Abstract
Purpose
Extensive correlative studies in human prostate cancer (PCa) as well as studies in vitro and in mouse models indicate that FGF receptor (FGFR) signaling plays an important role in PCa progression. In this study, we employed a probe compound for an FGFR inhibitor which potently inhibits FGFR1-3 and significantly inhibits FGFR-4. The purpose of this study is to determine if targeting FGFR signaling from all four FGFRs will have in vitro activities consistent with inhibition of tumor progression and will inhibit tumor progression in vivo.
Experimental Design
Effects of AZ8010 on FGFR signaling and invasion were analyzed using immortalized normal prostate epithelial (PNT1a) cells and PNT1a overexpressing FGFR-1 or FGFR-4. The effect of AZ8010 on invasion and proliferation in vitro was also evaluated in PCa cell lines. Finally, the impact of AZ8010 on tumor progression in vivo was evaluated using a VCaP xenograft model.
Results
AZ8010 completely inhibits FGFR-1 and significantly inhibits FGFR-4 signaling at 100 nM, which is an achievable in vivo concentration. These results in marked inhibition of ERK phosphorylation and invasion in PNT1a cells expressing FGFR-1 and FGFR-4 and all PCa cell lines tested. Treatment in vivo completely inhibited VCaP tumor growth and significantly inhibited angiogenesis and proliferation and increased cell death in treated tumors. This was associated with marked inhibition of ERK phosphorylation in treated tumors.
Conclusions
Targeting FGFR signaling is a promising new approach to treating aggressive PCa.
Keywords: prostate cancer, fibroblast growth factor receptor, extracellular signal related Kinase
INTRODUCTION
Prostate cancer (PCa) is the most common visceral malignancy and the second leading cause of cancer deaths in men in the United States. There is compelling evidence both from studies of human tumor samples and from animal models that fibroblast growth factors (FGFs) and FGF receptors (FGFRs) are important in PCa initiation and progression (reviewed in (1)). FGFs are a family of 19 different polypeptide ligands involved in a variety of biological and pathological processes. There are four distinct FGF receptors (FGFR 1–4) which have variable affinities for the different FGFs. FGFRs are transmembrane tyrosine kinase receptors. Upon binding to FGFs, FGFR dimerization is induced, which leads to FGFR phosphorylation and activation of various downstream signaling pathways including MAPKs, PI3K/AKT, PLC-γ and STATs (1–3).
FGFs play a key role in the growth and maintenance of normal prostatic epithelium and are expressed in normal prostatic stroma (reviewed in ((1)). FGFs are expressed as autocrine growth factors by PCa cells (4) and can also be expressed in the tumor microenvironment as paracrine growth factors (5–6). Multiple FGF ligands are expressed at increased levels in PCa (1, 4–5, 7–9) and increased expression has been shown to be associated with clinically aggressive disease (7, 10–11). Recent studies have shown high expression of FGF8 (10) and FGF9 (9) in PCa bone metastases. In all PCa cell lines examined to date one or more FGFs is expressed as an autocrine growth factor ((1) and unpublished data).
Our laboratory has shown that FGFR-1 is expressed in 20% of moderately differentiated cancers and 40% of poorly differentiated localized PCas based on immunohistochemistry (IHC) (5) and other groups have made similar observations (12–13). Studies in transgenic mice have linked FGFR-1 activation to cancer initiation and progression (14–16) and chronic FGFR-1 activation can lead to adenocarcinoma and epithelial-mesenchymal transition (17).
Changes in alternative splicing of FGFR-2 in PCa that enhance oncogenic signaling are well known. It has been shown by several groups (8, 18–19) including ours (20) that there is a change in alternative spicing favoring expression of the growth promoting FGFR-2 IIIc isoform with decreased levels of the IIIb isoform but high FGFR-2 protein expression is not strongly linked to PCa progression (21). FGFR-3 appears to play a less important role in PCa based on current data (21).
FGFR-4 is expressed at increased levels in PCa by IHC and this has been verified by quantitative RT-PCR (7, 21–23). Strong FGFR-4 expression is significantly associated with poor clinical outcome (7, 22). For example, Murphy et al (7) have shown that increased FGFR-4 expression is strongly associated with PCa specific death. Our group has shown that a germline polymorphism in the FGFR-4 gene, resulting in expression of FGFR-4 containing arginine at codon 388 (Arg388), instead of a more common glycine (Gly388), is associated with PCa incidence, recurrence after radical prostatectomy and metastatic disease (23). This allele was present in almost half of white PCa patients. These findings have been confirmed in a similar case control study (24) and in a meta-analysis of all published studies (25). Expression of the FGFR-4 Arg388 protein results in increased motility and invasion and is associated with prolonged receptor stability after ligand activation (23). In recently published studies we have shown that FGFR-4 expression leads to increased activity of the extracellular signal-related kinase (ERK) pathway, increased activity of serum response factor and AP-1 and transcription of multiple genes which are correlated with aggressive clinical behavior in PCa (26). Furthermore, stable knockdown of FGFR-4 via shRNA in PC3 PCa cells (26) resulted in inhibition of proliferation and invasion in vitro and decreased primary tumor growth and metastases in an orthotopic model in which cells are injected directly into the prostates of nude mice.
Finally, several groups, including ours, have shown that decreased expression of negative regulators of FGF signaling is common in human PCa and in some cases these alterations have been shown to be associated with aggressive disease (7, 27–31). These negative regulators include the Sprouty proteins as well as Sef. Loss of these negative signaling regulators is an important mechanism of enhancing FGF signaling in PCa. Thus both correlative studies in human tissues and mouse models strongly support the concept that FGFR signaling plays an important role in PCa
In PCa, FGF signaling can enhance PCa progression through both by increased proliferation and by preventing cell death (32). FGFs are well known angiogenic factors and can enhance angiogenesis through paracrine actions on endothelial and other stromal cells in the tumor microenvironment (1). Thus FGFs enhance tumor progression via multiple independent mechanisms.
