Abstract
One key objective of lifespan research is to examine how individual development is shaped by the historical time people live in. Secular trends favoring later-born cohorts on fluid cognitive abilities have been widely documented, but findings are mixed for well-being. It remains an open question whether secular increases in well-being seen in earlier phases of life also manifest in the last years of life. To examine this possibility, we made use of longitudinal data obtained from the mid 1980s until the late 2000s in two large national samples in the US (Health and Retirement Study, HRS) and Germany (German Socio-Economic Panel, SOEP). We operationally defined historical time from two complementary perspectives: birth-year cohorts based on the years people were born (earlier: 1930s vs. later: 1940s); and death-year cohorts based on the years people died (earlier: 1990s vs. later: 2000s). To control for relevant covariates, we used case-matched groups based on age (at death) and education and covaried for gender, health, and number of observations. Results from both countries revealed that well-being in old age was indeed developing at higher levels among later-born cohorts. However, for later-deceased cohorts, no evidence for secular increases in well-being was found. To the contrary, later-dying SOEP participants reported lower levels of well-being at age 75 years and 2 years prior to death and experienced steeper late-life declines. Our results suggest that secular increases in well-being observed in old age do not manifest in late-life where “manufactured” survival may be exacerbating age- and mortality-related declines.
Keywords: Well-being, Health and Retirement Study (HRS), German Socio-Economic Panel Study (SOEP), terminal decline, cohort differences
Lifespan research has long been interested in examining how individual development is shaped by historical and societal contexts (Baltes, Cornelius, & Nesselroade, 1979; Riley, 1973; Ryder, 1965; Schaie, 1965). For example, historical increases in performance measures of fluid intelligence are widely documented across birth-year cohorts (e.g., Flynn, 1987, 2007). Secular trends in measures of quality of life have received broader attention only recently (for notable exceptions, see Twenge, 2000; 2001; Twenge, Campbell, & Freeman, 2012). Thus far, empirical reports of historical increases in well-being are inconclusive, with some studies suggesting that later-born cohorts report higher levels of well-being (e.g., Sutin et al., 2013), whereas other studies found the opposite with later-born cohorts reporting lower levels of well-being (e.g., Schilling, 2005). It is also largely an open question whether secular trends favoring later cohorts seen in earlier phases of life generalize to the last years of life. Mortality-related processes may be so pervasive that they minimize cohort differences seen earlier in the lifespan. In the cognitive domain, there is initial evidence that historical trends favoring later cohorts do not exist in late-life (see Gerstorf, Ram, Hoppmann, Willis, & Schaie, 2011; Hülür, Infurna, Ram, & Gerstorf, 2013). In the present study, we examined (a) whether historical improvements in affective and cognitive-evaluative components of subjective well-being have taken place in the last decades, and (b) whether such improvements generalize to late-life trajectories of well-being.
Do Later-Born Cohorts Show Higher Levels of Well-Being?
What are the factors that shape development of well-being and how do these factors differ across cohorts? First, socio-economic conditions, typically operationalized using income and education, are highly relevant for individual development. Generally, individuals with higher socio-economic status report higher levels of subjective well-being (for a meta-analysis, see Pinquart & Sorensen, 2000). The last century was characterized by increasing economic prosperity in industrialized countries. For example, Cribier (2005) compared two cohorts of older adults from France who were born around 1907 vs. 1921 and found that the later-born cohort reported higher levels of material standards around the time when they entered retirement. Increased material standards may contribute to a higher well-being among later cohorts.
As a second factor, health also plays an important role in individual development. For example, recent reviews have documented robust associations between better health and positive emotions (Chida & Steptoe, 2008; Ong, 2010). Previous research documented mixed findings on historical improvements in the health domain (for an overview, see Crimmins & Beltran-Sanchez, 2011): On the one hand, common diseases such as arthritis and heart disease have become less disabling (Crimmins & Saito, 2000; Freedman, Schoeni, Martin, & Cornman, 2007). On the other hand, the prevalence of chronic health conditions such as cardio-vascular diseases and cancer has increased (Crimmins & Beltran-Sanchez, 2011), which are known to impair well-being (e.g., van Jaarsveld, Sanderman, Miedema, Ranchor, & Kempen, 2001; Siddiqi, Given, Given, & Sikorskii, 2009). These historical trends in the health domain have mixed implications for historical trends in well-being.
As a third factor, educational attainment improved significantly across cohorts over the last century within industrialized nations (e.g., Schaie, Willis, & Pennak, 2005). Schaie and colleagues (2005) reported an average increase of 5.5 years in length of schooling across individuals born between 1889 to 1973 in the US. Previous studies have found higher levels of education to be related to higher well-being (e.g., Blanchflower & Oswald, 2004), probably through higher income and better health (e.g., Bukenya, Gebremedhin, & Schaeffer, 2003). Taken together, it can be expected that historical increases in educational opportunities would lead to higher well-being among later cohorts.
In line with these arguments, a recent study based on two large-scale samples of US adults (in the Baltimore Longitudinal Study of Aging, BLSA and the National Health and Nutrition Examination Survey, NHANES) showed that later-born cohorts indeed reported higher levels of well-being compared with same-aged earlier-born cohorts (Sutin et al., 2013). For example, 70-year olds born in the year 1935 reported 0.20 SD higher well-being than 70-year olds born in the year 1925. Birth year did not moderate the rate of change in well-being, suggesting that the various cohorts followed parallel trajectories, but at different levels.
However, not all studies find historical increases in well-being. For example, in a longitudinal analysis of cohort differences in well-being, Schilling (2005) used 16 waves of data (1984-1999) from a national German sample (German Socio-Economic Panel Study, SOEP) born between 1909 and 1939 to report that later-born individuals showed lower levels of life satisfaction in old age than earlier-born individuals. For example, 75-year olds born in the year 1919 reported 0.16 SD lower well-being than 75-year olds born in the year 1909 (Schilling, 2005; Sample B). Interestingly, later-born individuals experienced less steep age-related declines in life satisfaction than earlier-born individuals, thereby reducing cohort differences with advancing age. For example, the above-mentioned disadvantage of 0.16 SD for later-born cohorts at age 75 years was reduced to 0.02 SD at age 80 years. This suggests that cohort differences in well-being are not stable across chronological age, but might differ at various points during the lifespan. In the present study, we examined whether cohort differences observed earlier in old age would also hold in late-life.
Are Improvements of Well-Being Maintained in the Last Years of Life? Cohort Differences across Death-Year Cohorts
Cohorts of people are not only differentially exposed to factors that shape their individual development in young adulthood and mid-life, they are also differentially exposed to factors that are central to late-life development. The second major objective of the present study was to investigate whether secular trends that exist in earlier phases of adult life still exist late in life. We approach the issue from a complementary perspective and examine differences between cohorts that are defined based on year of death – death-year cohorts. To our knowledge, no studies have yet examined differences in well-being across death-year cohorts. Previous research suggests that factors affecting late-life development have changed over the last several decades; for example historical trends in population health have been well-documented (Crimmins & Beltran-Sanchez, 2011). These changing conditions may also be altering late-life well-being.
We draw on notions of failure-of-success and success-of-success scenarios (see Christensen et al., 2013) to identify factors that shape development in late-life and how these factors differ across cohorts that died in different historical times. According to the failure-of-success hypothesis, successes in increasing longevity due to medical advances would lead to declines in the health of older adults (see Waidmann, Bound, & Schoenbaum, 1995; Christensen et al., 2013). Scenarios of manufactured survival (Olshansky, Hayflick, & Carnes, 2002) or an expansion of morbidity (Olshansky, Rudberg, Carnes, Cassel, & Brody, 1991) according to which increases in life expectancy would be gained at the cost of late-life functioning are in line with a failure-of-success hypothesis. An alternative scenario is known as the success-of-success effect (Christensen et al., 2013, see also Fries, 1980, for his compression of morbidity scenario): For example, later-born cohorts might benefit from advances in medical treatment and healthier lifestyles (e.g., reduction in smoking). Due to these historical developments, later-born cohorts might enter old age in better physical and cognitive health, which in turn also contributes to higher well-being.
Generalization across cohort studies is generally hampered by many differences in study design (e.g., use of different definitions of cohort). However, thus far, empirical evidence regarding a failure-of-success or success-of-success scenario in the health domain is inconclusive: In line with notions of success-of-success, a number of studies reported improved physical functioning among older adults from the 1980s through to the present (for an overview, see Crimmins & Beltran-Sanchez, 2011). On the other hand, the proportion of individuals with multiple diseases, as well as the number of diseases comorbid in an individual has been increasing (Crimmins & Beltran-Sanchez, 2011).
Findings are also mixed regarding success-of-success in the cognitive domain: Some studies indeed report evidence that later-born – when measured over the same ages – achieved higher cognitive test scores (Christensen et al., 2013; using studies of Danish nonagenarians; Gerstorf et al., in press), had lower prevalence rates of dementia (Matthews et al., 2013; using data from two studies of 65+ year olds from England and Wales), and showed more favorable age-related trajectories of cognitive change (i.e., higher levels and less decline: Dodge, Zhu, Lee, Chang, & Ganguli, 2014). Other studies report findings that speak against a success-of–success scenario in cognitive performance, and rather suggest a failure-of-success. For example, Gerstorf and Ram and their colleagues (2011) used multi-decade longitudinal data from the Seattle Longitudinal Study (SLS) to show that differences favoring later-born cohorts (1914-1947) over earlier-born cohorts (1883-1913) in trajectories of age-related cognitive change between ages 50 and 80 were not present anymore in the last years of life. Focusing on the role of late-life living conditions, Hülür and colleagues (2013) additionally showed that participants of the Asset and Health Dynamics Among the Oldest Old (AHEAD) study who died in the 2000s performed lower on memory tests 2 years prior to death and also experienced steeper declines as compared to participants who died in the 1990s. Based on medical and technological advances in recent decades, notions of success-of-success would predict higher levels of well-being for participants dying later in historical time, whereas notions of failure-of-success would predict the opposite.
