Abstract
This study examined the degree to which reinforcement, stimulant medication, and their combination impact response inhibition in children with Attention-Deficit/Hyperactivity Disorder (ADHD). Across three studies, participants with ADHD (n=111, 25 girls) and typically-developing (TD) controls (n=33, 6 girls) completed a standard version of the stop signal task (SST) and/or a reinforcement-manipulation SST with performance-contingent points. In two of these studies, these tasks were performed under placebo or 0.3 and 0.6 mg/kg methylphenidate (MPH) conditions. Cross-study comparisons were conducted to test hypotheses regarding the separate and combined effects of reinforcement and methylphenidate on response inhibition among children with ADHD relative to TD controls. Baseline response inhibition was worse among children with ADHD compared to controls. MPH produced dose-related improvements in response inhibition in children with ADHD; compared to non-medicated TD controls, 0.3 mg/kg MPH normalized deficient response inhibition, and 0.6 mg/kg MPH resulted in better inhibition in children with ADHD. Reinforcement improved response inhibition to a greater extent for children with ADHD than for TD children, normalizing response inhibition. The combination of MPH and reinforcement improved response inhibition among children with ADHD compared to reinforcement alone and MPH alone, also resulting in normalization of response inhibition despite repeated task exposure. Deficient response inhibition commonly observed in children with ADHD is significantly improved with MPH and/or reinforcement, normalizing inhibition relative to TD children tested under standard conditions.
Keywords: ADHD, reinforcement, methylphenidate, medication, inhibition
Deficient inhibitory control is central in leading models of attention-deficit hyperactivity disorder (ADHD; Barkley, 1997; Sonuga-Barke, Bitsakou, & Thompson, 2010). Impaired response inhibition is among the most common deficits in children with ADHD (Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). Response inhibition is commonly assessed with the Stop Signal Task (SST), involving occasional inhibition of a button press (when an auditory ‘stop’ signal occurs) during a discrimination task intended to yield a pre-potent ‘go’ response set. The primary metric from the SST is the stop signal reaction time (SSRT), an estimate of the speed of inhibitory processing (calculated as the difference between the mean ‘go’ reaction time (MRT) and the mean of the dynamically-adjusting stop signal delay (MSD)). SSRT tends to be slower (worse inhibition) in children with ADHD compared to typically-developing (TD) children (see meta-analytic reviews by Alderson, Rapport, & Kofler, 2007; Lijffijt, Kenemans, Verbaten, & van Engeland, 2005; Lipszyc & Schachar, 2010).
Deficits in basic processes such as response inhibition are natural targets for intervention – if poor response inhibition is causal in ADHD, then reversal of the inhibitory deficit should result in real-world clinical improvement. A central tenet of this treatment mechanism logic is that effective treatments should improve the hypothesized mediator (Froehlich et al., 2014; Jensen et al., 1999; MacKinnon, 2008). Thus, the present paper focuses on the extent to which response inhibition is improved by analogues of the evidence-based treatments for ADHD, which include stimulant medication, behavior therapy, and their combination (American Academy of Pediatrics, 2011). We extend and clarify the existing literature (discussed below) by integrating data collected across three studies to examine the separate and combined effects of stimulant medication and reinforcement on response inhibition, as measured by the SST, among children with ADHD. We also evaluate whether reinforcement and stimulant medication in the form of methylphenidate (MPH) normalize response inhibition relative to TD controls.
Medication Effects on Response Inhibition in ADHD
On average, acute doses of the stimulant MPH improve SSRT compared to placebo among children with ADHD (Bedard et al., 2003; DeVito et al., 2009; Konrad, Gunther, Hanisch, & Herpertz-Dahlmann, 2004; Lijffijt et al., 2006; Scheres et al., 2003; Tannock, Ickowicz, & Schachar, 1995; Tannock, Schachar, Carr, Chajczyk, & Logan, 1989); (c.f., Coghill, Seth, Pedroso, et al., 2013; Overtoom et al., 2003; Pliszka et al., 2007). In studies with multiple MPH doses, the dose-response function is quite varied, with reports of: comparable improvement for MPH doses 0.25 mg/kg and higher (Konrad et al., 2004; Scheres et al., 2003); step-wise improvement with increasing doses up to 1.0 mg/kg (Lijffijt et al., 2006; Tannock et al., 1989); and curvilinear dose-response functions, with maximal beneficial effect at 0.3 mg/kg MPH (Bedard et al., 2003) or 0.6 mg/kg MPH (Tannock et al., 1995). Although non-linear dose-response functions for response inhibition (in contrast to symptom reports) may have important theoretical and clinical implications (Coghill, Seth, Pedroso, et al., 2013; Tannock et al., 1995) the marked variability in dose-response functions across studies makes any interpretation tentative.
The studies differ in many ways, including whether participants are stimulant-naïve versus prior responders and whether predetermined or individually titrated optimal doses are employed. At a more basic level, only about 40-50% of ADHD youth exhibit poor response inhibition (Bedard et al., 2003; Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005), and about 70-80% show a positive clinical response to MPH (Pliszka, 2007). Among the children who show a positive clinical response, there are large, reliable individual differences in the magnitude of response and dose-response functions (Pelham & Smith, 2000). Across studies, these proportions likely vary due to chance and/or selection criteria, which could impact the pattern of results, particularly in the relatively small studies that characterize much of the literature (median ADHD n=21 [range 12-44] in the nine studies cited above). To clarify previous findings, we examined whether stimulant medication dose-dependently improved response inhibition in the largest sample of children with ADHD examined to date (n=87), with a typical range of externalizing comorbidities, and variability in response inhibition and prior exposure to stimulant medication.
Inclusion of a typically developing (TD) control group can provide valuable information about the relative impairment of the ADHD sample under placebo conditions, as well as the degree to which MPH normalizes response inhibition. Two such studies have been conducted to date. DeVito et al. (2009) found that, compared to controls, children with ADHD (all stabilized on MPH prior to study entry) exhibited much worse response inhibition (SSRT) under placebo conditions (d=1.28 compared to d=.61 in meta-analysis of Willcutt et al., 2005). In this study, a moderate dose of MPH (0.5 mg/kg) robustly improved SSRT for ADHD children compared to placebo (d=1.54), with a much larger effect size than is typically reported (d=.51 in meta-analysis by Coghill, Seth, Pedroso, et al., 2013), resulting in response inhibition that was comparable to that of TD children. These data suggest that moderate doses of MPH may normalize response inhibition in ADHD – at least for children with ADHD who exhibited particularly poor response inhibition under placebo conditions relative to TD controls (see effect sizes above) and are medication responsive. Thus, the effects of stimulant medication on cognitive function are most clearly interpretable in the context of baseline functioning and a normative comparison group. Therefore, in the current report, we examined whether and at what dosage MPH normalized response inhibition in a sample of children with ADHD who vary in the extent to which they exhibit impaired response inhibition.
Reinforcement Effects on Response Inhibition in ADHD
Behavior therapy, based on the systematic application of reinforcement and/or punishment to modify behavior, is the leading psychosocial treatment for ADHD (Evans, Owens, & Bunford, 2013; Pelham & Fabiano, 2008). Developing laboratory analogues of behavioral treatment is much more challenging than for medication. First, there are multiple target behaviors (go and stop), and an intervention that only targets one may not have the desired effect on the other (Leotti & Wager, 2010). For example, Scheres and colleagues (Scheres, Oosterlaan, & Sergeant, 2001) found that reinforcing inhibition improved SSRT; however, those data are difficult to interpret because mean go RT was slowed, as children presumably waited longer to respond in case a stop signal occurred (Konrad, Gauggel, Manz, & Scholl, 2000; Oosterlaan & Sergeant, 1998). In comparison, faster SSRT and equivalent go RT have been reported when contingencies were in place for going and stopping (Michel, Kerns, & Mateer, 2005; Stevens, Quittner, Zuckerman, & Moore, 2002), suggesting it is critical to have consequences for both go and stop trials.
