Abstract
Several forms of eye movement dysfunction (EMD) are regarded as promising candidate endophenotypes of schizophrenia. Discrepancies in individual study results have led to inconsistent conclusions regarding particular aspects of EMD in relatives of schizophrenia patients. To quantitatively evaluate and compare the candidacy of smooth pursuit, saccade and fixation deficits in first-degree biological relatives, we conducted a set of meta-analytic investigations. Among 16 measures of EMD, memory-guided saccade accuracy and error rate, global smooth pursuit dysfunction, intrusive saccades during fixation, antisaccade error rate and smooth pursuit closed loop gain emerged as best differentiating relatives from controls (standardized mean differences ranged from .46 to .66), with no significant differences among these measures. Anticipatory saccades, but no other smooth pursuit component measures were also increased in relatives. Visually-guided reflexive saccades were largely normal. Moderator analyses examining design characteristics revealed few variables affecting the magnitude of the meta-analytically observed effects. Moderate effect sizes of relatives v. controls in selective aspects of EMD supports their endophenotype potential. Future work should focus on facilitating endophenotype utility through attention to heterogeneity of EMD performance, relationships among forms of EMD, and application in molecular genetics studies.
There is a growing consensus that genes influence the development of many forms of psychopathology. The hunt for implicated genes is sweeping the natural and social sciences (e.g., Plomin & McGuffin, 2003). Arguably, this is nowhere more evident than in research on schizophrenia, a debilitating mental disorder for which a heritable component has long been recognized. But, in the “frustrating search for schizophrenia genes” (Tsuang & Faraone, 2000), progress has been slow. Genetic linkage studies have identified multiple susceptibility loci on many chromosomes (e.g., Badner & Gershon, 2002; Berrettini, 2000; Berry, Jobanputra, & Pal, 2003; Harrison & Owen, 2003; Jurewicz, Owen, O'Donovan, & Owen, 2001; Kato et al., 2002; Leboyer et al., 1998; Mowry & Nancarrow, 2001; Riley, 2004; Waterworth, Bassett, & Brzustowicz, 2002) and variations in particular genes have been identified in some individuals with schizophrenia. However, though evidence is accumulating that particular genes are implicated, studies have not yet consistently yielded associations between the gene aberrations and schizophrenia (for reviews see Harrison & Owen, 2003; Owen, Craddock, & O'Donovan, 2005; Riley, 2004; Waterworth, Bassett, & Brzustowicz, 2002; Wong, Buckle, & Van Tol, 2000). The identification of genes and their mechanisms will enhance our understanding of the brain abnormalities associated with psychopathology and enable researchers to develop more targeted and effective treatments (Hyman, 2000). As advances in molecular genetics strategies have catapulted us toward improved understanding of our basic genetic make-up, psychopathology researchers have strived to match the pace.
Unfortunately, there are several commonly recognized obstacles to the identification of disorder susceptibility genes. First, many disorders are likely etiologically heterogeneous, that is, each disorder may actually comprise several disorders, each with distinct genetic and/or non-genetic influences (e.g., Bray & Owen, 2001; Carpenter, Buchanan, Kirkpatrick, Tamminga, & Wood, 1993; Garver, 1997). Consequently, individuals presenting with the same clinical symptoms may not share the same underlying genetic etiology. The inclusion of such individuals together in studies seeking genes is thus likely to becloud genetic etiologies. Second, evidence from twin studies indicates that individuals may carry genetic risk for a disorder without actually manifesting the disorder (e.g., Cardno & Gottesman, 2000; Kringlen, 2000). If such “latent” gene carriers are unrecognized, sensitivity in genetic linkage studies is decreased. Third, it is widely suspected that most mental disorders are polygenic, that is, that multiple deleterious genetic variants contribute to disorder susceptibility. The involvement of multiple genes makes phenotypes (observed characteristics) like schizophrenia complex, composed of numerous quantitative or continuous characteristics. Genes contributing to complex traits are also called “quantitative trait loci” (QTL), reflecting the individual effects of multiple genes on quantitative traits. It has been suggested that there are direct relationships among the complexity of a phenotype, the number of genes involved in the phenotype, and the difficulty of genetic analyses (Gottesman & Gould, 2003). The more complex the phenotype like “schizophrenia”, the more genes are likely to be involved, and therefore, the more difficult it is to find those genes. Together, these three interrelated obstacles call for an innovative method of capturing an alternative quantitative phenotype that would (1) identify more genetically homogeneous groups than does the clinical diagnosis (2) identify all gene carriers, not just those with a clinical diagnosis, and arguably (Flint & Munafo, 2007; Tan, Callicott, & Weinberger, 2008), (3) have a simpler genetic architecture than the disorder.
Many contemporary discussions of the genetics of schizophrenia conclude that the use of “endophenotypes” will provide a method for refining the schizophrenia phenotype that will allow for the identification of schizophrenia vulnerability genes (e.g., Cannon, 2005; Gottesman & Gould, 2003; Iacono, 1998; Keefe, Silverman, Siever, & Cornblatt, 1991; Leboyer et al., 1998; Waterworth, Bassett, & Brzustowicz, 2002). Endophenotypes are characteristics, usually assessed in a laboratory, that appear to reflect the action of genes predisposing an individual to a specific disorder even in the absence of diagnosable pathology. As relatively simpler biobehavioral characteristics, there may be fewer genes involved in endophenotypes than in the more complex phenotype of schizophrenia. Thus, an endophenotype would (1) identify a more homogeneous subgroup of individuals who share susceptibility for schizophrenia (2) identify family members who may carry the gene(s) for a disorder without manifesting the disorder itself and (3) involve fewer genes than the schizophrenia phenotype, therefore reducing the complexity of genetic analyses (e.g., Gottesman & Gould, 2003)
Schizophrenia patients have long been documented to exhibit deficits in several laboratory-assessed abilities. Because healthy biological relatives share genes with schizophrenia patients without sharing the complications of chronic illness and medication exposure, the finding that some healthy relatives of schizophrenia patients exhibit deficits similar to those of schizophrenia patients has led to the suggestion that these characteristics could be endophenotypes. Growing research attention, accompanied by extensive research funding, is directed towards the search for endophenotypes of schizophrenia (e.g., Calkins et al., 2007). Indeed, the investigation of endophenotypes in the families of schizophrenia patients has been described as likely to be “the key to unlocking schizophrenia” (Holden, 2003, p, 334). Endophenotypes are being sought for other forms of psychopathology including substance related disorders (e.g., Hesselbrock, Begleiter, Porjesz, O'Connor, & Bauer, 2001; Iacono, Carlson, & Malone, 2000; Iacono, Malone, & McGue, 2003; Schuckit, 2000), mood disorders (e.g., Glahn, Bearden, Niendam, & Escamilla, 2004) and attention deficit hyperactivity disorder (e.g., Seidman, Biederman, Monuteaux, Weber, & Faraone, 2000). Indeed, although schizophrenia researchers have been studying characteristics now called endophenotypes for more than thirty years, their application in molecular genetic studies is regarded as a paradigm shift. Excitement about this model of psychopathology genetics research has piqued in part because a similar approach has been applied successfully to gene identification in particular complex medical conditions (for discussion, see Gottesman & Gould, 2003).
The most well-investigated candidate endophenotype of schizophrenia is smooth pursuit eye movement dysfunction (SPEMD), an impairment in the ability to visually track a smoothly moving target. SPEMD was first reported in schizophrenia patients in 1908 by Diefendorf and Dodge (Diefendorf & Dodge, 1908); because of its impact on oculomotor research, this paper gave rise to the current centenary special issue. SPEMD has since been viewed as one of the most promising candidate endophenotypes to assist in our search for schizophrenia genes (Erlenmeyer-Kimling & Cornblatt, 1987; Holzman, 1987; Iacono, 1983, 1988, 1998; Iacono & Grove, 1993; Lee & Williams, 2000; Siever & Coursey, 1985; Siever, Coursey, Alterman, Buchsbaum, & Murphy, 1982; Venables, 1991). In addition to SPEMD, several other forms of eye movement dysfunction (EMD) have been implicated as candidate endophenotypes of schizophrenia (e.g., Broerse, Holthausen, van den Bosch, & den Boer, 2001; Calkins & Iacono, 2000; Clementz, 1998; Curtis, Calkins, & Iacono, 2001). Altogether, there have been more than 900 scientific articles on EMD in schizophrenia; to our knowledge, no other class of candidate endophenotypes has been as extensively investigated.
To be considered an endophenotype of a disorder, a characteristic should have several properties that together indicate that it is a measurable, reliable manifestation of genetic risk for that disorder (e.g., Gottesman & Gould, 2003; Iacono, 1983, 1985, 1998; Iacono & Grove, 1993). One of the primary reasons that EMD has been of such interest is that several lines of evidence converge in support of a genetic influence on EMD, thereby implying that EMD will assist in gene identification. Indeed, a small number of molecular genetics studies have provided promising preliminary evidence in support of a relationship between EMD and particular genes (Ettinger et al., 2008; Haraldsson et al., 2008; Rybakowski & Borkowska, 2002; Rybakowski, Borkowska, Czerski, & Hauser, 2001; Thaker, Wonodi, Avila, Hong, & Stine, 2004) or chromosomal regions (Arolt et al., 1996; Arolt et al., 1999; Matthysse et al., 2004; Myles-Worsley et al., 1999). While these studies are important in their attempts to investigate the genetic underpinnings of EMD, they are few, difficult to replicate, typically employ small samples, cover few of the different forms of EMD that have been implicated in schizophrenia, and give us little to go on when faced with how to evaluate the candidacy of different aspects of EMD as endophenotypes.
There are several additional features of endophenotypes that can be evaluated that allow us to sort through different forms of EMD in a way that gets at their potential for being genetically informative. The primary and essential feature is that the characteristic is associated with a particular illness; it is observable in individuals with schizophrenia. If that characteristic is reflective of an active gene(s), then its manifestation should be ever-present, leading to the expectation that it will be independent of state and show trait-like properties. Such trait-like properties include stability over time, independence from stage of illness, and occurrence during symptom remission.
As a manifestation of genetic vulnerability, the candidate should be heritable and identify individuals who are at risk, but who do not evidence outward manifestations of psychopathology; results of family studies should indicate that the candidate is genetically transmissable, such that it is present in the biological relatives of affected individuals. If the characteristic taps “latent” gene carriers, that is, individuals who are carrying genes without manifesting the disorder, then the characteristic should be observable in healthy, unaffected relatives.
Evidence supporting the association between particular aspects of SPEMD and schizophrenia is presented by O'Driscoll and Callahan (this issue) and between saccade performance and schizophrenia and other disorders by Gooding and Basso (this issue). The purpose of the current investigation was to evaluate the endophenotype candidacy of smooth pursuit, saccade and fixation EMD based on evidence from family and genetic studies. Although several previous reviews have addressed the candidacy of SPEMD as an endophenotype of schizophrenia (Clementz & Sweeney, 1990; Erlenmeyer-Kimling & Cornblatt, 1987; Holzman, 1992; Iacono, 1983, 1985, 1988, 1998; Iacono & Grove, 1993; Keefe, Silverman, Siever, & Cornblatt, 1991; Keri & Janka, 2004; Lee & Williams, 2000; Levy, Holzman, Matthysse, & Mendell, 1993; Levy, Holzman, Matthysse, & Mendell, 1994; Lipton, Levy, Holzman, & Levin, 1983), the present review contributes in three ways to cumulative knowledge on the candidacy of several forms of eye movement dysfunction (EMD) as endophenotypes. First, in contrast to most previous reviews that have narratively summarized the literature, where possible, we employed meta-analysis, which confers several advantages. By cumulating effect sizes, we could estimate the magnitude of effect of particular comparisons. This allowed us to compare and contrast the relative discriminability of multiple forms of EMD. In addition, because we quantitatively combined the results of a number of studies, we could correct for the sampling error inherent to one study (Hunter & Schmidt, 1990). Such corrections for statistical artifacts are unavailable to the narrative reviewer. Further, unlike narrative reviewers who are typically limited to an appraisal of study results as reported by original investigators, we were able, by calculating effect sizes with reported data, to examine comparisons for which no statistical test was reported in original studies. Moreover, because meta-analysis provides for the cumulation of results of a set of studies in a manner that seeks to minimize reliance on judgment, we could avoid several problems that may affect conclusions drawn by narrative reviewers, including the selective inclusion of studies based on subjective judgments of study quality, differential weighting of studies in interpretations of findings (Wolf, 1986), over-reliance on significant results (Schmidt, 1996), and difficulty in weighing outcomes relative to sample size. Several meta-analyses including SPEMD in schizophrenia patients (O'Driscoll & Callahan, this issue, Heinrichs, 2001) or antisaccade EMD in relatives (Levy et al., this issue, Calkins, Curtis, Iacono, & Grove, 2004; Levy et al., 2004; Snitz, Macdonald, & Carter, 2006) have recently appeared, but in contrast to those reviews, the present review includes an examination and comparison of performance by biological relatives of schizophrenia patients on a complement of eye movements in which impairment in schizophrenia patients has been observed (i.e., smooth pursuit, saccade and fixation). In so doing, we evaluated the relative way that different forms of EMD fare, in order to determine which candidates, if any, emerge from the literature as “greatest prospects.” This approach also differentiates the current review from prior narrative reviews that evaluated only SPEMD as an endophenotype or biological marker (Iacono & Grove, 1993; Keri & Janka, 2004; Levy, Holzman, Matthysse, & Mendell, 1993).
Second, because the most recent comprehensive narrative reviews of SPEMD as a biological marker were published in 1993 (Iacono & Grove, 1993; Levy, Holzman, Matthysse, & Mendell, 1993), we could include a decade of investigations that were not available to previous reviewers. Finally, with the exception of a review on antisaccade performance (Hutton & Ettinger, 2006), subsequent reviews of other forms of EMD, though contributing valuable viewpoints, have not been framed in terms of the evaluation of performance of biological relatives (e.g., Broerse, Crawford, & den Boer, 2001; Hutton & Kennard, 1998; MacAvoy & Bruce, 1995) whereas the present review evaluated available evidence for multiple forms of EMD within this framework.
In order to evaluate EMD as a candidate endophenotype, we addressed the following questions that stem from the criteria for evaluating endophenotype candidacy: Is EMD deviant in biological relatives of schizophrenia patients? If so, what forms of EMD differentiate relatives from controls, and what variables moderate the magnitude of effect? Is there other evidence from family studies for the influence of genes on EMD (e.g., heritability)? We conclude with an overall discussion in which we compare and contrast the endophenotype potential of the different forms of EMD, discuss current evidence for gene associations between EMD and schizophrenia, and provide recommendations for future research in this area.
Methods
Identification of Literature
The location of appropriate literature was conducted in several recursive steps, in the context of a larger meta-analysis evaluating EMD in schizophrenia and mood disorder patients and family members. First, repeated (once a year between September 1996 and January 2003, and again through May 2008) searches were conducted of two computerized databases, Medline and PsycInfo, for articles published during the years covered by the databases (Medline: 1950 to 2008; PsycInfo: 1960 to 2008). The key words schizophrenia, psychosis, psychotic, affective, depression, depressive, bipolar, and manic were each used in combination with the following key words: eye movement, eye tracking, oculomotor, smooth pursuit, saccade, antisaccade, gain, fixation, global measures, signal to noise ratio, root-mean square error, mean square error, qualitative ratings, quantitative ratings, electro-oculography, electro-oculograph, electrooculogram, infrared oculography, eye tracking and infrared, eye tracking and high resolution, limbus boundary tracker. Certain of these words (e.g., eye movement dysfunction) were also used as key words alone. The relevance of identified articles (including book chapters and conference abstracts) was judged by inspection of the title and abstract of each, however, we conservatively retrieved and examined studies for which relevance was unclear, or for which there was no abstract. All articles, both empirical and non-empirical, identified in this manner were located and retrieved.
