Abstract
Personality traits contribute to variation in human behavior, including the propensity to take risk. Extant work targeted risk-taking processes with an explicit manipulation of reward, but it remains unclear whether personality traits influence simple decisions such as speeded versus delayed responses during cognitive control. We explored this issue in an fMRI study of the stop signal task, in which participants varied in response time trial by trial, speeding up and risking a stop error or slowing down to avoid errors. Regional brain activations to speeded versus delayed motor responses (risk-taking) were correlated to novelty seeking (NS), harm avoidance (HA) and reward dependence (RD), with age and gender as covariates, in a whole brain regression. At a corrected threshold, the results showed a positive correlation between NS and risk-taking responses in the dorsomedial prefrontal, bilateral orbitofrontal, and frontopolar cortex, and between HA and risk-taking responses in the parahippocampal gyrus and putamen. No regional activations varied with RD. These findings demonstrate that personality traits influence the neural processes of executive control beyond behavioral tasks that involve explicit monetary reward. The results also speak broadly to the importance of characterizing inter-subject variation in studies of cognition and brain functions.
Keywords: Anxiety, Impulsivity, TPQ, Reaction time, No-go
Introduction
There is a growing interest in individual variation in the neural bases of cognition, and personality represents an important factor contributing to such variations (Dambacher et al. 2014; Ibañez et al. 2013; Segalowitz et al.2012; Li et al. 2014; Gess et al. 2014). Personality traits are defined as habitual patterns of thoughts, emotions and behavioral tendencies that are considered to be relatively stable over time, to differ among individuals, and to influence behavior. One of the most influential trait theories is Cloninger’s tridimensional personality theory (Cloninger 1987, 1985), which describes three sets of behavioral manifestations. Novelty seeking (NS) characterizes a tendency to respond with intense excitement to novel stimuli, leading to pursuit of rewards. Harm avoidance (HA) characterizes a tendency to respond to previously established aversive stimuli and to passively avoid punishment. Reward dependence (RD) describes a tendency to respond to signals of reward and to maintain behavior previously associated with reward or with relief of punishment.
NS and HA are known to be associated with a number of neuropsychiatric disorders (Krueger et al. 2007; Lobo et al.2014; Meyer et al. 1999; Mitchell and Nelson-Gray 2006; Sher et al. 2005; Wills et al. 1994; Nordin and Nylander 2007). For instance, substance abuse was associated with a combination of high NS and low HA (Wills et al. 1994). Pathological gamblers scored higher on both NS and HA (Nordin and Nylander 2007). Apart from their clinical relevance, NS and HA each varies as a spectrum across healthy individuals (Ko et al. 2010; Lejuez et al. 2002; Martinotti et al. 2006; Aklin et al. 2005). Thus, for instance, individuals who are otherwise healthy may engage in risky behavior, deposing them toward negative consequences. It would therefore be useful to understand how NS and HA influence cognitive motor functions in a wide behavioral context.
Risk-taking is examined in the laboratory through a number of paradigms such as the Balloon Analog Risk Task (BART) (Lejuez et al. 2002; Fukunaga et al. 2012; Rao et al. 2008), Iowa Gambling Task (Fukui et al. 2005; Lawrence et al. 2009), and Cake Gambling Task (van Leijenhorst et al. 2006). BART measures risk-taking tendency, with risky decisions correlated with NS and self-reported risk-related behaviors, and inversely with HA (Lejuez et al. 2002, 2003a, b). Compared to a passive condition, decisions to take a risk in the BART engaged greater activity in the anterior cingulate cortex/medial frontal cortex, bilateral insulae and dorsolateral prefrontal cortices (Rao et al. 2008). In the Iowa Gambling Task, disadvantageous (risky) versus advantageous (safe) responses engaged greater activity in medial frontal gyrus, lateral orbitofrontal cortex and insula (Lawrence et al. 2009). Other studies have associated activation of the right insula (Paulus et al. 2003) and nucleus accumbens (Matthews et al. 2004) with HA in risk-taking decisions. Further, the influence of personality trait may be reflected in the mental set that dictates behavioral and cerebral responses to the stop signal task (Winkler et al. 2013). Together, these studies highlight the influence of personality traits on risk-taking behavior and its neural processes.
While extant work has largely focused on behavioral tasks with an explicit contingency of monetary compensation, it remains unclear whether the influence of personality traits extends to behavior that does not involve monetary reward. In a previous study, we showed that an anxiety personality trait, as assessed by Maudsley Obsessive Compulsive Inventory (MOCI), modulates cerebral activations during risk-taking decision in the stop signal task (SST) (Li et al. 2009). Successful performance in the SST requires prepotent, habitual behaviors to be inhibited. By dictating the participants to respond quickly and accurately, we also introduced a distinct element of risk in the SST. Thus, speeding up, as compared with slowing down, in response to a go stimulus, can be conceived as taking a risk that the stop signal would not appear (Li et al. 2009a, b; Yan and Li 2009). We observed that activity of the ventromedial prefrontal cortex during risk-taking is linearly modulated by MOCI score across subjects.
In this study, we sought to pursue this finding in a new and larger cohort of healthy participants assessed with Cloninger’s Tridimensional Personality Questionnaire (Cloninger 1987, 1985) with two specific goals; first, to investigate the influence of anxious personality beyond an obsessive compulsive trait; and second, to investigate the influence of novelty seeking, on risk-taking processes in the SST.
Methods
Participants and behavioral task
Sixty-one healthy adults (37 female; age 29.8 ± 10.0 years; all right-handed and using their right thumb to respond) were paid to participate in the study. All participants were free of major medical, neurological or psychiatric illnesses. None reported use of illicit substances. All participants signed a written informed consent, in accordance to a protocol approved by the Yale Human Investigation Committee.
We employed a simple reaction time task in this stop signal paradigm (Farr et al. 2012; Hendrick et al. 2010; Hu et al. 2014; Li et al. 2009a). There were two trial types, “go” and “stop”, randomly intermixed in presentation. A small dot appeared on the screen to engage attention at the beginning of a go trial. After a randomized time interval varying between 1 and 5 s (drawn from a uniform distribution), the dot turned into a circle, prompting participants to quickly press a button. The circle vanished at button press or after 1 s had elapsed, whichever came first, and the trial terminated. A premature button press prior to the appearance of the circle also terminated the trial. Approximately three quarters were go trials. The remaining one quarter were stop trials. In a stop trial, other than the fixation dot and go signal, an “X” (the stop signal) appeared after and replaced the go signal, instructing participants to withhold button press. Likewise, a trial terminated at button press or when 1 s had elapsed after the appearance of the stop signal. The stop signal delay (SSD) started at 200 ms and varied from one stop trial to the next according to a staircase procedure, increasing and decreasing by 67 ms each after a successful and failed stop (Levitt 1971). There was an inter-trial interval of 2 s. Participants were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up in a small number of trials, and both accuracy and response speed were emphasized (Li et al. 2008). Prior to the fMRI study, participants practiced on the same behavioral task outside the scanner. Each participant completed four 10-min runs of the task during fMRI. Depending on the actual stimulus timing (trials varied in fore-period duration) and speed of response, the total number of trials varied slightly across participants in an experiment. With the staircase procedure, we anticipated that the participants would succeed in withholding their response in approximately half of the stop trials.
