Abstract
Background
Unlike many other postnatal tissues, bone can regenerate and repair itself; nevertheless, this capacity can be overcome. Traditionally, surgical reconstructive strategies have implemented autologous, allogeneic and prosthetic materials. Autologous bone—the best option—is limited in supply, and also mandates an additional surgical procedure. In regenerative tissue engineering, there are myriad issues to consider in the creation of a functional, implantable replacement tissue. Importantly, there must exist an easily accessible, abundant cell source with the capacity to express the phenotype of the desired tissue, and a biocompatible scaffold to deliver the cells to the damaged region.
Methods
A literature review was performed using PubMed; peer-reviewed publications were screened for relevance in order to identify key advances in stem and progenitor cell contribution to the field of bone tissue engineering.
Results
In this review, we briefly introduce various adult stem cells implemented in bone tissue engineering such as mesenchymal stem cells (including bone marrow- and adipose-derived stem cells), endothelial progenitor cells and induced pluripotent stem cells. We then discuss numerous advances associated with their application and subsequently focus on technological advances in the field, before addressing key regenerative strategies currently used in clinical practice.
Conclusion
Stem and progenitor cell implementation in bone tissue engineering strategies have the ability to make a major impact on regenerative medicine and reduce patient morbidity. As the field of regenerative medicine endeavors to harness the body's own cells for treatment, scientific innovation has led to great advances in stem cell based therapies in the past decade.
Keywords: regenerative medicine, orthopedic surgery, reconstructive surgery, innovation
Introduction
Devastating deficits of bone can arise from an array of conditions such as congenital defects, cancer and trauma, resulting in significant patient morbidity. Unlike many other postnatal tissues, bone can regenerate and repair itself; nevertheless, in pathological fractures or critical-sized bone defects this capacity is overcome and bone healing can fail, thus posing a serious challenge to the reconstructive surgeon. In addition, further pathology such as insufficient blood supply [1], osteomyelitis [2] and systemic diseases (e.g. diabetes) [3] can negatively influence bone healing. It is interesting to note that bone is the second most commonly transplanted tissue after blood [1].
Traditionally, surgery for bone tissue reconstruction has implemented autologous, allogeneic and prosthetic materials for reconstruction of bone defects. Autologous bone—the best option—is limited in supply, and also mandates an additional surgical procedure, with associated donor site morbidity and risk of significant resorption [4, 5]. Each year in the United States, in excess of half a million patients require bone grafting procedures, with a total economic cost greater than $2.5 billion [6]. It is anticipated that this figure will double by 2020 in the United States due to a variety of factors, which include the growing needs of the baby-boomer population and increased life expectancy [6]. Thus, it is not surprising that there is immense interest in the development of bone graft substitutes and related products, an industry in itself with an estimated market value of $1 billion in the United States alone [7].
In reconstructive surgery, there is a wish to follow the old adage: “replace like with like”. As Ralph Millard, an eminent plastic surgeon, once said, “when a part of one's person is lost, it should be replaced in kind, bone for bone, muscle for muscle, hairless skin for hairless skin, an eye for an eye, a tooth for a tooth.” Unfortunately, as there is limited supply of available bone, we now search for new modalities to achieve reconstruction. Tissue engineering can perhaps be best defined as the use of a combination of cells, engineering materials and suitable biochemical factors to improve or replace biological functions. Thus, by engineering and delivering tissues and/or cells capable of replacing damaged bone, regenerative medicine and tissue engineering proper offer the potential to treat critical-sized bone defects, which pose considerable clinical dilemma.
In regenerative tissue engineering, there are myriad issues to consider in the creation of a functional, implantable replacement tissue. Importantly, there must exist an easily accessible, readily abundant cell source with the capacity to express the phenotype of the desired tissue, and a biocompatible scaffold to deliver the cells to the damaged region. As the field of regenerative medicine endeavors to harness the body's own cells for treatment, scientific innovation has led to great advances in stem cell based therapies in the past decade. Stem cells are defined as having the capacity for extensive self-renewal and for originating at least one type of highly differentiated descendant [8]. Stem cells, available as building blocks in tissue engineering, can be broken down into two main categories – adult and embryonic stem cells. The major difference between these cells pertains to the differentiation potential of the cells – embryonic cells are pluripotent, and thus, can differentiate into all cells, whereas adult stem cells are multipotent and their differentiation potential is thus restricted. Postnatal tissues have reservoirs of specific stem cells, which contribute to maintenance and regeneration. Adult stem cells have been isolated from myriad tissues including the gastrointestinal tract [9], central nervous system [10], skeletal muscle [11] and adipose tissue [12]. The adult bone marrow itself shelters various stem cells including hematopoietic [13] and mesenchymal stem cells [14].
In this review, we will briefly introduce various adult stem cells involved in bone tissue engineering and discuss the numerous advances associated with their application. We will then focus on advances in the field of bone tissue engineering including stem cell sheet technology and prospective isolation of cell subpopulations before addressing key regenerative strategies currently implemented in clinical practice. Research examining the role and potential application of stem cells in bone tissue engineering is diverse and continues to grow exponentially. While, we have strived to introduce multiple novel developments, the depth of research in this nascent field is staggering and thus cannot be fully summarized in this literature review (Table 1).
Table 1.
A PubMed search was utilized to identify available literature from 1991-April 2015. The initial search criteria included: Bone tissue engineering AND stem cells AND progenitor cells (+/− clinical). The number of studies screened is shown in parentheses. Due to the staggering volume of studies in this research area, the data cannot be fully summarized in this review. Thus, for refinement, additional search criteria included: scaffolds, bioengineering, bone regeneration, craniofacial and orthopedic. Search criteria were restricted to the English language, but acceptable English translations were sought for inclusion. Eligible studies were first identified by title and abstracts and then the full-text papers were retrieved. Additional studies were found after reviewing the related PubMed citations and references of the included papers.
| Pubmed analysis: 1991-2015 | Initial search criteria: Bone tissue engineering AND stem cells AND progenitor cells (n=5710) | Cell type [number of scientific studies screened) | Additional search criteria: scaffolds bioengineering bone regeneration craniofacial orthopedic |
| Mesenchymal stem cells. (n= 3676) | |||
| Adipose-derived stem cells (n=570) | |||
| Endothelial progenitor cells (n = 174) | Search criteria restrictions: English language, but acceptable English translations were sought for inclusion. | ||
| Induced pluripotent stem cells (n= 120) | |||
| Initial search criteria: Bone tissue engineering AND stem cells AND progenitor cells AND clinical (n=1333) | Mesenchymal stem tells (n=S31) |
Identification of eligible studies: 1. Title and Abstracts 2. Full-text papers then retrieved for further examination. |
|
| Adipose-derived stem cells (n= 131) | |||
| Endothelial progenitor cells (n=50) | Additional studies: Following review of the related PubMed citations and references of the included papers | ||
| Induced pluripotent-stem calls (nF40) |
TOTAL NUMBER OF STUDIES INCLUDED FOR FOCUSED REVIEW = 97
Stem/Progenitor Cells Applicable to Bone Tissue Engineering
Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells have been conventionally isolated from the bone marrow [15] and more recently from an array of other postnatal tissues including adipose tissue [12], synovium [16], periodontal ligaments [17] and the lung [18]. As the ultimate goal of regenerative medicine is to avoid in vitro expansion of cells and the associated complications, the adipose-derived stem cell (ASC) represents an ideal progenitor cell in bone tissue engineering. Intriguingly, previous studies have identified that bone marrow aspirate yields 6 × 106 nucleated cells per mL, of which 0.001 to 0.01% are stem cells [15]. Contrastingly, 2×106 nucleated cells can be isolated from 1 gram of adipose tissue, of which 10% are thought to be stem cells, thus one can easily discern the potential clinical implications of this abundant source of MSCs [19, 20]. In an effort to discern if there is a more favorable site from which to harvest MSCs for bone tissue engineering, researchers compared the in vivo osteogenic potential of adipose-derived, periosteal-derived and bone marrow-derived MSCs in a guided bone regeneration model in pig calvarial defects. Here, irrespective of the tissue source of MSCs, the speed and pattern of osseous healing after cell transplantations into monocortical bone defects were comparable, indicating that the efficiency of autologous ASC, periosteal derived-MSC and bone marrow derived-MSC (BM-MSCs) following ex vivo cell expansion is not significantly different for the guided regeneration of bone defects (Figure 1) [21].
Figure 1.
Microradiography of representative specimens of the different groups at specific time points (magnification = 2.5×). At early stage of bone healing up to 30 days after implantation there were only mild differences of bone regeneration visible among the three test defects (AD, PD, BM) and the control defect (CO). At day 60 and day 90 the area of newly formed bone inside the defect showed significant differences in comparison to control. (Reproduced with permission:[21]).
