Abstract
Background
The purpose of the MaxT algorithm is to provide a significance test algorithm that controls the family-wise error rate (FWER) during simultaneous hypothesis testing. However, the requirements in terms of computing time and memory of this procedure are proportional to the number of investigated hypotheses. The memory issue has been solved in 2013 by Van Lishout’s implementation of MaxT, which makes the memory usage independent from the size of the dataset. This algorithm is implemented in MBMDR-3.0.3, a software that is able to identify genetic interactions, for a variety of SNP-SNP based epistasis models effectively. On the other hand, that implementation turned out to be less suitable for genome-wide interaction analysis studies, due to the prohibitive computational burden.
Results
In this work we introduce gammaMAXT, a novel implementation of the maxT algorithm for multiple testing correction. The algorithm was implemented in software MBMDR-4.2.2, as part of the MB-MDR framework to screen for SNP-SNP, SNP-environment or SNP-SNP-environment interactions at a genome-wide level. We show that, in the absence of interaction effects, test-statistics produced by the MB-MDR methodology follow a mixture distribution with a point mass at zero and a shifted gamma distribution for the top 10 % of the strictly positive values. We show that the gammaMAXT algorithm has a power comparable to MaxT and maintains FWER, but requires less computational resources and time. We analyze a dataset composed of 106 SNPs and 1000 individuals within one day on a 256-core computer cluster. The same analysis would take about 104 times longer with MBMDR-3.0.3.
Conclusions
These results are promising for future GWAIs. However, the proposed gammaMAXT algorithm offers a general significance assessment and multiple testing approach, applicable to any context that requires performing hundreds of thousands of tests. It offers new perspectives for fast and efficient permutation-based significance assessment in large-scale (integrated) omics studies.
Electronic supplementary material
The online version of this article (doi:10.1186/s13040-015-0069-x) contains supplementary material, which is available to authorized users.
Keywords: Multiple testing, Genome-wide interaction studies, MaxT, Gamma distribution, SNP-environment interactions, 3-order interactions, Algorithmic
Background
Personalized medicine proposes to customize healthcare using molecular analysis [1–5]. However, for most human complex diseases, a deeper comprehension of the underlying biology is needed to make this approach workable. Since individual genes usually do not account for much of the heritability of phenotypes, the focus should be on the combined effect of all the genes in the background, rather than on the disease genes in the foreground [6–9]. MBMDR-4.2.2 is a software dedicated to genome-wide association interaction studies (GWAIs), the purpose of which is to identify pairs of SNPs and/or environmental factors that might regulate the susceptibility to the disease under investigation. The difficulty is to find a good balance between four main issues, that we summarise in the following objectives:
Minimize the amount of false discoveries.
Achieve sufficient statistical power to detect relevant pairs.
Reduce the computational burden implied by the high number of tests for interactions.
Provide a versatile software package that accommodates different study designs and study features, including flexibility in trait measurement types and the possibility to adjust for important predictor variables and confounders.
Available software
Among the numerous software designed for pair-wise or higher-order SNP-SNP interactions, we recall BOOST [10], BiForce [11], epiGPU [12], EpiBlaster [13], GLIDE [14], Multifactor Dimensionality Reduction (MDR) [15, 16] and Model-Based Multifactor Dimensionality Reduction (MB-MDR) [17, 18]. The following comparison of these approaches is mainly inspired from [19] who review and discuss several practical aspects GWAIs typically involve. BOOST is a software based on fast Boolean operations, to quickly search for epistasis associated with a binary outcome. Its main drawbacks are its inability to accommodate missing data and its necessity to perform a multiple testing correction outside the software package. BiForce is a regression-based tool handling binary and continuous outcomes, that can take account of missing genotypes and has a built-in multiple testing correction algorithm. Although, the latter is based on a fast Bonferroni correction implementation, it leads to reduced power for GWAIs, as further discussed in Multiple-testing correction Section. EpiBlaster, epiGPU and GLIDE are all GPU-based approaches. An obvious drawback of GPU-dependent software is that it is tuned for a particular GPU-infrastructure. Therefore, users are advocated to acquire the exact same infrastructure and only experts can adapt the code to specific needs. Note that users willing to work on dedicated hardware to speed up the computations can even turn to field-programmable gate array (FPGa) [20]. MDR is a non-parametric alternative to traditional regression-based methods that converts two or more variables into a single lower-dimensional attribute. The end goal is to identify a representation that facilitates the detection of non-linear or non-additive interactions. Over-fitting issues in MDR are solved via cross-validation and permutations. Since the design of MDR, several adaptations have been made [21]. MB-MDR breaks with the tradition of cross-validation and invests computing time in permutation-based multiple multilocus significance assessments and the implementation of the most appropriate association test for the data at hand. It is able to correct for important main effects. Its main asset compared to the other methods is its versatility. MB-MDR can for instance be used to highlight gene-environment or gene-gene-environment interactions in relation to a trait of interest, while efficiently controlling type I error rates. The trait can either be expressed on a binary or continuous scale, or as a censored trait. MDMDR-3.0.3 is a C++ software tool based on the MB-MDR methodology, achieving good results regarding objectives (1), (2) and (4) [22, 23]. However, concerns about computational efficiency remain when scaling up to exhaustive genome-wide interaction contexts. In this work we introduce a new version of the software, MDMDR-4.2.2, based on a novel multiple-testing correction algorithm, with the purpose of improving the performances along objective (3), with the same benefits as before regarding the other three ones.