On the basis of the race model (Logan et al. 1984), we computed for each participant the stop signal reaction time (SSRT), which represents the time one requires to stop the button press after the stop signal appears. We estimated the critical SSD, the delay that allows a participant to correctly inhibit response to a stop signal in half of the stop trials and computed the SSRT by subtracting the critical SSD from the median go trial reaction time (RT). Post-error slowing is computed as the RT difference between the go trials that followed an unsuccessful inhibition and those that followed another go trial (Li et al. 2008; Ide and Li 2011).
Tridimensional personality questionnaire
All participants were assessed with the Cloninger’s Tridimensional Personality Questionnaire—Short Form (TPQ-Short) (Sher et al. 1995). Derived from the 100-item long form of the TPQ (Cloninger 1987), the TPQ-Short demonstrated reliability and validity (Sher et al. 1995). It consists of 44 yes/no questions covering novelty seeking (NS; 13 items), harm avoidance (HA; 22 items) and reward dependence (RD; 9 items). Each personality subscale score was calculated by summing the item scores, reverse scoring where necessary. A higher subscore each represents a higher level of NS, HA and RD.
Imaging protocol
Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization using a 3T scanner (Siemens Trio) with a 12 channel head coil. Anatomical images of the functional slice locations were next obtained with spin echo imaging in the axial plane parallel to the AC-PC line with TR = 300 ms, TE = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field of view = 220 × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4 mm and no gap. Functional, blood oxygenation level dependent (BOLD) signals were then acquired with a single-shot gradient echo echo-planar imaging (EPI) sequence. Thirty-two axial slices parallel to the AC-PC line covering the whole brain were acquired with TR = 2000 ms, TE = 25 ms, bandwidth = 2004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, 32 slices with slice thickness = 4 mm and no gap. Slice scanning order was ascending interleaved. Three hundred images were acquired in each run for a total of four runs.
Data analysis and statistics
Data were analyzed with Statistical Parametric Mapping version 8 (SPM8, Wellcome Department of Imaging Neuroscience, University College London, UK). Images from the first five TRs at the beginning of each run were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Images of each individual participant were first corrected for slice timing and realigned (motion-corrected). A mean functional image volume was constructed for each participant for each run from the realigned image volumes. These mean images were normalized to an MNI (Montreal Neurological Institute) EPI template with affine registration followed by nonlinear transformation (Ashburner and Friston 1999; Friston et al. 1995). The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each participant. Finally, images were smoothed with a Gaussian kernel of 8 mm at full width at half maximum. The data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts.
Four main trial outcomes were distinguished: go success (G), go error (GE), stop success (SS), and stop error (SE) trial. G trials were divided into those that followed a G (pG), GE (pGE), SS (pSS), and SE (pSE) trial and pG trials were further divided into those that increased in RT (pGi) and those that did not increase in RT (pGni), as compared to the mean RT of all preceding pG trials. The pG trials that followed the pG/pSS/pSE trial were not included for comparison because these subsequent pG trials could not have a causal influence on the pG/pSS/pSE trial in question, in terms of how participants adjust their response speed. A single statistical analytical design was constructed for each individual participant, using the general linear model (GLM) with the onsets of go signal in each of these trial types convolved with a canonical hemodynamic response function (HRF) and with the temporal derivative of the canonical HRF and entered as regressors (Friston et al.1994). Realignment parameters in all 6 dimensions were also entered and serial auto-correlation was corrected by a first-degree autoregressive or AR(1) model (Della-Maggiore et al. 2002; Friston et al. 2000). The GLM estimated the component of variance that could be explained by each of the regressors.
In the first-level analysis, we contrasted pGni versus pGi for individual participants to identify the neural correlates of risk-taking (pGni > pGi). In the second-level analysis, the con or contrast (difference in β) images of the first-level analysis were used for random effects analysis. These images were correlated with the NS, HA and RD scores with age and gender as covariates in a simple regression across participants. Images were thresholded by using a voxelwise p < 0.005, combined with a cluster size of 29 contiguous voxels (783 mm3). This combined threshold was estimated with a Monte Carlo simulation using AlphaSim to give an overall threshold of p < 0.05, corrected for multiple comparisons for the entire brain (http://afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html). Functional regions of interest (ROIs) were defined based on activated clusters from whole brain analysis. All voxel activations were presented in MNI coordinates. We used MarsBaR (http://marsbar.sourceforge.net/) to derive the effect size (t statistic) of activity change for the ROIs for each individual participant.
Results
TPQ measures and stop signal task performance
Mean ± SD scores for NS, HA and RD were 4.2 ± 2.6, 6.6 ± 4.4, and 6.2 ± 2.4, respectively. NS, HA and RD did not show any significant pair-wise correlation across subjects (NS/HA, r = 0.169, p = 0.192; NS/RD, r = −0.063, p = 0.631; HA/RD, r = −0.074, p = 0.572). We compared men and women for each of the subscores and there was no significant gender difference in any subscore: NS, HA, and RD (p = 0.772, cohen’s d = 0.076; p = 0.068, cohen’s d = −0.487; p = 0.250, cohen’s d = −0.304, respectively).
In the stop signal task, the average go response rate was 98.1 ± 3.0 %, while the stop success rate was 52.3 ± 0.4 %. Average go trial reaction time (GoRT) and SSRT was 644 ± 100 ms and 206 ± 39 ms, respectively. Across participants, the RT of stop error trials (584 ± 116 ms) was significantly shorter than the mean RT of go success trials (644 ± 100 ms) (p < 0.001, paired t test). Furthermore, the RT and SSD of stop error trials were positively correlated (p < 0.01, r = 0.906, Pearson regression). Consistent with earlier work, we observed post-stop error slowing: RTs were significantly longer on pSE trials compared to pG trials (mean ± SD; 684 ± 98 versus 641 ± 104 ms; p < 0.0001, paired t test), suggesting that participants monitored error and adjusted behavior accordingly (Rabbit, 1966). By definition, RTs were significantly shorter on pGni trials compared to pGi trials (mean ± SD: 537 ± 97 versus 722 ± 102 ms; p < 0.0001, paired t test). Together, these findings suggest participants’ performance was well tracked by the staircase procedure and typical of what have been reported on the stop signal task.
