Abstract
Cataract is a common age-related condition that is caused by progressive clouding of the normally clear lens. Cataract can be effectively treated by surgery; however, like any surgery, there can be complications and the development of a secondary cataract, known as posterior capsule opacification (PCO), is the most common. PCO is caused by aberrant growth of lens epithelial cells that are left behind in the capsular bag after surgical removal of the fiber mass. An epithelial-to-mesenchymal transition (EMT) is central to fibrotic PCO and forms of fibrotic cataract, including anterior/posterior polar cataracts. Transforming growth factor β (TGFβ) has been shown to induce lens EMT and consequently research has focused on identifying ways of blocking its action. Intriguingly, recent studies in animal models have shown that EMT and cataract developed when a class of negative-feedback regulators, Sprouty (Spry)1 and Spry2, were conditionally deleted from the lens. Members of the Spry family act as general antagonists of the receptor tyrosine kinase (RTK)-mediated MAPK signaling pathway that is involved in many physiological and developmental processes. As the ERK/MAPK signaling pathway is a well established target of Spry proteins, and overexpression of Spry can block aberrant TGFβ-Smad signaling responsible for EMT and anterior subcapsular cataract, this indicates a role for the ERK/MAPK pathway in TGFß-induced EMT. Given this and other supporting evidence, a case is made for focusing on RTK antagonists, such as Spry, for cataract prevention. In addition, and looking to the future, this review also looks at possibilities for supplanting EMT with normal fiber differentiation and thereby promoting lens regenerative processes after cataract surgery. Whilst, it is now known that the epithelial to fiber differentiation process is driven by FGF, little is known about factors that coordinate the precise assembly of fibers into a functional lens. However, recent research provides key insights into an FGF-activated mechanism intrinsic to the lens that involves interactions between the Wnt-Frizzled and Jagged/Notch signaling pathways. This reciprocal epithelial-fiber cell interaction appears to be critical for the assembly and maintenance of the highly ordered three-dimensional architecture that is central to lens function. This information is fundamental to defining the specific conditions and stimuli needed to recapitulate developmental programs and promote regeneration of lens structure and function after cataract surgery.
Keywords: Fibrosis, lens epithelium, myofibroblasts, TGFß, RTK antagonists, EMT, Sprouty, lens regeneration
Cataract is defined as the loss of transparency of the eye lens, and accounts for much of the world's blindness. Cataract has widely variable phenotypes and hence several classifications, including nuclear, cortical, anterior and posterior polar and total (Francis et al., 1999). Cataracts can appear in association with various systemic diseases (Beiran et al., 1994), presenting diverse phenotypes; however, by far the most common contributing factor for cataract is ageing (Mukesh et al., 2006; Vinson, 2006). Over the years much effort has been directed towards understanding the etiology of human cataract. In addition to identifying a strong genetic component (as in congenital cataracts), recent progress has been made in identifying factors that influence the stability of the long-lived proteins in the lens as well as the sites on these proteins that show marked deterioration in age-related cataract. This has led to the view that lens opacification is the result of cumulative age-related modifications to lens proteins (Truscott and Friedrich, 2014). How to prevent or ameliorate these protein modifications provides a major challenge for lens researchers in the future.
To date, the only way to restore visual loss caused by cataract is surgery (Brian and Taylor, 2001). This is the most common ophthalmic procedure and involves removal of the opaque fiber mass followed by implantation of a synthetic intraocular lens (IOL) for restoration of vision (Awasthi et al., 2009). While modern surgery is largely effective, it is not without complications and the most frequent is posterior capsular opacification (PCO), also referred to as secondary cataract (Awasthi et al., 2009; Spalton et al., 2013; see Miyamoto et al., 2014). Consequently there is a strong drive towards gaining a greater understanding of the key cellular processes and molecular mechanisms responsible for PCO. This will provide the platform for devising molecular strategies for reducing the incidence of PCO and improving the outcome of cataract surgery. Here, we will consider some of the important molecules and signaling pathways leading to cataract formation, and the importance of tightly regulating them for maintenance of normal lens growth, architecture and function.
Epithelial mesenchymal transition
A common feature of fibrotic PCO and polar cataracts is the loss of lens epithelial cell integrity, associated with aberrant proliferation, migration and most significantly a change in cell morphology, with cells distancing themselves from their ectodermal epithelial origin and transforming into more mesodermal-derived mesenchymal-like cells (see Figure 1). This biological process, known as an epithelial to mesenchymal transition (EMT), is normal for the early gastrulating embryo, but also presents itself in tissue repair and pathology, including cancer and cataract (Kalluri and Neilson, 2003). EMT characteristics include the acquisition of a spindle-shaped cellular morphology that is accompanied by accumulation of α-smooth muscle actin (αSMA) and redistribution of actin stress fibers, loss of cell polarity and epithelial markers such as cytokeratin and ZO-1, loss of E-cadherin and expression of transcription factors including Snail (Snai1), Slug (Snai2) and Twist (Figure 2; Zeisberg and Neilson, 2009). αSMA is a common marker of active fibroblasts, but it is not specific for fibroblasts (Zeisberg and Neilson, 2009). It is one of six actin family members, and its expression has been shown to correlate with the EMT process that occurs during normal development, fibrosis or in cancer progression (Kalluri and Weinberg, 2009). E-cadherin is a calcium-dependent membrane-associated cell-cell adhesion molecule (van Roy and Berx, 2008), predominantly present in epithelial cells (Takeichi, 1991). Localised to the plasma membrane, E-cadherin complexes with β-catenin and αE-catenin, key functional components of adherens junctions (van Roy and Berx, 2008; Wijnhoven et al., 2000); hence, its loss or change in distribution promotes a loss of epithelial phenotype, characteristic of the EMT process. The newly established mesenchymal cell type also possesses elevated migratory and invasive properties, increased resistance to apoptosis, and exaggerated production of extracellular matrix (ECM) components (Kalluri and Weinberg, 2009). It is through this process that we see fibrosis in the lens, characterized primarily by the accumulation of excess connective tissue that obliterates not only normal lens structure but most importantly its function.
Figure 1.
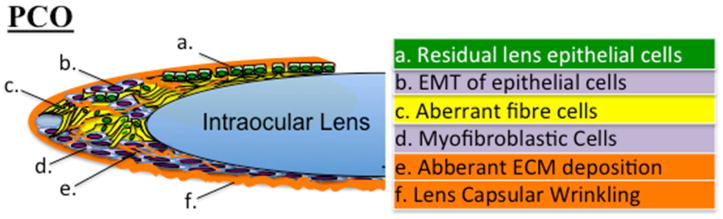
Complications following cataract surgery primarily lead to posterior capsular opacification (PCO). PCO results from residual lens epithelial cells (a), left behind following fiber cell extraction, that undergo an epithelial to mesenchymal transition (b) and/or aberrant differentiation into fiber cells (c) more commonly referred to as Soemmering's ring and Elschnig's pearls. The resultant myofibroblasts (d), also migrate posteriorly to populate and cover the posterior capsule, invading the visual axis as they further lay aberrant extracellular matrix (e) and modulate the underlying capsule, causing it to fold and wrinkle (f).
Figure 2.
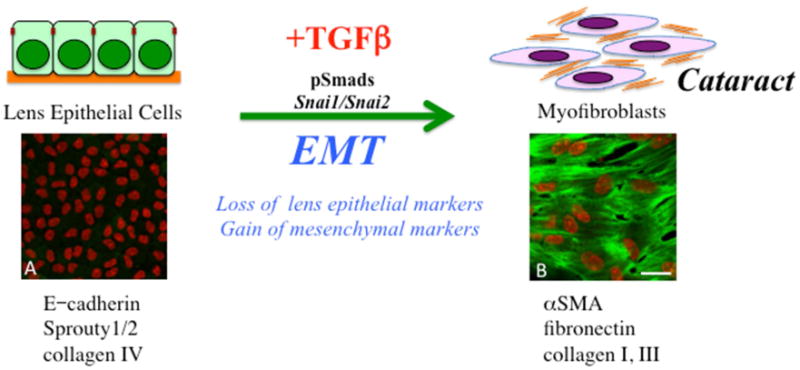
TGFß induces an epithelial to mesenchymal phenotype in lens epithelial cells. In the process, cells lose many of their normal epithelial markers and characteristics as they dissociate from each other to acquire a more myofibroblastic, migratory phenotype. Some of the specific markers now expressed include alpha smooth muscle actin (α-SMA), as well as excessive levels of extracellular matrix molecules, including fibronectin and collagens type I and III. It is this cellular process that contributes to lens pathology, especially the fibrotic changes leading to polar cataracts and posterior capsular opacification. Included are representative lens epithelial explants prepared from postnatal-day-15 murine lens exposed to either no TGFß (A) or 50pg/ml TGFß2 (B) for up to 5 days, immunolabeled for α-SMA (green), with cell nuclei counterstained with propidium iodide (red). With TGFß, lens epithelial cells undergo an EMT, highlighted by α-SMA-labeled myofibroblasts. Scale bar; 20μm.