Based on the above, FGF signaling is a promising therapeutic target in aggressive PCa. Several “FGF receptor” small molecule inhibitors have entered clinical trials but many inhibit multiple tyrosine kinases (2). AZ8010 is an ATP-competitive FGFR tyrosine kinase inhibitor. It is chemically related to AZD4547, with similar properties in vitro but has inferior pharmacokinetic properties. Recent studies have shown cellular IC50s for AZD4547 in Cos-1 cells for FGFR-1, 2, 3 and 4, of 12, 2, 40 and 142 nM, respectively(33) and AZ8010 has similar properties and potently inhibits FGFR1–3 at less than 100 nM and FGFR-4 at less than 200nM. The kinase domain of FGFR-4 is divergent from the kinase domains of FGFR 1–3, and many previously tested FGF receptor inhibitors do not effectively target FGFR-4. For example, PD173074, the only other specific small molecule FGFR inhibitor has a similar IC50 for FGFR1–3 but its IC50 for FGFR-4 is >1000 nM (34). The only other kinase inhibited at less than 500 nM by AZD4547 was VEGFR2 (IC50 258 nM in HUVEC cells). A recent report shows potent in vitro and/or in vivo activity of AZD4547 against cell lines from myeloid leukemia, myeloma and breast cancer (33). We show here that AZ8010 potently inhibits FGFR signaling, invasion in vitro and tumor growth in vivo in prostate cancer cells. These findings support the hypothesis that targeting FGFR signaling is a promising therapeutic approach to treating prostate cancer.
MATERIALS AND METHODS
Cell lines and tissue culture
Human prostate cancer cells PC3, LNCaP, and PNT1a immortalized normal prostate epithelial cells were maintained in RPMI-1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS, Invitrogen) and 1% penicillin/streptomycin (Invitrogen). VCaP cells were grown in DMEM under similar conditions. Luciferase expressing VCaP cells (VCaP-Luc) for in vivo studies have been described previously (35). PNT1a expressing FGFR-4 Arg388 has been described previously (26) and PNT1a cells overexpressing FGFR-1 were generated in a similar manner by subcloning the FGFR-1 cDNA from clone MGC:111078 into the pcDNA 3.1 vector and then subcloning into the pCDH lentiviral vector. Lentiviruses were generated and used to transduce PNT1a cells that were then selected with puromycin. The expression of FGFR-1 was similar to FGFR-4 Arg388 based on Western blots with anti-V5 antibodies (26) that detects the V5 tag on both receptors.
Invasion and cell proliferation assays
The Matrigel invasion assays were performed in triplicate using BD BioCoat Matrigel invasion chambers (BD Biosciences) as described previously (26). Cells were incubated with AZ8010 (100 nM or 500nM) or DMSO vehicle in the presence of FGF2 (50 ng/ml) in serum-free medium or in complete growth medium containing 10% FBS for either 24 hours (PC3), 48 hours (PNT1a, PNT1a-FGFR-1, PNT1a-FGFR-4) or 72 hours (LNCaP and VCaP). Non-invading cells in the upper chambers were removed and the invading cells on the lower surface of the membrane were fixed and stained with Diff-Quik Stain Set (Dade Behring, Inc.). The membranes were mounted on slides and scanned, photographed and all cells were counted. For cell proliferation analyses, cells were incubated with different concentrations of AZ8010 (0 nM, 100 nM, 500 nM) for 72 hours in serum free medium at the presence of FGF2 in 96-well plates. Cell proliferation was determined using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega) as described by the manufacturer.
Western blotting and immunoprecipitation
Protein extracts were prepared from cells in culture or VCaP xenograft tumors with modified RIPA buffer containing Tris 50 mM, NaCl 150 mM, Triton X-100 1%, SDS 0.1%, deoxycholate 0.5%, 2 mM sodium orthovanadate, 1mM sodium pyrophosphate, 50mM NaF, 5 mM EDTA, 1 mM PMSF and 1× protease inhibitor cocktail (Roach) and clarified by centrifugation. The protein concentrations of the lysates were determined using a BCA protein assay kit (Thermo Scientific). Western blots were performed as described previously (26). The antibodies were from Cell Signaling and included phospho-FGFR mouse monoclonal antibody (mAb, #3476) (26), phospho-p44/42 MAPK (p-Erk1/2) (#4370), p44/42 MAPK (Erk1/2) (#4695), phospho-MEK1/2 (#9154), MEK1/2 (#9122), phospho-AKT (T308, #4056), phospho-AKT (S473, #9271) and β-Tubulin (#2128) which were all used at 1:1000 dilution. β-actin mAb (Sigma A5316) was used at 1:5000 dilution. After incubation with primary antibodies for overnight at 4°C, horseradish peroxidase–labeled secondary antibodies were then applied to the membranes for 1 h at room temperature. Signals were visualized using enhanced chemiluminescence (Thermo).
To detect phosphorylated FGFR-1 in tumor extracts, immunoprecipitation assays were performed. Briefly, protein extract (500 µg) of xenograft tumors were precleared by incubating with 1 µg of normal mouse IgG together with 20 µl of resuspended protein A/G Plus-Agarose (Santa Cruz Biotechnology, Inc.) at 4°C for 30 minutes and were subsequently incubated with 2 µg of anti-human FGFR-1 mAb (Meridian Life Science Inc., P55213M) overnight at 4°C. 20 µl of resuspended protein A/G Plus-Agarose was then added to the lysate/antibody mixture. Following incubation for 1 hour at 4°C, the lysate/antibody/agarose mixture was centrifuged at 1000xg for 5 minutes at 4oC and the pellets were washed 4 times with 1.0 ml of RIPA buffer. Pellets were eluted in 40 µl of electrophoresis sample buffer and analyzed by Western blotting as described above with mouse anti-phospho-FGFR mAb (1:1000, Cell Signaling). Densitometry was performed using Image J program (National Institute of Health).
Subcutaneous VCaP xenografts
30 nude male mice (6–7 weeks old) were purchased from Charles River Laboratories International, Inc. and each animal was injected subcutaneously with 1 × 106 VCaP-Luc cells over the flank. Two weeks later those mice bearing subcutaneous tumors were divided randomly into 2 groups: the experimental group was treated with AZ8010 at 12.5 mg/kg/day in 1% polysorbate 80 by oral gavage; the control group was treated with vehicle only. Luciferase imaging of tumor growth was performed weekly after injection of D-luciferin using an IVIS imaging system as described previously (35). Body weights were monitored weekly. Four hours after the last treatment mice were euthanized and tumors were excised and weights and volumes measured. One portion of each tumor were fixed with buffered formalin, embedded in paraffin and processed for histological, IHC and TUNEL analysis; the other portion was snap frozen in liquid nitrogen and proteins extracted. All procedures were approved by the Baylor College of Medicine Institutional Animal Use and Care Committee.
