Abstract
Here, we present an inexpensive rapid-prototyping method that allows researchers and children to quickly assemble multi-layered microfluidic devices from easily pre-fabricated building blocks. We developed low-cost (<$2) kits based on laser-cut acrylic building block pieces and double-sided tape that allow users to generate water droplets in oil, capture living cells, and conduct basic phototaxis experiments. We developed and tested a 90-min lesson plan with children aged 12–14 yr and provide here the instructions for teachers to replicate these experiments and lessons. All parts of the kit are easy to make or order. We propose to use such easy to fabricate kits in labs with no access to current microfluidic tools as well as in classroom environments to get exposure to the powerful techniques of microfluidics.
I. INTRODUCTION
Over the last two decades, microfluidics evolved into a major field of research,1,2 and technologies based on microfluidic advances are rapidly emerging.3,4 The precise control of very small volumes of liquids allows for an array of new experiments, sensors,5,6 diagnostics,7 production units,8 and manipulation of single cells9 or even multicellular organisms.10,11 Even though many researchers at the university level have access to cleanroom facilities and various fast, accessible, and inexpensive fabrication methods have been proposed, microfluidics remains inaccessible to researchers across many fields due to complexity, time-, money-, and labor-intensive fabrication as well as unawareness of the power of microfluidics. As a result, the domain of microfluidics remains underutilized by research areas outside specialized research laboratories, and very little knowledge about miniaturization of fluids and benefits thereof is currently taught in schools and universities.12 Typically, a clean room is required where soft lithography tools are employed to fabricate silicon master molds to subsequently make poly(dimethylsiloxane) (PDMS) devices that are bonded to glass slides. Nowadays, such PDMS microfluidic devices (often called chips) are easily fabricated at well-equipped academic institutions at a graduate student level and above.13
However, to produce inexpensive chips in under an hour, other tools must be used. In addition to complex (traditional) clean room production, novel cheap, fast, and easy tools have been proposed. These approaches include use of paper,14 shrinky-dinks,15,16 wax,7 paraffin,17 or glass.18 Laser cut tape has been used for replica-molding19 and to fabricate cost-effective optical biochips.20 Also, advanced tape bonding techniques21 and low-cost prototyping methods with micro-milling,22 robotic cutter,23 or simple personal craft cutter (e.g., Silhouette) have been developed.18,24,25 Laser cutting also has been used to direct-write channels into polymethyl-methacrylate substrates.26 Using tape as a master removes the need for a cleanroom, but still requires PDMS and plasma bonding.27 For simple use, plug and play systems28 and even three dimensional building block type platforms have been proposed.29–32 Many of these rapid prototyping methods also are proven to be biocompatible and allow relevant biological experiments. For example, microfluidic stickers have recently been used for cell- and tissue-based assays in microchannels.33
While some of the mentioned tools and methods are convenient and powerful in a research environment, to our knowledge, very few educational approaches to microfluidics are available. Some setups employing shrinky-dinks,34 Jell-O chips,35 and PDMS36 have been proposed for classroom usage. Even given the availability of some fast prototyping and production methods, we note that quickly made devices are, even in well-equipped laboratories, still scarce albeit microfluidics will eventually be widely used in many labs. Furthermore, microfluidics will not only be an important platform in cell biology, materials science, chemistry, physics, fluid mechanics, and engineering disciplines but also giving its interdisciplinary nature, it is also a great learning opportunity in Science, Technology, Engineering, and Mathematics (STEM) education for students in K-12 and undergraduate levels.12 Hands-on teaching and gamification37 of learning has been proven beneficial,38 and experiments such as electrolysis of water39 or analytical devices40 have been proposed. Bardin and Lee41 recently proposed a whole series of experiments focusing on various age groups and featuring learning opportunities about fabrication, droplet generation, and flow characteristics.
To promote the accessibility of microfluidics, we present a platform to quickly and simply build microfluidic devices based on prefabricated building blocks without the need of specialized tools or knowledge. To introduce young researcher to microfluidics and in order to demonstrate the ease of use of our system, we distributed premade microfluidic building kits (Fig. 1, supplementary material Fig. S2)47 to middle-school students and asked them to build their own microfluidic devices. After a brief introduction and demonstration, the children built simple droplet generators within minutes, encapsulated the microorganism Euglena gracilis into droplets, and performed phototaxis experiments with the aid of a flashlight and a microscope. Overall, 35 students engaged with the system for an average of 1.5 h.
