Abstract
n-3 PUFA have been shown in many clinical studies to attenuate inflammatory responses. Although inflammatory responses are orchestrated by a wide spectrum of cells, CD4+ T cells play an important role in the etiology of many chronic inflammatory diseases such as inflammatory bowel disease and obesity. In light of recent concerns over the safety profiles of non-steroidal anti-inflammatory drugs (NSAIDs), alternatives such as bioactive nutraceuticals are becoming more attractive. In order for these agents to be accepted into mainstream medicine, however, the mechanisms by which nutraceuticals such as n-3 polyunsaturated fatty acids (PUFA) exert their anti-inflammatory effects must be fully elucidated. Lipid rafts are nanoscale, dynamic domains in the plasma membrane that are formed through favorable lipid-lipid (cholesterol, sphingolipids, and saturated fatty acids) and lipid-protein (membrane-actin cytoskeleton) interactions. These domains optimize the clustering of signaling proteins at the membrane to facilitate efficient cell signaling which is required for CD4+ T cell activation and differentiation. This review summarizes novel emerging data documenting the ability of n-3 PUFA to perturb membrane-cytoskeletal structure and function in CD4+ T cells. An understanding of these underlying mechanisms will provide a rationale for the use of n-3 PUFA in the treatment of chronic inflammation.
Keywords: Omega-3 fatty acids, Lipid rafts, T cell activation, T cell differentiation
1. Introduction
The mammalian immune system is critical in defending the host against foreign pathogens and malignant cells. Under normal conditions, T lymphocytes circulate throughout the body to survey for foreign antigens and transformed cells and target them for destruction. However, under certain pathophysiological conditions, the adaptive immune system may lose the ability to differentiate between self and foreign antigens, resulting in self-reactive T lymphocyte activation and effector function. The result of the loss of self-tolerance could be autoimmune diseases such as inflammatory bowel disease (IBD), e.g., Crohn’s disease and ulcerative colitis (Zenewicz et al., 2009). Alternatively, the adaptive immune system may become over-reactive against self-antigens and unable to resolve appropriately, resulting in chronic inflammatory diseases such as rheumatoid arthritis. The mammalian immune system is comprised of the innate and the adaptive system; this review will focus on the adaptive arm, specifically CD4+ T lymphocytes. These cells typically further differentiate into other effector cell types (TH1, TH2, Treg, TH17), which have opposing roles in autoimmune diseases such as IBD (Zenewicz et al., 2009).
The cell membrane, composed of a phospholipid bilayer and a myriad of proteins, constitutes the outer boundary of the cell. Not only does the cell membrane control molecular transport, but it also regulates communication between the cell and its environment by transducing signals. . The first model of the plasma membrane, the fluid mosaic model, was proposed in 1972 by Singer and Nicolson (Singer and Nicolson, 1972). In this model, the phospholipid bilayer is thought of as a fluid, dynamic, passive solvent in which proteins are either embedded in and span the membrane (i.e. integral proteins), or loosely associate (i.e. peripheral proteins), with the phospholipid bilayer. The plasma membrane contains three classes of amphiphilic lipids: phospholipids, glycolipids, and sterols. Phospholipids and glycolipids are further subdivided into various fatty acids and headgroups at the sn-1, sn-2, and sn-3 positions (Fahy et al., 2009). For example, a major species of phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] is composed of a saturated C18:0 fatty acid at the sn-1 position, an unsaturated C20:4 5,8,11,14 fatty acid at the sn-2 position, and myo-inositol 4,5-bisphosphate at the sn-3 position. The heterogeneity of the lipids in the plasma membrane is not well studied, with the potential to generate 9,000 – 100,000 different molecular species (Shevchenko and Simons, 2010; van Meer, 2005; Yetukuri et al., 2008). With all these layers of complexity, could lipids in the plasma membrane form local structures that can function to regulate cell signaling?
2. n-3 PUFA and lipid rafts in the CD4+ T cell plasma membrane
2.1 Lipid rafts
In a simple model system where two lipids (one high melting temperature, one low melting temperature) and cholesterol are mixed together, micron-scale domains phase separate and are easily visualized using conventional fluorescence microscopy (Nicolau et al., 2006). These micron-sized microdomains, one example of local structures in the plasma membrane, can be observed in epithelial cells, where the apical plasma membrane is enriched in sphingolipids, while the basolateral plasma membrane is enriched in phosphatidylcholine (Zidovetzki and Levitan, 2007). Small invaginations in the plasma membrane, enriched with cholesterol, sphingolipids, and the protein caveolin, can also be found in many cells such as endothelial and intestinal epithelial cells and adipocytes (Ma et al., 2004; Toulmay and Prinz, 2013). Smaller, highly dynamic, nanoscale lipid rafts enriched in sphingolipids, cholesterol, and saturated fatty acids, have been proposed to play a role in signal transduction (Fig. 1). In fact, stable nanodomains can be visualized in yeast vacuole membranes in response to various stresses such as nutrient deprivation and pH change; proteins that sort to these vacuolar membranes also segregate to one of two domains, similar to what would be predicted by the simple system composed of two lipids and cholesterol (Toulmay and Prinz, 2013). These nanodomains are thought to organize select proteins to optimize their signaling capacity upon ligand engagement. Computer simulations suggest that in order for lipid rafts to promote protein-protein interactions, these nanoscale domains must be small (6 to 14 nm in diameter) in order to operate as protein concentrators in the plasma membrane (Nicolau et al., 2006).
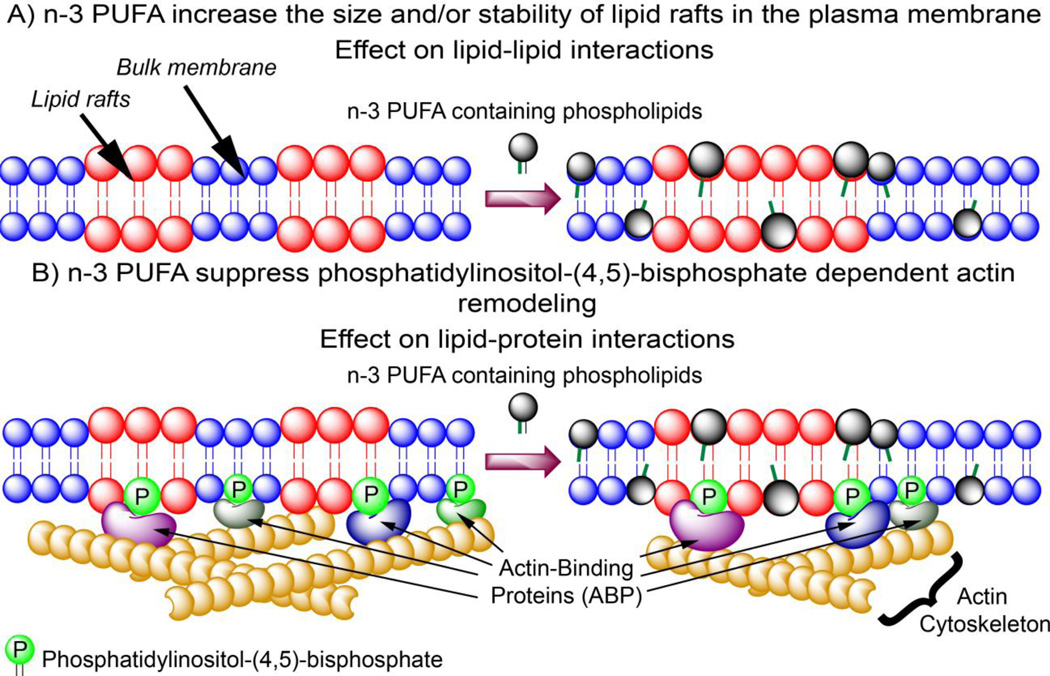
Figure 1.