Second, the reference lists of all retrieved articles were inspected to identify studies that were either unpublished or undetected via the automated searches. For each reference in a reference list, the context in which an article was cited was examined to determine the relevance of the cited article, but all articles were retrieved in cases in which relevance was unclear. In addition, the reference lists of all non-empirical articles (e.g., narrative reviews) were searched. Identified articles were then retrieved, and their reference lists inspected for additional articles, and so on.
Third, early in the data collection process, the identities of the twenty most active investigators in the area were determined, as estimated by the frequency of authorship of relevant publications. These researchers were then contacted via letter and asked to identify any missing studies from a list of their studies (response rate = 60%).
Finally, an expert (WGI) with thirty years experience in the field reviewed a preliminary list of all studies in order to identify any missing articles.
The entire process yielded a total of 949 articles on EMD in schizophrenia and mood disorders.
Study Inclusion Criteria
All articles, including those that appeared to be review articles, were inspected to determine whether data were reported that could be included. The following study inclusion criteria were employed. First, each study had to be written in English. Second, each study investigated smooth pursuit, saccadic, or fixation system functioning in the first-degree biological relatives of patients with schizophrenia and/or mood disorders. There were a few exceptions to this inclusion criterion. Studies were excluded in which the patient group was largely heterogeneous, e.g., in which results were reported for a combined group of schizophrenia patients and mood disorder patients. However, we did include a small number of studies in which the samples included fewer than three participants who fell outside the diagnostic group of interest. Relatives of schizophrenia patients included samples that were composed of any type of first-degree biological relative (parents, siblings and/or adult children).
Third, each study must have employed a control group.
Fourth, each study had to report sufficient data to calculate an effect size (i.e., mean, SD or SE, and n). In cases in which insufficient information was presented to allow the calculation of an effect size, a letter or e-mail was sent to the corresponding author that requested the necessary information. If no response was received from an author, the study could not be included.
Finally, in order to maximize independence of observations in our sample of studies, each study must have reported data that did not overlap with data reported in another study. In cases in which the authors reported that the relative groups consisted of participants for whom data were reported elsewhere, all studies reporting data from the group of subjects were examined; for any given variable, the data from the paper that reported the larger sample size were used.
All identified articles were retrieved and reviewed for relevance to the current set of analyses, and 52 were determined to be eligible for inclusion1.
Data Collection
Study Coding
The first author coded all studies. When coding decisions are uncomplicated as in the present investigation, the reliability of coded data has been reported as high for meta-analyses (Zakzanis, 1998). Nevertheless, the few problem cases were discussed with two experts, one in meta-analysis (DSO) and one in eye tracking (WGI) to arrive at consensus coding decisions. For each included study, four domains of characteristics were coded: study (e.g., authors, publication year), participant (e.g., group type, sample size, illness status), method (e.g., eye movement recording method, target waveform), and dependent measure (i.e., eye movement measure).
Effect Size Calculation
From the data in each report, an effect size was calculated for each group comparison on each eye tracking measure and task reported. Because estimates of effect size based on the means and SD's are more precise than indirect estimates based on the results of statistical tests, all attempts were made to extract information necessary to calculate effect sizes directly from the means and SD's. In cases in which the means and SD's were presented only in figures, best approximations were obtained by measuring with a ruler. Where means and SD's were provided, effect sizes were estimated using Cohen's d, which is the difference between the sample size weighted means of the two groups in the comparison, divided by the sample size weighted pooled standard deviation (Cohen, 1977). That is, d values index the standardized mean differences between the two groups being compared. A positive effect size indicates that the first group in the comparison had a greater value on the variable of interest than the second group. Although effect sizes can theoretically range between positive and negative infinity, given a normal distribution, 95.44% of all effect sizes are found between 2.00 and −2.00. In this study, effect sizes close to zero would indicate that the groups being compared scored similarly on eye tracking measures.
In cases in which only the results of statistical tests, but not means and SD's, were reported, effect sizes were calculated via conventional formulae if possible (Wolf, 1986). In studies of qualitative ratings of eye tracking performance in which frequencies of good and bad trackers were reported within groups, chi-square analyses were conducted, and the results converted to effect sizes.
In some cases, information necessary to calculate effect sizes were selectively reported for some but not all variables included in the study, most typically when the results for one or more variables were not significant. The frequency of narratively reported non-significant results for which effect sizes could not be calculated was tabulated for additional analyses to address potential biases in meta-analytic results.
Results from single studies frequently included those derived from multiple measures of eye tracking performance (e.g., a quantitative measure, a qualitative measure, different types of saccade rates), or from multiple tasks (e.g., two or more sine wave tasks of differing frequencies, two or more saccadic paradigms), or from both. In each of these cases, effect sizes were calculated for all measures and tasks reported. If the effect size for each comparison were entered into the relevant meta-analysis, the sample would be contributing disproportionately to, and potentially bias, the estimate of the population effect size. Thus, for the primary analysis of a given dependent measure, effect sizes were averaged when several forms of the dependent measure were reported for the same groups, so that each sample contributed only one effect size per dependent measure. However, in each such case, the individual effect sizes were retained in the database for use in moderator analyses.
In many cases, results were provided for two or more samples within a study. When the means and standard deviations were provided for the two samples combined, effect sizes from the combined sample were calculated. In such cases, the effect sizes for the sub-samples were also calculated for use in moderator analyses. However, when the means and standard deviations were provided for the two separate groups, and not for the combined sample, an effect size was calculated for each separate group, using the same control group, and entered into analyses as a unique sample. Thus, in some cases, the same control group data contributed to multiple effect sizes for the same comparison, but the index relatives never did.
Meta-Analyses
Meta-analyses of group comparisons were conducted according to the method of Hunter & Schmidt (1990; 2004), using a 1985 version of program software developed by Schmidt (http://www.testpublishers.org/Documents/FrankSchmidtSoftware.pdf). Bare bones meta-analyses of experimental effect sizes were conducted. A bare bones meta-analysis only corrects for one statistical artifact, sampling error. Bare bones meta-analysis results in the computation of an observed mean effect size across studies and also tests whether variation in effect sizes across studies is due to sampling error. Although at the level of the individual study, an effect size cannot be corrected for sampling error, at the meta-analytic level, the mean effect size can be corrected for this statistical artifact by pooling results across studies. Across a number of studies, the sampling error in the observed mean effect size is always smaller than the sampling error in any single observed effect size from any of the studies contributing to the meta-analysis.
Procedurally, for each group comparison, we first calculated the mean and variance of the effect sizes over the set of studies. Next, the variance was corrected for sampling error (i.e., error at the individual study level that leads the sample effect size to vary randomly from the population effect size). This yields D, the sample-size weighted mean of effect sizes that is interpreted as the estimate of the observed population effect size2. Estimates of the effect size are interpreted according to the guidelines of Cohen (1977); 0.8 = large (53% overlap of the two distributions), 0.5 = moderate (67% overlap), 0.2 = small (85% overlap).
Next, in order to estimate the accuracy of each mean effect size, a 95% confidence interval was calculated using the standard error of D (for discussion see Whitener, 1990). Confidence intervals that include zero suggest that the population effect size is not significantly different from zero. In addition, overlap between the confidence intervals constructed around two different comparisons suggests that the two population effect sizes (D) are not significantly different, whereas non-overlap indicates that the two population effect sizes significantly differ (Hunter & Schmidt, 1990).
As a summary statistic, significance of the difference between pairs of D's of group comparisons was examined with z statistic (Hunter & Schmidt, 1990, p. 438), one-tailed test with critical value of z = 1.96.
Several meta-analyses were conducted with small numbers of studies. We report these analyses in order to provide as comprehensive a picture as possible of the current EMD literature, but recognize the limitations of such an approach and have accordingly been conservative in our interpretations. It is important to note that for these variables, narrative reviews would also include summaries based on a small number of studies.
Moderator Analyses
The reasonable number of studies conducted with particular EMD variables provided the opportunity to construct credibility intervals in order to determine whether D is likely to represent the mean of several sub-populations, thereby indicating that moderator variables may be operating. As a reflection of the heterogeneity of the effect size distribution, wide credibility intervals or those overlapping zero suggest that subgroups of studies within the domain differ in characteristics that are associated with effect size magnitude. Such characteristics are said to moderate the magnitude of effect. Thus, for particular population effect size estimates, a 95% credibility interval was calculated using the corrected standard deviation around D. Because they are based on the standard deviation rather than the standard error of D, credibility intervals are distinct from confidence intervals (see Whitener, 1990, for detailed discussion of confidence v. credibility intervals). Where credibility intervals were large (here defined as intervals bracketing an effect size range >= 1.0) or included zero, follow-up moderator analyses were conducted to evaluate variables that have been suggested to influence estimates of the magnitude of group differences. Because we evaluated categorical participant design characteristics that varied across or within studies, the study domain subset method was used. This method entails dividing the study domain into subsets based on characteristics of interest, performing meta-analyses within each subset, and then testing the significance of the difference between the D's of domain subsets by z statistic, one-tailed test with critical value 1.96 (Hunter & Schmidt, 1990, p. 438).
File-drawer Analyses
The existence of unpublished studies, which are typically assumed to consist of non-significant results, would affect conclusions of meta-analyses. Thus, “file drawer analyses” were conducted for each primary and moderator analysis in order to determine how many missing non-significant studies must exist in order to reverse the conclusions suggested by the meta-analysis by bringing the value of D down or up to a specified level (here set at 0.1, −0.1, depending on the predicted relationship, specified in parentheses for each analysis) (Hunter & Schmidt, 1990). File drawer analyses are especially important for evaluating the results of meta-analyses to which a small number of studies contribute.
Smooth Pursuit Eye Movement Dysfunction in Families of Schizophrenia Patients
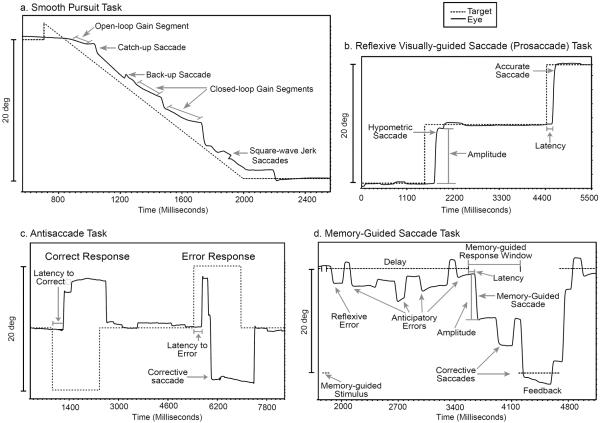
Smooth pursuit dysfunction has been the most widely investigated form of EMD in schizophrenia. The smooth pursuit system is evoked by moving objects; it maintains the image of the moving target on the fovea, the region of the retina containing the highest density of photoreceptors (Sharpe, 1998); Diefendorf & Dodge (1908) first observed that patients with dementia praecox had difficulty following an oscillating pendulum with their eyes. In the modern laboratory, smooth pursuit eye movements are typically elicited by visual stimuli presented on a computer monitor; a small dot target traverses the screen horizontally in a smooth, continuous motion while the participant, with head stable, follows the moving target with his/her eyes. Figure 1a presents an example of a trial of a smooth pursuit task; the target begins on the left side of the screen and travels at a constant speed to the right side of the screen, where it remains for a short interval before the next trial. Typically, multiple trials are administered. The participant's eye position relative to the target is recorded using specialized equipment, and subsequently analyzed for particular aspects of pursuit.
Figure 1.
Examples of EMD paradigms. a. One trial of a smooth pursuit task, in which the target begins on the right side of the computer monitor and travels at a constant velocity (16 deg/s) to the left side of the monitor. Open-loop gain segment = initial period during which pursuit is initiated, typically scored for average acceleration during that period. Closed-loop gain segments = two of several segments during which the accuracy of pursuit maintenance is estimated by examining the ratio of eye velocity to target velocity. Catch-up saccade = corrective saccade that takes the eyes from a position behind the target to a position on or near the target. Back-up saccade = corrective saccade that takes the eyes from the target to a position behind the target. Square wave jerk saccades =intrusive saccades consisting of a pair of small amplitude saccades separated by a brief intersaccadic interval, preceded and followed by pursuit. b. Two trials of a visually-guided reflexive saccade (prosaccade) task, in which the participant is required simply to generate a saccade in the direction of target motion. In the first trial, the saccade generated is hypometric; the eye position amplitude falls short of target amplitude. In the second trial, the saccade is accurate; the eye position amplitude closely matches target amplitude. Latency reflects the reaction time between stimulus presentation and saccade initiation. c. Two trials of an antisaccade task, in which the participant is instructed to make a saccade in the direction opposite target motion. In the first trial, the participant correctly generates a saccade in the opposite direction of target motion. In the second trial, an inappropriate reflexive saccade error is made to the target, followed quickly by a corrective saccade in the appropriate direction. Both trials are scored for latency between target appearance and the initiation of the primary saccade. d. A memory-guided saccade task in which the participant is instructed to maintain fixation during the presentation of a peripheral stimulus (memory-guided stimulus), to continue fixation during a delay period, and upon offset of the fixation stimulus, to generate a saccade to the remembered location of the memory-guided stimulus. This participant generates an inappropriate reflexive error to the memory-guided stimulus and makes several saccades in anticipation of the fixation offset during the delay period. Upon the cue (fixation offset) to look to the remembered location, during the memory-guided response window, the participant generates an inaccurate memory-guided saccade, subsequently generating a corrective saccade landing closer to the appropriate location. At the end of the memory-guided response window, a feedback stimulus shows the appropriate location, to which the participant generates a final corrective saccade.
What Aspects of Smooth Pursuit Are Deviant in Relatives of Schizophrenia Patients?
Since the earliest observations of smooth pursuit dysfunction in schizophrenia patients, methods of examining pursuit have proliferated. Many aspects of deviant smooth pursuit in schizophrenia patients have been investigated using indices of generalized dysfunction and characterization of particular eye movements observed during pursuit. The most widely used indices of deviance have been “global measures,” that is, measures that assess the overall extent to which participant eye position is congruent with the target position during a smooth pursuit task.
Because the traditional measures of EMD are “global,” they ostensibly reflect dysfunction in any of several types of eye movements that are invoked during a smooth pursuit task. In an influential article in the late 1980's, Abel and Ziegler (1988) suggested that the elucidation of the nature of the observed dysfunction could implicate etiological and neuropathological factors underlying schizophrenia. In particular, different measures of smooth pursuit might reflect correspondingly different pathophysiological processes, perhaps providing the opportunity to determine if schizophrenia patients have specific as opposed to general smooth pursuit deficits. Moreover, parsing the eye movements that occur during smooth pursuit could facilitate comparison of the performance of psychiatric patients to findings in neurological patients (e.g., Friedman et al., 1995; MacAvoy & Bruce, 1995). Subsequently, there has been widespread use of measures that attempt to characterize smooth pursuit performance on the basis of constituent eye movements. These types of measures have been referred to variously as specific quantitative, precise quantitative and neuro-ophthalmologically informed. Because none of these terms quite captures what these variables are measuring and all are attempts to parse the tracking response into component attributes, we will refer to them as “smooth pursuit component measures.” Despite the hope that smooth pursuit component measures would be neurophysiologically informative, it has not been clear from existing reviews whether all smooth pursuit component measures of pursuit integrity are deviant in schizophrenia patients, and no firm conclusions have been drawn regarding implicated neuropathology. Further, it is possible that some measures may work better than others according to some criterion related to serving as an endophenotype.