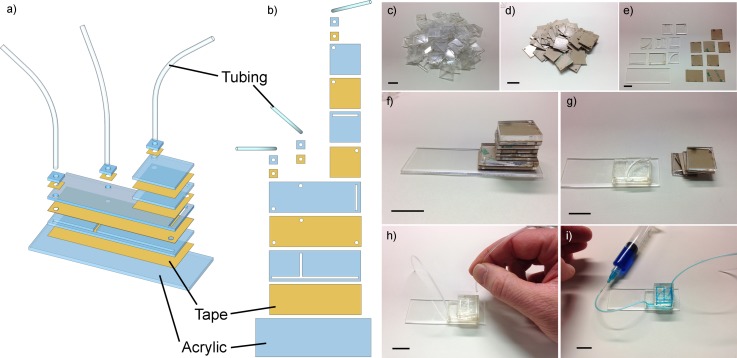
FIG. 1.
Premade building blocks allow the fabrication of a microfluidic device within minutes. (a) and (b) CAD (computer-aided design) representations of a stack of laser-cut acrylic (blue) and tape (yellow) layer. (c) and (d) Pile of acrylic parts and double-sided tape to select from. (e) Exemplary layout designed by a student and (f) the designed stack before building the device. (g) Pealing protective layers on tape and building of the device layer by layer. Holes in the tape allow for continuous channels and layer-to-layer transportation. After assembly and (h) attachment of tubes, (i) water (with added food-color) is flown through the device to visualize the channel's path. The whole building process and operation are shown in a time-lapse movie S1 and movie S2 in the supplementary material.47 All scale bars are 2 cm.
II. BUILDING BLOCKS
In this communication, we take it a step further and present an inexpensive modular approach to quickly build microfluidic devices. Simple building blocks were laser-cut in poly(methyl methacrylate) (a.k.a. PMMA or acrylic) and double-sided tape. Channel features were cut into the acrylic sheet, and holes were cut into the tape in order to allow fluids to flow from layer to layer. Figure 1 shows how such a device is build: After the selection of the acrylic building blocks and appropriate tape featuring specific laser-cut holes, the individual acrylic pieces are taped together layer by layer. Finally, tubing is attached to allow flow of liquids into and through the channels. See supplementary material movie S1 for device in action.47 While at low flow rates simple plugging of the tubes into the laser-cut holes was sufficient, we also prepared tube/seal-caps to prevent leakage at higher flow rates (see supplementary material Fig. S3).47 A small amount of glue (Gorilla Super Glue) was applied to the top of the small linking piece close to the tubes and cured overnight before use. A complete kit contains all needed materials including acrylic sheets (TAP Plastics, 1/16th in. thick) featuring simple laser-cut patterns (Epilog Laser Helix 24 × 18, 45 W), laser-cut double-sided adhesive tape (3M 300LSE), tubing with pre-attached connection pieces, blunt needles, and syringes filled with dyed water (food color, blue and yellow), oil (mineral oil with 5% Span 80), and an Euglena in water suspension. Our typical costs run below $2 per kit. Please refer to supplementary material Tables S1/S2 for a detailed part list and cost breakdown.47
III. SUGGESTED TEACHING STRUCTURE AND EXPERIMENTS
For classroom usage, we suggest the teaching structure shown in Table I. This structure was also used for the user-study presented in the second part of this communication. To start, a straight channel with continuous water flow (Fig. 2(a)) and a four-way-junction with multiple inlets for color mixing (Fig. 2(b)) can be demonstrated as the simplest microfluidic device. Demonstrating is an important step, as the children can see how syringes (with blunt needles) are operated and used to manually push liquids through the channels.
TABLE I.
Recommended teaching structure and user-study setup including tasks and learning objectives. Nos. 1–7 was conducted in a user-study during two sessions with a total of 35 students. No. 8 is only recommended for advanced students and if a laser-cutter is available.