Immunohistochemistry
IHC of mouse tissues was performed using the basic procedures described previously (28). Primary antibodies were used as follows: Ki67 (Thermo, RM-9106) at 1:400 for 30 minutes at room temperature and mouse anti-CD31 (BD Biosciences) at 1:10 overnight at 4° plus 3 hours at room temperature. TUNEL was performed using an ApopTag Peroxidase In Situ Apoptosis Kit (Millipore) according to the manufacturer’s instructions. Image analysis of stained sections was performed as described previously (36). Ki-67 and TUNEL were also carried out on cells grown on chamber slides and quantitated in a similar manner.
Quantitative RT-PCR
Copy numbers of all four FGFRs in prostate and PCa cell line RNAs was determined using quantitative RT-PCR using general procedures described previously (37). Primers and PCR conditions for FGFR-4 have been described. Primers and conditions for FGFR 1–3 are shown in Supplementary Table 1. In all cases exact copy number was determined in duplicate samples using a standard curve generated using purified PCR product cloned into plasmid or full length cDNA. HPRT levels were determined as described previously (6) and used to normalize expression levels across cell lines
RESULTS
Expression levels of FGFRs in prostate and prostate cancer cell lines
To better understand the impact of FGFR inhibition on prostate cancer cell lines we first sought to determine the relative expression of all four FGFR mRNAs in the immortalized prostate epithelial cell line PNT1a and the commonly used PCa cell lines PC3, LNCaP, VCaP and DU145 (Figure 1). All cell lines expressed detectable levels of all four FGFRs. FGFR-2 was expressed at relatively low levels in all cell lines compared to other FGFRs. FGFR-1 and FGFR-3 were expressed at similar levels while FGFR-4 was expressed at the highest level overall. Unfortunately the absence of high quality, specific antibodies with similar affinities for all four FGFRs precludes confirmation at the protein level. Our data indicates that there is ubiquitous expression of FGFRs in PCa, with significant but variable expression of FGFR-4.
Figure 1. Quantitation of FGFR mRNA expression in prostate and PCa cell lines.
RNAs from the indicated cell lines were used for quantitative RT-PCR and copy number of each FGFR determined by comparison to a standard curve. HPRT copy number was determined on the same RNAs and used to normalize data. FGFR copies per 100 HPRT copies are shown.
AZ8010 inhibits FGFR signaling in vitro
PNT1a are immortalized normal prostatic epithelial cells and when expressing exogenous FGFR most FGFR signaling can be attributed to the transfected receptor due to its high expression under a relatively strong promoter (37). We have previously established a cell line overexpressing FGFR-4 Arg388 and these cells express 90-fold higher levels of FGFR-4 than the parental PNT1a by quantitative RT-PCR (37). We have now established similar cell line expressing FGFR-1 which expresses FGFR-1 at similar levels to FGFR-4 in the FGFR-4 overexpressing cells (data not shown). In these cells, 100 nM AZ8010 inhibits FGFR-1 phosphorylation by 86% by quantitative Western blotting at 4 hours after treatment in serum free medium with FGF2 as the only growth factor (Fig 2A). This is equivalent to the 86% inhibition of FGFR-1 phosphorylation seen at 1000 nM AZ8010. ERK phosphorylation was also markedly inhibited (by 78% and 84%) at 100 and 1000 nM, respectively. In PNT1a cells overexpressing FGFR-4, phosphorylation was very significantly inhibited at 100 nM (75% by quantitative densitometry) although inhibition was somewhat less than that seen at 500 nM, which inhibits 89% of FGFR-4 phosphorylation (Fig 2B). More residual ERK phosphorylation seen in FGFR-4 expressing cells at 100nM AZ8010 (55% inhibition) and 200 nM AZ8010 (70% inhibition) when compared to the 90% inhibition at 500 nM AZ8010 by quantitative analysis of normalized band intensities. The FGFR-4 studies used 24 hr treatment since we have shown that FGFR-4 Arg388 phosphorylation can be sustained for up to 24 hrs after ligand stimulation (37). Control PNT1a cells also showed marked inhibition of ERK phosphorylation at both 100 and 500nM AZ8010. Note that while PNT1a express FGFR-1, FGFR-3 and FGFR-4, phosphorylated FGFRs cannot be detected by simple Western blotting in these cells, unlike the FGFR-1 and FGFR-4 transfected cells, confirming marked overexpression of the transfected receptor protein in the latter cell lines. Thus at 100nM AZ8010 FGFR-1 is markedly inhibited and FGFR-4 is significantly but not totally inhibited.
Figure 2. Inhibition of FGFR signaling by AZ8010.
FGFR-1 (A) or FGFR-4 (B) overexpressing PNT1a cells were serum starved overnight and stimulated with FGF2 (50 ng/ml) in the presence of the indicated concentration of AZ8010 (nM) or vehicle only (CON). Cell lysates were prepared after 4 hrs (FGFR-1) or 24 hrs (FGFR-4) and Western blot analyses were conducted using antibodies against a conserved phosphorylation site on all FGFRs (PFGFR) or phospho-ERK (P-ERK). β-actin or tubulin are loading controls. Similar experiments were performed using vector control PNT1a using 3 or 24 hrs treatment (C).