Second, in medication studies, the MPH doses that affect behavior clinically are well established and can be easily manipulated in the lab. With behavior therapy, an effective “dose” is not easily determined. Thus, it is critical to demonstrate that the reinforcers/punishers that are employed do affect behavior relative to a no-contingency control condition (analogous to no medication/placebo). Studies without a no-contingency condition may be of considerable interest for other purposes, such as to better understand the relative impact of contingency magnitude or type on task performance (e.g., Huang-Pollock, Mikami, Pfiffner, & McBurnett, 2007; Oosterlaan & Sergeant, 1998; Slusarek, Velling, Bunk, & Eggers, 2001). The reinforcement “dose” is complicated by the fact that there are typically three times as many go as stop trials, and therefore the magnitude of the reinforcer must be proportionate to the frequency of the target behavior. For example, Huang-Pollock et al (2007) found that increasing reinforcement for successful inhibition from two to 10 points improved SSRT (at least in those who received the smaller reinforcement first), but it may have done so by slowing go RT, which was reinforced with one point in both conditions (see also Shanahan, Pennington, & Willcutt, 2008).
Third, each target behavior may be modified by reinforcement, punishment, or a combination of the two. Studies examining the impact of contingencies on SST performance have used reinforcement either by itself (Huang-Pollock et al., 2007; Konrad et al., 2000; Scheres et al., 2001; Stevens et al., 2002), or in combination with or in comparison to punishment (Michel et al., 2005; Oosterlaan & Sergeant, 1998; Shanahan et al., 2008; Slusarek et al., 2001), resulting in mixed findings. In order to understand whether atypical response to reinforcement emphasized in theoretical models of ADHD (see review by Luman, Tripp, & Scheres, 2010) contributes to impairments in response inhibition, in the current study we examined the impact of reinforcement alone, rather than mixed with punishment, to isolate reinforcement effects.
Fourth, the order in which medication or contingencies are administered is also important to consider, given that studies have demonstrated order effects for behavioral contingencies (Huang-Pollock et al., 2007; Shanahan et al., 2008). In medication studies, order is typically counterbalanced, but that is not always the case for studies of behavioral contingencies (e.g., Konrad et al., 2000; Michel et al., 2005), making it difficult to disentangle the effects of contingencies versus practice or fatigue.
In sum, these studies vary considerably in the contingency structure that was used and the comparison conditions, resulting in mixed findings and limiting our understanding of how reinforcement impacts response inhibition as measured by the SST. Building on the previous literature, the current study is the first to address all four key issues described above by: (1) balancing reinforcement for go and stop trials, (2) using reinforcement only (not mixed with punishment) at a “dose” previously shown to be effective (Shiels et al., 2008), (3) repeatedly alternating reinforcement conditions within-subjects, (4) counterbalancing condition order, and (5) making comparisons to a no-contingency condition and a TD control group. Thus, we aim to clarify previous findings by examining whether children with ADHD show greater improvement in and normalization of response inhibition with reinforcement compared to TD children based on performance during a reinforcement-manipulation SST designed with these issues in mind.
Combined Effects of MPH and Reinforcement on Response Inhibition in ADHD
Combined treatment with stimulant medication and behavior therapy has generally been shown to be efficacious above and beyond either treatment alone (Fabiano et al., 2007; Pelham et al., 2014; Pelham et al., 1993) and is one of the recommended strategies in the pediatric guidelines for ADHD (American Academy of Pediatrics, 2011). Despite the evidence for the effectiveness of these treatments in reducing the behavioral symptoms of ADHD, only two studies have examined the impact of both stimulant medication and response contingencies on SST performance (Epstein et al., 2011; Tamm & Carlson, 2007). Surprisingly, neither MPH nor response contingencies significantly improved SSRT, making it difficult to interpret the non-significant interactions observed in both studies. In addition, methodological issues related to the structure of response contingencies (e.g., reinforce correct inhibition and punish failed inhibition without contingency for go process) and a lack of comparison to a typically developing control group preclude clear interpretation of the separate and combined effects of medication and reinforcement on inhibitory control in these studies.
In the current report, we examined whether the combination of effective “doses” of stimulant medication and reinforcement (as established in Studies 1 and 2) results in greater improvement in response inhibition than either reinforcement or MPH alone among an ADHD sample. Both methylphenidate (MPH) and reinforcement increase synaptic availability of dopamine in the ventral striatum (Arnsten & Rubia, 2012), an area with strong connections to frontal regions involved in cognitive and motor control (Haber & Knutson, 2010). Given the potential overlap in neurobiological mechanism, we predicted their combination to have a greater effect on response inhibition than either treatment alone, paralleling findings in the ADHD treatment outcome literature (Fabiano et al., 2007; Pelham et al., 2014; Pelham et al., 1993).
In sum, the proposed work aims to extend this literature and clarify the separate and combined effects of acute sustained-release MPH and reinforcement on response inhibition in ADHD by integrating data collected during three separate studies (see Figure 2 and detailed descriptions of each study below).1 The following hypotheses will be tested: (1) response inhibition will be weaker during a standard SST in children with ADHD relative to TD children, (2) stimulant medication will dose-dependently improve response inhibition in children with ADHD, (2a) stimulant medication will normalize response inhibition to be equivalent to that of TD participants, (3) although reinforcement will improve response inhibition in TD and ADHD children, children with ADHD will show a relatively greater improvement with reinforcement and (3a) normalization of response inhibition with reinforcement, and (4) the combination of stimulant medication and reinforcement will result in greater improvement in inhibition than either reinforcement or MPH alone among children with ADHD and (4a) will normalize inhibition to be equivalent to that of TD participants.
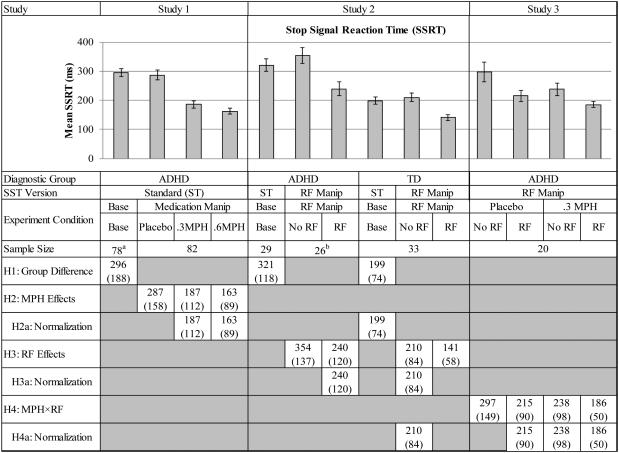
Figure 2. Diagnostic group differences and the separate and combined effects of methylphenidate and reinforcement on stop signal reaction time in children with ADHD compared to typically developing controls.
This figure illustrates the cross-study comparisons that were made to address each of the hypotheses. The cells containing values in the lower portion of the figure represent the data included in the analyses for each specific hypothesis. Notes. ADHD = Attention-Deficit/Hyperactivity Disorder group; TD = typically-developing control group; SST = stop signal task; SSRT = stop signal reaction time; ST = Standard; RF = Reinforcement; Manip = Manipulation; H = hypothesis; Base = baseline;. Values in the cells represent mean (SD) SSRT for each condition included in the comparison. Error bars represent standard error of the mean (SEM). aFour participants from Study 1 were missing SST data from the Baseline Day. bThree participants with ADHD from Study 2 were missing SST data from the Reinforcement Day.