| No. | Topic | Task | Time | Learning objective |
|---|---|---|---|---|
| 1 | Theory | Read/listen | 10 min | Mixing of liquids. Surfactants and emulsions. Hydro-/Lipo-phil/phobicity |
| 2 | Demonstration | Observe | 5 min | Understand device operation. Droplet formation. Syringe usage |
| 3 | Layout design | Select parts form kit | 10 min | 3-dimensional thinking. Planning. Teamwork. |
| 4 | Fabrication | Build devices | 15 min | Dexterity. 3-dimensional thinking |
| 5 | Flow/mixing | Use 2 water inlets and mix | 10 min | Operate device including syringes. Colour mixing. Blue + Yellow = Green |
| 6 | Droplets | Switch one water inlet to oil to make droplets | 15 min | Formation of droplets with oil, water, and surfactant. Adjust syringe pressures to generate various droplet sizes |
| 7 | Bioreactor | Include microorganisms into droplets. Apply light stimulus | 20 min | Recognize single Euglena cells. Understand concepts of photosynthesis and phototaxis. Observe single cells with microscope |
| 8 | Custom pieces | Laser-cutting | 2–4 h | Design and laser-cut alternative building blocks. For example, design maze with up and down streams |
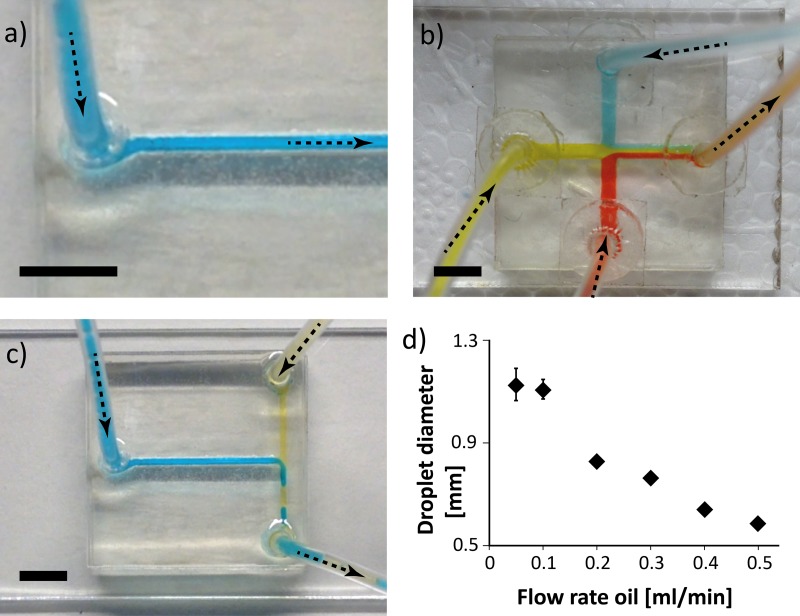
FIG. 2.
Examples of basic microfluidic devices built from the kit presented here. (a) A straight channel with laminar flow. (b) 3 colored water streams are flown into a four-way-junction simultaneously and only mix slowly towards the outlet due to diffusion. (c) T-junction generation of water-in-oil droplets. Drop size inside the channel shown here is about 2.4 mm long and contains 2.3 μl per drop. This drop will have a diameter of about 800 μm in an open well (see also Figs. 3 and 4). (d) Characterization of droplet generation in a T-junction: At steady aqueous flow rate (here 0.1 ml/min), an increased oil flow rate results in smaller drops. The arrows indicate the flow direction. Scale bars are 5 mm. n = 10 for each flow rate.
Concomitant to these basic devices, a basic calculation of the Reynolds number (Re) can be conducted together with the students and types of liquid flows discussed. In Equation (1), ρ is the density of the liquid, L is the characteristic length (hydraulic diameter, here 1 mm is used), μ is the dynamic viscosity, and v is the velocity which can be obtained from the volumetric flow rate Q and the cross-section A (Eq. (2))
| (1) |
| (2) |
The Reynolds number turns out to be small (<100), which indicates2 laminar flow with no turbulence and making diffusion the main force for mixing. Therefore, if separate water streams clash (Fig. 2(b)), they only mix after they travel some distance towards the outlet channel or if the flow is disturbed, e.g., when the streams reach the outlet and flow into the tube.
Next, to demonstrate the production of water-in-oil droplets, oil and water should be flowed through a T-junction (Fig. 2(c)). During the development of the kit, a droplet generating T-junction was calibrated by using syringe pumps to modulate the oil flow rate (0.05–0.1 ml/min) and hold the aqueous flow rate constant (0.1 ml/min) (Fig. 2(d)). Conforming to the theory, faster oil flow rates led to smaller droplets, and droplets ranging from 0.6 mm to 1.1 mm diameter were obtained. This corresponds to volumes of 100–700 nl.
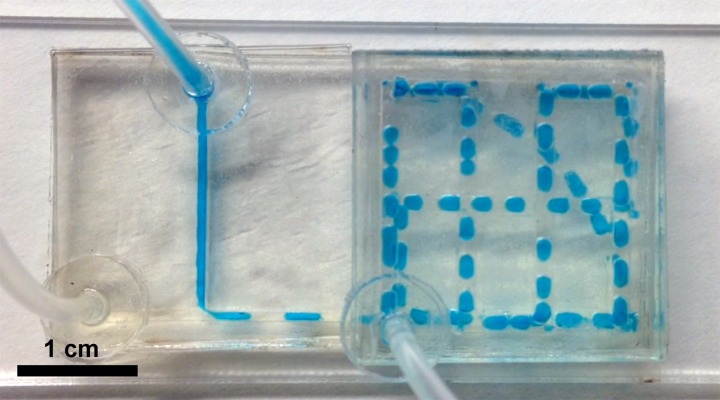
Thanks to the layer-by-layer design, this rapid prototyping approach allows fabrication of devices with multiple flow layers (Fig. 3 and movie supplementary material S1).47 All the channels are present in the acrylic layers, while the tape layers stick the acrylic together, separate the channels, and connect the channels through holes within the tape. This building block approach provides a large degree of freedom as selecting and assembling various building blocks lead to countless combinations and a broad range of devices are imaginable.
