
| 9-Hz power | −0.02 | 0.000 | 0.150* | 0.037* | 0.128 | 0.322* |
| 10-Hz power | −0.02 | 0.000 | 0.150* | 0.037* | 0.129 | 0.325* |
Data for all variables converted to z-scores (in units of SD with a mean=0 for individual animals). n-Hz power, power at individual frequency n expressed as a percentage of the total power.
The fraction of variance accounted for by each independent variable in the multiple regression analysis is smaller than that observed with simple regressions of void size on each of the independent variables alone (e.g., compare r2 for the simple regression (Table II) with that of the multiple regression (Table III)). The sum of r2 for interval, light-dark status, and each of the other EUS-related variables does not add up to the r2 for the entire model, indicating the existence of a modest degree of covariation among the variables (common r2 in Table III).
The data presented above are pooled across animals. We previously reported that there is considerable inter-animal variability as well as intra-animal variability in EUS activity during voiding7. Simple regression analyses of void size on each of the urodynamic and EUS-related variables described above performed within individual animals revealed this inter-animal variability. Table II shows the numbers of individual rats with significant correlations. The relationship between void size and inter-void interval was detected in all 23 rats. Not all the rats showed significant relationships with EUS-related variables, but the majority had significant relationships that were similar to those detected in the pooled data.
The wide range of correlation between EUS bursting-related variables and void size in many rats reflected the high variability in expression of EUS bursting within and between rats7. About 25% of voids were not associated with any significant bursting (as defined by bursting time=0); this ranged from 0–62% for individual rats. Thus, the relationships between void size and EUS bursting variables may have been influenced by a differential expression of bursting. Table IV shows results of simple regression analyses of void size on urodynamic and EUS properties using either voids with bursting (bursting time>0) or voids without bursting (bursting time=0). As expected, voids without bursting did not show significant relationships between void size and bursting-related properties such as bursting power or power at any of the individual bursting frequencies. For voids with significant bursting (i.e., bursting time>0), bursting time, bursting power, and power at the individual frequencies of 3–7 Hz were significantly and directly correlated with void size. That void size was negatively correlated with power at frequencies of 9 and 10 Hz highlights the importance of low-frequency bursting for voiding, and raises the possibility that high-frequency bursting is detrimental to voiding.
Table IV.
Simple regression analyses of voided urine weight on urodynamic and EUS EMG parameters for voids with different bursting times
| Independent variable | Bursting time>0 | Bursting time=0 | ||
|---|---|---|---|---|
|
| ||||
| Slope | r2 | Slope | r2 | |
| Inter-void interval | 0.49 | 0.264* | 0.49 | 0.254* |
| EMG amplitude | −0.18 | 0.029* | −0.20 | 0.045* |
| Bursting power | 0.13 | 0.015* | 0.01 | 0.000 |
| Dominant frequency | −0.26 | 0.064* | -- | -- |
| Bursting time | 0.33 | 0.111* | -- | -- |
| 3-Hz power | 0.18 | 0.032* | −0.03 | 0.001 |
| 4-Hz power | 0.27 | 0.084* | 0.06 | 0.001 |
| 5-Hz power | 0.20 | 0.043* | 0.06 | 0.001 |
| 6-Hz power | 0.12 | 0.016* | −0.06 | 0.002 |
| 7-Hz power | 0.06 | 0.004** | −0.08 | 0.002 |
| 8-Hz power | −0.03 | 0.001 | −0.06 | 0.001 |
| 9-Hz power | −0.09 | 0.009** | −0.06 | 0.001 |
| 10-Hz power | −0.10 | 0.009* | 0.01 | 0.000 |
Data for all variables converted to z-scores (in units of SD with a mean=0 for individual animals). n-Hz power, power at individual frequency n expressed as a percentage of the total power. Dashes indicate regression analyses that could not be performed for voids with bursting time=0.
