Abstract
Linkeropathies are a group of syndromes characterized by short stature, radio-ulnar synostosis, decreased bone density, congenital contractures and dislocations, joint laxity, broad digits, brachycephaly, small mouth, prominent eyes, short or webbed neck, congenital heart defects and mild developmental delay. Linkeropathies are due to enzymatic defects in the synthesis of the common linker region that joins the core proteins to their glycosaminoglycan side chains. The enzyme glucuronyltransferase 1, encoded by B3GAT3, adds the last of the four saccharides that comprise the linker region. Mutations in B3GAT3 have been reported in two unrelated families with the same homozygous mutation (c.830G>A, p.Arg277Gln). We report a patient with a novel homozygous B3GAT3 (c.667G>A, p.Gly223Ser) mutation and a history of multiple fractures, blue sclerae, and glaucoma. Our patient is a 12 month old boy born to consanguineous parents and, like previously reported patients, he has bilateral radio-ulnar synostosis, severe osteopenia, an increased gap between first and second toes, bilateral club feet, and atrial and ventricular septal defects. He also the additional features of bilateral glaucoma, hypertelorism, upturned nose with anteverted nares, a small chest, a diaphragmatic hernia, multiple fractures, arachnodactyly, overlapping fingers with ulnar deviation, lymphedema, hypotonia, hearing loss, and perinatal cerebral infarction with bilateral supra- and infratentorial subdural hematomas. We provide a clinical report to highlight the extended phenotypic range of B3GAT3 mutations and a comparative overview of the phenotypic features of the linkeropathies associated with mutations in XYLT1, B4GALT7, B3GALT6, and B3GAT3.
Keywords: Linkeropathy, B3GAT3, Larsen-like syndrome, proteoglycan disorder, congenital disorder of glycosylation, multiple fractures
INTRODUCTION
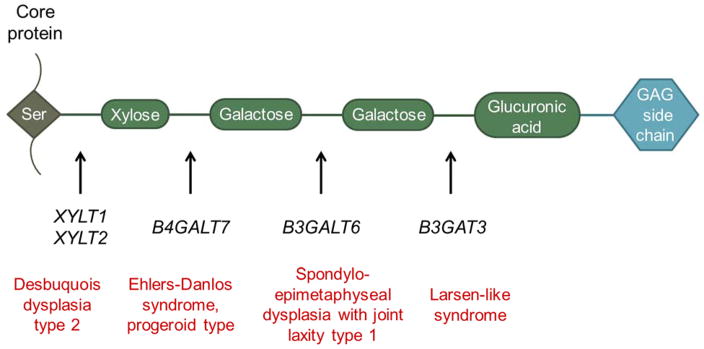
Proteoglycans (PGs) are cell surface molecules and essential components of extracellular matrices that help regulate cell-cell and cell-matrix interactions, cell proliferation, differentiation and development. PGs are composed of a protein core with variable glycosaminoglycan (GAG) side chains, which are attached to serine residues via a tetrasaccharide linker (Fig. 1). Recently, a group of related disorders that are due to defects in one of the four steps involved in synthesis of the common linker region has been described [Nakajima et al., 2013]. These multisystem disorders are referred to as “linkeropathies” [Freeze 2006, Nakajima et al., 2013].
Fig. 1.
Diagram of the linkeropathy genes and their associated syndromes. Proteoglycans are highly glycosylated proteins with covalently attached glycosaminoglycan (GAG) chain. The GAG is attached via a tetrasaccharide bridge (indicated by the four green ovals) to a serine (Ser) residue in the core protein. Human disorders caused by mutations affected each step of tetrasaccharide synthesis.
The enzymes involved in the synthesis of the common linker region and their corresponding genes are xylosyltransferases 1 and 2 (XYLT1 and XYLT2), galactosyltransferase 1 (B4GALT7) and galactosyltransferase 2 (B3GALT6) and glucuronosyltransferase 1 (B3GAT3) [Sugahara et al., 2003; Haltiwanger and Lowe 2003; Häcker et al., 2005; Bishop et al., 2007], and bi-allelic mutations in most genes have been reported to cause human disease. Mutations in XYLT1 cause Desbuquois dysplasia type 2 (OMIM # 615777), which has been previously reported in 9 patients [Schreml et al., 2014; Bui et al., 2014]. Mutations in B4GALT7 are associated with the progeroid type of Ehlers-Danlos syndrome (OMIM# 130070), which has been previously reported in 26 patients [Kresse et al., 1987; Faiyaz-Ul-Haque et al., 2004; Guo et al., 2013; Cartault et al., 2015]. Mutations in B3GALT6 are associated with spondyloepimetaphyseal dysplasia with joint laxity, type 1 (OMIM # 271640) and have been reported in 16 patients [Malfait et al., 2013; Nakajima et al., 2013]. A single homozygous mutation in B3GAT3, involved in the final step of linker region synthesis, has been previously reported in 6 patients with Larsen-like syndrome and multiple joint dislocations (OMIM #245600) in two families [Baasanjav et al., 2011; von Oettingen et al., 2014].