Proposed mechanisms by which n-3 PUFA modulate adaptive immune responses by A) modulating lipid-lipid interactions in the plasma membrane; and B) altering plasma membrane lipid-protein interactions by decreasing PI(4,5)P2 level, thereby lowering the recruitment of actin-binding proteins and suppressing actin cytoskeleton remodeling. Consequently, incorporation of n-3 PUFA into the plasma membrane increase the size and/or stability of the mesoscale lipid rafts and physiologically. This translates into suppressed CD4+ T cell activation and differentiation. Red highlight indicates liquid ordered lipid rafts; Blue indicates bulk membrane.
Although lipid rafts can associate and dissociate as a mechanism to regulate the formation of raft phases in the plasma membrane, one way to achieve a stabilized raft phase (i.e., stabilize the size and/or lifetime of the raft) is the presence of the actin cytoskeleton. Monomeric actin (G-actin) protein is capable of polymerization to form long, complex filamentous actin (F-actin) that can provide the force required for organelle movement, and the scaffold required for stabilization of membrane raft phases. F-actin is connected to the plasma membrane by interacting with integral and membrane-associated proteins; e.g., various protein-actin cytoskeleton interactions in erythrocytes (Luna and Hitt, 1992). One current model is that these “membrane skeletons” form the “fences” in the plasma membrane, impeding the diffusion of membrane proteins and lipids (Kusumi et al., 2012).
The participation of the actin cytoskeleton in the formation of nanoscale domains was first postulated when it was observed that the coefficient of diffusion of phospholipid probes were significantly lower in live cells (Lee et al., 1993; Swaisgood and Schindler, 1989), than those estimated in artificial membranes (Ladha et al., 1996; Sonnleitner et al., 1999). This difference was also observed for transmembrane protein markers and glycosylphosphatidylinositol-anchored protein markers (Kusumi et al., 2012). One suggestion to explain the difference in the diffusion coefficient was the presence of the membrane cytoskeleton in live cells compared to artificial membranes. The requirement for the actin cytoskeleton in maintaining the properties of nanoscale domains was also observed using secondary ion mass spectrometry, in which treatment of NIH 3T3 mouse fibroblasts with latrunculin A (prevents G-actin polymerization) resulted in a random distribution of sphingomyelin instead of sphingomyelin-enriched microdomains in control fibroblasts (Frisz et al., 2013).
The actin cytoskeleton is bridged to membranes through interactions with integral proteins, or through proteins recruited to the plasma membrane by specific phospholipids. One such phospholipid is phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2], which comprises 5% of the total phosphatidylinositol species, and 1% of the total plasma membrane phospholipid in mammalian cells (Di Paolo and De Camilli, 2006; Kwiatkowska, 2010). Classically, PI(4,5)P2 is important in signal transduction because of the hydrolysis products diacylglycerol (DAG), and inositol(1,4,5)-trisphosphate (IP3) produced by phospholipase C. However, an additional function of PI(4,5)P2 is to recruit actin remodeling proteins to localized sites at the plasma membrane. PI(4,5)P2 is thought to activate actin-regulatory proteins that induce actin polymerization, while concurrently inhibiting those proteins that promote actin disassembly. Indeed, the increase in local PI(4,5)P2 results in stress-fiber formation, indicative of increased actin filament formation (Kwiatkowska, 2010). Due to multiple acidic phosphate groups on PI(4,5)P2, basic protein domains are thought to play a role in recruiting proteins to the plasma membrane. For example, the Wiskott-Aldrich syndrome protein (WASP) is released from its autoinhibitory conformation when the basic domain binds to PI(4,5)P2 (Padrick et al., 2008). Similarly, PI(4,5)P2 can recruit and activate Rho family GTPase through its polybasic region. Some small GTPases play a role in cytoskeletal remodeling (Saarikangas et al., 2010).
A third interaction to consider in the formation and organization of optimal nanoscale lipid rafts is the role of higher-ordered protein complexes (i.e., protein-protein interactions), as proposed by Kai Simons and Akihiro Kusumi (Kusumi et al., 2011; Simons and Sampaio, 2011). As an example, epidermal growth factor receptor (EGFR) undergoes conformational change and dimerization upon binding to its ligand (EGF), resulting in downstream signaling (Turk et al., 2012; Turk and Chapkin, 2013). Optimal EGFR signaling, however, takes place in lipid rafts (Turk and Chapkin, 2013; 2015), highlighting the role of protein-protein interactions in driving the formation and organization of optimal nanoscale domains for cellular signaling.
2.2 Effects of n-3 PUFA on lipid rafts in CD4+ T cell plasma membrane
Eicosapentaenoic acid (EPA, 20:5 Δ5,8,11,14,17) and docosahexaenoic acid (DHA, 22:6 Δ 4,7,10,13,16,19) are thought to be the major bioactive n-3 PUFA in fish oil. EPA and DHA are incorporated into the two major classes of phospholipids in the plasma membrane, phosphatidylenthanolamine and phosphatidylcholine at the sn-2 position (Fan et al., 2004; Fan et al., 2003), consistent with the observation that saturated fatty acids occupy the sn-1 position of phospholipids, while unsaturated fatty acids are inserted at the sn-2 position. DHA is highly disordered and adopts various conformations on the subnanosecond time scale (Gawrisch and Soubias, 2008; Soubias and Gawrisch, 2007). By eliminating the double bond at the n-3 position to generate docosapentaenoic acid (DPA, 22:5 Δ 4,7,10,13,16), chain dynamics of the fatty acid are reduced (Gawrisch and Soubias, 2008), suggesting that the unsaturation at the n-3 position affords unique properties to EPA and DHA in the plasma membrane.
Studies on ion channels have demonstrated that EPA and DHA can modulate membrane protein properties. The incorporation of DHA into the lipid bilayer resulted in a decrease in bilayer stiffness without changing the negative curvature of the bilayer (Bruno et al., 2007). DHA increased the appearance and lifetime of gramicidin channels, and decreased the free energy for channel formation. Oleic acid (OA, 18:1 Δ 9) that intercalated into the plasma membrane at a greater rate, had no effect on gramicidin channel formation, suggesting that the effect of DHA was due to changes in bilayer properties and not due to specific binding (Bruno et al., 2007; Bruno et al., 2013). EPA and DHA inhibit cardiac Na+ and L-type Ca2+ channels (Xiao et al., 1997; Xiao et al., 1995), activate TRAAK-1 and TRPV1 channels (Fink et al., 1998; Matta et al., 2007), and increase desensitization of nAChR and GABAa channels (Bouzat and Barrantes, 1993; Nabekura et al., 1998).
