Abstract
Background and Purpose
Cardiac abnormalities occur commonly after subarachnoid hemorrhage (SAH) and may be caused by excessive release of catecholamines from the myocardial sympathetic nerves. We hypothesized that adrenoceptor polymorphisms resulting in greater catecholamine sensitivity would be associated with an increased risk of cardiac injury.
Methods
This was a prospective cohort study. The primary outcome variables were the serum level of cardiac troponin I (cTi, abnormal if >1.0 µg/L) and the left ventricular ejection fraction (LVEF, abnormal if <50%). Six adrenoceptor polymorphisms were genotyped: β1AR Arg389Gly, β1AR Ser49Gly, β2AR Gly16Arg, β2AR Gln27Glu, β2AR Thr164Ile, and α2AR del322–325. The effect of each polymorphism on the risk of developing cardiac abnormalities was quantified using multivariable logistic regression.
Results
The study included 182 patients. The CC genotype (Arg/Arg) of β1AR Arg389Gly (odds ratio [OR] 3.4, P=0.030) and the CC genotype (Gln/Gln) of β2AR Gln27Glu (OR 3.1, P=0.032) were predictive of cTi release. The presence of the α2AR deletion was predictive of reduced LVEF (OR 4.2, P=0.023). The combination of the β1AR 389 CC and the β2AR 27 CC genotypes resulted in a marked increase in the odds of cTi release (OR 15.5, P=0.012). The combination of the β1AR 389 CC and the α2AR deletion genotypes resulted in a marked increase in the odds of developing a reduced LVEF (OR 10.3, P=0.033).
Conclusions
Genetic polymorphisms of the adrenoceptors are associated with an increased risk of cardiac abnormalities after SAH. These data support the hypothesis that cardiac dysfunction after SAH is a form of neurocardiogenic injury.
Keywords: cerebral hemorrhage, congestive heart failure, echocardiography
Cardiac abnormalities such as myocardial necrosis1,2 and left ventricular (LV) systolic dysfunction3,4 are known to occur after subarachnoid hemorrhage (SAH) and are associated with adverse neurological outcomes.5,6 The cause of cardiac dysfunction after acute brain injury remains controversial, though experimental data indicate that catecholamine toxicity is a key mechanism.7,8
It is unknown why only a subgroup of SAH patients develop cardiac abnormalities. Genetic polymorphisms of the adrenoceptors have been shown to affect cardiac responsiveness to catecholamines9–11 and are associated with adverse cardiovascular outcomes.12,13 The hypothesis of this study is that specific adrenoceptor polymorphism genotypes are associated with a higher prevalence of cardiac injury and dysfunction after SAH.
Materials and Methods
Subjects
This is a prospective genetics substudy from a cohort of 300 patients. The inclusion criteria for the parent cohort study were age over 21 years and a confirmed diagnosis of SAH by computed tomography of the head or lumbar puncture. Patients were excluded if they had a history of prior myocardial infarction or congestive heart failure. After the first 100 patients were enrolled into the parent study, additional approval was obtained from the institutional review board (IRB) to bank DNA. Therefore, consent for DNA banking was an additional inclusion criterion for the genetics substudy. Consent was obtained from each patient or an appropriate surrogate. The patients were enrolled as soon as possible after admission and demographic and clinical data were collected.
Measurement of Cardiac Troponin I and Echocardiographic Parameters
On day 1, 3 and 6 after enrollment, serum specimens were collected for measurement of cardiac troponin I (cTi; fluorescent enzyme immunoassay, Abbot Diagnostics). The lowest detectable level was 0.3 µg/L. In addition, echocardiography was performed on each subject on each of the 3 study days, using an Acuson Sequoia 6.0 ultrasound system. All echocardiographic analyses were performed off-line (ProSolv) by a blinded observer. Left ventricular ejection fraction (LVEF) was measured and regional wall motion abnormalities (RWMA) were defined using standard methods.14
Polymorphism Selection and Genotyping
Six adrenoceptor polymorphisms previously reported to be functionally significant were chosen for analysis (Table 1). Genomic DNA was extracted from peripheral blood lymphocytes using a salt modification method (Gentra Systems). Polymorphisms were genotyped by template-directed dye-terminator incorporation assay with fluorescence polarization detection (FP-TDI),15 using the AcycloPrime-FP kit (Perkin-Elmer). We used published polymerase chain reaction primer sequences for β1AR1165C>G,16 β1AR145A>G,17 and α2AR del322–32512 and designed primers for other assays. Polymerase chain reaction conditions and primer sequences are shown in supplemental Table, available online at http://stroke.ahajournals.org. Genotyping was performed by investigators blinded to clinical status.
