Abstract
BACKGROUND
Twenty-four-hour ambulatory blood pressure (BP) patterns have been associated with diminished cognitive function in hypertensive and very elderly populations. The relationship between ambulatory BP patterns and cognitive function in community-living older adults is unknown.
METHODS
We conducted a cross-sectional study in which 24-hour ambulatory BP, in-clinic BP, and cognitive function measures were obtained from 319 community-living older adults.
RESULTS
The mean age was 72 years, 66% were female, and 13% were African-American. We performed linear regression with performance on the Montreal Cognitive Assessment (MoCA) as the primary outcome and 24-hour BP patterns as the independent variable, adjusting for age, sex, race/ethnicity, education, and comorbidities. Greater nighttime systolic dipping (P = 0.046) and higher 24-hour diastolic BP (DBP; P = 0.015) were both significantly associated with better cognitive function, whereas 24-hour systolic BP (SBP), average real variability, and ambulatory arterial stiffness were not.
CONCLUSIONS
Higher 24-hour DBP and greater nighttime systolic dipping were significantly associated with improved cognitive function. Future studies should examine whether low 24-hour DBP and lack of nighttime systolic dipping predict future cognitive impairment.
Keywords: aging, ambulatory blood pressure, blood pressure; cognitive function, hypertension.
Associations between in-clinic blood pressure (BP) and cognitive function have been the subject of much interest. Higher in-clinic systolic BP (SBP) and pulse pressure1,2 have each been associated with diminished cognitive function independent of age and traditional cardiovascular risk factors. Some studies have associated higher diastolic BP (DBP) with poorer cognitive function,3,4 while others have found both high and low DBP associated with poorer cognitive function.5 One study has explained these inconsistent findings based on gender, with higher in-clinic mean arterial pressures being associated with better cognition in men and poorer cognition in women.6 While the pathways remain uncertain, a number of pathophysiological mechanisms may account for these associations, including the development of atherosclerosis,7 silent cerebral infarcts, and white matter lesions.8,9
In addition to in-clinic BP, BP patterns over 24 hours may also have implications for cognitive function, but these have received less attention. 24-hour ambulatory BP monitoring (ABPM) offers the opportunity for more precise estimation of average BP over the day than in-clinic readings, and can provide valuable information about 24-hour BP patterns, including 24-hour BP averages, nocturnal dipping, average real variability (ARV), and ambulatory arterial stiffness index (AASI). Two studies in populations of hypertensive Japanese patients have found that greater BP variability was independently associated with worse cognitive function.10,11 DBPs may be associated with cognitive function in that hypotension throughout the day may lead to brain hypoperfusion, contributing to the progression of vascular dementia.10 Greater AASI—derived from ABPM measurements—has been found to predict stroke but has not been studied in relation to cognitive function.12,13 Another marker of variability derived from ABPM measurements—the ARV—has been associated with magnetic resonance imaging evidence of small vessel disease in community-living patients.14 The association of nighttime systolic dipping pattern and cognitive function has been inconsistent in prior studies, with some finding associations between blunted nighttime dipping and cognitive function or stroke and others finding no such associations.15–20 The majority of this prior work on ABPM patterns and cognition has been done in small, hypertensive referral populations or in a Japanese community-living cohort of 70-year olds14; the association between ABPM patterns and cognition in older community-living individuals is, to our knowledge, largely unexplored. The US Preventive Services Task Force is presently considering inclusion of ABPM as a general recommendation for screening and diagnosis of hypertension21; if indeed this becomes more widely used, understanding its associations with important aging-related outcomes such as cognitive function will be valuable.
We thus examined the relationship of ABPM parameters, in particular dipping, 24-hour average BPs, and ARV and AASI, with cognitive function in a cohort of community-dwelling older adults. We examined the associations of both ABPM and in-clinic BPs with cognitive function to provide a clinical reference point since ABPM is infrequently measured in current clinical practice and to determine if ABPM could reveal associations with cognitive function that in-clinic measurements did not. Our hypotheses were that abnormal ABPM parameters (nondipping, high 24-hour systolic and low 24-hour diastolic, high ARV, and high AASI) would be independently associated with decreased cognitive function on the Montreal Cognitive Assessment (MoCA).
METHODS
Study population
The University of California, San Diego (UCSD) Ambu latory Blood Pressure Study recruited from participants in the parent San Diego Population Study. The methods of the San Diego Population Study have previously been described.22 In brief, the San Diego Population Study is a study cohort originally assembled to examine the prevalence of chronic peripheral arterial and venous disease in asymptomatic, healthy individuals. The original cohort included 2,211 free-living white, African-American, Hispanic, and Asian volunteers aged 29–91; follow-up of these participants included 1,103 adults who returned for a visit between 2009 and 2011. The original participants were recruited from UCSD employees and spouses of UCSD employees, with a target of a minimum of 15% from each ethnic group.