DHA is thought to play a major role in altering the size and/or stability of nanoscale lipid rafts in the plasma membrane. As a result of its highly disordered nature, DHA may modify the lateral organization of the plasma membrane by forming a distinct non-raft DHA domain (Shaikh et al., 2014; Wassall and Stillwell, 2008). This DHA domain is distinct from the sphingolipid and cholesterol enriched lipid rafts due to the highly flexible DHA, which is incompatible with the rigidity of sphingolipids and cholesterol. Incorporation of DHA into the plasma membrane results in the distinct non-raft DHA domain inserting itself into lipid rafts, increasing the size of lipid rafts. This mechanism would explain nuclear magnetic resonance (NMR) data which indicated that EPA and DHA are incorporated into both raft and non-raft domains in a phosphatidylethanolamine/sphingomyelin/cholesterol membrane mixture (Williams et al., 2012). Similar results have also been observed in vivo by analyzing phospholipids of CD4+ T cells after isolating the detergent-resistant and –soluble membrane fractions (Fan et al., 2004; Fan et al., 2003), as well as utilizing lipid-sensitive fluorescent probes (Kim et al., 2014; Kim et al., 2008).
An alternative mechanism proposed to explain the effects of n-3 PUFA on lipid raft organization comes from the flexibility of n-3 PUFA which is incompatible with the rigid cholesterol. This effect promotes the aggregation of cholesterol in the plasma membrane, resulting in the coalescence of sphingolipid and cholesterol lipid rafts from the bulk membrane (Wassall and Stillwell, 2009). Indeed, it has been shown that EPA and DHA can increase the size of lipid rafts in HEK cells, CD4+ T cells, and B cells (Chapkin et al., 2008; Kim et al., 2008; Rockett et al., 2012). The resulting alteration in the optimal size of nanoscale lipid rafts could perturb the cellular signaling required for CD4+ T cell activation and differentiation.
3. CD4+ T cell activation
The engagement of the CD4+ T cell receptor (TCR) by an antigenic peptide presented by the major histocompatibility complex II (MHCII) molecule results in the formation of the immunological synapse (IS, Fig. 2). Proteins essential for propagating the signal are enriched at the IS to form the central supramolecular activation cluster (cSMAC). Adhesion molecules necessary to stabilize the IS form the peripheral supramolecular activation cluster (pSMAC) leading to the “bull’s eye” pattern of the IS (Lee et al., 2003; Monks et al., 1998). Proteins such as phosphatases that can abrogate signaling are excluded from the cSMAC and pSMAC in order for T cell activation to take place. At the IS, tyrosine kinases Lck and ZAP70 are activated and subsequently phosphorylate the adaptor protein linker for activation of T cells (LAT), leading to the assembly of the signalsome comprised of many proteins, including GADS, SLP76, NCK, ITK, VAV1, PAK, and PLC-γ1 (Tybulewicz and Henderson, 2009). The proper formation of the IS is required for sustained T cell activation and is stabilized by the actin cytoskeleton (Gomez and Billadeau, 2008; Huang and Burkhardt, 2007; Meiri, 2005). When the adhesion proteins coalesce to form the pSMAC, the actin cytoskeleton is connected to the IS by proteins such as talin, vinculin, and WASp. The dynamics of the actin cytoskeleton, however, are also important for sustained T cell activation. It is thought that the centripetal flow of F-actin to the initial engagement site between TCR and MHCII is important for the strength and duration of T cell activation. Indeed, T cell activation can be disrupted by inhibiting actin polymerization (Campi et al., 2005; DeMond et al., 2008; Kaizuka et al., 2007; Yokosuka et al., 2005).
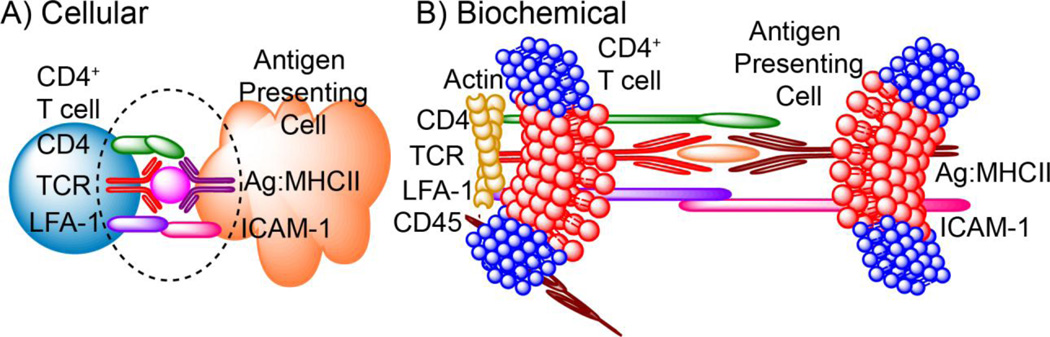
Figure 2.
Immunological synapse at the A) cellular; and B) biochemical level. When CD4+ T cells recognize cognate antigen presented by the antigen presenting cell, an immunological synapse is formed at the cellular level. At the biochemical level, major lipidomic and proteomic rearrangement occurs to propagate T cell activation. At the lipidomic level, lipids associated with lipid rafts are found to coalesce at the immunological synapse (represented in red). Adhesion molecules, such as LFA-1, are important for the stabilization of the immunological synapse, while CD45, which is required to terminate T cell activation, is excluded from the immunological synapse. Additionally, the actin cytoskeleton is important for T cell activation by stabilizing the immunological synapse.
3.1 Role of lipid rafts in CD4+ T cell activation
Lipid rafts in the plasma membrane are thought to be critical in CD4+ T cell activation The interaction between Ag:MHCII and TCR results in a major reorganization of the nanoscale lipid rafts and signaling proteins to form the IS (Jury et al., 2007; Lillemeier et al., 2010; Meiri, 2005). IS formation involves changes in lipid-lipid interactions in the membranes. Liquid ordered (Lo) lipids such as cholesterol and sphingolipids accumulate at the IS (Burack et al., 2002; Fan et al., 2003; Kim et al., 2008; Zech et al., 2009). The involvement of lipid rafts in CD4+ T cell activation is conclusive; disruption of lipid rafts with various agents such as methyl-β-cyclodextrin (Xavier et al., 1998), 7-ketocholesterol (Rentero et al., 2008), or n-3 PUFA such as EPA (discussed below, Zech et al., 2009) resulted in suppressed T cell activation. Importantly, these data suggest that an alteration in the size of rafts can impact IS formation, and thus regulate T cell activation (Rentero et al., 2008; Zech et al., 2009).
3.2 Effects of n-3 PUFA on CD4+ T cell activation
Since it has been shown that n-3 PUFA can alter the size and/or stability of lipid rafts in CD4+ T cells (Fan et al., 2004; Fan et al., 2003; Kim et al., 2014; Kim et al., 2008; Zech et al., 2009), it is not surprising that many studies have shown downstream perturbations in T cell activation when the CD4+ T cell plasma membrane is enriched with n-3 PUFA. Studies using the immortalized Jurkat T cell line demonstrated that n-3 PUFA displaced many of the signaling proteins necessary for T cell activation, including the Src family kinases Lck and Fyn (Stulnig et al., 1998) and LAT (Stulnig et al., 2001) from detergent-resistant membrane fractions. Recruitment and activation of signaling proteins such as PKC , LAT, Fas, PLC-γ1, and F-actin were also altered (Fan et al., 2004; Kim et al., 2008; Sanderson and Calder, 1998). n-3 PUFA, however, did not universally inhibit protein localization; one study observed an enhancement of surface CTLA-4 expression, a negative regulator of T cell activation, in CD4+ T cells isolated from mice fed an EPA-enriched diet (Ly et al., 2006). It is now appreciated that n-3 PUFA broadly suppress downstream activation signaling in CD4+ T cells, including mitochondrial translocation (Yog et al., 2010), IL-2 secretion (Arrington et al., 2001; Chapkin et al., 2002; Fan et al., 2004; Jolly et al., 1997; Ly et al., 2006; McMurray et al., 2000), and lymphoproliferation (Fan et al., 2008; Kim et al., 2008; McMurray et al., 2000).