TABLE 1.
Functional Rationale for Polymorphism Selection
| Gene | Variant | dbSNP ID | Functional Rationale for Polymorphism |
Clinical Rationale for Polymorphism | References |
|---|---|---|---|---|---|
| β1AR (ADRB1) | 1165C>G, Arg389Gly | rs1801253 | • C allele: ↑ response to β agonist stimulation | • CC genotype associated with ↑ risk of AMI | 9,12,23 |
| • CC genotype associated with ↑ risk of CHF (in association with α2CAR deletion) | |||||
| 145A>G, Ser49Gly | rs1801252 | • G allele: ↓ response to β agonist stimulation | • GG or AG genotype associated with improved survival in CHF | 16,24 | |
| β2AR (ADRB2) | 491C>T, Thr164Ile | rs1800888 | • T allele: ↓ response to β agonist stimulation | • CT genotype associated with worsened survival in CHF | 25,26 |
| 46G>A, Gly16Arg | rs1042713 | • G allele: ↑ agonist-promoted downregulation | • GG genotype associated with ↑ left ventricular ejection fraction | 13,27,28 | |
| 79C>G, Gln27Glu | rs1042714 | • G allele: absent agonist-promoted downregulation | • CC genotype associated with ↑ risk of CAD events | 10,13,27,28 | |
| α2CAR (ADRA2C) | 964del12bp del322–325 | … | • Deletion: ↑ presynaptic release of norepinephrine | • OO (homozygous deletion) genotype associated with ↑ risk of CHF | 12,18 |
CHF indicates congestive heart failure; CAD, coronary artery disease; AMI, acute myocardial infarction.
Statistical Analysis
The primary outcome variables in this study were cTi and LVEF. Because of a skewed distribution, cTi was treated as a dichotomous variable and a value >1.0 µg/L on any of the 3 study days was considered abnormal because this level of myocardial necrosis was considered significant for this assay. LVEF was treated as a dichotomous variable and was considered abnormal if <50% on any study day. The secondary outcome variables were the presence of RWMA on any study day and inpatient mortality.
Each polymorphism was tested for adherence to Hardy-Weinberg equilibrium (HWE) by χ2 (P<0.05), for the cohort and within each racial group. The prevalence of each outcome was quantified according to the genotype of each polymorphism. β-adrenoceptor single-nucleotide polymorphisms (SNPs) were coded as dichotomous predictor variables (most frequent homozygous genotype versus other). Because the α2AR 322 to 325 deletion results in enhanced presynaptic release of norepinephrine, this variable was coded as any deletion (homozygous OO or heterozygous XO) versus no deletion (XX). Multivariable regression models were created to quantify the effect of each polymorphism on the outcomes. The cocovariates in these models were age, gender, and race/ethnicity. Multivariable odds ratios (OR) based on average effects and probability values were reported.
For each model, we tested for significant (P<0.05) interactions among the predictor variables. If there was inadequate sample size to quantify interactions with race, it was dichotomized to white versus nonwhite. In cases where individual polymorphisms were significantly associated with the cardiac outcomes, additional models were developed to measure the effect of the polymorphism in the largest racial group(s).
Finally, the combined effects of adrenoceptor polymorphisms were measured. All genotypes shown to be individually predictive of cTi release or a reduced LVEF were included in these analyses. Using the approach described above, multivariable regression models were created to quantify the combined effect of at-risk genotypes on cTi release and a reduced LVEF.
Results
Subjects
A total of 182 out of 200 eligible patients provided consent for the genetics substudy and were enrolled an average of 4.2±3.6 days after SAH symptom onset. The clinical characteristics of the cohort are shown in Table 2. There were no statistically significant differences in these characteristics between the study cohort and the 122 subjects who were not genotyped, except for a lower rate of stimulant drug use (8% versus 17%, χ2 P=0.03). Sixteen subjects (9%) died during the hospitalization.
TABLE 2.