From January 2012 to June 2013, we sent 944 letters inviting participating adults from the follow-up study who were over 60 years of age for an additional visit, the UCSD Ambulatory Blood Pressure Study, directed at evaluating measures of ambulatory BP, physical function, and cognitive function; these letters were followed by reminder cards and a phone call/voice message. Initial letters were not sent to those who were known to have moved out of San Diego County (N = 14), had requested not to be contacted again (N = 102), or who were known to be deceased (N = 32) or who learned about the repeat study through their spouse or friends (N = 11). Of those who received initial letters, 354 completed some portion of the study protocol (response rate 37.5%); the majority of those receiving letters did not reply to repeated inquiries, but those who did decline participation by postcard (N = 25) cited other commitments or lack of interest as major factors. Eligible participants were independently living adults over 60 years of age, although if a participant’s spouse had been a previous San Diego Population Study participant and was 60 years or younger, they were invited as well (N = 2). We did not specifically exclude participants based on diminished cognitive function but all participants had to be able to provide their own informed consent. Participants who lived outside San Diego County were excluded. The study received institutional review board approval at the University of California, San Diego, and all participants provided informed consent; no financial incentive was provided to participants.
BP measurements
At the initial visit, participants’ in-clinic BP was recorded using a Dynapulse device (Dynapulse Corp, Vista, CA). After 5 minutes of rest, 3 seated BP measurements were taken 2 minutes apart in the right arm; clinic BP was considered to be the average of these 3 recordings. One standing BP was recorded after 3 minutes of equilibration to the standing position. Orthostatic BP was calculated as the difference between the standing clinic BP and the average of the 3 sitting BPs. Participants were then provided with an automated ambulatory BP cuff (Spacelabs) to wear for 24 hours. During this time, the cuff automatically recorded BP measurements every 20 minutes while the participant was awake and every 60 minutes while the participant was asleep.
Several measures of 24-hour BP patterns were calculated from the ABPM cuff data. We considered an adequate ABPM exam to be one that included at least 14 readings during the day and 6 at night. We calculated the ARV of the SBPs—a measure of BP variability over 24 hours)—as the mean of the absolute values of the difference between subsequent BP readings.23 We calculated the AASI as one minus the slope of the DBP reading over the SBP reading.24 We calculated nighttime systolic dipping as the percentage change from average daytime SBP to average nighttime SBP, basing the time windows for sleep and wake times on individual patient diary reports.
Other measurements
Baseline demographics and medical history were established by questionnaire and interview. Participants’ height and weight were measured at the visit. Blood specimens were obtained and serum creatinine was measured; the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to determine estimated glomerular filtration rate (eGFR),25 and participants provided urine samples for albumin-to-creatinine ratio (ACR). History of cardiovascular disease (CVD), hypertension, and diabetes was obtained through interview, as were questionnaires regarding medication use. The Geriatric Depression Scale,26 a 15-question instrument developed as a basic screening measure for depression in older adults, was administered to all participants.
Cognitive function measures
At the clinic visit, trained personnel administered several standard, validated measures of cognitive function. A priori, we chose to examine the MoCA as our primary endpoint because the MoCA has been validated with a high sensitivity for detecting mild cognitive impairment and tests a variety of cognitive domains.27 The MoCA is a 10-minute, 1-page written test measuring visuospatial, naming, memory, attention, language, abstraction, delayed recall, and orientation domains, and is scored out of 30 points, with a score less than 26 indicating the presence of at least mild cognitive impairment in prior studies with a sensitivity of 100% and specificity of 87%.28
Additional cognitive function measures were also administered so that specific domains of interest could be assessed in secondary analyses. The Hopkins Verbal Learning Test—Revised is a brief test of verbal learning and memory.29 The Trail-Making Test covers a number of domains, including attention, visual scanning, cognitive flexibility, and executive function.30,31 The Boston Naming Test (Short Form) evaluates aphasia and impairment of word retrieval, which requires participants to name pictures of varying levels of familiarity.32 The Digit Symbol Substitution Test measures nonspecific cognitive function and the speed of information processing.33 The Stroop Color–Word Test measures cognitive flexibility, attention, and response to cognitive stress.34
Statistical analysis
For baseline characteristics, we categorized participants based on MoCA scores ≥26 vs. MoCA scores <26. Differences in baseline characteristics were compared with the use of t-test and Mann–Whitney U-test for continuous variables and chi-square test for categorical variables. To graphically examine associations of BP measures and cognitive function and ensure linear relationships prior to multivariate modeling, we created unadjusted plots quartiles of BP variables against mean MoCA score. To test for trend in these plots, we used a Jonckheere–Terpstra trend test to evaluate the ordered differences in MoCA score across quartiles of the ABPM parameter.
We used linear regression to examine the association between BP patterns and cognitive function. Our predictor variables included some obtained from in-clinic (SBP, DBP, pulse pressure, and orthostatic hypotension) and 24-hour (SBP, DBP, pulse pressure, nighttime systolic dipping, systolic ARV, and AASI) BP measurements. We performed a separate linear regression for each of these predictor variables with MoCA as the outcome variable.