Multiple-testing correction
In GWAIs, the most global null hypothesis is that none of the SNPs pairs, nor their main effects, are associated with the trait. Testing each pair independently at level α does not control the overall FWER at level α; an adjustment is needed for the fact that multiple tests are performed. One such adjustment can be realized via a Bonferroni correction [24]. This is a so called single-step procedure for strong FWER control. Single-step methods tend to be conservative though and improvements in power can be achieved by so called step-down procedures [25]. Among these we recall step down minP adjusted p-values (minP) and step down maxT adjusted p-values (maxT). These methods guarantee strong control of the FWER under the subset pivotality assumption and weak control under all conditions [26]. Both procedures are available in MDMDR-3.0.3, the adjusted p-values being estimated by permutation. Since a high number of pairs of SNPs are tested, minP tends to be more conservative than maxT [25]. Furthermore, minP requires more computations than maxT. For these reasons, maxT is the default choice in MDMDR-3.0.3. Note that the drawback of maxT compared to minP, is that when the test statistics are not identically distributed unbalanced adjustments can be observed because not all tests contribute equally to the computed adjusted p-values.
Figure 1(a) describes the classical implementation of maxT in MB-MDR. Test-statistics are computed for all m pairs of SNPs and sorted in decreasing order in vector Real Data. The trait is permuted B times and test-statistics are computed for all pairs of SNPs and stored in vectors Permutation i, i=1,…,B. The latter are browsed from right to left and any value higher than its left neighbor’s value overwrites the latter value. This step is an algorithmic trick to reach efficiently an idea that is best explained the other way around. Let T i,max be the maximum of Permutation i, i=1,…,B. The T i,max values can be used to approximate the distribution of the highest observed value when testing m pairs under the global null hypothesis (no pair of SNPs associated to the disease). Comparing T 0,1 to this distribution enables the computation of adjusted p-value p 1, i.e. the probability of observing a value as extreme as T 0,1 for the most promising pair of SNPs. Removing the latter from the data and restarting the whole procedure would obviously allow the computation of adjusted p-value p 2 and so on for the remaining ones. From an algorithmic point of view, this would be a waste of time, hence the aforementioned procedure leading to the same result. Finally, the adjusted p-values are browsed from left to right and any value higher than its right neighbors’s value overwrites the latter. This procedure obviously aims at controlling the FWER. A particular hypothesis can indeed now only be rejected if all hypotheses were rejected beforehand. The problem of the original maxT is that it is both time and memory consuming.
Fig. 1.
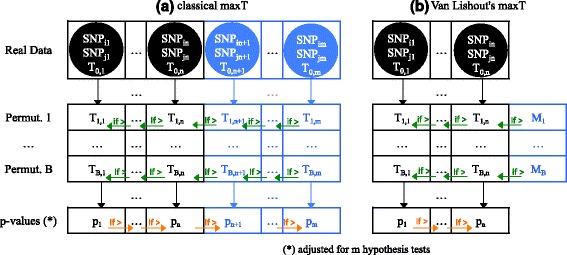
classical MaxT versus Van Lishout’s implementation of MaxT. In the classical implementation of MaxT, all T i,j values are computed and stored in memory, , ∀j=1…m. Then, T i,j is overwritten by T i,j+1 whenever T i,j+1>T i,j, , ∀j=m−1…1. Finally, p j+1 is overwritten by p j whenever p j>p j+1, ∀j=1…m−1. In Van Lishout’s implementation of MaxT, the [T i,n+1,…,T i,m] values are computed as before , but only the maximum values M i are stored in memory (for i>0)
Van Lishout’s implementation of maxT solves the latter issue [23]. It is based on the observation that in practice, only a few adjusted p-values will point towards interesting interactions to investigate. With this in mind, it adapts the original method such that it still calculates the test-statistics of all pairs, but only computes the adjusted p-values of the n best pairs, i.e. the ones with the n lowest adjusted p-values. The default value is n=1000 and can be tuned without loss of generality according to the researcher’s needs. Note that despite the fact that only n adjusted p-values are produced, they are still adjusted at the overall level, i.e. for the m association tests. Figure 1(b) describes Van Lishout’s MaxT implementation. The different steps are reported in Table 1.
Table 1.
Van Lishout’s MaxT
| (1) Compute the test-statistics for all pairs, but only store the n highest tests values. The result is a Real data |
| vector where T 0,1≥T 0,2≥…≥T 0,n. |
| (2) Initialise a vector p of size n with 1’s. |
| (3) Perform the following operations for i=1,…,B: |
| (a) Generate a random permutation of the trait column. |
| (b) Compute T i,1,…,T i,n and store them in a Permutation i vector. |
| (c) Compute the maximum M i of the test-statistics values T i,n+1,…,T i,m. |
| (d) Replace T i,n by M i if T i,n<M i. |
| (e) Force the monotonicity of the Permutation i vector: for j=n−1,…,1 replace T i,j by T i,j+1 if T i,j<T i,j+1. |
| (f) For each j=1,…,n, if T i,j≥T 0,j increment p j by one. |
| (4) Divide all values of vector p by B+1 to obtain the p-values vector. Force monotonicity: for j=1,…,n−1, |
| replace p j+1 by p j if p j+1<p j. |
Bottlenecks of Van Lishout’s maxT
Van Lishout’s implementation of maxT still leaves room for improvement. In what follows, we identify its main bottlenecks, in order to improve the overall performance on large-scale data. In Table 2 we report the number of operations performed (with the default parameters of the software n=1000 and B=999) on a dataset containing 106 SNPs, which is equivalent to m≈5×1011 pairs of SNPs.
Table 2.