Method
Participants
ADHD participants were primarily clinic-referred. Control participants were recruited through flyers in pediatricians’ offices and schools and advertisements in local periodicals. Diagnoses were made based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 2000). Parents completed a structured computerized clinical interview (Diagnostic Interview Schedule for Children; DISC-IV; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000). Both parents and teachers completed rating scales of symptoms of ADHD, oppositional defiant disorder (ODD), and conduct disorder (CD) (Disruptive Behavior Disorder Rating Scale; DBD; Pelham, Gnagy, Greenslade, & Milich, 1992) and impairment (Fabiano et al., 2006). The DISC-IV, DBD, and IRS rating scales have previously been shown to have sound psychometric properties and have been used extensively in studies of children with ADHD (Pelham, Fabiano, & Massetti, 2005). To meet diagnostic criteria, children were required to exhibit six or more symptoms of inattention and/or six or more symptoms of hyperactivity/impulsivity according to the DISC and/or DBD rating scale on the basis of parent and teacher reports. In addition, cross-situational impairment had to be present according to the IRS, and/or the DISC. Children eligible for the control group could not meet criteria for any behavioral disorder on the DISC, could not exhibit more than three symptoms of inattention or hyperactivity/impulsivity on the DBD, and were free of clinically significant impairment on the IRS (i.e., rating of 3 or below in all functional domains).
Exclusion criteria for both groups included estimated full scale intelligence quotient (FSIQ) < 80 based on the vocabulary and block design subtests from the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler, 2003), diagnosis of pervasive developmental disorder, schizophrenia or other psychotic disorders, and uncorrected visual or auditory problems. In Studies 1 and 3, children with ADHD were screened for contraindications for stimulant medications (Study 2 did not involve medication), and children taking atomoxetine were excluded from participation in Studies 2 and 3 due to the extended washout period for this medication and the short duration of these studies (two days) in comparison to Study 1 (1 week).
Procedures
Study 1
Study 1 involved a baseline day followed by a placebo-controlled dose-response examination of the effects of MPH on neurocognitive measures of response inhibition (SST), attention (Spencer et al., 2009), working memory, pre-pulse inhibition (Ashare et al., 2010), and delay discounting (Shiels et al., 2009) in a large sample of children with ADHD. The current report includes data from the SST only. All Study 1 participants were diagnosed with ADHD (n=82) with each subtype represented (see Table 1). Participants attended a week-long camp from 7:30am-5:00pm in cohorts of four to five children per week. The SST was one of several neurocognitive computer tasks completed during individual testing sessions interspersed with three 30-minute academic periods and breaks for meals, snacks, and recreational activities. An initial baseline day consisted of non-medicated testing (n=78; 4 participants were missing baseline data), the medication manipulation occurred on days two to four, and day five consisted of recreational activities and make-up testing. Participants earned a nominal prize for following the rules of the testing environment (e.g., stay in your seat); this prize was not contingent on actual task performance and was consistently applied across all days of all three studies.
Table 1.
Sample characteristics.
|
TD
Controls (n=33) |
ADHD-1a
(n=82) |
ADHD-2a
(n=29) |
ADHD-3a
(n=20) |
|
|---|---|---|---|---|
| Age in years, mean (SD) | 10.9 (1.0) | 10.8 (1.1) | 10.9 (1.1) | 10.3 (0.9) |
| Sex, ratio M:F | 27:6 | 61:21 | 25:4 | 15:5 |
|
| ||||
| Ethnicity, % Caucasian:African American:Other |
85:9:6 | 81:12:7 | 90:7:3 | 90:10:0 |
|
| ||||
| ADHD Subtype, %COMB:INA:HI | n/a | 77:19:4 | 100:0:0 | 100:0:0 |
|
| ||||
| Comorbid ODD | n/a | 44% | 45% | 45% |
| Comorbid CD | n/a | 27% | 31% | 30% |
|
| ||||
| Stimulant Naïve | 100% | 21% | 24% | 15% |
| IQ, mean (SD) b | 112.5 (11.5) | 103.4 (13.7) | 106.9 (11.5) | 107.9 (9.9) |
|
| ||||
| Inattention Symptoms c | 0.09 (0.4) | 6.6 (2.5) | 7.9 (1.5) | 7.8 (1.9) |
| Hyperative/Impulsive Symptoms c | 0.03 (0.2) | 5.2 (2.6) | 6.8 (2.0) | 6.5 (2.0) |
| ODD Symptoms c | 0.0 (0.0) | 3.2 (2.6) | 4.1 (2.6) | 3.9 (2.5) |
| CD Symptoms c | 0.0 (0.0) | 0.7 (1.1) | 0.9 (1.0) | 1.0 (0.9) |
Notes.
ADHD-1=Study 1 ADHD participants, ADHD-2=Study 2 ADHD participants, ADHD-3=Study 3 ADHD participants.
Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) estimated full-sale IQ.
Number of symptoms rated as “pretty much” or “very much” on the DBD-RS.
Sustained-release MPH in the form of OROS-MPH was administered rather than immediate-release MPH to allow us to test over the course of the entire day without the peaks and valleys that may be evident with repeated immediate-release dosing. The nearest commercially-available dose of OROS-MPH equivalent to 0.3 and 0.6 mg/kg of immediate-release MPH dosed TID and placebo were administered in a double-blind, placebo-controlled fashion. A single morning dose of MPH or placebo was given in a counterbalanced order across participants. Counterbalancing was modified for stimulant naïve children (n=24) who received a dose of 0.3 mg/kg MPH before 0.6 mg/kg MPH, although the placebo dose could occur on any of the three medication days. The average low and high doses were 39mg (SD=9) and 74mg (SD=13), respectively. Participants currently taking a stimulant medication completed a 24-hr washout period prior to participating in the study, and those taking non-stimulants completed a 1-week washout period. Upon arrival to camp at 7:30 AM, children were administered their daily dose of OROS-MPH by study staff. Testing began 90 minutes after dose administration to allow MPH to reach therapeutic levels and ended at 5:00 PM to ensure testing occurred during times when OROS-MPH was active. Blood pressure and heart rate were taken at mid-day, and counselors monitored side effects.
Study 2
In Study 2, a second sample of children with ADHD and a TD control group completed the same battery of standard tasks as in Study 1, then returned 1 week later to complete reinforcement-manipulation versions of these tasks. Study 2 included children with ADHD Combined type (ADHD-C, n=29) and a group of TD controls (n=33). Participants completed two days of testing, a baseline day and reinforcement-manipulation day, approximately 1 week apart with a similar structure to Study 1. On the baseline day, children completed the identical cognitive tasks as in Study 1. On the reinforcement-manipulation day (n=26; two children with ADHD did not return for day two and one child with ADHD took stimulant medication on day two, resulting in three children with missing data), participants were able to earn points for task performance during the reinforcement-manipulation SST, described in detail below. At the end of the day, participants exchanged points at a “points store” containing prizes that were specifically requested by the child (ranging from small toys like balls and cards to large prizes such as action figures, video games, and gift cards).