There are a multitude of studies reporting the beneficial use of BM-MSCs or ASCs alone in bone tissue engineering in various models of bone regeneration including distraction osteogenesis [22-25], segmental long bone defects [26-28] and calvarial defects [29-31]. Moving forward, stem cell therapy in combination with cytokines or genetic modification has the potential to further improve bone repair. For example, cytokines can lead to increased migration and homing of stem cells to the defect site. Stromal derived factor-1 (SDF-1) is thought to act to increase migration of stem cells to the site of injury [32]. Ho and colleagues hypothesized that BM-MSCs transfected with SDF-1 would not only directly enhance bone repair, but that they would also indirectly augment bone repair by increasing migration of native cells to the site of fracture. Here, they found that BM-MSCs overexpressing SDF-1 showed significantly greater new bone formation during the early stage of fracture healing in comparison to unmodified BM-MSCs, thus affording the authors to conclude that SDF1 played an additional role by leading to increased recruitment of host stem cells to the defect site and encouraging osteogenic differentiation and production of bone [33]. Interestingly, in another example of dual delivery of genetic material and stem cells to aid bone regeneration, Park et al. combined recombinant human platelet derived growth factor-BB (PDGF-BB) and BM-MSCs transfected with bone morphogenetic protein (BMP)-2 in a critical-sized bone defect in rats and observed an improved quantity and quality of bone regenerate formed in comparison to BM-MSCs transfected with BMP-2 alone, which they attributed to the modulation of PDGF-BB on BMP2-induced osteogenesis [34]. In addition, there have been favorable results reported in bone engineering applications following implementation of MSCs transfected with genes implicated in fracture healing; for example, osterix [35, 36], hypoxia inducible factor-1 [37, 38] and BMP-7 [39].
Focusing on the potential curative applications of MSCs implemented in bone tissue engineering, a group in Imperial College London showed that prenatal transplantation of human first trimester fetal blood MSCs led to phenotypic improvement in a mouse model of osteogenesis imperfecta (OI), a genetic disorder of type I collagen resulting in fragile bones and skeletal deformities, with reduced fracture rate secondary to improvements in the bone matrix [40]. Nevertheless, in this initial study the authors proposed that the observed therapeutic effect was incomplete and attributed this finding to the limited level of MSC engraftment in bone. Thus, they exploited the known mechanism of BM-MSC migration, reported to be CX-C chemokine receptor type 4 (CXCR4)-SDF1 dependent [32], and primed the BM-MSC with SDF1 prior to in vivo administration, with resultant increased CXCR4 cell surface expression. This led to improved chemotaxis in vitro and enhanced engraftment in vivo (at least threefold) in the OI and wild-type bone and bone marrow with higher engraftment of MSCs in the OI bones and more importantly, to a reduction in bone brittleness [41].
Endothelial Progenitor Cells
Bone is a highly vascularized tissue, which relies on the close spatial and temporal association between blood vessels and bone cells to maintain skeletal integrity. As early as 1763, the importance of blood vessels in bone formation was noted: “the origin of bone is the artery carrying the blood and in it the mineral elements” [42, 43]. It is thought that adequate vascularization is an essential pre-requisite that allows stem cells to reach the site of tissue repair and allows the delivery of oxygen, nutrients and morphogens. Angiogenesis is, thus, of pivotal importance in successful bone regeneration. Previous reports illustrated that the rate of delayed union or non-union of fracture can be as high as 46% in fracture patients with concomitant vascular injuries [44].
In 1997, Asahara and colleagues identified endothelial progenitor cells (EPCs) in the peripheral blood and reported their ability to initiate neovascularization [45]. These cells, isolated from purified hematopoietic progenitor cells, expressed endothelial-associated markers (i.e. cluster of differentiation molecule, CD34) and were shown to differentiate into an endothelial phenotype. While there are contrasting descriptions in the literature with respect to the origin and the surface markers of these cells, EPCs can be defined as bone-marrow-derived precursor cells with the ability to differentiate into endothelial cells and to participate in the formation of new blood vessels [43].
The major role of EPCs in new vessel formation and the ability of EPCs to proliferate and differentiate into endothelial cells present them as an ideal therapeutic strategy for amelioration of the ischemic environment of a critical-sized bone defect in bone tissue engineering. New vessel formation is an important element of the biological response to bone injury and in keeping with this trend, Laing and colleagues studied the mobilization of EPCs in response to closed diaphyseal tibia fractures [46]. Here, they reported that EPCs increased sevenfold on day 3 post injury, suggesting that a systemic pro-vascular response is initiated in response to musculoskeletal trauma [46]. Furthermore, Matsumoto observed that the frequency of EPCs increased in the bone marrow and peripheral blood in the early stages of fracture repair and further illustrated incorporation of EPCs into developing blood vessels at the site of bone injury. Further histological results demonstrated that neovascularization did not exclusively involve the EPC population, however, thus supporting the hypothesis that paracrine signaling from EPCs may also contribute to neovascularization at the ischemic site [47].
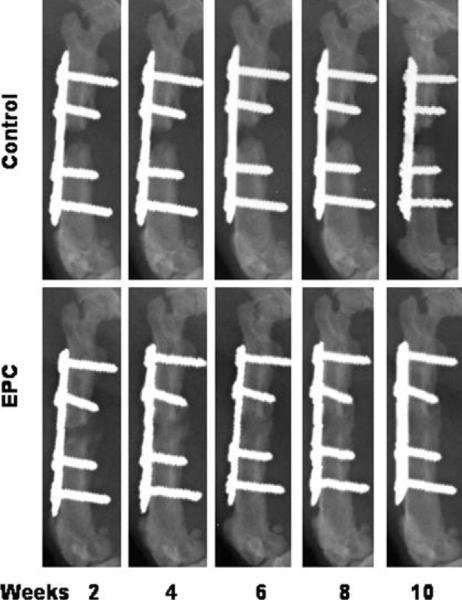
In a nude mouse calvarial defect model, Zigdon-Giladi et al. recently illustrated that human EPCs derived from peripheral blood could augment vasculogenesis and osteogenesis [48]. They reported that there was a seven-fold increase in blood vessel density in addition to increased extra-cortical bone height and bone area fraction in the bony regenerate following human EPC transplantation in comparison to control (β-tricalcium phosphate biomaterial dome alone). Furthermore, EPCs have also shown promising results when administered following a segmental bone defect creation in a rat model; here, Li et al. seeded EPCs on a gelfoam scaffold locally at the site of injury and compared this to gelfoam scaffold application alone. All of the animals in the EPC-treatment arm healed with bridging callus formation, whereas animals in the control group developed nonunion of the defect (Figure 2). Thus, unsurprisingly, the EPC treated femora had significantly higher torsional strength and stiffness in comparison to control [49]. Similar beneficial effects of EPC therapy have also been seen in murine models of long bone fracture [50-52]. An interesting finding that has been consistent in many studies is that the incorporation rate of EPCs in the developing vasculature in ischemic tissue is quite low, or at least not enough to explain the observed increase in re-/neovascularization. One possible explanation is that the efficiency of new blood vessel formation may combine the incorporation of EPCs in newly formed vessels and the release of proangiogenic factors in a paracrine manner [53, 47, 50, 54, 55].
Figure 2.
Plain radiographs showing the bone healing in endothelial progenitor cell (EPC)-treated and control group animals at 2, 4, 6, 8, and 10 weeks postfracture. (Reproduced with permission: [49]).
Moving from pre-clinical studies, Kuroda and colleagues first explored the use of putative EPCs in a clinical case of tibial non-union, whereby the patient had a successful outcome after receipt of autologous, granulocyte colony stimulating factor (GCSF)-mobilized CD34(+) cells accompanied with autologous bone grafting [56]. Following this success, this group progressed to a phase I/IIa clinical trial, in which autologous local transplantation of GCSF-mobilized CD34 positive cells was utilized in addition to autologous bone grafting [57]. While the overall results of this clinical pilot study were indeed positive, further randomized controlled trials need to be performed before definitive conclusions can be made [57].
Induced Pluripotent Stem Cells
In 2006 Yamanaka first proposed that pluripotency could be induced through the recapitulation of early biomolecular events after somatic cell fusion [58]. The ability to generate induced pluripotent stem cells (iPSC) is one of the major developments in the stem cell arena in recent years, for which Yamanaka was awarded a Nobel Prize in Physiology or Medicine [59]. Somatic cells from human adult fibroblasts can be reprogrammed into a primordial embryonic stem cell like state, with the capacity to develop into all three germ layers by forcing expression of four classical transcription factors (Oct4, Sox2, Klf4, and c-myc, “Yamanaka factors”) [58]. Subsequent studies have demonstrated the capacity to generate iPSCs using even fewer factors [60, 61]. Human-induced pluripotent stem-cell-derived mesenchymal stem cells (iPSC-MSCs) are thus a promising source of patient-specific stem cells with great regenerative potential. While clinical translation is currently delayed, as there are still issues that need to be addressed (e.g. risk of teratoma), pre-clinical investigation is currently generating considerable momentum.