Global Pursuit EMD
Global qualitative measures are ratings based on visual inspection of a tracking record and categorization of the impairment according to predetermined criteria, e.g., dichotomous ratings (e.g., good/bad) or scales of 4 or 5 anchors of increasing or decreasing impairment. For example, if the trial depicted in Figure 1a were representative of most other trials in the record, the tracking would be considered “bad.” Global quantitative measures, such as root-mean square error (RMSE) and signal to noise ratio (S/N), use digitized representations of eye movements and the mathematical quantification of the overall difference between the position of the eye and the position of the target during a tracking task. The trial depicted in Figure 1a, for example, would produce a high RMSE score.
The earliest indication that global SPEMD was a possible endophenotype of schizophrenia was the observation that healthy biological relatives of patients with schizophrenia also exhibited dysfunctional eye movements (e.g., Holzman et al., 1974). Deviance in relatives is viewed as an indicator of the heritability of the characteristic. Subsequent investigations with relatives have led reviewers to conclude that there is strong evidence for the familial association of global SPEMD in schizophrenia families (Holzman, 1985; Iacono & Grove, 1993; Keefe, Silverman, Siever, & Cornblatt, 1991; Levy, Holzman, Matthysse, & Mendell, 1993; Lipton, Levy, Holzman, & Levin, 1983).
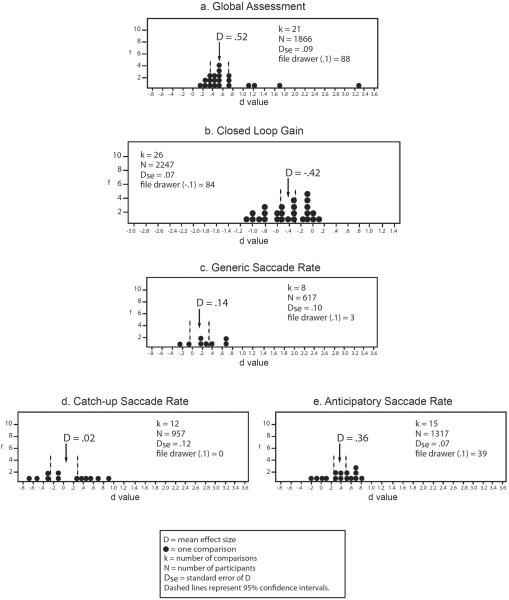
Results of comparisons involving schizophrenia patients' relatives are presented in Figure 23. The relatives of schizophrenia patients' evidence impairment in their global tracking proficiency in comparison with non-psychiatric controls at a moderate size of effect (see Figure 2a). Thus, like schizophrenia patients, the relatives of schizophrenia patients evidence global smooth pursuit eye movement dysfunction.
Figure 2.
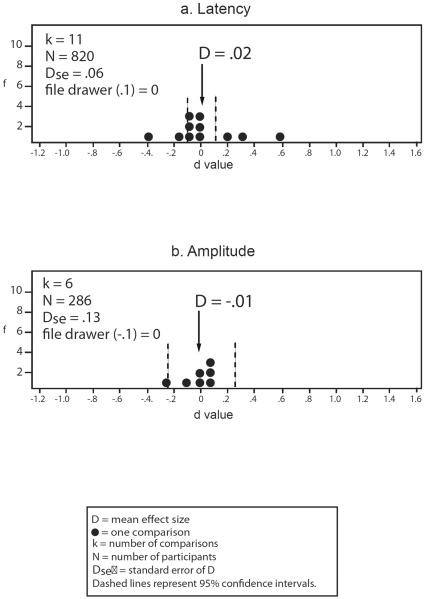
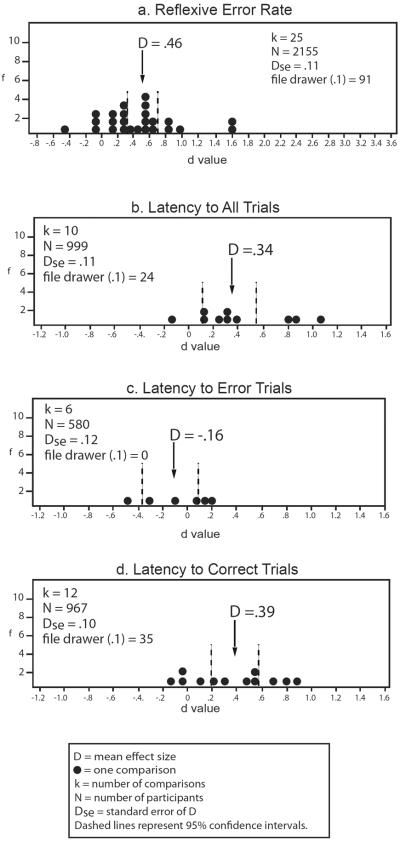
Smooth pursuit eye movement dysfunction in relatives of schizophrenia patients v. non-psychiatric controls. Frequency distributions of individual study effect sizes for group comparisons, with mean D and 95% confidence intervals. f = frequency of effect size. File drawer = number of studies required to reverse the conclusions suggested by the meta-analysis, by reducing or increasing the estimate of the population effect size to either 0.1 or −0.1, depending on the predicted relationship, specified in parentheses for each analysis. A negative effect size indicates relatives had a lower mean than controls. Effect sizes are interpreted as: .2 = small, .5 = moderate, .8 = large.
Smooth Pursuit Component Measures of EMD
Prior to the review of Clementz and Sweeney (1990), no review had addressed findings regarding smooth pursuit component measures. Moreover, many studies in this domain have been published since subsequent reviews (Levy, Holzman, Matthysse, & Mendell, 1993; MacAvoy & Bruce, 1995). A more recent review touched on conclusions drawn by some researchers in this domain, but did not review particular studies (Lee & Williams, 2000). Thus, the extant literature on smooth pursuit component measures of EMD in schizophrenia patients' relatives has heretofore been unreviewed.
It has been suggested that if SPEMD is genetically influenced, the nature of the deviation should be the same in both schizophrenia patients and their biological relatives (Levy, Holzman, Matthysse, & Mendell, 1993). On the basis of their review of studies available at the time, Levy and colleagues (1993) concluded that first-degree biological relatives of schizophrenia patients appeared to exhibit deviant closed-loop gain, but not increased rates of intrusive saccades, a pattern they believed consistent with the pattern implicated in schizophrenia patients (there were no studies of corrective saccades in relatives at that time). However, as noted by Levy et al. (1993), two investigations had reported excess intrusive saccades in schizophrenia patients' relatives. Moreover, subsequent investigations that yielded increased anticipatory saccade rates in relatives have led to the suggestion that such saccades may be a form of SPEMD that is specifically related to genetic risk for schizophrenia (Ross et al., 1998a). Thus, as with schizophrenia patients, the nature of SPEMD in relatives has been ambiguous.
Reduced pursuit gain
The integrity of the maintenance of smooth pursuit is most commonly assessed by the quantification of closed-loop gain, which is an index of the temporal synchrony of the eye and the target during pursuit, estimated by the ratio of the eye velocity to target velocity. Ideally, the ratio is 1.0, indicating that eye velocity closely matches target velocity. When eye velocity is unable to maintain target velocity, the ratio falls below 1.0, reflective of low gain pursuit. Figure 1a depicts two of the segments of the pursuit record from which closed-loop gain would be derived. Reviewers have tended to agree that low gain is an identifiable deviation in schizophrenia patients (Clementz & Sweeney, 1990; Hutton & Kennard, 1998; Iacono & Grove, 1993; Levy, Holzman, Matthysse, & Mendell, 1993), consistent with an abnormality somewhere in the smooth pursuit eye movement system.
Meta-analytic results of relative studies are presented in Figure 2b. Low closed-loop gain scores indicate that eye velocity was unable to maintain target velocity; hence, negative effect sizes indicate that the first group in the comparison demonstrated lower pursuit gain than the second group in the comparison. Relatives of patients with schizophrenia evidence moderately lower mean gain than non-psychiatric controls. Thus, meta-analytic results are consistent with the conclusion that relatives of schizophrenia patients are identifiably deviant in their smooth pursuit functioning as assessed by closed-loop gain.
Low gain can result from impingement in numerous brain regions, as evidenced by its occurrence in individuals with neurological conditions or lesions affecting diverse brain regions (Abel & Ziegler, 1988; MacAvoy & Bruce, 1995). The initiation and maintenance of smooth pursuit in response to a target begins with transmission of visual information from the eye to the thalamus (lateral geniculate nucleus), and on to a complex oculomotor system including visual cortex (striate and prestriate cortex, medial temporal and medial superior temporal), parietal cortex (posterior parietal), frontal cortex (frontal eye fields), pons, cerebellum, medulla and ultimately to the ocular motor nuclei (III, IV and VI) that generate the smooth pursuit eye movements (MacAvoy & Bruce, 1995; Sharpe, 1998). Therefore, the low gain observed in schizophrenia patients has been regarded as a non-specific smooth pursuit dysfunction that may not, in and of itself, shed light on the nature of the pursuit dysfunction (Hutton & Kennard, 1998). In addition, the possibility has been raised that reduced closed-loop gain is not reflective of a smooth pursuit dysfunction per se, and instead, may relate to other phenomena, such as expectancy effects, given that targets used to investigate closed-loop gain are generally predictable (Clementz & McDowell, 1994; Sweeney et al., 1999). As a result, some investigators have recommended combining the examination of closed-loop gain with the investigation of other aspects of pursuit functioning to more precisely shed light on the nature of pursuit dysfunction in schizophrenia (e.g., Clementz, Reid, McDowell, & Cadenhead, 1995).
In contrast to closed-loop gain, which assesses the maintenance of pursuit, open-loop gain is the average acceleration during the first 100 milliseconds of pursuit initiation (see Figure 1a). It is termed open-loop as visual feedback does not occur during this epoch; because the pursuit system cannot be updated about its performance, eye movements that occur during pursuit initiation are controlled solely by sensory input of visual motion signals (Sweeney et al., 1999). Consequently, open-loop gain has been described as potentially a better index of pursuit dysfunction than closed-loop gain (Clementz & McDowell, 1994; Clementz, Reid, McDowell, & Cadenhead, 1995; Farber, Clementz, & Swerdlow, 1997; Leigh & Zee, 1999). Relatively few investigations have been conducted on open-loop gain in schizophrenia; only two investigations of open-loop gain in schizophrenia patients' relatives have been reported, and the results were conflicting. Clementz et al. (1995) reported that relatives are impaired in pursuit initiation, whereas Thaker et al (2003) found largely unimpaired pursuit initiation. Reduced open-loop gain acceleration reported in schizophrenia patients has been interpreted as reflective of an abnormality in pursuit initiation (Clementz & McDowell, 1994; Levin et al., 1988), most likely mediated by frontal (Clementz & McDowell, 1994; Farber, Clementz, & Swerdlow, 1997; Sweeney et al., 1999), and/or possibly posterior parietal (Sweeney et al., 1999) oculomotor circuitry, but it is unclear whether relatives share this deficit.
In order to clarify the mechanisms of deviant pursuit, investigators have recommended the delineation of other eye movements observed during pursuit. Saccades are high-velocity eye movements that move both eyes from one position to another. Typically, the purpose of saccadic eye movements is to rapidly bring the image of a target onto the fovea (Avanzini & Villani, 1994). Spontaneous saccades, however, can occur in other contexts, including smooth pursuit (Pierrot-Deseilligny, 1994), as can be seen in Figure 1a.
Increased saccade rates
Early studies of saccades that occurred during smooth pursuit in schizophrenia patients typically counted "generic" saccades to arrive at an index of impairment (Levy, Holzman, Matthysse, & Mendell, 1993), that is, saccades were not sub-classified based on their characteristics or roles. Thus, in the illustration in Figure 1a, all events labeled “saccade” would be counted. Reviewers have concluded that increased generic saccade frequency appears to be characteristic of the smooth pursuit tracking of schizophrenia patients (Clementz & Sweeney, 1990; Iacono, 1988; Levy, Holzman, Matthysse, & Mendell, 1993), though again noting studies that have not found increased saccade rates (Levy, Holzman, Matthysse, & Mendell, 1993).
Generic Saccade Rates
To examine the magnitude of group differences in rates of generic saccades, we aggregated effect sizes when researchers reported generic saccade rates or three or more of the four subtypes of saccades (defined in Figure 1a caption). The results are presented in Figure 2c. Only eight studies provided generic saccade rates for relatives of schizophrenia patients in comparison with non-psychiatric controls; meta-analysis yielded a small magnitude of effect, with only three studies of no effect needed to reduce the effect size to 0.10 (see Figure 2c). The range of individual study effect sizes is wide and the confidence interval overlaps zero, suggesting that relatives of schizophrenia patients do not evidence reliably replicable abnormalities in the frequency of generic saccades that occur during their smooth pursuit tracking.
However, it has been argued that generic saccade frequencies provide limited information about the nature of the saccades that appear during pursuit (Abel & Ziegler, 1988; Levy, Holzman, Matthysse, & Mendell, 1993). In particular, the occurrence of saccades could reflect supplementation by the saccadic system to the smooth pursuit system when the smooth pursuit system is unable to maintain the velocity of the target. Alternatively, increased saccade rates could reflect a disinhibition of the saccade system, leading to the disruptive intrusion of saccades into smooth pursuit. Thus, although impairment during smooth pursuit tasks does appear to be an identifiable deviation in schizophrenia patients as evidenced by robust global dysfunction, decreased gain and increased generic saccade rates, it remains unclear whether the EMD in schizophrenia is a deviation of (1) the smooth pursuit system, perhaps with compensatory actions of the saccadic system (e.g. Abel, Friedman, Lesberger, Maliki, & Meltzer, 1991), (2) the saccadic system (e.g., disinhibition) (Levin, 1984), (3) both the saccadic and smooth pursuit systems (MacAvoy & Bruce, 1995), (4) either the saccade system or the smooth pursuit system, such that each anomaly is a pleiotropic manifestation of the same neuropathology (Clementz, Iacono, & Grove, 1996).
Corrective and Intrusive Saccades
To address perceived limitations in a generic saccade classification scheme, researchers have classified saccades that occur during smooth pursuit based largely on the putative role each plays, differentiating saccades that appear to correct for smooth pursuit deficiencies (corrective saccades) from saccades that disrupt tracking (intrusive saccades) (Leigh & Zee, 1999). Corrective or compensatory saccades include catch-up and back-up saccades; they serve to refoveate the eye to the target during pursuit when the eye position is not temporally synchronous with the target, and position error has thereby accumulated. Examples of catch-up and back-up saccades are given in Figure 1a. As implied by the nomenclature, catch-up saccades take the eyes from a position behind the target to a position on or near the target. Back-up saccades take the eyes from a position ahead of the target back to the target, or take the eyes from the target to a position behind the target (see Figure 1a). In an interactive manner, when the smooth pursuit system is unable to maintain the velocity of the target, the saccadic system generates a corrective saccade that serves to bring the eyes back to the target (Abel, Friedman, Jesberger, & Meltzer, 1988). Thus, low gain pursuit accompanied by increased catch-up saccade frequency could be reflective of an impaired smooth pursuit system but intact tolerance for eye position error (Hutton & Kennard, 1998). Conversely, low gain pursuit unaccompanied by increased rates of catch-up saccades could implicate a defect in smooth pursuit functioning accompanied by an increased tolerance for eye position error (Hutton & Kennard, 1998).