Growth factors trigger EMT
EMT can be triggered by aberrant signaling of various molecules, such as epidermal growth factor (EGF), fibroblast growth factor (FGF), insulin-like growth factor (IGF-II), hepatocyte growth factor (HGF), and Notch (Savagner et al., 1997; Morali et al., 2001; Strutz et al., 2002; Timmerman et al., 2004; Ahmed et al., 2006); however, it is transforming growth factor beta (TGFβ) that is the most well-known EMT inducer in both normal and pathological conditions (Figure 2; Zavadil and Bottinger, 2005). The TGFβ superfamily comprises over 30 TGFβ-related members, that include TGFβ isoforms, activins, inhibins, bone morphogenic proteins (BMPs), and many other structurally related factors, in vertebrates, insects and nematodes (Massague et al., 1994; Moustakas et al., 2001). TGFβ is involved in the regulation of cell growth, differentiation, migration, adhesion, organization, senescence and extracellular matrix production. Its signaling normally promotes growth and development during early embryogenesis, whereas in mature tissues, they usually induce either cytostatic or apoptotic responses, depending on the type and state of the cell (Massague and Wotton, 2000). There are 28 genes encoding these members (Venter et al., 2001) and they all show sequence similarity to the prototype TGFβ 1 (Massague et al., 1994), which naturally occurs as a secreted homodimeric protein. Three isoforms of TGFβ, namely TGFβ1, TGFβ2 and TGFβ3, have been identified in mammals, and all 3 have been localised in the lens (Jampel et al., 1990; Cousins et al., 1991; Pelton et al., 1991, de Iongh et al., 2001a), with TGFβ2, the predominant isoform in the ocular media (Connor et al., 1989).
TGFβ is secreted as a biologically inactive complex, comprised of a disulfide-bonded homodimer of the mature TGFβ, and another disulfide-bonded homodimer of a prodomain peptide termed the TGFβ latency-associated peptide (LAP; Zhu and Burgess, 2001). The biological activity of TGFβ in the aqueous and vitreous has been reported to be variable in different species, and also in different states of health (Connor et al., 1989; Granstein et al., 1990; Cousins et al., 1991; de Boer et al., 1994; Kurosaka and Nagamoto, 1994; Tripathi et al., 1994). Members of the TGFβ superfamily mediate important processes involved in the development of the eye, including the lens, promoting various stages of lens development and differentiation of lens fiber cells (Belecky-Adams et al., 2002; de Iongh et al., 2001a; de Iongh et al., 2001b; Obata et al., 1999; Yoshikawa et al., 2000). TGFβ1 and TGFβ2 are expressed by lens cells and are abundantly available in the ocular media (Gordon-Thomson et al., 1998) in their latent, inactive forms (Cousins et al., 1991). The activation of these TGFβs is tightly regulated (see Tocharus et al., 2004) and upon deregulation TGFβ has been shown to induce pathological changes in lens epithelial cells similar to those seen in human anterior subcapsular cataract (Srinivasan et al., 1998; Lovicu et al., 2002), the focus of this review.
TGFß Signaling
TGFβ family signaling is propagated by the combinatorial interactions of the heteromeric type I (TβRI) and type II (TβRII) serine/threonine kinase receptors and downstream phosphorylation of receptor-regulated Smads (R-Smads), including Smad2 and Smad3 (Massague, 1998; 2000), that acquire an elevated affinity for Smad4 forming heteromeric complexes (Baker and Harland, 1997; Xu et al., 2000) to induce an assembly of active nuclear transcriptional complexes (Massague, 2000; Massague and Wotton, 2000; Moustakas et al., 2001; Derynck and Zhang, 2003; Dijke and Hill, 2004). The Smads shuttle between the cytoplasm and the nucleus, with the cytoplasmic distribution of Smad2 and Smad3 mediated by interactions with a protein termed Smad anchor for receptor activation (SARA; Tsukazaki et al., 1998; Itoh et al., 2000). SARA physically limits Smad movement, and also occludes a region of Smad2 that is responsible for its nuclear translocation (Xu et al., 2000). In accordance, phosphorylation of Smad2 decreases its affinity for SARA, allowing its release. Once translocated upon TGFβ simulation, the Smad complexes remain in the nucleus for several hours, with the activation of TGFβ receptor signaling sustained for at least three hours following stimulation (Inman et al., 2002). Two other molecules, Snai1 and Snai2, that belong to the Snail family of zinc-finger transcription factors are downstream effector proteins of the TGFβ signaling pathway, functioning as repressors (Nieto et al., 1992; Savagner et al., 1997; Kataoka et al., 2000; Nakakura et al., 2001). A conserved function of the Snail proteins is regulation of cell motility. Snai1 is known to be located both in the nucleus and cytoplasm of a cell, with its nuclear location controlling its activity (Dominguez et al., 2003). In contrast, Snai2 is mostly detected in the cell nucleus; however, may also shuttle between the cell nucleus and cytoplasm (Mingot et al., 2009). This Smad signaling pathway can be regulated by a distinct subclass of inhibitory Smads (I-Smads), namely Smad6 and Smad7, that act as antagonists, directly interfering with the phosphorylation of the R-Smads (Massague, 2000).
Marked increases in TGFβ levels occur in some eye diseases (Connor et al., 1989), and during cataract surgery (Jampel et al., 1990), and it is elevated TGFβ signaling that causes lens epithelial cells to undergo an EMT that bears morphological and molecular resemblance to some forms of human cataract, including anterior subcapsular cataract (ASC) and fibrotic PCO, respectively. Many in vitro studies have reported that TGFβ can induce a cataractous phenotype in lens cultures, characterized by an EMT of the lens epithelial cells (Hales et al., 1995; Hales et al., 1994; Liu et al., 1994; Saika et al., 2004), mimicking cells of ASC that acquire a spindle-shaped morphology (Font and Brownstein, 1974). This aberrant TGFβ signaling in lens epithelial cells also induces the expression of α-SMA (a marker for myofibroblastic cells), normally absent in these cells (Hales et al., 1995; Hales et al., 1994), as well as extensive accumulation of extracellular matrix (ECM; including expression of Type I and III collagens and fibronectin), hallmarks of a fibrotic response (Figure 2). The ECM intersperses between these newly transdifferentiated lens cells, resulting in lens capsule remodeling and wrinkling and apoptotic cell death (Hales et al., 1995; Liu et al., 1994). Many of these characteristics were similarly observed in human ASC and PCO (Font and Brownstein, 1974; Novotny and Pau, 1984; Hatae et al., 1993; Miyamoto et al., 2014).
In vivo models confirmed that aberrant TGFβ signaling in the lens results in the induction of lens EMT to form a multilayered plaque of myofibroblastic cells, accompanied also by capsule remodeling, that closely resembled human ASC and features of PCO. Whether it was ectopic overexpression of the active form of TGFβ in transgenic mouse lens (Srinivasan et al., 1998; Lovicu et al., 2002), intravitreal injection of TGFβ in the rat eye (Hales et al., 1999), or adenoviral gene delivery of TGFβ into the anterior chamber of the mouse eye (Robertson et al., 2007), all resulted in cataract formation, displaying key EMT features. Interestingly, more recent studies in mice demonstrated a very similar phenotype to TGFß-induced EMT and cataract when a class of negative-feedback regulators, Sprouty1 and Sprouty2, were conditionally deleted from the lens (see Shin et al., 2012).
Sprouty: Negative Regulator of RTK signaling pathways
Members of the Sprouty (Spry) family act as general antagonists of the receptor tyrosine kinase (RTK)-mediated MAPK signaling pathway, involved in many physiological and developmental processes (reviewed in Li et al., 2003; Cabrita and Christofori, 2008; Mason et al., 2006). Numerous studies have indicated that the interacting partners of Spry remain variable depending on the biological context. In general, gain- or loss-of-function of various components of the MAPK pathway have revealed that Spry acts downstream of receptor tyrosine kinases (RTK) and upstream of the extracellular-regulated MAPKs, ERK1/2 (Casci et al., 1999; Kim and Bar-Sagi, 2004). However, such RTK-ERK/MAPK signaling can be linked to several other pathways (Kim and Bar-Sagi, 2004; Mason et al., 2006). The strength and duration of RTK/MAPK activation plays an important role in determining cell fate, and this has been clearly shown in the lens (see Iyengar et al., 2007; 2009). As a mediator of this important signaling pathway, Spry proteins have been shown to regulate cell proliferation, migration and differentiation in multiple cell types and many developmental processes (Minowada et al., 1999; Tefft et al., 1999; Gross et al., 2001; Yigzaw et al., 2001; Mailleux et al., 2001; Lee et al., 2004; Chi et al., 2004). Moreover, deregulation of Spry expression is also seen in many different cancer types (Lo et al., 2006).
Members of the Spry family are expressed throughout lens morphogenesis, with strong expression retained in the lens epithelium postnatally (Boros et al., 2006). As mentioned briefly above, conditional knockout of Spry from the lens of mice using different independent Crerecombinase expressing lines, leads to ASC (Shin et al., 2012), very similar to that seen with overexpression of TGFß (Srinivasan et al., 1998; Lovicu et al., 2004). The Spry-deficient anterior subcapsular plaques that formed in the postnatal lens of these mice were comprised of disorganised myofibroblastic cells with an abundant, aberrant accumulation of ECM. Subpopulations of ASC cells expressed αSMA, with a concurrent loss of E-cadherin, phenotypes shared with lens cells of TGFβ-induced ASC and PCO (Font and Brownstein, 1974; Novotny and Pau, 1984; Hatae et al., 1993). TGFβ induces αSMA expression in lens epithelial cells that undergo EMT (Hales et al., 1995; Srinivasan et al., 1998; Wormstone et al., 2004) and can repress E-cadherin expression, indicating loss of epithelial phenotype (de Iongh et al., 2005). Such downregulation of E-cadherin by TGFβ1 can be mediated through elevation of Snai1 and Snai2 transcription, in a Smad-independent manner (Dominguez et al., 2003; Peinado et al., 2003; Choi et al., 2007; Mingot et al., 2009; Li et al., 2013). Similarly, an established wound healing (lens puncture injury) model in murine lens also presents a lens epithelial-derived EMT and fibrotic response, which is reported to be Smad3-dependent (Saika et al., 2004); in contrast to other murine models of TGFß-induced ASC where Smad3-signaling is required (Banh et al., 2006). Alternate modes of TGFß-signaling accounting for these differences have recently been proposed (see Shirai et al., 2014). It was reported that ASC can also result from the absence of lens Spry proteins, with aberrant TGFβ signaling prior to cataractogenesis, evident by increased nuclear localization of phosphorylated Smad2, Snai1 and Snai2 in the lens epithelial cells (Shin et al., 2012). Taken together, the aberrant onset of EMT in Spry-deficient lenses resulting from dysregulation of the TGFß signaling pathway, suggests that Spry may be involved in the direct and/or indirect regulation of TGFβ signaling.
