Abstract
Hypertension is a significant global health burden associated with an increased risk of stroke, atherosclerosis and other cardiovascular diseases. Several risk factors including high dietary salt, obesity, genetics, race as well as behavioral and psychological factors contribute to development of this complex disease. Various hypertensive stimuli enhance sympathetic drive and promote autonomic dysfunction leading to elevated blood pressure. In further understanding the pathogenesis and end organ damage associated with hypertension, mounting evidence also highlights the role of inflammation in this process, and in particular the role of the adaptive immune system and T cells in this process. This review will discuss recent findings regarding the role of the central nervous system, T lymphocytes and the impact of cardiovascular risk factors such as psychological stress in hypertension.
Keywords: Inflammation, stress, hypertension
Introduction
Despite extensive study, the etiology of most cases of human hypertension remains unknown. Increasing evidence suggests that inflammation and adaptive immunity play a significant role in the pathogenesis of hypertension (Harrison et al., 2011; Muller et al., 2011). Previous studies from our laboratory have shown that mice lacking lymphocytes (RAG1−/−) are resistant to various forms of experimental hypertension and that adoptive transfer of T cells but not B cells restores their hypertensive phenotype (Guzik et al., 2007; Marvar et al., 2010). In these and other studies we demonstrate that hypertensive stimuli such as angiotensin II, high salt, catecholamines and more recently chronic psychological stress, cause T cell activation and entry of activated T cells into the peripheral blood vessels and kidney (Lob et al., 2010; Madhur et al., 2010; Marvar et al., 2010; Marvar et al., 2012). Recent work from other groups have extended these findings and provide further evidence of a role for T lymphocytes in salt-sensitive, pregnancy related, and pulmonary forms of hypertension (Rodriguez-Iturbe, 2010; Cuttica et al., 2011; De Miguel et al., 2011; Wallace et al., 2011). The severe combined immunodeficiency (SCID) mouse model also exhibits a blunted hypertensive response to chronic angiotensin II (Crowley et al., 2010). Therefore, independent of the type of experimental hypertension or the experimental model studied the adaptive immune response is important. The precise mechanism for how T cells become activated and contribute to hypertension is unknown and is currently being investigated by a number of research groups.
Neural Control of Blood Pressure in Hypertension and Inflammation
The central nervous system (CNS) is essential for processing and integrating neurohumoral signals from the periphery, modulating autonomic nervous activity to maintain blood pressure and fluid homeostasis (Guyenet, 2006). In both human hypertension and animal models of hypertension there are several lines of evidence supporting neural mechanisms that can be considered a contributing cause or consequence of neurogenic hypertension (Grassi et al., 2010). For example, elevated sympathetic nerve activity (Anderson et al., 1989), altered baroreceptor sensitivity (Matsukawa et al., 1991; Chapleau et al., 2001), enhanced norepinephrine turnover (Rumantir et al., 2000), angiotensin II and dietary salt (Osborn et al., 2007), inflammation (Waki et al., 2011) as well as environmental factors such as psychological stress (Esler et al., 2008; Davern & Head, 2011). Although these are all considered important factors in neurogenic hypertension their complex interactions and precise mechanisms are a matter of great debate. This brief review will be focused on the renin angiotensin system, psychological stress and implications in immune cell signaling and vascular inflammation in hypertension.
Autonomic control of blood pressure is in largely regulated by distinct nuclei in the hypothalamic forebrain (ie; circumventricular organs) and brainstem (McKinley et al., 2003) including the subfornical organ, the organum vasculosum of the lamina terminalis, the area postrema, and the median eminence (Figure 1). The circumventricular organs have an incompletely formed blood brain barrier and can therefore sense circulating hormones like angiotensin II. Importantly, circulating angiotensin II activates angiotensin type 1 (AT1) receptors in circumventricular organs (Brody, 1988), which in turn sends projections to hypothalamic areas, leading to long-term activation of hypothalamic neurons and increased sympathetic vasomotor activity (Davern & Head, 2007). This has been previously demonstrated in both animal (DiBona & Jones, 2001) and humans (Johansson et al., 1999) suggesting that circulating angiotensin II is a major signal to the CNS contributing to neurogenic hypertension.
Figure 1.