Here, we describe a patient from a consanguineous family with a novel homozygous B3GAT3 mutation and a very severe phenotype and who expands the range of phenotypic features associated with this disorder. We also review the phenotypic features of the linkeropathies associated with mutations in XYLT1, B4GALT7, B3GALT6 and B3GAT3.
CLINICAL REPORT
The patient is a 12-month-old Mexican boy born to parents who were second cousins once removed and whose family history was negative for any congenital or skeletal anomalies. Routine ultrasound at 19 weeks estimated gestation identified bilateral clubfeet, clenched hands, and increased nuchal translucency. A structural heart defect that was initially thought to be a hypoplastic left heart was also noted. Cell-free DNA aneuploidy screen was negative.
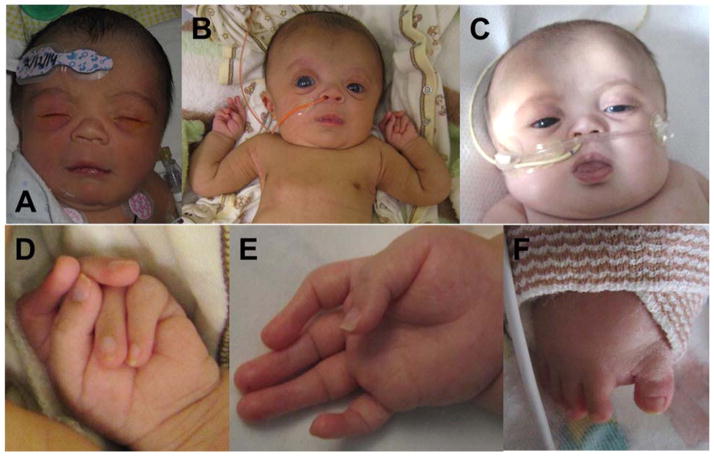
The infant was delivered vaginally at term and had a birth weight of 2.56 kg (4th centile), length of 44.5 cm (−2.93 standard deviations) and head circumference of 33 cm (11th centile). At birth, the previously identified clubfeet and clenched hands were present along with a large anterior fontanelle, hypertelorism, bilateral glaucoma, short and upturned nose with a flat nasal bridge, anteverted nares, and a short neck with redundant tissue (Fig. 2 A–C). His upper extremities had decreased flexion creases on interphalangeal joints, camptodactyly, arachnodactyly, ulnar deviation of fingers with the left 2nd and 3rd fingers overlapping the 4th and 5th (Fig. 2D and E). His lower extremities showed bilateral sandal gaps (Fig. 2F). He also had significant hypotonia and lymphedema.
Fig. 2.
A–C: Face at 1 day old (A), 2 months (B) and 9 months (C): Prominent forehead, prominent eyes with right greater than left, blue sclerae with corneal clouding, flat nasal bridge with short nose, small mouth, short neck. Feeding tube present at 2 and 9 months and nasal cannula present at 9 months. D: Left hand at 2 months: overlapping digits. E: Right hand at 2 months: Camptodactyly with absent distal flexion creases, broad tips of digits. F: Right foot at 6 months: Sandal gap between 1st and 2nd toes.