We selected potential confounding factors a priori from relevant demographic factors and data that we hypothesized might confound the relationship of BP with cognitive function. We chose variables that would be commonly available to a practicing clinician in most routine clinical practices. Covariates included demographic factors (age, sex, race/ethnicity, and education), medical history (alcohol use, CVD, history of treated hypertension, family history of CVD, diabetes, and smoking history), and in-clinic and laboratory measurements (body mass index (BMI), eGFR, and albumin-to-creatinine ratio).
For BP measures that found to be associated with MoCA score, we carried those measures forward and examined their association with the 5 other cognitive function tests in secondary analysis to provide a more comprehensive picture of the domains of cognitive function that might be most affected. These models included all potential confounding factors as covariates. Last, we tested for multiplicative interactions on the basis of age (split into 2 groups by median age), gender, and history of treated hypertension. When statistically significant interactions were observed, stratified analyses were evaluated. Finally, as a sensitivity analysis, we examined results in the subset of participants without chronic disease (no hypertension, diabetes, or CVD) to test the robustness of these findings in healthy older adults. All analyses were conducted using STATA SE version 11 statistical software (Statacorp, College Station, TX) and SAS version 9.3 (SAS Institute, Cary, NC), and P-values <0.05 were considered statistically significant for all analyses including interaction terms.
RESULTS
We enrolled 354 older adults in the study, of whom 319 had full data available for this analysis. The 35 participants who did not have complete data were slightly older (average age 76 vs. 72 years) and slightly more were women (80% vs. 66%); 14 participants were unable to complete the ABPM. The mean age of the 319 participants was 72±7 years, 66% were female and 13% were African-American. The mean in-clinic SBP and DBP were 141±16 and 75±10mm Hg, respectively, while the mean 24-hour SBP and DBP were 127±13 and 73±8mm Hg. The mean nighttime systolic dipping was an 11% relative to the daytime SBP. The mean MoCA score was 25±3. Baseline characteristics stratified by MoCA score are shown in Table 1. Participants with a MoCA score <26 were older, more likely to be African-American, and less likely to have postsecondary education. They were also more likely to be diabetic and less likely to use alcohol. Participants with a MoCA score <26 also had higher in-clinic and 24-hour SBPs, higher in-clinic and 24-hour pulse pressures, and lower 24-hour DBPs and less nighttime systolic dipping.
Table 1.
Baseline characteristics of participants by cognitive impairment
| Characteristic | MoCA category | ||
|---|---|---|---|
| I | II | P-value | |
| Cut points, MoCA score | <26 | ≥26 | |
| N | 166 | 153 | |
| Demographics | |||
| Age, years, mean ± SD | 73.5±7 | 70±6 | <0.01 |
| Female | 111 (67) | 100 (65) | 0.77 |
| Caucasian | 80 (48) | 110 (72) | <0.01 |
| African-American | 30 (18) | 12 (8) | 0.007 |
| Hispanic | 26 (16) | 21 (14) | 0.62 |
| Asian | 21 (13) | 9 (6) | 0.04 |
| Postsecondary education | 148 (89) | 146 (95) | 0.04 |
| Medical history | |||
| Cardiovascular disease | 30 (18) | 21 (14) | 0.29 |
| Family history of cardiovascular disease | 137 (86) | 129 (82) | 0.67 |
| History of hypertension | 77 (50) | 85 (52) | 0.83 |
| Diabetic | 25 (15) | 12 (7) | 0.04 |
| Alcohol user | 100 (60) | 118(77) | 0.001 |
| Current or former smoker | 47 (28) | 65 (36) | 0.071 |
| Antihypertensive medications used | |||
| angiotensin converting enzyme inhibitor/angiotensin receptor blocker | 56 (33) | 62 (41) | 0.2 |
| Beta-blocker | 34 (20) | 23 (15) | 0.2 |
| Calcium channel blockers | 31 (18) | 19 (12) | 0.12 |
| Diuretics | 43 (25) | 37 (24) | 0.72 |
| Mean number of BP medications (among users) | 1.9 | 1.9 | 0.98 |
| Measurements | |||
| Body mass index, kg/m2 ± SD | 27±5 | 27±5 | 0.9 |
| CKD-EPI eGFR, ml/min/1.73 m2 ± SD | 74±16 | 77±15 | 0.1 |
| Urine albumin-to-creatinine ratioa | 22 (13, 32) | 16 (10, 33) | 0.15 |
| Mean in-clinic systolic blood pressure, mm Hg ± SD | 142±17 | 139±15 | 0.11 |
| Mean in-clinic diastolic blood pressure, mm Hg ± SD | 74±10 | 76±9 | 0.14 |
| Mean in-clinic pulse pressure, mm Hg ± SD | 68±13 | 63±12 | 0.002 |
| Orthostatic hypotension, 10mm Hg | −3.4±12 | −2.8±11 | 0.65 |
| Mean 24-hour systolic blood pressure, mm Hg ± SD | 128±13 | 126±11 | 0.18 |
| Mean 24-hour diastolic blood pressure, mm Hg ± SD | 72±8 | 74±7 | 0.05 |
| Mean 24-hour pulse pressure, mm Hg ± SD | 56±12 | 52±10 | 0.004 |
| Mean wake systolic blood pressure, mm Hg ± SD | 130 (13) | 129 (12) | 0.31 |
| Mean sleep systolic blood pressure, mm Hg ± SD | 117 (16) | 113 (13) | 0.01 |
| Mean wake diastolic blood pressure, mm Hg ± SD | 74 (8) | 76 (8) | 0.04 |
| Mean sleep diastolic blood pressure, mm Hg ± SD | 64 (9) | 65 (8) | 0.51 |
| Nighttime systolic dipping, % | 10±8 | 12±7 | 0.02 |
| AASI | 0.53±0.14 | 0.50±0.14 | 0.06 |
| ARV (systolic) | 11±2 | 11±2 | 0.31 |
| Average geriatric depression score (range 0–15) | 1.2 | 1.3 | 0.14 |
Abbreviations: AASI, ambulatory arterial stiffness index; ARV, average real variability; BP, blood pressure; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; MoCA, Montreal Cognitive Assessment.