Study 3
In Study 3, a subset of ADHD-C participants from Studies 1 and 2 returned to the laboratory to complete the battery of reinforcement-manipulation tasks from Study 2 under placebo and MPH conditions in a fully within-subjects design for examination of the separate and combined effects of reinforcement and MPH on the same neurocognitive processes. All participants with ADHD-C from Studies 1 and 2 (except for those children taking non-stimulants due to the extended washout period) who remained in the 9-12 year-old age range were invited to participate in Study 3 (n=20). Study 3 was also conducted in a camp-like setting, and testing occurred on two consecutive days. The daily structure of tasks and activities was similar to the second day of Study 2. A single morning dose of placebo or OROS MPH at the nearest commercially-available equivalent of 0.3 mg/kg of immediate-release MPH dosed TID were administered in a double-blind, counterbalanced order. On both days, participants completed the reinforcement-manipulation SST.
Relation of Individual Studies to Cross-Study Hypotheses
In order to address all of our hypotheses, we made comparisons across each of these studies (see Figure 2). The structure of the testing day and the battery of neurocognitive tasks were similar for all three studies. A relatively equal number of participants were randomly assigned to the various task, medication dosage, and reinforcement condition orders. Data collected in Study 1 from children with ADHD and in Study 2 from ADHD-C and TD children during the standard SST performed on the baseline day were used to evaluate diagnostic group differences in response inhibition (Hypothesis 1). Data collected in Study 1 from children with ADHD during the standard SST performed on the medication trial days were used to examine dose-dependent effects of MPH on response inhibition (Hypothesis 2) and normalization of response inhibition with MPH in comparison to Study 2 TD controls during the standard SST completed on the baseline day (Hypothesis 2a). In addition, Study 2 data from children with ADHD-C and TD controls on the reinforcement-manipulation day were used to examine the effects of reinforcement on response inhibition (Hypothesis 3) and normalization of response inhibition with reinforcement (Hypothesis 3a). Study 3 data from children with ADHD-C during the reinforcement-manipulation SST completed on both testing days (placebo and 0.3 mg/kg MPH) were used to examine the separate and combined effects of reinforcement and MPH on response inhibition (Hypothesis 4). Finally, Study 3 data from children with ADHD-C during the reinforcement-manipulation SST under placebo and 0.3 mg/kg MPH conditions was compared to that of Study 2 TD controls during the no-reinforcement condition of the reinforcement-manipulation SST to assess normalization of response inhibition with reinforcement and MPH separately and in combination (Hypothesis 4a).
Stop Signal Paradigm
The SST was utilized to measure children’s ability to inhibit responding once a prepotent go response had been established. The task was presented on a 38 cm CRT computer monitor connected to a response box (Psychology Software Tools, Pittsburgh, PA). Task stimuli consisted of 2 × 2 cm arrows pointing left or right, and the child was instructed to press the left button for left-facing arrows and the right button for right-facing arrows. A 32-trial go practice was followed by a 32-trial stop practice, for which children were instructed to inhibit responding whenever the go stimulus presentation was followed by the auditory stop signal (25% of trials). The stop signal was a 1000 Hz tone presented for 100 ms. On the first stop trial, the tone onset was 350 ms (changed to 250 ms for Study 3 after observing mean stop signal delays for the Study 2 reinforcement-manipulation SST were 250-300 ms; see Table S1) after the onset of the go stimulus and adjusted dynamically. If a participant correctly inhibited, the latency between stimulus presentation and stop signal increased by 50 ms (i.e., became more difficult). If the participant failed to inhibit, the latency decreased by 50 ms (i.e., became easier).
Standard SST
The standard version of the SST was employed for Study 1 and the baseline day of Study 2 (see Figure 1A). After the practice trials, four test blocks of 64 trials each were administered (256 test trials total).
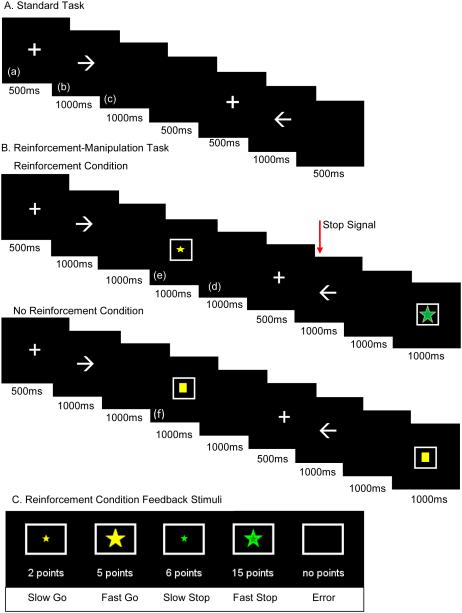
Figure 1. Task structure for the standard and reinforcement-manipulation versions of the stop signal task.
(A) Standard and (B) reinforcement-manipulation stop signal task structure and (C) feedback stimuli. Trial structure for all tasks included the (a) fixation point, (b) stimulus presentation, (c) response window, and (d) intertrial interval. The (e) reinforcement and (f) and no-reinforcement conditions included feedback stimuli. During the reinforcement condition, an empty square appeared when an error occurred, indicating no points were earned on the trial, and a square with stars of various sizes and colors appeared for a correct response indicating points were earned on the trial. During the no-reinforcement condition, no points could be earned and a yellow box always appeared inside the square.
Reinforcement-manipulation SST
Day two of Study 2 and both days of Study 3 employed a version of the SST that included a reinforcement manipulation (Figure 1B). The reinforcement-manipulation SST alternated between reinforcement and no-reinforcement conditions (order counterbalanced across participants), each of which consisted of two blocks with 64 trials per block. Continuous reinforcement was provided via a square presented for 1000 ms after the response period. The square contained stars of varying sizes to indicate how many points were earned on that trial (see Figure 1C). To maintain response speed, children earned five points for a correct, fast go response (faster than their go practice mean RT) and two points for a correct but slow go response. To balance the magnitude of reinforcement for go and stop trials, stop trials were worth three times as much as go trials; inhibition on a stop trial preceded by a fast go response earned 15 points, and inhibition on a stop trial preceded by a slow go response earned six points. Each point was worth approximately $0.01 that was later exchanged for prizes. During no-reinforcement blocks, participants were told to “try their best,” and an uninformative square was presented during the feedback period that contained no information regarding task performance.
Data Analysis
Stop task data were aggregated within trial block and then averaged across trial blocks within medication and/or reinforcement condition.2 Analyses focused on SSRT (Mean GoRT–Mean Stop Signal Delay). Descriptive statistics for the additional performance measures are presented in Table S1, available in the online supplementary material, including percent inhibition and percent accuracy for go trials, reaction time mean and standard deviation, and mean stop signal delay. Univariate ANOVAs were used to test for differences between diagnostic groups and among reinforcement and medication conditions. The analytic approach was tailored to address each specific question and is described below. Cohen’s d is reported as an estimate of effect size with small, medium, and large effect sizes as 0.3-0.5, 0.5-0.8, and ≥ 0.8, respectively (Cohen, 1988).
Results
Sample Characteristics
Demographic information for participants from each study is presented in Table 1. Control participants did not significantly differ from the ADHD samples in Studies 1, 2, or 3 in age (ps=.78, .97, and .06) or sex (ps=.47, .74, and .73), although the ADHD sample in Study 3 tended to be younger than the TD group. Average IQ was four to nine points higher among the TD controls compared to the ADHD samples from each study (ps=.01, .06, and .14). The ADHD samples in Studies 1 and 2 did not differ from each other in terms of age (p=.76), sex (p=.19), or IQ (p=.22), and typical comorbidity patterns for the disruptive behavior disorders (ODD, CD) were observed (see Table 1). The ADHD sample in Study 3 included participants from Study 1 (n=7) and Study 2 (n=13).