The Current Study
Based on lifespan notions of cohort (Baltes et al., 1979; Riley, 1973; Ryder, 1965; Schaie, 1965), we examined cohort differences in well-being – a developmental outcome that can be considered a summary measure of how well an individual is doing in multiple domains of functioning, including health, cognition, and social relationships. To examine whether differences existed in trajectories of well-being across birth-year and death-year cohorts, we used data from two national samples of the US (Health and Retirement Study; HRS) and German (SOEP) populations. Both studies included multi-year longitudinal assessments of subjective well-being. Previous research differentiated between cognitive-evaluative and affective components of subjective well-being (Diener, 1984): While the cognitive-evaluative component reflects general evaluations of quality of life, the affective component refers to experiences of positive and negative affect at a specific time. We examined cohort differences in affective (HRS) and cognitive-evaluative (SOEP) aspects of subjective well-being. To examine whether subjective well-being improved historically, we compared trajectories of age-related well-being change between birth-year cohorts who were born earlier (between 1930 and 1939) or later in historical time (between 1940 and 1947). To evaluate success-of-success vs. failure-of-success scenarios (see Christensen et al., 2013), we examined whether differences in age-related and mortality-related trajectories of well-being existed between death-year cohorts who died earlier (between 1990 and 1999) or later in historical time (between 2000 and 2009). We chose these years of birth and death to achieve similar sized birth-year and death-year cohorts, respectively. In order to control for known individual and cohort differences, we used propensity score matching procedures to equate cohorts on age (age at baseline for birth-year cohorts, and age at death for death-year cohorts) and education and covaried for gender, number of observations, and health. For death-year cohorts, comparison of trajectories of mortality-related change in well-being also controlled for age at baseline and age at death. In line with historical improvements in economic prosperity, healthcare, and educational attainment, we hypothesized that the cohort born in the 1940s would show higher levels of well-being than the cohort born in the 1930s. Based on previous findings on death-year cohorts (Hülür et al., 2013), we hypothesized that the cohort dying in the 2000s would show lower levels of well-being than those dying in the 1990s.
Our study extends previous reports by using a case-matched control design. Specifically, we used propensity score matching procedures (Coffman, 2011; Jackson, Thoemmes, Jonkmann, Lüdtke, & Trautwein, 2012; Stuart, 2010; Thoemmes & Kim, 2011) to match the birth-year and death-year cohorts as closely as possible on characteristics that differ between cohorts and are known to be relevant for well-being. Applying propensity score matching procedures based on length of life and education takes a step toward causal inference by incorporating notions of random assignment to cohort groups into an observational study design (Foster, 2010, p. 1455).
Method
To examine longitudinal changes in well-being, we used data from the HRS (10 biannual waves from 1993 to 2012) and the SOEP (27 annual waves from 1984 to 2010). Detailed descriptions of participants, variables, and procedures can be found in previous publications (HRS: Burkhauser & Gertler, 1995; SOEP: Haisken-De New & Frick, 2006; Wagner, Frick, & Schupp, 2007). Select details relevant to the present study are given below.
Participants and Procedure
Health and Retirement Study (HRS)
The HRS is an ongoing longitudinal study that started with a nationally representative probability sample of households in the United States that included a non-institutionalized individual of age 50 years or more in 1992. Data are collected approximately every two years. In analyses of birth-year cohorts, we included data from all participants who were born in the 1930s or 1940s and had provided (a) at least one observation of well-being when they were 65 years or older and (b) information on the covariates. This subsample included 11,010 participants (56% women) who were on average 66.0 years old (SD = 1.64, range: 65-81) at the first wave utilized in the present study (hereafter referred to as T1, for details see the paragraphs on data preparation). In analyses of death-year cohorts, we included data from all deceased participants who died in the 1990s (1993-1999) or in the 2000s (2000-2009), provided at least one observation of well-being when they were 65 years or older and in the last four years prior to their death, and provided information on the covariates. This subsample included 6,915 participants (54% women) who were on average 74.7 years old at T1 (SD = 7.12, range: 65-104).
German Socio-Oeconomic Panel Study (SOEP)
The SOEP is an ongoing longitudinal study that started with a representative sample of German households in 1984. Data are collected annually. The samples in the SOEP were defined in an analogous fashion. Our analyses of birth-year cohorts of individuals born in the 1930s or in the 1940s included data from all participants who had provided (a) at least one observation of well-being when they were 65 years or older and (b) information on the covariates. This subsample included 7,190 participants (50% women) who were on average 66.7 years old at T1 (SD = 3.17, range: 65-81). In analyses of death-year cohorts, we included data from all deceased participants who died in the 1990s (1990-1999) or in the 2000s (2000-2009), provided at least one observation of well-being when they were 65 years or older and in the last four years prior to their death, and provided information on covariates. This subsample included 2,270 participants (51% women) who were on average 71.7 years old at T1 (SD = 7.35, range: 65-98).
Measures
Well being
In the HRS, (affective) well-being is measured using depressive symptoms, indexed using a sum score of 8 items (0 – 8) from the CES-D (Radloff, 1977; see Wallace, 2000) asking whether participants experienced depressive symptoms much of the time during the past week (coded as 1) or not much of the time during the past week (coded as 0).
In the SOEP, (cognitive-evaluative) well-being is measured using life satisfaction, indexed by the response to the question, “Wie zufrieden sind Sie gegenwärtig, alles in allem, mit ihrem Leben?” (“How satisfied are you with your life, all things considered?”) on a 0 (totally unsatisfied) to 10 (totally satisfied) scale. Further details of the life satisfaction item as used in the SOEP and its measurement properties can be found in Fujita and Diener (2005), Kroh (2006), Schimmack, Schupp, and Wagner (2008), and Schilling (2005).
We standardized well-being scores to a T metric (M = 50, SD = 10) using the first utilized measurement occasion of our birth-year and death-year samples as reference frames (HRS birth-year cohorts: M = 1.31, SD = 1.87, range = 0-8; HRS death-year cohorts: M = 1.87, SD = 2.02, range = 0-8; SOEP birth-year cohorts: M = 7.15, SD = 1.76, range = 0-10; SOEP death-year cohorts: M = 7.04, SD = 2.21, range = 0-10).
Time metrics: Age and time-to-death
Chronological age at each measurement occasion was calculated as the number of years since an individual's birth. We calculated time-to-death at each occasion as the difference between the year of assessment and the year of an individual's death obtained either (a) from interviews at the biennial (HRS) or yearly (SOEP) assessments (i.e., from household members or, in the case of one-person households, neighbors) or (b) from city or national registries or offices (e.g., U.S. National Death Index).
Covariates
We included a number of variables known to differ between individuals and cohorts as covariates into our models. Gender was indicated by a binary variable. Education was measured as the number of years the individual had spent in formal schooling. Number of observations was the total number of observations from an individual that were included in the data. In the HRS, health status was indicated by number of health conditions assessed at each wave as the sum index of self-reported physician-diagnosed current health problems, including high blood pressure, diabetes, cancer, lung disease, heart condition, stroke, psychiatric problems, and arthritis (Wilson-Genderson, Pruchno, & Cartwright, 2009). For our analyses, we used the average number of health conditions across all utilized waves. In the SOEP, health status was indicated by a measure of disability, assessed at each wave with a single item asking participants whether they had been “officially certified as having a reduced capacity to work or being severely handicapped” (for details, see Lucas, 2007). We calculated age at death as the difference between the year of an individual's death and his or her year of birth and age at T1 as the difference between an individual's birth year and the year at their first utilized assessment.
Data preparation for analyses of birth-year cohorts
Four procedures were used to minimize possible confounds and to equate the cohorts as closely as possible. First, to focus our analyses on well-being in old age, we discarded all observations obtained prior to age 65 years. Second, to balance the opportunity to contribute observations to the data stream across cohorts, only the first 4 (HRS) or the first 7 (SOEP) observations for each participant were selected.
Third, we used propensity score matching procedures (Coffman, 2011; Stuart, 2010; Jackson et al., 2012; Thoemmes, & Kim, 2012) to obtain matched cohort groups. In particular, 1:1 matching methods were used to select for each participant from the later-born cohort (n = 3,999 in the HRS and n = 2,806 in the SOEP) a ‘twin’ participant from the earlier-born cohort (n = 7,011 in the HRS and n = 4,384 in the SOEP) who had the same (or as similar as possible) age at baseline and education. We estimated the probability of being in the earlier or later-born cohort for each individual by using a logistic regression that included the variables age at baseline and education. As recommended in the propensity score matching literature (e.g., Rosenbaum & Rubin, 1985), we then used logit-transformed propensity scores to calculate a between-group distance matrix. Nearest neighbors were matched via a caliper matching algorithm with a caliper (maximum allowable distance between matched participants) of c = .2 SD. Each participant in the earlier-born cohort was allocated the nearest neighbor from the later-born cohort only if the neighbor fell within the caliper distance. For all 3,999 HRS participants and 2,712 out of 2,806 SOEP (97%) in the later-born cohort, a suitable neighbor in the earlier-born cohort could be identified. Compared to the 2,712 participants in the later-born cohort for whom a suitable neighbor could be identified, the 94 unmatched participants were younger (age: M = 65.54; SD = 1.32 in the matched group vs. M = 65.00; SD = 0.00 in the unmatched group) and had spent more years in formal education (M = 11.78; SD = 2.56 in the matched group vs. M = 18.00; SD = 0.00 in the unmatched group). If the exclusion of 3% of the later-born cohort biased the possibility for identifying historical changes in well-being, it provides for more conservative test of historical improvement (by removing some young, educated, presumably high well-being, persons).