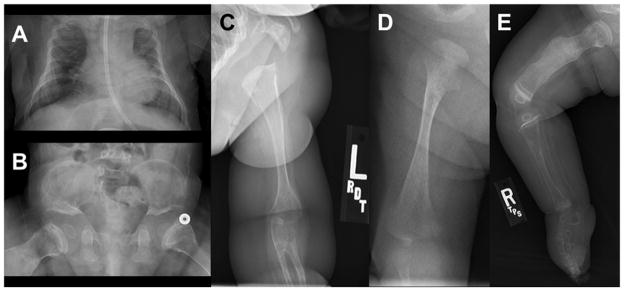
A skeletal survey revealed diffuse demineralization with bowed humeri, bilateral radio-ulnar synostosis, metaphyseal widening, femur fractures, and clubfeet; no joint dislocations were noted. The survey also showed a large anterior fontanelle, 11 pairs of ribs and a small thorax (Fig. 3A, 3C and 3D). The small thorax resulted in restrictive lung disease that required significant respiratory support with upper respiratory infections. At 3 months of age, our patient began to require supplemental oxygen that ultimately resulted in a 2 month intubation. He was slowly weaned to minimal respiratory support at night when he developed rhinovirus at 10 months of age. This resulted in another intubation and slow respiratory wean. During the first year of life, the patient has had approximately 25 fractures in all four extremities and vertebrae (Fig. 3E); some fractures were incurred during routine blood pressure monitoring. Pamidronate administration began around six months of age. After two months, zones of calcification with sclerotic metaphyseal bands were evident, consistent with bisphosphonate therapy; however, our patient continued to have fractures after six months of therapy. By 12 months of age, plain films of the patient’s abdomen and pelvis showed short ilia with flat acetabular angles (Fig. 3B).
Fig. 3.
A: Small chest with nasogastric tube at 3 months of age. B: Small ilia with flat acetabular angles. C: Radioulnar synostosis of left arm. D: Right femur at 3 weeks of age with fracture and metaphyseal flaring. E: Right lower extremity at 8 months of age with healing fractures. All five radiographs show bone demineralization.
Postnatal echocardiogram revealed that the patient did not have a hypoplastic left heart but rather a large atrial septal defect, ventricular septal defect and a patent ductus arteriosus. At about 3 months of age, brainstem auditory evoked response evaluation showed moderate conductive hearing loss bilaterally. At 6 months of age, trabeculotomy for the patient’s bilateral glaucoma was attempted but discontinued because of uveal prolapse due to marked scleral thinning. A brain MRI at 2 days of age revealed multiple foci of infarction in the left cerebral hemisphere and bilateral supra- and infratentorial subdural hematomas overlying the bilateral parietooccipital lobes and bilateral cerebellar hemispheres. A subsequent thrombophilia work up was negative. At 12 months of age, a chest x-ray revealed a Morgagni type of diaphragmatic hernia. Development at 9 months of age showed that our patient was beginning to bat objects and have some brief consonant babbling, but he was unable to roll over or sit independently.
GENETIC TESTING
The genetic evaluation began with analysis of very long chain fatty acids given the large fontanelle, hypotonia and flat nasal bridge; however, this test was negative. Other biochemical testing included a sterol panel and transferrin and apolipoprotein CIII isoform analysis for disorders of glycosylation and these tests were also negative. SNP microarray revealed a 121–201 kb deletion of PARK2 that was not thought to contribute to the patient’s phenotype. In addition, multiple long stretches of homozygosity of at least 3 Mb were noted, which comprised approximately 265 Mb or 13% of the autosomal genome. Because of the significant degree of homozygosity, the possibility of at least one autosomal recessive condition was considered. All OMIM genes with an autosomal recessive phenotype were identified using the Santa Cruz Genome Browser (http://www.genome.ucsc.edu). Consequently, a number of interesting candidate genes were considered, initially focusing on the genes with associated with multiple fractures and radio-ulnar synostosis. WNT1 (multiple fractures and hypotonia), B4GALT7 (glaucoma and radio-ulnar synostosis in progeroid Ehlers-Danlos syndrome), B3GALT6 (multiple fractures and spatulate terminal phalanges), and IFITM5 (multiple fractures with marked density at the epiphyseal-metaphyseal boundaries) sequences were all normal. Sequence of B3GAT3 revealed a novel homozygous missense mutation p.Gly223Ser, which was located within a 10.55Mb region of homozygosity. Because our patient’s phenotype was more severe than reported in the literature, there was concern that the B3GAT3 mutation may not have explained all of his features and that our patient may have an additional autosomal recessive syndrome. Consequently, clinical exome sequence analysis identified the same B3GAT3 mutation but did not reveal any additional causative mutations. Functional studies could not be performed because the family declined any further genetic analyses.