Values are in number (%) unless otherwise indicated.
aMedian (quartile 1, quartile 3).
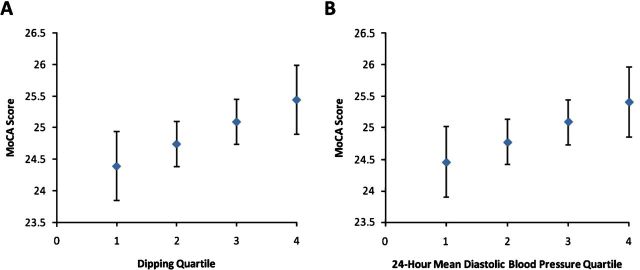
Considering MoCA as a continuous variable in unadjusted analysis, all in clinic and 24-hour BP parameters were associated with MoCA performance except for orthostatic BP (Table 2). After adjusting for age, sex, race, and education, none of the in-clinic BP parameters remained associated with the MoCA. Age adjustment was responsible for most of the attenuation. In contrast, on the ABPM, higher 24-hour DBP and greater nighttime systolic dipping remained significantly associated with a higher MoCA score. These results remained largely unchanged with additional adjustment for cardiovascular risk factors and kidney function. In the fully adjusted model, each 10mm Hg higher 24-hour mean DBP was associated with a 0.58 point higher MoCA score, and each 10% greater nighttime systolic dipping was associated with a 0.40 point higher MoCA score. Separating 24-hour DBP into sleep and wake components, we found that fully adjusted beta coefficients of wake and sleep DBP with MoCA were 0.54 (95% confidence interval (CI) 0.1 to 0.99) and 0.12 (95% CI −0.02 to 0.58) respectively, thus a slightly stronger association with wake-time DBP. When participants were categorized by quartile, higher systolic dipping quartiles and higher 24-hour DBP quartiles were both associated with higher MoCA scores (Figure 1A,B, Jonckheere–Terpstra trend tests significant for both figures).
Table 2.
Association of blood pressure measurements with MoCA score
| Blood pressure measure | Unadjusted | Age-adjusted | Model 1a | Model 2b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | P-value | Beta | 95% CI | P-value | Beta | 95% CI | P-value | Beta | 95% CI | P-value | |
| In-clinic measurements | ||||||||||||
| Systolic blood pressure, 10mm Hg | −0.3 | −0.48 to −0.02 | 0.03 | −0.11 | −0.33 to 0.10 | 0.3 | −0.01 | −0.23 to 0.21 | 0.92 | 0.01 | −0.23 to 0.22 | 0.93 |
| Diastolic blood pressure, 10mm Hg | 0.41 | 0.04 to 0.79 | 0.03 | 0.12 | −0.25 to 0.45 | 0.53 | 0.23 | −0.01 to 0.60 | 0.2 | 0.18 | −0.21 to 0.55 | 0.38 |
| Pulse pressure, 10mm Hg | −0.61 | −0.89 to −0.34 | <0.001 | −0.28 | −0.57 to 0.01 | 0.06 | −0.16 | −0.45 to 0.12 | 0.25 | −0.12 | −0.41 to 0.18 | 0.43 |
| Orthostatic hypotension, 10mm Hg | 0.09 | −0.23 to 0.41 | 0.58 | 0.14 | −0.17 to 0.44 | 0.37 | 0.14 | −0.15 to 0.43 | 0.48 | 0.12 | −0.17 to 0.40 | 0.41 |
| 24-hour measurements | ||||||||||||
| Systolic blood pressure, 10mm Hg | −0.06 | −0.34 to 0.22 | 0.67 | −0.02 | −0.24 to 0.30 | 0.85 | 0.07 | −0.19 to 0.38 | 0.58 | 0.13 | −0.15 to 0.39 | 0.37 |
| Diastolic blood pressure, 10mm Hg | 1.1 | 0.64 to 1.55 | <0.001 | 0.67 | 0.20 to 1.1 | 0.005 | 0.67 | 0.21 to 1.1 | 0.004 | 0.58 | 0.12 to 1.05 | 0.015 |
| Pulse pressure, 10mm Hg | −0.6 | −0.92 to −0.28 | 0.002 | −0.28 | −0.60 to 0.04 | 0.09 | −0.21 | −0.52 to 0.10 | 0.18 | −0.11 | −0.44 to 0.23 | 0.53 |
| Nighttime systolic dipping, % | 0.07 | 0.02 to 0.11 | 0.005 | 0.05 | 0.03 to 0.10 | 0.03 | 0.05 | .003 to 0.09 | 0.03 | 0.04 | 0.00 to 0.09 | 0.046 |