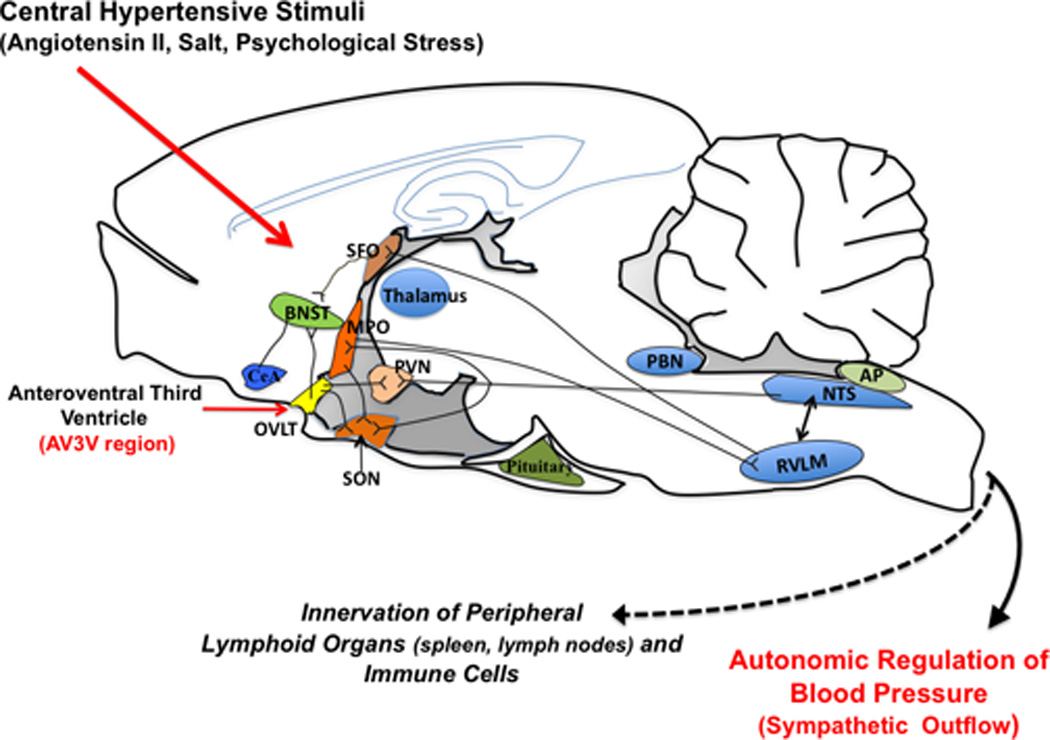
Schematic showing location and connections of some of the primary hypothalamic structures responsible for central angiotensin II signaling and the integration of the stress response. SFO, Subfornical organ; BNST, Bed nucleus stria terminalis; MPO, Median preoptic nucleus; PVN, paraventricular nucleus; CeA, Central amygdala; NTS, the nucleus of the solitary tract; RVLM, rostral ventrolateral medulla; PBN, parabrachial nucleus; AP, area postrema.
Importantly, activation of the above mentioned central pathways have also been shown to elicit a peripheral immune response. Ganta et al demonstrated that administration of angiotensin II in the lateral cerebral ventricles increased mRNA expression of pro-inflammatory splenic cytokines such as IL-1 beta and IL-6 (Ganta et al., 2005). Interestingly, splenic sympathetic denervation abrogated these responses, suggesting that central angiotensin II can elicit a peripheral immune response via the autonomic nervous system.
There is evidence to suggest bi-directional communication between the CNS and immune systems (Nance & Sanders, 2007). The spleen and lymph nodes receive substantial sympathetic innervation and most immune cells possess adrenergic receptors (Sanders & Straub, 2002). Studies have demonstrated that neuro-immune mechanisms contribute to the neurogenic component of hypertension (Paton & Waki, 2009; Shi et al., 2010; Yu et al., 2010). Paton and colleagues have shown that inflammatory cells and cytokines are increased in the brainstem in experimental hypertension, and that these inflammatory cells can impair central autonomic control of blood pressure regulation (Paton & Waki, 2009), (Waki et al., 2010). Moreover, angiotensin II increases blood brain barrier permeability and causes cerebral microvasculature inflammation, which could allow inflammatory cells and cytokines to gain entry to the brain (Zhang et al., 2010). Microglial cells, a type of specialized macrophage in the CNS, are also activated in hypertension, and inhibition of these cells reduces blood pressure (Shi et al., 2010). Taken together these data support the concept that the CNS can serve as both a target for inflammatory cells in hypertension as well as mediator of inflammation through its communication with the immune system. Angiotensin II is a key contributor to these processes in the setting of hypertension. Figure 2 illustrates the relationships between hypertensive stimuli, their sites of action in the CNS, (ie; circumventricular organs), and the neuroimmune system in hypertension.