FIG. 3.
A student-made device featuring a T-junction is used to generate water-in-oil droplet, which is then flown through a downstream 3-dimensional maze. The continuous phase (clear, straight channel) is mineral oil with 5% added Span 80 to prevent coalescence of the droplets; the dispersed phase (blue, from top) is dyed water. Channel width is about 0.6 mm in T-junction and 1.3 mm in the maze. Refer to the supplementary material S2 for a movie of this device in action.47
As a biological experiment, we suggest incorporating E. gracilis, a widely studied single-celled flagellate protist often used in class experiments,42 into droplets. Euglena are well suited for children as they are already commonly used in schools and safe to use, big enough to see with a low magnification microscope including subcellular detail (Fig. 4), easy to cultivate, ethical to handle,43 and readily available from commercial suppliers (e.g., Carolina, living culture, #151351). Due to their phototactic behavior, Euglena cells optimize their light exposure and hence avoid very bright light due to negative phototaxis.44 As a result, they agglomerate on one side of the droplets if light is shone from the side. The droplets containing Euglena shown in Figure 4 were exposed to 12 000 lx for 15 s with a flashlight (Maglite XL200, illuminance was measured with Reed LM-81LX light meter). It is worthwhile mentioning that the droplet produced here is stable for several days, but the Euglena inside remain motile and responsive to light inside these droplets only for about the duration of the experiment (2 h). (Note: the Euglena stock solution as supplied by Carolina is fine to use for multiple weeks.) For long-term experiments, oil/surfactant combinations with increased biocompatibility should be chosen. Water-in-fluorocarbon drops have shown great potential for compartmentalizing both in vitro and in vivo biological systems, and surfactants such as Pluronic F-68 (MP Biochemicals)45 or Krytox-PEG600-Krytox46 (RAN Biotechnologies) are commercially available.
FIG. 4.
Using a T-junction as shown in Fig. 2(c), E. gracilis can be captured into aqueous droplets surrounded by oil and assessed for their response to light stimuli. Upon illumination with a strong flashlight (12k lx) from the right side, Euglena exhibited negative phototaxis and agglomerated on the left side of each droplet within 15 s. The continuous phase was mineral oil with 5% added Span 80 surfactant.
IV. USER STUDY
To test the suitability of our building block based kit for educational purposes and in order to demonstrate the kit's ease of use, a user-study with 35 7th and 8th grade middle-school students (typically 12–13 yr old) was conducted in two sessions (16 and 19 students). To probe interest, success, and overall excitement, a short questionnaire was distributed after the conclusion of each session, and the instructors took notes and pictures during both sessions. In the following, only results from the second session are shown, since in the second session revised assembly instructions were provided thanks to feedback from the first session, and hence improved devices were fabricated.
Following a brief 10-min introduction on the topics of fluids, mixing behavior, hydro/lipo-phil/phobicity, and emulsions, the instructors demonstrated for about 5 min a preassembled device featuring a T-junction and showed how water-in-oil droplets are generated (Fig. 2). Then, the two instructors provided pairs of students (there was also one group of 3) sets of prefabricated building blocks and needed components in order to allow them to create their own microfluidic devices.
First, the children designed their devices and laid out all pieces in the right order. The instructors checked the functionality (i.e., connectivity from layer to layer). On average laying out the pieces consumed 10 min, and about 80% of devices were error-free and did not need any correction from the instructors. In the following 15 min, the children fabricated the devices by using the double-sided tape to stick the acrylic pieces together (Fig. 5(a)). Only one team misaligned the pieces during assembly. The difficulty of this activity was rated by the children as 2.6 for layout and 2.7 for building on average (1–5 scale: very easy–very difficult). Such moderate difficulty scores are desirable as overly simple activities are boring/non-instructive, whereas overly difficult ones are only frustrating and lead to less engagement. For their initial experiments, the children were asked to inject blue and yellow dyed water into the two inlets and observe what happens. Even though some devices were leaking (32%) due to poor sealing of the tape in at least one layer, 79% of the students observed laminar mixing of blue and yellow water to a green mixture in their devices.