| Average real variability, mm Hg | −0.27 | −0.44 to −0.09 | 0.003 | −0.14 | −0.31 to 0.03 | 0.11 | −0.12 | −0.29 to 0.04 | 0.15 | −0.12 | −0.29 to 0.05 | 0.16 |
| AASIc | −0.41 | −0.77 to −0.06 | 0.02 | −0.28 | −0.61to 0.06 | 0.11 | −0.26 | −0.58 to 0.07 | 0.12 | −0.18 | −0.51 to 0.15 | 0.28 |
Abbreviations: AASI, ambulatory arterial stiffness index; CI, confidence interval; MoCA, Montreal Cognitive Assessment.
aAdjusted for age, sex, race/ethnicity, and education.
bAdjusted for age, sex, race/ethnicity, education, alcohol use, cardiovascular disease, family history of cardiovascular disease, hypertension, diabetes, smoking, body mass index, eGFR, and ACR.
cAASI per standard deviation.
Figure 1.
Unadjusted relationship between dipping quartiles and 24-hour diastolic BP quartiles and MoCA score (Jonckheere–Terpstra trend test for dipping quartiles, P = 0.02; for 24-hour diastolic BP quartiles, P < 0.001). Abbreviations: BP, blood pressure; MoCA, Montreal Cognitive Assessment.
As 24-hour mean DBP and nighttime dipping percentage were independently associated with MoCA scores, we examined the associations of these BP measures with other measures of cognitive function. In these secondary analyses, we found that greater nighttime dipping was modestly associated with better performance on the immediate recall portion of the Hopkins Verbal Learning Test—Revised, which measures memory, and on the Trail-Making Test Part A, which measures a number of domains including cognitive flexibility, visuospatial processing, and executive function (Table 3). A higher 24-hour mean DBP was significantly associated with better performance only on the color portion of the Stroop Word–Color Association test.
Table 3.
Adjusted association of nighttime systolic dipping and 24-hour diastolic blood pressure with cognitive function
| Cognitive function measure | Systolic dipping, % | 24-hour diastolic bood pressure, 10mm Hg | ||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | P-value | Beta | 95% CI | P-value | |
| MoCA | 0.05 | 0.00 to 0.09 | 0.033 | 0.58 | 0.12 to 1.05 | 0.015 |
| Hopkins Verbal Learning Test | ||||||
| Immediate recall | 0.10 | 0.03 to 0.16 | 0.003 | 0.32 | −0.38 to 1.03 | 0.36 |
| Delayed recall | 0.04 | 0.00 to 0.08 | 0.051 | 0.18 | −0.24 to 0.60 | 0.39 |
| Retention % | 0.18 | −0.19 to 0.56 | 0.34 | 1.79 | −2.2 to 5.78 | 0.37 |
| Discrimination | 0.01 | −0.02 to 0.05 | 0.52 | 0.17 | −0.19 to 0.54 | 0.34 |
| Trail-Making Test | ||||||
| Part A | −0.27 | −0.46 to −0.07 | 0.007 | −1.94 | −4.02 to 0.14 | 0.07 |
| Part B | −0.54 | −1.09 to 0.02 | 0.06 | −4.14 | −10.1 to 1.8 | 0.17 |
| Stroop Word–Color Test | ||||||
| Word score | 0.12 | −0.11 to 0.35 | 0.31 | 2.02 | −0.45 to 4.5 | 0.11 |
| Color score | 0.03 | −0.14 to 0.20 | 0.75 | 2.09 | 0.23 to 3.9 | 0.03 |
| Word–color score | 0.10 | −0.03 to 0.22 | 0.12 | 0.26 | −1.09 to1.62 | 0.7 |
| Boston Naming Test | 0.00 | −0.04 to 0.03 | 0.91 | 0.00 | −0.36 to 0.37 | 0.97 |
| Digit symbol substitution | 0.09 | −0.07 to 0.25 | 0.29 | 0.29 | −1.4 to 2.0 | 0.73 |
Abbreviations: CI, confidence interval; MoCA, Montreal Cognitive Assessment.