DISCUSSION
B3GAT3 encodes the 335 amino acid glucuronosyltransferase I protein that catalyzes the addition of glucuronic acid, the final sugar moiety in the tetrasaccharide linker region [Kitagawa et al., 1998]. A p.Arg277Gln mutation has been previously reported in two consanguineous families, both from the United Arab Emirates. The first report described five affected siblings [Baasanjav et al., 2011] while the second report described a single affected child [von Oettingen et al., 2014]. Baasanjav et al. performed functional studies on two siblings with the homozygous p.Arg277Gln mutations and showed that the glucuronosyltransferase I activity was decreased to 3–5% of age-matched control levels. As hypothesized, they also showed that mutation resulted in a partial deficiency of all three O-glycanated proteoglycans (dermatan sulfate, chondroitin sulfate and heparan sulfate) [2011].
The homozygous p.Gly223Ser mutation has not been previously reported in humans. Review of the ExAC browser (http://exac.broadinstitute.org) revealed that this mutation was present in 1 out of 119,290 alleles with a frequency of 8.383e−6. In contrast, the p.Arg277Gln mutation was present in 3 out of 120,974 alleles with a frequency of 2.48e−5. There were no homozygotes for either of these mutations. PolyPhen2 [Adzhubei et al., 2010] analysis of the p.Gly223Ser was predicted to be “probably damaging” with a score of 0.935 while the p.Arg277Gln mutation was predicted to be “possibly damaging” with a score of 0.763. The GERP score for the p.Gly223Ser mutation was 3.78 while the p.Arg277Gln mutation was 5.31. Additionally, Gulberti and colleagues] found that substituting an alanine residue for either Gly222 or Gly223 markedly impaired the glucuronosyltransferase 1 activity of recombinant human glucuronosyltransferase 1 in yeast cells as part of a study investigating the specificity of glycosyltransferases [2005]. These analyses bolster the conclusion that both the p.Gly223Ser and the p.Arg277Gln mutations are likely pathogenic.
Our patient has many features that are also present in the 6 previously reported patients with B3GAT3 mutations (Table 1), including short stature, prominent forehead and eyes, broad fingers and toes, and cardiovascular abnormalities [Baasanjav et al., 2011; von Oettingen et al., 2014; this study]. He has the additional features of blue sclerae, glaucoma, arachnodactyly, hearing loss, multiple fractures, a diaphragmatic hernia and a small thorax. None of these features have been previously reported in an individual with a B3GAT3 mutation. Even at 12 months of age, it is clear that our patient has a more severe phenotype than reported previously and it is expected that he will have a significantly different clinical course. Consequently, we believe this represents a different syndrome with its own natural history and prognosis.
Table 1.
Comparison of Clinical Features in Patients with Linkeropathies
| Feature | XYLT1 (n=9)<br1>Bui et al. [2014], Schreml et al. [2014] | B4GALT7 (n=26)<br1>Kresse et al. [1987], Faiyaz-Ul-Haque [2004], Guo et al. [2013], Cartault et al. [2015] | B3GALT6 (n=16)<br1>Malfait et al. [2013], Nakajima et al. [2013] | B3GAT3 (n=6)<br1>Baasanjav et al. [2011], von Oettingen et al. [2014] | Our patient |
|---|---|---|---|---|---|
| Skeletal Abnormalities | |||||