Fourth, to control for the remaining known differences between the birth-year cohort groups, gender, number of observations, and health problems were included as additional covariates in our models. We note that these covariates might also have been included in the propensity score matching procedure. However, our preliminary analyses showed that there was a substantial tradeoff between the dimensionality of the propensity function and the potential to identify suitably similar neighbors. Thus, in order to balance the need for statistical control using covariates with sample-size based statistical power to find group differences, we chose the variable sets to minimize differences while maintaining groups with sample sizes of n = 3,999 (HRS) or n = 2,712 (SOEP) for each cohort. In analyses of birth-year cohorts, age was centered at 70 years. Other covariates were centered at sample means. An overview of descriptive statistics for the matched birth-year cohorts can be found in Table 1.
Table 1.
Descriptive Statistics of Study Measures for Birth-Year Cohorts.
| HRS | SOEP | |||
|---|---|---|---|---|
|
| ||||
| Earlier-born (1930s) | Later-born (1940s) | Earlier-born (1930s) | Later-born (1940s) | |
|
| ||||
| N | 3,999 | 3,999 | 2,712 | 2,712 |
| Year of birth | 1935 (3) | 1943 (2) | 1935 (3) | 1942 (2) |
| [1930 – 1939] | [1940 – 1947] | [1930 – 1939] | [1940 – 1946] | |
| Well-being at T1 a | 1.30 (1.81) | 1.32 (1.93) | 7.15 (1.79) | 7.15 (1.73) |
| [0 – 8] | [0 – 8] | [0 – 10] | [0 – 10] | |
| Years of education | 12.74 (3.09) | 12.75 (3.08) | 11.64 (2.58) | 11.78 (2.56) |
| [0 – 17] | [0 – 17] | [7 – 18] | [7 – 18] | |
| Age at individual T1 | 65.69 (0.98) | 65.69 (0.98) | 65.55 (1.31) | 65.54 (1.31) |
| [65 – 72] | [65 – 72] | [65 – 71] | [65 – 71] | |
| % women | 54 | 59 | 47 | 50 |
| Health problems | 2.16 (1.32) | 2.35 (1.44) | 0.38 (0.49) | 0.32 (0.47) |
| [0 – 8] | [0 – 8] | [0 – 1] | [0 – 1] | |
| Number of observations | 3.58 (0.90) | 2.34 (1.08) | 5.81 (2.01) | 3.41 (2.06) |
| [1 – 4] | [1 – 4] | [1 – 7] | [1 – 7] | |
Note. SD in parentheses. Ranges in square brackets.
Well-being is indicated by number of depressive symptoms in the HRS and a single-item measure of life satisfaction in the SOEP.
Health problems were indicated by the number of health conditions in the HRS and by the presence of disability in the SOEP.
Data preparation for analyses of death-year cohorts
Data was prepared in a similar way for analyses of death-year cohorts. That is, we discarded all observations obtained prior to age 65 years and only used the last 4 (HRS) or the last 15 (SOEP) observations per participant to balance the opportunity to contribute observations across cohorts. Again, we used 1:1 matching methods to select for each participant from the earlier-deceased cohort (n = 2,265 in the HRS and n = 769 in the SOEP) another participant from the later-deceased cohort (n = 4,650 in the HRS and n = 1,501 in the SOEP) based on age at death and education. A caliper matching algorithm matched nearest neighbors with a caliper of c = .2 SD. For all 2,265 (HRS) and all 769 (SOEP) participants in the earlier-deceased cohort, a suitably close neighbor in the later-deceased cohort could be identified. Furthermore, we controlled for the remaining known differences between the death-year cohort groups by controlling for age at T1, gender, number of observations, and health problems statistically in our models.
In analyses of death-year cohorts, age was centered at 75 years (vs. 70 in the birth-year cohorts because of differences in average age). Time-to-death was centered at two years prior to death, a point with sufficient density of observations to facilitate parameter estimation. Keeping the intercept relatively near to the time of death means that terminal decline effects were very likely to have set in (e.g. Gerstorf et al., 2010). Other covariates were centered at sample means. Table 2 gives an overview of descriptive statistics for the matched death-year cohorts.
Table 2.
Descriptive Statistics of Study Measures for Death-Year Cohorts.
| HRS | SOEP | |||
|---|---|---|---|---|
|
| ||||
| Earlier-deceased (1990s) | Later-deceased (2000s) | Earlier-deceased (1990s) | Later-deceased (2000s) | |
|
| ||||
| N | 2,265 | 2,265 | 769 | 769 |
| Year of death | 1997 (2) | 2004 (3) | 1995 (3) | 2005 (3) |
| [1993 – 1999] | [2000 – 2009] | [1990 – 1999] | [2000 – 2009] | |
| Well-being at T1 a | 1.94 (2.06) | 1.79 (1.99) | 5.85 (2.56) | 5.60 (2.53) |
| [0 – 8] | [0 – 8] | [0 – 10] | [0 – 10] | |
| Years of education | 10.56 (3.73) | 10.57 (3.72) | 10.55 (1.88) | 10.55 (1.89) |
| [0 – 17] | [0 – 17] | [7 – 18] | [7 – 18] | |
| Age at individual T1 | 78.25 (7.21) | 74.24 (6.64) | 78.45 (7.91) | 78.46 (7.86) |
| [65 – 103] | [65 – 97] | [65 – 99] | [65 – 98] | |
| Age at death | 81.72 (7.50) | 81.60 (7.62) | 79.76 (7.94) | 79.84 (7.89) |
| [65 – 107] | [65 – 104] | [66 – 100] | [66– 101] | |
| % women | 53 | 51 | 53 | 52 |
| Health problems | 2.06 (1.40) | 2.23 (1.42) | 0.46 (0.50) | 0.52 (0.50) |
| [0 – 8] | [0 – 7] | [0 – 1] | [0 – 1] | |
| Number of observations | 1.74 (0.75) | 3.45 (0.92) | 7.23 (3.61) | 8.37 (5.23) |
| [1 – 3] | [1 – 4] | [1 – 15] | [1 – 15] | |
Note. Sample-level Means with SD in parentheses. Ranges in square brackets.
Well-being is indicated by number of depressive symptoms in the HRS and a single-item measure of life satisfaction in the SOEP. Both converted into T-score for analysis.
Health problems were indicated by the number of health conditions in the HRS and by the presence of disability in the SOEP.
Data analysis
In order to examine cohort differences in how well-being changes over time, chronological age and time-to-death were used as the time metric in our growth curve models (see Ram, Gerstorf, Fauth, Zarit, & Malmberg, 2010; Ram & Grimm, 2015). At the within-person level (Level 1), the models were specified as
| (1) |
where well-beingti represents person i's life satisfaction or depressive symptoms at time t. β0i is a person-specific intercept parameter, β1i is a person-specific linear slope parameter that characterizes the rate of change per year in well-being. β2i is a quadratic slope parameter capturing the acceleration of change, and eti is residual error. Person-specific intercept (β0i) and linear (β1i) and quadratic slope (β2i) parameters were modeled at the between-person level (Level 2) for the age-related change model as
| (2) |
| (3) |
| (4) |
Cohort was indexed by a dichotomous variable indicating decade of birth or death. The time-to-death model included age at T1 and age at death as additional predictors,
| (5) |
| (6) |
| (7) |
With the covariates centered at sample means, γ00, γ10, and γ20 indicated the trajectory of the average person in the sample. Positive parameter estimates indicate differences favoring participants in later-born and later-deceased cohorts, women, those with more education, those with a higher number of health conditions, those who reported a disability during the course of the study, those who provided more data points, as well as participants with older ages at T1 and at death1. Residual between-person differences u0i and u1i were assumed to be multivariate normally distributed, correlated with each other and uncorrelated with the residual errors, eti. We note that between-person differences in the quadratic slope, u2i, were left un-modeled to keep the analyses consistent across studies (in several cases, models with three random effects did not converge). Models were estimated with SAS Proc Mixed (Littell, Miliken, Stoup, & Wolfinger, 1996) with incomplete data treated as missing at random (Little & Rubin, 1987) such that participants with only one observation still contribute to the estimation (e.g., of the intercept).
Results
Did Subjective Well-Being Improve Across Birth-Year Cohorts?
Table 3 presents results from our growth model examining whether birth-year cohorts differed in their age-related trajectories of well-being.
Table 3.
Growth Curve Models of Age-Related Change in Well-Being: The Role of Birth-Year Cohort and the Covariates.
| HRS (Depressive Affect) | SOEP (Life Satisfaction) | |||
|---|---|---|---|---|
| Lower levels = higher well-being | Higher levels = higher well-being | |||
|
|
||||
| Parameter | Estimate | SE | Estimate | SE |
| Fixed effects | ||||
| Intercept a, γ00 | 50.31* | 0.14 | 49.26* | 0.19 |
| Age, γ10 | 0.15* | 0.04 | −0.14* | 0.06 |
| Age2, γ20 | 0.02* | 0.01 | −0.01 | 0.01 |
| Birth-year cohort, γ01 | −1.08* | 0.24 | 0.70* | 0.33 |
| Gender, γ02 | 1.70* | 0.20 | −0.34 | 0.27 |
| Education (in years), γ03 | −0.60* | 0.03 | 0.44* | 0.05 |
| Number of observations, γ04 | −0.79* | 0.16 | −0.06 | 0.10 |
| Health problems b, γ05 | 1.99* | 0.08 | −4.46* | 0.28 |
| Birth-year cohort × Age, γ11 | 0.02 | 0.05 | 0.15* | 0.07 |
| Gender × Age, γ12 | −0.01 | 0.04 | −0.02 | 0.05 |
| Education × Age, γ13 | 0.00 | 0.01 | 0.01 | 0.01 |
| Number of observations × Age, γ14 | −0.05 | 0.04 | −0.06* | 0.02 |
| Health problems × Age, γ15 | −0.01 | 0.02 | −0.17* | 0.06 |
| Random effects | ||||
| Variance intercept, σ2u0 | 45.48* | 1.19 | 59.56* | 1.74 |
| Variance age, σ2u1 | 0.40* | 0.05 | 0.86* | 0.06 |
| Covariance intercept, age, σu0u1 | 1.13* | 0.18 | 2.24* | 0.25 |
| Residual variance, σ2e | 40.77* | 0.55 | 38.48* | 0.43 |
Note. Well-being score standardized to a T metric (M = 50, SD = 10) based on cross-sectional data at T1. Unstandardized estimates and standard errors are presented. Boldface type highlights cohort differences in well-being change. All covariates were effect coded/centered. Parameter estimates indicate the average trajectory and the extent of differences of a particular covariate. Health problems were indicated by the number of health conditions in the HRS and by the presence of disability in SOEP. Positive parameters indicate differences favoring later-born participants, women, participants with higher education, those who provided more observations, and those suffering more from health problems. HRS: Cohort = earlier-born (1930 –1939; n = 3,999) versus later-born (1940 – 1947; n = 3,999). SOEP: Cohort = earlier-born (1930 –1939; n = 2,712) versus later-born (1940 – 1946; n = 2,712).
