Abstract
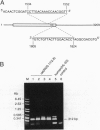
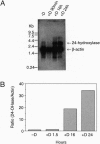
Human 1,25-dihydroxyvitamin D3 24-hydroxylase cDNA clones were isolated from an HL-60 cell cDNA library by using a reverse transcription/polymerase chain reaction-generated human cDNA probe. The 24-hydroxylase cDNA consists of a 1539-bp open reading frame encoding a 513-amino acid polypeptide. Protein sequence analysis shows that the human 24-hydroxylase is 90% homologous (82% identical) to that of the rat, with 100% homology in the 21-amino acid heme-binding region. Northern blot analysis showed that the 24-hydroxylase cDNA probe hybridized to a 3.4-kb mRNA species. Treatment of HL-60 cells with 0.1 microM 1,25-dihydroxyvitamin D3 for 24 hr produced a 30-fold increase in the 24-hydroxylase mRNA level. This result is consistent with previous studies in the same cell line, in which 24-hydroxylase activity was elevated to a maximum in 24 hr by a similar treatment with 1,25-dihydroxyvitamin D3. To verify the identity of these isolated cDNA clones, two polymerase chain reaction-amplified human 24-hydroxylase cDNA fragments containing the entire coding region were used to produce 24-hydroxylase enzyme activity in two genetic expression systems. Transient levels of 24-hydroxylase activity were measured in transfected mammalian COS-1 cells and in recombinant baculovirus-infected Spodoptera frugiperda (Sf21) insect cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brommage R., DeLuca H. F. Evidence that 1,25-dihydroxyvitamin D3 is the physiologically active metabolite of vitamin D3. Endocr Rev. 1985 Fall;6(4):491–511. doi: 10.1210/edrv-6-4-491. [DOI] [PubMed] [Google Scholar]
- Burgos-Trinidad M., Brown A. J., DeLuca H. F. A rapid assay for 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D 24-hydroxylase. Anal Biochem. 1990 Oct;190(1):102–107. doi: 10.1016/0003-2697(90)90141-u. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Vitamin D: recent advances. Annu Rev Biochem. 1983;52:411–439. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J. 1988 Mar 1;2(3):224–236. [PubMed] [Google Scholar]
- Gaudette M. F., Crain W. R. A simple method for quantifying specific mRNAs in small numbers of early mouse embryos. Nucleic Acids Res. 1991 Apr 25;19(8):1879–1884. doi: 10.1093/nar/19.8.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M., Burgos-Trinidad M., DeLuca H. F. Characteristics of the 25-hydroxyvitamin D3- and 1,25-dihydroxyvitamin D3-24-hydroxylase(s) from HL-60 cells. Arch Biochem Biophys. 1991 Feb 1;284(2):257–263. doi: 10.1016/0003-9861(91)90293-r. [DOI] [PubMed] [Google Scholar]
- Jones G., Kano K., Yamada S., Furusawa T., Takayama H., Suda T. Identification of 24,25,26,27-tetranor-23-hydroxyvitamin D3 as a product of the renal metabolism of 24,25-dihydroxyvitamin D3. Biochemistry. 1984 Jul 31;23(16):3749–3754. doi: 10.1021/bi00311a028. [DOI] [PubMed] [Google Scholar]
- Kuribayashi T., Tanaka H., Abe E., Suda T. Functional defect of variant clones of a human myeloid leukemia cell line (HL-60) resistant to 1 alpha,25-dihydroxyvitamin D3. Endocrinology. 1983 Dec;113(6):1992–1998. doi: 10.1210/endo-113-6-1992. [DOI] [PubMed] [Google Scholar]
- Mortensen B. T., Hartmann N. R., Christensen I. J., Larsen J. K., Kristensen T., Wieslander S. B., Nissen N. I. Synchronization of the human promyelocytic cell line HL 60 by thymidine. Cell Tissue Kinet. 1986 May;19(3):351–364. doi: 10.1111/j.1365-2184.1986.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Ohyama Y., Noshiro M., Okuda K. Cloning and expression of cDNA encoding 25-hydroxyvitamin D3 24-hydroxylase. FEBS Lett. 1991 Jan 28;278(2):195–198. doi: 10.1016/0014-5793(91)80115-j. [DOI] [PubMed] [Google Scholar]
- Pike J. W. Emerging concepts on the biologic role and mechanism of action of 1,25-dihydroxyvitamin D3. Steroids. 1987 Jan-Mar;49(1-3):3–27. doi: 10.1016/0039-128x(87)90077-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahaka Y., Lorenc R. S., DeLuca H. F. The role of 1,25-dihydroxyvitamin D3 and parathyroid hormone in the regulation of chick renal 25-hydroxyvitamin D3-24-hydroxylase. Arch Biochem Biophys. 1975 Dec;171(2):521–526. doi: 10.1016/0003-9861(75)90061-2. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Abe E., Miyaura C., Shiina Y., Suda T. 1 alpha,25-dihydroxyvitamin D3 induces differentiation of human promyelocytic leukemia cells (HL-60) into monocyte-macrophages, but not into granulocytes. Biochem Biophys Res Commun. 1983 Nov 30;117(1):86–92. doi: 10.1016/0006-291x(83)91544-9. [DOI] [PubMed] [Google Scholar]
- Tarella C., Ferrero D., Gallo E., Pagliardi G. L., Ruscetti F. W. Induction of differentiation of HL-60 cells by dimethyl sulfoxide: evidence for a stochastic model not linked to the cell division cycle. Cancer Res. 1982 Feb;42(2):445–449. [PubMed] [Google Scholar]
- Thomsen D. R., Stenberg R. M., Goins W. F., Stinski M. F. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):659–663. doi: 10.1073/pnas.81.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubaki M., Iwamoto Y., Hiwatashi A., Ichikawa Y. Inhibition of electron transfer from adrenodoxin to cytochrome P-450scc by chemical modification with pyridoxal 5'-phosphate: identification of adrenodoxin-binding site of cytochrome P-450scc. Biochemistry. 1989 Aug 22;28(17):6899–6907. doi: 10.1021/bi00443a019. [DOI] [PubMed] [Google Scholar]
- Tuls J., Geren L., Millett F. Fluorescein isothiocyanate specifically modifies lysine 338 of cytochrome P-450scc and inhibits adrenodoxin binding. J Biol Chem. 1989 Oct 5;264(28):16421–16425. [PubMed] [Google Scholar]
- Vaughn J. L., Goodwin R. H., Tompkins G. J., McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro. 1977 Apr;13(4):213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]