| Short stature | 9/9 (range: −5.5 to −9 SD) | 23/23 (range: −3.5 to −8.8 SD) | 13/15 (range: −1.6 to −10.7 SD) | 6/6 (<3rd %; −3.43 SD) | Yes (−2.13 SD at 9 months) |
| Joint laxity | 6/6 | 11/24 | 10/13 | 6/6 | No |
| Joint dislocations | 6/8 | 23/23 | 8/12 | 6/6 | No |
| Advanced bone age/carpal ossification | 5/5 | 12/16 | 5/10 | 0/1 | Unknown |
| Decreased bone density | NR | 2/3 | 2/2 | Yes but not specified | Yes |
| Multiple fractures | NR | 0/2 | 3/4 | NR | Yes |
| Joint contractures | NR | NR | 5/14 | 5/5 | Yes |
| Pectus abnormality | 3/3 | 7/22 | 4/4 | 1/1 | No |
| Kyphosis and/or scoliosis | NR | 6/22 | 14/14 | 0/1 | No |
| Radioulnar synostosis | 0/2 | 13/24 | NR | 0/1 | Yes |
| Restricted elbow movement | NR | 4/4 | 9/11 | 1/1 | Yes |
| Broad tips of fingers and/or toesa | 2/2 | 4/24 | 11/15 | 6/6 | Yes |
| Long fingers and/or toes | NR | 4/4 | NR | NR | Yes |
| Iliac abnormalitiesb | 1/1 | NR | 14/14 | 1/1 | Yes |
| Monkey wrench or Swedish key appearance of femora | 6/6* | 9/19 | NR | NR | No |
| Metaphyseal abnormalitiesc | NR | 3/3 | 12/13 | 1/1 | Yes |
| Foot abnormalitiesd | 4/4 | 3/3 | 8/16 | 6/6 | Yes |
| Sandal gap between toes | 1/1* | 1/1* | 1/1* | 1/1 | Yes |
| Craniofacial Features | |||||
| Prominent forehead | NR | 26/26 | 9/10 | 6/6 | Yes |
| Prominent/proptotic eyes | 2/2 | 24/24 | 8/11 | 6/6 | Yes |
| Blue sclerae | 3/3 | NR | 4/4 | NR | Yes |
| Hypertelorism | NR | 22/22 | NR | NR | No |
| Downslanting palpebral fissures | NR | 1/1 | 2/2 | 3/5 | No |
| Glaucoma/increased intraocular pressure | 0/2 | 5/21 | 1/1 | NR | Yes |
| Depressed nasal bridge | 1/1 | NR | 1/1 | 5/6 | Yes |
| Microstomia | NR | 25/25 | NR | 4/6 | Yes |
| Short and/or webbed neck | 1/1 | NR | NR | 6/6 | Yes |
| Dermatologic Abnormalities | |||||
| Hyperextensible skin | NR | 24/25 | 8/13 | 0/1 | No |
| Atrophic scars | NR | 2/2 | 2/2 | 0/1 | Not currently |
| Other findings | |||||
| Developmental delay, learning difficulties, or cognitive deficits | 8/9 | 16/26 | 6/6 | 1/6 | Yes |
| Hypotonia | 3/3 | 4/4 | 9/16 | 0/1 | Yes |
| Cardiovascular abnormalities | 0/2 | 0/1 | 0/2 | 6/6 | Yes |
“NR” indicates feature was not reported and *indicates feature was identified from picture.
Includes bifid thumbs, prominent digital pads.
Comprises of short ilia, flattened iliac wings or hypoplastic iliac bodies
Includes widening, flaring or other unspecified abnormalities
Includes clubfeet, broad feet, pes planus, talipes equinovarus, metatarsus varus
While our patient has features that have not been previously reported in patients with B3GAT3 mutations, some of these features have been reported in other types of linkeropathies (Table 1). For example, multiple fractures and blue sclerae have both been reported in patients with B3GALT6 mutations [Malfait et al., 2013]. Blue sclerae and small chests have also been reported in patients with XYLT1 mutations [Bui et al., 2014]. While hypotonia has not been previously reported in individuals with B3GAT3 mutations, it has been reported in all of the other linkeropathies. These similarities suggest a phenotypic overlap in individuals who have a deficiency in the biosynthesis of the common linker region [Kresse et al., 1987; Faiyaz-Ul-Haque et al., 2004; Guo et al., 2013; Malfait et al., 2013; Nakajima et al., 2013; Bui et al., 2014].