We tested for a correlation between personality traits and GoRT and SSRT, using an alpha of 0.05/6 = 0.0083 to guard against Type I error. GoRT and SSRT were not significantly correlated with NS, HA or RD across subjects (for GoRT, r = −0.151, p = 0.246; r = −0.205, p = 0.114; r = 0.279, p = 0.030, respectively; for SSRT, r = 0.150, p = 0.248; r = −0.048, p = 0.715; r = 0.061, p = 0.641, respectively).
fMRI results
Neural substrates of speeded versus delayed motor response during the SST
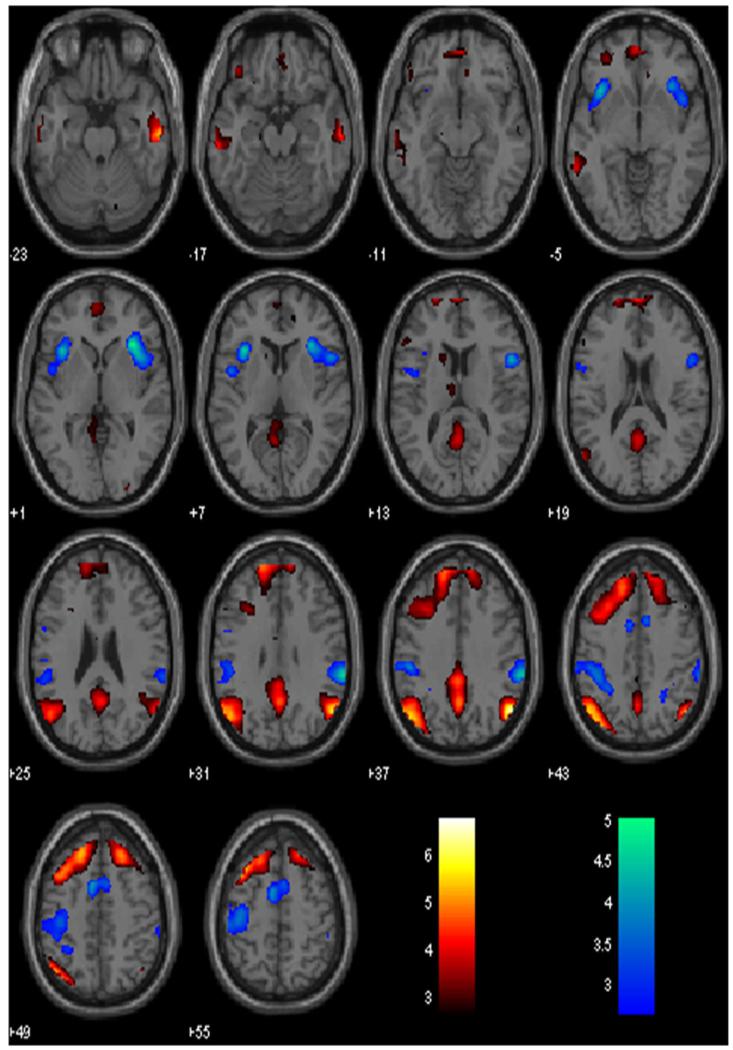
In a one sample t test across all participants for post-go go trials that did not increase in RT (pGni) as compared to post-go trials that increased in RT (pGi), we observed greater activity in bilateral angular gyri, bilateral middle and superior frontal gyri, posterior cingulate cortex and precuneus, middle temporal gyrus, and orbital frontal gyrus (Fig. 1; Table 1A). Conversely, compared with pGni, pGi engaged greater activity in right insula/inferior frontal gyrus, supramarginal gyrus, left insula, pre-supplementary motor area/supplementary motor area, and postcentral gyrus (Fig. 1; Table 1B). These findings replicated our previous work (Li et al. 2009a).
Fig. 1.
Neural correlates of speeded versus delayed motor responses in the stop signal task. Hot color shows increased activity during risk-taking responses (RT speeding > RT slowing) and winter color shows increased activity during risk-averting responses (RT slowing > RT speeding). BOLD contrasts were overlaid on a structural template in axial sections. Color bars indicate voxel T values
Table 1.
Regional activations during risk-taking (pGni > pGi) and risk-averting (pGi > pGni) responses in the SST
| Cluster size (voxels) | Voxel Z value | MNI coordinate (mm) |
Slide | Identified brain region | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| A. Risk-taking | ||||||
| 248 | 5.82 | 54 | −64 | 37 | R | Angular gyrus |
| 525 | 4.96 | −45 | −70 | 40 | L | Angular gyrus |
| 1674 | 4.95 | −33 | 20 | 55 | L | Middle frontal gyrus |
| 4.83 | −18 | 32 | 49 | L | Superior frontal gyrus | |
| 4.69 | 21 | 32 | 52 | R | Superior frontal gyrus | |
| 4.03 | 0 | 62 | 19 | R/L | Frontopolar cortex | |
| 3.95 | 3 | 53 | −11 | R | Ventromedial PFC | |
| 132 | 4.90 | 57 | −16 | −26 | R | Middle temporal gyrus |
| 614 | 4.45 | 0 | −58 | 37 | R/L | Precuneus |
| 4.32 | 0 | −40 | 37 | R/L | Post. cingulate cortex | |
| 166 | 3.99 | −63 | −25 | −17 | L | Inferior temporal gyrus |
| 3.78 | −66 | −43 | −5 | L | Middle temporal gyrus | |
| 3.66 | −63 | −13 | −20 | L | Inferior temporal gyrus | |
| 39 | 3.41 | −42 | 38 | −17 | L | Orbital frontal gyrus |
| B. Risk-averting | ||||||
| 360 | 4.57 | 33 | 26 | 1 | R | Insula gyrus/insula |
| 187 | 4.32 | 63 | −34 | 34 | R | Supramarginal gyrus |
| 268 | 4.21 | −33 | 20 | 7 | L | Insula |
| 3.41 | −48 | 2 | 7 | L | Insula | |
| 234 | 3.86 | −9 | 5 | 46 | L | Pre-SMA/SMA |
| 3.53 | 9 | 8 | 46 | R | Pre-SMA/SMA | |
| 569 | 3.70 | −48 | −28 | 37 | L | Supramarginal gyrus |
| 3.67 | −39 | −22 | 52 | L | Postcentral gyrus | |
All peak activations 8 mm apart are identified
PFC prefrontal cortex, SMA supplementary motor area
TPQ and risk-taking
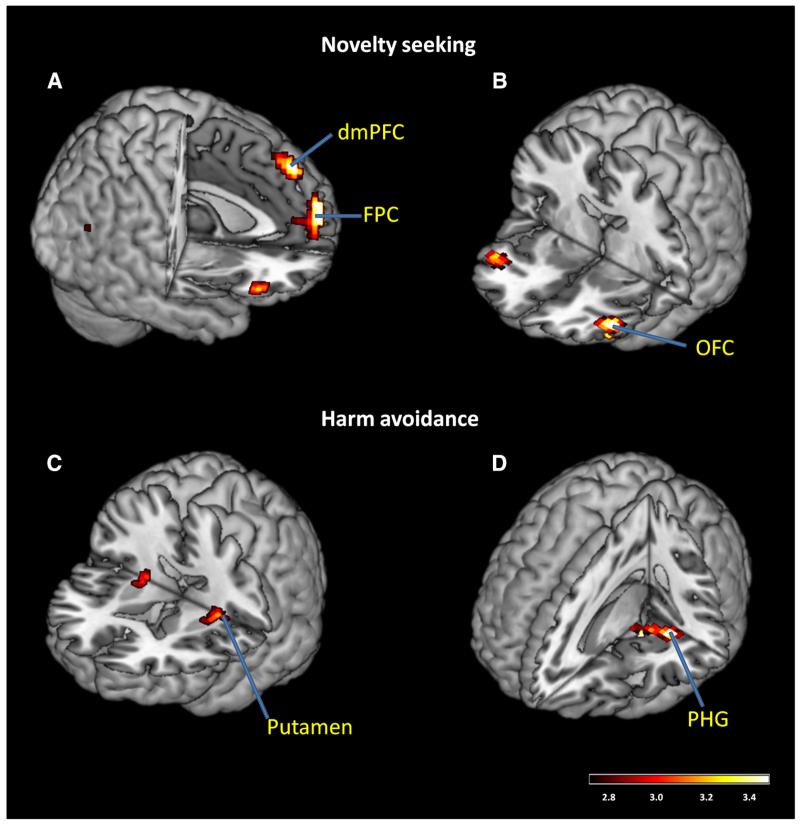
We carried out a whole brain multiple linear regression using NS, HA, RD, age, male and female as independent variables, and the contrast of pGni > pGi as the dependent variable. The results showed a significant positive correlation between NS and large clusters in the dorsomedial prefrontal cortex (dmPFC), bilateral orbitofrontal cortex (OFC), and frontopolar cortex (FPC; Fig. 2a, b; Table 2A). There were significant positive correlations between HA and putamen, and parahippocampal gyrus (PHG; Fig. 2c, d; Table 2B). There were no significant regional brain activations in association with RD.