Ardeshirylajimi led a recent study, which assessed the ability of polyethersulfone scaffolds seeded with iPSCs to regenerate bone in critical-sized calvarial defects. Here, the authors reported that polyethersulfone scaffolds seeded with iPSCs induced greater bone regeneration than scaffold alone [62], results which have also been replicated in periodontal bone regeneration [63]. Furthermore, Liu recently reported that NELL1 (which encodes a protein involved in cell growth regulation and differentiation) overexpression greatly enhanced the osteogenic differentiation and mineral synthesis of iPSC-MSCs on Arg-Gly-Asp-grafted calcium phosphate cement scaffold [64]. Ideally, implementing the many arms of tissue engineering, the design of simple and robust biomaterials with an innate ability to induce lineage-specificity of iPSCs is desirable for effective clinical implementation of iPSC-based bone tissue engineering. Kang and co-investigators questioned if osteogenic differentiation of iPSC can be achieved through cues provided by biomaterials alone [65]. They reported that osteogenic differentiation of iPSCs can be achieved by biomaterials containing calcium phosphate alone in an ex vivo model, thus presenting new avenues for personalized medicine and tissue engineering [65].
Interestingly, Lian and colleagues also reported the use of iPSCs in a mouse model of limb ischemia, where they found that the benefits of iPSC-MSCs were superior to those of adult BM-MSCs [66]. The authors found that the greater potential of iPSC-MSCs may be attributable to the superior survival and engraftment after transplantation to induce tissue regeneration via, both, de novo differentiation of the cells and paracrine mechanisms. As there is increasing interest in the interplay between osteogenesis and angiogenesis in bone tissue regeneration, this is an interesting finding, which deserves further attention in models of bone regeneration [48, 67, 68].
Looking further afield, iPSCs also offer a platform for investigation and modeling of rare skeletal diseases. For example, iPSCs have been derived from patients with fibrodysplasia ossificans progressiva, a rare genetic disorder characterized by progressive ossification in soft tissues, allowing researchers to develop a model in which to study therapeutic effects and basic mechanisms of disease [69]. While the implications of strategies such as these currently do not make an impressive impact on bone tissue regeneration, by developing further understanding of these rare skeletal diseases we can develop future strategies for bone regeneration in rare osteopathies.
Scientific Adventure: Prospective Isolation and Subpopulation Identification
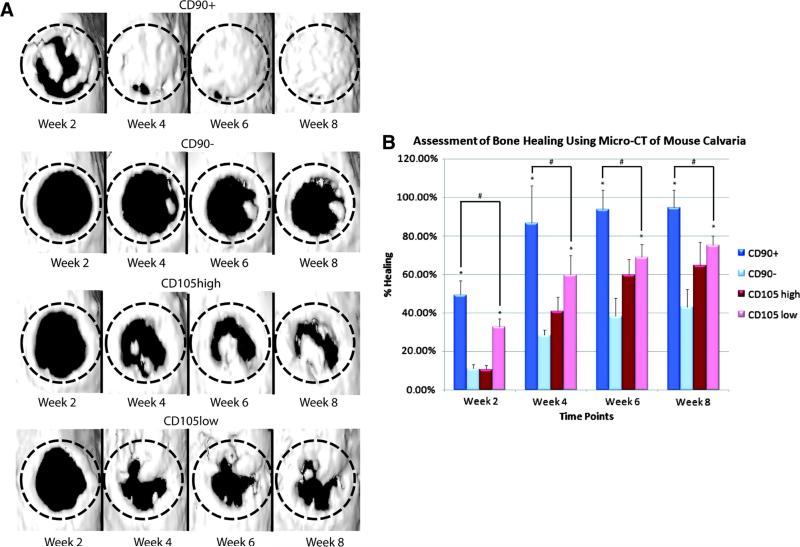
Drawbacks oft related to conventional stem cell isolation include issues related to purity, potency and availability. A useful strategy, which has emerged following scientific adventure, is that of prospective isolation and identification of certain cell subpopulations of stem cells with enhanced potential for osteogenesis or indeed angiogenesis [70]. This strategy allows researchers to purify stem and progenitor cell subpopulations based on the immunophenotype of the desired subgroup of cells by fluorescence-(FACS) or magnetic- (MACS) assisted cell sorting and avoids the necessity to wait for certain subpopulations of interest to emerge from long-term cultures of purified cells as per conventional methodology. For instance, Levi and colleagues enriched for an osteogenic subpopulation of cells derived from human subcutaneous adipose tissue utilizing microfluidic-based single cell transcriptional analysis and fluorescence-activated cell sorting. They demonstrated that a subpopulation of ASCs with low CD105 surface marker expression sorted using FACS significantly enhanced osteogenic differentiation both in vitro and in vivo when compared with unsorted ASCs or ASCs with high CD105 surface marker expression (Figure 3) [71]. In addition, Chung further explored the osteogenic potential of a subpopulation of ASCs expressing the surface marker CD90 [72]. Here CD90-expressing ASCs demonstrated greater osteogenic capacity both in vitro and in vivo, when compared to unsorted cells or those with either high or low CD105 surface marker expression, thus suggesting that CD90 may be a more effective marker than CD105 to isolate a highly osteogenic subpopulation for bone tissue engineering (Figure 4) [72].
Figure 3.
FACS analysis and gene expression of CD105 and osteogenic differentiation of CD105high, CD105low, and unsorted ASCs. (A), FACS analysis of CD105 expression 36 h after ASC harvest. (B), to confirm that cell surface marker sorting is indicative of transcriptional profile, we demonstrated that cells sorted with high CD105 do indeed have significantly higher expression of CD105 (*, p < 0.05). (C), gene expression of early (RUNX-2, alkaline phosphatase (ALP), and Collagen 1, (COL1)) and late (osteocalcin, OCN) osteogenesis as well as genes involved in the BMP pathway (BMP-2, BMP-4, and BMPR1B). Across all genes, CD105low cells had greater expression (*, p < 0.05). (D), alkaline phosphatase stain. (E), quantification of unsorted, CD105high, and CD105low ASCs (*, p < 0.05). (F), Alizarin red stain. (G), quantification comparing unsorted, CD105high, and CD105low hASCs. (*, p < 0.05). (H), gene expression of ALP (*, p < 0.05). (I), OCN during osteogenesis over time starting immediately after the sort and following for 7 days. The CD105low cells appear to maintain a higher expression profile of ALP and OCN over time (*, p < 0.05). Statistical analysis was performed with either a one-way ANOVA (cell population) or a two-way ANOVA (cell population and time) followed by post hoc individual comparisons. (Reproduced with permission: [71]).
Figure 4.
(A) Three-dimensional reconstruction of calvarial defects. Mice were scanned at 2, 4, 6, and 8 weeks following surgery. At each time point, the CD90+-treated defects demonstrated improved bone healing. (B) Quantification of osseous healing by micro-CT revealed significantly more healing with CD90+ cells relative to CD90− (*p<0.05) and CD105low cells (#p<0.05) cells at the 2-, 4-, 6-, and 8-week time points. (Reproduced with permission:[72]).
In addition, the degree of heterogeneity of stem cell populations and a better understanding of the contribution of a certain cell subpopulation to bone healing can be developed by prospective isolation of subpopulations of stem cells. Furthermore, stringent regulatory frameworks guiding approaches to cell-based therapies require that in vitro manipulation or culture must undergo stringent safety trials prior to approval for clinical use, while cells that can be directly implanted bypass much of this legislation [73, 70]. Higher degrees of purity of cell subpopulations obtained following prospective isolation best facilitate demonstration of the product safety and efficacy required for approval of treatment strategies by regulatory bodies, such as the Food and Drug Administration (FDA) in the United States [74, 70]. Looking to the future, one envisions a time when one can harvest subcutaneous adipose tissue from the ideal anatomic location, enrich for ASCs with enhanced osteogenic potential, treat the cells with ideal small molecules or cytokines, and implant these cells on a biomimetic scaffold into the skeletal defect in the same patient without leaving the operating room.