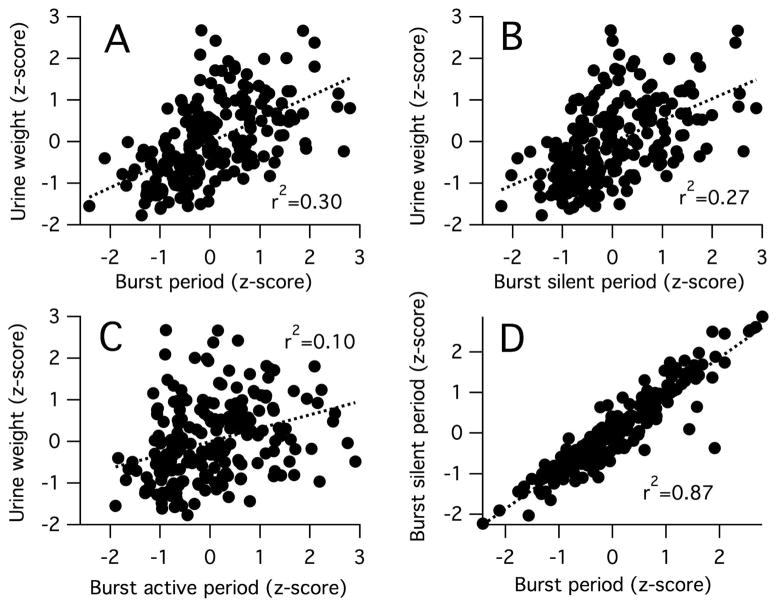
The importance of low-frequency bursting to productive voiding was further emphasized by the relationships detected between void size and the time course of single cycles of bursting. Void size was positively correlated with the bursting period (Figure 3A), demonstrating that in voids with bursting, void production is enhanced at lower frequencies of bursting. Void size was also positively correlated with bursting silent period (Figure 3B), suggesting that void production is enhanced by having longer segments of intermittent EUS relaxation during bursting. Bursting silent period is highly positively correlated with the bursting period (Figure 3D), suggesting that variation in void size reflects in part the frequency-dependent duration of the silent period. There was also a weaker, but still significant positive relationship between void size and bursting active period (Figure 3C). Multiple regression of void size on bursting silent and active periods revealed that both independent variables uniquely accounted for a significant amount of variation in void size (r2 for variance uniquely accounted for by the silent period or active period duration was 0.21 and 0.04, respectively, suggesting that while the silent period duration is an important contributor to void size, the active period plays a role (albeit weaker) in determining void size.
Figure 3.
Relationships between intra-burst activity timing and void size. Plots show relationships between z-transformed values of: void weight and bursting period (i.e., inverse of bursting frequency) (A), bursting silent period (B), and bursting active period (C); and bursting silent period and bursting period (D). Linear regressions (dashed lines) exhibited significant positive relationships (P<0.0001 for all).
Average intra-void EUS EMG was inversely related to void size in voids with or without bursting (Figure 1). For voids with bursting, this relationship likely reflects the significant covariation between bursting silent period and bursting frequency, in that average EUS EMG would be expected to be low during low bursting-frequency voids with proportionally long silent periods, and high during high bursting-frequency voids with proportionally short silent periods. For voids without evidence of bursting, the greater void size with smaller EUS EMG is consistent with a synergistic pattern of bladder contraction and EUS relaxation that is evident in all animals that do not employ EUS bursting as a voiding strategy (e.g., humans).
The strong relationship between void size and inter-void interval was comparable in voids with and without significant bursting (Table IV). Thus, EUS bursting was largely independent of the factors that determine how quickly and how much urine can be accumulated.