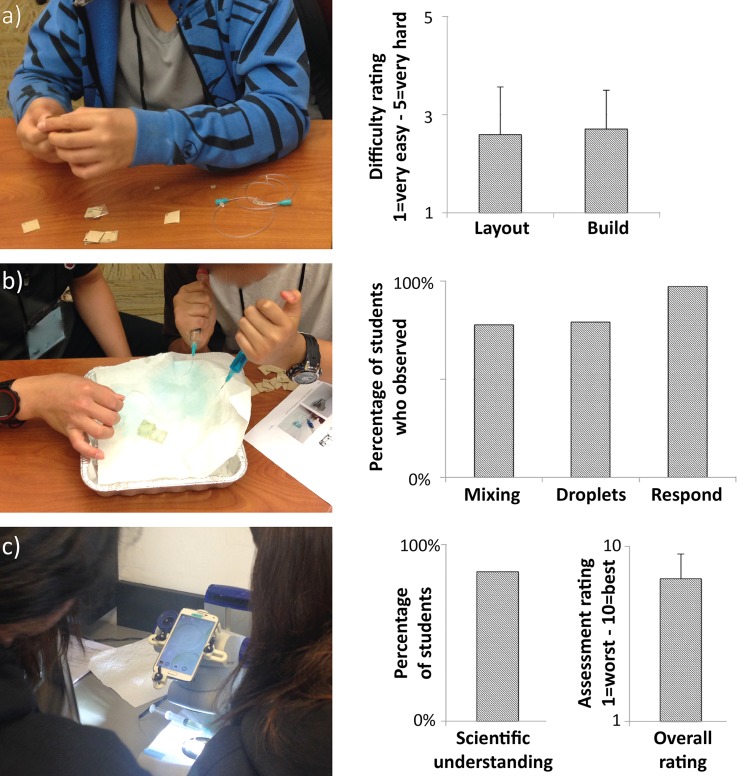
FIG. 5.
To assess the educational benefits of our kit, a user-study with two sessions with total of 35 7th and 8th graders (12–14 yr old) was conducted. (a) The assembly (including layout and building, Fig. 1) of a device was rated by the students at moderate difficulty. (b) A high percentage of students were able to observe color mixing, droplet formation, and Euglena responding to light (Figs. 2–4). (c) Students used a microscope with a mounted smartphone to conduct phototaxis experiments. 89% of the children showed some scientific understanding of the basics of Euglena phototaxis (Fig. 4) and got an idea of the benefits microfluidics may provide (estimate by the instructors based on interactions with the students and the final questionnaire). This activity received an average overall rating of 8.3 on a 1–10 scale. Percentage values show no error bars as binary values (observed/not observed, understood/did not understand) were used. (n = 19).
For the second experiment, the students were asked to exchange the water syringes connected to the straight channel with the oil syringe, to press equally to both inlets, and to observe what happens (Fig. 5(b)). 79% were able to generate droplets with their device and observe colored droplets flowing through the 3 dimensional maze. Judging from the excited interjecting comments, droplet formation was the most fascinating part of the whole microfluidic experience. After 15 min, we asked the children to exchange the remaining colored water syringe with the syringe containing the Euglena suspension, to form droplets once again, and to observe. We collected some of the successfully generated bio-droplets in a dish and presented the droplets to the children under a 10× microscope with a mounted smartphone for easy observation (Fig. 5(c)). Every child was able to see individual Euglena inside the droplets. In order to demonstrate phototaxis, we then placed a flashlight to the side and shone light from one side into the well. As the Euglena avoided this very bright light (12k lx), they agglomerated after 15 s on the far end of each droplet. See Figure 4 and supplementary material movie S3 for changing light conditions and the Euglena's response.47 All but one student were able to observe this behavior and showed at least some understanding of phototaxis.
Judging by the children's positive reactions and answers on the feedback form, most participants enjoyed this learning method (8.3 points of overall rating on a scale from 1 to 10 (super boring-super exciting)) and expressed a desire for more hands-on STEM learning in their school. In addition to user study with 7th and 8th grade middle-school students, our system was also tested with a total of 28 2nd and 3rd graders (typically 7–8 yr old). Even though some functional devices were made, we do not advise using this system with such young learners.