Intercept is centered at age 70 years.
Health problems were indicated by the number of health conditions in the HRS and by the presence of disability in SOEP.
p < .05.
In the HRS, the average level of depressive symptoms at 70 years (γ00 = 50.31) was close to the level of depressive symptoms at T1 for this sample (M = 50). The variance in average levels of depressive symptoms was reliably different from 0 after accounting for age trends and covariates (σ2u0 =45.48). Depressive symptoms increased slightly with advancing age. The linear component of the increase in depressive symptoms amounted to 15% of a SD unit per decade (γ10 = 0.15 per year) and the quadratic component was reliably different from 0 indicating accelerated increase (γ20 = 0.02 per year). Associations of birth-year cohort with the level and rates of change in depressive symptoms were of particular interest for our research question. Results revealed that – when compared to individuals born in the 1930s – those who were born in the 1940s showed lower levels of depressive symptoms at age 70 years (γ01 = −1.08). However, the birth-year cohorts did not differ in rates of age-related change in depressive symptoms. The birth-year cohort differences in age-related trajectories of depressive symptoms are illustrated in Panel A of Figure 1.
Figure 1.
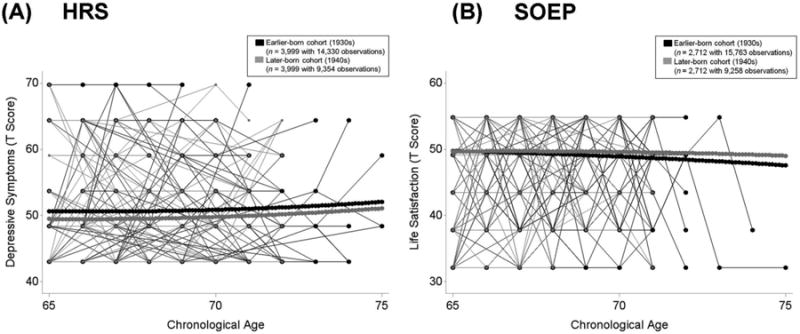
Illustrating differences in life satisfaction between birth-year cohorts in the HRS (Panel A) and in the SOEP (Panel B) from age 65 to age 80 years. Plot includes raw data from 100 earlier-born (1930s, black lines) and 100 later-born (1940s, grey lines) participants. Birth-year cohorts were matched on age at baseline and education and models additionally residualized for differences in gender, education, frequency of observations, and health problems (number of health conditions in the HRS and disability in SOEP). Results revealed secular trends favoring the later-born cohort. Panel A: HRS participants who were born in the 1940s (thick grey lines) showed lower levels of depressive symptoms at age 70 years than those who were born in the 1930s (thick black lines) Cohorts did not differ in rates of age-related change. Panel B: SOEP participants who were born in the 1940s (thick grey lines) showed higher levels of life satisfaction at age 70 years than those who were born in the 1930s (thick black lines) and less steep age-related declines.
In the SOEP, the average level of life satisfaction at 70 years (γ00 = 49.26) was a little lower than the level of life satisfaction at T1 for this sample (M = 50). Average levels of life satisfaction varied reliably across persons after accounting for age trends and covariates (σ2u0 = 59.56). In line with previous work (Gerstorf et al., 2010; Schilling, 2005), life satisfaction declined with advancing age. The linear component of age-related decline of life satisfaction again amounted to a little more than a tenth SD unit per decade (γ10 = −0.14 per year) and the quadratic component was not reliably different from 0 (γ20 = −0.01; p = 0.50). When compared to individuals born in the 1930s, those who were born in the 1940s reported higher levels of life satisfaction at age 70 years (γ01 = 0.70) and less steep age-related declines of life satisfaction (γ11 = 0.15). The birth-year cohort differences in age-related trajectories of life satisfaction are illustrated in Panel B of Figure 1. In sum, our findings showed evidence for secular increases in well-being across birth-year cohorts and provided support for notions of success-of-success.
Do Historical Improvements in Subjective Well-Being Generalize to Late-Life? Differences in Age-Related Trajectories between Death-Year Cohorts
Table 4 presents results from our growth model examining whether earlier and later death-year cohorts differed in age-related trajectories of well-being.
Table 4.
Growth Curve Models of Age-Related Change in Well-Being: The Role of Death-Year Cohort and the Covariates.
| HRS (Depressive Affect) | SOEP (Life Satisfaction) | |||
|---|---|---|---|---|
| Lower levels = higher well-being | Higher levels = higher well-being | |||
|
|
||||
| Parameter | Estimate | SE | Estimate | SE |
| Fixed effects | ||||
| Intercept a, γ00 | 50.41* | 0.15 | 47.89* | 0.22 |
| Age, γ10 | 0.11* | 0.02 | −0.35* | 0.03 |
| Age2, γ20 | 0.00 | <0.01 | −0.01* | <0.01 |
| Death-year cohort, γ01 | 0.67 | 0.38 | −1.41* | 0.41 |
| Gender, γ02 | 2.00* | 0.26 | −0.86* | 0.43 |
| Education (in years), γ03 | −0.41* | 0.04 | 0.69* | 0.11 |
| Number of observations, γ04 | −0.82* | 0.16 | 0.40* | 0.05 |
| Health problems b, γ05 | 1.99* | 0.10 | −4.94* | 0.41 |
| Death-year cohort × Age, γ11 | −0.06 | 0.05 | 0.05 | 0.05 |
| Gender × Age, γ12 | −0.13* | 0.03 | 0.02 | 0.05 |
| Education × Age, γ13 | 0.01 | <0.01 | 0.03* | 0.01 |
| Frequency of observations × Age, γ14 | 0.09* | 0.02 | −0.03* | 0.01 |
| Health problems × Age, γ15 | 0.00 | 0.01 | −0.07 | 0.05 |
| Random effects | ||||
| Variance intercept, σ2u0 | 38.48* | 1.61 | 43.54* | 2.24 |
| Variance age, σ2u1 | 0.06* | 0.02 | 0.28* | 0.03 |
| Covariance intercept, slope, σu0u1 | −0.01 | 0.12 | 0.53* | 0.18 |
| Residual variance, σ2e | 52.13* | 0.90 | 50.17* | 0.73 |
Note. Well-being score standardized to a T metric (M = 50, SD = 10) based on cross-sectional data at T1. Unstandardized estimates and standard errors are presented. Boldface type highlights cohort differences in well-being change. All covariates were effect coded/centered. Parameter estimates indicate the average trajectory and the extent of differences of a particular covariate. Health problems were indicated by the number of health conditions in the HRS and by the presence of disability in SOEP. Positive parameters indicate differences favoring later-dying participants, women, participants with higher education, those who provided more observations, and those suffering more from health problems. HRS: Cohort = earlier-deceased (1993 –1999; n = 2,265) versus later-deceased (2000 – 2009; n = 2,265). SOEP: Cohort = earlier-deceased (1990 –1999; n = 769) versus later-deceased (2000 – 2009; n = 769).
Intercept is centered at age 75 years.
Health problems were indicated by the number of health conditions in the HRS and by the presence of disability in the SOEP.
p < .05.
In the HRS, the average level of depressive symptoms at 75 years amounted to γ00 = 50.41 T units. Levels of depressive symptoms varied reliably across individuals (σ2u0 =38.48). Depressive symptoms increased slightly with advancing age, with a linear component amounting to a little more than a tenth SD unit per decade (γ10 = 0.11 per year) and a quadratic component of 0 (γ20 = 0.00, p = 0.37). These findings were in line with previous research showing relative stability of well-being with age (see Berg, 2014). Results revealed that cohorts did not reliably differ in levels of depressive symptoms at age 75 years (γ01 = 0.67, p = 0.07). The cohort difference in rates of age-related change was also not reliably different from 0 (γ11 = −0.06, p = 0.18). These findings are illustrated in Panel A of Figure 2.
Figure 2.