Noteworthy, in Spry-deficient ASC plaques, amongst the myofibroblastic cells that had undergone an EMT are subpopulations of cells negative for αSMA that accumulate β-crystallin, indicating aberrant differentiation into lens fiber-like cells (personal communication). This is consistent with earlier findings showing that TGFβ-induced ASC also contain β-crystallin-positive cells (Lovicu et al., 1998). PCO can be attributed to a fibrotic response leading to EMT as discussed earlier, as well as a ‘pearl-type’ PCO derived from aberrant fiber cell differentiation (see de Iongh and Duncan, 2014). Residual lens epithelial cells that do not undergo an EMT post-surgery can differentiate into fibers, resulting in the formation of Soemmering's ring or Elschnig's pearls (see Figure 1). Normal lens fiber differentiation depends on FGF-mediated RTK-ERK/MAPK signaling (see Lovicu and McAvoy, 2005; Wang et al., 2010) and hence it is not overly surprising that in the absence of RTK-antagonists that aberrant fiber differentiation ensues. It should also be noted that FGF2, in the absence of TGFβ stimulation, can induce αSMA expression in non-lens tissue (meniscal fibrochondrocytes; Cucchiarini et al., 2009), and that FGF2 can enhance the EMT responses of TGFβ in lens cells (Cerra et al., 2003). Thus Spry-mediated modification of FGF signaling may in itself contribute to EMT formation, which may or may not involve interactions with the TGFβ-Smad pathway.
It has been shown that in the Spry-deficient lens there is deregulation of TGFβ signaling that leads to EMT and cataract (see Figure 3; Shin et al., 2012), indicating that Spry normally plays a role in the negative regulation of the TGFβ signaling pathway. Given there is much ‘cross-talk’ between various intracellular signaling pathways, Spry may affect other signaling pathways like Smad signaling (either directly or indirectly), besides its known ERK1/2 signaling target (Kim and Bar-Sagi, 2004). It has been reported that overexpression of Spry in lens cells, both in vitro and in vivo, can block the effects of TGFß leading to an EMT and cataract. Epithelial cells overexpressing Spry1 in lens explants are less responsive to TGFß and do not undergo an EMT when compared to cells not overexpressing Spry (see Figure 3; Shin et al., 2012). This was validated by overexpressing Spry1 in transgenic mice that overexpress TGFß specifically in the lens. This co-expression of TGFß and Spry was shown to promote lens transparency, with TGFß failing to stimulate any EMT or cataract in these mice (Shin et al., 2012). These findings are supported by recent studies that indicate involvement of Spry proteins in the inhibition of EMT in other systems. In tooth, Spry proteins are able to prevent the establishment of FGF-mediated EMT for proper incisor morphogenesis (Klein et al., 2008). In lung cancer models, Spry4 can reverse the EMT phenotypes of tumour cells (Tennis et al., 2010). Conversely, Spry levels were reduced in an environment involving EMT. Spry2 was downregulated in fibrotic lung fibroblasts (Renzoni et al., 2004), where TGFβ is consistently associated with progressive fibrosis (Broekelmann et al., 1991; Sime et al., 1997). This indicates that Spry2 is a target of TGFβ-induced fibrosis. Consistent with this, Spry seems to have an inverse relationship with TGFβ. Spry1 and Spry2 transcripts were downregulated in response to TGFβ in human lens epithelial cells (Dawes et al., 2007). In mesenchymal cells, Spry2 expression was also reduced by TGFβ exposure (Ding et al., 2007). Furthermore, Spry1 (Kwabi-Addo et al., 2004; Lo et al., 2004), Spry2 (Lo et al., 2004; Tsavachidou et al., 2004; McKie et al., 2005; Fong et al., 2006; Sutterluty et al., 2007) and Spry4 (Wang et al., 2006; Tennis et al., 2010) are downregulated in a variety of cancer types, including breast, prostate, liver, lung and skin cancers, especially in the metastatic malignant stage involving EMT (Kwabi-Addo et al., 2004; Lo et al., 2004; McKie et al., 2005; Fong et al., 2006; Tennis et al., 2010; Assinder et al., 2014). Taken together, this indicates that Spry proteins possess tumour-suppressing ability (Lo et al., 2004; Shaw et al., 2007; Lee et al., 2008; Tennis et al., 2010).
Figure 3.
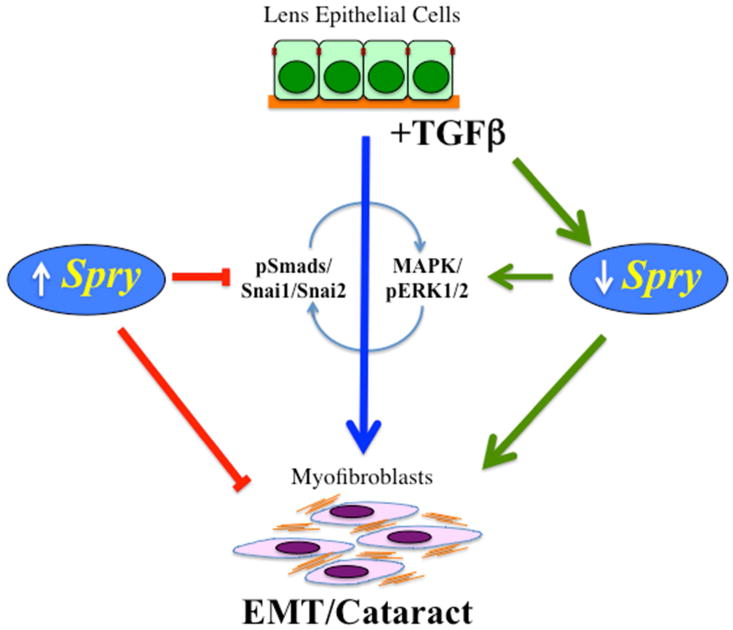
TGFß-signaling via the Smad/Snai molecules results in a lens EMT that contributes to fibrotic forms of cataract. This process also involves (either directly or indirectly) MAPK/ERK1/2 signaling, possibly by downregulating specific RTK antagonists, such as Spry that are normally expressed in the lens epithelium. In Spry-deficient lenses, phosphorylated ERK1/2 levels are elevated and pSmads and Snai1 and Snai2 are translocated to the cell nuclei, leading to an EMT/cataract, similar to that induced by TGFß. Overexpression of Spry in lens cells can effectively block TGFß-induced lens EMT (see Shin et al., 2012).
As the ERK/MAPK signaling pathway is a well established target of Spry proteins, and overexpression of Spry can block aberrant TGFβ-Smad signaling responsible for EMT and ASC, this indicates a role for the ERK/MAPK pathway in TGFß-induced EMT. Whilst TGFβ responses mostly occur through the canonical Smad signaling cascade, they become more complicated, versatile and diversified through interactions with other intracellular signaling pathways (Zhang, 2009), including ERK/MAPK, p38 MAPK (Bakin et al., 2002; Bhowmick et al., 2001; Hanafusa et al., 1999) and JNK cascades (Wang et al., 1997; Yamaguchi et al., 1995). It is known that the ERK/MAPK pathway can respond to TGFβ1 stimulation and coordinate the TGFβ-Smad signaling pathway in many cellular contexts (Ross et al., 2007), including that leading to EMT (Xie et al., 2004). The interaction between TGFβ and ERK signaling pathways appears to be cell type- and target gene-specific, since ERK signaling has been reported to be capable of enhancing (Blanchette et al., 2001; de Caestecker et al., 1998; Hartsough et al., 1996; Stratton et al., 2002; Watanabe et al., 2001; Yue and Mulder, 2000), as well as inhibiting (Calonge and Massague, 1999; Kretzschmar et al., 1999; Sowa et al., 2002) Smad2/3 activity TGFβ1 and TGFβ2 have been reported to rapidly and directly activate Ras (a known intermediate in TGFβ1-activation of MEK1; Ross et al., 2007) and ERK1, in a concentration-dependent manner in TGFβ-sensitive untransformed epithelial cells (Hartsough and Mulder, 1995; Mulder and Morris, 1992; Yan et al., 1994). Raf is also rapidly phosphorylated by TGFβ1 (Lee et al., 2007). In some cell lines; however, TGFβ-induced ERK activation was delayed, suggesting an involvement of an indirect response requiring protein translation (Simeone et al., 2001). TGFβ3 was shown to activate ERK2 in TGFβ-sensitive breast cancer cells, but not in TGFβ-resistant cells (Frey and Mulder, 1997). TGFβ1 was also reported to activate ERK in various cell types, including human mesangial cells (Hayashida et al., 2003) and human skin keratinocytes (Davies et al., 2005), with a relatively modest phosphorylation (2-fold increase) of TGFβ-induced-ERK sufficient for downstream cellular effects (Mulder, 2000).