Incorporating new technologies: stem cell sheets and bone tissue engineering
Bone tissue engineering is embracing new stem cell based technologies. For example, the presence of a functional periosteum is known to accelerate healing in bone defects by providing a source of progenitor cells that aid in repair. Thus, Syed-Picard and co-investigators hypothesized that cell sheets composed of BM-MSCs could be used to engineer functional periosteal tissue [75]. In order to generate the cell sheets, BM-MSCs were cultured to hyperconfluence so that they produced sufficient extracellular matrix to form robust cell sheets. The sheets were then wrapped around calcium phosphate pellets and implanted subcutaneously in mice for 8 weeks. Notably, they observed that the calcium phosphate pellets wrapped in BM-MSC sheets regenerated a bone-like tissue whereas the pellets lacking the cell sheet did not. Furthermore, the bone-like tissue seen on the calcium phosphate scaffolds wrapped with the BM-MSC sheets was enclosed within a periosteum-like tissue characterized morphologically and through expression of periostin [75]. This result was again echoed in a recent study reported by Chen and colleagues, in which the authors compared BM-MSC cell sheet technology with a control cell complex and found that the cell sheets resulted in greater levels of morphogens with known importance in skeletal biology, such as vascular endothelial growth factor, VEGF and PDGF [76]. Moving forward with cell sheet technology, Ren and coworkers recently prepared a three-dimensional vascularized stem cell sheet construct for tissue regeneration, whereby they used both BM-MSCs and human umbilical vein endothelial cells as cell sources with a resultant greater density of blood vessel formation than the control [77].
Looking to the future: understanding and manipulating the stem cell niche microenvironment
The concept of the stem cell niche, the natural microenvironment that surrounds stem cells, was first introduced in 1978. It can be defined as an anatomical and functional entity that plays a crucial role in maintaining tissue homeostasis and tissue repair and regeneration in the case of injury [78]. The stem cell niche provides a complex array of physical signals, including cell-cell contacts and cell matrix adhesions, and biochemical signals, such as growth factors, to stem cells in a temporospatial manner; with the integration of both local and systemic cues in the niche guiding these cells to proliferation and fate specification [79, 80]. It is intriguing to note that there is a reservoir of MSCs in myriad postnatal tissues, thus raising the question of whether therapeutic strategies could be employed to ‘recruit’ or accelerate the regenerative capacity of resident MSCs following injury. The ‘activation’ of adult stem cells is regulated, in part, by their neighboring cells in a niche microenvironment; manipulation of this communication through the local infiltration of growth factors or molecules integral to this communication may represent such a therapeutic strategy [70].
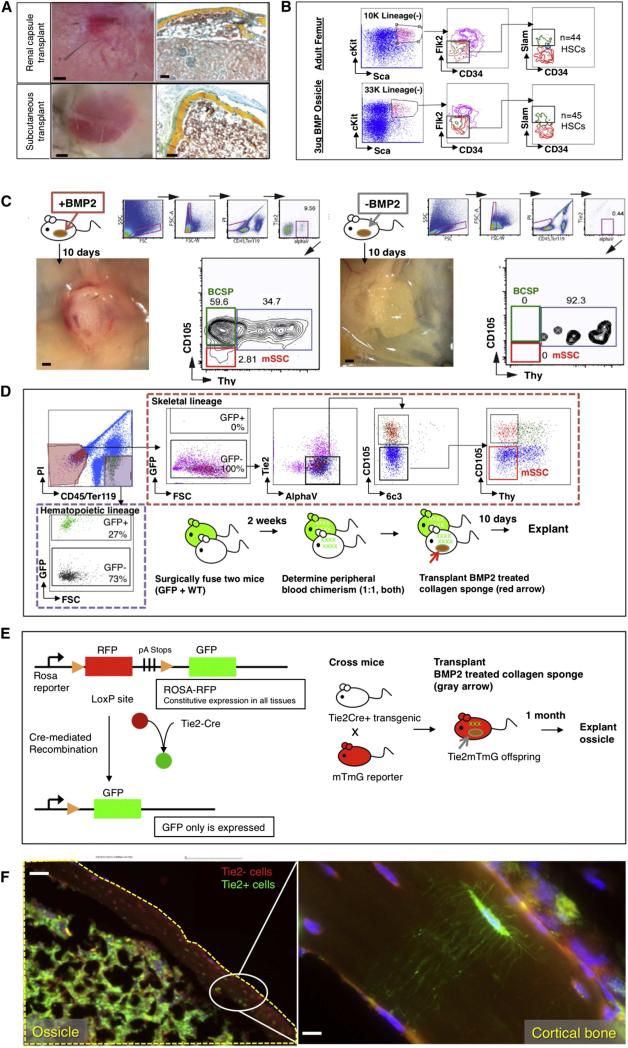
By developing a better understanding of the stem cell niche, we can determine new strategies for activation or migration of native stem and progenitor cells for bone tissue engineering. In situ activation of MSCs could be harnessed to accelerate healing and facilitate an early return to function after a variety of musculoskeletal injuries [70]. To this end, our laboratory, which has recently characterized the mouse skeletal stem cell and its downstream progeny [81], proposed that autocrine and paracrine BMP-2 signaling contributes to skeletal stem cell activity and regulation of its niche. Following placement of a collagen sponge impregnated with BMP-2 into adipose tissue, we induced de novo formation of ossicles, which consisted of skeletal stem and progenitor cells and were functionally similar to normal long bone in that the induced ossicles also engrafted circulating hematopoietic stem cells [81] (Figure 5). In addition, bone tissue engineering can be more unreliable in aging patients, due to age-related changes in the regenerative niche [82]. Helms and colleagues demonstrated that replenishment of Wnt3a signaling in bioengineered autografts resulted in superior bone forming capacity compared to standard of care in an aged mouse spinal fusion model[83]. By further exploring the stem cell niche environments of the skeleton, researchers can also develop novel strategies to re-create a niche microenvironment for the implanted stem and progenitor cells, which will hopefully increase stem cell viability and contribution to bone regeneration.
Figure 5.
(A) Collagen sponges containing 3 μg of lyophilized rhBMP2 were placed into extraskeletal sites in C57BL6 wild-type mice. One month later, the graft was explanted for analysis. Bright-field images of explants are shown, with renal capsule transplants shown above and subcutaneous transplants shown below (left). Transverse sections stained with Movat's pentachrome demonstrate that induced osseous osteoids formed a marrow cavity (red stain) (right).
(B) FACS analysis of cells within the induced osteoid marrow reveals that HSC engraftment occurs in the osteoids (bottom row) similar to that which occurs naturally in “normal” adult femurs (top row).
(C) (Left) Following explantation of rhBMP2-laced collagen sponges at day 10 post-extraskeletal placement, FACS analysis of constituent cell populations present within the graft revealed that mouse skeletal stem cells (red box on FACS plot) and their downstream progenitors (green box on FACS plot) are readily detectable in the rhBMP2-treated explants. (Right) In contrast, FACS analysis of adipose tissue in the absence of BMP2 does not detect either mSSC (red box on FACS plot) or BCSP (green box on FACS plot). mSSC = mouse skeletal stem cell; BCSP = bone, cartilage and stromal progenitor.
(D) A parabiosis model of GFP+ and non-GFP mouse shows that circulating skeletal progenitor cells did not contribute to BMP2-induced ectopic bones. A GFP+ mouse was parabiosed to a non-GFP mouse. Two weeks later, a collagen sponge containing 3 μg of lyophilized rhBMP2 was transplanted into the inguinal fat pad of the non-GFP mouse. Ten days later, the tissue was explanted and isolated the constituent cell populations of the ectopic bone tissue as described previously. The contribution of the GFP-labeled cells to ectopic bone formation in the non-GFP mouse was analyzed by FACS (broken red line; GFP+ = circulating cells, and nonfluorescent = local cells). GFP-labeled cells contributing to the graft were solely CD45+ hematopoietic cells (extreme left panel, broken purple line) and not consistent of the skeletal progenitor population (horizontal upper panel, mSSCs shown in red box on FACS plot).
(E) (Left) Diagram of reporter gene mouse model shows that Tie2 expression leads to GFP expression. Tie2+ cells turn green, but Tie2− cells remain red. (Right) Scheme of experiment: In order to determine the cell types, which could undergo BMP2-mediated reprogramming to mSSCs in extraskeletal sites, a collagen sponge containing rhBMP2 was placed into the subcutaneous inguinal fat pad of a Tie2Cre × MTMG reporter mouse.The ossicle was explanted 1 month later for histological analysis.
(F) Fluorescent micrographs: BMP2-derived ossicles (yellow broken line) clearly incorporate both GFP+ Tie2+-derived osteocytes with visible canaliculi and Tie2− RFP-labeled osteocytes. Area denoted by white oval is shown at higher magnification in the box on the extreme right, showing the presence of GFP+ Tie2+ canaliculi in the presence of RFP+ Tie2− cells. (Reproduced with permission: [81]).