Analysis of the computing times of the different steps of Van Lishout’s implementation of MaxT on a dataset containing 1 million SNPs
| Theoretical value | Numerical value | |
|---|---|---|
| Step 1 | O(m) | O(1011) |
| Step 2 | O(n) | O(103) |
| Step 3 (a) | O(B) | O(103) |
| Step 3 (b) | O(B n) | O(106) |
| Step 3 (c) | O(B m) | O(1014) |
| Step 3 (d) | O(B) | O(103) |
| Step 3 (e) | O(B n) | O(106) |
| Step 3 (f) | O(B n) | O(106) |
| Step 4 | O(n) | O(103) |
Table 2 reflects that in step 1 of Van Lishout’s maxT, as many elementary computations are carried out as there are SNP pairs to test. Although significance assessment can be based on fewer SNP pairs, this first step of computing test values and ordering them cannot be avoided nor simplified. However, the most computationally intensive part of the significance assessment procedure is step 3(c). With 106 inputted SNPs, the number of elementary computations required is proportional to 1014. Therefore, any improvement at this stage will lead to better overall performances. In “Methods” section, we introduce a novel algorithm for multiple testing, based on Van Lishout’s implementation of maxT. It is implemented in the software MBMDR-4.2.2 and resolves remaining concerns about maxT’s computation time in genome-wide screens for genetic interactions using the MB-MDR framework.
Methods
In MBMDR-4.2.2 the value of M i from Fig. 1 will be estimated from a sample from [ T i,n+1,…,T i,m] rather than calculated exactly. A detailed explanation of how we perform such an improvement is provided in the next section.
Distribution of MB-MDR statistics
We have indicated before that MB-MDR offers a flexible framework to test for SNP-SNP interactions. The software in which the framework is implemented has a modular built-up that allows a flexible choice of association test, depending on the input data. For instance, for quantitative traits, t-tests or non-parametric equivalents can be carried out. For binary traits, chi-squared test of independence can be chosen. The association test that best reflects the data at hand is used in both stage 1 and stage 2 of the MB-MDR framework [27]. After the data manipulation of combining cells using trait information, MB-MDR’s final test statistic no longer follows the theoretical sample distribution of the initially chosen test statistic. In fact, earlier work has shown that such sequential pooling may lead to permutation-based distributions of within MB-MDR test statistics that depend on the number of multi-locus genotype cells pooled [28] or on the minor allele frequencies (MAFs) of the SNP pair under consideration [29]. Rather than looking at the null distribution of the test statistic linked to a SNP-pair, we are now interested in the distribution of a number of test values over several SNP-pairs, from which to derive the maximum value M i. We hypothesize that test values in [ T i,n+1,…,T i,m], with i>0, follow a mixture distribution of a shifted gamma distribution [30] and a point mass at zero. Note that zero test values are induced by scenarios for which the MB-MDR test statistic cannot be computed. In MBMDR-4.2.2, whenever a group of subjects (e.g., in a 2-SNP interaction study, those subjects having two copies of the minor allele at each locus) is compared to the remaining subjects with respect to the trait under study and by using an appropriate association test statistic, this group can either be associated to a higher “risk” (“H” category), a lower “risk” (“L” category) or undecisive “risk” (nor “H”, nor “L”; “O” category) for the trait. Here, “risk” is used loosely. For instance for continuous traits, the “risk” categories above may rather refer to increased (“H” category), decreased (“L” category) mean trait values. Also, in the MB-MDR methodology, risk scales can be refined to incorporate multiple risk categories. It is important to realize that if all subjects are assigned the same label (in this scenario, most probably the “O” label), then MB-MDR will return an exact zero. It is not surprising that lack of power of GWAIs (which depends on sample size but also true effect size) will induce such technical zeros for a significant proportion of the tested SNP pairs. In order to take this important amount of zeros into account, we use the approach described in [31]. We assign a discrete probability mass to the exact zero value. Hence, if is a random variable returning a random value from [ T i,n+1,…,T i,m], with i>0, we can define the probabilities and . Therefore, the distribution of is semi-continuous with a discontinuity at zero. This implies that the probability density function is , where δ(x) is a point probability mass at x=0, is the distribution of the strictly positive values and is an indicator function taking the value 1 if x>0 and 0 otherwise. The parameter π depends on the data at hand and can be estimated with the Maximum Likelihood Estimation (MLE) method [32] from the observed frequency in a sample from [ T i,n+1,…,T i,m]. Due to the fact that our main goal consists in predicting a maximum, we are not particularly interested in fitting the distribution of on the entire set of strictly positive values. As a matter of fact, fitting the tail of should suffice. We show in the next section that focusing on the top 10 % strictly positive values is an acceptable practical choice. We consider this a good tradeoff between fitting on a large and a smaller range of positive values. The former might lead to a poor fit of the tail, because many samples might not belong to that range. The latter might lead to a poor fit of the tail due to an insufficient number of samples. The amount of values belonging to the top 10 % strictly positive values in [ T i,n+1,…,T i,m] is given by .
Assumption 1
We assume that the shifted gamma distribution is a good fit to the tail of . Hence, if is a random variable returning a value from the aforementioned top 10 % of strictly positive values, we postulate that its cumulative distribution function (CDF) is given by , where γ is the lower incomplete gamma function, y 0 is the location parameter, k is the shape parameter and θ the scale parameter. Some authors discourage the use of the gamma distribution for model fitting due to the difficulty of parameter estimation [33]. However, in the specific case of fitting the tail of the distribution of the MB-MDR statistics, we believe that simpler models would be consistently inaccurate. Moreover, the lack of knowledge regarding the shape of a plausible distribution and the diversity of the data we are performing our computations on, make a versatile distribution function like the gamma, a reasonable assumption. Note that the choice of shifting the gamma distribution comes naturally due to the fact that the smallest strictly positive value should not be in the neighborhood of zero. Indeed, a small value would represent a low-significant association between the interaction of the two loci and the phenotype. As previously mentioned, this would lead to the “O” category for all subjects and an exact zero. The CDF of the random variable returning the maximum of the q values belonging to the top 10 % strictly positive values in [ T i,n+1,…,T i,m] is given by . Indeed, if we take q independent and identically distributed (i.i.d.) values y 1,y 2,…,y q, then .