Comparisons of rates of catch-up saccades in patients with schizophrenia and healthy control subjects have yielded mixed results (Levy, Holzman, Matthysse, & Mendell, 1993). Nevertheless, several reviewers have concluded that the most commonly reported SPEM deficit exhibited by schizophrenia patients appears to involve impaired smooth pursuit functioning, characterized by low gain with consequent increased corrective action for position error by the saccadic system via corrective saccades (Hutton & Kennard, 1998; Levy, Holzman, Matthysse, & Mendell, 1993). In contrast, Clementz and Sweeney (1990) suggested that the observed high frequency of generic saccades could also be reflective of increased intrusive saccades. Intrusive saccades ostensibly disrupt tracking by purposelessly removing the target's image from the fovea (Leigh & Zee, 1999). Anticipatory saccades are large amplitude intrusive saccades occurring in the direction of target motion, moving the eye from a position on or near the target to a position ahead of the target. Square wave jerks are intrusive saccades consisting of a pair of small amplitude saccades separated by a brief intersaccadic interval, preceded and followed by pursuit. Examples of square wave jerk saccades are illustrated in Figure 1a (See Figure 1a). The observation of increased rates of intrusive saccades in schizophrenia patients has been interpreted as consistent with a failure of inhibitory input, possibly from the frontal eye fields, leading to a failure to suppress unnecessary saccades (e.g., by the superior colliculus and basal ganglia) (Levin, 1984). However, reviewers have not agreed on whether intrusive saccades represent an identifiable deviation in schizophrenia patients. MacAvoy and Bruce (1995) concluded that more than half of the studies they reviewed indicated that intrusive saccades played a role in the disrupted tracking of schizophrenia patients. Conversely, Hutton and Kennard (1998) described the evidence for increased saccadic intrusions as sparse, and Levy et al. (1993) concluded that intrusive saccades do not distinguish schizophrenia patients from non-psychiatric controls.
Thus, despite suggesting tentative conclusions, reviewers have frequently concluded that the research conducted on the nature, prevalence and characteristics of component smooth pursuit defects has been mixed (e.g., Hutton & Kennard, 1998; Iacono & Grove, 1993; Lee & Williams, 2000; Levy, Holzman, Matthysse, & Mendell, 1993). To assess performance in relatives, we compared rates of corrective and intrusive saccades in relatives and controls.
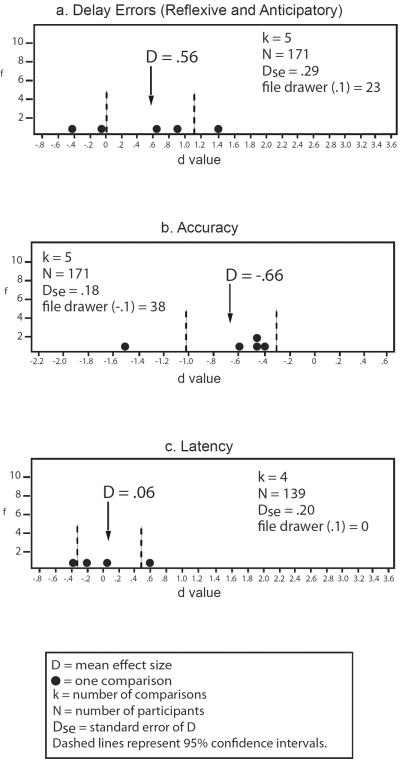
Meta-analytic results are presented in Figure 2d. Catch-up saccade rates do not well differentiate relatives from controls, although there is clearly great variability in individual study results (see Figure 2d). Surprisingly, relatives exhibit reduced rates of back-up saccades compared to controls [D = −.29, Dse = 0.05, k=2, n = 100, −0.39 < D < −.19, file drawer (−.1)=5] based on only two studies. Thus, the limited evidence on corrective saccades does not support reliably increased compensatory saccade production in relatives.
In contrast, schizophrenia patients' relatives demonstrate small to moderately increased rates of anticipatory saccades (see Figure 2e). The subtypes of intrusive saccades differ in discriminating schizophrenia patients' relatives from non-psychiatric controls; rates of square-wave jerks are actually lower in relatives [D = −.25, Dse = 0.21, k=3, n = 142, −0.66 < D < −.16, file drawer (−.1) =4], as suggested by three studies, but note the similarly small number of non-significant studies needed to reduce this magnitude to a non-significant level. Hence, where schizophrenia patients' relatives exhibit increased rates of intrusive saccades, they appear to be anticipatory saccades, rather than square-wave jerks.
Potential Moderators of SPEMD
The SPEMD meta-analyses presented in Figure 2 yielded credibility intervals that bracketed an effect size range > 1.0 and overlapped with zero, indicating that the mean effect size is likely to represent the mean of several sub-populations4. Thus, it is likely that particular study or participant characteristics are moderating the relationship between SPEMD and schizophrenia. Reviewers of the literature have often concluded that important characteristics have varied substantially from study to study rendering it difficult to interpret and compare study findings (e.g., Hutton & Kennard, 1998; Levy, Holzman, Matthysse, & Mendell, 1993). Though the reasonably small number of relative studies limits the power to conduct meaningful moderator analyses, we evaluated selected primary design characteristics that (1) have been hypothesized to contribute to inconsistent results, and; (2) exhibit sufficient, but not complete, variability across studies: measures of impairment measures, eye movement recording methods and task characteristics.
Measures of impairment
Debates over the most suitable measures of assessing smooth pursuit impairment have been characterized as “arguably the most vexing and polarized in the study of schizophrenia linked cognitive and information processing dysfunction” (Braff, 1998, p. 185). The appropriateness of global measures of smooth pursuit dysfunction has been particularly questioned, in large part because these measures do not distinguish smooth pursuit from saccade functioning (Abel & Ziegler, 1988). However, as discussed by Ross et al. (1998) and Lipton et al. (1983), global measures may play a role that is complementary to that of SPEM component measures. Furthermore, Iacono (1993) concluded that there was no empirical evidence, based on a review of relevant studies available at that time, to indicate that global measures like RMS are less preferable indices of an identifiable SPEM deviation than SPEM component measures. Among the global measures, which have been reported to highly inter-correlate (Iacono & Grove, 1993; Lindsey, Holzman, Haberman, & Yasillo, 1978; Siever, Coursey, Alterman, Buchsbaum, & Murphy, 1984), there has been discussion about the most suitable global measures for the detection of schizophrenia impairment (Clementz, Iacono, & Grove, 1996; Levy, Holzman, Matthysse, & Mendell, 1993).
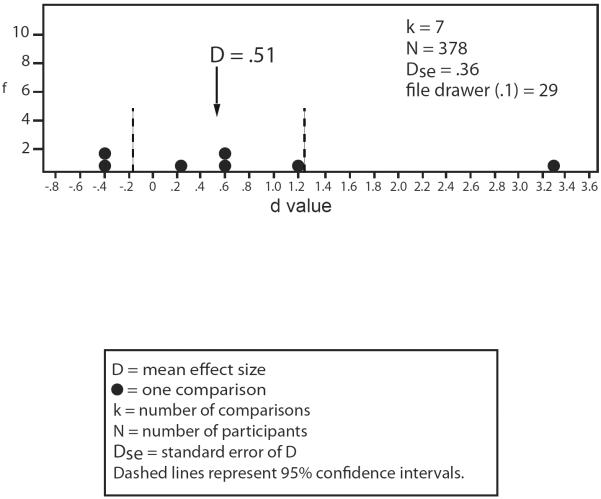
To shed additional empirical light on this issue, we evaluated effect sizes of different global measures. Both global quantitative [D = 0.40, Dse = 0.04, k = 13, n = 1476, 0.33 < D < 0.47, file drawer (0.1) = 39] and global qualitative [D = .74, Dse = 0.20, k = 10, n = 620, 0.36 < D < 1.13, file drawer (0.1) = 64] methods differentiated schizophrenia patients' relatives from non-psychiatric controls with a moderate magnitude of effect, though the effect size yielded by qualitative ratings was significantly greater (z = 2.15). Thus, regardless of method, schizophrenia patients' relatives evidence an identifiable deviation in their globally assessed pursuit functioning.
Among the SPEM component measures used in the schizophrenia EMD literature, closed-loop gain and the saccade subtypes have been identified and quantified in widely varying ways. Closed-loop gain is estimated using several methods that could differ in their discriminatory power (Levy, Holzman, Matthysse, & Mendell, 1993), including time weighted average gain (average of gain segments, weighted by duration of each segment, across the tracking record), average gain (average of gain segments, unweighted by duration of gain segments); frequency domain gain (gain calculated in the frequency domain through Fourier analysis); peak gain (average of trial-by-trial ratio of peak eye velocity to target velocity at that peak) ; other and unspecified gain (various other methods of calculating gain) or method was not identified. In subsets of studies formed on this basis, all of these methods yielded a small to moderate effect size difference between relatives and controls. The smallest effect was for “other and unspecified gain” [D = −.20, Dse = 0.07, k = 6, n = 520, −.34 < D < −.05, file drawer (−0.1) = 6] and the largest for frequency domain gain [D = −.73, Dse = 0.11, k = 3, n = 204, −.95 < D < −.51, file drawer (−0.1) = 19], which was a significant difference (z=2.89). Nine pairwise comparisons among the remaining effect sizes associated with each method yielded statistically different effect sizes among all pairs except frequency domain gain and time-weighted average gain [D = −.51, Dse = 0.17, k = 7, n = 656, −.84 < D < −.18, file drawer (−0.1) = 29]. Thus, though choice of impairment measures does not affect the ability to detect relative v. control differences, it may moderate the magnitude of the corresponding effect size. However, it is important to note that these analyses are based on a very small number of studies, and as such their results are particularly vulnerable to the influence of other characteristics on which the studies may vary.
We were unable to meta-analyze other dependent measure characteristics that vary considerably across studies. In particular, individual research team's criteria for the identification of saccade subtypes may affect estimates of saccade rates. For example, Ross and colleagues have presented evidence that varying amplitude and post-saccadic slowing criteria for the identification of anticipatory saccades affects the magnitude of the effect size obtained (Ross, Olincy, & Radant, 1999). Additional studies are required that share common criteria for classifying saccades to determine whether such results will routinely affect estimates of saccade subtypes.
Eye movement recording method
The two most frequently utilized eye movement recording methods in the schizophrenia EMD literature are horizontal electro-oculography (HEOG) and infrared oculography (IROG). HEOG, which measures changes in the corneo-retinal potential of the eyes as they move, was the predominant method of eye movement assessment in the earliest eye tracking studies. Current researchers most frequently employ IROG, which detects horizontal eye movements via scleral reflectance. It has been suggested that the ability to detect differences in the global SPEMD of schizophrenia patients and non-psychiatric controls is independent of the eye movement recording method (Holzman, 1985; Holzman, 1992; Iacono, 1988; Lipton, Levy, Holzman, & Levin, 1983). However, because IROG is better able to discern small eye movements than HEOG, several reviewers have suggested that oculographic measurement techniques employed among investigations of smooth pursuit may contribute to discrepancies in results (e.g., Hutton & Kennard, 1998). For global studies, which have a sufficient number of studies using both technologies, we therefore conducted analyses to determine whether the eye movement recording method affected the ability to identify a deviation in relatives compared to non-psychiatric controls. The magnitude of effect of global assessment was not significantly moderated by the recording method, indicating that both IROG [D = .65, Dse = 0.14, k = 16, n = 962, .38 < D < .93, file drawer (0.1) = 88] and HEOG [D = .42, Dse = 0.06, k = 7, n = 944, .31 < D < .54, file drawer (0.1) = 23] sufficiently differentiate relatives from non-psychiatric controls (z=1.51).
Task characteristics
In general, smooth pursuit tasks vary along several dimensions, including the waveform generated by the target, the degrees of visual angle spanned, the velocity of the target, and the predictability of target motion (several of these dimensions are interrelated.) Tasks designed to elicit smooth pursuit include sinusoidal and constant velocity tasks. Sinusoidal tasks vary in their frequencies during the course of target motion such that they mimic the movement of a pendulum, slowing down at the extremes of target motion and speeding up in the middle. Conversely, as the name implies, a constant velocity task consists of a target that maintains a steady velocity throughout the range of target motion (such as depicted in Figure 1a). Both the form and velocity of sinusoidal and constant velocity targets have varied across studies. While it has been suggested that global estimates of SPEMD are relatively invulnerable to task characteristics (Holzman, 1985; Iacono, 1988; Lipton, Levy, Holzman, & Levin, 1983), several reviewers have postulated that target speed and waveform can affect estimates of gain and/or saccade rates (Clementz, 1998; Hutton & Kennard, 1998; Levy, Holzman, Matthysse, & Mendell, 1993). As summarized by Hutton & Kennard (1998), one possible explanation for the inconsistencies noted across studies in discriminating ability of gain is “different research groups using different targets, moving at different speeds, in different waveforms” (p. 605). Therefore, by grouping investigations of SPEMD according to target waveform, we examined whether this particular target characteristic moderated the discriminability of relatives and controls. For global measures, comparably moderate effect sizes were obtained using both constant velocity [D = .49, Dse = 0.11, k = 7, n = 495, .27 < D < .70, file drawer (0.1) = 27] and sinusoidal [D = .56, Dse = 0.12, k = 16, n = 1411, .33 < D < .78, file drawer (0.1) = 73] tasks (z=.44). Similarly, closed-loop gain is substantially lower in relatives than in non-psychiatric controls during both constant velocity [D = −.36, Dse = 0.09, k = 17, n = 1549, −.54 < D < −.18, file drawer (−0.1) = 44] and sinusoidal [D = −.53, Dse = 0.08, k = 10, n = 740, −.69 < D < −.38, file drawer (−0.1) = 43] tasks, which do not significantly differ in effect sizes (z=1.43). Available evidence thus suggests that target waveform does not influence the magnitude of group differences for these measures of pursuit.
Participant Characteristics
We were unable to meta-analyze a number of participant characteristics that could impact study results, but for which there is too much variability among studies in relation to the number of family studies to provide for meaningful moderator analyses. A primary characteristic on which studies vary is the medical and psychiatric inclusion/exclusion criteria for relatives. Because some oculomotor impairments are observed in other non-psychotic diagnostic groups (e.g., mood disorders) (Clementz & Sweeney, 1990; Hutton & Kennard, 1998; Iacono & Grove, 1993; Levy, Holzman, Matthysse, & Mendell, 1993; Spohn & Larson, 1983; Spohn & Patterson, 1979), and because particular medical conditions may impact oculomotor performance (e.g., Leigh & Zee, 1999), estimates of EMD in relatives may be affected by inclusion of relatives with these conditions, especially if compared to medically and psychiatrically healthy controls (Snitz, Macdonald, & Carter, 2006). Inclusion of relatives with non-psychotic illness is common in schizophrenia family studies, and sensible for conditions like schizotypy that are believed to be genetically related to schizophrenia (e.g., Grove, Lebow, Clementz, Cerri, & Iacono, 1991), but there is no consensus on methods of investigating the relationship between neurocognition and psychopathology in such families.
Three primary strategies can be employed: 1) inclusion of all relatives without evaluating relationships between EMD and psychopathology/medical conditions; 2) a priori exclusion of relatives with psychopathology and/or medical conditions; and rarely, 3) post hoc exclusion of relatives with psychopathology and/or medical conditions, to determine whether differences between relatives and controls are upheld. Because other disorders may share genetic susceptibility (Craddock, O'Donovan, & Owen, 2006) and EMD with schizophrenia, the risk of strategy 1 is that relatives with psychopathology may contribute to a misinterpretation of deficits as specifically associated with schizophrenia genetic liability. This strategy obscures overlap among disorders. Conversely, strategy 2 risks “throwing the baby out with the bath water” by potentially excluding genetically informative individuals. Strategy 3 strengthens conclusions regarding EMD in psychiatrically healthy relatives, but does not explicitly inform understanding of EMD in relatives with non-psychotic psychopathology. A potential solution is to subgroup relatives according to psychopathology/medical conditions and directly compare across disorders, but this approach requires large samples.