Hypothesis 1: Diagnostic group differences
Figure 2 presents mean SSRT for all conditions across all three studies. To determine whether response inhibition was impaired in ADHD, we compared SSRT during the standard SST for the ADHD samples from the baseline day of Studies 1 and 2 to that of the TD sample during the standard SST from the baseline day of Study 2 using one-way ANOVA. The ADHD samples from Studies 1 and 2 had comparable SSRTs to one another, F(1,105)=0.9, p=.34. As expected, children with ADHD exhibited impaired response inhibition (slower SSRT) compared to control participants, F(1,138)=22.8, p<.001, d=1.06 (see Figure 2).
Hypothesis 2: Methylphenidate effects
To examine the effects of MPH on response inhibition, we analyzed SSRT data from the standard SST administered during the 3-day medication trial conducted in Study 1, which involved only children with ADHD. MPH effects were evaluated with a pair of orthogonal contrasts: a) active MPH (the average of the 0.3 and 0.6 mg/kg doses) versus placebo, and b) MPH dose (0.3 versus 0.6 mg/kg) (Ashare et al., 2010; Shiels et al., 2009; Spencer et al., 2009). As can be seen in Figure 2, active methylphenidate significantly reduced SSRT in children with ADHD, F(1,81)=61.3, p<.001, d=0.85. A significant dose effect also emerged, with better SSRT with 0.6 mg/kg MPH than with 0.3 mg/kg MPH, F(1,81)=6.7, p=.01, d=0.25. Thus, MPH dose-dependently improved response inhibition among children with ADHD.
Hypothesis 2a: Normalization of ADHD response inhibition with methylphenidate
To examine whether MPH normalized response inhibition among children with ADHD, SSRT during the standard SST of children with ADHD in Study 1 on each dose of MPH (0.3 and 0.6 mg/kg; n=82) was compared with that of the TD control group during the same task (standard SST) on the baseline day for Study 2. The diagnostic group difference in SSRT reported above (hypothesis 1) was eliminated with 0.3 and 0.6 mg/kg MPH (see Figure 2). Specifically, SSRT for children with ADHD taking 0.3 mg/kg MPH did not differ from the TD controls, F(1,113)=0.4, p=.55, d=0.13. Moreover, 0.6 mg/kg MPH resulted in SSRT that was, on average, lower than that of the control group, F(1,113)=4.4, p=.04, d=0.44, indicative of better response inhibition in children with ADHD when taking a dose of MPH at the high end of the clinical range (Pliszka, 2007) compared to TD children.
Hypothesis 3: Reinforcement effects
The impact of reinforcement on response inhibition was examined on the reinforcement-manipulation day of Study 2, in which ADHD and TD participants completed the reinforcement-manipulation SST. A 2 × 2 ANOVA included the between-subjects factor diagnostic group (ADHD versus TD) and the within-subjects factor reinforcement condition (no-reinforcement versus reinforcement). Reinforcement Order (reinforcement first versus second) was also included, but this counterbalancing factor did not interact with diagnostic group. The main effects of diagnostic group, F(1,55)=24.3, p<.001, d=1.16, and reinforcement condition, F(1,55)=78.5, p<.001, d=0.90, on SSRT were qualified by a Diagnostic Group × Reinforcement interaction, F(1,55)=4.8, p=.03 (see Figure 2). As predicted, the beneficial effect of reinforcement was greater for children with ADHD (mean difference=114.0 ms), F (1,57)=53.7, p<.001, than for TD children (mean difference=68.7ms), F(1,57)=24.7, p<.001.3
Hypothesis 3a: Normalization of ADHD response inhibition with reinforcement
To evaluate the extent to which reinforcement normalized response inhibition in children with ADHD, we compared SSRT for the ADHD group during the reinforcement condition of the reinforcement-manipulation SST to the controls during the no-reinforcement condition of the reinforcement-manipulation SST completed in Study 2. SSRT for children with ADHD during the reinforcement condition did not differ from that of controls when reinforcement was not provided, F(1,57)=1.3, p=.27, d=0.29 (see Figure 2), suggesting that, on average, reinforcement acutely normalized response inhibition for children with ADHD.
Hypothesis 4: Comparison of the separate and combined effects of 0.3 mg/kg MPH and reinforcement in children with ADHD
Whether the combination of MPH and reinforcement is more effective than either MPH or reinforcement alone was examined in Study 3, during which children with ADHD-C type completed the reinforcement-manipulation SST on two consecutive days under placebo and 0.3 mg/kg MPH conditions. Orthogonal contrasts were employed to directly test of the effects of interest: (1) no treatment (no-reinforcement + placebo) versus single treatment (average of reinforcement alone and 0.3 mg/kg MPH alone), (2) reinforcement alone versus 0.3 mg/kg MPH alone, and (3) single treatment (average of reinforcement alone and 0.3 mg/kg MPH alone) versus combined treatment (reinforcement + 0.3 mg/kg MPH). As shown in Figure 2 (Study 3), the single treatments (the average of MPH alone and reinforcement alone) decreased SSRT compared to no-reinforcement + placebo, F(1,19)=9.3, p=.01, d=0.57. SSRT was similar for reinforcement alone and MPH alone, F(1,19)=0.9, p=.37, d=0.24. Finally, the combination of reinforcement + MPH resulted in a further improvement in SSRT compared to the single treatments, F(1,19)=12.4, p=.002, d=0.54.
Hypothesis 4a: Normalization of ADHD response inhibition with the 0.3 mg/kg MPH and reinforcement
We also examined whether the combination of reinforcement and 0.3 mg/kg MPH normalized response inhibition by comparing the performance of children with ADHD in Study 3 during the reinforcement alone, 0.3 mg/kg MPH alone, and the reinforcement + 0.3 mg/kg MPH conditions of the reinforcement-manipulation SST to TD controls in Study 2 during the no-reinforcement condition of the reinforcement-manipulation SST. The performance of the TD group during the no-reinforcement condition was used for the normative comparison rather than performance during the standard SST completed on the baseline day of Study 2 due to differences in task structure. This analysis indicated that the SSRT of children with ADHD with each treatment alone did not differ from that of TD controls in Study 2 during the no-reinforcement condition of the same task, reinforcement alone: F(1,51)=0.05, p=.83, d = 0.06, and 0.3 mg/kg MPH alone: F(1,51)=1.2, p=.28, d = 0.31. In addition, SSRT for children with ADHD during the combined treatment (reinforcement+0.3 mg/kg MPH) condition of Study 3 was comparable to that of Study 2 controls during the no-reinforcement condition of the same task, F(1,51)=1.4, p=.25, d=0.36 (Figure 2).
Supplementary Analyses
The analyses reported above focused on SSRT, given our primary interest in response inhibition. Due to space constraints, we were unable to address several other interesting and important questions such as the contribution of slower and more variable responding to the go stimuli among children with ADHD to SSRT estimates (Alderson, Rapport, Sarver, & Kofler, 2008) and the impact of stimulant medication and reinforcement on other indicators of SST performance. This information is presented in Supplementary Materials available online, including additional results for each of the hypotheses for MRT and percent inhibition (S2) and the contribution of RT skew to the findings reported above (S3). In general, these supplementary analyses suggest that the adaptive algorithm worked as intended, with percent inhibition approximating 50% for all study samples and conditions, the strongest effects were found for SSRT compared to other SST measures, and greater RT skew among the ADHD samples did not account for the diagnostic group differences in SSRT or the effects of medication.
Discussion
In this series of studies, we examined the impact of laboratory analogues of evidence-based treatments for ADHD (behavior medication and stimulant medication) on response inhibition. Consistent with previous research, children with ADHD demonstrated weaker response inhibition, with a comparable effect size to that typically reported (d=1.06; Willcutt et al., 2005 meta-analysis average d=0.61). This finding supports models of ADHD that emphasize response inhibition in the pathophysiology of this disorder (Barkley, 1997; Coghill, Seth, & Matthews, 2013; Sonuga-Barke et al., 2010). Establishing deficient response inhibition in our sample of children with ADHD was important for interpreting the subsequent evaluation of MPH and reinforcement, as well as normalization of response inhibition relative to TD controls.