Assumption 2
We postulate that the parameters π, y 0, k and θ are stable from one permutation to another. This assumption is a plausible one, given the results in Table 3, which show low variance of these parameters across 999 permutations. An analogous observation has been noticed in a similar work [34], based on hypothesis testing with an extreme value distribution. In order to reduce the computational burden of the fitting, we estimate the parameters once every 20 permutations. We consider this a compromise between robustness and performance.
Table 3.
Mean and variance of the fitted parameters for datasets D 1−D 4
| D 1 | D 1 | D 2 | D 2 | D 3 | D 3 | D 4 | D 4 | |
|---|---|---|---|---|---|---|---|---|
| Mean | Var | Mean | Var | Mean | Var | Mean | Var | |
| π | 0.337 | 1.247×10−6 | 0.335 | 3.815×10−6 | 0.137 | 4.948×10−7 | 0.366 | 9.356×10−7 |
| y 0 | 7.742 | 5.566×10−4 | 7.825 | 8.778×10−4 | 6.189 | 6.472×10−4 | 7.788 | 3.805×10−4 |
| k | 1.017 | 2.612×10−4 | 1.012 | 2.534×10−4 | 0.990 | 3.580×10−4 | 1.017 | 1.725×10−4 |
| θ | 1.917 | 1.462×10−3 | 1.974 | 1.532×10−3 | 1.694 | 1.829×10−3 | 1.917 | 9.695×10−4 |
Estimating the parameters of the shifted gamma distribution
As mentioned in the introduction, the gammaMAXT algorithm only differs from Van Lishout’s implementation of maxT (Table 1) with respect to step 3(c). In the novel implementation the maximum M i is estimated from a sample of size S=106 of strictly positives values in [ T i,n+1,…,T i,m] rather than calculated directly. The parameter π is estimated on the fly using a variable z, counting the amount of zeros encountered during the sampling process. The new step 3(c) is described in Table 4.
Table 4.
Step 3(c) of gammaMAXT
| (1) If (i modulo 20 = 1) estimate π,y 0,k and θ: |
| (a) Set z=0. Create vector v of size S. |
| (b) Randomly select integer r in [n+1,m]. |
| (c) If T i,r=0, z=z+1, else store T i,r in v. |
| (d) Repeat steps (b) and (c) until v is full. |
| (e) Sort v. Remove the 90 % lowest values. The new size of v is . |
| (f) Estimate . |
| (g) Estimate y 0 by the minimum of v. |
| (h) Estimate k: see below. |
| (i) Estimate . |
| (2) If (i modulo 20 ≠ 1), use the latest estimated values of π,y 0,k and θ. |
| (3) Sample M i from the distribution of the maximum, whose CDF is . |
Whereas estimates in steps (1)(f), (1)(g) and (1)(i) are obtained via Maximum Likelihood, the estimation of the parameter k requires more elaboration. According to [35], an acceptable initial guess being within 1,5 % of the correct value is given by , with . This initial guess is updated iteratively via the Newton-Raphson method [36]. In particular, in every iteration, k is updated as until the difference between the new and the old value of k is lower than the desired precision (default: 0.000001). ψ(k) and ψ ′(k) are respectively the digamma and trigamma functions. Finally, Table 5 describes the procedure used at step (3) to compute the final M i estimation. Note that we have to sample and not take the expectation, in order to mimic the original maxT algorithm.
Table 5.
Sample M i when CDF is
| (a) Take a too high initial guess of M i (default: 1000). Initialize variable b to half of this value. |
| (a) Randomly select a real number r n∈[ 0,1]. |
| (c) If is lower than r n, M i=M i+b, else M i=M i−b. Divide b by 2. |
| (d) Repeat step (c) until b is below the desired precision (default: 0.000001). |
Parallel workflow
Figure 2 describes the four steps of the parallel workflow developed to further make MBMDR-4.2.2 suitable for GWAIs. The detailed algorithm is given in Table 6.
Fig. 2.
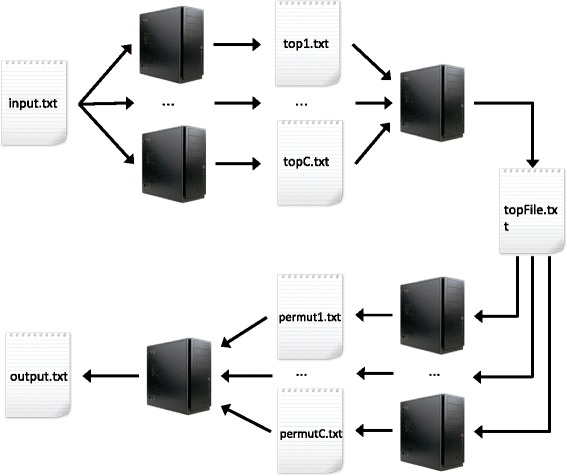
MBMDR-4.2.2 parallel workflow. First, each cluster node performs a fair proportion of the T 0,1,…,T 0,m values from Fig. 1 and saves the n highest into file top_c.txt. Second, a node aggregates all top_c.txt files and retrieves the overall n highest values, saved in topfile.txt. Third, each cluster node reads topfile.txt and performs an equitable fraction of the B permutations of Fig. 1, saving results into file permut_c.txt. Finally, a cluster node aggregates all permut_c.txt and produces the final output file
Table 6.