Last, we tested whether age (split at median age for cohort), gender, and history of hypertension or use of BP medications modified the relationship of dipping percentage and DBP with MoCA score. We found no evidence for effect modification across any of these subgroups (all P-values for interaction >0.05). In the 145 individuals who had no diabetes, hypertension, or CVD, our findings were comparable in direction and magnitude to the full cohort but underpowered for statistical significance (fully adjusted beta value for systolic dipping with MoCA score was 0.06 (−0.01 to 0.13), and for 24 diastolic mean BP with MoCA score 0.52 (−0.02 to 0.12).
DISCUSSION
In this cohort of community-living older adults, we observed that both greater nighttime systolic dipping and higher 24-hour mean DBP on the ABPM were associated with better cognition, whereas for 24-hour SBP, AASI, and ARV we did not find independent associations with cognitive function. In-clinic BP parameters did not independently associate with cognitive function.
Associations with MoCA score remained significant after adjustment for demographic factors, cardiovascular risk factors, and kidney function and appeared similar across age categories, gender, and in individuals with and without a history of hypertension.
Some studies have found an association between blunted nighttime systolic dipping and cognitive dysfunction,16 whereas in other studies, the association between nondipping and cognitive function was limited to particular cognitive domains (learning and memory).17 Our findings are consistent with a recent study of hypertensive patients, which found higher rates of strokes in both nondippers and dippers with a high morning BP surge.20 To our knowledge, all studies reporting no association between dipping and cognitive function were conducted in hypertensive populations, suggesting that study populations may account for this difference. Our findings are consistent with the subset of studies that found an association between dipping and cognitive function, and extend them to community-living older adults, with or without hypertension. By examining for effect modification, we are also able to conclude that the association between dipping and cognitive function is similar in both hypertensive and normotensive individuals, as well as across genders and in those without chronic diseases.
We also demonstrated an association between dipping across a number of cognitive function tests. The finding of an association with some tests, such as the MoCA, Hopkins Verbal Learning Test—Revised immediate recall, and Trail-Making Test, but not with others, such as the delayed recall portions of the Hopkins Verbal Learning Test—Revised and Boston Naming Test, suggests that dipping may be associated primarily with preservation of executive function, rather than other domains such as memory and delayed word retrieval. Furthermore, while extreme dipping, defined as a nighttime dip ≥20%, has previously been associated with stroke in older hypertensive patients,35 we found that the degree of dipping was associated with better cognitive function in a linear fashion across quartiles. Although we cannot draw causal inferences from this cross-sectional study, we hypothesize that dysregulation of the normal dipping pattern may be associated with dysfunctional cerebral autoregulation and therefore cognitive dysfunction.
We also found that higher 24-hour mean DBP was associated with better cognitive function. Interestingly, in-clinic DBP was only weakly associated with cognitive function in unadjusted analysis, an association that was attenuated and rendered no longer statistically significant when adjusted for age. Some studies have found that a higher in-clinic DBP is associated with poorer cognitive function and white matter lesions.8,36 These studies had large sample sizes and may have been statistically powered to detect an association that we did not detect. In-clinic measurements may also detect different physiological phenomena than ABPM. A person with a normal or relatively high in-clinic DBP may have a low DBP over 24 hours and be subject to periods of brain hypoperfusion, a potential contributing factor to vascular dementia.11 However, 1 study in a population-based cohort of 70-year-old men in Sweden found that higher ambulatory DBP was associated with poorer cognitive function.3 Thus, our results are in conflict. The exact reason for these differing findings is unclear, and may be attributable to differences in study populations, such as the inclusion of both genders and a wider age range in our population. Our data also did not suggest a U-shape or J-shape relationship between DBP and cognitive function, which has been previously found in the Baltimore Longitudinal Study of Aging.5 However, the range of DBP in our population did not include a substantial number of individuals with average DBP >90, so we are not able to investigate associations with the higher range of DBP; we hypothesize, however, that there is a point at which DBP is not sufficient for cerebral perfusion and that under that point low DBP is associated with cognitive dysfunction.
We found no association between SBP, or any measures of arterial stiffness—pulse pressure, AASI, or ARV—and cognitive function. Our finding of no association between SBP and cognitive function was surprising given the possibility of end-organ damage and recent findings by Kwon et al.37 that greater 24-hour BP is associated with more advanced white matter lesions in stroke patients. It is possible that community-living patients are better able to auto-regulate cerebral perfusion for systolic pressures throughout the day than for diastolic pressures, which better represent a patient’s baseline perfusion. This finding is consistent with the findings of Kilander et al.,3 who found no association between 24-hour SBP and cognitive function in community-living adults. With regard to measures of variability—2 prior studies have found associations with variability in SBP with cognitive function, but these studies were performed in referral populations of hypertensive patients and patients with chronic diseases.10,11 Other studies of community-living adults have associated higher pulse pressure with poorer cognitive function,2 an association that was not present in our data. Our results suggest that the importance of increased pulse pressure in relatively healthy adults may not be in arterial stiffness itself, but rather that lower diastolic pressure is the component of importance for cognitive function.