Embracing Clinical Translation: early days and innovation
Moving to therapies currently in use in clinical practice, there are a few examples of studies, which are attempting to bridge the divide between pre-clinical and clinical studies. For instance, osteoarthritis (OA) is a common degenerative joint disease affecting in excess of 27 million people in the USA where it is also the leading cause of chronic disability [84]. A group in Colorado has reported the safety and efficacy of their case series consisting of greater than 300 patients who had undergone treatment with culture-expanded, autologous, BM-MSC for osteoarthritis. Here, the cells were cultured in monolayer culture flasks using an autologous platelet lysate technique and re-injected into the peripheral joints or into the intervertebral discs under image guidance, with the majority of patients reporting a greater than or equal to 50% improvement in clinical symptoms [85]. A similar experience was reported by a group in South Korea following injection of infrapatellar fat pad-derived ASCs in the management of knee OA [86]. Of particular interest, the clinical and radiological improvement reported in this study following ASC therapy was positively correlated to the number of cells injected [86]. Drawing attention to a rarer condition, two patients with a diagnosis of OI have been reported in the literature following stem cell-based therapy. The patients received both prenatal human allogeneic fetal MSCs and postnatal boosting with same donor MSCs and had improved linear growth, mobility, and reduced fracture incidence. While the limited clinical experience to date means that it is not currently possible to be conclusive, these case studies support further investigation of this stem cell based treatment modality and bone engineering [87].
Shifting our focus to traumatic bone defects, non-unions and delayed fracture healing are ideal situations in which to harness the intrinsic regenerative potential of stromal and/or stem cells [88]. In a randomized controlled clinical study, which included 24 patients who were considered to be at low risk of non-unions of the tibia, the prophylactic effects of MSCs in expediting fracture healing were assessed[89]. Autologous MSCs isolated from the iliac crest and peripheral blood were injected into the fracture site together with platelet-rich plasma and allograft demineralized bone matrix. This treatment resulted in a significant reduction in the time to union, from 3.0 months to 1.5 months in the intervention group of patients who received the biological composite compared with the control group of patients who did not receive this treatment [89, 88]. Promising results have also been seen following the use of stem and progenitor cells in the treatment of osteonecrosis of the femoral head, a debilitating skeletal disorder that can lead to significant impairment of the activities of daily living and ultimately the need for total hip replacement [90-93].
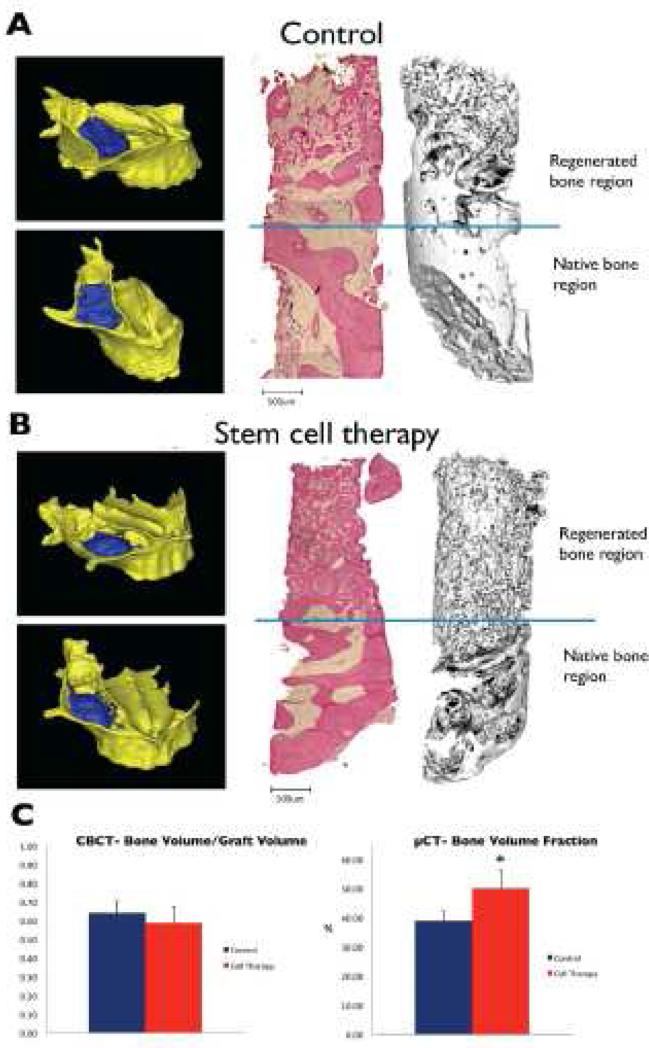
In addition, prospective isolation of subpopulations of stem / progenitor cells has begun to reach the translational arena. Tissue repair cells (TRC) isolated from the bone marrow, consisting of mixed stem and progenitor cell populations enriched in CD90- and CD14-positive cells, have recently been successfully implemented in a phase I/II randomized controlled feasibility trial to reconstruct localized craniofacial bone defects [94]. Here, twenty-four patients requiring localized osseous reconstruction were randomized to either guided bone regeneration (GBR) or TRC transplantation; and subsequently surveyed with clinical and radiographic assessments prior to dental reconstruction. The authors reported that TRC therapy accelerated alveolar bone regeneration compared to GBR therapy and that it also reduced the need for secondary bone grafting at the time of oral implant placement, supporting expanded studies of skeletal stem and progenitor cell therapy in the treatment of craniofacial deformities [94]. Building on these data, in a further phase I/II randomized controlled clinical trial, the authors further demonstrated that autologous cell transplantation with CD90-positive stem cells and CD14-positive monocytes led to superior bone regeneration in patients affected by maxillary sinus bone deficiency than control (Figure 6) [95].
Figure 6.
Representative images of 3-D reconstructions of occlusal and lateral open views into the maxillary sinus cavity of the skull show the bone volume which was grafted (blue) in the (A) control and (B) stem cell therapy groups in severe bone defects. Histological and corresponding micro-CT images of bone biopsies harvested from the grafted regions of the two groups show a greater degree of mineralized bone tissue in the stem cell therapy group. (C) CBCT analysis of the bone volume:graft volume ratio was no different between the control and stem cell therapy groups in treating severe defects; micro-CT analyses of the bone biopsies revealed that compared to the control, Bone Volume Fraction was significantly higher in the stem cell therapy group in treating severe defects. (Reproduced with permission: [95])
Stem cell-based products: moving to ease implementation of stem cell therapy
For ease of application, there are now several commercially available stem cell-based products, which have been utilized on a case-by-case basis in the treatment of osseous defects. For instance, Trinity evolution ® (Orthofix, Netherlands Antilles) is an implemented allograft of cancellous bone containing viable adult stem and osteoprogenitor cells used in orthopedic applications, with clinical trials currently ongoing in foot and ankle (NCT00988338), and spinal surgery (NCT00965380, NCT00951938) [96]. Furthermore, Osteocel (NuVasive, USA) ®, a similar allograft product, has shown promise upon implementation in anterior cervical discectomy and subsequent fusion [97] and transforaminal lumbar interbody fusion [98]. In order to truly affect clinical practice, allogeneic cell-based products must be readily available at an economic cost, which requires development and standardization of practice for the shipment and storage of cell-based therapeutics. Globally, much has been learnt following the development of blood donation and transfusion centers and these lessons will serve as a foundation for this budding industry. International standardization of stem cell isolation, culture and transportation also remains an obstacle to be overcome. Furthermore, as stem cells are often used in combination with morphogens and biomaterial scaffolds, this poses further difficulty in obtaining regulatory approval, such as that of the FDA in the United States [88].
Concluding Remarks
The highly orchestrated process of successful bone tissue engineering requires coordinated use of osteo-inductive morphogens and suitable innovative scaffolds, in addition to an optimal micro-environment that is specifically enriched with stem cells of osteogenic potential. Considerable progress has been made in understanding how stem and progenitor cells can accelerate bone tissue engineering. The developing knowledge concerning the intricacies of scaffold interaction with the local environment and the role of morphogens influencing osteogenic and chondrogenic differentiation have paralleled advancements in MSC biology. It is most important to remain aware of the fact that stem cells provide a valuable substrate for regeneration, but that success in clinical applications will require a holistic approach incorporating state-of-the-art scaffolds, which not only mimic the extracellular matrix of developing bone and provide a stem cell niche environment to transplanted cells, but that also can release multiple morphogens in a controlled and appropriate temporospatial sequence for successful bone regeneration. The field has advanced greatly in the past decade and one envisions that similar clinical success lies within reach.