gammaMAXT parallel workflow
| (1) Each cluster node performs an equitable fraction of the computations of the T 0,1,…,T 0,m |
| values from Fig. 1. The n highest values (and corresponding SNP pair indexes) from each node are saved |
| into file top_c.txt. |
| (2) Upon termination of all computations at the previous step, a cluster node aggregates all top_c.txt files and |
| retrieves the overall n highest values (and corresponding SNP pair indexes). Results are saved into topfile.txt. |
| (3) Each cluster node reads topfile.txt, initialize a vector V of size n with 0’s and performs an equitable fraction |
| of the B permutations of Fig. 1. For each permutation i attributed to node c: |
| (a) Generate a random permutation of the trait column. |
| (b) Compute T i,1,…,T i,n and store them in a Permutation i vector. |
| (c) Execute step (3)(c) of the gammaMAXT algorithm to estimate M i. |
| (d) Replace T i,n by M i if T i,n<M i. |
| (e) Force the monotonicity of the Permutation i vector: for j=n−1,…,1 replace T i,j by T i,j+1 if T i,j<T i,j+1. |
| (f) For each j=1,…,n, if T i,j≥T 0,j increment V j by one. |
| Upon completion of all computations on node c, save V into file permut_c.txt. |
| (4) A cluster node sums all vectors from the permut_c.txt files to obtain a vector p. All elements of p are |
| incremented by 1 and divided by B+1. The monotonicity is forced: for j=1,…,n−1, replace p j+1 by p j |
| if p j+1<p j. |
Results and discussion
In this section, we first show results supporting the two assumptions on which the novel algorithm is based. Then, we analyse the performances in terms of computing-time, power and control of the FWER.
Results supporting assumption 1
In this part, we investigate the hypothesis that the tail of follows a shifted gamma distribution and that fitting the top 10 % of strictly positive values is an acceptable choice. We use the following datasets for this experiment:
A simulated dataset D 1 expressed on a binary scale, composed of 1000 SNPs and 1000 individuals. Table 7 states the two-locus penetrance table used to generate it. A balanced number of cases and controls is sampled. The minor allele frequencies of the functional SNPs are fixed at 0.5 and those of the non-functional SNPs are randomly generated from a uniform distribution on [0.05, 0.5]. This corresponds to the first of six purely epistatic models discussed in [15]. Furthermore, any value in the dataset had a 5 % chance to be missing.
A simulated dataset D 2, with the same properties as D 1, except that the trait is expressed on a continuous scale.
A simulated dataset D 3, with the same properties as D 1, except that the MAF’s are on average lower, i.e. the non-functional SNPs were randomly generated from a uniform distribution on [0.05, 0.1].
A real-life dataset D 4 on Crohn’s disease, for which the trait is expressed on a binary scale [37, 38], reduced to 12471 SNPs and 1687 subjects as in [23].
Table 7.
Two-locus penetrance table used to create the simulated datasets D 1, D 2 and D 3
| b/b | b/B | B/B | |
|---|---|---|---|
| a/a | 0 | 0.1 | 0 |
| a/A | 0.1 | 0 | 0.1 |
| A/A | 0 | 0.1 | 0 |
For each of the aforementioned datasets, we first carry out the initial Van Lishout’s implementation of maxT based on 104 permutations to generate a reference distribution for M i. We second execute step (3)(c) of the gammaMAXT algorithm based on 104 permutations, with different values for the internal parameter defining the percentage of strictly positive values belonging to the tail of . Figure 3 is generated in R and shows the results for dataset D 1. We observe that focusing on respectively 25, 20, 15, 5 and 1 % of the strictly positive values leads to a good fit, but that 10 % is the optimal alternative. The curves of subfigure (d) are indeed close and the Kolmogorov-Smirnov (KS) distance is the lowest among these choices. This supports the hypothesis that the gammaMAXT algorithm produces accurate predictions of the M i values. Addditional file 1: Figure S1, Addditional file 2: Figure S2 and Addditional file 3: Figure S3 show that 10 % is consistently a good option, although not always the most optimal one.
Fig. 3.

Theoretical (green) versus predicted M i values for D 1. 10 % is the optimal choice, leading to the lowest Kolmogorov-Smirnov distance
Results supporting assumption 2
In this section, we show results supporting the hypothesis that parameters π, y 0, k and θ are stable across permutations. We perform MBMDR-4.2.2 analyses on datasets D 1 to D 4, using the default settings. For this experiment, we modified the gammaMAXT algorithm such that it fits new parameters for each of the 999 permutations (not only once every 20 as previously mentioned) and saves these into a file. We report their means and variances in Table 3. We observe that the variance is very low across all scenarios.
Computing-time of the gammaMAXT algorithm
In order to assess the speed performances of MBMDR-4.2.2, we created 4 different datasets with 1000 individuals each, of respectively 103, 104, 105 and 106 SNPs. All datasets were generated using GAMETES, a fast, direct algorithm for generating pure epistatic models with random architectures [39]. Another set of 4 datasets, containing the same number of individuals and SNPs, but expressing the trait on a continuous scale, was created using a similar strategy as for D 2. The parallel workflow of MBMDR-4.2.2 has been tested on a 256-core computer cluster (Intel L5420 2.5 GHz). The sequential version has been tested on a single core of this cluster. Table 8 shows the results. We observe that MBMDR-4.2.2 outperforms the computing times of MBMDR-3.0.3 reported in [23]. For instance, solving a continuous dataset of 104 SNPs on a single core takes about 56 min with MBMDR-4.2.2 and almost 12 days with MBMDR-3.0.3, i.e. about 300 times less. Solving a continuous dataset of 106 SNPs on a 256-core cluster takes about one day with MBMDR-4.2.2 and would take about 104 longer with MBMDR-3.0.3. In general, the theoretical computing time of step 3 (c), which was O(B m) in MBMDR-3.0.3 according to Table 2, is now independent from B and m. The computing time of MBMDR-4.2.2 is therefore asymptotically equal to the computing time of step 1, i.e. O(m) (a big improvement compared to O(B m), the asymptotic computing time of MBMDR-3.0.3). Note that the computing times reported in [23] are based on runs without any correction for the main effects of the SNPS. In this case, the times corresponding to a binary trait are about twice faster than those based on a continuous case. In our study, a codominant correction for the main effects of the SNPs has been performed, implying a regression framework. Since the latter is similar in the binary and continuous case, we logically observe similar computing times.