Strengths of the present study include its evaluation of a diverse cohort of free-living older adults, allowing us to extend findings to this population and compare findings to prior studies in hypertensive, chronic kidney disease, and very elderly patients. In addition, the availability of both clinic-based and 24-hour ABPM measurements and multiple tests of cognitive function allowed us to ascertain the degree to which additional information above and beyond clinic BP could be garnered with the ABPM. Considerable race/ethnic diversity, representation of both genders, a wide age spectrum, and availability of measures of multiple potential confounding variables are additional strengths.
Our study also has important limitations. The study population was recruited from employees at a university and had a high rate of postsecondary education, which may limit the generalizability of our findings to populations with less education. We used a cross-sectional design and we cannot determine the temporal direction of the associations or cause–effect relationships. We could neither obtain imaging studies to link cognitive function testing to abnormalities on brain magnetic resonance imaging, nor obtain formal pulse-wave velocity studies for arterial stiffness.
In conclusion, we demonstrate that higher 24-hour DBP and dipping percentage are both independently associated with better cognitive function in community-living older adults; associations that were stronger than those seen with in-clinic BP readings. If confirmed, and if 24-hour DBP and dipping patterns are shown to precede cognitive impairment in longitudinal studies, these findings suggest that addition of ABPM to clinic-based BP may ultimately allow identification older person at higher risk for cognitive impairment above and beyond other risk factors for cognitive decline. Secondary analyses of future BP intervention studies can be conducted to examine if aggressive lowering of DBP might worsen the trajectory of cognitive function, or if manipulation of dipping pattern with timed administration of antihypertensives might improve trajectory of cognitive function in older community-living persons.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by K23 DK091521 (D.E.R.). Mr. Conway was supported by a medical student research grant from the UCSD O’Brien Core Center for Acute Kidney Injury Research (NIH P30-DK079337). The investigators wish to thank T. Tran for her work as study coordinator and the participants in the UCSD ABPM study.
REFERENCES
- 1. Obisesan TO, Obisesan OA, Martins S, Alamgir L, Bond V, Maxwell C, Gillum RF. High blood pressure, hypertension, and high pulse pressure are associated with poorer cognitive function in persons aged 60 and older: the Third National Health and Nutrition Examination Survey. J Am Geriatr Soc 2008; 56:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 2008; 51:99–104. [DOI] [PubMed] [Google Scholar]
- 3. Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension 1998; 31:780–786. [DOI] [PubMed] [Google Scholar]
- 4. Tsivgoulis G, Alexandrov AV, Wadley VG, Unverzagt FW, Go RC, Moy CS, Kissela B, Howard G. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology 2009; 73:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension 2005; 45:374–379. [DOI] [PubMed] [Google Scholar]
- 6. Cherbuin N, Mortby ME, Janke AL, Sachdev PS, Abhayaratna WP, Anstey KJ. Blood pressure, brain structure, and cognition: opposite associations in men and women. Am J Hypertens 2015; 28:225–231. [DOI] [PubMed] [Google Scholar]
- 7. Arnett DK, Boland LL, Evans GW, Riley W, Barnes R, Tyroler HA, Heiss G. Hypertension and arterial stiffness: the Atherosclerosis Risk in Communities Study. ARIC Investigators. Am J Hypertens 2000; 13:317–323. [DOI] [PubMed] [Google Scholar]
- 8. Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, Tyroler HA. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Atherosclerosis Risk in Communities Study. Stroke 1996; 27:2262–2270. [DOI] [PubMed] [Google Scholar]
- 9. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003; 348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 10. Kanemaru A, Kanemaru K, Kuwajima I. The effects of short-term blood pressure variability and nighttime blood pressure levels on cognitive function. Hypertens Res 2001; 24:19–24. [DOI] [PubMed] [Google Scholar]
- 11. Sakakura K, Ishikawa J, Okuno M, Shimada K, Kario K. Exaggerated ambulatory blood pressure variability is associated with cognitive dysfunction in the very elderly and quality of life in the younger elderly. Am J Hypertens 2007; 20:720–727. [DOI] [PubMed] [Google Scholar]
- 12. Dolan E, Thijs L, Li Y, Atkins N, McCormack P, McClory S, O’Brien E, Staessen JA, Stanton AV. Ambulatory arterial stiffness index as a predictor of cardiovascular mortality in the Dublin Outcome Study. Hypertension 2006; 47:365–370. [DOI] [PubMed] [Google Scholar]