Acknowledgements
The authors acknowledge the following ongoing support for this work - National Institute of Health grants: R01DE02183, R21DE02423001, R01DE019434 and U01HL099776 (to M.T.L.), the Oak Foundation, the Hagey Laboratory for Pediatric Regenerative Medicine, the A.C.S. Franklin Martin Faculty Research Fellowship (to D.C.W.), the Stanford University Child Health Research Institute Faculty Scholar Award (to D.C.W), the Plastic Surgery Foundation/Plastic Surgery Research Council Pilot Grant, the Stanford University Transplant and Tissue Engineering Center of Excellence Fellowship and the American Society of Maxillofacial Surgeons Research Grant (to R.T.), the Stanford Medical Scientist Training Program and NIGMS training grant GM07365 (to G.G.W), the California Institute for Regenerative Medicine Clinical Fellow training grant TG2-01159 and the American Society of Maxillofacial Surgeons/Maxillofacial Surgeons Foundation Research Grant Award (M.S.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. Journal of orthopaedic surgery and research. 2014;9(1):18. doi: 10.1186/1749-799X-9-18. doi:10.1186/1749-799x-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calori GM, Mazza E, Colombo M, Ripamonti C. The use of bone-graft substitutes in large bone defects: any specific needs? Injury. 2011;42(Suppl 2):S56–63. doi: 10.1016/j.injury.2011.06.011. doi:10.1016/j.injury.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Wada K, Yu W, Elazizi M, Barakat S, Ouimet MA, Rosario-Melendez R, et al. Locally delivered salicylic acid from a poly(anhydride-ester): impact on diabetic bone regeneration. Journal of controlled release : official journal of the Controlled Release Society. 2013;171(1):33–7. doi: 10.1016/j.jconrel.2013.06.024. doi:10.1016/j.jconrel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tevlin R, McArdle A, Atashroo D, Walmsley GG, Senarath-Yapa K, Zielins ER, et al. Biomaterials for Craniofacial Bone Engineering. Journal of dental research. 2014 doi: 10.1177/0022034514547271. doi:10.1177/0022034514547271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neovius E, Engstrand T. Craniofacial reconstruction with bone and biomaterials: review over the last 11 years. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2010;63(10):1615–23. doi: 10.1016/j.bjps.2009.06.003. doi:10.1016/j.bjps.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Baroli B. From natural bone grafts to tissue engineering therapeutics: Brainstorming on pharmaceutical formulative requirements and challenges. Journal of pharmaceutical sciences. 2009;98(4):1317–75. doi: 10.1002/jps.21528. doi:10.1002/jps.21528. [DOI] [PubMed] [Google Scholar]
- 7.Flierl MA, Smith WR, Mauffrey C, Irgit K, Williams AE, Ross E, et al. Outcomes and complication rates of different bone grafting modalities in long bone fracture nonunions: a retrospective cohort study in 182 patients. Journal of orthopaedic surgery and research. 2013;8:33. doi: 10.1186/1749-799X-8-33. doi:10.1186/1749-799x-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science (New York, NY) 2000;287(5457):1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 9.Slack JM. Stem cells in epithelial tissues. Science (New York, NY) 2000;287(5457):1431–3. doi: 10.1126/science.287.5457.1431. [DOI] [PubMed] [Google Scholar]
- 10.Daniela F, Vescovi AL, Bottai D. The stem cells as a potential treatment for neurodegeneration. Methods in molecular biology (Clifton, NJ) 2007;399:199–213. doi: 10.1007/978-1-59745-504-6_14. doi:10.1007/978-1-59745-504-6_14. [DOI] [PubMed] [Google Scholar]
- 11.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiological reviews. 2004;84(1):209–38. doi: 10.1152/physrev.00019.2003. doi:10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 12.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell. 2002;13(12):4279–95. doi: 10.1091/mbc.E02-02-0105. doi:10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spangrude GJ, Smith L, Uchida N, Ikuta K, Heimfeld S, Friedman J, et al. Mouse hematopoietic stem cells. Blood. 1991;78(6):1395–402. [PubMed] [Google Scholar]
- 14.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science (New York, NY) 1997;276(5309):71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 15.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science (New York, NY) 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 16.De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis and rheumatism. 2001;44(8):1928–42. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. doi:10.1002/1529-0131(200108)44:8<1928::aid-art331>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–55. doi: 10.1016/S0140-6736(04)16627-0. doi:10.1016/s0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 18.Sabatini F, Petecchia L, Tavian M, Jodon de Villeroche V, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Laboratory investigation; a journal of technical methods and pathology. 2005;85(8):962–71. doi: 10.1038/labinvest.3700300. doi:10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- 19.Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6(1):7–14. doi: 10.1080/14653240310004539. doi:10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell biochemistry and function. 2008;26(6):664–75. doi: 10.1002/cbf.1488. doi:10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- 21.Stockmann P, Park J, von Wilmowsky C, Nkenke E, Felszeghy E, Dehner JF, et al. Guided bone regeneration in pig calvarial bone defects using autologous mesenchymal stem/progenitor cells - a comparison of different tissue sources. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2012;40(4):310–20. doi: 10.1016/j.jcms.2011.05.004. doi:10.1016/j.jcms.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Ma D, Mao T. [Cell-based approaches to promote bone regeneration in distraction osteogenesis]. Zhongguo xiu fu chong jian wai ke za zhi = Zhongguo xiufu chongjian waike zazhi = Chinese journal of reparative and reconstructive surgery. 2012;26(12):1512–5. [PubMed] [Google Scholar]
- 23.Nomura I, Watanabe K, Matsubara H, Hayashi K, Sugimoto N, Tsuchiya H. Uncultured Autogenous Adipose-derived Regenerative Cells Promote Bone Formation During Distraction Osteogenesis in Rats. Clinical orthopaedics and related research. 2014;472(12):3798–806. doi: 10.1007/s11999-014-3608-8. doi:10.1007/s11999-014-3608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Z, Tee BC, Kennedy KS, Kennedy PM, Kim DG, Mallery SR, et al. Scaffold-based delivery of autologous mesenchymal stem cells for mandibular distraction osteogenesis: preliminary studies in a porcine model. PloS one. 2013;8(9):e74672. doi: 10.1371/journal.pone.0074672. doi:10.1371/journal.pone.0074672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sunay O, Can G, Cakir Z, Denek Z, Kozanoglu I, Erbil G, et al. Autologous rabbit adipose tissue-derived mesenchymal stromal cells for the treatment of bone injuries with distraction osteogenesis. Cytotherapy. 2013;15(6):690–702. doi: 10.1016/j.jcyt.2013.02.004. doi:10.1016/j.jcyt.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Ng MH, Duski S, Tan KK, Yusof MR, Low KC, Rose IM, et al. Repair of segmental load-bearing bone defect by autologous mesenchymal stem cells and plasma-derived fibrin impregnated ceramic block results in early recovery of limb function. BioMed research international. 2014;2014:345910. doi: 10.1155/2014/345910. doi:10.1155/2014/345910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang SH, Chung YG, Oh IH, Kim YS, Min KO, Chung JY. Bone regeneration potential of allogeneic or autogeneic mesenchymal stem cells loaded onto cancellous bone granules in a rabbit radial defect model. Cell and tissue research. 2014;355(1):81–8. doi: 10.1007/s00441-013-1738-z. doi:10.1007/s00441-013-1738-z. [DOI] [PubMed] [Google Scholar]