Table 8.
Execution times of MBMDR-4.2.2. The parallel workflow was tested on a 256-core computer cluster (Intel L5420 2.5 GHz). The sequential executions were performed on a single core of this cluster
| SNPs | MBMDR-4.2.2 | MBMDR-4.2.2 | MBMDR-4.2.2 | MBMDR-4.2.2 |
|---|---|---|---|---|
| Binary trait | Binary trait | Continuous trait | Continuous trait | |
| sequential execution | parallel workflow | sequential execution | parallel workflow | |
| 103 | 13 min 33 sec | 20 sec | 13 min 18 sec | 18 sec |
| 104 | 52 min 15 sec | 1 min 05 sec | 56 min 14 sec | 53 sec |
| 105 | 64 h 35 min | 22 min 15 sec | 70 h 03 min | 20 min 28 sec |
| 106 | ≈ 270 days | 25 h 12 min | ≈ 290 days | 24 h 06 min |
The results prefixed by the symbol “ ≈” are extrapolated
FWER of the gammaMAXT algorithm
To study the control of the FWER, we run MBMDR-4.2.2 on four sets of datasets:
A set S 1 of 1000 datasets, each composed of 1000 SNPs and 1000 individuals, containing null data generated randomly from a uniform distribution on [0.05, 0.5]. A balanced number of cases and controls is sampled.
A set S 2 with the same properties as S 1, except that the trait is expressed on a continuous scale.
A set S 3 of 200 datasets, each composed of 104 SNPs and 1000 individuals, constructed in the same way as S 1.
A set S 4 with the same properties as S 3, except that the trait is expressed on a continuous scale.
We report the observed false-positive rates in Table 9. In practice, these are computed as the percentage of datasets containing at least one pair of SNPs that gave rise to an adjusted p-value below 5 %. On each set, we note that the estimated rates are within the interval [2,5 %−7,5 %] and satisfies thus Bradley’s liberal criterion of robustness for the significance level α=5 % [40]. This criterion specifies that the FWER are controlled for any significance level α, if the empirical rate is contained in the interval .
Table 9.
Observed FWER of MBMDR-4.2.2
| Set | Amount datasets | Observed FWER |
|---|---|---|
| S 1 | 1000 | 4.5 % |
| S 2 | 1000 | 6.2 % |
| S 3 | 200 | 7 % |
| S 4 | 200 | 6.5 % |
Power of the gammaMAXT algorithm
To evaluate the power, we create nine sets of data with GAMETES. Each set consists in 1000 datasets, all composed of 1000 individuals (500 cases and 500 controls) and 200 SNPs (out of which exactly one pair is linked with the trait). The heritability varies across the datasets from 0.03 to 0.01. In this way, we provide a range of decreasing effect sizes showing the power reduction. Table 10 indicates the percentage of time that the pair linked with the trait gave rise to an adjusted p-value below 5 %. We observe that the original MaxT and the new gammaMAXT algorithm leads to very similar power. By predicting the M i values instead of computing them explicitly, we can of course not win power, so that the power of the gammaMAXT algorithm is obviously equal or lower than the one of MaxT. However, we observe that the difference is small, the largest power reduction being of 1,7 %.
Table 10.
Power comparison between the gammaMAXT and the MaxT algorithms
| Heritability | gammaMAXT | MaxT |
|---|---|---|
| 0.0100 | 3.7 % | 4.2 % |
| 0.0125 | 17.9 % | 19.4 % |
| 0.0150 | 50.3 % | 51.5 % |
| 0.0175 | 67.0 % | 68.7 % |
| 0.0200 | 86.6 % | 87.9 % |
| 0.0225 | 94.3 % | 94.7 % |
| 0.0250 | 97.5 % | 97.8 % |
| 0.0275 | 99.2 % | 99.3 % |
| 0.0300 | 99.6 % | 99.6 % |
Conclusion
In this work we introduced gammaMAXT, a novel implementation of the maxT algorithm for multiple testing correction. The algorithm was implemented in software MBMDR-4.2.2, as part of the MB-MDR framework to screen for SNP-SNP, SNP-environment or SNP-SNP-environment interactions at a genome-wide level. In this context, we analyzed a dataset composed of 106 SNPs and 1000 individuals within one day on a 256-core computer cluster. The same analysis would take about 104 times longer with Van Lishout’s implementation of maxT, which was already an improvement of the classic Westfall and Young implementation [26]. These results are promising for future GWAIs. However, the proposed gammaMAXT algorithm offers a general significance assessment and multiple testing approach, applicable to any context that requires performing hundreds of thousands of tests. It offers new perspectives for fast and efficient permutation-based significance assessment in large-scale (integrated) omics studies.
Availability
MBMDR-4.2.2 can be downloaded for free at http://www.statgen.ulg.ac.be.