V. RECREATING THIS ACTIVITY
Replicating and extending these activities are straight forward as all parts can be ordered (Tables S1/S2);47 instructions for the teacher and student work sheet are in the supplementary material S5.47 In short: The acrylic and tape building blocks need to be fabricated, which can be easily achieved with an available laser cutter, or manually using a drill and saw, or by ordering from a commercial laser cut service (we successfully tested Pololu.com who provided a kit for 20 students for $100, where we had Amazon ship the tape to them). The .ai file provided in the supplementary material can be modified to design additional layouts.47 The safety concerns for this activity are low and in line with existing school experiments. Attention should be paid to the blunt needles; we recommend children to wear safety glasses (in the unlikely event of sudden pressurized fluid escape). While all liquids are commonly found in food, cosmetics, and ponds, we recommend proper hand washing afterwards.
VI. CONCLUSIONS
We presented an inexpensive kit with building blocks that allows children and researchers to rapidly fabricate functional microfluidic devices. With minimal instruction 7th and 8th graders build 3-dimensional devices typically within 30 min, conducted classic microfluidic operations, and encapsulated E. gracilis into droplets to conduct a basic phototaxis experiment. Given the simplicity and speed of preparing microfluidic devices from laser-cut materials, we believe that such a building block technique can also be used to quickly test microfluidics oriented hypotheses. This allows designing, cutting, and assembling of novel devices within about an hour at very low costs. Compared to typical (i.e., photolithographic) processes, where multiple steps may take several days, huge time-savings can be achieved. Nowadays, laser-cutters are widely available in research environments such as universities or can be purchased at relatively low cost ($10k). Given the total cost <$2/kit for this user-study, we expect cost to drop below $1/kit due to cheaper manufacturing methods (die-cutting or injection molding) and economies of scale if 1000 + kits are produced.
Most importantly, for students and children, this microfluidic kit constitutes an interesting and fun learning opportunity and getting to know the benefits of miniaturization might allow them to use this powerful research tool later in their lives. To address even younger students, additional features can be added to each layer, such as cut or printed numbers to indicate in which layer of the stack the piece goes and the piece's proper orientation. For older students, including college level device classes,38 more flexibility can be granted by, for instance, allowing the students to create novel designs of their choosing.
ACKNOWLEDGMENTS
This work has been supported in part by NSF Cyberlearning (NSF 1324753). L.C.G. acknowledges support from the Swiss National Science Foundation. Brogan Miller is kindly acknowledged for support with the laser-cutting, Stanford Splash for organizing the Splash 2015 event, and Nate J. Cira, Seung-Ah Lee, and Marc Anthony for helpful discussions and inspiration. Contribution: L.C.G. and I.R.K. designed the experiments. L.C.G. designed and fabricated the kits. L.C.G. and H.K. executed the user-study. L.C.G., H.K., and I.R.K. wrote the paper.
References
- 1. Whitesides G. M., Nature 442, 368 (2006). 10.1038/nature05058 [DOI] [PubMed] [Google Scholar]
- 2. Squires T. and Quake S., Rev. Mod. Phys. 77, 977 (2005). 10.1103/RevModPhys.77.977 [DOI] [Google Scholar]
- 3. Jang J. S., Simon V. A., Feddersen R. M., Rakhshan F., Schultz D. A., Zschunke M. A., Lingle W. L., Kolbert C. P., and Jen J., BMC Genomics 12, 144 (2011). 10.1186/1471-2164-12-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen C. H., Cho S. H., Chiang H.-I., Tsai F., Zhang K., and Lo Y.-H., Anal. Chem. 83, 7269 (2011). 10.1021/ac2013465 [DOI] [PubMed] [Google Scholar]