Figure 2.
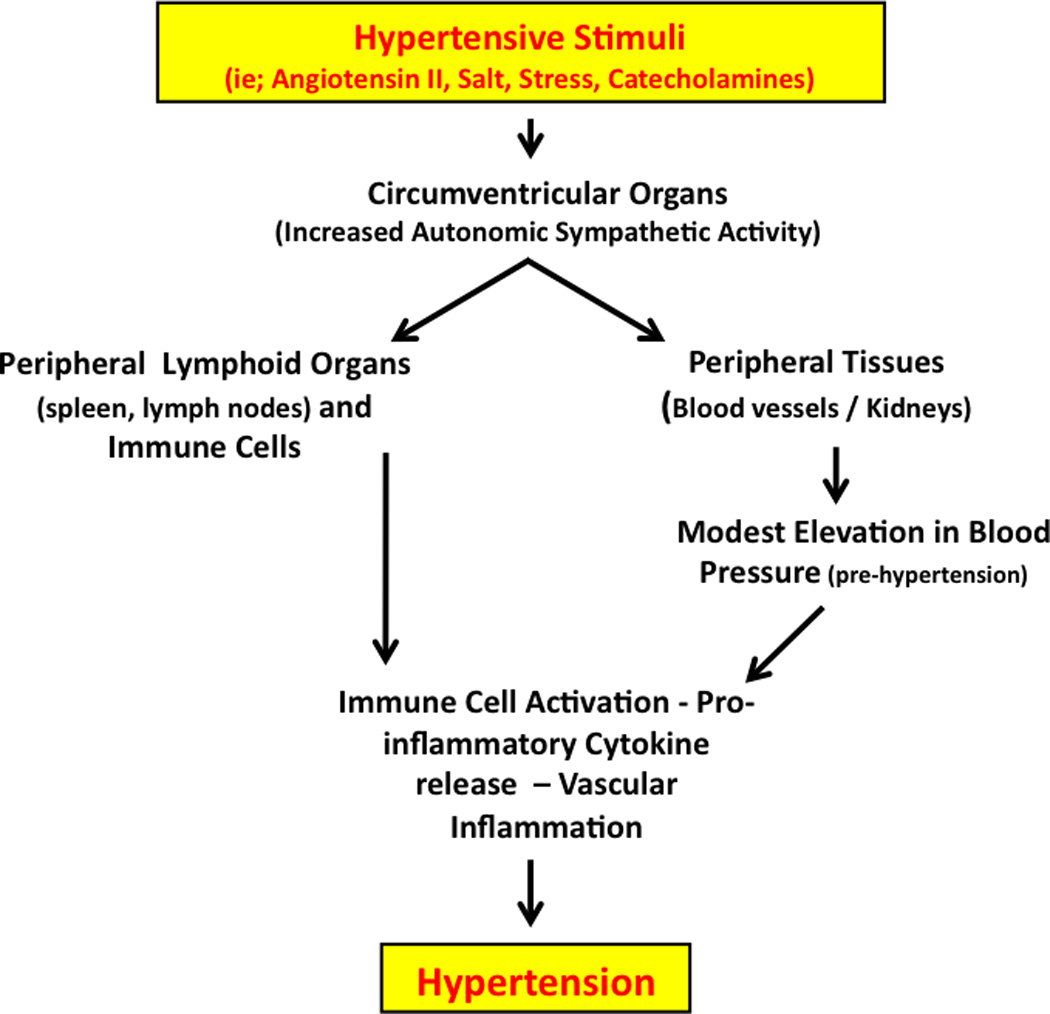
We propose that hypertensive stimuli such as angiotensin II, salt and chronic stress act on the CNS to increase sympathetic outflow in part via the circumventricular organs. Increased sympathetic outflow to the kidneys and vasculature leads to a modest elevation in blood pressure, which promotes the formation of unknown antigens that are processed by antigen presenting cells, leading to T cell activation. Direct autonomic innervation of lymphoid tissue and immune cells may also contribute to this pathway. Activated T cells infiltrate the vasculature and kidney, interacting with monocyte/macrophages and promote pro-inflammatory cytokine release, endothelial dysfunction, further vasoconstriction, salt /water retention and ultimately severe hypertension.