Acknowledgements
This research was in part funded by the Fonds de la Recherche Scientifique (F.N.R.S.), in particular “Integrated complex traits epistasis kit” (Convention 2.4609.11) [FVL, KVS]. We also acknowledge research opportunities offered by F.N.R.S., “Foresting in Integromics Inference” (Convention T.0180.13) [FG, KVS]. In addition, this paper presents research results of the Belgian Network DYSCO (Dynamical Systems, Control, and Optimization), funded by the Interuniversity Attraction Poles Programme, initiated by the Belgian State, Science Policy Office [FVL, FG, LW, KVS]. JHM was funded by National Institutes of Health (USA) grant LM009012. The scientific responsibility rests with the authors.
Additional files
Figure S1. Theoretical (green) versus predicted M i values for D 2. 10 % is again the optimal choice. (EPS 64 kb)
Figure S2. Theoretical (green) versus predicted M i values for D 3. 20 % is the optimal choice, but 10 a low Kolmogorov-smirnov distance and remains a good choice. (EPS 64 kb)
Figure S3. Theoretical (green) versus predicted M i values for D 4. 10 % is again the optimal choice. (EPS 57 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
FVL and JHM discussed the pros and cons of Van Lishout’s implementation of MaxT. This lead to the idea to try to predict most of the computations. FVL first tried to base the predictions on a normal distribution without success. FG proved that a gamma distribution is a much better choice than a poisson, a normal, an exponential or a Weibull distribution. FVL and LW found the idea to focus on the top part of the distribution. KVS suggested to try to improve power by using either an extreme value distribution or a generalized gamma distribution. FVL found that a shifted gamma distribution is the best choice. KVS provided a lot of useful information for the background section. FVL carried out the analyses. KVS, FVL and LW interpreted the results. FVL and FG are the main contributors of the manuscript. All authors read and approved the final manuscript.
References
- 1.Shastry BS. Pharmacogenetics and the concept of individualized medicine. Pharmacogenomics J. 2006;6(1):16–21. doi: 10.1038/sj.tpj.6500338. [DOI] [PubMed] [Google Scholar]
- 2.van’t Veer LJ, Bernards R. Enabling personalized cancer medicine through analysis of gene-expression patterns. Nature. 2008;452(7187):564–70. doi: 10.1038/nature06915. [DOI] [PubMed] [Google Scholar]
- 3.Galas DJ, Hood L. Systems biology and emerging technologies will catalyze the transition from reactive medicine to predictive, personalized, preventive and participatory (p4) medicine. Interdisc Bio Central. 2009;1:1–4. doi: 10.4051/ibc.2009.2.0006. [DOI] [Google Scholar]
- 4.Beevers CG, McGeary JE Therapygenetics: moving towards personalized psychotherapy treatment. Trends Cogn Sci. 2012;16(1):11–12. doi: 10.1016/j.tics.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lester KJ, Eley TC. Therapygenetics: Using genetic markers to predict response to psychological treatment for mood and anxiety disorders. Biology of mood and anxiety disorders. 2013;3(1):1–16. doi: 10.1186/2045-5380-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slatkin M. Epigenetic inheritance and the missing heritability problem. Genetics. 2009;182(3):845–50. doi: 10.1534/genetics.109.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichler EE, Flint J, Gibson G, Kong A, Lean S, Moore JH, et al. Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet. 2010;11(6):446–50. doi: 10.1038/nrg2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SH, Wray NR, Goddard ME, Visscher PM. Estimating missing heritability for disease from genome-wide association studies. Am J Hum Genet. 2011;88(3):294. doi: 10.1016/j.ajhg.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuk O, Hechter E, Sunyaev SR, Lander ES. The mystery of missing heritability: Genetic interactions create phantom heritability. Proc Natl Acad Sci. 2012;109(4):1193–98. doi: 10.1073/pnas.1119675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan X, Yang C, Yang Q, Xue H, Fan X, Tang NL, et al. Boost: A fast approach to detecting gene-gene interactions in genome-wide case-control studies. Am J Hum Genet. 2010;87:325–40. doi: 10.1016/j.ajhg.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gyenesei A, Moody J, Semple CA, Haley CS, Wei WH. High-throughput analysis of epistasis in genome-wide association studies with biforce. Bioinformatics. 2012;19:376–82. doi: 10.1093/bioinformatics/bts304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemani G, Theocharidis A, Wei W, Haley C. epigpu: exhaustive pairwise epistasis scans parallelized on consumer level graphics cards. Bioinformatics. 2011;27:1462–1465. doi: 10.1093/bioinformatics/btr172. [DOI] [PubMed] [Google Scholar]
- 13.Kam-Thong T, Czamara D, Tsuda K, Borgwardt K, Lewis C, Erhardt-Lehmann A, et al. epiblaster-fast exhaustive two-locus epistasis detection strategy using graphical pro- cessing units. Eur J Hum Genet. 2011;19:465–71. doi: 10.1038/ejhg.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kam-Thong T, Azencott C, Cayton L, Putz B, Altmann A, Karbalai N, et al. Glide: Gpu-based linear regression for detection of epistasis. Hum Hered. 2012;73:220–36. doi: 10.1159/000341885. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie MD, Hahn LW, Moore JH. Power of multifactor dimensionality reduction for detecting gene-gene interactions in the presence of genotyping error, missing data, phenocopy, and genetic heterogeneity. Genet Epidemil. 2003;24(2):150–7. doi: 10.1002/gepi.10218. [DOI] [PubMed] [Google Scholar]
- 16.Hahn LW, Ritchie MD, Moore JH. Multifactor dimensionality reduction software for detecting gene–gene and gene–environment interactions. Bioinformatics. 2002;19(3):376–82. doi: 10.1093/bioinformatics/btf869. [DOI] [PubMed] [Google Scholar]
- 17.Calle ML, Urrea V, Vellalta G, Malats N, Van Steen K. Improving strategies for detecting genetic patterns of disease susceptibility in association studies. Stat Med. 2008;27:6532–546. doi: 10.1002/sim.3431. [DOI] [PubMed] [Google Scholar]
- 18.Cattaert T, Calle ML, Dudek SM, Mahachie John JM, Van Lishout F, Urrea V, et al. Model-based multifactor dimensionality reduction for detecting epistasis in case-control data in the presence of noise. Ann Hum Genet. 2011;75:78–89. doi: 10.1111/j.1469-1809.2010.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gusareva E, Van Steen K. Practical aspects of genome-wide association interaction analysis. Hum Genet. 2014;133(11):1343–58. doi: 10.1007/s00439-014-1480-y. [DOI] [PubMed] [Google Scholar]
- 20.Wienbrandt L, Kässens JC, Gonzalez-Dominguez J, Schmidt B, Ellinghaus D, Schimmler M. FPGA-based Acceleration of Detecting Statistical Epistasis in GWAS In: Science PC, editor. 14th International Conference on Computational Science. Elsevier - Procedia Computer Science, vol 29;2014. p. 220–30. http://www.sciencedirect.com/science/article/pii/S1877050914001975.