Both lipid-lipid interactions and lipid-protein interactions (i.e., membrane-actin cytoskeleton interactions) are important for the formation of the IS. In a breakthrough finding, we recently demonstrated that n-3 PUFA directly perturb the membrane-actin cytoskeleton interactions by depleting the overall level of PI(4,5)P2 in CD4+ T cells, resulting in the suppression/normalization of actin cytoskeletal rearrangement upon T cell activation (Hou et al., 2012). This effect was rescued by exogenous incubation of n-3 PUFA-enriched CD4+ T cells with PI(4, 5)P2, demonstrating the direct perturbation of membrane-actin cytoskeleton interactions by n-3 PUFA. Perturbation of membrane-actin cytoskeleton interactions by n-3 PUFA may occur in other cell types. Colonocytes enriched with n-3 PUFA exhibited suppressed activation of cytosksletal remodeling proteins such as PLC-γ1, Rc1, and Cdc42 (Turk et al., 2013).
4. CD4+ T cell differentiation
Activated CD4+ T cells differentiate into pro-inflammatory effector subsets (TH1, TH17) in the presence of the cytokines such as interferon- (IFN-) and interleukin-12 (IL-12) for TH1 cells, and Transforming growth factor-β (TGFβ), interleukin-6 (IL-6), and interleukin 21 (IL-21) for TH17 cells (Zhu et al., 2010). CD4+ T cells can differentiate into anti-inflammatory TH2 cells in the presence of IL-4 and IL-2, and into regulatory Treg cells (Zhu et al., 2010). Typically these CD4+ T effector cell subsets are defined by their transcriptional signatures. However, the role of the plasma membrane in directing the differentiation of CD4+ T cells is underappreciated. Although the anti-inflammatory effects of EPA and DHA have been well documented, their precise biochemical mechanisms of action with respect to cell polarization/differentiation have yet to be clearly defined. We propose that the ability of n-3 PUFA to alter plasma membrane and cytoskeleton-dependent signaling may impact CD4+ T cell differentiation. These molecular targets may provide a novel therapeutic strategy for modulating the pathologic effector T cell subsets in autoimmune and chronic inflammatory diseases.
4.1 Role of lipid rafts in CD4+ T cell differentiation
Early reports suggested that different CD4+ effector T cell populations depend upon distinct plasma membrane characteristics. For example, treatment of T cells with methyl-β-cyclodextrin to disturb lipid rafts demonstrated that TH1, but not TH2,cells were more sensitive as detected by suppressed Ca2+ influx upon antigen stimulation (Balamuth et al., 2001). Indeed, later studies demonstrated that TH1 and TH2 cells have distinct IS. The TH1 cell IS is characterized by the bull’s eye pattern where the cSMAC, comprised of Ag:MHCII/TCR interactions, is surrounded by the pSMAC populated with adhesion molecules. In contrast, the TH2 cell IS is described as multifocal and dependent on the concentration of antigens (Thauland et al., 2008). Furthermore, individual human CD4+ T cells can be categorized into three classes based on the lipid order of their plasma membrane (low, intermediate, and high), as determined by the generalized polarization of di-4-ANEPPDHQ, a probe that changes fluorescence intensities at 570 nm and 620 nm based on the lipid order of its surrounding microenvironment upon incorporation into the plasma membrane (Miguel et al., 2011). CD4+ T cells with intermediate membrane order were associated with IFN- production (i.e. TH1 phenotype), while high membrane order was associated with IL-4 production (i.e. TH2 phenotype); low membrane order CD4+ T cells were associated with increased apoptosis. When CD4+ T cells were cultured in either TH1 or TH2-polarizing conditions, the membrane order of the CD4+ T cells changed to the corresponding T cell subset; intermediate membrane order was observed under TH1 conditions, while high membrane order was observed under TH2 conditions. To further link the role of membrane order with CD4+ T cell differentiation, the reduction of membrane order with 7-ketoxycholesterol resulted in an increase in the number of CD4+ T cells producing IFN-, indicative of a switch to an intermediate membrane order associated with the TH1 phenotype. Clinically, CD4+ T cells isolated from human patients with systemic lupus erythematosus, Sjogrens Syndrome, or rheumatoid arthritis have an increased population of intermediate membrane order CD4+ T cells, associated with IFN- production and pro-inflammatory phenotype (McDonald et al., 2014; Miguel et al., 2011). TH17 cells are also known to be affected by membrane order. Decreasing levels of glycosphingolipid, a lipid known to be associated with lipid rafts in CD4+ T cells, resulted in a reduction in TH17 differentiation (Zhu et al., 2011). These studies demonstrate the structural importance of membrane nanoscale domains in directing CD4+ T cell differentiation.
4.2 Effects of n-3 PUFA on CD4+ T cell differentiation
n-3 PUFA suppress CD4+ T cell activation and differentiation. Only TH1-like cells exhibited enhanced activation-induced cell death (AICD) upon enrichment with n-3 PUFA, whereas TH2-like cells were unaffected by n-3 PUFA (Switzer et al., 2004; Switzer et al., 2003). In addition, CD4+ T cells enriched with n-3 PUFA failed to polarize into TH1 or TH17 cells as efficiently when compared to control CD4+ T cells (Monk et al., 2013; Monk et al., 2012a; Zhang et al., 2005). These phenotypes were accompanied by changes in downstream signaling, such as reduced activation of TH17-related STAT3, reduced expression of TH17 signature cytokines (i.e., IL-17A), reduced expression of the TH17 transcription factor (i.e., RORγt), and reduced surface expressions of critical surface receptors for TH17 polarization (i.e., IL-6R and IL-23R) (Monk et al., 2013; Monk et al., 2012a; Zhang et al., 2005).
Interestingly, n-3 PUFA do not seem to affect the polarization of CD4+ T cells into TH2 or Treg subsets (Monk et al., 2013; Monk et al., 2012a; Monk et al., 2012b). This may be due to the different membrane properties optimized for polarization into these subsets (Miguel et al., 2011; Thauland et al., 2008). However, a recent study reported a decrease in CD4+ T cell differentiation under TH2 polarization conditions in cells isolated from Fat-1 mice, which incorporate n-3 PUFA into cell membranes endogenously (Jang et al., 2014). This highlights the need for more study into how n-3 PUFA affect CD4+ T cell differentiation, such as examining the dose of n-3 PUFA, and the differentiation environment the CD4+ T cells are selected under.