For the SPEMD literature, we aimed to conduct moderator analyses based on relative inclusion/exclusion criteria, but as can be seen in the summary of relative exclusion criteria presented in Table 1, relevant information is either not reported or is not clearly stated in a large percentage of SPEMD studies (42.86%) (see Table 1). Moreover, among those that do specify criteria, there is little overlap in strategies for including relatives for participation. This shortcoming in the literature not only limits the ability to meta-analyze the effects of psychopathology and medical conditions on EMD performance in schizophrenia, it also limits the ability to address the important endophenotype criteria that EMD co-segregates with illness in families, such that individuals with schizophrenia and related disorders are more likely to exhibit EMD than relatives with non-psychotic disorders or no psychopathology. At the same time, it does not allow a quantitative synthesis of performance in medically and psychiatrically healthy relatives who are potentially “latent” gene carriers.
Table 1.
Summary of psychopathology and medical exclusion criteria for relatives in schizophrenia EMD family studies
| Eye Movement Task |
||||
|---|---|---|---|---|
| Exclusion Criteria | SPEM | Antisaccade | ||
|
|
|
|||
| k | % | k | % | |
| Neither psychopathology nor medical conditions basis for exclusion | 7 | 16.67 | 1 | 4.55 |
| Both psychopathology and medical conditions basis for exclusion | 4 | 9.52 | 4 | 18.18 |
| Exclude for psychopathology, but not for medical conditions | 2 | 4.76 | 0 | 0.00 |
| Exclude for medical conditions, but not for psychopathology | 11 | 26.19 | 2 | 9.09 |
| Either medical or psychopathology status is basis for exclusion, the other is unclear | 6 | 14.29 | 11 | 50.00 |
| Either medical or psychopathology status is not basis for exclusion, the other is unclear | 1 | 2.38 | 3 | 13.64 |
| Both psychopathology and medical condition exclusions are unreported or unclear | 11 | 26.19 | 1 | 4.55 |
|
|
||||
| Total | 42 | 22 | ||
Note: k= number of studies, %= percent of studies with exclusion criteria
Few studies have specifically addressed the co-segregation of SPEMD with schizophrenia related disorders in families by studying relatives with and without schizophrenia spectrum conditions. Thaker et al. (1998) and Avila et al. (2002) presented two subgroups of relatives, divided based on presence of threshold schizophrenia spectrum symptoms. Because it would be predicted that EMD is present in relatives with disorders related to schizophrenia (e.g., Iacono, 1998), we included only the subgroups of relatives with schizophrenia spectrum symptoms in the meta-analyses of SPEMD depicted in Figure1. Also excluded were the relative subgroup described as “least likely genetic” carriers reported by Ross et al. (1998b). When all relatives from these investigations were included, D values were reduced, but were not significantly different: Global: D = .46, Dse = .10, k = 24, n = 2103, .29 < D < .63, file drawer (.1) = 86; closed-loop gain: D = −.38, Dse = .06, k = 30, n = 2499, −.51 < D < −.25, file drawer (−.1) = 85; anticipatory saccade rate: D = .32, Dse =.07, k = 18, n = 1484, .18 < D < .45, file drawer = 39. Within study follow-up analyses reported by individual research teams have been consistent with greater SPEMD in schizophrenia spectrum v. non-spectrum relatives in most (Arolt, Lencer, Nolte, Pinnow, & Schwinger, 1996; Clementz, Grove, Iacono, & Sweeney, 1992; Clementz, Sweeney, Hirt, & Haas, 1990; Lencer, Trillenberg-Krecker, Schwinger, & Arolt, 2003), but not all (Sporn et al., 2005) investigations. Thus, current evidence is tentatively, though not universally, in support of co-aggregation of SPEMD with illness in schizophrenia families.
Family Study Evidence for the Heritability of SPEM
Early twin studies of globally assessed EMD provided evidence that EMD was genetically influenced in families of individuals with psychosis (Holzman, Kringlen, Levy, & Haberman, 1980; Holzman, Kringlen, Levy, Proctor, & Haberman, 1978; Holzman et al., 1977), with significantly greater intraclass correlations or twin concordance for poor tracking in monozygotic compared to dizygotic twins. Three studies of healthy twins reported intraclass correlations in monozygotic twins ranging from .49 to .68 (Iacono & Lykken, 1979; Katsanis, Taylor, Iacono, & Hammer, 2000; Iacono, 1982) and from .14 to .35 in dizygotic twins (Katsanis, Taylor, Iacono, & Hammer, 2000; Iacono, 1982); indicating greater similarity in MZ twins. In the only study reporting heritability for globally assessed SPEM, Katsanis et al. obtained an estimate of h2=.57 for RMS in a sample of 11–12 and 17–18 year old healthy twins (Katsanis, Taylor, Iacono, & Hammer, 2000), suggesting a significant genetic influence on SPEM.
As with globally assessed SPEM, few twin studies have been conducted with SPEM component measures. Two studies of healthy twins obtained heritability estimates for closed-loop gain ranging from h2=.46 (Katsanis, Taylor, Iacono, & Hammer, 2000) to h2=.70 (Blekher, Miller, Yee, Christian, & Abel, 1997). Two additional studies reported significant healthy monozygotic twin intraclass correlations for closed loop gain of h2=.60 (Litman et al., 1997), and from h2=.91 to .98 depending on target waveform and velocity (Bell, Abel, Wei, Christian, & Yee, 1994). Though the results of these healthy twin investigations is consistent with a genetic influence on closed loop gain, three investigations of schizophrenia families reporting non-twin, discordant sibling intraclass correlations for closed loop gain are conflicting (Ettinger et al., 2004; Hong et al., 2006; Litman et al., 1997), reported as ranging from small and non-significant to significant (r=.44, Ettinger et al., 2004). The only reported heritability estimate in schizophrenia families for closed loop gain was small (h2=.27, n=92) (Hong et al., 2006). Notably, in that study, the heritability of another index of pursuit performance, predictive pursuit gain, was very high (h2=.90), supporting suggestions that predictive pursuit gain may be genetically mediated (Hong et al., 2006).
Only two studies, both of healthy twins, have estimated heritability of saccade component measures. In their study of young twins (n=112 pairs), Katsanis and colleagues (Katsanis, Taylor, Iacono, & Hammer, 2000) results suggested a genetic influence on generic saccade rate (h2=.66) and velocity (h2=.43), catch-up saccade rate (h2=.61), anticipatory saccade rate (h2=.62), and square-wave jerk rate (h2=.50). In contrast, low heritabilities were found for generic saccade amplitude (h2=.15) and back-up saccade rate (h2=0). The remaining study, which investigated only catch-up saccade amplitude in 47 monozygotic and dizygotic twin pairs, reported high heritabilities for two SPEM sinusoidal tasks (h2=.75 and h2=.78).
Together, the results from a small number of family studies are generally supportive of at least partial genetic control of global smooth pursuit, closed-loop gain, and rates of corrective and intrusive saccades, with the exception of back-up saccades. The finding of heritability of eye movements in healthy individuals is intriguing, suggesting that EMD may reflect an underlying genetically influenced dysfunctional process that is more prevalent in schizophrenia.
Summary: SPEMD in Families of Schizophrenia Patients
Together, the available evidence to date indicates that the smooth pursuit tracking of schizophrenia patients' relatives is characterized by global impairment, decreased closed-loop gain and increased anticipatory saccade rates. Moreover, heritability estimates from a small number of healthy twin and schizophrenia family studies are generally supportive of at least partial genetic control of these eye movements. Meta-analytic results are consistent with the suggestion that it is worthwhile to differentiate components of SPEMD; not all SPEM component measures are identifiably deviant in schizophrenia patients' relatives. The global SPEMD and low gain observed in schizophrenia patients' relatives is consistent with a smooth pursuit deviation. However, in contrast to conclusions of several previous reviewers (Hutton & Kennard, 1998; Levy, Holzman, Matthysse, & Mendell, 1993), the smooth pursuit deviation appears to be only part of the picture; there is a moderately increased rate of anticipatory saccades in schizophrenia patients' relatives. Thus, a disinhibition of the saccade system may be operating concurrently with a smooth pursuit system dysfunction, or neuropathologies of either smooth pursuit or saccade functioning manifest in different relatives. Two investigations of the association between anticipatory saccade rate and gain in relatives have yielded inconsistent results (r = −0.30, n = 99, Clementz, Grove, Iacono, & Sweeney, 1992; r = 0.41, n = 53, Clementz, Sweeney, Hirt, & Haas, 1991). Intriguingly, evidence to date suggests that the saccadic system does not compensate for low gain through the generation of corrective (catch-up or back-up) saccades, though lack of evidence for increased catch-up saccades in relatives may simply be due to the few studies in this domain. Based on limited evidence to date, back-up saccades are neither heritable nor deviant in relatives. However, square wave jerks, which also have not reliably been found to be deviant in relatives, are nonetheless reported as heritable in healthy families, perhaps suggesting a genetically influenced eye movement that is simply not more prevalent in schizophrenia families.
Analyses of variables that could potentially moderate the relationship between relative status and global EMD, and for which there were sufficient studies to meta-analyze, indicate that the magnitude of the relationship remains small to moderate regardless of measures of impairment, eye movement recording methods, and particular task characteristics. Other variables that could not be meta-analyzed include some participant characteristics stemming from inclusion/exclusion criteria applied to family members. Individual study results nonetheless are preliminarily consistent with a co-aggregation of SPEMD with schizophrenia spectrum conditions in families. Finally, because we cumulated results across studies, this review did not address several unique and illuminating experimental task manipulations aimed at investigating the underlying neuropathology of EMD in schizophrenia (e.g. Avila, Hong, Moates, Turano, & Thaker, 2006; Chen, Nakayama, Levy, Matthysse, & Holzman, 1999; Holzman, 2000; Thaker et al., 2003).
Saccade Dysfunction in Families of Schizophrenia Patients
In the 1990's, many schizophrenia EMD researchers expanded their focus to include investigations of saccades that occur during paradigms specifically designed to elicit saccades, in contrast to the spontaneous saccades that occur during smooth pursuit. Although the saccade and smooth pursuit systems are recognized as neuro-opthalmologically distinct in several regards (Sharpe, 1998), it has been suggested that the investigation of saccades during saccadic tasks are likely to provide more specific information regarding neuropathology in schizophrenia than are investigations of smooth pursuit functioning (e.g., Kennard, Crawford, & Henderson, 1994). Several types of saccades have been investigated. Reflexive visually guided saccades, or prosaccades, are triggered externally and, as their name implies, are characterized by a reflexive saccade generated towards a sudden stimulus (Leigh & Zee, 1999). Conversely, intentional or voluntary saccades are volitional saccadic eye movements that are internally triggered by the individual in response to the pursuit of a goal (Sharpe, 1998). Among several subtypes of volitional saccades are those that are made in the direction opposite to a visual or auditory target (antisaccade) and those directed toward the remembered location of a target (memory-guided). While conducting research on these types of saccades, investigators have obtained results bearing on the status of saccade impairments as potential endophenotypic markers.
Reflexive Visually-Guided Saccade Dysfunction in Families of Schizophrenia Patients
Because reflexive saccades are externally, rather than internally driven, they are considered to be a less complex subclass of saccades than intentional saccades (Leigh & Zee, 1999). Tasks designed to elicit reflexive saccades require simply that the participant generate a saccade to a visual stimulus that appears suddenly, usually in an unpredictable location. An example of two trials of a visually guided reflexive saccade task and measures of performance are presented in Figure 1b (see Figure 1b). The production of reflexive saccades appears to involve a neural pathway of motor and premotor eye movement systems that includes the thalamus (lateral geniculate body), occipital cortex, posterior parietal cortex (parietal eye fields), frontal cortex (frontal eye fields), superior colliculus and brainstem (reticular formation) (Leigh & Zee, 1999; Sharpe, 1998). Reviewers typically conclude that there are no identifiable deviations in the velocity, latency to initiation, and amplitude of reflexive saccades in schizophrenia patients as elicited by regular visually guided saccade paradigms (e.g., Broerse, Holthausen, van den Bosch, & den Boer, 2001; Holzman et al., 1987; Hutton & Kennard, 1998; Iacono, 1988; Lipton, Levy, Holzman, & Levin, 1983; McDowell et al., 2002), suggestive of intact motor and premotor systems of eye movement control (e.g., Broerse, Holthausen, van den Bosch, & den Boer, 2001; Clementz, 1996). Support for the normalcy of visually guided reflexive saccades also stems from imaging studies with schizophrenia patients, which have failed to find abnormalities in activation of frontal and supplementary eye field, and posterior parietal cortex during prosaccades (e.g., McDowell et al., 2002).
However, the conclusion of normal reflexive saccade parameters has been questioned by some authors (e.g., Crawford, Haeger, Kennard, Reveley, & Henderson, 1995), with exceptions noted in which schizophrenia patients were impaired in one or more parameters (Clementz & Sweeney, 1990). Still others have suggested that indeed schizophrenia patients have repeatedly been demonstrated to undershoot the target (hypometria) during reflexive saccade tasks (Arolt, Teichert, Steege, Lencer, & Heide, 1998) (see Figure 1b). Crawford (1995) attributed the inconsistencies across studies to “insensitive recording techniques and other methodological shortcomings.” Thus, as observed by Clementz (1990), there has been some ambiguity about the normality of the characteristics of reflexive saccades in schizophrenia. There has been little discussion about the familial nature of reflexive saccade parameters. We therefore included an examination of the reflexive saccade latency and amplitude of schizophrenia patients' relatives.
Figure 3 presents the results of this set of meta-analyses. Relatives of schizophrenia patients are differentiated from non-psychiatric controls in neither the latency nor amplitude of their reflexive saccades (see Figure 3a and 3b). The range of effect sizes in latency indicates that there is some variability among studies, but the majority of relatives v. control effect sizes fall at or below zero, suggesting that relatives do not tend to exhibit increased saccade latencies compared to controls.
Figure 3.
Reflexive visually guided saccades in relatives of schizophrenia patients v. non-psychiatric controls. Frequency distributions of individual study effect sizes for group comparisons, with mean D and 95% confidence intervals. f = frequency of effect size. File drawer = number of studies required to reverse the conclusions suggested by the meta-analysis, by reducing or increasing the estimate of the population effect size to either 0.1 or −0.1, depending on the predicted relationship, specified in parentheses for each analysis. A negative effect size indicates relatives had a lower mean than controls. Effect sizes are interpreted as: .2 = small, .5 = moderate, .8 = large.
Antisaccade Dysfunction in Schizophrenia Families
In the antisaccade task, the participant is required to generate a saccade in the direction opposite target movement, and thus to inhibit a reflexive saccade to the target. Failure to inhibit the prepotent reflexive saccade is considered an error response. Examples of correct and incorrect antisaccade responses are depicted in Figure 1c (see Figure 1c). The neural substrates involved in the production of antisaccades overlap substantially with those involved in the production of reflexive saccades, however unlike reflexive saccades, the correct performance of an antisaccade requires higher order cognitive processes (i.e., reflexive saccade inhibition) presumably governed by the prefrontal cortex (Curtis, Calkins, & Iacono, 2001; Guitton, Buchtel, & Douglas, 1985; Sharpe, 1998). Most investigators have concurred that schizophrenia patients exhibit increased error rates and longer latencies to the initiation of correct responses (e.g., Broerse, Holthausen, van den Bosch, & den Boer, 2001; Hutton & Kennard, 1998; Hutton & Ettinger, 2006; Turetsky et al., 2007) (see Figure 1c for illustration of latencies to correct and error responses).