Characteristics and Genotype Frequency for the Study Population
| Patients, n | 182 |
| Age, mean±SD | 56±13 |
| Female, n (%) | 130 (71) |
| Race/Ethnicity, n (%) | |
| White | 116 (64) |
| Latino | 28 (15) |
| Asian | 22 (12) |
| Black | 14 (8) |
| Pacific Islander | 2 (1) |
| Admission Hunt & Hess grade,29 n (%) | |
| I | 72 (40) |
| II | 24 (13) |
| III | 52 (29) |
| IV | 26 (15) |
| V | 5 (3) |
| Risk factors for coronary artery disease, n (%) | |
| History of hypertension | 76 (42) |
| History of diabetes | 9 (5) |
| History of hyperlipidemia | 20 (11) |
| History of smoking | 71 (39) |
| Family history of coronary artery disease | 17 (9) |
| History of amphetamine/cocaine use, n (%) | 14 (8) |
| History of coronary artery disease, n (%) | 9 (5) |
| β1AR1165C>G (Arg389Gly) genotype, n (%) | |
| CC | 95 (57) |
| CG | 65 (39) |
| GG | 7 (4) |
| β1AR145A>G (Ser49Gly) genotype, n (%) | |
| AA | 125 (79) |
| AG | 32 (20) |
| GG | 2 (1) |
| β2AR46G>A (Gly16Arg) genotype, n (%) | |
| GG | 64 (39) |
| AG | 70 (42) |
| AA | 31 (19) |
| β2AR79C>G (Gln27Glu) genotype, n (%) | |
| CC | 79 (46) |
| CG | 68 (40) |
| GG | 24 (14) |
| α2AR del322–325 (X=insertion, O=deletion), n (%) | |
| XX | 135 (83) |
| XO | 21 (13) |
| OO | 6 (4) |
Cardiac Outcomes
A total of 24 patients (13%) had a peak cTi level >1.0 µg/L. A total of 21 subjects (12%) had a LVEF <50%. Forty-six patients (25%) had RWMA. A total of 11 (52%) of the patients with a LVEF <50% and 16 (35%) of the patients with RWMA also had a cTi >1.0 µg/L. In addition, pulmonary infiltrates were observed on chest x-ray films in 14% of the subjects.
Adrenoceptor Genotypes
Genotypes for the 6 adrenoceptor polymorphisms are shown in Table 2. The β2AR 491C>T (Thr164Ile) SNP was very rare (only 1 minor allele carrier) and was not included in the analysis. For the cohort as a whole, genotype frequencies for all polymorphisms were consistent with HWE, except for the α2AR deletion (P<0.001). This finding was expected given the different frequency of the α2AR deletion (O) in different ethnic groups (4% in whites versus 22% in nonwhites in this cohort). Within specific race/ethnic groups, all polymorphisms were in HWE with 2 exceptions. Specifically, β2AR 79C>G was not in HWE in the Latino subgroup (P=0.010) and the α2AR deletion was not in HWE in the Asian subgroup (P=0.019).
Adrenoceptor Genotypes and Outcomes
As shown in Table 3, there were significant associations between specific andrenoceptor polymorphisms and cardiac outcomes. The CC genotype of β1AR 1165C>G was significantly associated with cTi release and RWMA, and there was a borderline association with a LVEF <50%. The CC genotype of β2AR 79C>G was associated with cTi release and the α2AR deletion was associated with a LVEF <50%. The associations between the CC genotypes of β1AR 1165 and β2AR 79 and cTi release were not statistically significant if a cutpoint of >0.3 µg/L (indicating any cTi release) was used for the analysis. There were no significant associations between any of the adrenoceptor polymorphisms and inpatient mortality.
TABLE 3.