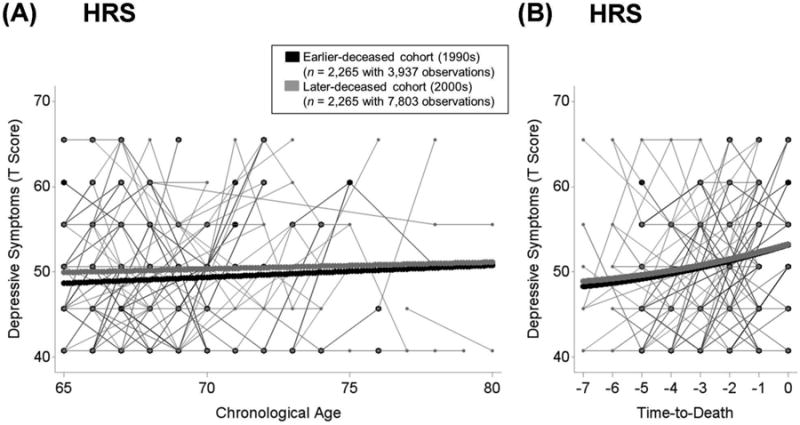
Illustrating differences in depressive symptoms between death-year cohorts in the HRS from age 65 to age 100 (Panel A) and in terminal decline across the last 7 years of life (Panel B). Plot includes raw data from 100 earlier-deceased (1990s, black lines) and 100 later-deceased (2000s, grey lines) participants. Death-year cohorts were matched on age at death and education and models additionally residualized for differences in gender, education, frequency of observations, and number of health conditions (mortality-related model also residualized for age at T1 and at death). Results revealed no evidence for secular trends favoring the later-deceased cohort. Panel A: HRS participants who died in the 2000s (thick grey lines) did not differ from those who died in the 1990s (thick black lines) in trajectories of well-being over age. Panel B: Over time to death, participants who died in the 2000s (thick grey lines) did not differ from participants who died in the 1990s (thick black lines).
In the SOEP, the average level of life satisfaction at 75 years amounted to γ00 = 47.89 T score units. Levels of life satisfaction showed reliable variation across participants (σ2u0 = 43.54). Life satisfaction declined with advancing age, with a linear component amounting to about a third SD unit per decade (γ10 = −0.35 per year) and a quadratic component indicating accelerated decline (γ20 = −0.01). The later death-year cohort showed lower levels of life satisfaction at age 75 years (γ01 = −1.41). The cohorts did not differ in the rate of age-related change in life satisfaction (γ11 = 0.05, p = 0.29). These findings are illustrated in Panel A of Figure 3. In sum, our findings did not show evidence for secular increases in well-being across death-year cohorts and failed to support notions of success-of-success. To the contrary, in the SOEP, later death-year cohort showed slightly lower levels of well-being at age 75 years.
Figure 3.
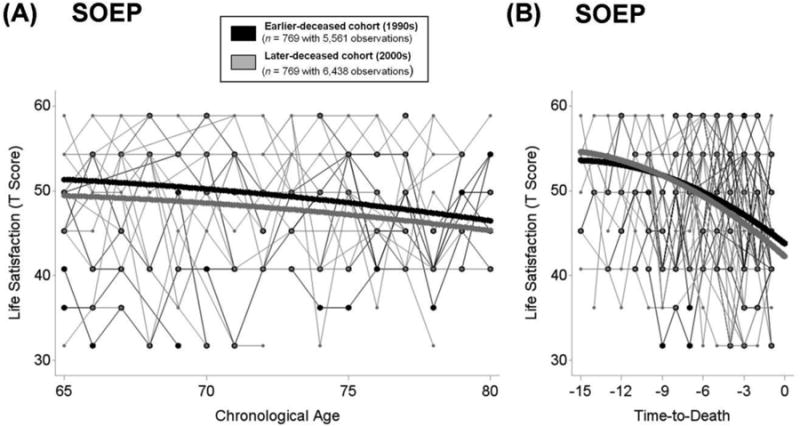
Illustrating differences in life satisfaction between death-year cohorts in the SOEP from age 65 to age 100 (Panel A) and in terminal decline across the last 15 years of life (Panel B). Plot includes raw data from 25 earlier-deceased (1990s, black lines) and 25 later-deceased (2000s, grey lines) participants. Death-year cohorts were matched on age at death and education and models additionally residualized for differences in gender, education, frequency of observations, and disability (mortality-related model also residualized for age at T1 and at death). Results revealed no evidence for secular trends favoring the later-deceased cohort. Panel A: To the contrary, SOEP participants who died in the 2000s (thick grey lines) showed lower levels of life satisfaction at age 75 years than those who died in the 1990s (thick black lines). Panel B: Over time to death, participants who died in the 2000s (thick grey lines) experienced lower levels of life satisfaction 2 years prior to death and steeper terminal declines than participants who died in the 1990s (thick black lines).
Differences in Terminal Decline between Death-Year Cohorts
Table 5 presents results from our mortality-related growth model that examined cohort differences in terminal decline of well-being.
Table 5.
Growth Curve Models of Mortality-Related Change in Well-Being: The Role of Death-Year Cohort and the Covariates.
| HRS (Depressive Affect) | SOEP (Life Satisfaction) | |||
|---|---|---|---|---|
| Lower levels = higher well-being | Higher levels = higher well-being | |||
|
|
||||
| Parameter | Estimate | SE | Estimate | SE |
| Fixed effects | ||||
| Intercept a, γ00 | 51.41* | 0.13 | 45.53* | 0.22 |
| Time-to-Death, γ10 | 0.81* | 0.07 | −1.18* | 0.06 |
| Time-to-Death2, γ20 | 0.05* | 0.02 | −0.04* | 0.01 |
| Death-year cohort, γ01 | 0.19 | 0.41 | −1.19* | 0.44 |
| Gender, γ02 | 1.82* | 0.27 | −1.58* | 0.47 |
| Education (in years), γ03 | −0.40* | 0.04 | 0.77* | 0.12 |
| Frequency of observations, γ04 | −0.87* | 0.25 | 0.08 | 0.27 |
| Health problems b, γ05 | 1.99* | 0.10 | −4.13* | 0.46 |
| Age at T1, γ06 | −0.44* | 0.11 | 0.04 | 0.27 |
| Age at death, γ07 | 0.45* | 0.11 | −0.07 | 0.27 |
| Death-year cohort × Time-to-Death, γ11 | −0.08 | 0.13 | −0.17* | 0.06 |
| Gender × Time-to-Death, γ12 | −0.03 | 0.07 | 0.01 | 0.06 |
| Education × Time-to-Death, γ13 | 0.01 | 0.01 | 0.04* | 0.02 |
| Frequency of observations × Time-to-Death, γ14 | −0.07 | 0.07 | 0.00 | 0.03 |
| Health problems × Time-to-Death, γ15 | 0.04 | 0.03 | −0.06 | 0.06 |
| Age at T1 × Time-to-Death, γ16 | −0.02 | 0.03 | −0.04 | 0.03 |
| Age at death × Time-to-Death, γ17 | 0.02 | 0.03 | 0.03 | 0.03 |
| Random effects | ||||
| Variance intercept, σ2u0 | 50.01* | 1.75 | 57.93* | 2.66 |
| Variance time-to-death, σ2u1 | 0.71* | 0.09 | 0.43* | 0.04 |
| Covariance intercept, slope, σu0u1 | 3.15* | 0.32 | 3.05* | 0.28 |
| Residual variance, σ2e | 46.49* | 0.93 | 48.23* | 0.70 |
Note. Well-being score standardized to a T metric (M = 50, SD = 10) based on cross-sectional data at T1. Unstandardized estimates and standard errors are presented. Boldface type highlights cohort differences in well-being change. All covariates were effect coded/centered. Parameter estimates indicate the average trajectory and the extent of differences of a particular covariate. Positive parameters indicate differences favoring later-dying participants, women, participants with higher education, those who provided more observations those suffering more from health problems, and those with a higher age at T1 and at death. HRS: Cohort = earlier-deceased (1993 –1999; n = 2,265) versus later-deceased (2000 – 2009; n = 2,265). SOEP: Cohort = earlier-deceased (1990 –1999; n = 769) versus later-deceased (2000 – 2009; n = 769).
Intercept is centered at 2 years prior to death.
Health problems were indicated by the number of health conditions in the HRS and by the presence of disability in SOEP.
p < .05.
In the HRS, the average level of depressive symptoms (γ00 = 51.41) two years prior to death (the centering point) was somewhat higher than the overall level at T1 for this subsample (M = 50). Depressive symptoms varied reliably across individuals after accounting for terminal increases and covariates (σ2u0 =50.01). Depressive symptoms increased proximate to death, the linear component of increase in depressive symptoms amounted to about 80% of a full SD per decade (γ10 = 0.81 per year) with some quadratic curvature (γ20 = 0.05), indicating accelerated increase proximate to death. In line with previous research (see Gerstorf & Ram, 2013), the mortality related increase in depressive symptoms was much stronger than the age-related increase. Cohort differences in level and change of depressive symptoms were again of primary interest for our research question. The levels of depressive symptoms two years prior to death (γ01 = 0.19; p = 0.64) and the rate of mortality-related change (γ11 = −0.08; p = 0.54) did not differ across cohorts. Panel A of Figure 2 illustrates the lack of cohort differences in terminal trajectories of depressive symptoms.
In the SOEP, the average level of life satisfaction (γ00 = 45.53) two years prior to death was about half a SD unit lower than the overall level at T1 for this subsample (M = 50). Levels of life satisfaction varied reliably across individuals after accounting for terminal decline and covariates (σ2u0 =57.93). In line with previous reports of decrements in life satisfaction associated with terminal decline (e.g., Gerstorf et al., 2010), the linear component of the decline in life satisfaction amounted to more than a full SD unit per decade (γ10 = −1.18 per year) with some negative quadratic curvature (γ20 = −0.04), indicating an accelerated decline. The level of life satisfaction two years prior to death was lower (γ01 = −1.19) and mortality-related declines were steeper (γ11 = −0.17) for the later death-year cohort. As a result, cohort differences increased with increasing proximity of death. Panel B of Figure 3 illustrates these cohort differences in mortality-related trajectories of life satisfaction. In sum, our findings did not reveal evidence for secular increases in mortality-related trajectories of well-being across death-year cohorts and failed to support notions of success-of-success. To the contrary, small differences favoring the earlier death-year cohort emerged in the SOEP.
Across all models examined (see Tables 3, 4, and 5), those with more education and those in better health reported higher levels of well-being. When gender differences emerged, they were to the favor of men. Taken together, these findings were in line with previous research showing higher levels of well-being for men (e.g., Tesch-Römer, Motel-Klingebiel, & Tomasik, 2008), those with more education (e.g., Blanchflower & Oswald, 2004) and those in better health (e.g., Chida & Steptoe, 2008).
