Abstract
We report on a case of potential envenomation caused by multiple bites by the aglyphous opisthodont snake Leioheterodon madagascariensis in the left thumb of a healthy adult man, which is among the most serious snakebites hitherto reported from Madagascar. The adult snake (total length > 1 meter) was unusually aggressive before and during capture. The symptoms included extensive bleeding, severe local pain, and substantial swelling of the hand and the distal part of the lower arm. The swelling disappeared entirely after five days, but pain in the thumb (when moved) was recognizable even longer. Although L. madagascariensis is widespread and common in anthropogenic habitats in eastern and western Madagascar, this case report seems to be the first description of long-lasting symptoms of its bite. Since aglyphous snakes are relatively rarely involved in “envenomation” and because hemolytic activity has been recorded in the secretions of the Duvernoy’s glands of Leioheterodon, we describe its dentition using microcomputed tomography and discuss the potential mode of envenomation in this case.
Electronic supplementary material
The online version of this article (doi:10.1186/s40409-015-0047-2) contains supplementary material, which is available to authorized users.
Keywords: Madagascar, Lamprophiidae, Pseudoxyrhophiinae, Leioheterodon madagascariensis, Envenomation, Dentition, Microcomputed tomography
Background
The caenophidian snake fauna of Madagascar consists mainly of a large radiation of lamprophiid (mostly pseudoxyrhophiine) snakes currently comprising more than 80 endemic species in 20 genera, and new species continue to be discovered and described regularly [1, 2]. Although relatively closely related to highly venomous snakes of the family Elapidae, which includes cobras, kraits, and sea snakes, lamprophiid snakes from Madagascar are comparatively harmless, and none of these snakes has venomous front fangs [3]. Several Malagasy genera are aglyphous, whereas others are opisthoglyphous, i.e., they have grooved rear fangs and are considered as mildly venomous, but little is known on the effects of their venoms to humans [4, 5]. Until now, mild “envenomation” caused by accidental bites by Malagasy snakes has been reported only for the psammophiine species Mimophis mahfalensis [6, 7] and six pseudoxyrhophiine species: two species of Madagascarophis [6, 8], Leioheterodon modestus [5], Leioheterodon madagascariensis [9], Ithycyphus miniatus [10], and Langaha madagascariensis [11], all of which can attain a relatively large size (≥1 m total length).
Leioheterodon comprises three robust species with terrestrial and diurnal habits [12, 13]. Although it is an aglyphous genus, it has a pair of enlarged teeth on the posterior end of the maxilla [4–6]. Leioheterodon madagascariensis, the Malagasy giant hognose snake, is one of the largest (total length up to > 1.50 m) and most common snakes in natural and anthropogenic habitats in eastern and western Madagascar, and is well known throughout the island as “menarana” [13, 14]. It is endemic to Madagascar and has been introduced to the island of Grand Comoro [15–17]. The recorded diet includes fish, frogs, reptiles (including snakes), reptile eggs, mammals and birds [14, 18–20]. According to Conant [21] one female of L. madagascariensis in captivity constricted larger prey and swallowed small animals directly without constriction. Mori and Tanaka [22] observed prey handling behavior of juvenile L. madagascariensis and reported that virtually all prey animals were swallowed alive. These observations suggest that the biological function of the secretions of the Duvernoy’s glands is not to kill prey during feeding. On the other hand, local pain, and mild swelling caused by the accidental bite by L. madagascariensis on humans is reported [9].
Herein, we report on a case of snakebite by L. madagascariensis in an adult man with a detailed description of the symptoms that were localized to the bite wound on the hand and the distal part of the arm. No other complications were observed. The potential mode of envenomation by Duvernoy’s gland secretions is discussed with reference to a study of its aglyphous dentition based on microcomputed tomography.
Case presentation
During intensive ecological fieldwork on snakes in Madagascar, the first author (hereafter referred to as patient) has been bitten several times by L. madagascariensis in the past decade. In all these cases only minor bleeding was observed after the bite, and only a small area around the puncture wounds became swollen. Only little pain was felt, and all symptoms had disappeared after about one hour.