Antisaccade Error Rate
Because patients with lesions of the dorsolateral prefrontal cortex and/or abnormalities of the caudate also exhibit increased antisaccade error rates, whereas it appears that patients with cortical lesions of the frontal eye fields, supplementary eye fields, posterior parietal cortex and temporal cortex do not (Gaymard, Ploner, Rivaud, Vermersch, & Pierrot-Deseilligny, 1998), the increased error rate observed in schizophrenia patients has been viewed as consistent with dorsolateral prefrontal cortical dysfunction in schizophrenia (e.g., Clementz, 1998; Curtis, Calkins, & Iacono, 2001). Imaging (McDowell et al., 2002; Nakashima et al., 1994; Pauler, Escobar, Sweeney, & Greenhouse, 1996) and event-related potential (Klein, Heinks, Andresen, Berg, & Moritz, 2000) studies in schizophrenia patients and healthy individuals have tended to support this conclusion, though not unanimously (Crawford et al., 1996).
The presence of increased antisaccade error rates in relatives has been described as inconsistently demonstrated (e.g., Brownstein et al., 2003; Crawford et al., 1998; Levy et al., 2004). Levy and colleagues (2004) conducted a meta-analysis of selected studies (k=9) using a particular (“standard” or step) version of the antisaccade task, and obtained a moderate mean magnitude of effect between relatives and controls (mean Cohen's d=0.43 Levy et al., 2004). Calkins et al. (Calkins, Curtis, Iacono, & Grove, 2004) conducted a meta-analysis of performance across antisaccade paradigms, and also obtained a moderate effect size (Cohen's d=0.61). However, Levy and colleagues conducted moderator analyses, based on a small number of studies, which were interpreted as suggesting that schizophrenia patients' relatives appear impaired in the antisaccade task because studies in this realm use more stringent inclusion criteria for controls than relatives, in effect leading to the spurious appearance of a deficit in relatives. Because there are so few studies in this realm, we (Calkins, Curtis, Iacono, & Grove, 2004) conducted a re-analysis of primary data from the largest family study at that time (Curtis, Calkins, Grove, Feil, & Iacono, 2001). Antisaccade performance in medically and psychiatrically healthy relatives (n=45), who were selected from a larger sample of relatives based on criteria applied to healthy controls, was significantly more impaired than in healthy control participants (d=0.81). Moreover, excluded (n=71) and included relatives did not differ (d=0.14). An independent group compared psychiatrically healthy controls with comparably screened siblings of schizophrenia patients and obtained an effect size of d=.49 (Ettinger et al., 2004), though there have since been two failures to replicate the effect in small sample studies of healthy relatives (Boudet et al., 2005; Raemaekers, Ramsey, Vink, van den Heuvel, & Kahn, 2006).
Results from the current meta-analysis, including studies published subsequent to the 2004 reviews, are depicted in Figure 4, and again suggest moderately increased antisaccade error rates in relatives v. controls5. Yet, consistent with the suggestion by Levy et al. (2004), the credibility interval for error rate (−.47 to 1.40) indicates that moderator variables are indeed operating (see also Levy et al., this issue). As shown in Table 1, the majority of reports (68%) have not included sufficient information regarding psychopathology and medical exclusions in relatives to subcategorize the studies on this basis, and few of the remaining use overlapping bases for inclusion/exclusion. Thus, as with SPEMD, these potential moderators cannot be meta-analyzed.
Figure 4.
Antisaccade Performance in relatives of schizophrenia patients v non-psychiatric controls. Frequency distributions of individual study effect sizes for group comparisons, with mean D and 95% confidence intervals. f = frequency of effect size. File drawer = number of studies required to reverse the conclusions suggested by the meta-analysis, by reducing or increasing the estimate of the population effect size to either 0.1 or −0.1, depending on the predicted relationship, specified in parentheses for each analysis. A negative effect size indicates relatives had a lower mean than controls. Effect sizes are interpreted as: .2 = small, .5 = moderate, .8 = large.
Antisaccade Latency and Amplitude
Anomalies in response latency in the antisaccade paradigm may provide evidence of visual processing inefficiencies. The results of meta-analyses of antisaccade latencies are depicted in Figure 4. A small number of comparisons suggests that relatives demonstrate longer latencies to all trials (see Figure 4b) and to correct trials (see Figure 4d)6, but not to error trials (see Figure 4c). Thus, longer latencies to correct antisaccade responses are observed in relatives, potentially reflective of compensatory slowing due to difficulties inhibiting unwanted reflexive saccades (Curtis, Calkins, & Iacono, 2001). In general, meta-analyses of response latency indicate that it will be important for future investigations to differentiate latency to error from correct responses. Only three studies have reported examinations of antisaccade amplitude in relatives. Based on these studies, there is only a slightly decreased mean accuracy in relatives compared to controls, with only three non-significant unpublished studies needed to reduce the effect size to −.1 [D = −0.19, Dse = 0.41, k = 3, n = 138, −1.00 < D < 0.62, file drawer (−0.1) = 39].
Memory-guided Saccade Dysfunction in Families of Schizophrenia Patients
Memory-guided saccades are volitional saccades that are directed toward the remembered location of a target. A trial of a prototypical task is presented in Figure 1d. Integrity of the memory-guided saccade control system is assessed by frequency of reflexive and anticipatory errors to the remembered location during a delay period, latency to initiation after the cue to look to the remembered location, and accuracy of the eye position resulting from the memory guided saccade with regard to the location of the initial stimulus (see Figure 1d). Like the antisaccade task, the performance of accurate and timely memory-guided saccades appears to require intact dorsolateral prefrontal cortical neurons, as suggested by several lines of evidence including that focal lesions in both humans and monkeys significantly impair the ability to generate this form of saccades (Pierrot-Deseilligny, 1994; Sharpe, 1998). There have been few studies of memory-guided saccades in schizophrenia; on the basis of these studies, Clementz (1998) suggested that schizophrenia patients generate memory-guided saccades of decreased accuracy, increased reflexive errors to the stimulus cue during the delay period, and increased latency in comparison with healthy controls. This result has been viewed as consistent with results of antisaccade studies and prefrontal cortical dysfunction in schizophrenia (Clementz, 1998). Few studies have evaluated occurrence in schizophrenia patients' relatives, so the results of meta-analyses of memory-guided saccade functioning, presented in Figure 5, should accordingly be considered as very preliminary.
Figure 5.
Memory-guided saccade performance in relatives of schizophrenia patients v. non-psychiatric controls. Frequency distributions of individual study effect sizes for group comparisons, with mean D and 95% confidence intervals. f = frequency of effect size. File drawer = number of studies required to reverse the conclusions suggested by the meta-analysis, by reducing or increasing the estimate of the population effect size to either 0.1 or −0.1, depending on the predicted relationship, specified in parentheses for each analysis. A negative effect size indicates relatives had a lower mean than controls. Effect sizes are interpreted as: .2 = small, .5 = moderate, .8 = large.
Relatives of schizophrenia patients evidence identifiable deviations in comparison with non-psychiatric controls in the increased frequency of errors to the remembered location occurring after stimulus offset but prior to a cue to initiate the memory-guided saccade (see Figure 5a). In addition, relative's evidence reduced accuracy of the eye position resulting from the memory guided saccade, with respect to the location of the initial stimulus (see Figure 5b). In contrast, relatives do not exhibit longer latency to initiation after the cue (see Figure 5c)7.
Clearly, more studies with this task are required to enable a full evaluation of familiality. The extant data suggest that relatives of patients with schizophrenia exhibit more errors and responses that are less accurate than the responses of non-psychiatric controls. They also generate more frequent saccades in anticipation of target appearance; these intrusions appear consistent with the saccadic disinhibition that is observed in other oculomotor paradigms. Thus, the few studies with relatives conducted thus far are suggestive that increased inhibitory errors and decreased accuracy in memory-guided saccade paradigm may be genetically informative characteristics worthy of further exploration. Because accuracy in the memory-guided saccade task is essentially a reflection of spatial working memory (e.g., Funahashi, Bruce, & Goldman-Rakic, 1993), cite funahashi these results are consistent with the replicated report that patients with schizophrenia exhibit deficiencies in spatial working memory as assessed by nonoculomotor tasks (e.g., Park & Holzman, 1992).
Family Study Evidence for the Heritability of Saccadic Eye Movements
The heritability of saccadic eye movements has rarely been investigated. Substantial heritabilities in a healthy twin sample have been reported for reflexive saccade accuracy and latency by one group (h2 range accuracy=.60 to .83, range latency = .54 to .98, n=45 pairs, Gale, Abel, Christian, Sorbel, & Yee, 1996), along with significant intraclass correlation coefficients of accuracy and latency in a small sample (n= 8 pairs) of monozygotic twins (accuracy r=.83, latency r=.64, Bell, Abel, Wei, Christian, & Yee, 1994). However, Iacono (1982) found much lower intraclass correlation coefficients for saccade latency in both healthy MZ (n=34 pairs, r=.35) and DZ twins (n=24 pairs; r=.23), suggesting limited influence of genetic factors in that sample.
In a large sample of healthy 11- and 17-year old twins (n of pairs=638), Malone & Iacono (Malone & Iacono, 2002) reported a significant heritability of antisaccade error rate (h2=.57 in the combined sample, 95% CI= .51–.63). More recently, the Consortium on the Genetics of Schizophrenia multi-site investigation of 525 family members ascertained through a proband with schizophrenia yielded a significant heritability of h2=.42 (95% CI=.27–.57) for antisaccade error rate (Greenwood et al., 2007). Two smaller studies of sibling pairs discordant for schizophrenia yielded small (r=.33, n=70, de Wilde, Bour, Dingemans, Boeree, & Linszen, 2008) and non-significant (r<.29, n=48, Ettinger et al., 2004) sibling intra-class correlations for error rate, with the former study also reporting a small intraclass correlation for latency to correct responses (r=.32) and a negligible intraclass correlation for antisaccade amplitude (r=.08, de Wilde, Bour, Dingemans, Boeree, & Linszen, 2008).
Sibling similarity in memory-guided saccade performance has been investigated in only one small sample (n=34) study of discordant sibling pairs, which reported no significant intraclass correlations for latency or accuracy of memory-guided saccades (Landgraf, Amado, Bourdel, Leonardi, & Krebs, 2008).
In sum, the available studies are limited in number, but suggestive of a genetic influence on antisaccade performance in healthy and schizophrenia ascertained families, and inconsistently, of reflexive saccade performance in healthy twins.
Summary: Saccade Dysfunction in Families of Schizophrenia Patients
Together, the meta-analyses of antisaccade performance indicate that saccadic disinhibition is an identifiable deviation in schizophrenia patients' relatives. Increased latencies to correct response in the antisaccade task are also observed in schizophrenia patients' relatives. Evidence has been presented indicating that the dysfunction observed in relatives of schizophrenia patients varies with the degree of inhibitory load placed on the saccade system, thus implicating an inhibitory processing deficit, at least in the oculomotor domain, as a core feature of schizophrenia liability (Curtis, Calkins, Grove, Feil, & Iacono, 2001). This result has been viewed as consistent with disinhibition observed in schizophrenia patients in nonoculomotor paradigms (e.g., Clementz, 1998). Meta-analytic results indicate that, based on a limited number of studies, relatives of schizophrenia patients also evidence saccade disinhibition in the context of memory-guided saccade tasks. Thus, current meta-analytic results of saccade disinhibition in the antisaccade and memory-guided saccade paradigms, as well as decreased memory-guided saccade accuracy, are seemingly consistent with several lines of evidence suggestive of prefrontal cortical dysfunction in schizophrenia (e.g., Bertolino et al., 2000; Braver, Barch, & Cohen, 1999; Bunney & Bunney, 2000). However, the ongoing scientific debate regarding the presence of antisaccade impairment in relatives underscores the importance of careful attention to the potential impact of participant in/exclusion criteria on endophenotype performance, and begs for researchers to be explicit in reporting and critically evaluating their criteria.
Because the dorsolateral prefrontal cortex does not appear to be involved in the production of reflexive saccades (Leigh & Zee, 1999), the reported normality of reflexive saccades in schizophrenia patients has been interpreted as evidence against a neurally diffuse impairment (McDowell et al., 2002), and as additional support for the hypothesis that the origins of schizophrenia antisaccade (e.g., McDowell et al., 2002) and smooth pursuit EMD (e.g., Levin, 1984) lie in higher cortical centers (e.g., Broerse, Crawford, & den Boer, 2001; Clementz, 1996). The largely normal visually guided reflexive saccade performance of relatives of schizophrenia patients appears consistent with this interpretation. This finding is especially notable in light of evidence from some twin studies that reflexive saccade performance may be under at least partial genetic control; these sets of findings combined might suggest specificity of genetically influenced eye movement dysfunctions, rather than simply function, in schizophrenia families. However, a significant genetic influence on prosaccade latency was not found in all twin samples, leaving this question unresolved.
Fixational Instability in Families of Schizophrenia Patients
Unlike smooth pursuit and saccadic eye movements, which are typically studied as responses to moving stimuli, fixation occurs in response to the demand of maintaining stability of gaze on one location. Fixation proficiency is assessed by either quantification of saccades that intrude into a fixation epoch or qualitative ratings of fixation performance. In healthy subjects, microsaccades occur during fixation at a rate of 120 per minute (Sharpe, 1998), but larger intrusive saccades can disrupt fixation. Gaze stability during fixation, which is distinct from smooth pursuit, is controlled by neurons in regions including the brainstem, superior colliculus, parietal cortex, and frontal cortex, notably the prefrontal cortex which appears to regulate the suppression of unwanted saccades during fixation (Sharpe, 1998).
There have been few investigations (k=7) of fixational stability in schizophrenia patients' relatives. This small number of studies with relatives is suggestive that relatives generate more frequent saccades off target than non-psychiatric controls during fixation (see Figure 6). However there is clearly variability in individual study results, and the confidence interval overlaps with zero suggesting that relatives may not significantly differ from controls. Thus, additional investigations are required to obtain a more stable estimate of the magnitude of effect. The frequency of intrusive saccades during fixation reportedly increases as a function of inhibitory load; patients with schizophrenia and their relatives demonstrate a greater difficulty in maintenance of fixation with the addition of distracting stimuli (Curtis, Calkins, & Iacono, 2001; but see Hutton, Joyce, Barnes, & Kennard, 2002). Most investigators have not included distracting stimuli in their fixation paradigms, so this effect could not be meta-analyzed, but the addition of this manipulation may moderate the magnitude of the relationship and accentuate the impairment in schizophrenia patients and relatives. Overall, the investigation of fixation stability in schizophrenia is in its infancy relative to other forms of EMD. Meta-analytic results suggest that it will be worthwhile to examine other endophenotype characteristics, such as deviance in schizophrenia patients, trait-like properties, and potential moderator variables.
Figure 6.
Fixation stability in relatives of schizophrenia patients v. non-psychiatric controls. Frequency distributions of individual study effect sizes for group comparisons, with mean D and 95% confidence intervals. f = frequency of effect size. File drawer = number of studies required to reverse the conclusions suggested by the meta-analysis, by reducing or increasing the estimate of the population effect size to either 0.1 or −0.1, depending on the predicted relationship, specified in parentheses for each analysis. A negative effect size indicates relatives had a lower mean than controls. Effect sizes are interpreted as: .2 = small, .5 = moderate, .8 = large.