Circumventricular Organs (CVOs): Important CNS sites in T lymphocyte Mediated Hypertension
The anteroventral third ventricle (AV3V) region contains a high density of AT1 receptors and receives input from the SFO, a CVO, and sends efferent projections to key cardiovascular regulatory brainstem sites such as the nucleus of the solitary tract (NTS) and rostral ventrolateral medulla (RVLM) (Brody et al., 1978) (Figure 1). Earlier studies have shown that electrolytic ablation of this region abolishes all central actions of angiotensin II including drinking behavior, sympathetic outflow, vasopressin release as well as preventing and/or reversing several forms of experimental hypertension (Brody et al., 1978; Brody & Johnson, 1980). In support of these early studies, we demonstrated that the hypertensive response to a 2-week infusion of angiotensin II is attenuated by AV3V lesioning (Marvar et al., 2010). Interestingly, AV3V lesions also completely prevented activation and vascular infiltration of T cells. This demonstrated that the central pressor actions of angiotensin II are required for T cell activation and peripheral vascular inflammation in the setting of hypertension caused by this octapeptide. We went on to show that this effect was specific to the AV3V region as norepinephrine infusion, a peripherally acting hypertensive stimulus, caused hypertension and T cell activation independent of the lesion. To determine whether these effects were due to the central actions of angiotensin II or a result of blood pressure lowering from the lesion, we blocked the rise in blood pressure to angiotensin II with hydralazine, which prevented the T cell activation and vascular inflammation. Our findings are compatible with a pathway in which central stimuli such as angiotensin II cause modest elevations of blood pressure, which lead to T cell activation, and ultimately more severe hypertension. These data also suggest that the adaptive immune response can be triggered in hypertension irrespective of the type of hypertensive stimulus.
Psychological Stress, Angiotensin II and Inflammation
Psychological stress has been considered for years to be a risk factor for hypertension (Esler et al., 2008). Many clinical and epidemiological studies have shown that behavioral and psychological factors are involved in the pathogenesis of human hypertension (Matthews et al., 2004; Gasperin et al., 2009). In addition, psychological stress can profoundly affect the immune system, promoting inflammation and cardiovascular disease (Glaser & Kiecolt-Glaser, 2005; Black, 2006; Pickering, 2007). Despite substantial clinical data, the mechanisms by which stress causes hypertension remain poorly understood. Current evidence suggests that increased sympathetic neural activity and central actions of angiotensin II likely have an important role (Saiki et al., 1997; Esler et al., 2008; Groeschel & Braam, 2011). Moreover, recent pre-clinical data show that angiotensin II receptor blockers reduce manifestation of of stress, including brain inflammation and ischemia (Saavedra et al., 2011).
Given our previous data supporting a role of inflammation and adaptive immunity in hypertension, we recently asked the question of whether stress-related hypertension could promote T cell activation in a similar fashion and whether angiotensin II could augment these effects (Marvar et al., 2012). Repeated exposure of mice to the stressors of repeated cage switching and restraint produced anxiety like behavior and modest elevations in blood pressure after 1 week. Interestingly, circulating T cells these animals exhibited a greater percentage of activation markers and their vascular tissue contained an increased number of leukocytes including total leukocytes and T cells. Interestingly, we found that RAG-1−/− mice, which lack lymphocytes, were protected from stress-induced hypertension and that reconstitution of T cells into the RAG1−/− mice by adoptive transfer restored the hypertensive response to repeated stress. These data support our previous findings in other hypertensive models and suggest that the hypertensive stimulus of chronic stress contributes to T cell mediated vascular inflammation in hypertension (Marvar et al., 2012).