Fig. 2.
Neural correlates of risk-taking responses that vary with novelty seeking (NS, a, b) and harm avoidance (HA, c, d) personality traits. Color bars shows voxel T values. dmPFC dorsomedial prefrontal cortex, FPC frontopolar cortex, OFC orbitofrontal cortex, PHG parahippocampal gyrus
Table 2.
Modulation of risk-taking activations by novelty seeking (NS) and harm avoidance (HA)
| Cluster size (voxels) | Voxel Z value | MNI coordinate (mm) |
Slide | Identified brain region | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| A. NS | ||||||
| 125 | 4.00 | −9 | 62 | 19 | L | Frontopolar cortex |
| 3.29 | 3 | 59 | 10 | R | Frontopolar cortex | |
| 64 | 3.58 | −42 | 35 | −14 | L | Orbitofrontal cortex |
| 2.73 | −45 | 41 | 1 | L | Orbitofrontal cortex | |
| 59 | 3.36 | −3 | 47 | 46 | L | Dorsomedial PFC |
| 31 | 3.11 | 48 | 32 | −11 | R | Orbitofrontal cortex |
| B. HA | ||||||
| 56 | 3.44 | −33 | −31 | −17 | L | Parahippocampal gyrus |
| 3.30 | −15 | −19 | −20 | L | ||
| 40 | 3.05 | −27 | −13 | 7 | L | Putamen |
All peak activations 8 mm apart are identified
PFC prefrontal cortex
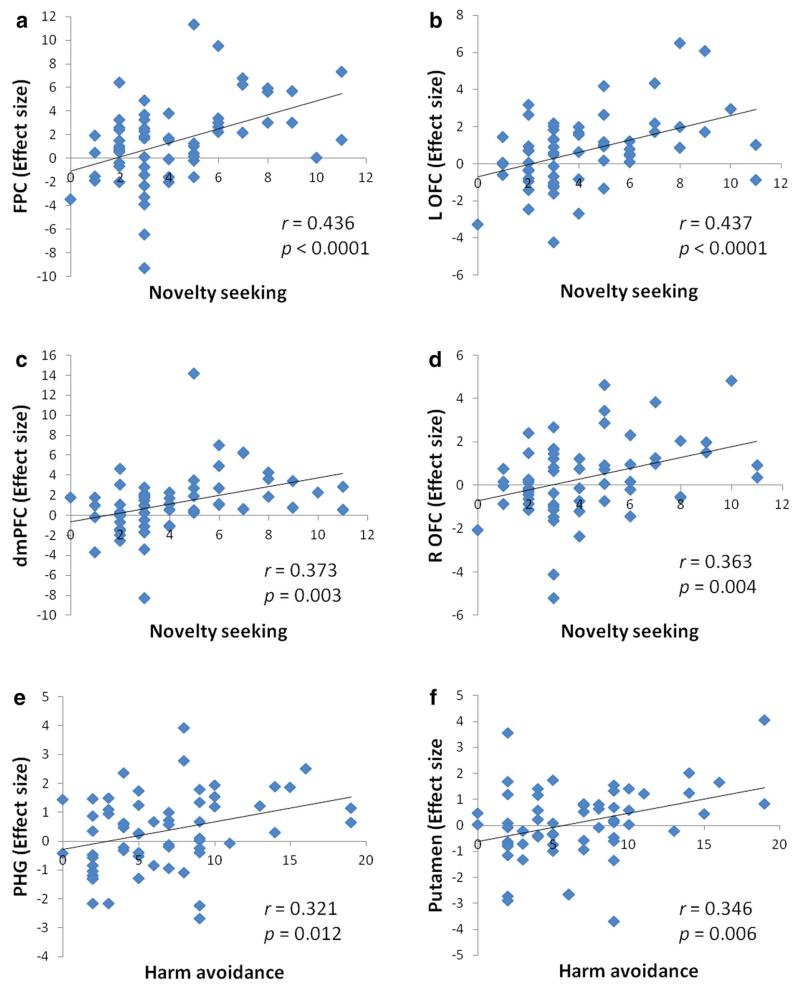
We derived the effect sizes for all of these activity clusters (see Methods). Four linear regression analyses were conducted, with NS as the independent variable and the effect size of FPC, left OFC, dmPFC and right OFC as the dependent variable, respectively. NS accounted for 19.0 % (r = 0.436, p < 0.0001, Fig. 3a), 19.1 % (r = 0.437, p < 0.0001, Fig. 3b), 13.9 % (r = 0.373, p = 0.003, Fig. 3c), and 13.2 % (r = 0.363, p = 0.004; Fig. 3d) of the variance each for the effect size of the FPC, left OFC, dmPFC and right OFC. Two linear regression analyses were conducted, with HA as the independent variable and the effect size of PHG and putamen as the dependent variable, respectively. HA accounted for 10.3 % (r = 0.321, p = 0.012; Fig. 3e) and 12.0 % (r = 0.346, p = 0.006; Fig. 3f) of the variance each, for the effect size of the PHG and putamen. There appeared to be 3 outliers (more than two standard deviations away from the HA mean). After these three subjects were removed, the correlation between HA and putamen activity was no longer significant (r = 0.187, p = 0.159). In addition, because of the skewed distribution, we performed a non-parametric Spearman regression and showed that the correlation between HA and putamen activity was significant (rho = 0.330, p = 0.009). Note that these correlations did not provide any new information in addition to the whole brain analyses but simply served to help readers visualize and better understand the inter-subject variability.