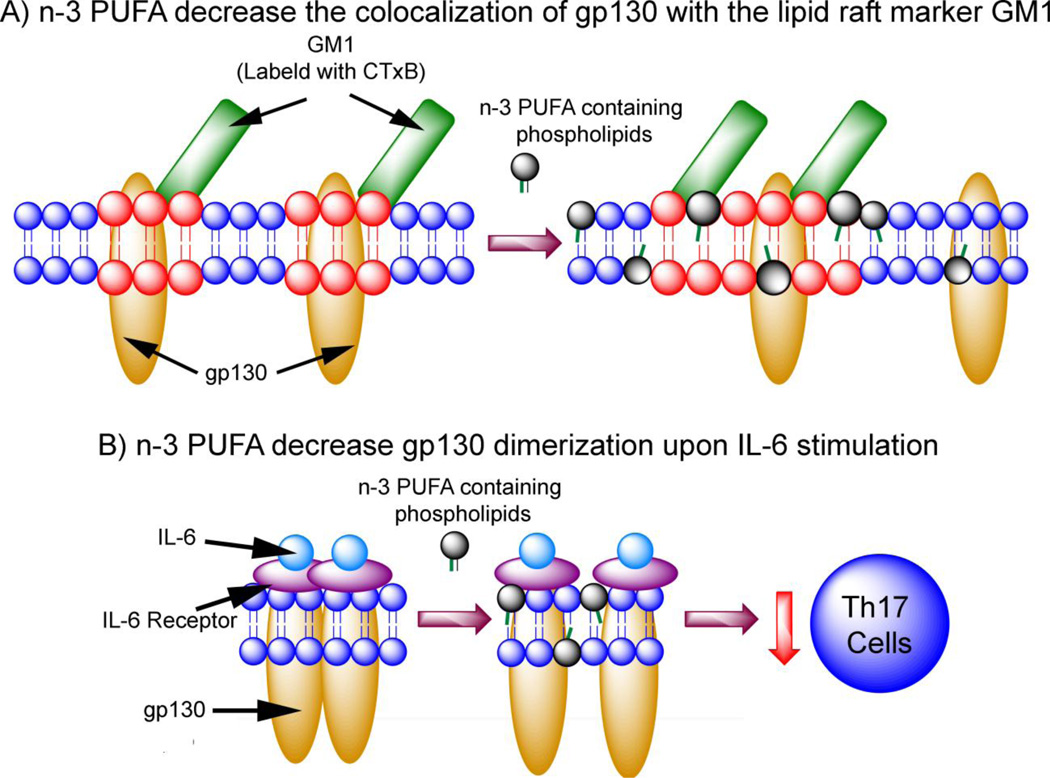
One mechanism by which long chain n-3 PUFA (i.e., EPA and DHA) suppress CD4+ T cell polarization into TH17 cells involves the IL-6-gp-130-STAT3 signaling axis which regulates the earliest events in TH17 cell differentiation (Bettelli et al., 2006; Jones et al., 2013; Jones et al., 2010; Nishihara et al., 2007; Veldhoen et al., 2006). A hexameric signaling complex composed of two IL-6 binding to two membrane bound IL-6 receptors (mIL-6R) and two molecules of glycoprotein 130 (gp130) is formed, leading to the phosphorylation of STAT3, translocation to the nucleus, and subsequent activation of RORγt, the master regulator of TH17 transcription (Briso et al., 2008; Jones et al., 2010). As mentioned above, phosphorylation of STAT3 and activation of RORγt are both suppressed by n-3 PUFA in CD4+ T cells (Monk et al., 2013; Monk et al., 2012a). While the IL-6 receptor (IL-6R) is an 80 kDa surface protein expressed on naive CD4+ T cells (Betz and Muller, 1998), gp130 is ubiquitously expressed and mediates signal transduction for IL-6 and other cytokines (Silver and Hunter, 2010). Both IL-6R and gp130 are associated with lipid rafts; the localization of IL-6R at the plasma membrane is regulated by cholesterol (Matthews et al., 2003), while gp130 has been found to reside in lipid rafts of kidney (Buk et al., 2004) and neuroepithelial (Yanagisawa et al., 2004) cell plasma membranes. Based upon the essential nature of the IL-6-gp130-STAT3 signaling axis in TH17 differentiation (Veldhoen et al., 2006) and the fact that membrane modulation disrupts TH17 development (Zhu et al., 2011), Allen and colleagues hypothesized that n-3 PUFA reduce TH17 differentiation by interfering with IL-6 signaling in a lipid raft-dependent fashion (Allen et al., 2014). The presence of n-3 PUFA decreased surface expression of IL-6R and the association of gp130 with lipid rafts (Fig. 3). Specifically, the homodimerization of gp130 was reduced in activated CD4+ T cells enriched with n-3 PUFA. The lipid raft perturbations caused by n-3 PUFA led to downstream STAT3 phosphorylation. These results demonstrate that membrane alterations induced by n-3 PUFA suppress TH17 differentiation by altering the IL6/gp130/Stat3 pathway.
Figure 3.
Proposed mechanism by which n-3 PUFA suppress TH17 cell differentiation. n-3 PUFA directly modulate IL-6/gp130 signaling at the plasma membrane, thus decreasing TH17 differentiation. A) n-3 PUFA decrease colocalization of gp130 in lipid rafts, as assessed by colocalization with cholera toxin (CTxB), a marker of lipid rafts. Red highlight indicates liquid ordered lipid rafts; Blue indicates bulk membrane. B) n-3 PUFA decrease the dimerization of gp130 upon IL-6 stimulation, suppressing downstream TH17 differentiation (Allen et al., 2014).
5. Future Directions
A third potential target for n-3 PUFA in the formation and organization of optimal mesoscale lipid rafts are higher-ordered protein complexes (i.e., protein-protein interactions), as proposed by Kai Simons and Akihiro Kusumi (Kusumi et al., 2011; Simons and Sampaio, 2011). Similar to EGFR, Fas receptor (FasR) localizes in lipid rafts, and upon engagement with its ligand, Fas ligand (FasL), FasR undergoes oligomerization to induce favorable interactions between lipid rafts, again highlighting how protein-protein interactions can mediate lipid raft formation and organization (Muppidi and Siegel, 2004; Wang et al., 2010; Wilson et al., 2009). The TCR itself forms nanoclusters in the plasma membrane in a cholesterol-dependent fashion, requiring both cholesterol and sphingomyelin for the formation of TCR dimers (Molnar et al., 2012). Thus, the hierarchical organization of the plasma membrane is aided by lipid-lipid, lipid-protein, and protein-protein interactions. With advances in super-resolution microscopy to visualize nanoclusters at the plasma membrane, it will be possible to determine whether n-3 PUFA can also affect protein-protein interactions in forming TCR nanoclusters at the plasma membrane of CD4+ T cells.
Not all n-3 PUFA are effective at perturbing plasma membrane lipid rafts. Phenotypes are not interchangeable when cells are treated with EPA or DHA (Corsetto et al., 2012; Turk et al., 2013), and biophysical studies have demonstrated that EPA and DHA do not disrupt lipid rafts with equal efficiency (Williams et al., 2012). Adding to the complication, 1-palmitoyl-2-docosahexaenoylphosphatidylethanolamine preferentially segregates into the non-raft plasma membrane, while 1-palmitoyl-2-docosahexaenoylphosphatidylcholine prefers the mesoscale raft domains of the plasma membrane (Shaikh et al., 2014), demonstrating that the headgroups of phospholipids can also affect the mechanisms by which these n-3 PUFA modulate plasma membrane properties.
Although it is appreciated that n-3 PUFA are pleotropic and can act upon targets outside of the plasma membrane, cogent data demonstrate that incorporation of n-3 PUFA can alter the biochemical and biophysical properties of CD4+ T cell plasma membranes, favorably modulating cytoskeletal dependent CD4+ T cell activation and differentiation (Fig. 1). In conclusion, n-3 PUFA target the plasma membrane of immune cells, and may contribute in the attenuation of autoimmune and chronic inflammatory diseases. However, in order to provide further rationale for their potential use as therapeutics, additional research is required to further elucidate how n-3 PUFA affect the nanoscale organization of the plasma membrane, and how this mediates the suppression of CD4+ T cell activation and differentiation into pathologic effector subsets.