- 5. Nie Z., Nijhuis C. A., Gong J., Chen X., Kumachev A., Martinez A. W., Narovlyansky M., and Whitesides G. M., Lab Chip 10, 477 (2010). 10.1039/B917150A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerber L. C., Rosenfeld L., Chen Y., and Tang S. K. Y., Lab Chip 14, 4324 (2014). 10.1039/C4LC00640B [DOI] [PubMed] [Google Scholar]
- 7. Martinez A. W., Phillips S. T., Whitesides G. M., and Carrilho E., Anal. Chem. 82, 3 (2010). 10.1021/ac9013989 [DOI] [PubMed] [Google Scholar]
- 8. Chan E. M., Mathies R. A., and Alivisatos A. P., Nano Lett. 3, 199 (2003). 10.1021/nl0259481 [DOI] [Google Scholar]
- 9. Baret J.-C., Miller O. J., Taly V., Ryckelynck M., El-Harrak A., Frenz L., Rick C., Samuels M. L., Hutchison J. B., Agresti J. J., Link D. R., Weitz D. A., and Griffiths A. D., Lab Chip 9, 1850 (2009). 10.1039/b902504a [DOI] [PubMed] [Google Scholar]
- 10. Chronis N., Lab Chip 10, 432 (2010). 10.1039/B919983G [DOI] [PubMed] [Google Scholar]
- 11. Carr J. A., Parashar A., Gibson R., Robertson A. P., Martin R. J., and Pandey S., Lab Chip 11, 2385 (2011). 10.1039/c1lc20170k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fintschenko Y., Lab Chip 11, 3394 (2011). 10.1039/c1lc90069b [DOI] [PubMed] [Google Scholar]
- 13. Friend J. and Yeo L., Biomicrofluidics 4, 026502 (2010). 10.1063/1.3259624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez A. W., Phillips S. T., and Whitesides G. M., PNAS 105, 19606 (2008). 10.1073/pnas.0810903105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grimes A., Breslauer D. N., Long M., Pegan J., Lee L. P., and Khine M., Lab Chip 8, 170 (2008). 10.1039/B711622E [DOI] [PubMed] [Google Scholar]
- 16. Nguyen D., McLane J., Lew V., Pegan J., and Khine M., Biomicrofluidics 5, 022209 (2011). 10.1063/1.3576930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Renaudot R., Agache V., Fouillet Y., Laffite G., Bisceglia E., Jalabert L., Kumemura M., Collard D., and Fujita H., Lab Chip 13, 4517 (2013). 10.1039/c3lc50850a [DOI] [PubMed] [Google Scholar]
- 18. Yuen P. K. and Goral V. N., J. Chem. Educ. 89, 1288 (2012). 10.1021/ed3000292 [DOI] [Google Scholar]
- 19. Luo L. W., Teo C. Y., Ong W. L., Tang K. C., Cheow L. F., and Yobas L., J. Micromech. Microeng. 17, N107 (2007). 10.1088/0960-1317/17/12/N02 [DOI] [Google Scholar]
- 20. Patko D., Mártonfalvi Z., Kovacs B., Vonderviszt F., Kellermayer M., and Horvath R., Sens. Actuators, B 196, 352 (2014). 10.1016/j.snb.2014.01.107 [DOI] [Google Scholar]
- 21. Thompson C. S. and Abate A. R., Lab Chip 13, 632 (2013). 10.1039/c2lc40978j [DOI] [PubMed] [Google Scholar]
- 22. Guckenberger D. J., de Groot T. E., Wan A. M. D., Beebe D. J., and Young E. W. K., Lab Chip 15, 2364 (2015). 10.1039/C5LC00234F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Z., Zhao Z., and Lo J. F.-J., in SPIE MOEMS-MEMS, edited by Gray B. L. and Becker H. ( SPIE, 2014), p. 89760B. [Google Scholar]
- 24. Yuen P. K. and Goral V. N., Lab Chip 10, 384 (2010). 10.1039/B918089C [DOI] [PubMed] [Google Scholar]
- 25. Ragavendar M. S., Jayaraman S., Ramya V. M., Roy R., and Manwani H., in 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (2014), p. 6609. [DOI] [PubMed] [Google Scholar]
- 26. Hong T.-F., Ju W.-J., Wu M.-C., Tai C.-H., Tsai C.-H., and Fu L.-M., Microfluid. Nanofluid. 9, 1125 (2010). 10.1007/s10404-010-0633-0 [DOI] [Google Scholar]
- 27. Kim J., Surapaneni R., and Gale B. K., Lab Chip 9, 1290 (2009). 10.1039/b818389a [DOI] [PubMed] [Google Scholar]
- 28. Lim J., Maes F., Taly V., and Baret J.-C., Lab Chip 14, 1669 (2014). 10.1039/c3lc51399h [DOI] [PubMed] [Google Scholar]
- 29. Bhargava K. C., Thompson B., and Malmstadt N., Proc. Natl. Acad. Sci. 111, 15013 (2014). 10.1073/pnas.1414764111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang M., Wu J., Wang L., Xiao K., and Wen W., Lab Chip 10, 1199 (2010). 10.1039/b923101c [DOI] [PubMed] [Google Scholar]
- 31. Rhee M. and Burns M. A., Lab Chip 8, 1365 (2008). 10.1039/b805137b [DOI] [PubMed] [Google Scholar]
- 32. Hsieh Y.-F., Yang A.-S., Chen J.-W., Liao S.-K., Su T.-W., Yeh S.-H., Chen P.-J., and Chen P.-H., Sens. Actuators, B 204, 489 (2014). 10.1016/j.snb.2014.07.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morel M., Bartolo D., Galas J.-C., Dahan M., and Studer V., Lab Chip 9, 1011 (2009). 10.1039/B819090A [DOI] [PubMed] [Google Scholar]