Fig. 3.
Correlations between novelty seeking and the effect size of FPC (a), left OFC (b), dmPFC (c), right OFC (d); between harm avoidance and the effect size of PHG (e), putamen (f). FPC frontopolar cortex, OFC orbitofrontal cortex, dmPFC dorsomedial prefrontal cortex, PHG parahippocampal gyrus
To examine gender differences, we compared the regression slopes between men and women for each correlation. For all clusters, the slopes of linear regressions did not differ between men and women (FPC, p = 0.484; left OFC, p = 0.834; dmPFC, p = 0.838; right OFC, p = 0.924; PHG, p = 0.670; putamen, p = 0.223).
We also examined whether the reverse contrast pGi > pGni showed any regional activities in correlation with the personality traits. The results showed no regional activities in correlation with NS, HA, or RD at the same threshold.
Discussion
“Risk-taking” during the SST and novelty seeking
Activation of the dorsomedial prefrontal cortex (dmPFC; x = −3, y = 47, z = 46) during post-go speeding as compared to slowing, in the area of anterior pre-supplementary motor area (pre-SMA) and frontopolar cortex (FPC; x = −9, y = 62, z = 19) correlated positively with novelty seeking trait, as assessed by the TPQ. Both regions have been implicated in risk-taking. In the Iowa Gambling Task, FPC (x = −2, y = 57, z = 21) responds to risky as compared to safe decisions (Fukui et al. 2005). Using a computerized gambling task, Xue et al. (2009) reported enhanced dmPFC (x = 4, y = 48, z = 26) activity when participants were making risky as compared to safe choices.
Risk-taking refers to the willingness to accept a possible negative consequence to potentially achieve a desirable outcome (Juhasz et al. 2009). Thus, dmPFC and FPC activation during a speeded response in the SST supports a risk-taking process in cognitive motor decisions—to speed up while incurring the risk of stop failure. Forstmann et al. (2008) manipulated the speed-accuracy trade-off in a reaction time decision task. Speed emphasis led to activation in the posterior pre-SMA (x = 4, y = 5, z = 45). Importantly, activity of the posterior pre-SMA was negatively associated with individual variation in response caution, a psychological construct in contrast to novelty seeking and risk-taking. Together, these findings suggest a role of the dmPFC in modulating action readiness to maintain a balance between fast and accurate decisions (Kanai and Rees 2011), in accord with its cortical and subcortical connectivity (Zhang et al. 2012).
The novelty seeking trait is also associated with greater activation of bilateral (albeit greater on the left) lateral orbitofrontal cortex (lOFC) during risk-taking decisions in the SST. The lOFC processes stimuli or behavioral outcomes of negative incentive/affective values (Kringelbach and Rolls 2004; O’Doherty et al. 2001; Gottfried et al. 2002; Mohanty et al. 2008; O’Doherty 2007; Munar et al. 2012). It appears that novelty or sensation seekers may experience risk-taking as a motivationally negative event, suggesting that risk-taking is not intrinsically rewarding, even for individuals who tend to seek risk. On the other hand, neurophysiological studies failed to confirm a distinction in medial and lateral OFC in processing positive and negative reward (Rich and Wallis 2014). Functional connectivity of the medial and lateral OFC also did not appear to support such differences (Zald et al. 2014). An earlier imaging study suggested that the lOFC may process implicit motivational value, and lOFC activation diminishes along with decreasing stimulus valuation or saliency (Rothkirch et al. 2012). Thus, an alternative explanation for the current finding is that greater lOFC activation reflects sustained saliency of risk-taking decisions in novelty seekers.
“Risk-taking” during the SST and harm avoidance
Harm avoidance, as assessed by the TPQ, is associated with increased putamen activation during risk-taking in the SST. Adolescents with high behavioral inhibition demonstrated stronger putamen activation during reward anticipation in a monetary incentive delay task, as compared to those characterized as non-inhibited (Guyer et al. 2006). In a juice delivery task, unexpected as compared with expected juice reward, elicited stronger putamen activity in monkeys (McClure et al. 2003). According to the Expectancy Violation theory (Jussim et al. 1987; Weber and Mayer 2008), if a positive violation of a negative expectation occurs, individual’s initial negative reaction is replaced by a more positive reaction than if a positive expectation had been confirmed. Furthermore, harm avoidance has been described as a heritable tendency to learn to avoid punishment or errors (Cloninger 1987). One is tempted to speculate that, in the SST, people higher in harm avoidance may be more likely to anticipate the stop signal. When the stop signal does not appear, the violation of a negative expectation occurs and the psychological consequences of such a violation are greater during a speeded response. As a result, the affective response derived from the violation of a negative expectation may increase in individuals with greater behavioral inhibition or harm avoidance.
More broadly, an unexpected reward is highly salient. An emerging perspective is that the striatum, including the caudate, putamen, and nucleus accumbens, responds to saliency (Clauss et al. 2014; Zink et al. 2003). In the study of Clauss et al. (2014), inhibited individuals showed greater caudate activation when viewing both novel and recently familiarized faces, suggesting sustained responses to familiar stimuli as though they were still novel (Blackford et al. 2011). Thus, increased putamen activation associated with HA may reflect that adults with higher HA have increased and sustained sensitivity to salient stimuli during risk-taking in the SST. Other studies have implicated structural variation of the putamen in association with HA (Laricchiuta et al. 2014), although its relevance to the current findings needs to be established.
Harm avoidance is also associated with greater activation of the parahippocampal gyrus (PHG) during risk-taking as compared to risk-averting decisions. The PHG is known for its role in processing contextual associations (Aminoff et al. 2013) and forming episodic memory (Eichenbaum et al. 2012). Other studies have also implicated the PHG in cognitive processes, including those involved in decision making to take or avoid a risk. In an inter-temporal choice task where the outcome was revealed immediately or after a delay, the PHG showed greater activation during the delayed as compared to immediate condition and the extent of its activation was modulated by reward uncertainty (Luhmann et al. 2008). The latter finding suggests that greater activation of the PHG may be related to hesitation and/or cognitive assessment of uncertainty in individuals with harm avoidance trait. In particular, a large body of literature supports the anterior/ventral PHG in anxiety-related behavior, including aversive associative learning (Bannerman et al. 2004; Moser and Moser 1998). Glucose metabolism in the anterior PHG predicts anxious temperament and is a heritable trait in non-human primates (Oler et al. 2010). Other recent studies have reported a positive correlation between PHG gray matter volume and anxiety traits or disorders (Wei et al. 2014; Talati et al. 2013; Yang et al. 2013), reduced functional connectivity between the anterior cingulate cortex and left PHG during threat disengagement in anxious youth (Price et al. 2014), altered activity and connectivity of the PHG in anxiety disorders (Arnold Anteraper et al. 2014; Lemche et al. 2013; Schlumpf et al. 2013), all of which speaks broadly to a link between PHG and anxiety trait. The current observation of greater PHG activation during risk-taking in harm avoidant individuals supports this association.