Acknowledgements
T.Y.H. was supported by pre-doctoral fellowships from the National Science and Engineering Research Council (NSERC) of Canada and Texas A&M University System Health Science Center Microbial Pathogenesis Training Grant Faculty. We also acknowledge support from U.S. Department of Agriculture CSREES (Cooperative State Research, Education, and Extension Service) Special Grant, “Designing Foods for Health” [2008-34402-17121] and NIH grants CA129444 and P30ES023512.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen MJ, Fan YY, Monk JM, Hou TY, Barhoumi R, McMurray DN, Chapkin RS. n-3 PUFAs reduce T-helper 17 cell differentiation by decreasing responsiveness to interleukin-6 in isolated mouse splenic CD4(+) T cells. J. Nutr. 2014;144:1306–1313. doi: 10.3945/jn.114.194407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrington JL, McMurray DN, Switzer KC, Fan YY, Chapkin RS. Docosahexaenoic acid suppresses function of the CD28 costimulatory membrane receptor in primary murine and Jurkat T cells. J.Nutr. 2001;131:1147–1153. doi: 10.1093/jn/131.4.1147. [DOI] [PubMed] [Google Scholar]
- Balamuth F, Leitenberg D, Unternaehrer J, Mellman I, Bottomly K. Distinct patterns of membrane microdomain partitioning in Th1 and th2 cells. Immunity. 2001;15:729–738. doi: 10.1016/s1074-7613(01)00223-0. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Betz UA, Muller W. Regulated expression of gp130 and IL-6 receptor alpha chain in T cell maturation and activation. Int. Immunol. 1998;10:1175–1184. doi: 10.1093/intimm/10.8.1175. [DOI] [PubMed] [Google Scholar]
- Bouzat CB, Barrantes FJ. Effects of long-chain fatty acids on the channel activity of the nicotinic acetylcholine receptor. Receptors Channels. 1993;1:251–258. [PubMed] [Google Scholar]
- Briso EM, Dienz O, Rincon M. Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells. J. Immunol. 2008;180:7102–7106. doi: 10.4049/jimmunol.180.11.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno MJ, Koeppe RE, 2nd, Andersen OS. Docosahexaenoic acid alters bilayer elastic properties. Proc. Natl. Acad. Sci. U. S. A. 2007;104:9638–9643. doi: 10.1073/pnas.0701015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno MJ, Rusinova R, Gleason NJ, Koeppe RE, 2nd, Andersen OS. Interactions of drugs and amphiphiles with membranes: modulation of lipid bilayer elastic properties by changes in acyl chain unsaturation and protonation. Faraday Discuss. 2013;161:461–480. doi: 10.1039/c2fd20092a. discussion 563–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buk DM, Waibel M, Braig C, Martens AS, Heinrich PC, Graeve L. Polarity and lipid raft association of the components of the ciliary neurotrophic factor receptor complex in Madin-Darby canine kidney cells. J. Cell. Sci. 2004;117:2063–2075. doi: 10.1242/jcs.01049. [DOI] [PubMed] [Google Scholar]
- Burack WR, Lee KH, Holdorf AD, Dustin ML, Shaw AS. Cutting edge: quantitative imaging of raft accumulation in the immunological synapse. J. Immunol. 2002;169:2837–2841. doi: 10.4049/jimmunol.169.6.2837. [DOI] [PubMed] [Google Scholar]
- Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapkin RS, Arrington JL, Apanasovich TV, Carroll RJ, McMurray DN. Dietary n-3 PUFA affect TcR-mediated activation of purified murine T cells and accessory cell function in co-cultures. Clin. Exp. Immunol. 2002;130:12–18. doi: 10.1046/j.1365-2249.2002.01951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapkin RS, Wang N, Fan YY, Lupton JR, Prior IA. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim. Biophys. Acta. 2008;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsetto PA, Cremona A, Montorfano G, Jovenitti IE, Orsini F, Arosio P, Rizzo AM. Chemical-physical changes in cell membrane microdomains of breast cancer cells after omega-3 PUFA incorporation. Cell. Biochem. Biophys. 2012;64:45–59. doi: 10.1007/s12013-012-9365-y. [DOI] [PubMed] [Google Scholar]
- DeMond AL, Mossman KD, Starr T, Dustin ML, Groves JT. T cell receptor microcluster transport through molecular mazes reveals mechanism of translocation. Biophys. J. 2008;94:3286–3292. doi: 10.1529/biophysj.107.119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CR, Shimizu T, Spener F, van Meer G, Wakelam MJ, Dennis EA. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009;(50 Suppl):S9–S14. doi: 10.1194/jlr.R800095-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YY, Kim W, Callaway E, Smith R, Jia Q, Zhou L, McMurray DN, Chapkin RS. fat-1 transgene expression prevents cell culture-induced loss of membrane n-3 fatty acids in activated CD4+ T-cells. Prostaglandins Leukot. Essent. Fatty Acids. 2008;79:209–214. doi: 10.1016/j.plefa.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J. Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- Fan YY, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J. Nutr. 2003;133:1913–1920. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisz JF, Lou K, Klitzing HA, Hanafin WP, Lizunov V, Wilson RL, Carpenter KJ, Kim R, Hutcheon ID, Zimmerberg J, Weber PK, Kraft ML. Direct chemical evidence for sphingolipid domains in the plasma membranes of fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E613–E622. doi: 10.1073/pnas.1216585110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawrisch K, Soubias O. Structure and dynamics of polyunsaturated hydrocarbon chains in lipid bilayers-significance for GPCR function. Chem. Phys. Lipids. 2008;153:64–75. doi: 10.1016/j.chemphyslip.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TS, Billadeau DD. T cell activation and the cytoskeleton: you can’t have one without the other. Adv. Immunol. 2008;97:1–64. doi: 10.1016/S0065-2776(08)00001-1. [DOI] [PubMed] [Google Scholar]
- Hou TY, Monk JM, Fan YY, Barhoumi R, Chen YQ, Rivera GM, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress phosphatidylinositol 4,5-bisphosphate-dependent actin remodelling during CD4+ T-cell activation. Biochem. J. 2012;443:27–37. doi: 10.1042/BJ20111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Burkhardt JK. T-cell-receptor-dependent actin regulatory mechanisms. J. Cell Sci. 2007;120:723–730. doi: 10.1242/jcs.000786. [DOI] [PubMed] [Google Scholar]
- Jang HY, Lim K, Lee SM, Park BH. Effects of n-3 PUFA on the CD4(+) type 2 helper T-cell-mediated immune responses in Fat-1 mice. Mol. Nutr. Food Res. 2014;58:365–375. doi: 10.1002/mnfr.201300194. [DOI] [PubMed] [Google Scholar]
- Jolly CA, Jiang YH, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids suppress murine lymphoproliferation, interleukin-2 secretion, and the formation of diacylglycerol and ceramide. J. Nutr. 1997;127:37–43. doi: 10.1093/jn/127.1.37. [DOI] [PubMed] [Google Scholar]
- Jones GW, Greenhill CJ, Williams JO, Nowell MA, Williams AS, Jenkins BJ, Jones SA. Exacerbated inflammatory arthritis in response to hyperactive gp130 signalling is independent of IL-17A. Ann. Rheum. Dis. 2013;72:1738–1742. doi: 10.1136/annrheumdis-2013-203771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GW, McLoughlin RM, Hammond VJ, Parker CR, Williams JD, Malhotra R, Scheller J, Williams AS, Rose-John S, Topley N, Jones SA. Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells. J. Immunol. 2010;184:2130–2139. doi: 10.4049/jimmunol.0901528. [DOI] [PubMed] [Google Scholar]