Association of Adrenoceptor SNP Genotypes With Cardiac Injury and Dysfunction
| β1AR 1165C>G (Arg389Gly) Genotype | ||||||
| Cardiac Abnormality | CC n=95 |
CG n=65 |
GG n=7 |
OR CC vs Any G |
95% CI | P Value |
| cTi >1 µg/L | 16 (17%) | 4 (6%) | 0 | 3.9 | 1.1–13.6 | 0.030 |
| LVEFM<50% | 11 (12%) | 4 (6%) | 0 | 4.8 | 1.0–23.8 | 0.052 |
| RWMA | 25 (26%) | 12 (18%) | 1 (14%) | 2.5 | 1.0–6.2 | 0.040 |
| β1AR 145A>G (Ser49Gly) Genotype | ||||||
| AA n=125 |
AG n=32 |
GG n=2 |
OR AA vs Any G |
|||
| cTi >1 µg/L | 17 (14%) | 2 (6%) | 0 | 2.2 | 0.5–10.4 | 0.32 |
| LVEF<50% | 12 (10%) | 3 (10%) | 0 | 1.4 | 0.3–5.7 | 0.65 |
| RWMA | 31 (25%) | 6 (19%) | 1 (50%) | 1.6 | 0.6–4.3 | 0.37 |
| β2AR 46G>A (Gly16Arg) Genotype | ||||||
| GG n=64 |
AG n=70 |
AA n=31 |
OR GG vs Any A |
|||
| cTi >1 µg/L | 8 (13%) | 7 (10%) | 5 (16%) | 1.2 | 0.4–3.3 | 0.70 |
| LVEF<50% | 7 (11%) | 5 (7%) | 4 (13%) | 1.2 | 0.4–3.5 | 0.73 |
| RWMA | 15 (23%) | 15 (21%) | 7 (23%) | 1.1 | 0.5–2.5 | 0.73 |
| β2AR 79C>G (Gln27Glu) Genotype | ||||||
| CC n=79 |
CG n=68 |
GG n=24 |
OR CC vs Any G |
|||
| cTi >1 µg/L | 14 (18%) | 5 (7%) | 2 (8%) | 3.1 | 1.1–8.6 | 0.032 |
| LVEF<50% | 10 (13%) | 4 (6%) | 3 (13%) | 1.4 | 0.5–4.4 | 0.54 |
| RWMA | 23 (29%) | 10 (15%) | 7 (29%) | 1.5 | 0.7–3.3 | 0.30 |
| α2AR del322–325 | ||||||
| XX n=135 |
XO n=21 |
OO n=6 |
OR Any O vs XX |
|||
| cTi >1 µg/L | 16 (12%) | 3 (14%) | 1 (17%) | 2.0 | 0.5–7.7 | 0.33 |
| LVEF<50% | 10 (8%) | 6 (29%) | 1 (17%) | 4.2 | 1.2–14.5 | 0.023 |
| RWMA | 32 (24%) | 7 (33%) | 1 (17%) | 1.1 | 0.4–3.1 | 0.91 |
The largest numbers of subjects with CC genotypes of the β1AR 1165C>G and β2AR 79C>G SNPs were of white race. Restricting the multivariable analysis of the CC genotype of β1AR 1165C>G to the white group did not have a large effect on the ORs, though statistical significance was reduced in some cases (OR 5.5 and P=0.03 for cTi >1.0 µg/L; OR 5.0 and P=0.15 for LVEF <50%; OR 2.7 and P=0.08 for RWMA). Restricting the analysis of the CC genotype of β2AR 79C>G to whites resulted in an OR of 2.2 for cTi >1.0 µg/L, P=0.17. Excluding only the Latino group, which was not in HWE, from this analysis resulted in an OR of 2.9, P=0.044.
The α2AR deletion (≥1 copy) was more prevalent in blacks and Asians (42%) in comparison with the other subjects (10%, χ2 P<0.001). Restricting the multivariable analysis of the α2AR deletion to the black and Asian groups did not significantly affect the OR for LVEF <50% (OR 5.0, P=0.21). Excluding only the Asian group, which was not in HWE, from this analysis resulted in an OR of 3.7, P=0.046.
There were no significant interactions in the models predictive of cardiac outcomes, though 5 interaction terms could not be modeled because of limited sample size.