On 10 May 2014, during fieldwork in northern Madagascar, the patient (41 years old, 58 kg) captured an adult L. madagascariensis (total length > 1 m) at 11:40 a.m. While his left hand was wrapped around the snake’s neck, the snake bit dorsolaterally (on the right side) the middle segment of the left thumb three times with increasing severity. Total bite duration was approximately 90 s. Shortly after the removal of the snake, the first series of photographs were taken to document the symptoms (Fig. 1). After removing the blood and disinfecting the thumb with an alcoholic solution (Octenisept®), three puncture wounds became visible. Two of them appeared rather deep with a distance of about 2 mm from each other, whereas the third one was relatively shallower with a distance of about 4 mm to the next one.
Fig. 1.
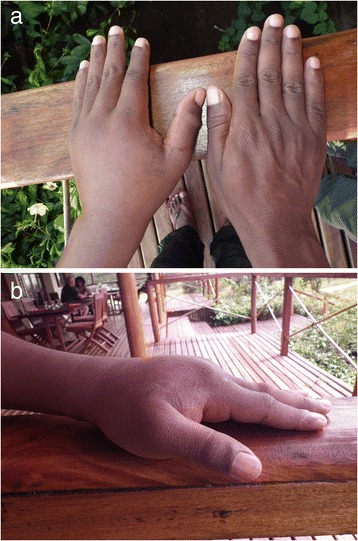
Photographs showing the swollen left hand after the bite of Leioheterodon madagascariensis. Approximately (a) 5 h and 30 min and (b) 24 h after the accident
Initial symptoms included partial loss of feeling in the fingers and distal half of the arm. Bleeding lasted for more than three hours after the initial bite. Swelling extended throughout the hand and to the distal half of the arm, and reached its maximum extent in 24 h. Slight pain was recognized in the left axillary lymph nodes five hours after the bite and persisted until the third day. Swelling lasted five days, but the last symptoms, the pain in the thumb and in the hand, only completely disappeared by the evening of 19 May 2014, nine days after the bite. During the whole event, only local symptoms were recognized. There were no signs of fever, diarrhea, headaches, sweating, bad sleep, or lethargy, and appetite remained normal. Treatment only consisted of cleaning up of the blood, disinfecting the wound, and application of a brayed local medicinal plant (called “tamotamo” in Malagasy language) to the injured region of the thumb eight hours after the first bite.
Dentition
The head of a L. madagascariensis was subjected to microtomographic analysis at the Zoologische Staatssammlung München (ZSM) using a phoenix nanotom m (GE Sensing & Inspection Technologies, Billerica, MA, USA) at 130 kV and 100 μA for 18 min, generating 1440 projections per scan. The data were visualized in VG Studio Max 2.2 (Visual Graphics GmbH, Heidelberg, Germany). Surface meshes of the skull were generated using the threshold tool in the segmentation editor of the software AMIRA 5.4.5 (FEI Visualization Sciences Group, Burlington MA, USA). The PDF-3D model (see Additional file 1) was then prepared following the procedures outlined by Ruthensteiner and Hess [23].
The following dentitional description is based on one adult male (ZSM 806/2001), of which separated dentigerous bones are shown in Fig. 2. On the maxilla, the 12 teeth increase in size posteriorly and are separated from the paired rear teeth by a diastema. Maxillary diastema width is more than the width of the preceding tooth. The paired rear teeth are greatly enlarged, approximately 4 mm in length (1.6 times the size of the preceding tooth), and are ungrooved with a knifelike posterior edge for a majority of teeth. The tips of the leading edge are slightly compressed. The nine palatine teeth are subequal. The 22 pterygoid teeth decrease in size. The dentary has 17 teeth, with the anterior dentary teeth slightly larger than the posterior.
Fig. 2.