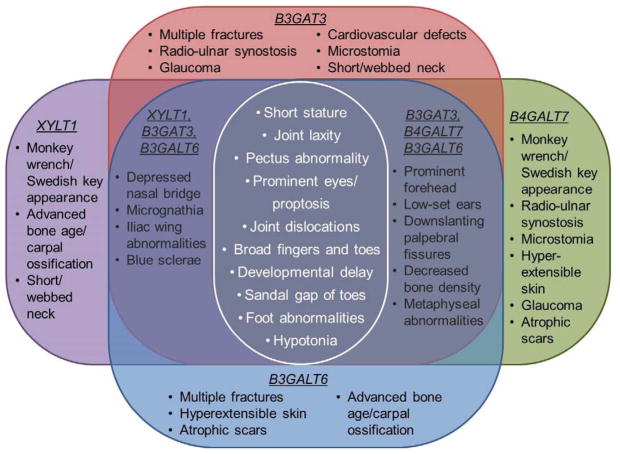
This conclusion is further supported by the presence of phenotypic features that are shared among all of the linkeropathies (Fig. 4). These features include short stature, prominent foreheads and eyes, pectus abnormalities, joint laxity and dislocations, broad tips of digits, foot abnormalities, hypotonia, and developmental delays [Kresse et al., 1987; Faiyaz-Ul-Haque et al., 2004; Guo et al., 2013; Cartault et al., 2015; Malfait et al., 2013; Nakajima et al., 2013; Schreml et al., 2014; Bui et al., 2014; Baasanjav et al., 2011; von Oettingen et al., 2014]. Other features are present in some but not all of the disorders, such as cardiac defects, radio-ulnar synostosis, monkey wrench or Swedish key appearance of the femora, and atrophic scars. This phenotypic disparity may be due to varying levels of gene expression in different tissues as suggested by Nakajima [2013]. Another consideration may be that the linkeropathies have not been fully characterized. Several previous reports have focused on a particular aspect of the phenotype, such as the skeletal features, and may not have described other systemic features that were present.
Fig. 4.
Diagram of the features shared by all of the linkeropathies along with other common features shared by several of the linkeropathies.
In summary, this report describes a new syndrome caused by a homozygous c.667G>A mutation in B3GAT3, resulting in the p.Gly223Ser change in glucuronosyltransferase I. This expands the number of syndromes caused by homozygous mutations in B3GAT3. Based on our experience with this patient, we suggest considering B3GAT3 mutations in a patient with multiple fractures, prominent forehead and eyes, hypotonia and developmental delays and who has had a negative evaluation for osteogenesis imperfecta. Furthermore, a clinician should consider the linkeropathies when a patient presents with short stature, prominent foreheads and eyes, joint laxity and dislocations, iliac and metaphyseal abnormalities, broad tips to digits, foot abnormalities and developmental delays. As additional clinical descriptions of these disorders are reported, further insight will help characterize the linkeropathies individually and as a group of disorders.
Acknowledgments
The authors would like to thank the patient and family for their participation. We also would like to thank Anita E. Beck, MD, PhD for her thoughtful and helpful discussions.
References
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baasanjav S, Al-Gazali L, Hashiguchi T, Mizumoto S, Fischer B, Horn D, Seelow D, Ali BR, Aziz SA, Langer R, Saleh AA, Becker C, Nürnberg G, Cantagrel V, Gleeson JG, Gomez D, Michel J-B, Stricker S, Lindner TH, Nürnberg P, Sugahara K, Mundlos S, Hoffmann K. Faulty initiation of proteoglycan synthesis causes cardiac and joint defects. Am J Hum Genet. 2011;89:15–27. doi: 10.1016/j.ajhg.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M, Götte M, Park P, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparin sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- Bui C, Huber C, Tuysuz B, Yasemin A, Bole-Feysot C, Leroy JG, Mortier G, Nitschke P, Munnich A, Cormier-Daire V. XYLT1 mutations in Desbuquois dysplasia type 2. Am J Hum Genet. 2014;94:205–414. doi: 10.1016/j.ajhg.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartault F, Munier P, Jacquemont M-L, Vellayoudom J, Doray B, Payet C, Randiranaivo H, Laville J-M, Munnich A, Cormier-Daire V. Expanding the clinical spectrum of B4GALT7 deficiency: homozygous p.R270C mutation with founder effect causes Larsen of Reunion Island syndrome. Eur J Hum Genet. 2015;23:49–53. doi: 10.1038/ejhg.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiyaz-Ul-Haque M, Zaidi SHE, Al-Ali M, Al-Mureikhi MS, Kennedy S, Al-Thani G, Tsui L-C, Teebi AS. A novel missense mutation in the galactosyltransferase-I (B4GALT7) gene in a family exhibiting facioskeletal anomalies and Ehlers-Danlos syndrome resembling the progeroid type. Am J Med Genet. 2004;128A:39–45. doi: 10.1002/ajmg.a.30005. [DOI] [PubMed] [Google Scholar]