Thus, MAPK signaling can enhance TGFβ-Smad signaling and in this context both appear to be necessary to induce EMT. Consistent with this, we highlight that Spry, a negative regulator of FGF-mediated ERK/MAPK signaling pathway, is able to also inhibit TGFβ-induced EMT in lens epithelial cells, and consequent ASC in situ. This is confirmed by a reverse relationship between TGFβ and Spry. Spry does not directly inhibit TGFβ receptors nor the Smad proteins; instead, it directly binds and inhibits Grb2 and Raf, which in turn represses the linking of ShcA (phosphorylated by activated TGFβ receptor) and to Grb2-Sos-Ras-Raf-MEK-ERK/MAPK signaling pathway. In addition, to being able to prevent ASC formation, Spry may also be a promising target gene of study in impeding other eye diseases involving markedly elevated TGFβ signaling (Connor et al., 1989; Srinivasan et al., 1998; Wormstone et al., 2004), with some concomitant increases in FGF signaling, such as PCO. As FGF potentiates the cellular effects of TGFβ (Hayashida et al., 2003), FGF inhibitors (that indirectly block ERK1/2 signaling), may also have the potential to protect the lens against TGFβ-induced eye pathologies. Furthermore, Spry may also be a promising investigative target for addressing various tumour types and anti-fibrosis diseases involving aberrant TGFβ signaling.
Whilst a good case can be made for focusing on RTK antagonists, such as Spry, for cataract prevention, the pathological processes involved in TGFß-induced EMT unquestionably involves other levels of regulation and these should be considered as potential targets. For example, tighter control of downstream mediators and regulators of TGFß/Smad-signaling, including connective tissue growth factor (CTGF) and gremlin (Ma et al., 2014) may have a role in controlling lens pathology. Similarly, interfering with other downstream molecules involved in TGFß-mediated EMT, such as matrix metalloproteinases (Korol et al., 2014), the production of reactive oxygen species (Chamberlain et al., 2009; Wang et al., 2014) and even specific integrins (αv; Mamuya et al., 2014) may also contribute to prevention strategies. Other gene regulators, such as miRNA-204-5p and miRNA-26b, which are reduced in PCO and cataract, and have been shown to repress TGFß-induced EMT in human lens epithelial cells (Dong et al., 2014; Wang et al., 2013), may also serve as effective modulators of pathological events.
Maintenance of the epithelial phenotype after cataract surgery and possibilities for promoting normal regenerative processes
Continued growth in understanding the role of various key molecules such as Spry, that can modulate pathological events, will open up possible strategies for maintaining the epithelial phenotype after cataract surgery. The challenge will then be to take the next logical step and promote lens regenerative processes. It is already clear that fiber differentiation can occur after cataract surgery, as evidenced by the formation of Soemmering's ring or Elschnig's pearls (see Figure 1); however, for effective regeneration of function the differentiating fibers need to assemble into the three-dimensional ordered arrangement as in the normal lens. It is now well established that epithelial to fiber differentiation is initiated by FGF (Lovicu and McAvoy, 2005; Robinson et al., 2006; Zhao et al., 2008; Qu et al., 2011; Cvekl and Ashery-Padan, 2014). Whilst, much progress has been made in elucidating FGF triggered signaling pathways and regulation of key events in the fiber differentiation process, little is known about the mechanisms that regulate the coordinated behavior of the differentiating fibers so that they assemble into the characteristic spheroidal lens structure.
Whilst this area of research is relatively new, there are now several reports that illustrate the feasibility of generating nearly normal sized lenses from the residual epithelial cells after mock cataract surgery in animal models (e.g., Call et al., 2004; Huang and Xie, 2010; Gwon and Gruber, 2010; Lois et al., 2010). As already alluded to, one of the challenges in regenerating a functional lens is to consistently reproduce the orderly alignment/orientation of the differentiating fibers so that the lens develops the correct curvature that is required for its optical function. Research in this area has been fragmentary and, at least until recently, little information was available on molecular mechanisms that regulate these key morphogenetic processes that are critically important for lens function. Now, recent research points to the planar cell polarity (PCP) signaling pathway, with Wnt ligands and Frizzled receptors playing critical roles in regulating an interaction between epithelial and fiber cells that influences the polarized behavior of differentiating fibers (Sugiyama et al, 2010). Rat lens epithelial explant studies show that FGF upregulates Wnt-Fz signaling and that this involves translocation of Fz and the centrosome to the leading edge (apical tip) of similarly polarized groups of elongating fiber cells (Dawes et al, 2013). This polarized/oriented behaviour of elongating fibers in FGF-treated epithelial explants is coordinated by islands of epithelial cells (Dawes et al, 2014). Moreover, these epithelial cells express Wnt5A (Dawes et al., 2014; Hoang et al, 2014), and there are indications that Wnt5A (or another member of the Wnt family) could be the source of the fiber-polarizing cue. In turn, studies both in vivo and in vitro have revealed a reciprocal interaction between differentiating fibers and epithelial cells. Early differentiating fibers express the Notch signaling pathway ligand, Jagged1, and this drives the Notch signaling in the epithelium that is required for maintaining the proliferating population of epithelial cells in the germinative zone (Jia et al, 2007; Rowan et al., 2008; Le et al, 2009; Saravanamuthu et al, 2009; Saravanamuthu et al., 2012; Dawes et al., 2014). Taken together, this provides key insights into a self-regulatory mechanism intrinsic to the lens. These reciprocal interactions between epithelial cells and fiber cells appear to be critical for the formation and maintenance of the two lens cell compartments. Furthermore, evidence from earlier experiments with rat lens epithelial explant pairs (O'Connor and McAvoy, 2008), indicate that through their interactions (probably mediated by the Wnt-Fz and Notch signaling pathways described above) lens epithelial and fiber cells can assemble into the highly ordered three-dimensional spheroidal polarized structure that is central to lens function.
In this context it is also interesting that Gwon and Gruber (2010) noted that the regeneration of a complete epithelial layer after mock cataract surgery in rabbits was the key to regenerating the most functional lenses. This observation fits with the results from the explant experiments described earlier, i.e. that epithelial cells provide an important polarizing cure for the differentiating fibers. Therefore, to achieve the goal of regenerating functional lens structure, two steps may be envisaged. First, it will be critical to block EMT and maintain a normal epithelial layer. This blockade would need to be applied early because lens epithelial cells are exquisitely sensitive to TGFß, and sensitivity increases with age (Hales et al., 2000). Given the success of fiber differentiation after mock surgery in animal models (presumably from the FGF-induced differentiation stimulus provided by the FGF-rich vitreous), in humans the main requirement will be to block TGFß until the fiber differentiation response is underway. One way of achieving this would be to impregnate the IOL with a slow release form of an appropriate TGFß blocking agent. The in vivo environment would then promote fiber differentiation and the epithelium would provide the polarizing cue needed to coordinate the assembly of aligned and similarly polarized fibers (see Figure 4). The IOL would also need to be designed to facilitate this process as some IOL materials have been shown to be better than others at blocking PCO (Eldred et al., 2014), and promoting normal regenerative processes (Gwon and Gruber, 2010). In this case the partial regeneration of the equatorial region of the lens would serve the important function of holding the IOL in place and in addition, unlike the progressive fibrosis that can lead to PCO, maintain lens clarity.
Figure 4.
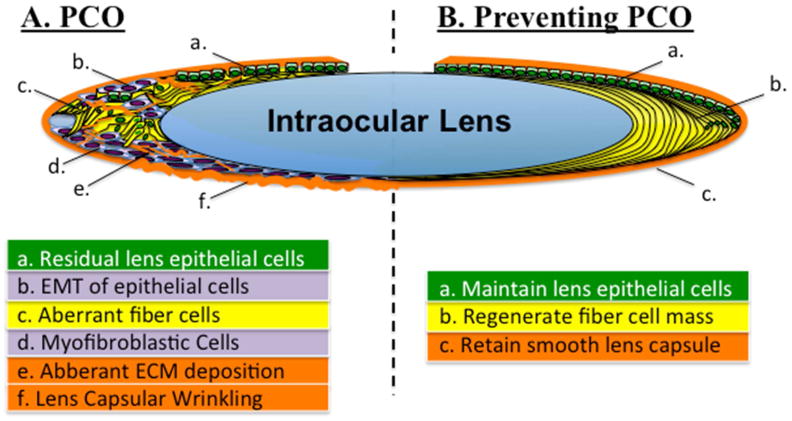
Whilst complications following cataract surgery may lead to PCO (A), an alternative approach to preventing PCO (B) is to promote normal lens architecture and growth. This latter approach would first require blocking aberrant TGFß-signaling at the time of surgery (that may maintain Spry levels in the lens), hence maintain the normal phenotype of lens epithelia (a). The normal in vivo ocular environment could then regenerate the lens fiber mass (b) by promoting the coordinated assembly and alignment of the differentiating secondary fiber cells. This partial regeneration of the equatorial region of the lens may also serve to hold the intraocular lens in place and in addition, unlike the progressive fibrosis that leads to PCO and capsular wrinkling, maintain lens clarity (c).