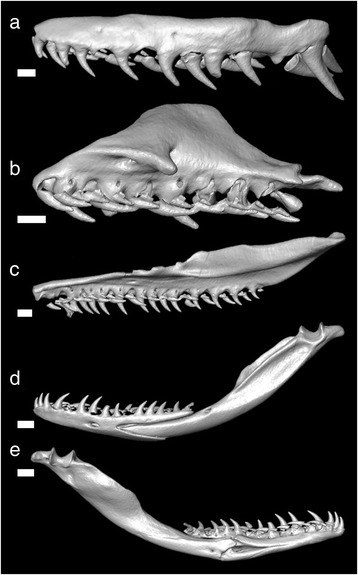
Dentition of Leioheterodon madagascariensis (ZSM 806/2001) based on microcomputed tomography scans of the skull. Outer lateral views of: (a) maxilla, (b) palatine, (c) pterygoid, (d) mandible (outer lateral view); (e) inner lateral view of mandible. Scale bars = 1 mm for (a–c). Scale bars = 2 mm for (d) and (e)
An interactive, 3D reconstructed skull of another L. madagascariensis (ZSM 806/2001) can be found in Additional file 1.
Discussion and conclusions
The case of snakebite by L. madagascariensis described herein is among the most serious hitherto reported from Madagascar. The swelling lasted for more than four days, and the last symptoms (local pain in the thumb) disappeared only after nine days. The comparatively strong swelling and heavy pain may be partially attributed to the amount of blunt trauma experienced by the patient (three bites total, with two deep bites). Additionally, the patient has a prior history of snakebites during which he could have developed a hypersensitivity reaction to the gland secretions, which then in combination with the prolonged duration of the bite, could have further contributed to the relative severity of the case. The snake was rather aggressive prior to capture, and although speculative, an increasing stress level may have resulted in more secretion delivery in the moment of biting.
It is likely that some of the symptoms could be attributed to secretions of the Duvernoy’s glands. Although no description of these glands in L. madagascariensis is available, it was recently described in Mimophis mahfalensis, a species of psammophiine lamprophiid snake [7]. Hemolytic activity has been recorded when the Duvernoy’s gland secretion from L. geayi was mixed with blood from mice, rabbit, and chicken, and injections of gland secretion from L. geayi into mice resulted in paralysis and, ultimately, death within twenty minutes [24]. Thus, Domergue [6] considered Leioheterodon to be capable of “envenomation” [5]. However, it appears unlikely that the “venom” of the “menarana” is mainly used for defense of potential predators. Instead it might be used primarily to aid in feeding by incapacitating large prey before it is swallowed or by facilitating digestion [25]. Further study is required to evaluate the composition of Duvernoy’s gland secretions and their toxicity to humans.
The dentition of L. madagascariensis appears to be relatively generalized and lacks specialized teeth for specific prey types found in previous studies [26–29]. This is supported by its generalist diet of prey ranging from frogs to birds to iguana eggs [14]. The left maxilla of L. modestus has been figured by Mori [5], which resembles that of L. madagascariensis. Examinations of the “venom” delivery mechanism of an opisthoglyphous snake, Boiga irregularis, which possesses Duvernoy’s glands and grooved, enlarged maxillary teeth, clarified the low-pressure system and found it to be much less efficient in venom delivery than the high-pressure system of viperids and elapids [30, 31]. The mechanism of “venom” delivery in snakes with ungrooved, enlarged teeth is not clear, but is probably similar to the low-pressure system of opisthoglyphous snakes in which the saliva flows into the bite wound. Further investigations of the mechanism of envenomation may reveal the functional role of ungrooved but enlarged maxillary teeth of Leioheterodon.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal.
Ethics committee approval
The CT-scanned snake specimen was collected and exported with all necessary permits of the Ministère de l’Environnement, de l’Ecologie, de la Mer et des Forêts, Madagascar. Approval by an ethics committee is not required by Malagasy laws for observations of accidental snakebites.
Acknowledgments
We are grateful to Yohei Suzuki (GenTV, Tokyo) who financed the field work in the framework of a film project. Manuscript preparation was partially supported by a Grant-in-Aid for International Scientific Research (B) (no. 24405008) from the Japan Ministry of Education, Culture, Sports, Science, and Technology.
Additional file
Interactive PDF-3D model based on the computed tomography scanned skull of Leioheterodon madagascariensis (ZSM 806/2001). Click on the image to enable the 3D content using Adobe Acrobat Reader (version IX or later). (PDF 1376 kb)
Footnotes
Competing interests
The authors declare that there are no competing interests.