Impact of Narrative Reports of Non-Significance
The file drawer analyses associated with the majority of the primary meta-analyses yielding moderate effects (Figures 2–6) indicated that between 24–89 unretrieved studies would need to exist to reverse the conclusions suggested by the meta-analyses. Typically, it is assumed that non-significant results are unlikely to be published. However, in the schizophrenia EMD literature, non-significant group differences on EMD variables have been described, often in the context of significant group differences on other EMD variables within a study. Where such non-significant comparisons were merely narratively described, effect sizes could not be calculated and therefore the comparison is not reflected in the meta-analytically derived estimate of the population effect size. In addition, in some cases, tasks are described in the methods as having been administered, but the relative v. control comparison is not reported, or is not reported in a codeable form. Therefore, if for any one EMD group comparison, the number of published “non-significant” or “not reported” comparisons approached or exceeded the file drawer estimate of unretrieved studies of null effect required to reverse the conclusion suggested by the meta-analysis, the accuracy of meta-analytic results would clearly be called into question. Consequently, for each of the primary comparisons, we tallied the number of investigations that qualitatively described group comparisons as “non-significant,” or were otherwise not reported, and calculated the percent of the file drawer number that was represented by the non-significant/not reported comparisons. For example, there were two studies that collected but did not report the difference in global SPEMD between relatives and controls, while the corresponding file drawer analysis for that meta-analysis yielded 88 studies of null effect needed to reverse the conclusion of the meta-analysis. Thus, the non-reported studies accounted for only approximately 2% of the file drawer number. There were very few narrative reports of non-significant results for any given comparison (k range = 0 to 2), and for all comparisons, their existence represents a small percentage (range = 0.00% to 6.9%) of the corresponding file drawer analysis. Thus, the existence of retrieved non-significant and unreported results is unlikely to weaken the conclusions drawn from the meta-analyses.
General Discussion
This meta-analytic review examined fifteen measures of oculomotor performance in the relatives of schizophrenia patients, in order to evaluate their endophenotype candidacy: Is EMD deviant in biological relatives of schizophrenia patients? If so, what forms of EMD differentiate relatives from controls, and what features moderate the magnitude of effect? Is there other evidence from family studies for the influence of genes on EMD (e.g., heritability)? The most promising candidate EMD endophenotypes, ranked by order of relative v. control effect size, are summarized in Table 2 (see Table 2).
Table 2.
Comparisons Among Eye Movement Dysfunction Measure Effect Sizes of Differences Between Relatives of Schizophrenia Patients and Controls
| k | D | MGS AMP |
MGS ERR |
SPEM Global |
FIX INT |
ANT ERR |
SPEM CLG |
ANT LAT COR |
SPEM ASR |
ANT AMP |
SPEM SACR |
MGS LAT |
PRO LAT |
SPEM CUSR |
PRO AMP |
ANT LAT ERR |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||
| MGS AMP | 5 | 0.66 | ** | ||||||||||||||
| MGS ERR | 5 | 0.56 | 0.28 | ** | |||||||||||||
| SPEM Global | 21 | 0.52 | 0.69 | 0.15 | ** | ||||||||||||
| FIX INT | 7 | 0.51 | 0.36 | 0.11 | 0.01 | ** | |||||||||||
| ANT ERR | 24 | 0.47 | 0.95 | 0.34 | 0.43 | 0.14 | ** | ||||||||||
| SPEM CLG | 26 | 0.42 | 1 22 | 0.47 | 0.85 | 0.24 | 0.28 | ** | |||||||||
| ANT LAT COR | 12 | 0.39 | 1.33 | 0.58 | 1.00 | 0.34 | 0.50 | 0.31 | ** | ||||||||
| SPEM ASR | 15 | 0.36 | 1.55 | 0.69 | 1.41 | 0.42 | 0.79 | 0.67 | 0.25 | ** | |||||||
| ANT AMP | 3 | 0.19 | 1.04 | 0.74 | 0.78 | 0.59 | 0.64 | 0.56 | 0.47 | 0.41 | ** | ||||||
| SPEM SACR | 8 | 0.14 | 2.53* | 1.40 | 2.83* | 1.01 | 2.20* | 2.36* | 1.80 | 1.79 | 0.12 | ** | |||||
| MGS LAT | 4 | 0.06 | 2.27* | 1.46 | 2.15* | 1.12 | 1.82 | 1.78 | 1.53 | 1.46 | 0.29 | 0.36 | ** | ||||
| PRO LAT | 11 | 0.02 | 3.40* | 1.86 | 4.72* | 1.36 | 3.67* | 4.59* | 3.32* | 3.73* | 0.41 | 1.01 | 0.19 | ** | |||
| SPEM CUSR | 12 | 0.02 | 2.95* | 1.75 | 3.31* | 1.31 | 2.73* | 2.91* | 2.39* | 2.41* | 0.40 | 0.75 | 0.17 | 0.02 | ** | ||
| PRO AMP | 6 | 0.01 | 2.94* | 1.75 | 3.27* | 1.31 | 2.71* | 2.87* | 2.37* | 2.38* | 0.40 | 0.76 | 0.18 | 0.03 | 0.01 | ** | |
| ANT LAT ERR | 6 | −0.16 | 3.82* | 2.33* | 4.60* | 1.79 | 3.92* | 4.32* | 3.62* | 3.78* | 0.81 | 1.91 | 0.95 | 1.37 | 1.04 | 1.01 | ** |
| SPEM SWJR | 3 | −0.25 | 3.31* | 2.29* | 3.40* | 1.84 | 3.05* | 3.09* | 2.79* | 2.78* | 0.95 | 1.68 | 1.08 | 1.25 | 1.11 | 1.09 | 0.41 |
Note: Significance of differences between pairs of D's of group comparisons examined with z-statistic, one-tailed test with critical value of z=1.96. Table values are z-values. EMD measures in the first column are arranged from largest D Value (indicating greatest separation between relatives and controls) to smallest. MGS: AMP = Memory Guided Saccade Amplitude; MGS: ERROR = memory guided saccade delay errors; SPEM: Global = Smooth pursuit, global measures; FIX: INT = fixation, intrusive saccades; ANT: ERR = Antisaccade, reflexive errors; SPEM: CLG = smooth pursuit, closed-loop gain; ANT: LAT COR = Antisaccade latency to correct responses; SPEM: ASR, smooth pursuit, anticipatory saccade rate; ANT: AMP = Antisaccade amplitude; SPEM: SACR = Smooth pursuit, saccade rate; MGS: LAT = memory-guided saccade latency; PRO: LAT = prosaccade latency; SPEM: CUSR = Smooth pursuit catch-up saccade rate; PRO AMP = Prosaccade amplitude; ANT: LAT = antisaccade, latency to reflexive errors; SPEM: SWJR = smooth pursuit, square wave jerk rate.
Evaluation of Endophenotype Potential in Schizophrenia
Memory-guided saccade amplitude emerged as the form of EMD that best differentiates schizophrenia patients from controls, followed by memory-guided saccade error rate, globally assessed smooth pursuit, fixation instability, SPEM closed-loop gain, latency to correct antisaccade responses, and anticipatory saccades during pursuit. Importantly, the effect sizes of relatives v. controls are not significantly different among these forms of EMD. Thus, in so far as apparent deviation in schizophrenia families, these aspects of EMD are moderate in relatives, and none clearly outperforms the others. The finding that global SPEM measures are comparable to SPEM component measures suggests that though they may be less able to provide information regarding underlying pathophysiology of pursuit, they nonetheless capture genetic risk for schizophrenia. In addition to impairment in relatives, global SPEM, antisaccade error, closed loop gain, and anticipatory saccade rates also show evidence of heritability, further supporting their endophenotype potential. Notably, EMD effect sizes obtained here equal meta-analytically derived effect sizes from comparisons of relatives vs. controls on measures of neurocognitive functions like executive dysfunction, verbal memory and attention reported previously (Sitskoorn, Aleman, Ebisch, Appels, & Kahn, 2004; Snitz, Macdonald, & Carter, 2006). EMD in relatives appears to be selective: square-wave jerks, back-up and catch-up saccades, and reflexive visually guided saccades do not differentiate relatives from controls, despite that all but square-wave jerks appear to be genetically influenced in healthy twins.
Though several EMD variables appear to be replicable deviations in relatives of schizophrenia patients, a full evaluation of endophenotype characteristics is limited because few relevant studies have been conducted. In particular, family studies are needed to further investigate the familiality of memory-guided saccade performance, smooth pursuit generic and corrective saccade rates, and prosaccade hypometria. Moreover, important theoretical advancements can occur through experimental manipulations of smooth pursuit task parameters (e.g., Allen, Matsunaja, Hacisalihzade, & Stark, 1990; Clementz & McDowell, 1994; Thaker et al., 1996; Thaker et al., 1998), visually guided saccades (e.g., Broerse, Holthausen, van den Bosch, & den Boer, 2001; Clementz, 1996; Currie et al., 1993; Hutton et al., 2001; Matsue, Osakabe et al., 1994; Sereno & Holzman, 1991), antisaccades (e.g., Barton et al., 2002; Curtis, Calkins, & Iacono, 2001; McDowell & Clementz, 1997; McDowell, Myles-Worsley, Coon, Byerley, & Clementz, 1999), as well as little-studied eye movements like predictive saccades (e.g., Clementz, McDowell, & Zisook, 1994; Hutton et al., 2001).
The focus of nearly all EMD research thus has either explicitly or implicitly dealt with establishing the endophenotype potential of EMD. The current investigation suggests that this line of research does support the endophenotype potential of particular aspects of EMD. But, it also invokes an obvious and nagging question. Endophenotypes though they might be, how exactly will they be useful in the search for schizophrenia genes and their neurocognitive characterization?
Evaluation of Endophenotype Utility in Schizophrenia
Following the rapid rise of awareness of the endophenotype construct in psychiatric genetics research, some recent authors have raised questions about the utility of endophenotypes in their current form (e.g., Flint & Munafo, 2007). To address the question of utility, we return to the contention that endophenotypes can address several problems inherent to schizophrenia genetics research, by (1) identifying a more homogeneous subgroup of individuals who share susceptibility for schizophrenia (2) identifying family members who may carry the gene(s) for a disorder without manifesting the disorder itself and (3) reflecting fewer genes than the schizophrenia phenotype, therefore reducing the complexity of genetic analyses. We believe that the literature is suggestive that EMD will be useful to address these problems, but a full actualization will require work as yet undone.
Does EMD identify a more homogenous subgroup of individuals than does the diagnosis of schizophrenia?
It is in large part because the diagnostic category of schizophrenia is likely etiologically heterogeneous that researchers seek to identify endophenotypes (e.g., Cadenhead, Light, Geyer, McDowell, & Braff, 2002; Leboyer et al., 1998). Most EMD research is conducted on group differences. Ironically, if there is heterogeneity in schizophrenia, such that not all individuals with schizophrenia manifest EMD, then the group differences focus of EMD research may actually obscure EMD deficits existing in a more homogeneous subgroup of schizophrenia patients.
Evidence for Heterogeneity of EMD in Schizophrenia
Multiple lines of evidence converge in support of the heterogeneity of oculomotor performance in schizophrenia. First and most fundamentally, the vast majority of studies indicate that EMD does not occur in all patients with schizophrenia. Reports of the percentage of schizophrenia patients evidencing global SPEMD range from 12% (Blackwood, St. Clair, Muir, & Duffy, 1991) to 96% (Allen et al., 1990), with the majority of studies falling in the range of 50–86% (Holzman, Kringlen, Levy, & Haberman, 1980), compared to a range of less than 1% (Lencer, Trillenberg-Krecker, Schwinger, & Arolt, 2003) to 8% (Campion et al., 1992) in controls. Decreased closed loop gain has been reported as occurring in 20% (Kathmann, Hochrein, Uwer, & Bondy, 2003) to 75% (Campion et al., 1992)of schizophrenia patients, vs. 2% (Ross et al., 2002) to 19% (Louchart-de la Chapelle et al., 2005) in controls. Increased antisaccade error rates have been reported in 20% (Brownstein et al., 2003) to 92% (Fukushima, Fukushima, Morita, & Yamashita, 1990) of schizophrenia patients, with most studies falling in the range of 49–69%. In contrast, percentages in controls range from 16.5% (Louchart-de la Chapelle et al., 2005) to 31% (Fukushima et al., 1990). Between 14% (Blackwood, St. Clair, Muir, & Duffy, 1991) to 64% (Myles-Worsley et al., 1991) of relatives have been reported to exhibit global SPEMD, and between 9% (Kathmann, Hochrein, Uwer, & Bondy, 2003) to 55% (Louchart-de la Chapelle et al., 2005) to show low closed loop gain. Antisaccade impairment has been reported in between 5% (Brownstein et al., 2003) to 48% (Myles-Worsley et al., 1991) of relatives. Thus, although estimates vary depending on a number of variables, including impairment criteria (e.g., Iacono & Clementz, 1992), the percentage ranges clearly indicate that not all patients with schizophrenia, or their relatives, evidence EMD, leading some investigators to theorize that SPEMD is a discrete trait (Levy et al., 2000; Matthysse et al., 2004). Intriguingly, three studies that divided schizophrenia patients into good and impaired groups based on antisaccade error rates, and then compared the performance of their relatives, found higher error rates in the relatives of the impaired groups than in the relatives of the “good performers” (Brownstein et al. 2004; Curtis et al. 2001a, Crawford et al. 1998; but see MacCabe et al. 2005). Thus, there is some evidence that heterogeneity of at least antisaccade EMD is familial.
Second, mixture analysis, a statistical technique that can be used to test whether continuously distributed data are better fit by a single or two mixing distributions, has suggested that the SPEMD scores of schizophrenia patients and their relatives reflect the presence of two mixing distributions (Clementz, Grove, Iacono, & Sweeney, 1992; Iacono, Moreau, Beiser, Fleming, & Lin, 1992; Ross, Ochs, Pandurangi, Thacker, & Kendler, 1996; Ross, 2003; Ross et al., 2002). This result is consistent with heterogeneity of SPEMD by indicating that there are two groups of patients and relatives: those with poor pursuit and those with good pursuit. Thus, SPEMD appears to occur in only a subset of schizophrenia patients and their relatives, possibly reflecting a more genetically homogeneous subgroup of schizophrenia patients within the larger sample of individuals diagnosed with schizophrenia (e.g., Clementz, Grove, Iacono, & Sweeney, 1992; Iacono, Moreau, Beiser, Fleming, & Lin, 1992).
Third, a small number of studies, increasing in recent years, in which groups of schizophrenia patients or their relatives have been divided by salient clinical or neuropsychological characteristics have suggested that particular subgroups of patients and/or relatives exhibit greater EMD than other subgroups of patients and relatives (e.g., Broerse, Holthausen, van den Bosch, & den Boer, 2001; Hong, Avila, Adami, Elliot, & Thaker, 2003; Ross, 2000; Ross et al., 1998b; Sporn et al., 2005; Thaker et al., 2000).
Finally, the large credibility intervals observed in the current investigation suggest that there are moderating variables at play, but none of the hypothesized variables that we could test accounted for this effect, with the exception of closed-loop gain calculation method. We were necessarily limited to known characteristics with sufficient, but not complete, variability across studies, and we suggested potential moderators that could not be analyzed due to the small number of studies in a given study domain. Heterogeneity of EMD is a potential moderator that cannot be meta-analyzed but that could account for the observed pattern of results (e.g., Broerse, Holthausen, van den Bosch, & den Boer, 2001).
Together, these areas of investigation suggest that the group difference focus of most EMD research, though providing vital initial support for EMD as candidate endophenotypes, is likely to obscure meaningful information about heterogeneity in schizophrenia families, in turn potentially obscuring underlying genetics and neuropathology.
Evidence for Heterogeneity of Multiple Forms of EMD in Schizophrenia
In addition to heterogeneity of each form of EMD within schizophrenia samples, it is possible that there is heterogeneity of multiple forms of EMD, such that particular forms of EMD (e.g., increased antisaccade error rate and reduced gain) occur in different relatives. There have been surprisingly few investigations of the relationships among EMD deficits in schizophrenia patients despite that smooth pursuit, saccade and fixation deficits have been discussed as all occurring in schizophrenia patients. Indeed, for example, while the saccadic disinhibition observed in smooth pursuit, antisaccade, memory-guided saccade and fixation paradigms may be reflective of a common underlying inhibitory deficit, it is unclear whether we could expect a particular patient or relative evidencing one form of disinhibition to also exhibit all of the others. There are at least two methods that can be used to address this question.