AZ8010 inhibits invasion in vitro
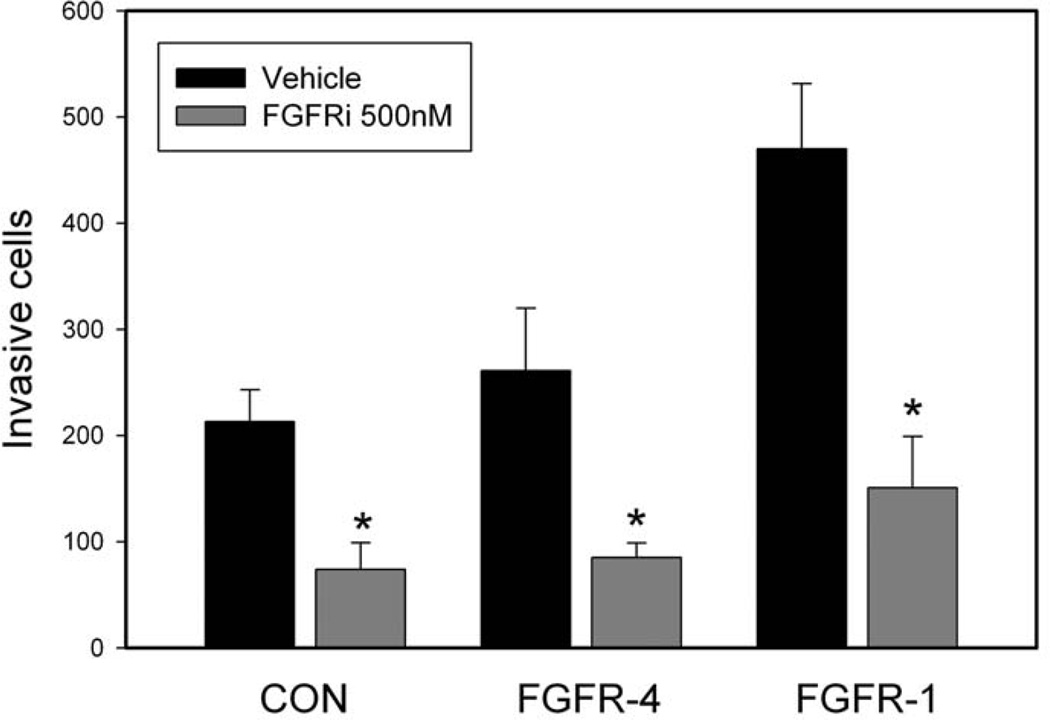
We next examined the impact of AZ8010 on invasion using these same two PNT1a derived cell lines and the PNT1a control cells in defined medium with FGF2 as the only growth factor since we have previously shown that ERK dependent invasion is a major phenotype driven by FGFR-4 in PCa cells. In these experiments we used 500 nM AZ8010 to maximally suppress either FGFR-1 or FGFR-4 activity. AZ8010 markedly inhibited invasion (Fig 3) in both FGFR-4 (67%, p=0.04, t-test) and FGFR-1 (68%, p=0.02) expressing cells. Invasion of control PNT1a cells, which showed lower numbers of invasive cells, was also potently inhibited (65%, p=.01) so that the effects seen in the overexpressing cell lines are probably partly due to inhibition of endogenous FGFRs and partially due to inhibition of the overexpressed FGFR. Thus AZ8010 can potently inhibit invasion of immortalized prostate epithelial cells and cells overexpressing either FGFR-1 or FGFR-4.
Figure 3. Inhibition of FGFR signaling inhibits invasion in PNT1a cells expressing FGFR-1 or FGFR-4.
PNT1a cells or PNT1a cells expressing FGFR-1 or FGFR-4 were plated in the upper chamber of Matrigel transwell chambers in defined medium with FGF2 as the only growth factor and 500nM AZD8010 or vehicle only. 48 hours later cells invading through the filter were stained and counted. Mean +/− SEM of triplicates is shown. * p<.05
We then evaluated the impact of AZ8010 on PCa cell invasion in defined medium with FGF2 as the only growth factor (Figure 4A) and in serum containing medium (Figure 4B). For both LNCaP and PC3 cells invasion in FGF2 defined medium was markedly reduced by 100 nM AZ8010 (LNCaP 78%; PC3: 56%, both p<0.01, t-test). Thus, in the face of saturating quantities of FGF2, the majority of invasion can be accounted for by FGFR signaling. Results with 500 nM AZ8010 were essentially the same as with 100nM. Somewhat surprisingly invasion was markedly inhibited in serum containing medium by 45–62% in LNCaP, PC3 and VCaP cell lines at 100 nM AZ8010 (all p<0.01, t-test). This result indicates that FGFs in serum and/or autocrine FGFs from cancer cells drive a significant fraction of invasion by PCa cells, even in serum, which contains other growth factors. Treatment with 500 nM AZ8010 further decreased invasion somewhat compared 100 nM AZ8010 but the differences were not statistically significant.
Figure 4. AZ8010 inhibits prostate cancer cell invasion and proliferation.
A. PCa cell lines were plated and serum starved overnight and pre-incubated with either AZ8010 (100 nM or 500 nM) or vehicle for 1 hour before stimulation with FGF2 (50 ng/ml) for 24 hour (PC3) and 72 hours (LNCaP). The invading cells on the lower surface of the membranes of the invasion chambers were fixed, stained, scanned, photographed and all cells were counted. B. Cells were incubated with either AZ8010 or vehicle for 24 hour (PC3) or 72 hour (LNCaP and VCaP) in growth medium containing 10% FBS and invasive cells enumerated as above. C. PCa cells were incubated with different concentrations of AZ8010 or vehicle for 72 hours in serum free medium at the presence of FGF2. Cell proliferation was determined using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay. All values expressed as percent of vehicle control. Mean +/− SEM is shown. * p<.05; ** p<.01; ***p<.001
Proliferation was decreased in FGF2 defined medium (Figure 4C) at both 100 nM and 500 nM AZ8010 but effects on proliferation were less pronounced than those on invasion (11–38% inhibition of proliferation). Analysis of AZ8010 treated VCaP cells with Ki67 immunohistochemistry and TUNEL showed statistically significant decreases in Ki67 staining and increases in TUNEL at both doses (Supplementary Figure 1). Similar results were seen with PC3 and LNCaP cells (data not shown). No statistically significant effect on proliferation was seen on PCa cell lines in serum containing medium (data not shown). Of note, PC3 which express higher levels of FGFR-4 than VCaP (Fig 1), showed similar responses to both 100 nM and 500nM AZ8010, indicating that higher FGFR-4 expression does not contribute significantly to resistance to AZ8010 at these levels of drug.