Authors’ contributions
BR experienced snake envenomation and contributed to writing. CW carried out the microcomputed tomography study and contributed to writing. AM contributed to writing. FG conceived of the study, collected data, and drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Bertrand Razafimahatratra, Email: razbertrand@gmail.com.
Cynthia Wang, Email: cynthia.yh.wang@gmail.com.
Akira Mori, Email: gappa@ethol.zool.kyoto-u.ac.jp.
Frank Glaw, Email: frank.glaw@zsm.mwn.de.
References
- 1.Nagy ZT, Glaw F, Vences M. Systematics of the snake genera Stenophis and Lycodryas from Madagascar and the Comoros. Zool Scr. 2010;39(5):426–35. doi: 10.1111/j.1463-6409.2010.00435.x. [DOI] [Google Scholar]
- 2.Glaw F, Kucharzewski C, Nagy ZT, Hawlitschek O, Vences M. New insights into the systematics and molecular phylogeny of the Malagasy snake genus Liopholidophis suggest at least one rapid reversal of extreme sexual dimorphism in tail length. Org Divers Evol. 2014;14:121–32. doi: 10.1007/s13127-013-0152-4. [DOI] [Google Scholar]
- 3.Vidal N, Delmas AS, David P, Cruaud C, Couloux A, Hedges SB. The phylogeny and classification of caenophidian snakes inferred from seven nuclear protein-coding genes. C R Biol. 2007;330(2):182–7. doi: 10.1016/j.crvi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Guibé J. Les serpents de Madagascar. Mem Inst Sci Madagascar. 1958;12:189–260. [Google Scholar]
- 5.Mori A. A case of envenomation by the Madagascan colubrid snake, Leioheterodon modestus. Snake. 2002;29:7–8. [Google Scholar]
- 6.Domergue CA. Un serpent venimeux de Madagascar. Observation de deux cas de morsure par Madagascarophis (Colubridé opisthoglyphe) Arch Inst Pasteur Madagascar. 1989;56:299–311. [PubMed] [Google Scholar]
- 7.Rosa GM, Boistel R, Campantico E, Gillet B, Eusebio Bergò P, Andreone F. Case solved: presence of toxin-secreting oral glands in the lamprophiid snake Mimophis mahfalensis (Grandidier, 1867) from Madagascar. Zoomorphology. 2014;133(4):417–23. doi: 10.1007/s00435-014-0234-7. [DOI] [Google Scholar]
- 8.Domergue CA. Un serpent venimeux de Madagascar: Madagascarophis colubrina. Bull Acad Malgache. 1962;40:97–8. [Google Scholar]
- 9.Malina T, Krecsák L, Korsós Z, Takács Z. Snakebites in Hungary – epidemiological and clinical aspects over the past 36 years. Toxicon. 2008;51(6):943–51. doi: 10.1016/j.toxicon.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Mori A, Mizuta T. Envenomation by the Madagascan colubrid snake, Ithycyphus miniatus. J Venom Anim Toxins incl Trop Dis. 2006;12(3):512–20. doi: 10.1590/S1678-91992006000300013. [DOI] [Google Scholar]
- 11.D'Cruze NC. Envenomation by the Malagasy colubrid snake Langaha madagascariensis. J Venom Anim Toxins incl Trop Dis. 2008;14:546–51. doi: 10.1590/S1678-91992008000300014. [DOI] [Google Scholar]
- 12.Brygoo ER. Les ophidiens de Madagascar. Mem Inst Butantan. 1982;46:19–58. [Google Scholar]
- 13.Cadle JE. Colubridae, snakes. In: Goodman SM, Benstead JP, editors. The natural history of Madagascar. Chicago and London: The University of Chicago Press; 2003. pp. 997–1004. [Google Scholar]
- 14.Glaw F, Vences M. A field guide to the amphibians and reptiles of Madagascar. 3. Köln: Vences & Glaw Verlag GbR; 2007. [Google Scholar]