- Freeze HH. Genetic defects in the human glycome. Nature Rev. 2006;7:537–551. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- Guo MH, Stoler J, Lui J, Nilsson O, Bianchi DW, Hirschhorn JN, Dauber A. Redefining the progeroid form of Ehlers-Danlos syndrome: Report of the fourth patient with B4GALT7 deficiency and review of the literature. Am J Med Genet A. 2013;161A:2519–2527. doi: 10.1002/ajmg.a.36128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulberti S, Lattard V, Fondeur M, Jacquinet J-C, Mulliert G, Netter P, Magdalou J, Ouzzine M, Fournel-Gigleux Phosphorylation and sulfation of oligosaccharide substrates critically influence the activity of human β1,4-galactosyltransferase 7 (GalT-I) and β1,3-glucuronosyltransferase I (GlcAT-I) involved in the biosynthesis of the glycosaminoglycan-protein linkage region of proteoglycans. J Biol Chem. 2005;280:1417–1425. doi: 10.1074/jbc.M411552200. [DOI] [PubMed] [Google Scholar]
- Häcker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: The sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annu Rev Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Tone Y, Tamura J-I, Neumann KW, Ogawa T, Oka S, Kawasaki T, Shugahara K. Molecular cloning and expression of glucuronyltransferase I involved in the biosynthesis of the glycosaminoglycan-protein linkage region of proteoglycans. J Biol Chem. 1998;273:6615–6618. doi: 10.1074/jbc.273.12.6615. [DOI] [PubMed] [Google Scholar]
- Kresse H, Rosthøj S, Quentin E, Hollmann J, Glössl J, Okada S, Tønnesen T. Glycosaminoglycan-free small proteoglycan core protein is secreted by fibroblasts from a patient with a syndrome resembling progeroid. Am J Hum Genet. 1987;41:436–453. [PMC free article] [PubMed] [Google Scholar]
- Malfait F, Kariminejad A, Van Damme T, Gauche C, Syx D, Merhi-Soussi F, Gulberti S, Symoens S, Vanhauwaert S, Willaert A, Bozorgmehr B, Kariminejad MH, Ebrahimiadib N, Hausser I, Huysseune A, Fournel-Gigleux S, De Paepe A. Defective initiation of glycosaminoglycan synthesis due to B3GALT6 mutations causes a pleiotropic Ehlers-Danlos syndrome-like connective tissue disorder. Am J Hum Genet. 2013;92:935–945. doi: 10.1016/j.ajhg.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Mizumoto S, Miyake N, Kogawa R, Iida A, Ito H, Kitoh H, Hirayama A, Mitsubuchi H, Miyazaki O, Kosaki R, Horikawa R, Lai A, Mendoza-Londono R, Dupuis L, Chitayat D, Howard A, Leal GF, Cavalcanti D, Tsurusaki Y, Saitsu H, Watanabe S, Lausch E, Unger S, Bonafé L, Ohashi H, Superti-Furga A, Matsumoto N, Sugahara K, Nishimura G, Ikegawa S. Mutations in B3GALT6, which encodes a glycosaminoglycan linker region enzyme, cause a spectrum of skeletal and connective tissue disorders. Am J Hum Genet. 2013;92:927–934. doi: 10.1016/j.ajhg.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pönighaus C, Ambrosius M, Casanova JC, Prante C, Kuhn J, Esko JD, Kleesiek K, Götting C. Human xylosyltransferase II is involved in the biosynthesis of the uniform tetrasaccharide linkage region in chondroitin sulfate and heparan sulfate proteoglycans. J Biol Chem. 2007;282:5201–5206. doi: 10.1074/jbc.M611665200. [DOI] [PubMed] [Google Scholar]
- Schreml J, Durmaz B, Cogulu O, Keupp K, Beleggia F, Pohl E, Milz E, Coker M, Ucar SK, Nürnberg G, Nürnberg P, Kuhn J, Ozkinay The missing “link”: an autosomal recessive short stature syndrome caused by a hypofunctional XYLT1 mutation. 2014 doi: 10.1007/s00439-013-1351-y. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Mikami T, Uyama T, Mizuguchi S, Nomura K, Kitagawa H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr Opin Struct Biol. 2003;13:612–620. doi: 10.1016/j.sbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Von Oettingen JE, Tan W-H, Dauber A. Skeletal dysplasia, global developmental delay and multiple congenital anomalies in a 5-year-old boy – Report of the second family with B3GAT3 mutation and expansion of the phenotype. Am J Med Genet A. 2014;164A:1580–1586. doi: 10.1002/ajmg.a.36487. [DOI] [PMC free article] [PubMed] [Google Scholar]