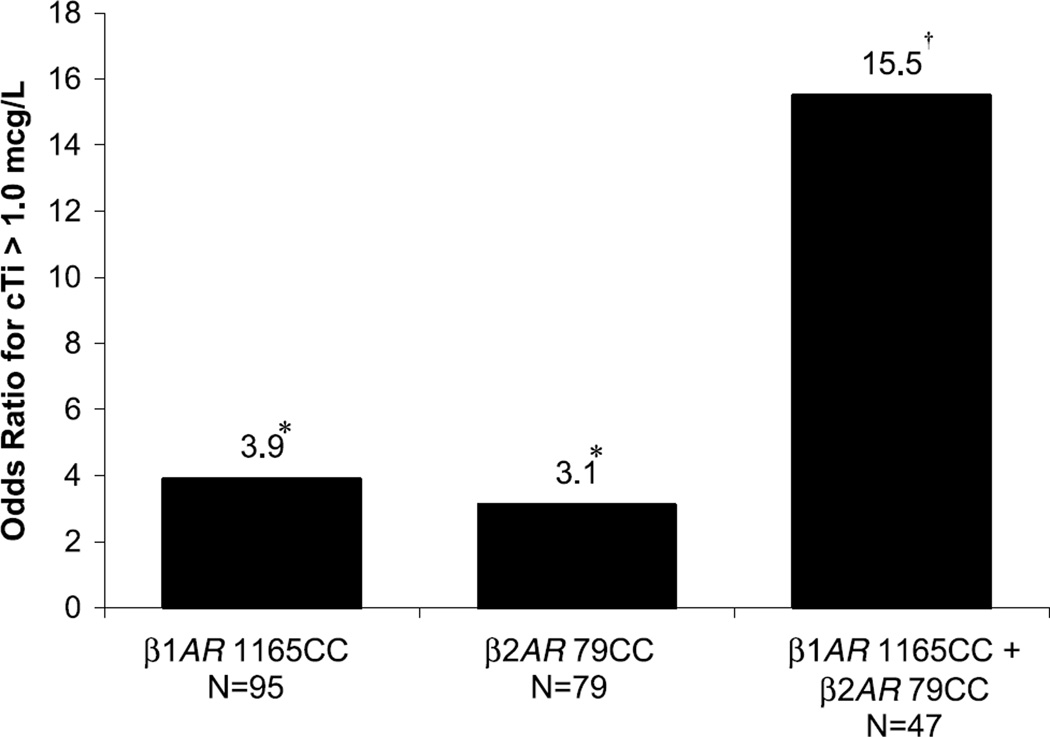
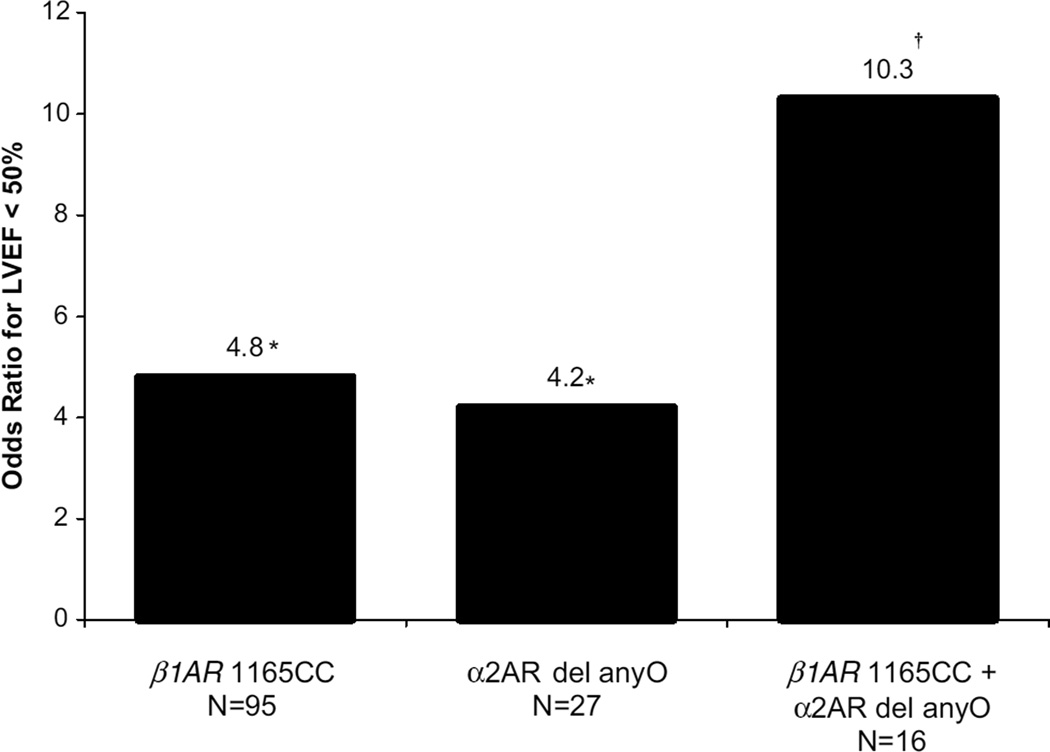
The individual and combined effects of the CC genotypes of β1AR 1165C>G and β2AR 79C>G on the odds of cTi release are shown in Figure 1. Thirty percent of the subjects had both CC genotypes and 23% of these individuals had cTi release, versus 2% in the subjects with neither at-risk genotype. After adjustment for covariates, patients with both at-risk genotypes had an OR of 15.5 for a cTi level >1.0 µg/L in comparison to subjects with neither at-risk genotype. As shown in Figure 2, a similar effect on LVEF was observed in patients with both the CC genotype of β1AR 1165C>G and the α2AR deletion. Ten percent of the subjects had both at-risk genotypes and 19% of these individuals had a LVEF <50%, versus 4% in the subjects with neither at-risk genotype. In each model, the interaction between the 2 polymorphisms was not significant, implying that their effects are independent from each other.