DISCUSSION
The present analysis extends and expands upon the findings of our previous methodological study7. The previous study demonstrated the chronic EUS recording methodology, analyzed the spectral content of EUS EMG during voiding, and identified the prevalence of EUS bursting during voiding. The novel results of the present analysis are that EUS temporal and spectral properties vary with void size during spontaneous micturition in conscious, freely moving female rats. Void size is inversely correlated with bursting frequency and positively correlated with: the duration of bursting silent and active periods, the overall duration of bursting, and the spectral power of EUS EMG at low frequencies within the bursting range. These data indicate that bursting—particularly at low frequencies—enhances void size in unanesthetized rats. After accounting for the contribution of interval and light-dark status, EUS properties still account for a significant amount of the variation in void size. These results are consistent with bursting being important for voiding in the unanesthetized rat, as it is in the anesthetized rat2, 3, 5.
The mechanism by which bursting enhances voiding in rats is not entirely clear. EUS bursting was initially suggested to provide a pumping action that ejects urine12. Subsequent analyses demonstrated that urine flow during bursting occurs during EMG silent periods and not active periods, as would be the case if it were being actively pumped13. The role of this mechanism is supported by the parallel effect of serotonergic agents on silent period duration and voiding efficiency8, 14. The strong positive correlation between void size and bursting silent period seen in the present study is consistent with the importance of the intra-burst EUS EMG silence to voiding.
Transection of the sensory branch of the pudendal nerve also reduces voiding efficiency4, suggesting that at least part of the contribution of bursting may be to enhance afferent drive to LUT circuitry during voiding reflexes. The positive correlation between void size and bursting active period is consistent with this mechanism.
Despite the clear importance of bursting to voiding in rats, our previous study demonstrated that many sizable voids occurred without bursting7. The present study detected an inverse relationship between void size and intra-void EMG amplitude, suggesting that the EUS can relax tonically to favor voiding and that high tonic activity restricts voiding. Thus, in the absence of bursting, tonic EUS relaxation may contribute to voiding in much the same way that it does in humans (i.e., synergically with bladder contraction).
Inter-void interval was as strongly related to void size as were the best-correlated EUS-related factors (i.e., bursting period and bursting silent period). The EUS is only one part of the multi-component system that comprises the LUT, which also includes the smooth muscle of the bladder and urethra. Furthermore, bladder filling rate, and thus LUT output, depends upon the rate of urine production by the kidney. The diurnal variation in voiding frequency and volume reflects diurnal variation in glomerular filtration rate, bladder capacity, and autonomic function11, 15, 16. Thus, many other factors contribute to voiding that were not assessed directly in the present study. The strength of the relationship between void size and inter-void interval likely reflects the contribution of a number of these factors, including: the rate of production of urine (reflecting in large part glomerular filtration rate), which dictates how fast the bladder fills; bladder compliance (influenced by tissue biomechanics and autonomic tone) which affects bladder capacity; and the sensitivity of afferent and central pathways involved in transduction, transmission and integration of sensory information that contribute to setting the threshold for reflex control of bladder activity. Diurnal variation in these properties could in theory introduce an artificial relationship between void size and inter-void interval. However, multiple regression analysis including inter-void interval, lighting status, and EUS-related variables did not occlude the significant relationship between inter-void interval and void size. Thus, this strong relationship reflects the intrinsic dependence of void size on non-EUS-related factors that determine LUT function.
These results provide new insights into EUS function during voiding in rats and other species. Rats can exhibit EUS bursting even in the absence of supraspinal input; this is consistent with the existence of spinal circuitry that acts as a bursting pattern generator6, 10, 17. The observation that urine flow can occur with or without EUS bursting in the rat suggests that the bursting pattern generator can be suppressed or that it is simply not activated during some voids at physiological rates of bladder filling.
EUS bursting is not unique to rats. Phasic EUS activity during voiding has also been reported in the mouse18, hamster19, cat20 and non-human primate21 with intact nervous systems and after damage to the nervous system in the rabbit22 and dog23. The guinea pig EUS does not normally exhibit phasic activation during voiding19, but noxious experimental conditions (e.g., instillation of slightly acidic6 or ice-cold solutions24 into the bladder) can elicit EUS bursting. These observations, plus one anecdotal report of bursting-like EUS activity in brain-dead humans25, support the hypothesis that bursting is a phylogenetically conserved mechanism6. Thus, bursting may represent a primitive and/or energetically costly mode of voiding that is used differentially among mammals, and that can be recruited into action in some species when neural circuitry that controls LUT function is perturbed, e.g., by neural injury, noxious sensory input, or anesthesia.