Follow-up analyses
Our aim in using propensity score matching procedure was to control for potential confounding variables and processes of study selection that may differ across cohorts. To examine whether our use of propensity score matching concealed patterns of cohort differences available in raw data, we repeated our analyses of cohort differences using all available observations after the age of 65 and using year of birth and year of death as a continuous indicator of cohort membership. We used the same sets of covariates as in previous analyses.
Analyses of birth-year cohorts
The HRS included data from 21,354 individuals born between the years 1890 and 1947. Year of birth was not related to levels of (γ01 = 0.01; p = 0.42) and changes in (γ11 = 0.00; p = 0.10) depressive symptoms. The SOEP included data from 11,999 individuals born between the years 1882 and 1946. In the SOEP, a later birth year was associated with lower levels of life satisfaction (γ01 = −0.11 per year) in line with previous findings (Schilling, 2005). However, cohorts did not differ in rates of age-related change in well-being (γ11 = 0.00; p = 0.69). Taken together, cohort effects favoring the later-born cohort were only found in analyses involving propensity score matching.
Analyses of death-year cohorts
The HRS included data from 7,347 now deceased individuals who have died between the years 1993 and 2011. A later year of death was associated with a higher level of depressive symptoms at age 75 (γ01 = 0.15 per year), however year of death was not associated with age-related change in depressive symptoms (γ11 = 0.00; p = 0.41). Year of death did not relate to levels of depressive symptoms two years prior to death (γ01 = −0.02; p = 0.65), however, a later death year was associated with less steep terminal increase in depressive symptoms (γ11 = −0.04). The SOEP included data from 2,861 now deceased individuals who have died between the years 1984 and 2011. In the SOEP, a later year of death was associated with a lower level of life satisfaction at age 75 (γ01 = −0.12 per year), however a later year of death predicted less steep age-related declines of subjective well-being (γ11 = 0.01). A later year of death was also associated with lower levels of life satisfaction two years prior to death (γ01 = −0.08 per year), but was unrelated to rates of terminal decline (γ11 = 0.00; p = 0.48). Taken together, these findings showed mixed evidence favoring earlier-dying cohorts in levels of well-being and favoring later-dying cohorts in rates of well-being change late in life. However, it is important to note that these analyses involving full samples did not take different processes of study selection across cohorts into account.
Discussion
In the present study, we examined whether secular trends favoring later cohorts also manifest in the last years of life. Taken together, our findings gathered from national samples in the US and in Germany revealed evidence of historical increases in well-being in old age across birth-year cohorts born in the 1930s vs. in the 1940s. However, among death-year cohorts dying in the 1990s vs. in the 2000s we did not find evidence for secular increases in well-being. It is important to note that we chose a liberal criterion (p < 0.05) for significance so that even small positive secular trends would be identified. Despite this liberal criterion, no differences emerged favoring the later-dying. Instead, individuals in the later death-year cohort reported slightly lower levels of well-being and steeper terminal declines of well-being in the SOEP. We discuss possible factors underlying these findings suggesting that secular trends in well-being favoring later cohorts do not manifest in the last years of life.
Differences among Birth-Year Cohorts
Our findings on birth-year cohorts show some evidence for historical improvements that are in line with previous research showing historical increases in well-being (e.g., Sutin et al., 2013) and in other domains of functioning (cognitive performance: Gerstorf et al., in press; control beliefs and loneliness: Hülür et al., submitted) in old age. However, our findings differed from a previous report from the SOEP (Schilling, 2005) that found life satisfaction to be lower and show less-steep age-related declines among later-born individuals. We note two possible explanations for this discrepancy. First, our analyses focused on different birth-year cohorts than in this previous report: Schilling (2005) analyzed data from three subsamples born in the years from 1909 to 1939. In contrast, we focused on two birth-year cohorts born in the 1930s or in the 1940s. It is possible that different historical trends are invoked through the different operational definitions of cohort. A second explanation relates to population selection. Participants who were born in earlier historical times entered the SOEP at later ages. Thus, selection effects might have played a role: For example, a participant born in 1909 entered the SOEP in 1984 when he or she was 75 years old. A sizeable part of the birth-year cohort might have already died by then. In contrast, a participant born in 1939 entered the SOEP in 1984 when he or she was 45 years old, when the majority of his or her birth-year cohort was still alive. Thus, participants in earlier-born cohorts in the SOEP might be a more highly select subsample than the later-born cohorts. The use of propensity score matching procedures to equate the cohorts on education and age at baseline might have reduced the impact of population selection in the present study. In line with this interpretation, we note that in follow-up analyses we replicated the findings of Schilling et al. (2005) with the full sample of the SOEP (i.e., we corroborated the reported finding that later-born individuals showed lower levels of life satisfaction). In a similar manner, cohort differences favoring those born in the 1940s only emerged in the HRS when cohorts were matched on age at baseline and education. Taken together, our findings revealed some evidence for higher well-being among those born in the 1940s when compared to those born in the 1930s, supporting scenarios of success-of-success.
Why Are Historical Increases in Well-Being Not Manifesting In the Last Years of Life? – Possible Mechanisms
Our study is, to the best of our knowledge, one of the first to examine differences between cohorts who had died during different decades in age- and mortality-related trajectories of well-being. Noting explicitly that our study is descriptive and does not provide for strong inferences regarding the underlying mechanisms, we highlight possible explanations for the lack of secular increases in well-being across the death-year cohorts and in the last years of life. The first set of explanations revolves around differences in health and functional capacities of older adults belonging to different death-year cohorts (see Gerstorf et al., 2011; Hülür et al., 2013). According to notions of failure-of-success (see Christensen et al., 2013) and manufactured survival (Olshansky et al., 2002), an extension of life based on medical and technical improvements would diminish or even reverse historical trends favoring later cohorts in late-life functioning. Later-dying cohorts are possibly benefiting from medical and technological advances and thus are able to live with impairments that might have been mortal among earlier-dying cohorts (Christensen, Doblhammer, Rau, & Vaupel, 2009). Because of resulting decreases in the quality of late life, older adults in later-dying cohorts might be somewhat less satisfied with their lives, thereby offsetting historical improvements in well-being observed among birth-year cohorts.
A second possible mechanism that could lead to a stop of historical increases in well-being is based on lifespan notions suggesting that cultural means become less efficient to compensate for frailties and losses (Baltes & Smith, 2003; see Gerstorf et al., 2011; Hülür et al., 2013). If more individuals are experiencing chronic conditions (Crimmins & Beltran-Sanchez, 2011), and the length of life spent in diseased states is increasing, the necessity for cultural means to outweigh these developments is also increasing. However, current intervention and rehabilitation efforts to maintain prior levels of functioning may reach their limits in old age. For example, mobility limitations related to decreasing functional health might make it more and more difficult to obtain the needed (increasing) amount of medical care, especially in areas that are not densely populated and where healthcare facilities are sparse.
As a third explanation, population selection might lead to a reduction of historical increases in well-being among older adults (see Gerstorf et al., 2011; Hülür et al., 2013). As more and more individuals reach higher ages, the relative proportion of older adults from less healthy population segments is increasing. By implication, these less healthy people may also be less satisfied with their lives relative to the more positively select earlier-dying cohort.
The fourth set of explanations revolves around individuals' perceptions of their living circumstances and subjective standards when evaluating their overall satisfaction with one's life. According to Michalos' multiple discrepancies theory (Michalos, 1985), standards for comparisons can be formed based on past experiences. Earlier cohorts, who have possibly spent parts of their earlier life under more adverse living circumstances, may have had lower subjective standards (see Schilling, 2005). However, three arguments can be brought forward against differences in subjective standards being the main source of cohort differences in well-being: First, based on this explanation, one could expect earlier-born individuals to report higher levels of well-being than later-born cohorts. This was however not the case for the birth-year cohorts examined in the present study. Only among death-year cohorts later-deceased individuals reported lower levels of well-being in the SOEP. Second, previous work (Hülür et al., 2013) revealed that later-deceased cohorts experienced less favorable late-life trajectories of cognitive aging and terminal decline, suggesting that in part different late-life living circumstances indeed exist. Third, in the HRS, well-being was indexed by depressive symptoms, a measure that is less likely to be based on subjective standards than life satisfaction. However, also in the HRS, we did not observe any evidence indicating historic increases in well-being among death-year cohorts. Taken together, our findings showed that historical improvements in well-being do not extend to the last years of life and suggested that historical increases observed in other domains such as cognitive performance, or psychosocial function (e.g., Gerstorf et al., in press; Hülür et al., submitted) may also get smaller late in life.
At the individual level, factors other than cohort may be more relevant for a person's subjective well-being: For example, in the SOEP, having been certified as having a disability through the course of the study was associated with almost half a standard deviation unit lower subjective well-being (see Tables 3, 4, and 5). Although cohort differences in well-being were generally small, they may nevertheless be relevant from a societal perspective, especially when considering that birth-year and death-year cohorts were close in years of birth and death, respectively (see Tables 1, and 2). For example, this study allowed for a test between success-of-success and failure-of-success scenarios (see Christensen et al., 2013). When examining trajectories of well-being across birth-year cohorts in old age, our findings supported success-of-success scenarios. However, there was no evidence for such a scenario in late-life trajectories of well-being among death-year cohorts. It has been proposed that data on well-being collected at the national level should be shaping policy decisions (see Diener & Seligman, 2004). The findings of the present study suggest that public policy should be aimed at improving subjective well-being in the last years of life. For example, it may be argued that availability and quality of services offered to local residents can have a considerable impact on older adults' developmental trajectories (see Gerstorf & Ram, 2012). Thus, the services that are especially relevant in the last years of life (e.g., ambulatory and stationary care facilities) can play an important role in maintaining cohort-related advantages that are apparent in earlier phases of life.