- 15.Wallach V. Lioheterodon madagascariensis an addition to the snake fauna of the Comoro islands. J Herpetol Assoc Afr. 1986;32:24–5. [Google Scholar]
- 16.Meirte D. New records of Leioheterodon madagascariensis (Reptilia, Colubridae) from the Comoros. J Herpetol Assoc Afr. 1993;42:21–3. [Google Scholar]
- 17.Hawlitschek O, Brückmann B, Berger J, Green K, Glaw F. Integrating field surveys and remote sensing data to study distribution, habitat use and conservation status of the herpetofauna of the Comoro Islands. ZooKeys. 2011;144:21–79. doi: 10.3897/zookeys.144.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preston-Mafham K. Madagascar: a natural history. New York: Facts on file; 1991. pp. 76–108. [Google Scholar]
- 19.Mori A, Randriamahazo H. Leioheterodon madagascariensis (Madagascar Menarana snake) diet. Herp Rev. 2002;33:57. [Google Scholar]
- 20.Furrer S. Erfahrungsbericht einer Gemeinschaftshaltung zwischen Madagassischen Hakennattern (Leioheterodon madagascariensis) und madagassischen Hundskopfboas (Sanzinia madagascariensis) im Zoo Zürich. Draco. 2004;5:91–2. [Google Scholar]
- 21.Conant R. A note on eggs and young of Leioheterodon madagascariensis (Duméril & Bibron) Zoologica. 1938;23:389–92. [Google Scholar]
- 22.Mori A, Tanaka K. Preliminary observations on chemical preference, antipredator responses, and prey-handling behavior of juvenile Leioheterodon madagascariensis (Colubridae) Curr Herpetol. 2001;20:39–49. doi: 10.5358/hsj.20.39. [DOI] [Google Scholar]
- 23.Ruthensteiner B, Hess M. Embedding 3D models of biological specimens in PDF publications. Microsc Res Tech. 2008;71(11):778–86. doi: 10.1002/jemt.20618. [DOI] [PubMed] [Google Scholar]
- 24.Domergue CA, Richaud J. Activité hémolytique des secrétions des glandes de Duvernoy chez Lioheterodon (Colubridé aglyphe) Arch Inst Pasteur Madagascar. 1971;40:145–8. [Google Scholar]
- 25.Kardong KV. Colubrid snakes and Duvernoy's ‘venom’ glands. Toxin Rev. 2002;21(1–2):1–19. doi: 10.1081/TXR-120004739. [DOI] [Google Scholar]
- 26.Savitzky AH. Hinged teeth in snakes: an adaptation for swallowing hard-bodied prey. Science. 1981;212(4492):346–9. doi: 10.1126/science.212.4492.346. [DOI] [PubMed] [Google Scholar]
- 27.Savitzky AH. Coadapted character complexes among snakes: fossoriality, piscivory, and durophagy. Am Zool. 1983;23(2):397–409. doi: 10.1093/icb/23.2.397. [DOI] [Google Scholar]
- 28.Jackson K, Fritts TH. Dentitional specialisations for durophagy in the Common Wolf snake, Lycodon aulicus capucinus. Amphibia-Reptilia. 2004;25:247–54. doi: 10.1163/1568538041975134. [DOI] [Google Scholar]
- 29.Jackson K, Underwood G, Arnold EN, Savitzky AH. Hinged teeth in the enigmatic colubrid, Iguanognathus werneri. Copeia. 1999;1999(3):815–8. doi: 10.2307/1447621. [DOI] [Google Scholar]
- 30.Hayes WK, Lavín-Murcio P, Kardong K. Delivery of Duvernoy's secretion into prey by the brown tree snake, Boiga irregularis (Serpentes: Colubridae) Toxicon. 1993;31(7):881–7. doi: 10.1016/0041-0101(93)90223-6. [DOI] [PubMed] [Google Scholar]
- 31.Kardong K, Lavin-Murcio PA. Venom delivery of snakes as high-pressure and low-pressure systems. Copeia. 1993;1993:644–50. doi: 10.2307/1447225. [DOI] [Google Scholar]