Medication Effects on Response Inhibition in ADHD
Sustained-release MPH improved response inhibition on the standard SST in a dose-dependent fashion, with 0.6 mg/kg MPH resulting in additional benefit beyond 0.3 mg/kg. These findings inform the inconsistent existing literature, which includes failures to observe beneficial effects at moderate doses of MPH (Coghill, Seth, Pedroso, et al., 2013; Overtoom et al., 2003; Pliszka et al., 2007) and variable dose-response functions (Konrad et al., 2004; Lijffijt et al., 2006; Scheres et al., 2003; Tannock et al., 1995; Tannock et al., 1989), and extends this literature to sustained-release MPH, which has become the standard in clinical practice. To enhance both power and generalizability, we examined the dose-response function in a large sample (roughly three times larger than that typical of prior studies) with a typical range of externalizing comorbidities, variability in response inhibition ability, and variability in prior exposure to stimulant medication. Thus, in the largest MPH dose-response study of a representative sample of children with ADHD on laboratory cognitive task performance conducted to date, there was a clear dose-dependent improvement in SSRT that nicely parallels clinical dose-response data.
Furthermore, comparisons to TD controls indicated that 0.3 mg/kg MPH normalized response inhibition in children with ADHD. Not only was the ADHD-control difference in response inhibition no longer statistically significant, but the group effect size (Cohen’s d) dropped from 1.06 in the absence of medication to 0.13 when children with ADHD received 0.3 mg/kg MPH. Moreover, 0.6 mg/kg MPH resulted in significantly better response inhibition in children with ADHD compared to TD controls (d = 0.44). These findings replicate and extend DeVito et al.’s (2009) observations of normalization for ADHD children stabilized on MPH to a sample that included stimulant-naïve children. However, the examination of acute effects of MPH on SSRT, measured only once for each dose, limits our ability to determine whether these effects persist over time and relate to improvement in ADHD symptoms.
Reinforcement Effects on Response Inhibition in ADHD
Reinforcement contingencies also improved response inhibition in children with ADHD and this effect was greater among the ADHD group. The question of whether disinhibition in children with ADHD is under executive or motivational control has been raised (Nigg, 2001), with theories of ADHD postulating that atypical motivation is primary in children with ADHD (Luman et al., 2010). Our findings suggest that children with ADHD can perform as well as TD children on a measure of response inhibition when they receive performance-based contingencies, suggesting that motivation and cognition interact to produce behavior.
Few studies have demonstrated a differential effect of response contingencies on SSRT for children with ADHD compared to controls (Konrad et al., 2000; Slusarek et al., 2001) and some have not found improved SSRT in children with ADHD with contingencies. Previous studies varied greatly in the targets, magnitude, and valence of their contingencies. Building on the existing literature, the reinforcement manipulation applied here was carefully designed to balance consequences for stopping and going. This resulted in lower SSRT (compared to the no-reinforcement condition) while also maintaining approximately 50% response inhibition and speeded responses to the go stimuli (see Table S1 and S2). This is particularly important, given the tendency for response slowing over the course of the SST in order to increase the probability of inhibition, which has been shown to impact estimates of SSRT (Leotti & Wager, 2010; Verbruggen, Chambers, & Logan, 2013). In addition, we compared performance on the SST during a condition with powerful, continuous reinforcement and near-immediate feedback in comparison to a no-contingency and no-feedback condition, providing a strong test of the impact of reinforcement on response inhibition. Under these conditions, response inhibition in children with ADHD did not significantly differ from that of controls without reinforcement (Study 2); the large ADHD-control group difference observed under baseline testing (d=1.06) was reduced to a small effect size by reinforcement (d=.29), suggesting nearly complete normalization of response inhibition.
While these findings are promising, they should be considered in the context of the study’s limitations. First, the reinforcement contingencies in our study are not feasible to implement in a classroom setting, although a less intensive behavior modification system may be just as effective when combined with MPH (Fabiano et al., 2007; Pelham et al., 2014). Second, the acute analogue model does not capture whether reinforcers lose their effectiveness over time (Lloyd, Medina, Hawk, Fosco, & Richards, 2014). However, part of this issue is addressed by Study 3, which included children with ADHD who had prior exposure to the SST under MPH (completed four times in Study 1) or reinforcement (completed twice in Study 2) conditions. Comparison with Study 2 TD children suggests that 0.3 mg/kg MPH and reinforcement each normalized SSRT, despite Study 3 children being younger and having repeated task exposure.
Combined Effects of MPH and Reinforcement on Response Inhibition in ADHD
Although reinforcement and 0.3 mg/kg MPH each improved SSRT in Study 3, the combination of 0.3 mg/kg MPH and reinforcement resulted in further improvement. The combination not only normalized response inhibition but actually resulted in comparable SSRT to that of Study 2 TD children (d = .36), a finding similar to that observed for the high dose (0.6 mg/kg) of MPH. This is the first study to demonstrate such an effect on response inhibition (c.f., two prior studies: Epstein et al., 2011; Tamm & Carlson, 2007, failed to observe significant effects of either treatment component). These findings are particularly important because they parallel clinical findings that the combination of low doses of MPH (0.15 and 0.3 mg/kg) in combination with a low dose behavior modification system were better than either treatment alone and just as effective as a high dose of MPH (0.6 mg/kg) in reducing classroom rule violations and improving academic productivity and behavior in social settings (Fabiano et al., 2007; Pelham et al., 2014).
We examined the impact of effective treatments for ADHD on response inhibition as a component of a treatment mechanism framework. The basic tenet of this approach is that improvement in response inhibition with MPH and reinforcement accounts for the clinical impact of these treatments. Here, we demonstrated that both treatments improve the hypothesized mediator (response inhibition), establishing the first component of the mediational frame (treatment impacts mediator) (MacKinnon, 2008). Future work should test the full meditational model to determine the extent to which individual differences in clinical treatment response are accounted for by treatment effects on response inhibition. In addition, the parallel effects of MPH and reinforcement observed in the present studies, combined with the literature demonstrating that both stimulants and reinforcement increase striatal dopamine availability, may suggest a common mechanism at the neurobiological level as well. A similar hypothesis has previously been proposed in regard to classroom behavior and academic performance based on findings that boys with ADHD who responded to behavior modification also responded to stimulant medication (Pelham et al., 1993).
Summary
In this study, we replicated previous research demonstrating deficient response inhibition in children with ADHD and improvement in response inhibition with MPH. We also clarified the results from the mixed literature on dose-response relationships in the largest sample of children with ADHD examined to date with a typical range of externalizing comorbidities, and variability in response inhibition and prior exposure to stimulant medication. In addition, we showed that balanced, continuous reinforcement for going and stopping with immediate feedback and preferred prizes simultaneously improved response inhibition and execution, particularly for children with ADHD, clarifying findings from an inconsistent literature on the impact of motivational contingencies. Finally, we demonstrated normalization of response inhibition with MPH and reinforcement, with the combination resulting in greater improvement than either treatment alone. Collectively, these studies encourage future research examining inhibition as a mechanism by which behavioral and pharmacological treatments improve real-world behavior. It will be important for future research to examine whether other cognitive deficits implicated in ADHD, such as working memory and sustained attention, are similarly impacted by reinforcement and MPH and whether improvement in these deficits is related to improvement in ADHD symptoms and associated impairment.