Limitations and Outlook
In closing, we note several limitations of our study. To begin with, we examined cohort differences in well-being indexed by measures of life satisfaction (SOEP) and depressive symptoms (HRS). An implicit assumption of our approach was that the meaning of well-being did not change across cohorts. However, it is possible that not only the level, but also the structure and correlates of well-being change across historical time. For example, a recent study has shown that age-related declines in the proportion of non-relatives in one's social network was smaller in later cohorts than it was in earlier cohorts (Suanet, van Tilburg, & Broese van Groenou, 2013). It is possible that older adults from later cohorts place more importance on friendships when rating their well-being. Different operational definitions of well-being in the HRS (depressive symptoms) and in the SOEP (life satisfaction) precluded direct comparisons between the samples and/or pooling of the data. Also, we used different operational definitions of health in each study (HRS: number of health conditions, SOEP: being certified as having a disability). We note that differences in results across these two samples might potentially be based on these methodological differences. Nevertheless, integrated analysis and broad replication across multiple studies allowed for both broader operationalization of subjective well-being and more robust potential for generalization.
In a recent study on cohort differences in cognitive functioning (Christensen et al., 2013), the authors were able to approach all individuals in the population in question, i.e., all Danish individuals born in 1905 and in 1915. The response rate was similar for both cohorts (63%). In contrast, in the present study a possible bias could have been introduced by the sampling schemes used by the HRS and the SOEP. Because it was not possible to study the entire cohorts in question, our analyses depend on the assumption that the selection into the study samples was equivalently representative. Even if selection into the parent samples of the HRS and the SOEP was truly representative, this does not necessarily generalize to the subsamples included in our analyses. We moved in the direction of addressing this point explicitly with propensity score matching procedures, but some caution is warranted because such procedures only cover the variables selected for the procedure, and ignore potential differences in other unobserved variables.
To be able to compare a similar number of participants in each cohort, we defined the birth-year cohorts as born in the 1930s vs. in the 1940s, and the death-year cohorts as dying in the 1990s vs. in the 2000s, which are arbitrary distinctions. As a result, the birth-year and death-year cohorts were close in years of birth and death, respectively (see Table 1, and 2). Thus, the birth-year cohorts may have experienced similar historical events, such as the World War II and Great Depression, and the death year-cohorts may have experienced similar late-life circumstances. Cohort differences may be even stronger in cohorts with a greater distance between years of birth and death, respectively. Alternatively, future studies could focus on specific sets of events or transitions that profoundly influence the circumstances of individual development (see Gerstorf et al., 2011; Hülür et al., 2013) such as the Great Depression (Elder, 1974) or the implementation of the GI Bill (Laub & Sampson, 2005), and the specific ages at which those events/affordances are experienced.
In the present study, age- and mortality-related changes in well-being were modeled using a growth model with linear and quadratic change components. With this model, we were only partially able to test notions of compressed morbidity (Fries, 1980; 2000). In the present study, compressed morbidity would manifest as a delayed onset of age-related and/or terminal decline in well-being (see also Vaupel, 2010: deceleration or right-shifting of aging). This prediction could be examined more directly with a two-phase model of change that specifically includes a transition point toward a phase of terminal decline (Fauth, Gerstorf, Ram, & Malmberg, 2014; Gerstorf et al., 2010). Well-being data in late life over a longer time span and with more dense assessments would allow examining the notion of compressed morbidity more directly (see Hülür et al., 2013).
Conclusions
In the present study, we examined cohort differences in trajectories of well-being among birth-year and death-year cohorts. As can be expected based on improvements in life conditions over the last century, we found evidence of secular trends favoring the later-born cohort (1940s) in trajectories of well-being. Focusing on effects of conditions that shape late-life development, our findings revealed no evidence for cohort differences favoring the later-deceased cohort (2000s) as compared to the earlier-deceased cohort (1990s). To the contrary, some of our findings revealed small secular trends in the opposite direction. Taken together, secular trends favoring later cohorts in well-being during the recent decades do not generalize to late-life. These findings highlight the necessity for social policy and interventions that aim at improving older adults' well-being in their last years of life.
Footnotes
Follow-up analyses showed that including interaction terms between covariates and cohort effects on levels of and changes in well-being led to substantively the same findings.
References
- Baltes PB, Cornelius SW, Nesselroade JR. Cohort effects in developmental psychology. In: Nesselroade JR, Baltes PB, editors. Longitudinal research in the study of behavior and development. New York, NY: Academic Press; 1979. pp. 61–87. [Google Scholar]
- Baltes PB, Smith J. New frontiers in the future of aging: From successful aging of the young old to the dilemmas of the fourth age. Gerontology. 2003;49:123–135. doi: 10.1159/000067946. [DOI] [PubMed] [Google Scholar]
- Berg AI. Life satisfaction in the oldest old. In: Michalos AC, editor. Encyclopedia of quality of life and well-being research. New York: Springer; 2014. pp. 3589–3591. [DOI] [Google Scholar]
- Blanchflower DG, Oswald AJ. Well-being over time in Britain and the USA. Journal of Public Economics. 2004;88:1359–1386. doi: 10.1016/S0047-2727(02)00168-8. [DOI] [Google Scholar]
- Bukenya JO, Gebremedhin TG, Schaeffer PV. Analysis of rural quality of life and health: A spatial approach. Economic Development Quarterly. 2003;17:280–293. doi: 10.1177/0891242403255325. [DOI] [Google Scholar]
- Burkhauser RV, Gertler PJ. Introduction: Special Issue on the Health and Retirement Survey: Data quality and early results. Journal of Human Resources. 1995;30:S1–S6. [Google Scholar]
- Chida Y, Steptoe A. Positive psychological well-being and mortality: A quantitative review of prospective observational studies. Psychosomatic Medicine. 2008;70:741–756. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. The Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Thinggaard M, Oksuzyan A, Steenstrup T, Andersen-Ranberg K, Jeune B, McGue M, Vaupel JW. Physical and cognitive functioning of people older than 90 years: a comparison of two Danish cohorts born 10 years apart. The Lancet. 2013;382:1507–1513. doi: 10.1016/S0140-6736(13)60777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman DL. Estimating causal effects in mediation analysis using propensity scores. Structural Equation Modeling. 2011;18:357–369. doi: 10.1080/10705511.2011.582001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribier F. Changes in the experiences of life between two cohorts of Parisian pensioners, born in circa 1907 and 1921. Ageing and Society. 2005;25:637–654. doi: 10.1017/S0144686X05004009. [DOI] [Google Scholar]
- Crimmins EM, Beltrán-Sánchez H. Mortality and morbidity trends: is there compression of morbidity? Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2011;66:P75–P86. doi: 10.1093/geronb/gbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E. Subjective well-being. Psychological Bulletin. 1984;95:542–575. doi: 10.1037/0033-2909.95.3.542. [DOI] [PubMed] [Google Scholar]
- Diener E, Oishi S, Ryan K. Universal and cultural differences in the causes and structure of “happiness” – A multilevel review. In: Keyes C, editor. Mental well-being: International contributions to the study of positive mental healths. Dordrecht, Netherlands: Springer; 2013. pp. 153–176. [Google Scholar]
- Diener E, Seligman MEP. Beyond money: Toward an economy of well-being. Psychological Science in the Public Interest. 2004;5:1–31. doi: 10.1111/j.0963-7214.2004.00501001.x. [DOI] [PubMed] [Google Scholar]
- Dodge HH, Zhu J, Lee CW, Chang CCH, Ganguli M. Cohort effects in age-associated cognitive trajectories. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2014;69:M687–M694. doi: 10.1093/gerona/glt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterlin RA, McVey LA, Switek M, Sawangfa O, Zweig JS. The happiness-income paradox revisited. Proceedings of the National Academy of the Sciences of the United States of America. 2010;107:22463–22468. doi: 10.1073/pnas.1015962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GH., Jr . Children of the great depression. Chicago, IL: University of Chicago Press; 1974. [Google Scholar]
- Elder GH, Jr, Hareven TK. Rising above life's disadvantages: From the Great Depression to global war. In: Modell J, Elder GH Jr, Parke R, editors. Children in time and place. New York: Cambridge University Press; 1992. pp. 47–72. [Google Scholar]
- Fauth EB, Gerstorf D, Ram N, Malmberg B. Comparing changes in late-life depressive symptoms across aging, disablement, and mortality processes. Developmental Psychology. 2014;50:1584–1593. doi: 10.1037/a0035475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JR. Massive IQ gains in 14 nations: What IQ tests really measure. Psychological Bulletin. 1987;101:171–191. doi: 10.1037/0033-2909.101.2.171. [DOI] [Google Scholar]
- Flynn JR. Solving the IQ Puzzle. Scientific American Mind. 2007;18:24–31. doi: 10.1038/scientificamericanmind1007-24. [DOI] [Google Scholar]
- Foster E. Inference and developmental psychology. Developmental Psychology. 2010;46:1454–1480. doi: 10.1037/a0020204. [DOI] [PubMed] [Google Scholar]
- Freedman VA, Schoeni R, Martin LG, Cornman J. Chronic conditions and the decline in late life disability. Demography. 2007;44:459–477. doi: 10.1353/dem.2007.0026. [DOI] [PubMed] [Google Scholar]