Figure 1.
Combined effects of adrenoceptor genotypes on release of cTi. *See Table 3 for statistics; †logistic regression OR, P=0.012, 95% CI, 1.8 to 131.7.
Figure 2.
Combined effects of adrenoceptor genotypes on reduced LVEF. *See Table 3 for statistics; †logistic regression OR, P=0.033, 95% CI, 1.2 to 87.4.
Discussion
This study has identified associations between polymorphisms of the β-1-, β-2-, and α-adrenoceptors and the presence of cardiac injury and dysfunction after SAH. Polymorphism genotypes known to be associated with increased sensitivity to and release of catecholamines were associated with a 3- to 4.8-fold increase in the risk of cardiac injury and dysfunction. Two genotype combinations, present in 30% and 10% of the patients in this study, were associated with 15- and 10-fold increase in the risk of cardiac injury or dysfunction, respectively.
The β1-adrenoceptors are positioned at the cell membrane of cardiomyocytes. The C allele of β1AR 1165C>G encodes arginine at amino acid 389, whereas the G allele encodes glycine. In vitro studies of isoproterenol stimulation showed that the Arg-389 receptors produce higher levels of adenylyl cyclase activity in comparison to Gly-389 receptors,9 resulting in enhanced cardiac sensitivity to catecholamines. For this reason, the association between the CC genotype and cardiac injury and dysfunction in the present study is consistent with the hypothesis that SAH results in catecholamine-mediated cardiotoxicity.
The α2-adrenoceptors regulate the release of norepinephrine from cardiac sympathetic nerves.18 The α2AR del322–325 four-amino acid deletion results in a loss of normal synaptic autoinhibitory feedback and enhanced presynaptic release of norepinephrine.19 For this reason, the finding that patients with SAH and the α2AR deletion are more likely to have impaired LV contractile function also supports the catecholamine cardiotoxicity hypothesis.
It has previously been shown that patients who are homozygous for both the CC genotype of β1AR 1165C>G and the α2AR deletion have an increased risk of congestive heart failure.12 Our findings in the SAH population are consistent with this report. Specifically, patients with the β1AR 1165CC genotype who were either homozygous or heterozygous for the α2AR deletion were 10 times more likely to have a LVEF <50% in comparison to patients with neither at-risk genotype.
β-2 adrenoceptors are also present in human myocardium, and their stimulation by catecholamines appears to lead to complicated downstream effects.20–22 The CC genotype of β2AR 79C>G has previously been associated with an increased risk of adverse cardiovascular events in an elderly population.13
Our study has limitations, the most important of which is its relatively small sample size and statistical power. For this reason, the CIs around the ORs are relatively large. Our findings should be confirmed in a larger, independent cohort. We did not perform a Bonferroni correction because there were a priori reasons for treating the polymorphisms independently based on their pathophysiologic mechanisms (Table 1). Also, the at-risk genotypes have additive and independent effects on the odds of developing myocardial necrosis or LV contractile dysfunction. Although the study’s results were adjusted for self-reported race/ethnicity, it remains possible that underlying population stratification could contribute to the findings. However, restricting the analyses to the largest racial groups and excluding the groups not in HWE did not have large effects on the ORs. Because of the small percentage of Hunt & Hess Grade V patients in the cohort, the results may not be generalizable to patients with severe SAH.