- 13. Kikuya M, Staessen JA, Ohkubo T, Thijs L, Metoki H, Asayama K, Obara T, Inoue R, Li Y, Dolan E, Hoshi H, Hashimoto J, Totsune K, Satoh H, Wang JG, O’Brien E, Imai Y. Ambulatory arterial stiffness index and 24-hour ambulatory pulse pressure as predictors of mortality in Ohasama, Japan. Stroke 2007; 38:1161–1166. [DOI] [PubMed] [Google Scholar]
- 14. Yamaguchi Y, Wada M, Sato H, Nagasawa H, Koyama S, Takahashi Y, Kawanami T, Kato T. Impact of ambulatory blood pressure variability on cerebral small vessel disease progression and cognitive decline in community-based elderly Japanese. Am J Hypertens 2014; 27:1257–1267. [DOI] [PubMed] [Google Scholar]
- 15. Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens 2008; 26:1636–1641. [DOI] [PubMed] [Google Scholar]
- 16. Bellelli G, Frisoni GB, Lucchi E, Guerini F, Geroldi C, Magnifico F, Bianchetti A, Trabucchi M. Blunted reduction in night-time blood pressure is associated with cognitive deterioration in subjects with long-standing hypertension. Blood Press Monit 2004; 9:71–76. [DOI] [PubMed] [Google Scholar]
- 17. van Boxtel MP, Gaillard C, Houx PJ, Buntinx F, de Leeuw PW, Jolles J. Is nondipping in 24h ambulatory blood pressure related to cognitive dysfunction? J Hypertens 1998; 16:1425–1432. [DOI] [PubMed] [Google Scholar]
- 18. Van Boxtel MPJ, Henskens LHG, Kroon AA, Hofman P a. M, Gronenschild EHBM, Jolles J, de Leeuw PW. Ambulatory blood pressure, asymptomatic cerebrovascular damage and cognitive function in essential hypertension. J Hum Hypertens 2006; 20:5–13. [DOI] [PubMed] [Google Scholar]
- 19. Cicconetti P, Ciotti V, Monteforte G, Moisè A, Chiarotti F, Piccirillo G, Cacciafesta M. Circadian blood pressure pattern and cognitive function in newly diagnosed older hypertensives. Blood Press 2003; 12:168–174. [PubMed] [Google Scholar]
- 20. Pierdomenico SD, Pierdomenico AM, Cuccurullo F. Morning blood pressure surge, dipping, and risk of ischemic stroke in elderly patients treated for hypertension. Am J Hypertens 2014; 27:564–570. [DOI] [PubMed] [Google Scholar]
- 21. US Preventive Services Task Force. High Blood Pressure in Adults: Screening http://www.uspreventiveservicestaskforce.org/ Page/Document/RecommendationStatementDraft/hypertension-in-adults-screening-and-home-monitoring Accessed 4 July 2015.
- 22. Criqui MH, Jamosmos M, Fronek A, Denenberg JO, Langer RD, Bergan J, Golomb BA. Chronic venous disease in an ethnically diverse population: the San Diego Population Study. Am J Epidemiol 2003; 158:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mena L, Pintos S, Queipo NV, Aizpúrua JA, Maestre G, Sulbarán T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens 2005; 23:505–511. [DOI] [PubMed] [Google Scholar]
- 24. Li Y, Wang JG, Dolan E, Gao PJ, Guo HF, Nawrot T, Stanton AV, Zhu DL, O’Brien E, Staessen JA. Ambulatory arterial stiffness index derived from 24-hour ambulatory blood pressure monitoring. Hypertension 2006; 47:359–364. [DOI] [PubMed] [Google Scholar]
- 25. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17:37–49. [DOI] [PubMed] [Google Scholar]
- 27. Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can J Psychiatry 2007; 52:329–332. [DOI] [PubMed] [Google Scholar]
- 28. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699. [DOI] [PubMed] [Google Scholar]
- 29. Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test Revised: normative data and analysis of interform and test -retest reliability. Clin Neuropsychol 1998;12:43–55. [Google Scholar]
- 30. Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Motor Skills 1958;8:271–276. [Google Scholar]
- 31. Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci 1996; 17:305–309. [DOI] [PubMed] [Google Scholar]
- 32. Joan C., Borod HG. Normative data on the boston diagnostic aphasia examination, parietal lobe battery, and the Boston Naming Test. J Clin Exp Neuropsychol 1980; 2:209–215. [Google Scholar]
- 33. Salthouse TA. The role of memory in the age decline in digit-symbol substitution performance. J Gerontol 1978; 33:232–238. [DOI] [PubMed] [Google Scholar]
- 34. Golden CJ, Freshwater SM. The Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, IL: Stoelting. [Google Scholar]
- 35. Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 2001; 38:852–857. [DOI] [PubMed] [Google Scholar]
- 36. Guo X, Pantoni L, Simoni M, Bengtsson C, Björkelund C, Lissner L, Gustafson D, Skoog I. Blood pressure components and changes in relation to white matter lesions: a 32-year prospective population study. Hypertension 2009; 54:57–62. [DOI] [PubMed] [Google Scholar]
- 37. Kwon HS, Lim YH, Kim HY, Kim HT, Kwon HM, Lim JS, Lee YJ, Kim JY, Kim YS. Association of ambulatory blood pressure and heart rate with advanced white matter lesions in ischemic stroke patients. Am J Hypertens 2014; 27:177–183. [DOI] [PubMed] [Google Scholar]