- 28.Berner A, Reichert JC, Woodruff MA, Saifzadeh S, Morris AJ, Epari DR, et al. Autologous vs. allogenic mesenchymal progenitor cells for the reconstruction of critical sized segmental tibial bone defects in aged sheep. Acta biomaterialia. 2013;9(8):7874–84. doi: 10.1016/j.actbio.2013.04.035. doi:10.1016/j.actbio.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Yang R, Shi S. Systemic Infusion of Mesenchymal Stem Cells Improves Cell-Based Bone Regeneration via Upregulation of Regulatory T Cells. Tissue engineering Part A. 2014 doi: 10.1089/ten.tea.2013.0673. doi:10.1089/ten.TEA.2013.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Im JY, Min WK, You C, Kim HO, Jin HK, Bae JS. Bone regeneration of mouse critical-sized calvarial defects with human mesenchymal stem cells in scaffold. Laboratory animal research. 2013;29(4):196–203. doi: 10.5625/lar.2013.29.4.196. doi:10.5625/lar.2013.29.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, et al. Craniofacial tissue engineering by stem cells. Journal of dental research. 2006;85(11):966–79. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yellowley C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. BoneKEy reports. 2013;2:300. doi: 10.1038/bonekey.2013.34. doi:10.1038/bonekey.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho CY, Sanghani A, Hua J, Coathup MJP, Kalia P, Blunn G. Mesenchymal stem cells with increased SDF-1 expression enhanced fracture healing. Tissue engineering Part A. 2014 doi: 10.1089/ten.tea.2013.0762. doi:10.1089/ten.TEA.2013.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park SY, Kim KH, Shin SY, Koo KT, Lee YM, Seol YJ. Dual delivery of rhPDGF-BB and bone marrow mesenchymal stromal cells expressing the BMP2 gene enhance bone formation in a critical-sized defect model. Tissue engineering Part A. 2013;19(21-22):2495–505. doi: 10.1089/ten.tea.2012.0648. doi:10.1089/ten.tea.2012.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tu Q, Valverde P, Li S, Zhang J, Yang P, Chen J. Osterix overexpression in mesenchymal stem cells stimulates healing of critical-sized defects in murine calvarial bone. Tissue engineering. 2007;13(10):2431–40. doi: 10.1089/ten.2006.0406. doi:10.1089/ten.2006.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang B, Huang S, Pan L, Jia S. Enhancement of bone formation by genetically engineered human umbilical cord-derived mesenchymal stem cells expressing osterix. Oral surgery, oral medicine, oral pathology and oral radiology. 2013;116(4):e221–9. doi: 10.1016/j.oooo.2011.12.024. doi:10.1016/j.oooo.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 37.Zou D, He J, Zhang K, Dai J, Zhang W, Wang S, et al. The bone-forming effects of HIF-1alpha-transduced BMSCs promote osseointegration with dental implant in canine mandible. PloS one. 2012;7(3):e32355. doi: 10.1371/journal.pone.0032355. doi:10.1371/journal.pone.0032355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou D, Zhang Z, He J, Zhang K, Ye D, Han W, et al. Blood vessel formation in the tissue-engineered bone with the constitutively active form of HIF-1alpha mediated BMSCs. Biomaterials. 2012;33(7):2097–108. doi: 10.1016/j.biomaterials.2011.11.053. doi:10.1016/j.biomaterials.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Li Y, Ma S, Gao Y, Zuo Y, Hu J. Enhancement of bone formation by BMP-7 transduced MSCs on biomimetic nano-hydroxyapatite/polyamide composite scaffolds in repair of mandibular defects. Journal of biomedical materials research Part A. 2010;95(4):973–81. doi: 10.1002/jbm.a.32926. doi:10.1002/jbm.a.32926. [DOI] [PubMed] [Google Scholar]
- 40.Vanleene M, Saldanha Z, Cloyd KL, Jell G, Bou-Gharios G, Bassett JH, et al. Transplantation of human fetal blood stem cells in the osteogenesis imperfecta mouse leads to improvement in multiscale tissue properties. Blood. 2011;117(3):1053–60. doi: 10.1182/blood-2010-05-287565. doi:10.1182/blood-2010-05-287565. [DOI] [PubMed] [Google Scholar]
- 41.Jones GN, Moschidou D, Lay K, Abdulrazzak H, Vanleene M, Shefelbine SJ, et al. Upregulating CXCR4 in human fetal mesenchymal stem cells enhances engraftment and bone mechanics in a mouse model of osteogenesis imperfecta. Stem cells translational medicine. 2012;1(1):70–8. doi: 10.5966/sctm.2011-0007. doi:10.5966/sctm.2011-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haller A. Experimentorum de ossiem formatione. Opera Minora. 1763;2(400):400. [Google Scholar]
- 43.Atesok K, Matsumoto T, Karlsson J, Asahara T, Atala A, Doral MN, et al. An emerging cell-based strategy in orthopaedics: endothelial progenitor cells. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2012;20(7):1366–77. doi: 10.1007/s00167-012-1940-7. doi:10.1007/s00167-012-1940-7. [DOI] [PubMed] [Google Scholar]
- 44.Dickson KF, Katzman S, Paiement G. The importance of the blood supply in the healing of tibial fractures. Contemporary orthopaedics. 1995;30(6):489–93. [PubMed] [Google Scholar]
- 45.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science (New York, NY) 1997;275(5302):964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 46.Laing AJ, Dillon JP, Condon ET, Street JT, Wang JH, McGuinness AJ, et al. Mobilization of endothelial precursor cells: systemic vascular response to musculoskeletal trauma. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25(1):44–50. doi: 10.1002/jor.20228. doi:10.1002/jor.20228. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto T, Mifune Y, Kawamoto A, Kuroda R, Shoji T, Iwasaki H, et al. Fracture induced mobilization and incorporation of bone marrow-derived endothelial progenitor cells for bone healing. Journal of cellular physiology. 2008;215(1):234–42. doi: 10.1002/jcp.21309. doi:10.1002/jcp.21309. [DOI] [PubMed] [Google Scholar]
- 48.Zigdon-Giladi H, Michaeli-Geller G, Bick T, Lewinson D, Machtei EE. Human Blood-Derived Endothelial Progenitor Cells Augment Vasculogenesis and Osteogenesis. Journal of clinical periodontology. 2014 doi: 10.1111/jcpe.12325. doi:10.1111/jcpe.12325. [DOI] [PubMed] [Google Scholar]
- 49.Li R, Atesok K, Nauth A, Wright D, Qamirani E, Whyne CM, et al. Endothelial progenitor cells for fracture healing: a microcomputed tomography and biomechanical analysis. Journal of orthopaedic trauma. 2011;25(8):467–71. doi: 10.1097/BOT.0b013e31821ad4ec. doi:10.1097/BOT.0b013e31821ad4ec. [DOI] [PubMed] [Google Scholar]
- 50.Fukui T, Mifune Y, Matsumoto T, Shoji T, Kawakami Y, Kawamoto A, et al. Superior Potential of CD34-Positive Cells Compared to Total Mononuclear Cells for Healing of Nonunion Following Bone Fracture. Cell transplantation. 2014 doi: 10.3727/096368914X681586. doi:10.3727/096368914x681586. [DOI] [PubMed] [Google Scholar]
- 51.Rao RR, Stegemann JP. Cell-based approaches to the engineering of vascularized bone tissue. Cytotherapy. 2013;15(11):1309–22. doi: 10.1016/j.jcyt.2013.06.005. doi:10.1016/j.jcyt.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toupadakis CA, Granick JL, Sagy M, Wong A, Ghassemi E, Chung DJ, et al. Mobilization of endogenous stem cell populations enhances fracture healing in a murine femoral fracture model. Cytotherapy. 2013;15(9):1136–47. doi: 10.1016/j.jcyt.2013.05.004. doi:10.1016/j.jcyt.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li R, Nauth A, Li C, Qamirani E, Atesok K, Schemitsch EH. Expression of VEGF gene isoforms in a rat segmental bone defect model treated with EPCs. Journal of orthopaedic trauma. 2012;26(12):689–92. doi: 10.1097/BOT.0b013e318266eb7e. doi:10.1097/BOT.0b013e318266eb7e. [DOI] [PubMed] [Google Scholar]
- 54.Kuroda R, Matsumoto T, Kawakami Y, Fukui T, Mifune Y, Kurosaka M. Clinical impact of circulating CD34-positive cells on bone regeneration and healing. Tissue engineering Part B, Reviews. 2014;20(3):190–9. doi: 10.1089/ten.teb.2013.0511. doi:10.1089/ten.TEB.2013.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosell A, Morancho A, Navarro-Sobrino M, Martinez-Saez E, Hernandez-Guillamon M, Lope-Piedrafita S, et al. Factors secreted by endothelial progenitor cells enhance neurorepair responses after cerebral ischemia in mice. PloS one. 2013;8(9):e73244. doi: 10.1371/journal.pone.0073244. doi:10.1371/journal.pone.0073244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuroda R, Matsumoto T, Miwa M, Kawamoto A, Mifune Y, Fukui T, et al. Local transplantation of G-CSF-mobilized CD34(+) cells in a patient with tibial nonunion: a case report. Cell transplantation. 2011;20(9):1491–6. doi: 10.3727/096368910X550189. doi:10.3727/096368910x550189. [DOI] [PubMed] [Google Scholar]
- 57.Kuroda R, Matsumoto T, Niikura T, Kawakami Y, Fukui T, Lee SY, et al. Local transplantation of granulocyte colony stimulating factor-mobilized CD34+ cells for patients with femoral and tibial nonunion: pilot clinical trial. Stem cells translational medicine. 2014;3(1):128–34. doi: 10.5966/sctm.2013-0106. doi:10.5966/sctm.2013-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. doi:10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. doi:10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 60.Kim JB, Sebastiano V, Wu G, Arauzo-Bravo MJ, Sasse P, Gentile L, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136(3):411–9. doi: 10.1016/j.cell.2009.01.023. doi:10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 61.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature biotechnology. 2008;26(1):101–6. doi: 10.1038/nbt1374. doi:10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 62.Ardeshirylajimi A, Dinarvand P, Seyedjafari E, Langroudi L, Adegani FJ, Soleimani M. Enhanced reconstruction of rat calvarial defects achieved by plasma-treated electrospun scaffolds and induced pluripotent stem cells. Cell and tissue research. 2013;354(3):849–60. doi: 10.1007/s00441-013-1693-8. doi:10.1007/s00441-013-1693-8. [DOI] [PubMed] [Google Scholar]
- 63.Hynes K, Menicanin D, Han J, Marino V, Mrozik K, Gronthos S, et al. Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. Journal of dental research. 2013;92(9):833–9. doi: 10.1177/0022034513498258. doi:10.1177/0022034513498258. [DOI] [PubMed] [Google Scholar]