There are clinical implications to the present study. First, there is increasing evidence that cardiac injury and dysfunction occurring after SAH may contribute to poor outcomes. A previous study by Mayer and colleagues showed that a reduced cardiac index was associated with an increased risk of symptomatic cerebral vasospasm/DIND.5 Recently, an elevated level of cTi has been associated with poor neurological outcomes, after adjusting for clinical factors.6
The results also provide a rationale for future research exploring the potential cardioprotective benefits of adrenergic blockade in SAH patients. Because it is likely that genotyping of specific polymorphisms will become clinically available in the future, it may be feasible to treat selected at-risk individuals using a pharmacogenetic approach.
In conclusion, genetic polymorphisms which modulate the catecholamine sensitivity of the adrenoceptors are associated with an increased risk of cardiac injury and dysfunction after SAH. Specific combinations of adrenoceptor SNP genotypes result in a 10- to 15-fold increase in the odds of developing myocardial necrosis and LV contractile dysfunction after SAH. These data provide novel evidence for the hypothesis that neurocardiogenic injury occurs in humans with SAH.
Acknowledgments
Sources of Funding
This study was supported by the National Institutes of Health (NHLBI K23 HL04054-01A1, NINDS 1R21 NS050551-01, PI Zaroff and R01 NS41877, PI Young), the Charles A. Dana Foundation, and a gift from The Pritzker Cousins Foundation, John A. Pritzker, Director.
Footnotes
Disclosures
None.
References
- 1.Fabinyi G, Hunt D, McKinley L. Myocardial creatine kinase isoenzyme in serum after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1977;40:818–820. doi: 10.1136/jnnp.40.8.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horowitz MB, Willet D, Keffer J. The use of cardiac troponin-I (cTnI) to determine the incidence of myocardial ischemia and injury in patients with aneurysmal and presumed aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 1998;140:87–93. doi: 10.1007/s007010050063. [DOI] [PubMed] [Google Scholar]
- 3.Davies KR, Gelb AW, Manninen PH, Boughner DR, Bisnaire D. Cardiac function in aneurysmal subarachnoid haemorrhage: A study of electrocardiographic and echocardiographic abnormalities. Br J Anaesth. 1991;67:58–63. doi: 10.1093/bja/67.1.58. [DOI] [PubMed] [Google Scholar]
- 4.Sato K, Masuda T, Izumi T. Subarachnoid hemorrhage and myocardial damage clinical and experimental studies. Jpn Heart J. 1999;40:683–701. doi: 10.1536/jhj.40.683. [DOI] [PubMed] [Google Scholar]
- 5.Mayer S, Lin J, Homma S, Solomon R, Lennihan L, Sherman D, Fink M, Beckford A, Klebanoff L. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke. 1999;30:780–786. doi: 10.1161/01.str.30.4.780. [DOI] [PubMed] [Google Scholar]
- 6.Naidech AM, Kreiter KT, Janjua N, Ostapkovich ND, Parra A, Commichau C, Fitzsimmons BF, Connolly ES, Mayer SA. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112:2851–2856. doi: 10.1161/CIRCULATIONAHA.105.533620. [DOI] [PubMed] [Google Scholar]
- 7.Mertes P, Carteaux J, Jaboin Y, Pinelli G, el Abassi K, Dopff C, Atkinson J, Villemot J, Burlet C, Boulange M. Estimation of myocardial interstitial norepinephrine release after brain death using cardiac microdialysis. Transplantation. 1994;57:371–377. doi: 10.1097/00007890-199402150-00010. [DOI] [PubMed] [Google Scholar]
- 8.Hachinski VC, Smith KE, Silver MD, Gibson CJ, Ciriello J. Acute myocardial and plasma catecholamine changes in experimental stroke. Stroke. 1986;17:387–390. doi: 10.1161/01.str.17.3.387. [DOI] [PubMed] [Google Scholar]