A recent study (Liang et al. 2014) applied transcranial direct current stimulation (tDCS) with anodal electrode over pre-SMA and reported that tDCS increased the EEG complexity of the frontal lobe during the SST. In addition, low-performing participants benefitted more from this facilitating effect than high-performing participants. These results reflect people’s natural ability to adapt to the environmental change. The neural associations between “risk-taking” during the SST and TPQ may reflect adaptation to environmental change. Future studies may investigate the differential effects of brain stimulation of the target regions, such as the pre-SMA, on risk-taking behavior in individuals with high and low novelty seeking.
Risk-taking between reward-related versus cognitive motor decisions
Numerous studies have reported that the insula plays an important role in risk-taking decisions that involve monetary reward (Galvan and Peris 2014; Helfinstein et al. 2014; Lawrence et al. 2009; Paulus et al. 2003; Rao et al. 2008). However, the insula did not respond to post-go speeding as compared to slowing in the SST. A possible explanation is that these behavioral tasks engage different types of reward and motivational processes. For instance, in an incentive delay task offering either money or social approval, social stimuli were mainly associated with amygdala activation, while the thalamus was more strongly activated by the presentation of monetary reward (Rademacher et al. 2010). Sescousse et al. (2013) performed an activation likelihood estimation meta-analysis of 87 studies comparing the brain responses to monetary, erotic, and food reward outcomes. They reported that, money-specific responses were observed in the anterior portion of the OFC, while food and erotic reward were more strongly represented in the anterior insula. These results suggest distinct cerebral responses to different types of reward.
On the other hand, the insula is known to play a role in a vast number of cognitive and affective processes (Nelson et al. 2010). Besides reward processing, the insula responds to error (Ullsperger et al. 2010) and prediction error (Bossaerts 2010), represents internal bodily states including arousal (Singer et al. 2009) and engages in intuitive decision making possibly based on perceptually salient information (Kuo et al. 2009; Hu and Yu 2014). The lack of insula activation presumably could reflect the absence of these processes during risk-taking in the SST (Li et al.2009a).
Limitations of the study and conclusions
There are several limitations that need to be considered. First, while the current study demonstrated that the neural processes of risk-taking during a cognitive motor decision appear to be modulated by personality traits differently from those involved in a behavioral task with explicit reward contingencies, a direct comparison within the same group of individuals is required to confirm these findings. Second, there are many other instruments that may capture personality traits in dimensions not covered by the TPQ. For instance, future work may consider the revised NEO Personality Inventory (NEO-PI-R, Costa and MacCrae 1992) and UPPS (Whiteside and Lynam 2003) in addition to TPQ and employ principal component analyses to identify inter-subject variability in risk-taking and harm avoidance traits. Third, we did not conduct a power calculation and thus many of the negative findings (e.g., lack of gender difference in harm avoidance) should be confirmed in future work. Finally, personality is known to have a robust genetic basis (e.g., Wang et al. 2014). Future work incorporating genotyping will help evaluate whether neural phenotypes as revealed by fMRI are related to inter-subject variation in genetic predispositions.
In conclusion, we reported how novelty seeking and harm avoidance personality traits influence the neural processes of risk-taking in the stop signal task. Individuals who are novelty seekers demonstrate greater dorsomedial and lateral orbitofrontal activation, and those who are more harm avoidant demonstrate greater parahippocampal and putamen activation during cognitive motor decisions that increased error risk. These findings add to our understanding of the neural basis of inter-subject variation in behavior and cognition.
Acknowledgments
This study was supported by NIH grants K02DA026990, R01DA023248, R01AA021449. Jianping Hu was supported by the Scientific Research Foundation of Graduate School of South China Normal University (Grant 2013kyjj025).
References
- Aklin WM, Lejuez C, Zvolensky MJ, Kahler CW, Gwadz M. Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behav Res Ther. 2005;43(2):215–228. doi: 10.1016/j.brat.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Aminoff EM, Kveraga K, Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn Sci. 2013;17(8):379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold Anteraper S, Triantafyllou C, Sawyer AT, Hofmann SG, Gabrieli JD, Whitfield-Gabrieli S. Hyper-connectivity of subcortical resting-state networks in social anxiety disorder. Brain Connect. 2014;4(2):81–90. doi: 10.1089/brain.2013.0180. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7(4):254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman D, Rawlins J, McHugh S, Deacon R, Yee B, Bast T, Zhang W-N, Pothuizen H, Feldon J. Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev. 2004;28(3):273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Cowan RL, Shelton RC, Zald DH. Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Soc Cogn Affect Neurosci. 2011;6(5):621–629. doi: 10.1093/scan/nsq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaerts P. Risk and risk prediction error signals in anterior insula. Brain Struct Funct. 2010;214(5-6):645–653. doi: 10.1007/s00429-010-0253-1. [DOI] [PubMed] [Google Scholar]
- Clauss JA, Seay AL, VanDerKlok RM, Avery SN, Cao A, Cowan RL, Benningfield MM, Blackford JU. Structural and functional bases of inhibited temperament. Soc Cogn Affect Neurosci. 2014 doi: 10.1093/scan/nsu019. doi:10.1093/scan/nsu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiat Develop. 1985;4(3):167–226. [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants: a proposal. Arch Gen Psychiatry. 1987;44(6):573. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Costa PT, MacCrae RR. Revised NEO personality inventory (NEO PI-R) and NEO five-factor inventory (NEO FFI): professional manual. Psychological Assessment Resources; Odessa: 1992. [Google Scholar]
- C-sR Li, Luo X, Yan P, Bergquist K, Sinha R. Altered impulse control in alcohol dependence: neural measures of stop signal performance. Alcohol Clin Exp Res. 2009;33(4):740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambacher F, Sack AT, Lobbestael J, Arntz A, Brugman S, Schuhmann T. Out of control evidence for anterior insula involvement in motor impulsivity and reactive aggression. Soc Cogn Affect Neurosci. 2014 doi: 10.1093/scan/nsu077. doi:10.1093/scan/nsu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, Chau W, Peres-Neto PR, McIntosh AR. An empirical comparison of SPM preprocessing parameters to the analysis of fMRI data. Neuroimage. 2002;17(1):19–28. doi: 10.1006/nimg.2002.1113. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36(7):1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr OM, Hu S, Zhang S, Chiang-shan RL. Decreased saliency processing as a neural measure of Barratt impulsivity in healthy adults. Neuroimage. 2012;63(3):1070–1077. doi: 10.1016/j.neuroimage.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Dutilh G, Brown S, Neumann J, Von Cramon DY, Ridderinkhof KR, Wagenmakers E-J. Striatum and pre-SMA facilitate decision-making under time pressure. Proc Natl Acad Sci. 2008;105(45):17538–17542. doi: 10.1073/pnas.0805903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2(4):189–210. [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RS. Spatial registration and normalization of images. Hum Brain Mapp. 1995;3(3):165–189. [Google Scholar]