- 64.Liu J, Chen W, Zhao Z, Xu HH. Effect of NELL1 gene overexpression in iPSC-MSCs seeded on calcium phosphate cement. Acta biomaterialia. 2014 doi: 10.1016/j.actbio.2014.08.016. doi:10.1016/j.actbio.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang H, Shih YR, Hwang Y, Wen C, Rao V, Seo T, et al. Mineralized gelatin methacrylate-based matrices induce osteogenic differentiation of human induced pluripotent stem cells. Acta biomaterialia. 2014 doi: 10.1016/j.actbio.2014.08.010. doi:10.1016/j.actbio.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121(9):1113–23. doi: 10.1161/CIRCULATIONAHA.109.898312. doi:10.1161/circulationaha.109.898312. [DOI] [PubMed] [Google Scholar]
- 67.Rachmiel A, Leiser Y. The Molecular and Cellular Events That Take Place during Craniofacial Distraction Osteogenesis. Plastic and reconstructive surgery Global open. 2014;2(1):e98. doi: 10.1097/GOX.0000000000000043. doi:10.1097/gox.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nature medicine. 2014 doi: 10.1038/nm.3668. doi:10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsumoto Y, Hayashi Y, Schlieve CR, Ikeya M, Kim H, Nguyen TD, et al. Induced pluripotent stem cells from patients with human fibrodysplasia ossificans progressiva show increased mineralization and cartilage formation. Orphanet journal of rare diseases. 2013;8:190. doi: 10.1186/1750-1172-8-190. doi:10.1186/1750-1172-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murray IR, Corselli M, Petrigliano FA, Soo C, Peault B. Recent insights into the identity of mesenchymal stem cells: Implications for orthopaedic applications. The bone & joint journal. 2014;96-b(3):291–8. doi: 10.1302/0301-620X.96B3.32789. doi:10.1302/0301-620x.96b3.32789. [DOI] [PubMed] [Google Scholar]
- 71.Levi B, Wan DC, Glotzbach JP, Hyun J, Januszyk M, Montoro D, et al. CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor beta1 (TGF-beta1) signaling. The Journal of biological chemistry. 2011;286(45):39497–509. doi: 10.1074/jbc.M111.256529. doi:10.1074/jbc.M111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chung MT, Liu C, Hyun JS, Lo DD, Montoro DT, Hasegawa M, et al. CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue engineering Part A. 2013;19(7-8):989–97. doi: 10.1089/ten.tea.2012.0370. doi:10.1089/ten.TEA.2012.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mahalatchimy A, Rial-Sebbag E, Tournay V, Faulkner A. The legal landscape for advanced therapies: material and institutional implementation of European Union rules in France and the United Kingdom. Journal of law and society. 2012;39(1):131–49. doi: 10.1111/j.1467-6478.2012.00574.x. [DOI] [PubMed] [Google Scholar]
- 74.Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC medicine. 2011;9:52. doi: 10.1186/1741-7015-9-52. doi:10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Syed-Picard FN, Shah GA, Costello BJ, Sfeir C. Regeneration of periosteum by human bone marrow stromal cell sheets. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2014;72(6):1078–83. doi: 10.1016/j.joms.2014.02.005. doi:10.1016/j.joms.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 76.Chen T, Wang Y, Bu L, Li N. Construction of functional tissue-engineered bone using cell sheet technology in a canine model. Experimental and therapeutic medicine. 2014;7(4):958–62. doi: 10.3892/etm.2014.1514. doi:10.3892/etm.2014.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ren L, Ma D, Liu B, Li J, Chen J, Yang D, et al. Preparation of three-dimensional vascularized MSC cell sheet constructs for tissue regeneration. BioMed research international. 2014;2014:301279. doi: 10.1155/2014/301279. doi:10.1155/2014/301279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walker MR, Patel KK, Stappenbeck TS. The stem cell niche. The Journal of pathology. 2009;217(2):169–80. doi: 10.1002/path.2474. doi:10.1002/path.2474. [DOI] [PubMed] [Google Scholar]
- 79.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116(6):769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 80.Moore KA, Lemischka IR. Stem cells and their niches. Science (New York, NY) 2006;311(5769):1880–5. doi: 10.1126/science.1110542. doi:10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 81.Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160(1-2):285–98. doi: 10.1016/j.cell.2014.12.002. doi:10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leucht P, Jiang J, Cheng D, Liu B, Dhamdhere G, Fang MY, et al. Wnt3a reestablishes osteogenic capacity to bone grafts from aged animals. The Journal of bone and joint surgery American volume. 2013;95(14):1278–88. doi: 10.2106/JBJS.L.01502. doi:10.2106/jbjs.l.01502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jing W, Smith AA, Liu B, Li J, Hunter DJ, Dhamdhere G, et al. Reengineering autologous bone grafts with the stem cell activator WNT3A. Biomaterials. 2015;47:29–40. doi: 10.1016/j.biomaterials.2014.12.014. doi:10.1016/j.biomaterials.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 84.Centers for Disease Control and Prevention C Prevalence and most common causes of disability among adults--United States, 2005. MMWR Morbidity and mortality weekly report. 2009;58(16):421–6. [PubMed] [Google Scholar]
- 85.Centeno CJ, Schultz JR, Cheever M, Freeman M, Faulkner S, Robinson B, et al. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Current stem cell research & therapy. 2011;6(4):368–78. doi: 10.2174/157488811797904371. [DOI] [PubMed] [Google Scholar]
- 86.Koh YG, Jo SB, Kwon OR, Suh DS, Lee SW, Park SH, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2013;29(4):748–55. doi: 10.1016/j.arthro.2012.11.017. doi:10.1016/j.arthro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 87.Gotherstrom C, Westgren M, Shaw SW, Astrom E, Biswas A, Byers PH, et al. Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: a two-center experience. Stem cells translational medicine. 2014;3(2):255–64. doi: 10.5966/sctm.2013-0090. doi:10.5966/sctm.2013-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grayson WL, Bunnell BA, Martin E, Frazier T, Hung BP, Gimble JM. Stromal cells and stem cells in clinical bone regeneration. Nature reviews Endocrinology. 2015;11(3):140–50. doi: 10.1038/nrendo.2014.234. doi:10.1038/nrendo.2014.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liebergall M, Schroeder J, Mosheiff R, Gazit Z, Yoram Z, Rasooly L, et al. Stem cell-based therapy for prevention of delayed fracture union: a randomized and prospective preliminary study. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21(8):1631–8. doi: 10.1038/mt.2013.109. doi:10.1038/mt.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year follow-up of a prospective controlled study. Bone. 2011;49(5):1005–9. doi: 10.1016/j.bone.2011.07.032. doi:10.1016/j.bone.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 91.Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. The Journal of bone and joint surgery American volume. 2004;86-a(6):1153–60. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 92.Mao Q, Jin H, Liao F, Xiao L, Chen D, Tong P. The efficacy of targeted intraarterial delivery of concentrated autologous bone marrow containing mononuclear cells in the treatment of osteonecrosis of the femoral head: a five year follow-up study. Bone. 2013;57(2):509–16. doi: 10.1016/j.bone.2013.08.022. doi:10.1016/j.bone.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325–30. doi: 10.1016/j.bone.2011.11.002. doi:10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 94.Kaigler D, Pagni G, Park CH, Braun TM, Holman LA, Yi E, et al. Stem cell therapy for craniofacial bone regeneration: a randomized, controlled feasibility trial. Cell transplantation. 2013;22(5):767–77. doi: 10.3727/096368912X652968. doi:10.3727/096368912x652968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaigler D, Avila-Ortiz G, Travan S, Taut AD, Padial-Molina M, Rudek I, et al. Bone engineering of maxillary sinus bone deficiencies using enriched CD90+ stem cell therapy: A randomized clinical trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015 doi: 10.1002/jbmr.2464. doi:10.1002/jbmr.2464. [DOI] [PubMed] [Google Scholar]
- 96.Rush SM. Trinity Evolution: mesenchymal stem cell allografting in foot and ankle surgery. Foot & ankle specialist. 2010;3(3):140–3. doi: 10.1177/1938640010369638. doi:10.1177/1938640010369638. [DOI] [PubMed] [Google Scholar]
- 97.Eastlack RK, Garfin SR, Brown CR, Meyer SC. Osteocel plus cellular allograft in anterior cervical discectomy and fusion: evaluation of clinical and radiographic outcomes from a prospective multicenter study. Spine. 2014;39(22):E1331–7. doi: 10.1097/BRS.0000000000000557. doi:10.1097/brs.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 98.Ammerman JM, Libricz J, Ammerman MD. The role of Osteocel Plus as a fusion substrate in minimally invasive instrumented transforaminal lumbar interbody fusion. Clinical neurology and neurosurgery. 2013;115(7):991–4. doi: 10.1016/j.clineuro.2012.10.013. doi:10.1016/j.clineuro.2012.10.013. [DOI] [PubMed] [Google Scholar]