- 21.Van Steen K. Traveling the world of gene-gene interactions. Brief Bioinform. 2011;13(1):1–19. doi: 10.1093/bib/bbr012. [DOI] [PubMed] [Google Scholar]
- 22.Mahachie John JM, Cattaert T, Van Lishout F, Gusareva E, Van Steen K. Lower-order effects adjustment in quantitative traits model-based multifactor dimensionality reduction. PLoS ONE. 2012;7(1):29594–1013710029594. doi: 10.1371/journal.pone.0029594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Lishout F, Mahachie John JM, Gusareva ES, Urrea V, Cleynen I, Théâtre E, et al. An efficient algorithm to perform multiple testing in epistasis screening. BMC Bioinforma. 2013;14(138). http://www.biomedcentral.com/1471-2105/14/138. [DOI] [PMC free article] [PubMed]
- 24.Dunn OJ. Multiple comparisons among means. J Am Stat Assoc. 1961;56(293):52–64. doi: 10.1080/01621459.1961.10482090. [DOI] [Google Scholar]
- 25.Ge Y, Dudoit S, Speed TP. Resampling-based multiple testing for microarray data analysis. Technical Report 633. Berkley: Department of Statistics, University of California; 2003. [Google Scholar]
- 26.Westfall PH, Young SS. Resampling-base Multiple Testing. New York: Wiley; 1993. [Google Scholar]
- 27.Mahachie John JM, Van Lishout F, Van Steen K. Model-based multifactor dimensionality reduction to detect epistasis for quantitative traits in the presence of error-free and noisy data. Eur J Hum Genet. 2011;19(6):696–703. doi: 10.1038/ejhg.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calle ML, Urrea V, Malats N, Van Steen K. Mb-mdr: model-based multifactor dimensionality reduction for detecting interactions in high-dimensional genomic data. Technical Report 24. 2008.
- 29.Mahachie John JM. Genomic association screening methodology for high-dimensional and complex data structures: Detecting n-order interactions. 2012. http://orbi.ulg.ac.be/handle/2268/136086.
- 30.Kotz S, Balakrishnan N, Johnson N. Continuous Multivariate Distributions, Models and Applications: Wiley; 2000.
- 31.Hautsch N, Malec P, Schienle M. Capturing the zero: A new class of zero- augmented distributions and multiplicative error processes. J Financ Econ. 2013;12(1):89. [Google Scholar]
- 32.Bickel P, Doksum K. Mathematical Statistics, Basic Ideas and Selected Topics: Prentice-Hall, Inc; 1977.
- 33.Allenby GM, Leone RP, Jen LC. A dynamic model of purchase timing with application to direct marketing. J Am Stat Assoc. 1999;94:365–74. doi: 10.1080/01621459.1999.10474127. [DOI] [Google Scholar]
- 34.Pattin KA, White BC, Barney N, Gui J, Nelson HH, Kelsey KT, et al. A computationally efficient hypothesis testing method for epistasis analysis using multifactor dimensionality reduction. Genet Epidemiol. 2009;33(1):87–94. doi: 10.1002/gepi.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minka TP. Estimating a gamma distribution. 2002. http://research.microsoft.com/en-us/um/people/minka/papers/minka-gamma.pdf.
- 36.Choi SC, Wette R. Maximum likelihood estimation of the parameters of the gamma distribution and their bias. Technometrics. 1969;11(4):683–90. doi: 10.1080/00401706.1969.10490731. [DOI] [Google Scholar]
- 37.Libioulle C, Louis E, Hansoul S, Sandor C, Farnir F, Franchimont D, et al. Novel crohn disease locus identified by genome-wide association maps to a gene desert on 5p13.1 and modulates expression of ptger4. Plos Genetics. 2007;3(4):58. doi: 10.1371/journal.pgen.0030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, et al. Genome-wide association defines more than 30 distinct susceptibility loci for crohn’s disease. Nat Genet. 2008;40(8):955–62. doi: 10.1038/ng.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urbanowicz RJ, Kiralis J, Sinnott-Armstrong NA, Heberling T, Fisher JM, Moore JH Gametes: a fast, direct algorithm for generating pure, strict, epistatic models with random architectures. BioData Mining. 2012;5(1):16. doi: 10.1186/1756-0381-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradley J. Robustness? Br J Math Stat Psychol. 1978;31:144–52. doi: 10.1111/j.2044-8317.1978.tb00581.x. [DOI] [Google Scholar]