AZ8010 inhibits tumor growth in vivo
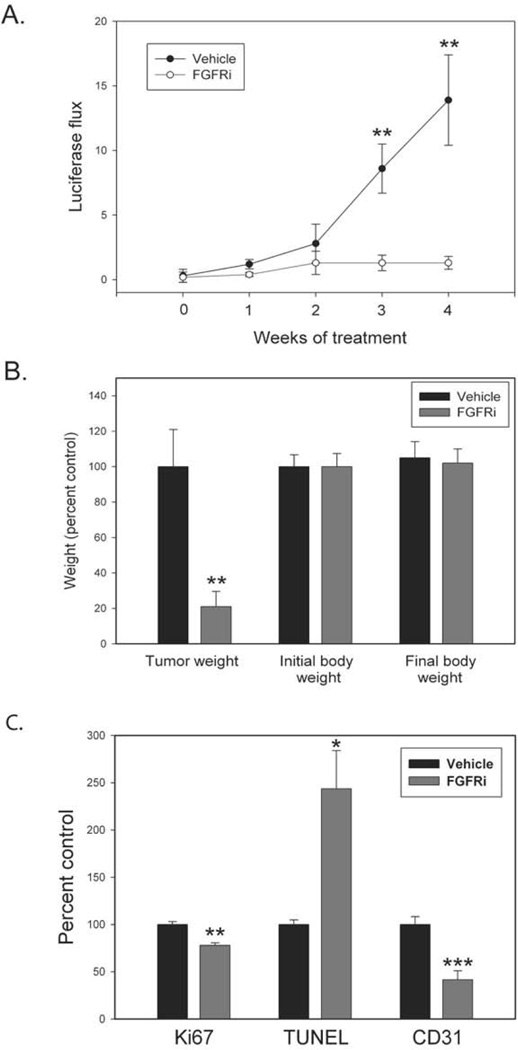
We then tested the anti-tumor activity of the AZ8010 using VCaP cells expressing luciferase in vivo. Two weeks after subcutaneous injection in nude mice, animals were treated with AZ8010 at 12.5 mg/kg/day by oral gavage or vehicle only. Tumors were collected 4 hours after the last drug treatment. As seen in Fig 5A, this treatment resulted in nearly complete inhibition of tumor growth by luciferase imaging. Mean tumor weight after 4 weeks of treatment was significantly decreased (Fig 5B); 194 mg for treated tumors Vs 910 mg for controls, (p=.01, Mann-Whitney). No toxicity was detected and mouse weights were stable throughout this experiment and no differences were seen in body weight between the treated and control groups (Fig 5B). Tumor sections were then analyzed using IHC for Ki67 to evaluate proliferation and CD31 to evaluate angiogenesis. Apoptosis was evaluated by TUNEL and all three markers were quantitated using image analysis (Fig 5C). Ki67 staining was decreased by 22% (p<0.01, Mann-Whitney) while TUNEL was increased by almost 250% (p<0.02, Mann-Whitney). Blood vessel area as determined by CD31 immunostaining and image analysis was decreased by 58% (p<0.001, Mann-Whitney). See Supplementary Figure 2 for representative images of stained slides. These findings are concordant with the decreased tumor growth observed.
Figure 5. AZ8010 treatment inhibits tumor progression in vivo.
A. Nude mice were injected subcutaneously with VCaP expressing luciferase. After two weeks (0 timepoint), luciferase flux in tumors was measured using a Xenogen imager after luciferin injection. Mice were then treated by oral gavage with 12.5 mg/kg/day AZ8010 or vehicle and tumor luciferase flux measured weekly for 4 weeks. Values Mean +/− SEM (n=21, treated; n=24, control). B. Left: Mean tumor weights +/− SEM in AZ8010 treated and control mice at termination of treatment. Values expressed as percent of tumor size of control mice; right: Mean body weights (+/− SEM) of mice treated with AZ8010 or vehicle at the initiation and termination of treatment. Values expressed as percent of weight of control mice at the initiation of treatment. C. Mean percent nuclei stained with Ki67 or TUNEL or mean tumor area stained with CD31 in treated and control tumors. Values expressed as percent control +/− SEM. * p<.05; ** p<.01; ***p<.001
In vivo targets of AZ8010
To evaluate inhibition of activation of key signaling targets by AZ8010 in vivo, protein lysates of VCaP xenografts treated with AZ8010 or controls were analyzed. Equal quantities of xenograft extract protein were immunoprecipitated with anti-FGFR-1 and immunoblotted with anti-phospho-FGFR antibodies. Phosphorylated FGFR-1 was markedly decreased (Fig 6A) and quantitative analysis of Western blots showed a 95% decrease in band intensity relative to controls (p<.04, t test). Western blots of tumor extracts were also analyzed for alterations of MEK phosphorylation (Fig 6B), which is upstream of ERK. MEK phosphorylation was visibly decreased and by quantitative analysis of Western blots there was a 56% decrease in band intensity relative to controls (p<.01, t-test). ERK phosphorylation (Fig 6C) was also significantly inhibited and, by quantitative analysis, band intensity was decreased by 84% (p<0.02, t-test). Thus the predicted targets show significant inhibition in vivo in tumors treated with AZ8010. Interestingly, we saw no alteration in AKT phosphorylation in treated tumors (Fig 6D), although in some systems AKT activation is downstream of FGFR signaling. Concordant with this observation, we observed no decrease in AKT activation upon treatment with AZ8010 in PC3 and FGFR-4 expressing PNT1a cells (Supplementary Fig 3). Thus ERK, rather than AKT, seems to be the critical target of AZ8010 in vivo.
Figure 6. Treatment with AZ8010 inhibits FGFR-1 and ERK signaling in vivo.
A. Equal quantities of VCaP xenograft extracts from AZD8010 treated group and vehicle control group were immunoprecipitated with anti-FGFR-1 and immunoblotted with anti-phospho-FGFR antibodies. B. VCaP xenograft tumor protein lysates were analyzed for the phosphorylation of MEK1/2. C. VCaP xenograft tumor protein lysates were analyzed for the phosphorylation of ERK1/2. D. VCaP tumors protein lysates were analyzed for expression of P-AKT. Note that numbers above lanes represent loading order and do not correspond between the different analyses due to the limited amount of extract in many treated tumors.