Another important factor in development of stress-related hypertension is the renin-angiotensin system (Raasch et al., 2006; Groeschel & Braam, 2010; Liu et al., 2012). Angiotensin II not only exerts the central effects mentioned above to increase sympathetic outflow, but has direct effects on post-ganglionic fibers to enhance release of catecholamines (Weiner, 1979; Dendorfer et al., 2002). It also promotes vasoconstriction (Evans et al., 2010) and is a potent stimulus for pro-inflammatory and pro-oxidative events (Suzuki et al., 2003; Guzik et al., 2007) (Ferrario & Strawn, 2006). Circulating levels of renin and angiotensin II are elevated during stress (Clamage et al., 1976; Carrasco & Van de Kar, 2003) and AT1 receptors are located in a number of brain regions related to the emotional stress response (Aguilera et al., 1995). For example, the amygdala, bed nucleus stria terminalis and other limbic brain regions involved in the stress response contain AT1 receptors (von Bohlen und Halbach & Albrecht, 1998). Interestingly, projections from these regions extend to critical autonomic control sites including OVLT and SFO (Sunn et al., 2003) (Figure 1). Moreover, Krause et al., recently demonstrated that restraint stress increases circulating levels of angiotensin II which in turn activates neurons in the SFO. The authors also showed that lentiviral knockdown of the AT1 receptor in the SFO attenuated the humoral and behavioral responses to the stress paradigm (Krause et al., 2011). Mice lacking the AT1 receptor, exposed to aversive stress exhibit decreased cardiovascular reactivity, and this effect appears to be centrally mediated as well (Davern et al., 2009). These data highlight the central actions of angiotensin II as an important neurohumoral stimulus in stress related hypertension.
Angiotensin II can affect T-cell–mediated inflammation in hypertension (Hoch et al., 2009) and has anxiogenic properties that can influence behavior (Gard, 2002). To further understand the interactions between chronic stress and angiotensin II, we recently examined the effects of a chronic low dose infusion of angiotensin II combined with chronic stress on T cell mediated inflammation (Marvar et al., 2012). Low dose angiotensin II infusion markedly augmented the blood pressure response to repeated stress as well as the vascular inflammation. Studies in both humans (Nyklicek et al., 2005) and animals (McDougall et al., 2005) have shown that following repeated chronic stress, exposure to additional acute stressors elicits an exaggerated acute pressor response likely due to increased cardiovascular/autonomic sensitivity. Similarly, the animals exposed to stress and angiotensin II in our study displayed an exaggerated hypertensive response to a bout of acute cage switch stress (Marvar et al., 2012). In these studies we did not examine indices of autonomic function to determine if greater sympathetic activity was the driving force behind these enhanced effects, however, Moretti et al., recently showed that chronic low dose angiotensin II enhances renal sympathetic nerve activity in rabbits. These authors also showed that animals exposed to angiotensin II and stress displayed greater neuronal Fos-related antigen immunoreactivity in circumventricular organs, suggesting that these enhanced peripheral autonomic responses are likely mediated within the CNS via AT1 receptors (Moretti et al., 2012). Overall, these data provide evidence that the circumventricular organs are key targets for angiotensin II and that external stimuli such as stress converge on these sites to enhance sympathetic activity, contributing to elevations in blood pressure and vascular inflammation.
Taken together, we propose that hypertensive stimuli such as angiotensin II, salt and chronic stress act on the CNS to increase sympathetic outflow in part via the circumventricular organs. Increased sympathetic outflow to the kidneys and vasculature leads to a modest elevation in blood pressure, which promotes the formation of unknown antigens that are processed by antigen presenting cells, leading to T cell activation. Activated T cells infiltrate the vasculature and kidney, interacting with monocyte/macrophages and promote pro-inflammatory cytokine release, endothelial dysfunction, further vasoconstriction, salt /water retention and ultimately severe hypertension.
Conclusion
Blood pressure control is influenced by many factors including vasoactive substances that control vascular tone, renal regulation of electrolytes and volume and the central nervous system. We and others have shown that the CNS, in particular the CVOs play a critical role in the setting of hypertension, and the accompanied T cell activation and vascular inflammation. These hypothalamic brain regions are also heavily influenced by psychological stress, which on its own can influence the immune response. Further understanding the role of psychological stress on central control of blood pressure and the associated inflammatory responses could provide the potential for the development of new treatment strategies for hypertension.