EUS bursting does not occur during normal LUT function in humans. The extensive cross-species expression of bursting described above raises the possibility that humans may also have a central pattern generator that is capable of producing this pattern of activity, but it is too weak or too strongly inhibited to be expressed under normal circumstances. Pharmacological or electrophysiological activation of this pattern generator to elicit EUS bursting could represent a therapeutic approach to improving voiding in pathological conditions such as detrusor-sphincter dyssynergia.
CONCLUSIONS
In unanesthetized unrestrained female rats, EUS bursting (particularly bursting frequency and intra-burst timing) influences void size as strongly as non-EUS-related factors. Non-bursting voiding can also occur with synergic coordination, suggesting that studies of the role of EUS activity during voiding in conscious rats may be more relevant to non-bursting species (notably humans) than previously appreciated.
Acknowledgments
This study was supported by the Craig H. Neilsen Foundation (J. S. Carp), NIH (NS22189, J. R. Wolpaw; HD36020, X. Y. Chen; NS061823, J. R. Wolpaw and X. Y. Chen), and the New York State Spinal Cord Injury Trust Fund (X. Y. Chen).
References
- 1.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conte B, Maggi CA, Parlani M, Lopez G, Manzini S, Giachetti A. Simultaneous recording of vesical and urethral pressure in urethane-anesthetized rats: effect of neuromuscular blocking agents on the activity of the external urethral sphincter. J Pharmacol Methods. 1991;26:161–171. doi: 10.1016/0160-5402(91)90041-3. [DOI] [PubMed] [Google Scholar]
- 3.Cruz Y, Downie JW. Sexually dimorphic micturition in rats: relationship of perineal muscle activity to voiding pattern. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1307–1318. doi: 10.1152/ajpregu.00088.2005. [DOI] [PubMed] [Google Scholar]
- 4.Peng CW, Chen JJ, Cheng CL, Grill WM. Role of pudendal afferents in voiding efficiency in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R660–672. doi: 10.1152/ajpregu.00270.2007. [DOI] [PubMed] [Google Scholar]
- 5.Yoshiyama M, deGroat WC, Fraser MO. Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin, a neuromuscular junction blocking agent, on voiding dysfunction in the rat with spinal cord injury. Urol. 2000;55:956–960. doi: 10.1016/s0090-4295(00)00474-x. [DOI] [PubMed] [Google Scholar]
- 6.Fraser MO. New insights into the pathophysiology of detrusor-sphincter dyssynergia. Curr Bladder Dysfunct Rep. 2011;6:93–99. [Google Scholar]
- 7.LaPallo BK, Wolpaw JR, Chen XY, Carp JS. Long-term recording of external urethral sphincter EMG activity in unanesthetized, unrestrained rats. Am J Physiol Renal Physiol. 2014;307:F485–497. doi: 10.1152/ajprenal.00059.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SC, Cheng CL, Fan WJ, Chen JJ, Lai CH, Peng CW. Effect of a 5-HT1A receptor agonist (8-OH-DPAT) on external urethral sphincter activity in a rat model of pudendal nerve injury. Am J Physiol Regul Integr Comp Physiol. 2011;301:R225–235. doi: 10.1152/ajpregu.00260.2010. [DOI] [PubMed] [Google Scholar]
- 9.Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol. 2004;187:445–454. doi: 10.1016/j.expneurol.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Dolber PC, Gu B, Zhang X, Fraser MO, Thor KB, Reiter JP. Activation of the external urethral sphincter central pattern generator by a 5-HT(1A) receptor agonist in rats with chronic spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1699–1706. doi: 10.1152/ajpregu.00142.2006. [DOI] [PubMed] [Google Scholar]