DISCUSSION
Based on correlative studies in human tissue samples and animal model studies, FGFR signaling is a promising therapeutic target in PCa. Our studies with AZ8010 support this concept. It should be noted that reported analyses to date do not show high level amplification or point mutations of FGFRs in PCa tissues, in contrast to the findings in other malignancies such as gastric cancer (amplification) or bladder cancer (point mutation). In PCa there is overexpression of multiple FGF ligands, increased receptor expression, association of progression with germline polymorphisms that enhance signaling and downregulation of FGF signaling inhibitors (2). Thus while somatic DNA structural alterations are reliable indicators of susceptibility to targeted agents in many cases, other alterations can also be indicative of involvement of a specific signaling pathway in cancer progression.
One interesting aspect of our in vitro studies is our finding that the FGFR inhibitor had significant effects on invasion in all cell lines tested while effects on proliferation were significantly weaker. However, net cell growth in vivo was markedly inhibited by FGFR inhibition. It is interesting to note that the TMPRSS2/ERG fusion gene, which is present in 40–60% of human PCas, strongly promotes invasion in vitro but has more limited affects on proliferation in vitro and yet when it is knocked down with shRNA, tumor progression in vivo is significantly inhibited (35). One interpretation of these findings is that invasive capacity is required for tumor growth in vivo and that effects on proliferation in vitro may not necessarily reflect the ability of a drug or knockdown of a gene target to inhibit tumor progression in vivo.
In addition to direct effects on tumor invasion in PCa, inhibition of FGFR signaling has significant effects on the tumor microenvironment, either directly or indirectly. One major target is angiogenesis, which was decreased by almost 60% in treated tumors. This may reflect the well known direct affects of FGF signaling on endothelial cells and other vascular cells to promote angiogenesis (2). In addition, there may be indirect affects on tumor cells of FGFR inhibition that could inhibit secretion of paracrine factors that promote angiogenesis. For example, VEGF has been shown to be induced by FGF signaling in some systems (38). Similarly, FGF signaling also plays a role in myofibroblast promotion of PCa progression, in part by enhancing angiogenesis (39). It is likely that the decreased proliferation and increased cell death seen in the treated tumors in vivo is in part due to inhibition of angiogenesis and other microenvironmental effects and this accounts for some of the difference between in vitro and the in vivo affects on net proliferation. It is also possible that these effects may be due to changes in the biology of the cancer cells themselves when growing in an in vivo context. One potential explanation is that in tumors the effective FGF concentration is higher due to binding of secreted FGFs by extracellular matrix proteins within the tumor. Further studies are needed to understand in detail the importance of different activities in the observed tumor growth inhibition.
As noted above, AZ8010 inhibits VEGFR2 activation at an IC50 of greater than 200 nM. VEGFR2 is expressed on endothelial cells and promotes angiogenesis (40) so that it is possible that some of the effects seen on angiogenesis are a result of inhibit of endothelial VEGFR2. However, two hours after oral administration of AZ8010 in nude mice free serum levels of the drug are approximately 170 nM and 64 nM by 4 hours, with levels following to 3 nM at 24 hours after treatment (unpublished data). Thus any inhibition of endothelial VEGFR2 (and VEGFR2 on PCa cells) is likely to be quite transient using a daily drug administration. Thus while inhibition of endothelial VEGFR2 may play a role in the effects seen in vivo, it is likely to be minor. Overall, it is likely that the vast majority of the antitumor effects of AZD81010 in vivo can be accounted for by FGFR inhibition but further studies are needed to clarify this point. Of course, from a clinical point of view, some VEGFR2 inhibition is not a negative attribute for a cancer therapeutic.
We have previously shown that ERK activation is a major downstream target of activated FGFRs and ERK activation strongly promotes PCa cell invasion in vitro (26). A striking result of our studies is that the vast majority of ERK activation in VCaP cells in vivo (>80%) can be attributed to FGFR activation. The extent of this inhibition is surprising given that many growth factor receptors can activate ERK. However, this finding implies that FGFs are the major growth factor receptor ligands in VCaP cells that activate ERK in vivo. This is clinically relevant since our previous studies have shown that an ERK driven gene signature is associated with aggressive disease in PCa (26). Equally striking was the lack of effect on AKT activation. To date, we have not seen major direct effects of FGFR signaling on AKT activation in PCa cell lines, even in cells with PTEN inactivation (unpublished data). Of note, studies with AZD4547 show variable impact of FGFR inhibition on AKT activation, with breast cancer cell lines showing inhibition of AKT activation while myeloma and myeloid leukemia cells did not show any effect (33). Thus FGFR activation of AKT seems to be highly context dependent. This implies that in PCa FGFR inhibition and targeted inhibition of the AKT pathway may be a rationale therapeutic strategy in cancer subtypes not showing inhibition of AKT activation by FGFR inhibitors. The extent to which other signaling pathways activated by FGFRs, such as PLC-γ and STATs (1–3), contribute to the anti-tumor efficacy of FGFR inhibition in PCa in vivo will need to be determined.
AZ8010 is highly chemically related to a newer generation FGFR inhibitor AZD4547 (33), and its properties in vitro are almost identical to AZD4547, but it has inferior pharmacokinetic properties. As described above, two hours after administration of AZD8010 serum levels are approximately 170 nM and falls to 3 nM by 24 hours after administration. Thus effective drug concentrations that can inhibit FGFR-4, and to a lesser extent FGFR-1, are not maintained for the entire 24 hours between drug administrations in our studies. This almost certainly decreases its potential efficacy and it is likely that AZD4547 will be more potent in vivo in targeting PCa expressing higher levels of FGFR-4. AZD4547 is currently undergoing Phase I clinical trials in patients with advanced cancers. Our studies suggest that the AZD4547 may be useful in the treatment of aggressive PCa at various clinical stages.
Supplementary Material
STATEMENT OF CLINICAL RELEVANCE.