It could be argued that such a prospect of partial lens regeneration after cataract surgery is overly optimistic, particularly at the advanced ages when most cataract surgery is conducted; however, some evidence from analysis of capsular bags with implanted IOLs indicates that it may indeed be a feasible proposition. Marcantonio et al (2000) conducted a detailed light and electron microscopic analysis of capsular bags from patients ranging from 57-87 years of age that were collected 4 months to 13 years following cataract surgery. In any one capsular bag they identified a variety of cell types, with fibroblastic cells and associated extracellular matrix common in some regions, whereas in other regions the normal epithelial phenotype predominated. At the equatorial region of each bag variable amounts of fiber-like cells were also commonly found. Significantly, in some cases, the cells were well organized and similarly polarized in apposition to an overlying layer of cobblestone-packed epithelial cells; also fiber cells in these regions appeared structurally similar to fibers in the bow region of normal lenses. In short, this morphological analysis showed that conditions in the equatorial niche of the capsular bag favored normal patterns of growth and differentiation. This observation is consistent with the finding that the epithelial cells provide a polarizing cue that is important for the alignment and orderly arrangement of lens fibers. With this in mind, and given continued progress towards understanding of mechanisms and molecules that drive this orderly assembly of lens cells, it is foreseeable that it will eventually be possible to replicate the conditions needed to recapitulate normal developmental processes. Thus, the ultimate aim would be to supplant the fibrotic growth that leads to PCO after cataract surgery with normal growth that leads to regeneration of lens structure and function.
Highlights.
EMT and cataract developed when a class of negative-feedback regulators, Spry1 and Spry2, were conditionally deleted from the lens.
As the ERK/MAPK signaling pathway is a target of Spry proteins, and overexpression of Spry can block aberrant TGFβ-Smad signaling responsible for EMT and cataract, this indicates a role for the ERK/MAPK pathway in TGFß-induced EMT.
Reciprocal interactions between the Wnt-Frizzled and Jagged/Notch signaling pathways between epithelial-fiber cells appears to be critical for the assembly and maintenance of the highly ordered three-dimensional architecture that is central to lens function.
A better understanding of TGFß-signaling regulation, together with promoting regeneration of lens structure and function after cataract surgery, may lead to the prevention of PCO.
Acknowledgments
We would like to acknowledge funding by the NIH (R01 EY03177), USA, the National Health & Medical Research Council (NHMRC), and the Sydney Foundation for Medical Research, Australia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Assinder S, Beniamen D, Lovicu FJ. Co-suppression of Sprouty (SPRY) and Sprouty-related (SPRED) negative regulators of FGF signalling in prostate cancer: A working hypothesis. In “Signal Transduction Inhibitors as Promising Anticancer Agents”. Biomedical Research International. 2014 in press, Nov, 2014. [Google Scholar]
- Awasthi N, Guo S, Wagner BJ. Posterior capsular opacification: a problem reduced but not yet eradicated. Archives of Ophthalmology. 2009;127:555–62. doi: 10.1001/archophthalmol.2009.3. [DOI] [PubMed] [Google Scholar]
- Baker JC, Harland RM. From receptor to nucleus: the Smad pathway. Current Opinion in Genetics & Development. 1997;7:467–73. doi: 10.1016/s0959-437x(97)80072-x. [DOI] [PubMed] [Google Scholar]
- Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115:3193–206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- Beiran I, Scharf J, Tamir A, Miller B. Influence of systemic diseases and environmental factors on age at appearance, location and type of acquired cataract. Metabolic, Pediatric & Systemic Ophthalmology. 1994;17:34–7. [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem. 2001;276:46707–13. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- Blanchette F, Rivard N, Rudd P, Grondin F, Attisano L, Dubois CM. Cross-talk between the p42/p44 MAP kinase and Smad pathways in transforming growth factor beta 1-induced furin gene transactivation. J Biol Chem. 2001;276:33986–94. doi: 10.1074/jbc.M100093200. [DOI] [PubMed] [Google Scholar]
- Boros J, Newitt P, Wang Q, McAvoy JW, Lovicu FJ. Sef and Sprouty expression in the developing ocular lens: Implications for regulating lens cell proliferation and differentiation. Seminars in Cell & Developmental Biology. 2006;17:741–52. doi: 10.1016/j.semcdb.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian G, Taylor H. Cataract blindness: challenges for the 21st century. Bulletin of the World Health Organization. 2001:79, 249–56. [PMC free article] [PubMed] [Google Scholar]
- Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA. 1991;88:6642–6. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrita MA, Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- Call MK, Grogg MW, Del Rio-Tsonis K, Tsonis PA. Lens regeneration in mice: im plications in cataracts. Exp Eye Res. 2004;78:297–9. doi: 10.1016/j.exer.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Calonge MJ, Massagué J. Smad4/DPC4 silencing and hyperactive Ras jointly disru pt transforming growth factor-beta antiproliferative responses in colon cancer cells. J Biol Chem. 1999;274:33637–43. doi: 10.1074/jbc.274.47.33637. [DOI] [PubMed] [Google Scholar]
- Casci T, Vinos J, Freeman M. Sprouty, an intracellular inhibitor of Ras signaling. Cell. 1999;96:655–65. doi: 10.1016/s0092-8674(00)80576-0. [DOI] [PubMed] [Google Scholar]
- Cerra A, Mansfield KJ, Chamberlain CG. Exacerbation of TGF-beta-induced cataract by FGF-2 in cultured rat lenses. Mol Vis. 2003;9:689–70. [PubMed] [Google Scholar]
- Chamberlain CG, Mansfield KJ, Cerra A. Glutathione and catalase suppress TGFbeta-induced cataract-related changes in cultured rat lenses and lens epithelial explants. Mol Vis. 2009;15:895–905. [PMC free article] [PubMed] [Google Scholar]
- Chi L, Zhang S, Lin Y, Prunskaite-Hyyrylainen R, Vuolteenaho R, Itaranta P, Vainio S. Sprouty proteins regulate ureteric branching by coordinating reciprocal epithelial Wnt11, mesenchymal Gdnf and stromal Fgf7 signalling during kidney development. Development. 2004;131:3345–56. doi: 10.1242/dev.01200. [DOI] [PubMed] [Google Scholar]
- Choi J, Park SY, Joo CK. Transforming Growth Factor-beta1 represses E-cadherin production via slug expression in lens epithelial cells. Invest Ophthalmol & Vis Sci. 2007;48:2708–18. doi: 10.1167/iovs.06-0639. [DOI] [PubMed] [Google Scholar]
- Connor TB, Roberts AB, Sporn MB, Danielpour D, Dart LL, Michels RG, de Bustros S, Enger C, Kato H, Lansing M, et al. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. Journal of Clinical Investigation. 1989;83:1661–6. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol & Vis Sci. 1991;32:2201–11. [PubMed] [Google Scholar]
- Cucchiarini M, Schetting S, Terwilliger EF, Kohn D, Madry H. rAAV-mediated overexpression of FGF-2 promotes cell proliferation, survival, and alpha-SMA expression in human meniscal lesions. Gene Therapy. 2009;16:1363–72. doi: 10.1038/gt.2009.91. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Ashery-Padan R. The cellular and molecular mechanisms of vertebrate lens development. Development. 2014;141:4432–47. doi: 10.1242/dev.107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M, Robinson M, Smith E, Huntley S, Prime S, Paterson I. Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways. J Cell Biochem. 2005;95:918–31. doi: 10.1002/jcb.20458. [DOI] [PubMed] [Google Scholar]
- Dawes LJ, Sugiyama Y, Lovicu FJ, Harris CG, Shelley EJ, McAvoy JW. Interactions between lens epithelial and fiber cells reveal an intrinsic self-assembly mechanism. Dev Biol. 2014;385:291–303. doi: 10.1016/j.ydbio.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes LJ, Sugiyama Y, Tanedo AS, Lovicu FJ, McAvoy JW. Wnt-frizzled signaling is part of an FGF-induced cascade that promotes lens fiber differentiation. Invest Ophthalmol Vis Sci. 2013;54:1582–90. doi: 10.1167/iovs.12-11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes LJ, Elliott RM, Reddan JR, Wormstone YM, Wormstone IM. Oligonucleotide microarray analysis of human lens epithelial cells: TGFbeta regulated gene expression. Molecular Vision. 2007;13:1181–97. [PubMed] [Google Scholar]
- de Boer JH, Limpens J, Orengo-Nania S, de Jong PT, La Heij E, Kijlstra A. Low mature TGF-beta 2 levels in aqueous humor during uveitis. Invest Ophthalmol & Vis Scie. 1994;35:3702–10. [PubMed] [Google Scholar]