New Findings.
This review will discuss:
Recent findings regarding the central nervous system in hypertension, the role of T lymphocytes, angiotensin II and the impact of cardiovascular risk factors such as psychological stress.
This review highlights data that provides new insight for our understanding in the role of inflammation in hypertension.
Acknowledgements
This work was supported by NIH funding NIH K99HL107675, R01HL039006, P01HL058000, P01HL095070, P01GM015431, R01HL105294-02
References
- Aguilera G, Kiss A, Luo X, Akbasak BS. The renin angiotensin system and the stress response. Ann N Y Acad Sci. 1995;771:173–186. doi: 10.1111/j.1749-6632.1995.tb44679.x. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypotheses. 2006;67:879–891. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Brody M, Fink G, Buggy J, Haywood J, Gordon F, Knuepfer M, Mow M, Mahoney L, Johnson A. Critical role of the AV3V region in development and maintenance of experimental hypertension. In: Perspectives in Nephrology and Hypertension. In: Schmitt H, Meyers P, editors. Perspectives in Nephdogy and Hypertension. New York, NY: Wiley and Flammarion; 1978. pp. 76–84. [Google Scholar]
- Brody MJ. Central nervous system and mechanisms of hypertension. Clin Physiol Biochem. 1988;6:230–239. [PubMed] [Google Scholar]
- Brody MJ, Johnson AK. Role of the anteroventral third ventricle region in fluid and electrolyte balance, arterial pressure regulation, and hypertension. New York: Frontiers in Neuroendocrinology Raven Press; 1980. [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Chapleau MW, Li Z, Meyrelles SS, Ma X, Abboud FM. Mechanisms determining sensitivity of baroreceptor afferents in health and disease. Ann N Y Acad Sci. 2001;940:1–19. doi: 10.1111/j.1749-6632.2001.tb03662.x. [DOI] [PubMed] [Google Scholar]
- Clamage DM, Sanford CS, Vander AJ, Mouw DR. Effects of psychosocial stimuli on plasma renin activity in rats. Am J Physiol. 1976;231:1290–1294. doi: 10.1152/ajplegacy.1976.231.4.1290. [DOI] [PubMed] [Google Scholar]
- Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1089–R1097. doi: 10.1152/ajpregu.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttica MJ, Langenickel T, Noguchi A, Machado RF, Gladwin MT, Boehm M. Perivascular T-cell infiltration leads to sustained pulmonary artery remodeling after endothelial cell damage. Am J Respir Cell Mol Biol. 2011;45:62–71. doi: 10.1165/rcmb.2009-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davern PJ, Chen D, Head GA, Chavez CA, Walther T, Mayorov DN. Role of angiotensin II Type 1A receptors in cardiovascular reactivity and neuronal activation after aversive stress in mice. Hypertension. 2009;54:1262–1268. doi: 10.1161/HYPERTENSIONAHA.109.139741. [DOI] [PubMed] [Google Scholar]
- Davern PJ, Head GA. Fos-related antigen immunoreactivity after acute and chronic angiotensin II-induced hypertension in the rabbit brain. Hypertension. 2007;49:1170–1177. doi: 10.1161/HYPERTENSIONAHA.106.086322. [DOI] [PubMed] [Google Scholar]
- Davern PJ, Head GA. Role of the medial amygdala in mediating responses to aversive stimuli leading to hypertension. Clin Exp Pharmacol Physiol. 2011;38:136–143. doi: 10.1111/j.1440-1681.2010.05413.x. [DOI] [PubMed] [Google Scholar]
- De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol. 2011;300:F734–F742. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendorfer A, Thornagel A, Raasch W, Grisk O, Tempel K, Dominiak P. Angiotensin II induces catecholamine release by direct ganglionic excitation. Hypertension. 2002;40:348–354. doi: 10.1161/01.hyp.0000028001.65341.aa. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Jones SY. Sodium intake influences hemodynamic and neural responses to angiotensin receptor blockade in rostral ventrolateral medulla. Hypertension. 2001;37:1114–1123. doi: 10.1161/01.hyp.37.4.1114. [DOI] [PubMed] [Google Scholar]
- Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Dawood T, Kaye D, Barton D, Pier C, Guo L, Brenchley C, Jennings G, Lambert E. Chronic mental stress is a cause of essential hypertension: presence of biological markers of stress. Clin Exp Pharmacol Physiol. 2008;35:498–502. doi: 10.1111/j.1440-1681.2008.04904.x. [DOI] [PubMed] [Google Scholar]
- Evans RG, Head GA, Eppel GA, Burke SL, Rajapakse NW. Angiotensin II and neurohumoral control of the renal medullary circulation. Clin Exp Pharmacol Physiol. 2010;37:e58–e69. doi: 10.1111/j.1440-1681.2009.05233.x. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- Ganta CK, Lu N, Helwig BG, Blecha F, Ganta RR, Zheng L, Ross CR, Musch TI, Fels RJ, Kenney MJ. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289:H1683–H1691. doi: 10.1152/ajpheart.00125.2005. [DOI] [PubMed] [Google Scholar]
- Gard PR. The role of angiotensin II in cognition and behaviour. Eur J Pharmacol. 2002;438:1–14. doi: 10.1016/s0014-2999(02)01283-9. [DOI] [PubMed] [Google Scholar]
- Gasperin D, Netuveli G, Dias-da-Costa JS, Pattussi MP. Effect of psychological stress on blood pressure increase: a meta-analysis of cohort studies. Cad Saude Publica. 2009;25:715–726. doi: 10.1590/s0102-311x2009000400002. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Quarti-Trevano F. The 'neuroadrenergic hypothesis' in hypertension: current evidence. Exp Physiol. 2010;95:581–586. doi: 10.1113/expphysiol.2009.047381. [DOI] [PubMed] [Google Scholar]
- Groeschel M, Braam B. Connecting chronic and recurrent stress to vascular dysfunction: no relaxed role for the renin-angiotensin system. Am J Physiol Renal Physiol. 2010 doi: 10.1152/ajprenal.00208.2010. [DOI] [PubMed] [Google Scholar]
- Groeschel M, Braam B. Connecting chronic and recurrent stress to vascular dysfunction: no relaxed role for the renin-angiotensin system. Am J Physiol Renal Physiol. 2011;300:F1–F10. doi: 10.1152/ajprenal.00208.2010. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296:R208–R216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Elam M, Rundqvist B, Eisenhofer G, Herlitz H, Lambert G, Friberg P. Increased sympathetic nerve activity in renovascular hypertension. Circulation. 1999;99:2537–2542. doi: 10.1161/01.cir.99.19.2537. [DOI] [PubMed] [Google Scholar]
- Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, Ulrich-Lai YM, Woods SC, Wilson SP, Reagan LP, Herman JP, Sakai RR. Blood-borne angiotensin II acts in the brain to influence behavioral and endocrine responses to psychogenic stress. J Neurosci. 2011;31:15009–15015. doi: 10.1523/JNEUROSCI.0892-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Havens J, Yu Q, Wang G, Davisson RL, Pickel VM, Iadecola C. The link between angiotensin II-mediated anxiety and mood disorders with NADPH oxidase-induced oxidative stress. Int J Physiol Pathophysiol Pharmacol. 2012;4:28–35. [PMC free article] [PubMed] [Google Scholar]
- Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–283. doi: 10.1161/HYPERTENSIONAHA.109.142646. 276p following 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvar PJ, Vinh A, Thabet S, Lob HE, Geem D, Ressler KJ, Harrison DG. T lymphocytes and vascular inflammation contribute to stress-dependent hypertension. Biol Psychiatry. 2012;71:774–782. doi: 10.1016/j.biopsych.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa T, Gotoh E, Hasegawa O, Shionoiri H, Tochikubo O, Ishii M. Reduced baroreflex changes in muscle sympathetic nerve activity during blood pressure elevation in essential hypertension. J Hypertens. 1991;9:537–542. doi: 10.1097/00004872-199106000-00009. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- McDougall SJ, Lawrence AJ, Widdop RE. Differential cardiovascular responses to stressors in hypertensive and normotensive rats. Exp Physiol. 2005;90:141–150. doi: 10.1113/expphysiol.2004.028308. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol. 2003;172:III–XII. 1–122. doi: 10.1007/978-3-642-55532-9. back cover. [DOI] [PubMed] [Google Scholar]
- Moretti JL, Burke SL, Davern PJ, Evans RG, Lambert GW, Head GA. Renal sympathetic activation from long-term low-dose angiotensin II infusion in rabbits. J Hypertens. 2012;30:551–560. doi: 10.1097/HJH.0b013e328350133a. [DOI] [PubMed] [Google Scholar]