Prostate cancer (PCa) is the most common visceral malignancy and the second leading cause of cancer deaths in men in the United States. There is compelling evidence both from studies of human tumor samples and from animal models that fibroblast growth factors (FGFs) and FGF receptors (FGFRs) are important in PCa initiation and progression. In this study we demonstrate that inhibition of FGFR signaling using a novel small molecule inhibitor inhibits PCa cell invasion in vitro and tumor progression in vivo. These results indicate that targeting FGFR signaling is a promising new therapeutic approach for treating aggressive PCa.
ACKNOWLEDGEMENTS
The technical assistance of Billie Smith with immunohistochemistry is gratefully acknowledged. Astra Zeneca provided AZ8010 but no direct funding for these studies.
Grant Support: This work was supported by the Dept of Veterans Affairs Merit Review program (MI), the Prostate Cancer Foundation (MI) and the NIH U01 Mouse Models of Human Cancer (U01CA141497; MI) and by the use of the facilities of the Michael E. DeBakey VAMC.
REFERENCES
- 1.Kwabi-Addo B, Ozen M, Ittmann M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer. 2004;11:709–724. doi: 10.1677/erc.1.00535. [DOI] [PubMed] [Google Scholar]
- 2.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 3.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Ropiquet F, Giri D, Kwabi-Addo B, Mansukhani A, Ittmann M. Increased expression of fibroblast growth factor 6 in human prostatic intraepithelial neoplasia and prostate cancer. Cancer Res. 2000;60:4245–4250. [PubMed] [Google Scholar]
- 5.Giri D, Ropiquet F, Ittmann M. Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res. 1999;5:1063–1071. [PubMed] [Google Scholar]
- 6.Dakhova O, Ozen M, Creighton CJ, Li R, Ayala G, Rowley D, et al. Global gene expression analysis of reactive stroma in prostate cancer. Clin Cancer Res. 2009;15:3979–3989. doi: 10.1158/1078-0432.CCR-08-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy T, Darby S, Mathers ME, Gnanapragasam VJ. Evidence for distinct alterations in the FGF axis in prostate cancer progression to an aggressive clinical phenotype. J Pathol. 2010;220:452–460. doi: 10.1002/path.2657. [DOI] [PubMed] [Google Scholar]
- 8.Valve EM, Nevalainen MT, Nurmi MJ, Laato MK, Martikainen PM, Harkonen PL. Increased expression of FGF-8 isoforms and FGF receptors in human premalignant prostatic intraepithelial neoplasia lesions and prostate cancer. Lab Invest. 2001;81:815–826. doi: 10.1038/labinvest.3780291. [DOI] [PubMed] [Google Scholar]
- 9.Li ZG, Mathew P, Yang J, Starbuck MW, Zurita AJ, Liu J, et al. Androgen receptornegative human prostate cancer cells induce osteogenesis in mice through FGF9-mediated mechanisms. J Clin Invest. 2008;118:2697–2710. doi: 10.1172/JCI33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valta MP, Tuomela J, Bjartell A, Valve E, Vaananen HK, Harkonen P. FGF-8 is involved in bone metastasis of prostate cancer. Int J Cancer. 2008;123:22–31. doi: 10.1002/ijc.23422. [DOI] [PubMed] [Google Scholar]
- 11.Heer R, Douglas D, Mathers ME, Robson CN, Leung HY. Fibroblast growth factor 17 is over-expressed in human prostate cancer. J Pathol. 2004;204:578–586. doi: 10.1002/path.1668. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi H. [Studies on the expression of fibroblast growth factors and fibroblast growth factor receptors in human prostate cancer] Nippon Hinyokika Gakkai Zasshi. 1998;89:836–845. doi: 10.5980/jpnjurol1989.89.836. [DOI] [PubMed] [Google Scholar]
- 13.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. Expression of bFGF/FGFR-1 and vascular proliferation related to clinicopathologic features and tumor progress in localized prostate cancer. Virchows Arch. 2006;448:68–74. doi: 10.1007/s00428-005-0075-3. [DOI] [PubMed] [Google Scholar]
- 14.Jin C, McKeehan K, Guo W, Jauma S, Ittmann MM, Foster B, et al. Cooperation between ectopic FGFR1 and depression of FGFR2 in induction of prostatic intraepithelial neoplasia in the mouse prostate. Cancer Res. 2003;63:8784–8790. [PubMed] [Google Scholar]
- 15.Polnaszek N, Kwabi-Addo B, Peterson LE, Ozen M, Greenberg NM, Ortega S, et al. Fibroblast growth factor 2 promotes tumor progression in an autochthonous mouse model of prostate cancer. Cancer Res. 2003;63:5754–5760. [PubMed] [Google Scholar]
- 16.Wang F, McKeehan K, Yu C, Ittmann M, McKeehan WL. Chronic activity of ectopic type 1 fibroblast growth factor receptor tyrosine kinase in prostate epithelium results in hyperplasia accompanied by intraepithelial neoplasia. Prostate. 2004;58:1–12. doi: 10.1002/pros.10311. [DOI] [PubMed] [Google Scholar]
- 17.Acevedo VD, Gangula RD, Freeman KW, Li R, Zhang Y, Wang F, et al. Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer Cell. 2007;12:559–571. doi: 10.1016/j.ccr.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Carstens RP, Eaton JV, Krigman HR, Walther PJ, Garcia-Blanco MA. Alternative splicing of fibroblast growth factor receptor 2 (FGF-R2) in human prostate cancer. Oncogene. 1997;15:3059–3065. doi: 10.1038/sj.onc.1201498. [DOI] [PubMed] [Google Scholar]
- 19.Naimi B, Latil A, Fournier G, Mangin P, Cussenot O, Berthon P. Down-regulation of (IIIb) and (IIIc) isoforms of fibroblast growth factor receptor 2 (FGFR2) is associated with malignant progression in human prostate. Prostate. 2002;52:245–252. doi: 10.1002/pros.10104. [DOI] [PubMed] [Google Scholar]