- 34. Hemling M., Crooks J. A., Oliver P. M., Brenner K., Gilbertson J., Lisensky G. C., and Weibel D. B., J. Chem. Educ. 91, 112 (2014). 10.1021/ed4003018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang C. W. T., Ouellet E., and Lagally E. T., Anal. Chem. 82, 5408 (2010). 10.1021/ac902926x [DOI] [PubMed] [Google Scholar]
- 36. Chia M. C., Sweeney C. M., and Odom T. W., J. Chem. Educ. 88, 461 (2011). 10.1021/ed1008624 [DOI] [Google Scholar]
- 37. Riedel-Kruse I. H., Chung A. M., Dura B., Hamilton A. L., and Lee B. C., Lab Chip 11, 14 (2011). 10.1039/C0LC00399A [DOI] [PubMed] [Google Scholar]
- 38. Cira N. J., Chung A. M., Denisin A. K., Rensi S., Sanchez G. N., Quake S. R., and Riedel-Kruse I. H., PLoS Biol. 13, e1002110 (2015). 10.1371/journal.pbio.1002110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davis T. A., Athey S. L., Vandevender M. L., Crihfield C. L., Kolanko C. C. E., Shao S., Ellington M. C. G., Dicks J. K., Carver J. S., and Holland L. A., J. Chem. Educ. 92, 116 (2015). 10.1021/ed400757m [DOI] [Google Scholar]
- 40. Piunno P. A. E., Zetina A., Chu N., Tavares A. J., Noor M. O., Petryayeva E., Uddayasankar U., and Veglio A., J. Chem. Educ. 91, 902 (2014). 10.1021/ed400728a [DOI] [Google Scholar]
- 41. Bardin D. and Lee A. P., Lab Chip 14, 3978 (2014). 10.1039/C4LC00424H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Littleford R. A., Am. Biol. Teach. 22, 551 (1960). 10.2307/4439448 [DOI] [Google Scholar]
- 43. Harvey H., Havard M., Magnus D., Cho M. K., and Riedel-Kruse I. H., Hastings Cent. Rep. 44, 38 (2014). 10.1002/hast.386 [DOI] [PubMed] [Google Scholar]
- 44. Colombetti G., Häder D.-P., Lenci F., and Quaglia M., Curr. Microbiol. 7, 281 (1982). 10.1007/BF01566863 [DOI] [Google Scholar]
- 45. Brouzes E., Medkova M., Savenelli N., Marran D., Twardowski M., Hutchison J. B., Rothberg J. M., Link D. R., Perrimon N., and Samuels M. L., Proc. Natl. Acad. Sci. 106, 14195 (2009). 10.1073/pnas.0903542106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holtze C., Rowat A. C., Agresti J. J., Hutchison J. B., Angilè F. E., Schmitz C. H. J., Köster S., Duan H., Humphry K. J., Scanga R. A., Johnson J. S., Pisignano D., and Weitz D. A., Lab Chip 8, 1632 (2008). 10.1039/b806706f [DOI] [PubMed] [Google Scholar]
- 47.See supplementary material at http://dx.doi.org/10.1063/1.4935593E-BIOMGB-9-003506 for movies, additional figures, and cost estimations. Movie S1 shows how building blocks are assembled to a functional droplet generator. Movie S2 shows the device from Figure 3 in action: Blue water droplets in oil are generated and subsequently flown through a 3D maze. Movie S3 shows Euglena inside droplets as produced by the students. First, a flashlight is shone from SW, later from NE. The changing light conditions will make the Euglena move inside the droplets. Table S1 displays the cost for one kit as used in the user-study. Table S2 estimates cost for a set of 20 kits for class of 20 students. Figure S1 shows the drawings for the laser cutter. Figure S2 shows complete kit including dyed water and oil. Figure S3 shows a connector close-up with seal cap. Additionally, the CAD drawings and instructions to recreate the activity are provided.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1063/1.4935593E-BIOMGB-9-003506 for movies, additional figures, and cost estimations. Movie S1 shows how building blocks are assembled to a functional droplet generator. Movie S2 shows the device from Figure 3 in action: Blue water droplets in oil are generated and subsequently flown through a 3D maze. Movie S3 shows Euglena inside droplets as produced by the students. First, a flashlight is shone from SW, later from NE. The changing light conditions will make the Euglena move inside the droplets. Table S1 displays the cost for one kit as used in the user-study. Table S2 estimates cost for a set of 20 kits for class of 20 students. Figure S1 shows the drawings for the laser cutter. Figure S2 shows complete kit including dyed water and oil. Figure S3 shows a connector close-up with seal cap. Additionally, the CAD drawings and instructions to recreate the activity are provided.