- 9.Mason DA, Moore JD, Green SA, Liggett SB. A gain-of-function polymorphism in a G-protein coupling domain of the human β1-adrenergic receptor. J Biol Chem. 1999;274:12670–12674. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- 10.Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human β2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 11.Bruck H, Leineweber K, Buscher R, Ulrich A, Radke J, Insel PA, Brodde OE. The Gln27Glu β2-adrenoceptor polymorphism slows the onset of desensitization of cardiac functional responses in vivo. Pharmacogenetics. 2003;13:59–66. doi: 10.1097/00008571-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of β1- and α2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med. 2002;347:1135–1142. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- 13.Heckbert SR, Hindorff LA, Edwards KL, Psaty BM, Lumley T, Siscovick DS, Tang Z, Durda JP, Kronmal RA, Tracy RP. β2-adrenergic receptor polymorphisms and risk of incident cardiovascular events in the elderly. Circulation. 2003;107:2021–2024. doi: 10.1161/01.CIR.0000065231.07729.92. [DOI] [PubMed] [Google Scholar]
- 14.Schiller N, Shah P, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 15.Hsu TM, Kwok PY. Homogeneous primer extension assay with fluorescence polarization detection. Methods Mol Biol. 2003;212:177–187. doi: 10.1385/1-59259-327-5:177. [DOI] [PubMed] [Google Scholar]
- 16.Ranade K, Jorgenson E, Sheu WH, Pei D, Hsiung CA, Chiang FT, Chen YD, Pratt R, Olshen RA, Curb D, Cox DR, Botstein D, Risch N. A polymorphism in the β1-adrenergic receptor is associated with resting heart rate. Am J Hum Genet. 2002;70:935–942. doi: 10.1086/339621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maqbool A, Hall AS, Ball SG, Balmforth AJ. Common polymorphisms of β1-adrenoceptor: Identification and rapid screening assay. Lancet. 1999;353:897. doi: 10.1016/s0140-6736(99)00549-8. [DOI] [PubMed] [Google Scholar]
- 18.Hein L, Altman JD, Kobilka BK. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- 19.Small KM, Forbes SL, Rahman FF, Bridges KM, Liggett SB. A four amino acid deletion polymorphism in the third intracellular loop of the human α2C-adrenergic receptor confers impaired coupling to multiple effectors. J Biol Chem. 2000;275:23059–23064. doi: 10.1074/jbc.M000796200. [DOI] [PubMed] [Google Scholar]
- 20.Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999;51:651–690. [PubMed] [Google Scholar]
- 21.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by β(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson AJ, Zhu W, Chow A, Agrawal R, Kosek J, Xiao RP, Kobilka B. Protecting the myocardium: A role for the β2 adrenergic receptor in the heart. Crit Care Med. 2004;32:1041–1048. doi: 10.1097/01.ccm.0000120049.43113.90. [DOI] [PubMed] [Google Scholar]
- 23.Iwai C, Akita H, Kanazawa K, Shiga N, Terashima M, Matsuda Y, Takai E, Miyamoto Y, Shimizu M, Kajiya T, Hayashi T, Yokoyama M. Arg389Gly polymorphism of the human β1-adrenergic receptor in patients with nonfatal acute myocardial infarction. Am Heart J. 2003;146:106–109. doi: 10.1016/S0002-8703(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 24.Borjesson M, Magnusson Y, Hjalmarson A, Andersson BA. Novel polymorphism in the gene coding for the β(1)-adrenergic receptor associated with survival in patients with heart failure. Eur Heart J. 2000;21:1853–1858. doi: 10.1053/euhj.1999.1994. [DOI] [PubMed] [Google Scholar]
- 25.Green SA, Cole G, Jacinto M, Innis M, Liggett SB. A polymorphism of the human β2-adrenergic receptor within the fourth transmembrane domain alters ligand binding and functional properties of the receptor. J Biol Chem. 1993;268:23116–23121. [PubMed] [Google Scholar]
- 26.Liggett SB, Wagoner LE, Craft LL, Hornung RW, Hoit BD, McIntosh TC, Walsh RA. The Ile164 β2-adrenergic receptor polymorphism adversely affects the outcome of congestive heart failure. J Clin Invest. 1998;102:1534–1539. doi: 10.1172/JCI4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanki H, Yang P, Xie HG, Kim RB, George AL, Jr, Roden DM. Polymorphisms in β-adrenergic receptor genes in the acquired long QT syndrome. J Cardiovasc Electrophysiol. 2002;13:252–256. doi: 10.1046/j.1540-8167.2002.00252.x. [DOI] [PubMed] [Google Scholar]
- 28.Cockcroft JR, Gazis AG, Cross DJ, Wheatley A, Dewar J, Hall IP, Noon JP. β(2)-adrenoceptor polymorphism determines vascular reactivity in humans. Hypertension. 2000;36:371–375. doi: 10.1161/01.hyp.36.3.371. [DOI] [PubMed] [Google Scholar]
- 29.Hunt W, Hess R. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1967;28:14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]