- Jury EC, Flores-Borja F, Kabouridis PS. Lipid rafts in T cell signalling and disease. Semin. Cell. Dev. Biol. 2007;18:608–615. doi: 10.1016/j.semcdb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104:20296–20301. doi: 10.1073/pnas.0710258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Barhoumi R, McMurray DN, Chapkin RS. Dietary fish oil and DHA down-regulate antigen-activated CD4+ T-cells while promoting the formation of liquid-ordered mesodomains. Br. J. Nutr. 2014;111:254–260. doi: 10.1017/S0007114513002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J. Immunol. 2008;181:6236–6243. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Fujiwara TK, Chadda R, Xie M, Tsunoyama TA, Kalay Z, Kasai RS, Suzuki KG. Dynamic organizing principles of the plasma membrane that regulate signal transduction: commemorating the fortieth anniversary of Singer and Nicolson’s fluid-mosaic model. Annu. Rev. Cell. Dev. Biol. 2012;28:215–250. doi: 10.1146/annurev-cellbio-100809-151736. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Suzuki KG, Kasai RS, Ritchie K, Fujiwara TK. Hierarchical mesoscale domain organization of the plasma membrane. Trends Biochem. Sci. 2011;36:604–615. doi: 10.1016/j.tibs.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska K. One lipid, multiple functions: how various pools of PI(4,5)P(2) are created in the plasma membrane. Cell. Mol. Life Sci. 2010;67:3927–3946. doi: 10.1007/s00018-010-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladha S, Mackie AR, Harvey LJ, Clark DC, Lea EJ, Brullemans M, Duclohier H. Lateral diffusion in planar lipid bilayers: a fluorescence recovery after photobleaching investigation of its modulation by lipid composition, cholesterol, or alamethicin content and divalent cations. Biophys. J. 1996;71:1364–1373. doi: 10.1016/S0006-3495(96)79339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GM, Zhang F, Ishihara A, McNeil CL, Jacobson KA. Unconfined lateral diffusion and an estimate of pericellular matrix viscosity revealed by measuring the mobility of gold-tagged lipids. J. Cell Biol. 1993;120:25–35. doi: 10.1083/jcb.120.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, Kanagawa O, Markiewicz M, Allen PM, Dustin ML, Chakraborty AK, Shaw AS. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat. Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna EJ, Hitt AL. Cytoskeleton--plasma membrane interactions. Science. 1992;258:955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- Ly LH, Smith R, Switzer KC, Chapkin RS, McMurray DN. Dietary eicosapentaenoic acid modulates CTLA-4 expression in murine CD4+ T-cells. Prostaglandins Leukot. EssentFatty Acids. 2006;74:29–37. doi: 10.1016/j.plefa.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Ma DW, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004;18:1040–1042. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- Matta JA, Miyares RL, Ahern GP. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J. Physiol. 2007;578:397–411. doi: 10.1113/jphysiol.2006.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews V, Schuster B, Schutze S, Bussmeyer I, Ludwig A, Hundhausen C, Sadowski T, Saftig P, Hartmann D, Kallen KJ, Rose-John S. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE) J. Biol. Chem. 2003;278:38829–38839. doi: 10.1074/jbc.M210584200. [DOI] [PubMed] [Google Scholar]
- McDonald G, Deepak S, Miguel L, Hall CJ, Isenberg DA, Magee AI, Butters T, Jury EC. Normalizing glycosphingolipids restores function in CD4+ T cells from lupus patients. J. Clin. Invest. 2014;124:712–724. doi: 10.1172/JCI69571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray DN, Jolly CA, Chapkin RS. Effects of dietary n-3 fatty acids on T cell activation and T cell receptor-mediated signaling in a murine model. J. Infect. Dis. 2000;182(Suppl. 1):S103–S107. doi: 10.1086/315909. [DOI] [PubMed] [Google Scholar]
- Meiri KF. Lipid rafts and regulation of the cytoskeleton during T cell activation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:1663–1672. doi: 10.1098/rstb.2005.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel L, Owen DM, Lim C, Liebig C, Evans J, Magee AI, Jury EC. Primary human CD4+ T cells have diverse levels of membrane lipid order that correlate with their function.. J. Immunol. 2011;186:3505–3516. doi: 10.4049/jimmunol.1002980. [DOI] [PubMed] [Google Scholar]
- Molnar E, Swamy M, Holzer M, Beck-Garcia K, Worch R, Thiele C, Guigas G, Boye K, Luescher IF, Schwille P, Schubert R, Schamel WW. Cholesterol and sphingomyelin drive ligand-independent T-cell antigen receptor nanoclustering. J. Biol. Chem. 2012;287:42664–42674. doi: 10.1074/jbc.M112.386045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk JM, Hou TY, Turk HF, McMurray DN, Chapkin RS. n3 PUFAs reduce mouse CD4+ T-cell ex vivo polarization into Th17 cells. J. Nutr. 2013;143:1501–1508. doi: 10.3945/jn.113.178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk JM, Hou TY, Turk HF, Weeks B, Wu C, McMurray DN, Chapkin RS. Dietary n-3 polyunsaturated fatty acids (PUFA) decrease obesity-associated Th17 cell-mediated inflammation during colitis. PLoS One. 2012a;7:e49739. doi: 10.1371/journal.pone.0049739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk JM, Jia Q, Callaway E, Weeks B, Alaniz RC, McMurray DN, Chapkin RS. Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice. J. Nutr. 2012b;142:117–124. doi: 10.3945/jn.111.147058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Muppidi JR, Siegel RM. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat. Immunol. 2004;5:182–189. doi: 10.1038/ni1024. [DOI] [PubMed] [Google Scholar]
- Nabekura J, Noguchi K, Witt MR, Nielsen M, Akaike N. Functional modulation of human recombinant gamma-aminobutyric acid type A receptor by docosahexaenoic acid. J. Biol. Chem. 1998;273:11056–11061. doi: 10.1074/jbc.273.18.11056. [DOI] [PubMed] [Google Scholar]
- Nicolau DV, Jr, Burrage K, Parton RG, Hancock JF. Identifying optimal lipid raft characteristics required to promote nanoscale protein-protein interactions on the plasma membrane. Mol. Cell. Biol. 2006;26:313–323. doi: 10.1128/MCB.26.1.313-323.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara M, Ogura H, Ueda N, Tsuruoka M, Kitabayashi C, Tsuji F, Aono H, Ishihara K, Huseby E, Betz UA, Murakami M, Hirano T. IL-6-gp130-STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state. Int. Immunol. 2007;19:695–702. doi: 10.1093/intimm/dxm045. [DOI] [PubMed] [Google Scholar]
- Padrick SB, Cheng HC, Ismail AM, Panchal SC, Doolittle LK, Kim S, Skehan BM, Umetani J, Brautigam CA, Leong JM, Rosen MK. Hierarchical regulation of WASP/WAVE proteins. Mol. Cell. 2008;32:426–438. doi: 10.1016/j.molcel.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentero C, Zech T, Quinn CM, Engelhardt K, Williamson D, Grewal T, Jessup W, Harder T, Gaus K. Functional implications of plasma membrane condensation for T cell activation. PLoS One. 2008;3:e2262. doi: 10.1371/journal.pone.0002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett BD, Teague H, Harris M, Melton M, Williams J, Wassall SR, Shaikh SR. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. J Lipid Res. 2012;53:674–685. doi: 10.1194/jlr.M021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarikangas J, Zhao H, Lappalainen P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol. Rev. 2010;90:259–289. doi: 10.1152/physrev.00036.2009. [DOI] [PubMed] [Google Scholar]