- de Caestecker MP, Parks WT, Frank CJ, Castagnino P, Bottaro DP, Roberts AB, Lechlei der RJ. Smad2 transduces common signals from receptor serine-threonine and tyrosine kinases. Genes Dev. 1998;12:1587–92. doi: 10.1101/gad.12.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iongh RU, Gordon-Thomson C, Chamberlain CG, Hales AM, McAvoy JW. TGFbeta receptor expression in lens: implications for differentiation and cataractogenesis. Exp Eye Res. 2001a;72:649–59. doi: 10.1006/exer.2001.1001. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Lovicu FJ, Overbeek PA, Schneider MD, Joya J, Hardeman ED, McAvoy JW. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development. 2001b;128:3995–4010. doi: 10.1242/dev.128.20.3995. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Duncan M. Growth factor signaling in lens fiber differentiation. In: Saika S, Werner L, Lovicu FJ, editors. Lens epithelium and posterior capsular opacification. Springer; Japan: 2014. pp. 81–104. [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dijke PT, Hill CS. New insights into TGF-beta-smad signalling. Trends in Biochemical Sciences. 2004;29:265–73. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Ding W, Shi W, Bellusci S, Groffen J, Heisterkamp N, Minoo P, Warburton D. Sprouty2 downregulation plays a pivotal role in mediating crosstalk between TGF-beta1 signaling and EGF as well as FGF receptor tyrosine kinase-ERK pathways in mesenchymal cells. J Cell Physiol. 2007;212:796–806. doi: 10.1002/jcp.21078. [DOI] [PubMed] [Google Scholar]
- Dominguez D, Montserrat-Sentis B, Virgos-Soler A, Guaita S, Grueso J, Porta M, Puig I, Baulida J, Franci C, Garcia de Herreros A. Phosphorylation regulates the subcellular location and activity of the Snail transcriptional repressor. Mol Cell Biol. 2003;23:5078–89. doi: 10.1128/MCB.23.14.5078-5089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Xu B, Benya SR, Tang X. MiRNA-26b inhibits the proliferation, migration, and epithelial-mesenchymal transition of lens epithelial cells. Mol Cell Biochem. 2014;396:229–38. doi: 10.1007/s11010-014-2158-4. [DOI] [PubMed] [Google Scholar]
- Eldred JA, Spalton DJ, Wormstone IM. An in vitro evaluation of the Anew Zephyr Open-Bag IOL in the prevention of posterior capsule opacification using a human capsular bag model. Invest Ophthalmol Vis Sci. 2014;55:7057–64. doi: 10.1167/iovs.14-15302. [DOI] [PubMed] [Google Scholar]
- Fong CW, Chua MS, McKie AB, Ling SH, Mason V, Li R, Yusoff P, Lo TL, Leung HY, So SK, Guy GR. Sprouty 2, an inhibitor of mitogen-activated protein kinase signaling, is down-regulated in hepatocellular carcinoma. Cancer Research. 2006;66:2048–58. doi: 10.1158/0008-5472.CAN-05-1072. [DOI] [PubMed] [Google Scholar]
- Font RL, Brownstein S. A light and electron microscopic study of anterior subcapsular cataracts. Am J Ophthalmol. 1974;78:972–84. doi: 10.1016/0002-9394(74)90811-3. [DOI] [PubMed] [Google Scholar]
- Francis PJ, Berry V, Moore AT, Bhattacharya S. Lens biology: development and human cataractogenesis. Trends in Genetics. 1999;15:191–196. doi: 10.1016/s0168-9525(99)01738-2. [DOI] [PubMed] [Google Scholar]
- Frey RS, Mulder KM. Involvement of extracellular signal-regulated kinase 2 and stress-activated protein kinase/Jun N-terminal kinase activation by transforming growth factor beta in the negative growth control of breast cancer cells. Cancer Res. 1997;57:628–33. [PubMed] [Google Scholar]
- Gordon-Thomson C, de Iongh RU, Hales AM, Chamberlain CG, McAvoy JW. Differential cataractogenic potency of TGF-beta1, -beta2, and -beta3 and their expression in the postnatal rat eye. Invest Ophthalmol & Vis Sci. 1998;39:1399–409. [PubMed] [Google Scholar]
- Granstein RD, Staszewski R, Knisely TL, Zeira E, Nazareno R, Latina M, Albert DM. Aqueous humor contains transforming growth factor-beta and a small (less than 3500 daltons) inhibitor of thymocyte proliferation. Journal of Immunology. 1990;144:3021–7. Erratum appears in J Immunol. 146(10):3687. [PubMed] [Google Scholar]
- Gross I, Bassit B, Benezra M, Licht JD. Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. J of Biol Chem. 2001;276:46460–8. doi: 10.1074/jbc.M108234200. [DOI] [PubMed] [Google Scholar]
- Gwon A, Gruber L. Engineering the crystalline lens with a biodegradable or non-de gradable scaffold. Exp Eye Res. 2010;91:220–8. doi: 10.1016/j.exer.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Hales AM, Chamberlain CG, Dreher B, McAvoy JW. Intravitreal injection of TGFbeta induces cataract in rats. Invest Ophthalmol & Vis Sci. 1999;40:3231–6. [PubMed] [Google Scholar]
- Hales AM, Chamberlain CG, McAvoy JW. Susceptibility to TGFbeta2-induced cat aractincreases with aging in the rat. Invest Ophthalmol Vis Sci. 2000;41:3544–51. [PubMed] [Google Scholar]
- Hales AM, Chamberlain CG, McAvoy JW. Cataract induction in lenses cultured with transforming growth factor- beta. Invest Ophthalmol Vis Sci. 1995;36:1709–13. [PubMed] [Google Scholar]
- Hales AM, Schulz MW, Chamberlain CG, McAvoy JW. TGF-beta 1 induces lens cells to accumulate alpha-smooth muscle actin, a marker for subcapsular cataracts. Curr Eye Res. 1994;13:885–90. doi: 10.3109/02713689409015091. [DOI] [PubMed] [Google Scholar]
- Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsum oto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase path way in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274:27161–7. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- Hartsough MT, Mulder KM. Transforming growth factor beta activation of p44mapk in proliferating cultures of epithelial cells. J Biol Chem. 1995;270:7117–24. doi: 10.1074/jbc.270.13.7117. [DOI] [PubMed] [Google Scholar]
- Hartsough MT, Frey RS, Zipfel PA, Buard A, Cook SJ, McCormick F, Mulder KM. Altered transforming growth factor signaling in epithelial cells when ras activation is blocked. J Biol Chem. 1996;271:22368–75. doi: 10.1074/jbc.271.37.22368. [DOI] [PubMed] [Google Scholar]
- Hatae T, Ishibashi T, Yoshitomi F, Shibata Y. Immunocytochemistry of types I-IV collagen in human anterior subcapsular cataracts. Graefes Archive for Clinical & Experimental Ophthalmology. 1993;231:586–90. doi: 10.1007/BF00936523. [DOI] [PubMed] [Google Scholar]
- Huang Y, Xie L. Expression of transcription factors and crystallin proteins during rat lens regeneration. Mol Vis. 2010;16:341–352. [PMC free article] [PubMed] [Google Scholar]
- Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J. 2003;17:1576–8. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- Hoang TV, Kumar PKR, Sutharzan S, Tsonis PA, Liang C, Robinson ML. Comparative transcriptome analysis of epithelial and fiber cells in newborn mouse lenses with RNA sequencing. Mol Vis. 2014;20:1491–1517. [PMC free article] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Hill CS. Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of TGF-beta receptor activity. Molecular Cell. 2002;10:283–94. doi: 10.1016/s1097-2765(02)00585-3. [DOI] [PubMed] [Google Scholar]
- Itoh S, Itoh F, Goumans MJ, Dijke PT. Signaling of transforming growth factor-beta family members through Smad proteins. Eur J Biochem. 2000;267:6954–67. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- Iyengar L, Patkunanathan B, McAvoy JW, Lovicu FJ. Growth factors involved in aqueous humour-induced lens cell proliferation. Growth Factors. 2009;27:50–62. doi: 10.1080/08977190802610916. [DOI] [PubMed] [Google Scholar]
- Iyengar L, Wang Q, Rasko JE, McAvoy JW, Lovicu FJ. Duration of ERK1/2 phosphorylation induced by FGF or ocular media determines lens cell fate. Differentiation. 2007;75:662–8. doi: 10.1111/j.1432-0436.2007.00167.x. [DOI] [PubMed] [Google Scholar]
- Jampel HD, Roche N, Stark WJ, Roberts AB. Transforming growth factor-beta in human aqueous humor. Curr Eye Res. 1990;9:963–9. doi: 10.3109/02713689009069932. [DOI] [PubMed] [Google Scholar]
- Jia J, Lin M, Zhang L, York JP, Zhang P. The Notch signaling pathway controls the size of the ocular lens by directly suppressing p57Kip2 expression. Mol Cell Biol. 2007;27:7236–47. doi: 10.1128/MCB.00780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–84. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clinical Investigation. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka H, Murayama T, Yokode M, Mori S, Sano H, Ozaki H, Yokota Y, Nishikawa S, Kita T. A novel snail-related transcription factor Smuc regulates basic helix-loop-helix transcription factor activities via specific E-box motifs. Nucleic Acids Research. 2000;28:626–33. doi: 10.1093/nar/28.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nature Reviews Molecular Cell Biology. 2004;5:441–50. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- Klein OD, Lyons DB, Balooch G, Marshall GW, Basson MA, Peterka M, Boran T, Peterkova R, Martin GR. An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development. 2008;135:377–85. doi: 10.1242/dev.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol A, Pino G, Dwivedi D, Robertson JV, Deschamps PA, West-Mays JA. Matrix metalloproteinase-9-null mice are resistant to TGF-β-induced anterior subcapsular cataract formation. Am J Path. 2014;184:2001–12. doi: 10.1016/j.ajpath.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGF beta/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–16. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuracha MR, Burgess D, Siefker E, Cooper JT, Licht JD, Robinson ML, Govindarajan V. Spry1 and Spry2 are necessary for lens vesicle separation and corneal differentiation. Invest Ophthalmol & Vis Sci. 2011;52:6887–97. doi: 10.1167/iovs.11-7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaka D, Nagamot T. Inhibitory effect of TGF-beta 2 in human aqueous humor on bovine lens epithelial cell proliferation. Invest Ophthalmol & Vis Sci. 1994;35:3408–12. [PubMed] [Google Scholar]
- Kwabi-Addo B, Wang J, Erdem H, Vaid A, Castro P, Ayala G, Ittmann M. The expression of Sprouty1, an inhibitor of fibroblast growth factor signal transduction, is decreased in human prostate cancer. Cancer Research. 2004;64:4728–35. doi: 10.1158/0008-5472.CAN-03-3759. [DOI] [PubMed] [Google Scholar]