Supplementary Material
Acknowledgements
We thank Rosemary Tannock for her guidance on task and study design, Jerry Richards for his assistance with data analysis and interpretation, Dominica Vito and Brian Gangloff for project coordination, Mark Kutgowski for computer programming, and Sarah Spencer and Michael Strand for their assistance with data collection. We appreciate the time and effort taken by the families who participated in these studies. This research was supported by grants from the National Institute of Mental Health (NIMH) awarded to Larry W. Hawk (R01 MH069434, 3R01 MH069434-04S1) with additional support of co-author contributions from grants from the NIMH awarded to Keri S. Rosch (K23 MH101322), James G. Waxmonsky (MH080791), and William E. Pelham (MH62946, MH069614, MH53554, MH69434, MH65899, MH78051, MH062946, NS39087, AA11873, DA12414) and by grants from the Institute of Education Sciences awarded to William E. Pelham (R324B060045, L03000665A).
Footnotes
Although we have published data from other tasks completed in one or more of these studies (Ashare et al., 2010; Bubnik, Hawk, Pelham, Waxmonsky, & Rosch, 2015; Shiels et al., 2009; Spencer et al., 2009; Strand et al., 2012) the present manuscript is the first to report the SST data.
Previous studies with the SST have varied in their criteria for exclusion of task blocks, with some studies reporting no exclusions (Epstein et al., 2011; Huang-Pollock et al., 2007; Shanahan et al., 2008) and other studies eliminating blocks with percent inhibition <20% or >80% or percent accuracy <70-80% (Nigg, 1999). Our primary analyses included all task blocks. However, we conducted supplementary analyses in which we eliminated blocks with percent inhibition <20% or >80% or percent accuracy <80%. Except where noted, the results did not change.
When analyses were restricted to include only blocks with percent inhibition between 20-80% and percent accuracy of at least 80%, 5 participants are excluded (4 ADHD, 1 TD); the Diagnostic Group × Reinforcement interaction was no longer significant, F(1, 50) = 1.8, p = .18, although the pattern of means was similar.
References
- Alderson RM, Rapport MD, Kofler MJ. Attention-deficit/hyperactivity disorder and behavioral inhibition: a meta-analytic review of the stop-signal paradigm. Journal of Abnormal Child Psychology. 2007;35:745–758. doi: 10.1007/s10802-007-9131-6. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- Alderson RM, Rapport MD, Sarver DE, Kofler MJ. ADHD and behavioral inhibition: a re-examination of the stop-signal task. Journal of Abnormal Child Psychology. 2008;36:989–998. doi: 10.1007/s10802-008-9230-z. doi: 10.1007/s10802-008-9230-z. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007–1022. doi: 10.1542/peds.2011-2654. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:356–367. doi: 10.1016/j.jaac.2012.01.008. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Ashare RL, Hawk LW, Shiels K, Rhodes JD, Pelham WE, Waxmonsky JG. Methylphenidate enhances prepulse inhibition during processing of task-relevant stimuli in attention-deficit/hyperactivity disorder. Psychophysiology. 2010;47:838–845. doi: 10.1111/j.1469-8986.2010.01001.x. doi: 10.1111/j.1469-8986.2010.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Ickowicz A, Logan GD, Hogg-Johnson S, Schachar R, Tannock R. Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. Journal of Abnormal Child Psychology. 2003;31:315–327. doi: 10.1023/a:1023285614844. [DOI] [PubMed] [Google Scholar]
- Bubnik MG, Hawk LW, Pelham WE, Waxmonsky JG, Rosch KS. Reinforcement Enhances Vigilance Among Children With ADHD: Comparisons to Typically Developing Children and to the Effects of Methylphenidate. Journal of Abnormal Child Psychology. 2015;43:149–161. doi: 10.1007/s10802-014-9891-8. doi: 10.1007/s10802-014-9891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill DR, Seth S, Matthews K. A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: advancing beyond the three-pathway models. Psychological Medicine. 2013:1–13. doi: 10.1017/S0033291713002547. doi: 10.1017/S0033291713002547. [DOI] [PubMed] [Google Scholar]
- Coghill DR, Seth S, Pedroso S, Usala T, Currie J, Gagliano A. Effects of Methylphenidate on Cognitive Functions in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: Evidence from a Systematic Review and a Meta-Analysis. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.10.005. doi: 10.1016/j.biopsych.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Cohen D. Statistical power analyses for the behavioral sciences. 2nd Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- DeVito EE, Blackwell AD, Clark L, Kent L, Dezsery AM, Turner DC, Sahakian BJ. Methylphenidate improves response inhibition but not reflection-impulsivity in children with attention deficit hyperactivity disorder (ADHD) Psychopharmacology. 2009;202:531–539. doi: 10.1007/s00213-008-1337-y. doi: 10.1007/s00213-008-1337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Brinkman WB, Froehlich T, Langberg JM, Narad ME, Antonini TN, Altaye M. Effects of stimulant medication, incentives, and event rate on reaction time variability in children with ADHD. Neuropsychopharmacology. 2011;36:1060–1072. doi: 10.1038/npp.2010.243. doi: 10.1038/npp.2010.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SW, Owens JS, Bunford N. Evidence-Based Psychosocial Treatments for Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Journal of Clinical Child and Adolescent Psychology. 2013 doi: 10.1080/15374416.2013.850700. doi: 10.1080/15374416.2013.850700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE, Gnagy EM, Burrows-MacLean L, Coles EK, Chacko A, Robb JA. The single and combined effects of multiple intensities of behavior modification and methylphenidate for children with attention deficit hyperactivity disorder in a classroom setting. School Psychology Review. 2007;36:195–216. [Google Scholar]
- Fabiano GA, Pelham WE, Waschbusch Daniel A., Gnagy Elizabeth M., Lahey Benjamin B., Chronis Andrea M., Burrows-MacLean Lisa. A Practical Measure of Impairment: Psychometric Properties of the Impairment Rating Scale in Samples of Children With Attention Deficit Hyperactivity Disorder and Two School-Based Samples. Journal of Clinical Child & Adolescent Psychology. 2006;35:369–385. doi: 10.1207/s15374424jccp3503_3. doi: 10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Antonini TN, Brinkman WB, Langberg JM, Simon JO, Adams R, Epstein JN. Mediators of methylphenidate effects on math performance in children with attention-deficit hyperactivity disorder. Journal of Developmental and Behavioral Pediatrics. 2014;35:100–107. doi: 10.1097/DBP.0000000000000025. doi: 10.1097/DBP.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang-Pollock CL, Mikami AY, Pfiffner L, McBurnett K. ADHD Subtype Differences in Motivational Responsivity but not Inhibitory Control: Evidence From a Reward-Based Variation of the Stop Signal Paradigm. Journal of Clinical Child and Adolescent Psychology. 2007;36:127–136. doi: 10.1080/15374410701274124. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Arnold LE, Richters JE, Severe JB, Vereen D, Vitiello B, Grp, MTA Cooperative Moderators and mediators of treatment response for children with attention-deficit/hyperactivity disorder - The multimodal treatment study of children with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 1999;56:1088–1096. doi: 10.1001/archpsyc.56.12.1088. [DOI] [PubMed] [Google Scholar]
- Konrad K, Gauggel S, Manz A, Scholl M. Lack of inhibition: a motivational deficit in children with attention deficit/hyperactivity disorder and children with traumatic brain injury. Child Neuropsychology. 2000;6:286–296. doi: 10.1076/chin.6.4.286.3145. doi: 10.1076/chin.6.4.286.3145. [DOI] [PubMed] [Google Scholar]
- Konrad K, Gunther T, Hanisch C, Herpertz-Dahlmann B. Differential effects of methylphenidate on attentional functions in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:191–198. doi: 10.1097/00004583-200402000-00015. [DOI] [PubMed] [Google Scholar]