- Sanderson P, Calder PC. Dietary fish oil appears to prevent the activation of phospholipase C-gamma in lymphocytes. Biochim. Biophys. Acta. 1998;1392:300–308. doi: 10.1016/s0005-2760(98)00044-7. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Kinnun JJ, Leng X, Williams JA, Wassall SR. How polyunsaturated fatty acids modify molecular organization in membranes: Insight from NMR studies of model systems. Biochim. Biophys. Acta. 2014;1848:211–219. doi: 10.1016/j.bbamem.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Simons K. Lipidomics: coming to grips with lipid diversity. Nat Rev Mol. Cell. Biol. 2010;11:593–598. doi: 10.1038/nrm2934. [DOI] [PubMed] [Google Scholar]
- Silver JS, Hunter CA. gp130 at the nexus of inflammation, autoimmunity, and cancer. J. Leukoc. Biol. 2010;88:1145–1156. doi: 10.1189/jlb.0410217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011;3:a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Sonnleitner A, Schutz GJ, Schmidt T. Free Brownian motion of individual lipid molecules in biomembranes. Biophys. J. 1999;77:2638–2642. doi: 10.1016/S0006-3495(99)77097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubias O, Gawrisch K. Docosahexaenoyl chains isomerize on the sub-nanosecond time scale. J. Am. Chem. Soc. 2007;129:6678–6679. doi: 10.1021/ja068856c. [DOI] [PubMed] [Google Scholar]
- Stulnig TM, Berger M, Sigmund T, Raederstorff D, Stockinger H, Waldhausl W. Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains. J. Cell Biol. 1998;143:637–644. doi: 10.1083/jcb.143.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulnig TM, Huber J, Leitinger N, Imre EM, Angelisova P, Nowotny P, Waldhausl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J. Biol. Chem. 2001;276:37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- Swaisgood M, Schindler M. Lateral diffusion of lectin receptors in fibroblast membranes as a function of cell shape. Exp. Cell. Res. 1989;180:515–528. doi: 10.1016/0014-4827(89)90078-5. [DOI] [PubMed] [Google Scholar]
- Switzer KC, Fan YY, Wang N, McMurray DN, Chapkin RS. Dietary n-3 polyunsaturated fatty acids promote activation-induced cell death in Th1-polarized murine CD4+ T-cells. J. Lipid Res. 2004;45:1482–1492. doi: 10.1194/jlr.M400028-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer KC, McMurray DN, Morris JS, Chapkin RS. (n-3) Polyunsaturated fatty acids promote activation-induced cell death in murine T lymphocytes. J. Nutr. 2003;133:496–503. doi: 10.1093/jn/133.2.496. [DOI] [PubMed] [Google Scholar]
- Thauland TJ, Koguchi Y, Wetzel SA, Dustin ML, Parker DC. Th1 and Th2 cells form morphologically distinct immunological synapses. J. Immunol. 2008;181:393–399. doi: 10.4049/jimmunol.181.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmay A, Prinz WA. Direct imaging reveals stable, micrometer-scale lipid domains that segregate proteins in live cells. J. Cell Biol. 2013;202:35–44. doi: 10.1083/jcb.201301039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk HF, Barhoumi R, Chapkin RS. Alteration of EGFR spatiotemporal dynamics suppresses signal transduction. PLoS One. 2012;7:e39682. doi: 10.1371/journal.pone.0039682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk HF, Chapkin RS. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fatty Acids. 2013;88:43–47. doi: 10.1016/j.plefa.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk HF, Chapkin RS. Analysis of epidermal growth factor receptor dimerization by BS(3) cross-linking. Methods Mol. Biol. 2015;1233:25–34. doi: 10.1007/978-1-4939-1789-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk HF, Monk JM, Fan YY, Callaway ES, Weeks B, Chapkin RS. Inhibitory effects of omega-3 fatty acids on injury-induced epidermal growth factor receptor transactivation contribute to delayed wound healing. Am. J. Physiol. Cell Physiol. 2013;304:C905–917. doi: 10.1152/ajpcell.00379.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat. Rev. Immunol. 2009;9:630–644. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G. Cellular lipidomics. EMBO J. 2005;24:3159–3165. doi: 10.1038/sj.emboj.7600798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Wang L, Yang JK, Kabaleeswaran V, Rice AJ, Cruz AC, Park AY, Yin Q, Damko E, Jang SB, Raunser S, Robinson CV, Siegel RM, Walz T, Wu H. The Fas-FADD death domain complex structure reveals the basis of DISC assembly and disease mutations. Nat. Struct. Mol. Biol. 2010;17:1324–1329. doi: 10.1038/nsmb.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassall SR, Stillwell W. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem. Phys. Lipids. 2008;153:57–63. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Wassall SR, Stillwell W. Polyunsaturated fatty acid-cholesterol interactions: domain formation in membranes. Biochim. Biophys. Acta. 2009;1788:24–32. doi: 10.1016/j.bbamem.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Williams JA, Batten SE, Harris M, Rockett BD, Shaikh SR, Stillwell W, Wassall SR. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys. J. 2012;103:228–237. doi: 10.1016/j.bpj.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat. Immunol. 2009;10:348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- Xiao YF, Gomez AM, Morgan JP, Lederer WJ, Leaf A. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4182–4187. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YF, Kang JX, Morgan JP, Leaf A. Blocking effects of polyunsaturated fatty acids on Na+ channels of neonatal rat ventricular myocytes. Proc. Natl. Acad. Sci. U. S. A. 1995;92:11000–11004. doi: 10.1073/pnas.92.24.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Nakamura K, Taga T. Roles of lipid rafts in integrin-dependent adhesion and gp130 signalling pathway in mouse embryonic neural precursor cells. Genes Cells. 2004;9:801–809. doi: 10.1111/j.1365-2443.2004.00764.x. [DOI] [PubMed] [Google Scholar]
- Yetukuri L, Ekroos K, Vidal-Puig A, Oresic M. Informatics and computational strategies for the study of lipids. Mol. Biosyst. 2008;4:121–127. doi: 10.1039/b715468b. [DOI] [PubMed] [Google Scholar]
- Yog R, Barhoumi R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress mitochondrial translocation to the immunologic synapse and modulate calcium signaling in T cells. J. Immunol. 2010;184:5865–5873. doi: 10.4049/jimmunol.0904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat. Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- Zech T, Ejsing CS, Gaus K, de Wet B, Shevchenko A, Simons K, Harder T. Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J. 2009;28:466–476. doi: 10.1038/emboj.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol. Med. 2009;15:199–207. doi: 10.1016/j.molmed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Zhang P, Smith R, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids modulate murine Th1/Th2 balance toward the Th2 pole by suppression of Th1 development. J. Nutr. 2005;135:1745–1751. doi: 10.1093/jn/135.7.1745. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Gumlaw N, Karman J, Zhao H, Zhang J, Jiang JL, Maniatis P, Edling A, Chuang WL, Siegel C, Shayman JA, Kaplan J, Jiang C, Cheng SH. Lowering glycosphingolipid levels in CD4+ T cells attenuates T cell receptor signaling, cytokine production, and differentiation to the Th17 lineage. J. Biol. Chem. 2011;286:14787–14794. doi: 10.1074/jbc.M111.218610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]