- 11.Herrera GM, Meredith AL. Diurnal variation in urodynamics of rat. PLoS One. 2010;5:e12298. doi: 10.1371/journal.pone.0012298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruse MN, Belton AL, de Groat WC. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am J Physiol. 1993;264:R1157–1163. doi: 10.1152/ajpregu.1993.264.6.R1157. [DOI] [PubMed] [Google Scholar]
- 13.Streng T, Santti R, Andersson KE, Talo A. The role of the rhabdosphincter in female rat voiding. BJU Int. 2004;94:138–142. doi: 10.1111/j.1464-4096.2004.04875.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheng CL, de Groat WC. Role of 5-HT1A receptors in control of lower urinary tract function in anesthetized rats. Am J Physiol Renal Physiol. 2010;298:F771–778. doi: 10.1152/ajprenal.00266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakatua DJ, Haus E, Halberg F, et al. Circadian characteristics of urinary epinephrine and norepinephrine from healthy young women in Japan and U.S. A Chronobiol Int. 1986;3:189–195. doi: 10.3109/07420528609066366. [DOI] [PubMed] [Google Scholar]
- 16.Pons M, Tranchot J, L’Azou B, Cambar J. Circadian rhythms of renal hemodynamics in unanesthetized, unrestrained rats. Chronobiol Int. 1994;11:301–308. doi: 10.3109/07420529409057246. [DOI] [PubMed] [Google Scholar]
- 17.Chang HY, Cheng CL, Chen JJ, de Groat WC. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am J Physiol Renal Physiol. 2007;292:F1044–1053. doi: 10.1152/ajprenal.00175.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith PP, Kuchel GA. Continuous uroflow cystometry in the urethane-anesthetized mouse. Neurourol Urodyn. 2010;29:1344–1349. doi: 10.1002/nau.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maggi CA, Giuliani S, Santicioli P, et al. Species-related variations in the effects of capsaicin on urinary bladder functions: relation to bladder content of substance P-like immunoreactivity. Naunyn Schmiedebergs Arch Pharmacol. 1987;336:546–555. doi: 10.1007/BF00169312. [DOI] [PubMed] [Google Scholar]
- 20.Sackman JE, Sims MH. Electromyographic evaluation of the external urethral sphincter during cystometry in male cats. Am J Vet Res. 1990;51:1237–1241. [PubMed] [Google Scholar]
- 21.Lee U, Chang HH, Christe K, Havton L. Analysis of evoked voiding contractions and corresponding urethral sphincter electromyography in non human primates. Abstract 33. J Urol. 2013;189:e13. [Google Scholar]
- 22.Hiraizumi Y, Hisamitsu T, Ichikawa S, Fujimaki E. Long term observation of micturition by spinal cord transected rabbits. Physiol Behav. 1987;41:331–339. doi: 10.1016/0031-9384(87)90397-0. [DOI] [PubMed] [Google Scholar]
- 23.Nishizawa O, Fukuda T, Matsuzaki A, Moriya I, Harada T, Tsuchida S. Role of the sympathetic nerve in bladder and urethral sphincter function during the micturition cycle in the dog evaluated by pressure flow EMG study. J Urol. 1985;134:1259–1261. doi: 10.1016/s0022-5347(17)47707-x. [DOI] [PubMed] [Google Scholar]
- 24.Gardiner JC, McMurray G, Westbrook S. A bladder-cooling reflex in the anaesthetised guinea-pig: a model of the positive clinical ice-water test. J Pharmacol Toxicol Methods. 2007;55:184–192. doi: 10.1016/j.vascn.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Schalow G. Oscillatory firing of single human sphincteric alpha 2 and alpha 3-motoneurons reflexly activated for the continence of urinary bladder and rectum. Restoration of bladder function in paraplegia. Electromyogr Clin Neurophysiol. 1991;31:323–355. [PubMed] [Google Scholar]