- Leotti LA, Wager TD. Motivational influences on response inhibition measures. Journal of Experimental Psychology: Human Perception and Performance. 2010;36:430–447. doi: 10.1037/a0016802. doi: 10.1037/a0016802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, ter Wal A, Quik EH, Kemner C, Westenberg H, van Engeland H. Dose-related effect of methylphenidate on stopping and changing in children with attention-deficit/hyperactivity disorder. European Psychiatry. 2006;21:544–547. doi: 10.1016/j.eurpsy.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans L, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in Attention-Deficit/Hyperactivity Disorder: deficient inhibitory motor control? Journal of Abnormal Psychology. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Lipszyc J, Schachar R. Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. Journal of the International Neuropsychological Society. 2010;16:1064–1076. doi: 10.1017/S1355617710000895. doi: 10.1017/S1355617710000895. [DOI] [PubMed] [Google Scholar]
- Lloyd DR, Medina DJ, Hawk LW, Fosco WD, Richards JB. Habituation of reinforcer effectiveness. Frontiers in Integrative Neuroscience. 2014;7:107. doi: 10.3389/fnint.2013.00107. doi: 10.3389/fnint.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neuroscience and Biobehavioral Reviews. 2010;34:744–754. doi: 10.1016/j.neubiorev.2009.11.021. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. Lawrence Erlbaum Associates; New York: 2008. [Google Scholar]
- Michel JA, Kerns KA, Mateer CA. The effect of reinforcement variables on inhibition in children with ADHD. Neuropsychology, Development, and Cognition: Section C, Child Neuropsychology. 2005;11:295–302. doi: 10.1080/092970490911270. [DOI] [PubMed] [Google Scholar]
- Nigg JT. The ADHD response-inhibition deficit as measured by the stop task: replication with DSM-IV combined type, extension, and qualification. Journal of Abnormal Child Psychology. 1999;27:393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychological Bulletin. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Sergeant JA. Effects of reward and response cost on response inhibition in AD/HD, disruptive, anxious, and normal children. Journal of Abnormal Child Psychology. 1998;26:161–174. doi: 10.1023/a:1022650216978. [DOI] [PubMed] [Google Scholar]
- Overtoom CC, Verbaten MN, Kemner C, Kenemans JL, van Engeland H, Buitelaar JK, Koelega HS. Effects of methylphenidate, desipramine, and l-dopa on attention and inhibition in children with Attention Deficit Hyperactivity Disorder. Behavioural Brain Research. 2003;145:7–15. doi: 10.1016/s0166-4328(03)00097-4. doi: 10.1016/s0166-4328(03)00097-4. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Burrows-Maclean L, Gnagy EM, Fabiano GA, Coles EK, Wymbs BT, Waschbusch DA. A Dose-Ranging Study of Behavioral and Pharmacological Treatment in Social Settings for Children with ADHD. Journal of Abnormal Child Psychology. 2014;42:1019–1031. doi: 10.1007/s10802-013-9843-8. doi: 10.1007/s10802-013-9843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Carlson C, Sams SE, Vallano G, Dixon MJ, Hoza B. Separate and combined effects of methylphenidate and behavior modification on boys with attention deficit-hyperactivity disorder in the classroom. Journal of Consulting and Clinical Psychology. 1993;61:506–515. doi: 10.1037/0022-006X.61.3.506. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. Journal of Clinical Child & Adolescent Psychology. 2008;37:184–214. doi: 10.1080/15374410701818681. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA, Massetti GM. Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34:449–476. doi: 10.1207/s15374424jccp3403_5. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Smith BH. Prediction and Measurement of individual responses to Ritalin by children and adolescents with attention deficit hyperactivity disorder. In: Greenhill LL, Osman BB, editors. Ritalin: theory and practice, second edition. Mary Ann Liebert, Inc.; NY: 2000. pp. 193–218. [Google Scholar]
- Pliszka SR. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Liotti M, Bailey BY, Perez R, 3rd, Glahn D, Semrud-Clikeman M. Electrophysiological effects of stimulant treatment on inhibitory control in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology. 2007;17:356–366. doi: 10.1089/cap.2006.0081. doi: 10.1089/cap.2006.0081. [DOI] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Sergeant JA. Response inhibition in children with DSM-IV subtypes of AD/HD and related disruptive disorders: the role of reward. Child Neuropsychology. 2001;7:172–189. doi: 10.1076/chin.7.3.172.8746. [DOI] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Swanson J, Morein-Zamir S, Meiran N, Schut H, Sergeant JA. The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. Journal of Abnormal Child Psychology. 2003;31:105–120. doi: 10.1023/a:1021729501230. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shanahan MA, Pennington BF, Willcutt EW. Do motivational incentives reduce the inhibition deficit in ADHD? Developmental Neuropsychology. 2008;33:137–159. doi: 10.1080/87565640701884238. doi: 791652918 [pii] 10.1080/87565640701884238. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Lysczek CL, Tannock R, Pelham WE, Spencer SV, Waschbusch DA. The effects of incentives on visual-spatial working memory in children with attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2008;36:903–913. doi: 10.1007/s10802-008-9221-0. doi: 10.1007/s10802-008-9221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Reynolds B, Mazzullo RJ, Rhodes JD, Pelham WE, Gangloff BP. Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Experimental and Clinical Psychopharmacology. 2009;17:291–301. doi: 10.1037/a0017259. doi: 10.1037/a0017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarek M, Velling S, Bunk D, Eggers C. Motivational effects on inhibitory control in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:355–363. doi: 10.1097/00004583-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Bitsakou P, Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Spencer SV, Hawk LW, Richards JB, Shiels K, Pelham WE, Waxmonsky JG. Stimulant treatment reduces lapses in attention among children with ADHD: the effects of methylphenidate on intra-individual response time distributions. Journal of Abnormal Child Psychology. 2009;37:805–816. doi: 10.1007/s10802-009-9316-2. doi: 10.1007/s10802-009-9316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J, Quittner AL, Zuckerman JB, Moore S. Behavioral inhibition, self-regulation of motivation, and working memory in children with attention deficit hyperactivity disorder. Developmental Neuropsychology. 2002;21:117–139. doi: 10.1207/S15326942DN2102_1. [DOI] [PubMed] [Google Scholar]
- Strand MT, Hawk LW, Jr., Bubnik M, Shiels K, Pelham WE, Jr., Waxmonsky JG. Improving working memory in children with attention-deficit/hyperactivity disorder: the separate and combined effects of incentives and stimulant medication. Journal of Abnormal Child Psychology. 2012;40:1193–1207. doi: 10.1007/s10802-012-9627-6. doi: 10.1007/s10802-012-9627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Carlson CL. Task demands interact with the single and combined effects of medication and contingencies on children with ADHD. Journal of Attention Disorders. 2007;10:372–380. doi: 10.1177/1087054706289946. [DOI] [PubMed] [Google Scholar]
- Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without comorbid anxiety. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:886–896. doi: 10.1097/00004583-199507000-00012. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. Journal of Abnormal Child Psychology. 1989;17:473–491. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Chambers CD, Logan GD. Fictitious inhibitory differences: how skewness and slowing distort the estimation of stopping latencies. Psychological Science. 2013;24:352–362. doi: 10.1177/0956797612457390. doi: 10.1177/0956797612457390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler DL. Wechsler Intelligence Scale for Children - Fourth Edition (WISC-IV) The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.