- Le TT, Conley KW, Brown NL. Jagged 1 is necessary for normal mouse lens formation. Dev Biol. 2009;328:118–26. doi: 10.1016/j.ydbio.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Putnam AJ, Miranti CK, Gustafson M, Wang LM, Vande Woude GF, Gao CF. Overexpression of sprouty 2 inhibits HGF/SF-mediated cell growth, invasion, migration, and cytokinesis. Oncogene. 2004;23:5193–202. doi: 10.1038/sj.onc.1207646. [DOI] [PubMed] [Google Scholar]
- Lee SA, Ho C, Roy R, Kosinski C, Patil MA, Tward AD, Fridlyand J, Chen X. Integration of genomic analysis and in vivo transfection to identify sprouty 2 as a candidate tumor suppressor in liver cancer. Hepatology. 2008;47:1200–10. doi: 10.1002/hep.22169. [DOI] [PubMed] [Google Scholar]
- Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007;26:3957–67. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wheldon L, Heath JK. Sprouty: a controversial role in receptor tyrosine kinase signalling pathways. Biochemical Society Transactions. 2003;31:1445–6. doi: 10.1042/bst0311445. [DOI] [PubMed] [Google Scholar]
- Li P, Jing J, Hu J, Li T, Sun Y, Guan H. RNA interference targeting Snail inhibits the Transforming Growth Factor β 2-induced epithelial-mesenchymal transition in human Lens Epithelial Cells. J Ophthalmol. 2013;2013:869101. doi: 10.1155/2013/869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hales AM, Chamberlain CG, McAvoy JW. Induction of cataract-like changes in rat lens epithelial explants by transforming growth factor beta. Invest Ophthalmol & Vis Sci. 1994;35:388–401. [PubMed] [Google Scholar]
- Lo TL, Fong CW, Yusoff P, McKie AB, Chua MS, Leung HY, Guy GR. Sprouty and cancer: the first terms report. Cancer Letters. 2006;242:141–50. doi: 10.1016/j.canlet.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Lo TL, Yusoff P, Fong CW, Guo K, McCaw BJ, Phillips WA, Yang H, Wong ESM, Leong HF, Zeng Q, Putti TC, Guy GR. The ras/mitogen-activated protein kinase pathway inhibitor and likely tumor suppressor proteins, sprouty 1 and sprouty 2 are deregulated in breast cancer. Cancer Research. 2004;64:6127–36. doi: 10.1158/0008-5472.CAN-04-1207. [DOI] [PubMed] [Google Scholar]
- Lois N, Reid B, Song B, Zhao M, Forrester J, McCaig C. Electric currents and lens regeneration in the rat. Exp Eye Res. 2010;90:316–23. doi: 10.1016/j.exer.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Developmental Biology. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–77. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Schulz MW, Hales AM, Vincent LN, Overbeek PA, Chamberlain CG, McAvoy JW. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Brit J of Ophthalmol. 2002;86:220–6. doi: 10.1136/bjo.86.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, Steven P, Saika S, McAvoy JW. Aberrant lens fiber differentiation in anterior subcapsular cataract formation: a process dependent on reduced levels of Pax6. Invest Ophthalmol & Vis Sci. 2004;45:1946–53. doi: 10.1167/iovs.03-1206. [DOI] [PubMed] [Google Scholar]
- Ma B, Kang Q, Qin L, Cui L, Pei C. TGF-β2 induces transdifferentiation and fibrosis in human lens epithelial cells via regulating gremlin and CTGF. Biochem Biophys Res Commun. 2014;447:689–95. doi: 10.1016/j.bbrc.2014.04.068. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Tefft D, Ndiaye D, Itoh N, Thiery JP, Warburton D, Bellusci S. Evidence that SPROUTY2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mechanisms of Development. 2001;102:81–94. doi: 10.1016/s0925-4773(01)00286-6. [DOI] [PubMed] [Google Scholar]
- Mamuya FA, Wang Y, Roop VH, Scheiblin DA, Zajac JC, Duncan MK. The roles of αV integrins in lens EMT and posterior capsular opacification. J Cell Mol Med. 2014;18:656–70. doi: 10.1111/jcmm.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio JM, Rakic JM, Vrensen GF, Duncan G. Lens cell populations studied in human donor capsular bags with implanted intraocular lenses. Invest Ophthalmol Vis Sci. 2000;41:1130–41. [PubMed] [Google Scholar]
- Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends in Cell Biology. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFβ signal transduction. Annual Review of Biochemistry. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massague J. How cells read TGF-[beta] signals. Nature Reviews Molecular Cell Biology. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- Massague J, Attisano L, Wrana JL. The TGF-[beta] family and its composite receptors. Trends in Cell Biology. 1994;4:172–178. doi: 10.1016/0962-8924(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO Journal. 2000;19:1745–54. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKie AB, Douglas DA, Olijslagers S, Graham J, Omar MM, Heer R, Gnanapragasam VJ, Robson CN, Leung HY. Epigenetic inactivation of the human sprouty2 (hSPRY2) homologue in prostate cancer. Oncogene. 2005;24:2166–74. doi: 10.1038/sj.onc.1208371. [DOI] [PubMed] [Google Scholar]
- Mingot JM, Vega S, Maestro B, Sanz JM, Nieto MA. Characterization of Snail nuclear import pathways as representatives of C2H2 zinc finger transcription factors. Journal of Cell Science. 2009;122:1452–1460. doi: 10.1242/jcs.041749. [DOI] [PubMed] [Google Scholar]
- Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, Krasnow MA, Martin GR. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–75. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- Mulder KM. Role of Ras and Mapks in TGFbeta signaling. Cytokine Growth Factor Rev. 2000;11:23–35. doi: 10.1016/s1359-6101(99)00026-x. [DOI] [PubMed] [Google Scholar]
- Mulder KM, Morris SL. Activation of p21ras by transforming growth factor beta in epithelial cells. J Biol Chem. 1992;267:5029–31. [PubMed] [Google Scholar]
- Miyamoto T, Ishikawa N, Shirai K, Kitano-Izutani A, Tanaka S, Saika S. Histology of Posterior Capsular Opacification. In: Saika S, Werner L, Lovicu FJ, editors. Lens Epithelium and Posterior Capsular Opacification. Springer; Japan: 2014. pp. 177–188. [Google Scholar]
- Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. Journal of Cell Science. 2001;114:4359–69. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- Mukesh BN, Le A, Dimitrov PN, Ahmed S, Taylor HR, McCarty CA. Development of cataract and associated risk factors: The Visual Impairment Project. Arch Ophthalmol. 2006;124:79–85. doi: 10.1001/archopht.124.1.79. [DOI] [PubMed] [Google Scholar]
- Nakakura EK, Watkins DN, Schuebel KE, Sriuranpong V, Borges MW, Nelkin BD, Ball DW. Mammalian Scratch: a neural-specific Snail family transcriptional repressor. PNAS, USA. 2001;98:4010–5. doi: 10.1073/pnas.051014098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–9. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- Novotny GE, Pau H. Myofibroblast-like cells in human anterior capsular cataract. Virchows Archiv - A, Pathological Anatomy & Histopathology. 1984;404:393–401. doi: 10.1007/BF00695223. [DOI] [PubMed] [Google Scholar]
- Obata H, Kaji Y, Yamada H, Kato M, Tsuru T, Yamashita H. Expression of transforming growth factor-beta superfamily receptors in rat eyes. Acta Ophthalmologica Scandinavica. 1999;77:151–6. doi: 10.1034/j.1600-0420.1999.770207.x. [DOI] [PubMed] [Google Scholar]
- O'Connor MD, McAvoy JW. In vitro generation of functional lens-like structures with relevance to age-related nuclear cataract. Invest Ophthalmol Vis Sci. 2007;48:1245–52. doi: 10.1167/iovs.06-0949. [DOI] [PubMed] [Google Scholar]
- Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. Journal of Biological Chemistry. 2003;278:21113–23. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biology. 1991;115:1091–105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Hertzler K, Pan Y, Grobe K, Robinson ML, Zhang X. Genetic epistasis between heparan sulfate and FGF-Ras signaling controls lens development. Dev Biol. 2011;355:12–20. doi: 10.1016/j.ydbio.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzoni EA, Abraham DJ, Howat S, Shi-Wen X, Sestini P, Bou-Gharios G, Wells AU, Veeraraghavan S, Nicholson AG, Denton CP, et al. Gene expression profiling reveals novel TGFbeta targets in adult lung fibroblasts. Respiratory Research. 2004;5:24. doi: 10.1186/1465-9921-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JV, Nathu Z, Najjar A, Dwivedi D, Gauldie J, West-Mays JA. Adenoviral gene transfer of bioactive TGFbeta1 to the rodent eye as a novel model for anterior subcapsular cataract. Molecular Vision. 2007;13:457–69. [PMC free article] [PubMed] [Google Scholar]
- Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17:726–40. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KR, Corey DA, Dunn JM, Kelley TJ. SMAD3 expression is regulated by mitogen-activated protein kinase kinase-1 in epithelial and smooth muscle cells. Cell Signal. 2007;19:923–31. doi: 10.1016/j.cellsig.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Rowan S, Conley KW, Le TT, Donner AL, Maas AL, Brown NL. Notch signaling regulates growth and differentiation in the mammalian lens. Dev Biol. 2008;321:111–22. doi: 10.1016/j.ydbio.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Flanders KC, Yoo J, Anzano M, Liu CY, Kao WWY, Roberts AB. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. American Journal of Pathology. 2004;164:651–63. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanamuthu SS, Gao CY, Zelenka PS. Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fibre differentiation. Dev Biol. 2009;332:166–76. doi: 10.1016/j.ydbio.2009.05.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanamuthu SS, Le TT, Gao CY, Cojocaru RI, Pandiyan P, Liu C, Zhang J, Zelenka PS, Brown NL. Conditional ablation of the Notch2 receptor in the ocular lens. Dev Biol. 2012;362:219–29. doi: 10.1016/j.ydbio.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. Journal of Cell Biology. 1997;137:1403–19. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AT, Meissner A, Dowdle JA, Crowley D, Magendantz M, Ouyang C, Parisi T, Rajagopal J, Blank LJ, Bronson RT, Stone JR, Tuveson DA, Jaenisch R, Jacks T. Sprouty-2 regulates oncogenic K-ras in lung development and tumorigenesis. Genes & Development. 2007;21:694–707. doi: 10.1101/gad.1526207. Erratum appears in Genes Dev. 2008 Jan 15;22(2):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin EH, Basson MA, Robinson ML, McAvoy JW, Lovicu FJ. Sprouty is a negative regulator of transforming growth factor β-induced epithelial-to-mesenchymal transition and cataract. Mol Med. 2012;18:861–73. doi: 10.2119/molmed.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai K, Kitano-Izutani A, Miyamoto T, Tanaka S, Saika S. Wound healing and epithelial-mesenchymal transition in the lens epithelium: roles of growth factors and extracellular matrix. In: Saika S, Werner L, Lovicu FJ, editors. Lens Epithelium and Posterior Capsular Opacification. Springer; Japan: 2014. pp. 159–174. [Google Scholar]
- Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. Journal of Clinical Investigation. 1997;100:768–76. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone DM, Zhang L, Graziano K, Nicke B, Pham T, Schaefer C, Logsdon CD. Smad4 mediates activation of mitogen-activated protein kinases by TGF-beta in pancreatic acinar cells. Am J Physiol Cell Physiol. 2001;281:C311–9. doi: 10.1152/ajpcell.2001.281.1.C311. [DOI] [PubMed] [Google Scholar]
- Sowa H, Kaji H, Yamaguchi T, Sugimoto T, Chihara K. Activations of ERK1/2 and JNK by transforming growth factor beta negatively regulate Smad3-induced alkaline phosphatase activity and mineralization in mouse osteoblastic cells. J Biol Chem. 2002;277:36024–31. doi: 10.1074/jbc.M206030200. [DOI] [PubMed] [Google Scholar]
- Spalton D. Posterior capsule opacification: have we made a difference? Br J Ophth almol. 2013;97:1–2. doi: 10.1136/bjophthalmol-2012-302570. [DOI] [PubMed] [Google Scholar]
- Srinivasan Y, Lovicu FJ, Overbeek PA. Lens-specific expression of transforming growth factor beta1 in transgenic mice causes anterior subcapsular cataracts. Journal of Clinical Investigation. 1998;101:625–34. doi: 10.1172/JCI1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton R, Rajkumar V, Ponticos M, Nichols B, Shiwen X, Black CM, Abraham DJ, Leask A. Prostacyclin derivatives prevent the fibrotic response to TGF-beta by inhibiting the Ras/MEK/ERK pathway. FASEB J. 2002;16:1949–51. doi: 10.1096/fj.02-0204fje. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Stump RJ, Nguyen A, Wen L, Chen Y, Wang Y, Murdoch JN, Lovicu FJ, McAvoy JW. Secreted frizzled-related protein disrupts PCP in eye lens fiber cells that have polarised primary cilia. Dev Biol. 2010;338:193–201. doi: 10.1016/j.ydbio.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterluty H, Mayer CE, Setinek U, Attems J, Ovtcharov S, Mikula M, Mikulits W, Micksche M, Berger W. Down-regulation of Sprouty2 in non-small cell lung cancer contributes to tumor malignancy via extracellular signal-regulated kinase pathway-dependent and -independent mechanisms. Molecular Cancer Research: MCR. 2007;5:509–20. doi: 10.1158/1541-7786.MCR-06-0273. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–5. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Tefft JD, Lee M, Smith S, Leinwand M, Zhao J, Bringas P, Crowe DL, Warburton D. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Current Biology. 1999;9:219–22. doi: 10.1016/s0960-9822(99)80094-3. [DOI] [PubMed] [Google Scholar]
- Tennis MA, Van Scoyk MM, Freeman SV, Vandervest KM, Nemenoff RA, Winn RA. Sprouty-4 inhibits transformed cell growth, migration and invasion, and epithelial-mesenchymal transition, and is regulated by Wnt7A through PPARγ in non-small cell lung cancer. Molecular Cancer Research. 2010;8:833–843. doi: 10.1158/1541-7786.MCR-09-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocharus J, Tsuchiya A, Kajikawa M, Ueta Y, Oka C, Kawaichi M. Developmentally regulated expression of mouse HtrA3 and its role as an inhibitor of TGF-beta signaling. Development Growth & Differentiation. 2004;46:257–74. doi: 10.1111/j.1440-169X.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Experimental Eye Research. 1994;59:723–7. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- Truscott RJ, Friedrich MG. Old proteins and the Achilles heel of mass spectrometry. The role of proteomics in the etiology of human cataract. Proteomics Clin Appl. 2014;8:195–203. doi: 10.1002/prca.201300044. [DOI] [PubMed] [Google Scholar]
- Tsavachidou D, Coleman ML, Athanasiadis G, Li S, Licht JD, Olson MF, Weber BL. SPRY2 is an inhibitor of the ras/extracellular signal-regulated kinase pathway in melanocytes and melanoma cells with wild-type BRAF but not with the V599E mutant. Cancer Research. 2004;64:5556–9. doi: 10.1158/0008-5472.CAN-04-1669. [DOI] [PubMed] [Google Scholar]
- Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell. 1998;95:779–91. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cellular & Molecular Life Sciences. 2008;65:3756–88. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, et al. The sequence of the human genome. Science. 2001;291:1304–51. doi: 10.1126/science.1058040. [see comment][erratum appears in Science 2001 Jun 5;292(5523):1838] [DOI] [PubMed] [Google Scholar]
- Wang J, Thompson B, Ren C, Ittmann M, Kwabi-Addo B. Sprouty4, a suppressor of tumor cell motility, is down regulated by DNA methylation in human prostate cancer. Prostate. 2006;66:613–24. doi: 10.1002/pros.20353. [DOI] [PubMed] [Google Scholar]
- Wang Q, McAvoy JW, Lovicu FJ. Growth factor signaling in vitreous humor-induced lens fiber differentiation. Invest Ophthal & Vis Sci. 2010;51:3599–610. doi: 10.1167/iovs.09-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li W, Zang X, Chen N, Liu T, Tsonis PA, Huang Y. MicroRNA-204-5p regulates epithelial-to-mesenchymal transition during human posterior capsule opacification by targeting SMAD4. Invest Ophthalmol Vis Sci. 2013;54:323–32. doi: 10.1167/iovs.12-10904. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhou G, Hu MC, Yao Z, Tan TH. Activation of the hematopoietic progenitor kinase-1 (HPK1)-dependent, stress-activated c-Jun N-terminal kinase (JNK) pathway by transforming growth factor beta (TGF-beta)-activated kinase (TAK1), a kinase mediator of TGF beta signal transduction. J Biol Chem. 1997;272:22771–5. doi: 10.1074/jbc.272.36.22771. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Zhu J, Zheng GY. Role of glutathione and other antioxidants in the inhibition of apoptosis and mesenchymal transition in rabbit lens epithelial cells. Genet Mol Res. 2014;13:7149–56. doi: 10.4238/2014.September.1.1. [DOI] [PubMed] [Google Scholar]
- Watanabe H, de Caestecker MP, Yamada Y. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic ATDC5 cells. J Biol Chem. 2001;276:14466–73. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. British Journal of Surgery. 2000;87:992–1005. doi: 10.1046/j.1365-2168.2000.01513.x. [DOI] [PubMed] [Google Scholar]
- Wormstone IM, Tamiya S, Eldred JA, Lazaridis K, Chantry A, Reddan JR, Anderson I, Duncan G. Characterisation of TGF-beta2 signalling and function in a human lens cell line. Experimental Eye Research. 2004;78:705–14. doi: 10.1016/j.exer.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia. 2004;6:603–10. doi: 10.1593/neo.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Chen YG, Massague J. The nuclear import function of Smad2 is masked by SARA and unmasked by TGFbeta-dependent phosphorylation. Nature Cell Biology. 2000;2:559–62. doi: 10.1038/35019649. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270(5244):2008–11. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- Yan Z, Winawer S, Friedman E. Two different signal transduction pathways can be activated by transforming growth factor beta 1 in epithelial cells. J Biol Chem. 1994;269:13231–7. [PubMed] [Google Scholar]
- Yigzaw Y, Cartin L, Pierre S, Scholich K, Patel TB. The C terminus of Sprouty is important for modulation of cellular migration and proliferation. J Biol Chem. 2001;276:22742–47. doi: 10.1074/jbc.M100123200. [DOI] [PubMed] [Google Scholar]
- Yoshikawa SI, Aota S, Shirayoshi Y, Okazaki K. The ActR-I activin receptor protein is expressed in notochord, lens placode and pituitary primordium cells in the mouse embryo. Mechanisms of Development. 2000;91:439–44. doi: 10.1016/s0925-4773(99)00320-2. [DOI] [PubMed] [Google Scholar]
- Yue J, Mulder KM. Requirement of Ras/MAPK pathway activation by transforming growth factor beta for transforming growth factor beta 1 production in a Smad-dependent pathway. J Biol Chem. 2000;275:30765–73. doi: 10.1074/jbc.M000039200. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. Journal of Clinical Investigation. 2009;119:1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–39. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–88. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HJ, Burgess AW. Regulation of transforming growth factor-beta signaling. Molecular Cell Biology Research Communications. 2001;4:321–30. doi: 10.1006/mcbr.2001.0301. [DOI] [PubMed] [Google Scholar]