- Muller DN, Kvakan H, Luft FC. Immune-related effects in hypertension and target-organ damage. Curr Opin Nephrol Hypertens. 2011;20:113–117. doi: 10.1097/MNH.0b013e3283436f88. [DOI] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyklicek I, Bosch JA, Amerongen AV. A generalized physiological hyperreactivity to acute stressors in hypertensives. Biol Psychol. 2005;70:44–51. doi: 10.1016/j.biopsycho.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- Paton JF, Waki H. Is neurogenic hypertension related to vascular inflammation of the brainstem? Neurosci Biobehav Rev. 2009;33:89–94. doi: 10.1016/j.neubiorev.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Pickering TG. Stress, inflammation, and hypertension. J Clin Hypertens (Greenwich) 2007;9:567–571. doi: 10.1111/j.1524-6175.2007.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasch W, Wittmershaus C, Dendorfer A, Voges I, Pahlke F, Dodt C, Dominiak P, Johren O. Angiotensin II inhibition reduces stress sensitivity of hypothalamo-pituitary-adrenal axis in spontaneously hypertensive rats. Endocrinology. 2006;147:3539–3546. doi: 10.1210/en.2006-0198. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Iturbe B. Renal infiltration of immunocompetent cells: cause and effect of sodium-sensitive hypertension. Clin Exp Nephrol. 2010;14:105–111. doi: 10.1007/s10157-010-0268-1. [DOI] [PubMed] [Google Scholar]
- Rumantir MS, Kaye DM, Jennings GL, Vaz M, Hastings JA, Esler MD. Phenotypic evidence of faulty neuronal norepinephrine reuptake in essential hypertension. Hypertension. 2000;36:824–829. doi: 10.1161/01.hyp.36.5.824. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Sanchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology. 2011;36:1–18. doi: 10.1016/j.psyneuen.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki Y, Watanabe T, Tan N, Matsuzaki M, Nakamura S. Role of central ANG II receptors in stress-induced cardiovascular and hyperthermic responses in rats. Am J Physiol. 1997;272:R26–R33. doi: 10.1152/ajpregu.1997.272.1.R26. [DOI] [PubMed] [Google Scholar]
- Sanders VM, Straub RH. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav Immun. 2002;16:290–332. doi: 10.1006/brbi.2001.0639. [DOI] [PubMed] [Google Scholar]
- Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharmacol Physiol. 2010;37:e52–e57. doi: 10.1111/j.1440-1681.2009.05234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunn N, McKinley MJ, Oldfield BJ. Circulating angiotensin II activates neurones in circumventricular organs of the lamina terminalis that project to the bed nucleus of the stria terminalis. J Neuroendocrinol. 2003;15:725–731. doi: 10.1046/j.1365-2826.2003.00969.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Albrecht D. Mapping of angiotensin AT1 receptors in the rat limbic system. Regul Pept. 1998;78:51–56. doi: 10.1016/s0167-0115(98)00109-8. [DOI] [PubMed] [Google Scholar]
- Waki H, Gouraud SS, Maeda M, Paton JF. Evidence of specific inflammatory condition in nucleus tractus solitarii of spontaneously hypertensive rats. Exp Physiol. 2010;95:595–600. doi: 10.1113/expphysiol.2009.047324. [DOI] [PubMed] [Google Scholar]
- Waki H, Gouraud SS, Maeda M, Raizada MK, Paton JF. Contributions of vascular inflammation in the brainstem for neurogenic hypertension. Respir Physiol Neurobiol. 2011;178:422–428. doi: 10.1016/j.resp.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN, Jr, LaMarca B. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57:949–955. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner N. Multiple factors regulating the release of norepinephrine consequent to nerve stimulation. Fed Proc. 1979;38:2193–2202. [PubMed] [Google Scholar]
- Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 2010;55:652–659. doi: 10.1161/HYPERTENSIONAHA.109.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.09.029. [DOI] [PubMed] [Google Scholar]