- Friston KJ, Josephs O, Zarahn E, Holmes A, Rouquette S, Poline J-B. To smooth or not to smooth?: bias and efficiency in fMRI time-series analysis. Neuroimage. 2000;12(2):196–208. doi: 10.1006/nimg.2000.0609. [DOI] [PubMed] [Google Scholar]
- Fukui H, Murai T, Fukuyama H, Hayashi T, Hanakawa T. Functional activity related to risk anticipation during performance of the Iowa Gambling Task. Neuroimage. 2005;24(1):253–259. doi: 10.1016/j.neuroimage.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Fukunaga R, Brown JW, Bogg T. Decision making in the Balloon Analogue Risk Task (BART): anterior cingulate cortex signals loss aversion but not the infrequency of risky choices. Cogn Affect Behav Neurosci. 2012;12(3):479–490. doi: 10.3758/s13415-012-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Peris TS. Neural correlates of risky decision making in anxious youth and healthy controls. Depress Anxiety. 2014;31(7):591–598. doi: 10.1002/da.22276. doi:10.1002/da.22276. [DOI] [PubMed] [Google Scholar]
- Gess JL, Fausett JS, Kearney-Ramos TE, Kilts CD, James GA. Task-dependent recruitment of intrinsic brain networks reflects normative variance in cognition. Brain Behav. 2014;4(5):650–664. doi: 10.1002/brb3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci. 2002;22(24):10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Bjork JM, Henderson HA, Pine DS, Fox NA. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci. 2006;26(24):6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein SM, Schonberg T, Congdon E, Karlsgodt KH, Mumford JA, Sabb FW, Cannon TD, London ED, Bilder RM, Poldrack RA. Predicting risky choices from brain activity patterns. Proc Natl Acad Sci. 2014;111(7):2470–2475. doi: 10.1073/pnas.1321728111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick OM, Ide JS, Luo X, Chiang-shan RL. Dissociable processes of cognitive control during error and non-error conflicts: a study of the stop signal task. PLoS One. 2010;5(10):e13155. doi: 10.1371/journal.pone.0013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Yu R. The neural correlates of the decoy effect in decisions. Front Behav Neurosci. 2014;8:271. doi: 10.3389/fnbeh.2014.00271. doi:10.3389/fnbeh.2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Tseng YC, Winkler AD, Li CSR. Neural bases of individual variation in decision time. Hum Brain Mapp. 2014;35(6):2531–2542. doi: 10.1002/hbm.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez A, Aguado J, Baez S, Huepe D, Lopez V, Ortega R, Sigman M, Mikulan E, Lischinsky A, Torrente F. From neural signatures of emotional modulation to social cognition: individual differences in healthy volunteers and psychiatric participants. Soc Cogn Affect Neurosci. 2013 doi: 10.1093/scan/nst067. doi:10.1093/scan/nst067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, C-sR Li. A cerebellar thalamic cortical circuit for error-related cognitive control. Neuroimage. 2011;54(1):455–464. doi: 10.1016/j.neuroimage.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz G, Downey D, Hinvest N, Thomas E, Chase D, Toth ZG, Lloyd-Williams K, Mekli K, Platt H, Payton A. Risk-taking behavior in a gambling task associated with variations in the tryptophan hydroxylase 2 gene: relevance to psychiatric disorders. Neuropsychopharmacology. 2009;35(5):1109–1119. doi: 10.1038/npp.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jussim L, Coleman LM, Lerch L. The nature of stereotypes: a comparison and integration of three theories. J Pers Soc Psychol. 1987;52(3):536. [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci. 2011;12(4):231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Ko C-H, Hsiao S, Liu G-C, Yen J-Y, Yang M-J, Yen C-F. The characteristics of decision making, potential to take risks, and personality of college students with internet addiction. Psychiatry Res. 2010;175(1):121–125. doi: 10.1016/j.psychres.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychol. 2007;116(4):645. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo WJ, Sjöström T, Chen YP, Wang YH, Huang CY. Intuition and deliberation: two systems for strategizing in the brain. Science. 2009;324(5926):519–522. doi: 10.1126/science.1165598. [DOI] [PubMed] [Google Scholar]
- Laricchiuta D, Petrosini L, Piras F, Cutuli D, Macci E, Picerni E, Chiapponi C, Caltagirone C, Spalletta G. Linking novelty seeking and harm avoidance personality traits to basal ganglia: volumetry and mean diffusivity. Brain Struct Funct. 2014;219(3):793–803. doi: 10.1007/s00429-013-0535-5. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Jollant F, O’Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions in the Iowa Gambling Task. Cereb Cortex. 2009;19(5):1134–1143. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- Lejuez C, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8(2):75. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lejuez C, Aklin WM, Jones HA, Richards JB, Strong DR, Kahler CW, Read JP. The balloon analogue risk task (BART) differentiates smokers and nonsmokers. Exp Clin Psychopharmacol. 2003a;11(1):26. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- Lejuez C, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J Adol. 2003b;26(4):475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lemche E, Surguladze SA, Brammer MJ, Phillips ML, Sierra M, David AS, Williams SC, Giampietro VP. Dissociable brain correlates for depression, anxiety, dissociation, and somatization in depersonalization-derealization disorder. CNS Spect. 2013;23:1–8. doi: 10.1017/S1092852913000588. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acous Soc Am. 1971;49(2B):467–477. [PubMed] [Google Scholar]
- Li C-sR, Huang C, Yan P, Paliwal P, Constable RT, Sinha R. Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. J Cogn Neurosci. 2008;20(6):1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-sR, Chao HH-A, Lee T-W. Neural correlates of speeded as compared with delayed responses in a stop signal task: an indirect analog of risk taking and association with an anxiety trait. Cereb Cortex. 2009;19(4):839–848. doi: 10.1093/cercor/bhn132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li X, Huang L, Kong X, Yang W, Wei D, Li J, Cheng H, Zhang Q, Qiu J. Brain structure links trait creativity to openness to experience. Soc Cogn Affect Neurosci. 2014 doi: 10.1093/scan/nsu041. doi:10.1093/scan/nsu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W-K, Lo M-T, Yang AC, Peng C-K, Cheng S-K, Tseng P, Juan C-H. Revealing the brain’s adaptability and the transcranial direct current stimulation facilitating effect in inhibitory control by multiscale entropy. Neuroimage. 2014;90:218–234. doi: 10.1016/j.neuroimage.2013.12.048. [DOI] [PubMed] [Google Scholar]