- Fries JF. Aging, natural death, and the compression of morbidity. New England Journal of Medicine. 1980;303:130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- Fries JF. Compression of morbidity in the elderly. Vaccine. 2000;18:1584–1589. doi: 10.1016/s0264-410x(99)00490-9. [DOI] [PubMed] [Google Scholar]
- Fujita F, Diener E. Life satisfaction set point: Stability and change. Journal of Personality and Social Psychology. 2005;88:158–164. doi: 10.1037/0022-3514.88.1.158. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Hülür G, Drewelies J, Eibich P, Duezel S, Demuth I, et al. Lindenberger U. Secular changes in late-life cognition and well-being: Towards a long bright future with a short brisk ending? Psychology and Aging. doi: 10.1037/pag0000016. in press. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Ram N. Late-life: A venue for studying the mechanisms by which contextual factors influence individual development. In: Whitbourne SK, Sliwinski MJ, editors. The Wiley-Blackwell Handbook of Adulthood and Aging. New York, NY: Wiley; 2012. pp. 49–71. [DOI] [Google Scholar]
- Gerstorf D, Ram N. Inquiry into terminal decline: Five objectives for future study. The Gerontologist. 2013;53:727–737. doi: 10.1093/geront/gnt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D, Ram N, Hoppmann CA, Willis SL, Schaie KW. Cohort differences in cognitive aging and terminal decline in the Seattle Longitudinal Study. Developmental Psychology. 2011;47:1026–1041. doi: 10.1037/a0023426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D, Ram N, Mayraz G, Hidajat M, Lindenberger U, Wagner GG, Schupp J. Late-life decline in well-being across adulthood in Germany, the United Kingdom, and the United States: Something is seriously wrong at the end of life. Psychology and Aging. 2010;25:477–485. doi: 10.1037/a0017543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haisken-De New JP, Frick R. Desktop companion to the German Socio-Economic Panel Study (SOEP) Berlin: German Institute for Economic Research (DIW Berlin); 2006. [Google Scholar]
- Hülür G, Gerstorf D, Drewelies J, Eibich P, Duezel S, Demuth I, et al. Lindenberger U. Cohort differences in psychosocial function: Older adults nowadays feel less lonely and less dependent on external circumstances. Gerontology. doi: 10.1159/000438991. submitted. [DOI] [PubMed] [Google Scholar]
- Hülür G, Infurna FJ, Ram N, Gerstorf D. Cohorts based on decade of death: No evidence for secular trends favoring later cohorts in cognitive aging and terminal decline in the AHEAD study. Psychology and Aging. 2013;28:115–127. doi: 10.1037/a0029965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JJ, Thoemmes F, Jonkmann K, Lüdtke O, Trautwein U. Military training and personality trait development: Does the military make the man or does the man make the military? Psychological Science. 2012;23:270–277. doi: 10.1177/0956797611423545. [DOI] [PubMed] [Google Scholar]
- Kroh M. DIW Discussion Papers No 546. Berlin: German Institute for Economic Research (DIW Berlin); 2006. An experimental evaluation of popular well-being measures. [Google Scholar]
- Laub JH, Sampson RJ. Coming of age in wartime: how World War II and the Korean War changed lives. In: Schaie KW, Elder GH, editors. Historical influences on lives and aging. New York: Springer Publishing Company; 2005. pp. 208–228. [Google Scholar]
- Littell RC, Miliken GA, Steoup WW, Wolfinger RD. SAS for mixed models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. J. Wiley & Sons; New York: 1987. [Google Scholar]
- Lucas RE. Long-term disability is associated with lasting changes in subjective well-being: Evidence from two nationally representative longitudinal studies. Journal of Personality and Social Psychology. 2007;92:717–730. doi: 10.1037/0022-3514.92.4.717. [DOI] [PubMed] [Google Scholar]
- Martin L, Schoeni RF, Andreski PM. Trends in health of older adults in the United States: Past, present, future. Demography. 2010;47:S17–S40. doi: 10.1353/dem.2010.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, Brayne C. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. The Lancet. 2013;382:1405–1412. doi: 10.1016/S0140-6736(13)61570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalos AC. Multiple discrepancies theory (MDT) Social Indicators Research. 1985;16:347–413. [Google Scholar]
- Olshansky SJ, Hayflick L, Carnes BA. Position statement on human aging. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2002;57:M292–M297. doi: 10.1093/gerona/57.8.B292. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Rudberg MA, Carnes BA, Cassel CK, Brody JA. Trading off longer life for worsening health: The expansion of morbidity hypothesis. Journal of Aging and Health. 1991;3:194–216. doi: 10.1177/089826439100300205. [DOI] [Google Scholar]
- Ong AD. Pathways linking positive emotion and health in later life. Current Directions in Psychological Science. 2010;19:358–362. doi: 10.1177/0963721410388805. [DOI] [Google Scholar]
- Pinquart M, Sorensen S. Influences of socioeconomic status, social network, and competence on subjective well-being in later life: A meta-analysis. Psychology and Aging. 2000;15:187–224. doi: 10.1037/0882-7974.15.2.187. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Ram N, Gerstorf D, Fauth E, Zarit S, Malmberg B. Aging, disablement, and dying: Using time-as-process and time-as-resources metrics to chart late-life change. Research in Human Development. 2010;7:27–44. doi: 10.1080/15427600903578151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram N, Grimm K. Growth curve modeling and longitudinal factor analysis. In: Damon W, Lerner RM, Overton W, Molenaar PCM, editors. Handbook of child psychology: Vol 1 Theoretical models of human development. 7th. Hoboken, NJ: Wiley; 2015. [Google Scholar]
- Riley MW. Aging and cohort succession: Interpretations and misinterpretations. Public Opinion Quarterly. 1973;37:35–49. [Google Scholar]
- Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. The American Statistician. 1985;39:33–38. doi: 10.2307/2683903. [DOI] [Google Scholar]
- Ryder NB. The cohort as a concept in the study of social changes. American Sociological Review. 1965;30:843–861. doi: 10.1007/978-1-4613-8536-3_2. [DOI] [PubMed] [Google Scholar]
- Schaie KW. A general model for the study of developmental problems. Psychological Bulletin. 1965;64:92–107. doi: 10.1037/h0022371. [DOI] [PubMed] [Google Scholar]
- Schaie KW, Willis SL, Pennak S. An historical framework for cohort differences in intelligence. Research in Human Development. 2005;2:43–67. doi: 10.1080/15427609.2005.9683344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling OK. Cohort- and age-related decline in elder"s life satisfaction: is there really a paradox? European Journal of Ageing. 2005;2:254–263. doi: 10.1007/s10433-005-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmack U, Schupp J, Wagner GG. The influence of environment and personality on the affective and cognitive component of subjective well-being. Social Indicators Research. 2008;89:41–60. doi: 10.1007/s11205-007-9230-3. [DOI] [Google Scholar]
- Siddiqi A, Given C, Given B, Sikorskii A. Quality of life among primary, metastatic and recurrent cancer patients. European Journal of Cancer Care. 2009;18:84–96. doi: 10.1111/j.1365-2354.2008.01021.x. [DOI] [PubMed] [Google Scholar]
- Stuart EA. Matching methods for causal inference: a review and a look forward. Statistical Science. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suanet B, van Tilburg TG, Broese Van Groenou MI. Nonkin in older adults' personal networks: more important among later cohorts? Journals of Gerontology Series B Psychological Sciences and Social Sciences. 2013;68:633–643. doi: 10.1093/geronb/gbt043. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Terracciano A, Milaneschi Y, An Y, Ferrucci L, Zonderman AB. Cohort effect on well-being: The legacy of economic hard times. Psychological Science. 2013;24:379–385. doi: 10.1177/0956797612459658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch-Römer C, Motel-Klingebiel A, Tomasik MJ. Gender differences in subjective well-being: comparing societies with respect to gender equality. Social Indicators Research. 2008;82:329–349. doi: 10.1007/s11205-007-9133-3. [DOI] [Google Scholar]
- Thoemmes F, Kim ES. A systematic review of propensity score methods in the social sciences. Multivariate Behavioral Research. 2011;46:90–118. doi: 10.1080/00273171.2011.540475. [DOI] [PubMed] [Google Scholar]
- Twenge JM. The age of anxiety? Birth cohort change in anxiety and neuroticism, 1952-1993. Journal of Personality and Social Psychology. 2000;79:1007–1021. doi: 10.1037/0022-3514.79.6.1007. [DOI] [PubMed] [Google Scholar]
- Twenge JM. Birth cohort changes in extraversion: a cross-temporal meta-analysis, 1966–1993. Personality and Individual Differences. 2001;30:735–748. doi: 10.1016/S0191-8869(00)00066-0. [DOI] [Google Scholar]
- Twenge JM, Campbell WK, Freeman EC. Generational differences in young adults" life goals, concern for others, and civic orientation, 1966–2009. Journal of Personality and Social Psychology. 2012;102:1045–1062. doi: 10.1037/a0027408. [DOI] [PubMed] [Google Scholar]
- van Jaarsveld CHM, Sanderman R, Miedema I, Ranchor AV, Kempen GIJM. Changes in health-related quality of life in older patients with acute myocardial infarction or congestive heart failure: a prospective study. Journal of the American Geriatrics Society. 2001;49:1052–1058. doi: 10.1046/j.1532-5415.2001.49208.x. [DOI] [PubMed] [Google Scholar]
- Vaupel JW. Biodemography of human ageing. Nature. 2010;464:536–542. doi: 10.1038/nature08984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waidmann TA, Bound J, Schoenbaum M. The illusion of failure: trends in self-reported health of the U.S. elderly. The Milbank Quarterly. 1995;73:253–287. doi: 10.2307/3350259. [DOI] [PubMed] [Google Scholar]
- Wagner GG, Frick JR, Schupp J. The German Socio-Economic Panel Study (SOEP) - Scope, Evolution and Enhancements. Schmollers Jahrbuch. 2007;127:139–169. [Google Scholar]
- Wallace RB, Herzog AR, Ofstedal MB, Steffick DC, Fonda S, Langa K. Documentation of affective functioning measures in the Health and Retirement Study. Ann Arbor: Survey Research Center, University of Michigan; 2000. [Google Scholar]
- Wilson-Genderson M, Pruchno RA, Cartwright FP. Effects of caregiver burden and satisfaction on affect of older end-stage renal disease patients and their spouses. Psychology and Aging. 2009;24:955–967. doi: 10.1037/a0017368. [DOI] [PMC free article] [PubMed] [Google Scholar]