- 20.Kwabi-Addo B, Ropiquet F, Giri D, Ittmann M. Alternative splicing of fibroblast growth factor receptors in human prostate cancer. Prostate. 2001;46:163–172. doi: 10.1002/1097-0045(20010201)46:2<163::aid-pros1020>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 21.Sahadevan K, Darby S, Leung HY, Mathers ME, Robson CN, Gnanapragasam VJ. Selective over-expression of fibroblast growth factor receptors 1 and 4 in clinical prostate cancer. J Pathol. 2007;213:82–90. doi: 10.1002/path.2205. [DOI] [PubMed] [Google Scholar]
- 22.Gowardhan B, Douglas DA, Mathers ME, McKie AB, McCracken SR, Robson CN, et al. Evaluation of the fibroblast growth factor system as a potential target for therapy in human prostate cancer. Br J Cancer. 2005;92:320–327. doi: 10.1038/sj.bjc.6602274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Stockton DW, Ittmann M. The fibroblast growth factor receptor-4 Arg388 allele is associated with prostate cancer initiation and progression. Clin Cancer Res. 2004;10:6169–6178. doi: 10.1158/1078-0432.CCR-04-0408. [DOI] [PubMed] [Google Scholar]
- 24.Ma Z, Tsuchiya N, Yuasa T, Inoue T, Kumazawa T, Narita S, et al. Polymorphisms of fibroblast growth factor receptor 4 have association with the development of prostate cancer and benign prostatic hyperplasia and the progression of prostate cancer in a Japanese population. Int J Cancer. 2008;123:2574–2579. doi: 10.1002/ijc.23578. [DOI] [PubMed] [Google Scholar]
- 25.Xu B, Tong N, Chen SQ, Hua LX, Wang ZJ, Zhang ZD, et al. FGFR4 Gly388Arg polymorphism contributes to prostate cancer development and progression: A meta-analysis of 2618 cases and 2305 controls. BMC Cancer. 2011;11:84. doi: 10.1186/1471-2407-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu W, Feng S, Dakhova O, Creighton CJ, Cai Y, Wang J, et al. FGFR-4 Arg(3) enhances prostate cancer progression via extracellular signal-related kinase and serum response factor signaling. Clin Cancer Res. 2011;17:4355–4366. doi: 10.1158/1078-0432.CCR-10-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fritzsche S, Kenzelmann M, Hoffmann MJ, Muller M, Engers R, Grone HJ, et al. Concomitant down-regulation of SPRY1 and SPRY2 in prostate carcinoma. Endocr Relat Cancer. 2006;13:839–849. doi: 10.1677/erc.1.01190. [DOI] [PubMed] [Google Scholar]
- 28.Kwabi-Addo B, Wang J, Erdem H, Vaid A, Castro P, Ayala G, et al. The expression of Sprouty1, an inhibitor of fibroblast growth factor signal transduction, is decreased in human prostate cancer. Cancer Res. 2004;64:4728–4735. doi: 10.1158/0008-5472.CAN-03-3759. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Thompson B, Ren C, Ittmann M, Kwabi-Addo B. Sprouty4, a suppressor of tumor cell motility, is down regulated by DNA methylation in human prostate cancer. Prostate. 2006;66:613–624. doi: 10.1002/pros.20353. [DOI] [PubMed] [Google Scholar]
- 30.Darby S, Murphy T, Thomas H, Robson CN, Leung HY, Mathers ME, et al. Similar expression to FGF (Sef) inhibits fibroblast growth factor-induced tumourigenic behaviour in prostate cancer cells and is downregulated in aggressive clinical disease. Br J Cancer. 2009;101:1891–1899. doi: 10.1038/sj.bjc.6605379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darby S, Sahadevan K, Khan MM, Robson CN, Leung HY, Gnanapragasam VJ. Loss of Sef (similar expression to FGF) expression is associated with high grade and metastatic prostate cancer. Oncogene. 2006;25:4122–4127. doi: 10.1038/sj.onc.1209428. [DOI] [PubMed] [Google Scholar]
- 32.Ozen M, Giri D, Ropiquet F, Mansukhani A, Ittmann M. Role of fibroblast growth factor receptor signaling in prostate cancer cell survival. J Natl Cancer Inst. 2001;93:1783–1790. doi: 10.1093/jnci/93.23.1783. [DOI] [PubMed] [Google Scholar]
- 33.Gavine PR, Mooney L, Kilgour E, Thomas AP, Al-Kadhimi K, Beck S, et al. AZD4547: An orally bioavailable, potent and selective inhibitor of the Fibroblast Growth Factor Receptor tyrosine kinase family. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-3034. [DOI] [PubMed] [Google Scholar]
- 34.Ho HK, Pok S, Streit S, Ruhe JE, Hart S, Lim KS, et al. Fibroblast growth factor receptor 4 regulates proliferation, anti-apoptosis and alpha-fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. J Hepatol. 2009;50:118–127. doi: 10.1016/j.jhep.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Cai Y, Yu W, Ren C, Spencer DM, Ittmann M. Pleiotropic biological activities of alternatively spliced TMPRSS2/ERG fusion gene transcripts. Cancer Res. 2008;68:8516–8524. doi: 10.1158/0008-5472.CAN-08-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Cai Y, Shao LJ, Siddiqui J, Palanisamy N, Li R, et al. Activation of NF-{kappa}B by TMPRSS2/ERG Fusion Isoforms through Toll-Like Receptor-4. Cancer Res. 2011;71:1325–1333. doi: 10.1158/0008-5472.CAN-10-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Yu W, Cai Y, Ren C, Ittmann MM. Altered fibroblast growth factor receptor 4 stability promotes prostate cancer progression. Neoplasia. 2008;10:847–856. doi: 10.1593/neo.08450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claffey KP, Abrams K, Shih SC, Brown LF, Mullen A, Keough M. Fibroblast growth factor 2 activation of stromal cell vascular endothelial growth factor expression and angiogenesis. Lab Invest. 2001;81:61–75. doi: 10.1038/labinvest.3780212. [DOI] [PubMed] [Google Scholar]
- 39.Yang F, Strand DW, Rowley DR. Fibroblast growth factor-2 mediates transforming growth factor-beta action in prostate cancer reactive stroma. Oncogene. 2008;27:450–459. doi: 10.1038/sj.onc.1210663. [DOI] [PubMed] [Google Scholar]
- 40.Tahir SA, Park S, Thompson TC. Caveolin-1 regulates VEGF-stimulated angiogenic activities in prostate cancer and endothelial cells. Cancer Biol Ther. 2009;8:2286–2296. doi: 10.4161/cbt.8.23.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.