- Lobo D, Quilty L, Martins S, Tavares H, Vallada H, Kennedy J, Bagby R. Pathological gambling subtypes: a comparison of treatment-seeking and non-treatment-seeking samples from Brazil and Canada. Addict Behav. 2014;39(7):1172–1175. doi: 10.1016/j.addbeh.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10(2):276. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Luhmann CC, Chun MM, Yi D-J, Lee D, Wang X-J. Neural dissociation of delay and uncertainty in intertemporal choice. J Neurosc. 2008;28(53):14459–14466. doi: 10.1523/JNEUROSCI.5058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti G, Andreoli S, Giametta E, Poli V, Bria P, Janiri L. The dimensional assessment of personality in pathologic and social gamblers: the role of novelty seeking and self-transcendence. Compr Psychiatry. 2006;47(5):350–356. doi: 10.1016/j.comppsych.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Simmons AN, Lane SD, Paulus MP. Selective activation of the nucleus accumbens during risk-taking decision making. NeuroReport. 2004;15(13):2123–2127. doi: 10.1097/00001756-200409150-00025. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38(2):339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Carver CS. Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. J Psychopathol Behav Assess. 1999;21(4):275–292. doi: 10.1023/A:1022119414440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JT, Nelson-Gray RO. Attention-deficit/hyperactivity disorder symptoms in adults: relationship to Gray’s behavioral approach system. Personality Individ Differ. 2006;40(4):749–760. [Google Scholar]
- Mohanty A, Gitelman DR, Small DM, Mesulam MM. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb Cortex. 2008;18(11):2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Munar E, Nadal M, Rosselló J, Flexas A, Moratti S, Maestú F, Marty G, Cela-Conde CJ. Lateral orbitofrontal cortex involvement in initial negative aesthetic impression formation. PLoS One. 2012;7(6):e38152. doi: 10.1371/journal.pone.0038152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214(5-6):669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin C, Nylander P-O. Temperament and character in pathological gambling. J Gambl Stud. 2007;23(2):113–120. doi: 10.1007/s10899-006-9049-x. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Ann N Y Acad Sci. 2007;1121(1):254–272. doi: 10.1196/annals.1401.036. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, Kalin NH. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466(7308):864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19(4):1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Price RB, Siegle GJ, Silk JS, Ladouceur CD, McFarland A, Dahl RE, Ryan ND. Looking under the hood of the dot-probe task: an fMRI study in anxious youth. Depress Anxiety. 2014;31(3):178–187. doi: 10.1002/da.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49(4):3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI Study of the Balloon Analog Risk Task (BART) Neuroimage. 2008;42(2):902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich E, Wallis J. Medial-lateral organization of the orbitofrontal cortex. J Cogn Neurosci. 2014;26(7):1347–1362. doi: 10.1162/jocn_a_00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkirch M, Schmack K, Schlagenhauf F, Sterzer P. Implicit motivational value and salience are processed in distinct areas of orbitofrontal cortex. Neuroimage. 2012;62(3):1717–1725. doi: 10.1016/j.neuroimage.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Schlumpf YR, Nijenhuis ER, Chalavi S, Weder EV, Zimmermann E, Luechinger R, La Marca R, Reinders A, Jäncke L. Dissociative part-dependent biopsychosocial reactions to backward masked angry and neutral faces: An fMRI study of dissociative identity disorder. NeuroImage Clin. 2013;3:54–64. doi: 10.1016/j.nicl.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Willoughby T, Reker DL, Campbell K, Chalmers H, Rose-Krasnor L. Adolescent peer interaction and trait surgency weaken medial prefrontal cortex responses to failure. Soc Cogn Affect Neurosci. 2012;7(1):115–124. doi: 10.1093/scan/nsq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Caldú X, Segura B, Dreher J-C. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37(4):681–696. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Wood MD, Crews TM, Vandiver P. The Tridimensional Personality Questionnaire: reliability and validity studies and derivation of a short form. Psychol Assess. 1995;7(2):195. [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annu Rev Clin Psychol. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Talati A, Pantazatos SP, Schneier FR, Weissman MM, Hirsch J. Gray matter abnormalities in social anxiety disorder: primary, replication, and specificity studies. Biol Psychiatry. 2013;73(1):75–84. doi: 10.1016/j.biopsych.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR. Conscious perception of errors and its relation to the anterior insula. Brain Struct Funct. 2010;214(5-6):629–643. doi: 10.1007/s00429-010-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44(11):2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Wang J, Qin W, Liu B, Zhou Y, Wang D, Zhang Y, Jiang T, Yu C. Neural mechanisms of oxytocin receptor gene mediating anxiety-related temperament. Brain Struct Funct. 2014;219(5):1543–1554. doi: 10.1007/s00429-013-0584-9. [DOI] [PubMed] [Google Scholar]
- Weber L, Mayer K. The benefits of combining psychology and economics theory in strategy research: the contract’s simultaneous role as safeguard and relationship management tool. Benefits. 2008 [Google Scholar]
- Wei D, Du X, Li W, Chen Q, Li H, Hao X, Zhang L, Hitchman G, Zhang Q, Qiu J. Regional gray matter volume and anxiety-related traits interact to predict somatic complaints in a non-clinical sample. Soc Cogn Affect Neurosci. 2014 doi: 10.1093/scan/nsu033. doi:10.1093/scan/nsu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: application of the UPPS impulsive behavior scale. Exp Clin Psychopharmacol. 2003;11(3):210. doi: 10.1037/1064-1297.11.3.210. [DOI] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: an application of Cloninger’s theory. J Subst Abuse. 1994;6(1):1–20. doi: 10.1016/s0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Winkler AD, Hu S, Li CS. The influence of risky and conservative mental sets on cerebral activations of cognitive control. Int J Psychophysiol. 2013;87:254–261. doi: 10.1016/j.ijpsycho.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cereb Cortex. 2009;19(5):1019–1027. doi: 10.1093/cercor/bhn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P, Li C-SR. Decreased amygdala activation during risk taking in non-dependent habitual alcohol users: a preliminary fMRI study of the stop signal task. Am J Drug Alcohol Abuse. 2009;35(5):284–289. doi: 10.1080/00952990902968569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Kendrick KM, Wu Q, Chen T, Lama S, Cheng B, Li S, Huang X, Gong Q. Structural and functional connectivity changes in the brain associated with shyness but not with social anxiety. PLoS One. 2013;8(5):e63151. doi: 10.1371/journal.pone.0063151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, McHugo M, Ray KL, Glahn DC, Eickhoff SB, Laird AR. Meta-analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cereb Cortex. 2014;24(1):232–248. doi: 10.1093/cercor/bhs308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ide JS, Chiang-shan RL. Resting-state functional connectivity of the medial superior frontal cortex. Cereb Cortex. 2012;22(1):99–111. doi: 10.1093/cercor/bhr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23(22):8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]