First, when two or more EMD paradigms have been administered within a sample, deficit intercorrelations can be examined. Only two studies have reported correlations among EMD paradigms in relatives of schizophrenia patients. Antisaccade error rate and closed loop gain were reported to be significantly negatively correlated in two investigations(r=−.42, Karoumi et al., 2001; r= −.31, Thaker et al., 2000), indicating that higher antisaccade error rates were associated with reduced SPEM gain. Correlations between antisaccade latency and closed loop gain were not significant (Karoumi et al., 2001; Thaker et al., 2000), and neither were correlations among antisaccade performance and other smooth pursuit component measures (Karoumi et al., 2001).Thus, the relationship among multiple forms of EMD in relatives is largely unexplored using correlational approaches.
A second approach, also employed by only a few investigators to date, involves subgrouping individuals based on EMD performance, and examining performance on other forms of EMD. Two investigations have reported that in small samples of schizophrenia patients subgrouped on the basis of SPEM performance, patients with SPEMD exhibit higher antisaccade error rates than patients with normal SPEM (Matsue, Saito et al., 1994; Sereno & Holzman, 1995). In another investigation, patients identified as exhibiting SPEMD did not have more impaired fixational stability than patients without SPEMD (Gooding, Grabowski, & Hendershot, 2000). No similar investigations have been reported in samples including schizophrenia patients' relatives. However, in the Ross et al. (1998b) examination of antisaccade and memory-guided saccade performance in parents of schizophrenia patients, parents judged to be more likely genetic carriers (positive family history of schizophrenia) displayed greater inhibitory errors than controls, while parents judged to be least likely genetic carriers (negative family history) exhibited significantly reduced accuracy. These results suggest that there may be particular aspects of antisaccade and memory-guided saccade deficits that are independently genetically transmitted.
Hence, although it is possible that various forms of EMD co-aggregate within individuals or families, and as such are reflective of the same underlying neuropathology (Sereno & Holzman, 1995) and/or genetic vulnerability, at present there is little evidence that they necessarily do. Consequently, although investigations of different forms of EMD from independent samples of schizophrenia patients and/or their relatives have been interpreted as pointing to frontal or prefrontal cortical dysfunction and normal posterior functioning in schizophrenia, there has been little evidence bearing on whether any given individual with schizophrenia, or any given relative, could be expected to exhibit disinhibition in smooth pursuit, antisaccade, memory-guided saccade AND fixation tasks, but normal prosaccade functioning. Moreover, even within SPEMD, the association between closed-loop gain and anticipatory saccades occurring during pursuit remains unclear; meta-analytic results of group differences suggest that both forms of EMD occur in schizophrenia patients' relatives, but correlations between them are mixed.
In summary, in answer to our question as to whether EMD identifies a more homogeneous subgroup of individuals than does the diagnosis of schizophrenia, at this point, the literature can at the very least respond “most likely, yes.” However, given the very goal of reducing heterogeneity of schizophrenia, and in light of the evidence for heterogeneity of EMD, we recommend that investigations of heterogeneity of EMD move to the forefront.
Does EMD identify family members who may carry the genes for schizophrenia without manifesting the disorder itself?
Candidate endophenotypes should assist in identifying individuals who might carry the genetic vulnerability for schizophrenia without manifesting overt psychopathology (e.g., Iacono, 1998). Otherwise, the candidate confers little advantage over the clinical diagnosis. As Table 2 indicates, a number of EMD indices differentiate relatives, as a general group, from controls, but as suggested by Table 1, the literature varies considerably in whether and how relative's psychopathology status is obtained, described, and analyzed. Nevertheless, at least three lines of evidence indicate that EMD occurs in family members who do not manifest schizophrenia. First, among family studies, a number of studies have reported EMD in relatives specifically identified as unaffected with schizophrenia (Blackwood, St. Clair, Muir, & Duffy, 1991; Holzman et al., 1974; Myles-Worsley et al., 1991; Siegel, Waldo, Mizner, Adler, & Freedman, 1984), schizophrenia spectrum disorders (Blackwood, St. Clair, Muir, & Duffy, 1991; Clementz, Sweeney, Hirt, & Haas, 1990), or any psychiatric disorders (Arolt, Lencer, Nolte, Pinnow, & Schwinger, 1996; Blackwood, St. Clair, Muir, & Duffy, 1991; Calkins, Curtis, Iacono, & Grove, 2004; Clementz, Reid, McDowell, & Cadenhead, 1995; Holzman, Solomon, Levin, & Waternaux, 1984; Schlenker et al., 1994). Second, historic twin studies suggest higher concordance for SPEMD than for schizophrenia (Holzman et al., 1977). Third, classical high risk studies of offspring of schizophrenia patients (e.g., Erlenmeyer-Kimling & Cornblatt, 1987) and more recent “prodromal” studies (e.g., Gschwandtner et al., 2003; Nieman et al., 2007) support the occurrence of EMD in young people apparently at risk for developing schizophrenia. Thus, there is converging evidence supporting the contention that EMD can identify genetic risk carriers who do not manifest the disorder.
Does EMD Reflect Fewer Genes Than the Schizophrenia Phenotype, Thereby Reducing the Complexity of Genetic Analyses?
This is the ultimate question. How much closer are we to finding any EMD based genes for schizophrenia than we were thirty years ago when modern EMD research began? Relatively few research teams have employed EMD in genetic association designs. Weinberger, Egan and colleagues hypothesized that catechol-O-methyltransferase (COMT), which is an enzyme that metabolizes dopamine, mediates performance on candidate endophenotypes that appear to reflect prefrontal cortex abilities in schizophrenia patients and their relatives (Egan et al., 2001). Five investigations of COMT and oculomotor function have been published. Consistent with the presumed influence of DLPFC on SPEMD, Rybakowski and colleagues examined SPEMD as a function of COMT genotype in schizophrenia patients (n=117) and found that smooth pursuit performance was improved in schizophrenia patients with the Met/Met genotype (2002). However, the association was found only in male patients, leading the authors to suggest that the prefrontal dopamine system may play a sex-specific role in the oculomotor functioning of schizophrenia patients. Subsequently, Thaker and colleagues (Thaker, Wonodi, Avila, Hong, & Stine, 2004) reported that patients with the COMT Met/Met genotype exhibited reduced predictive pursuit gain than patients with Val/Val and Val/Met genotypes (total n patients=62), but healthy subjects with the Met/Met genotype had significantly better predictive pursuit gain (total n healthy subjects=53). COMT genotype explained approximately 10% of variance in predictive pursuit performance. Conversely, there was no significant effect of genotype on closed-loop gain performance. Thaker and colleagues hypothesized that Met/Met genotype and other etiological factors may jointly affect predictive pursuit performance, but in contrast to Rybakowski et al., Met/Met did not appear to affect maintenance pursuit.
On the antisaccade, Haraldsson and colleagues (Haraldsson et al., 2008) reported that, in a sample of schizophrenia patients (n=105) and controls (n=95), an increasing number of Val alleles was associated with better antisaccade performance using indices of both error rate and latency. Importantly, in a second investigation reporting COMT genotype associations with fMRI assessed brain function , Ettinger and colleagues (Ettinger et al., 2008) reported that the Val allele was associated with a pattern of BOLD response believed to reflect better performance: enhanced deactivations of prefrontal cortex (ventromedial and dorsomedial) during antisaccades when compared to prosaccades. However, no significant relationship between COMT genotype and antisaccade performance was reported in another investigation of healthy participants (n=543, Stefanis et al., 2004).
In three additional reports, Rybakowski's group reported that SPEMD was associated with polymorphisms of three other genes (Bogacki, Borkowska, Wojtanowska-Bogacka, & Rybakowski, 2005; Rybakowski, Borkowska, Czerski, Dmitrzak-Weglarz, & Hauser, 2003; Rybakowski, Borkowska, Czerski, & Hauser, 2001). First, they examined the dopamine receptor D3 (DRD3) gene located on chromosome 3q (Rybakowski, Borkowska, Czerski, & Hauser, 2001). The polymorphism is a Ser9 to Glycine9 substitution in exon 1 of the gene; excess of homozygosity has arguably been associated with schizophrenia (for reviews, see Dubertret et al., 1998; Schwartz, Diaz, Pilon, & Sokoloff, 2000; Waterworth, Bassett, & Brzustowicz, 2002; Williams et al., 1998). The functional significance of the DRD3 polymorphism is unknown. However, Rybakowski and colleagues (2001) reported that schizophrenia patients and controls with the ser-ser genotype exhibited the most impaired pursuit, those with ser/gly exhibited an intermediate level, and those with gly/gly were least impaired. The authors next reported an association between EMD and a chromosome 1q25 cytosolic PLA2 (cPLA2) gene polymorphism in their schizophrenia patient sample (controls were not reported) (Rybakowski, Borkowska, Czerski, Dmitrzak-Weglarz, & Hauser, 2003); their index of EMD (mean intensity of fixation and smooth pursuit disturbances) was reportedly higher in individuals with the A2/A2 genotype than in heterozygotes. Most recently, in a small sample of schizophrenia patients (n=40), this group reported correlations between intensity of fixation and smooth pursuit disturbances and the presence of class I and II human leukocyte antigens (HLA), which are coded for by a region on chromosome 6q21(Bogacki, Borkowska, Wojtanowska-Bogacka, & Rybakowski, 2005).
Though important in the attempt to assess gene effects on EMD, further investigation is required for several reasons. Most notably, despite that four polymorphisms were investigated in one sample, each report appeared in isolation without analysis of the relative contributions of each gene to eye tracking performance. Thus, the degree of overlap among individuals in the poor performing polymorphism identified groups is unknown. In addition, because indices of EMD were employed that are not used in other investigations in this field, rather than an index for which endophenotype properties have been well established (e.g., closed loop gain), the results may not generalize to other aspects of EMD.
In 1996, Arolt and colleagues (1996) conducted the first investigation employing SPEMD in a linkage study, using multiply affected families and reporting linkage of closed-loop gain or generic saccade frequency to chromosome 6p21-23, a chromosome that has been associated with schizophrenia in some but not all investigations (e.g., Nurnberger & Foroud, 1999; Waterworth, Bassett, & Brzustowicz, 2002). After adding two more families to their sample, Arolt et al. (1999) subsequently tested but did not find linkage of SPEMD to chromosome 22 (or 8, 9, or 20), confirming only their initial report of linkage to chromosome 6p. More recently, Matthysse and colleagues (2004), using qualitatively defined SPEMD examined in two extended pedigrees, reported linkage to a proximal region on chromosome 6p. Furthermore, Bogacki and colleagues (2005) reported that some HLA antigens coded at the 6p21-23 band were associated with EMD, interpreted as also supporting the results of Arolt et al.
Together, available studies thus implicate genes on chromosomes 1q, 3q, 6p and 22q as playing a role in SPEMD. Moreover, because there is evidence that nicotine improves smooth pursuit gain (e.g., Depatie et al., 2002; Sherr et al., 2002), other indices of pursuit (e.g., Avila, Sherr, Hong, Myers, & Thaker, 2003; Olincy, Ross, Young, Roath, & Freedman, 1998), and antisaccade performance (Depatie et al., 2002; Larrison-Faucher, Matorin, & Sereno, 2004), it has been hypothesized that cholinergic receptor dysfunction may contribute to EMD in schizophrenia patients (Olincy, Ross, Young, Roath, & Freedman, 1998), possibly via alpha7 nicotinic receptor dysfunction (Olincy, Johnson, & Ross, 2003). Thus, further investigation of multiple gene effects on EMD is warranted.
The only genetic linkage study to include the antisaccade task reported linkage at D22S315 on chromosome 22q11-12 in eight schizophrenia multiplex families when relatives were identified by a disinhibitory endophenotype of either antisaccade deficit, or the P50 sensory gating deficit (Myles-Worsley et al., 1999). The composite endophenotype identified substantially more relatives as affected than did either of the endophenotypes alone, likely enhancing the power to detect linkage. While the linkage finding has yet to be replicated, the results are particularly notable because the linked region is the site of the COMT gene. Given the apparent involvement of the DLPFC in the accurate performance of the antisaccade task, the association between antisaccade performance (and/or P50) and chromosome 22q could be partially explicable by COMT effects. However, the P50 sensory gating deficit has received considerable attention because it has been linked to a dinucleotide polymorphism at chromosome 15q13-14, the site of the alpha 7-nicotinic receptor (Freedman et al., 1997; Freedman et al., 2001). Leonard and colleagues reported that the alpha7 promoter polymorphism, a -86-bp C to T variant, in healthy individuals was associated with the P50 deficit (Leonard et al., 2002). The finding of linkage between the P50 endophenotype and chromosome 15 is supportive of the utility of employing phenotypes other than schizophrenia in linkage analysis (e.g., Waterworth, Bassett, & Brzustowicz, 2002). Moreover, this result is consistent with other lines of evidence indicating that nicotinic receptor expression and function is abnormal in schizophrenia (e.g., Adler et al., 1998; Leonard et al., 2000) and associated with EMD (Avila, Sherr, Hong, Myers, & Thaker, 2003; Depatie et al., 2002; Larrison-Faucher, Matorin, & Sereno, 2004; Olincy, Johnson, & Ross, 2003; Sherr et al., 2002). Although the functional role of nicotinic receptors is yet unknown (for review, see Weiland, Bertrand, & Leonard, 2000), the association between P50 and antisaccade performance raises the possibility that cholinergic systems could influence antisaccade performance. Thus, both COMT and alpha7 genes could affect antisaccade performance, once again highlighting the importance of considering the possible effects of multiple genes on endophenotype performance.
Overall, despite widespread agreement that EMD will assist in the schizophrenia gene hunt, and though progress clearly has been made in the last ten years, no EMD related genes have been definitively identified. Converging lines of evidence are most supportive for the role of genes on chromosome 6p and 22q, but lack of support for other genes may simply be due to a lack of studies in these areas. Ironically, endophenotype researchers must be wary of some of the same pitfalls that have thwarted the search for schizophrenia genes, including phenotypic (or perhaps “endophenotypic”) heterogeneity. Moreover, the dearth of knowledge regarding the relationships among forms of EMD invokes the possibility that the observed saccade, smooth pursuit and fixation deficits may constitute unrelated risk factors that identify different types of genetic risk (Calkins & Iacono, 2000). An alternative to the use of a single endophenotype is to combine endophenotypes or to create a composite, multivariate phenotype in order to better identify genetic risk (Grove, Lebow, Clementz, Cerri, & Iacono, 1991; Iacono & Grove, 1993; Iacono, Carlson & Malone, 2000). The results of this meta-analytic review indicate that several forms of EMD are individually promising candidate endophenotypes that might together form genetically informative composite endophenotypes. Large family studies utilizing comprehensive batteries of EMD tasks would provide a mechanism to determine whether the observed smooth pursuit, antisaccade, memory-guided saccade and fixation deficits could be combined to identify a homogeneous set of patients with schizophrenia, or whether different subsets of patients are identified by each deficit. If the deficits tend to co-aggregate in schizophrenia patients, then it would suggest that the combined set of deficits may mark a single variant of genetic risk for schizophrenia. However, if the deficits are largely independent of one another, they may assist in the identification of different variants of genetic risk (Calkins & Iacono, 2000). The validity of different variants of genetic risk would depend in part on the co-aggregation of deficits in schizophrenia families, and ultimately on their success in genetic analyses. Thus, multivariate studies with large samples that examine the interrelatedness of EMD measures are needed as they have the potential to better address the questions regarding possible heterogeneity (clusters) and underlying etiologic mechanisms (factors or components of correlated EMDs). Large-scale family studies will be in an ideal position to perform such analyses.
Moreover, as an adjunct to studies investigating individual gene effects on individual candidate endophenotypes, it is important to assess the effect of multiple candidate genes on multiple candidate endophenotypes. First, although each endophenotype may have a simpler genetic basis than schizophrenia, and although evidence has been presented that single major genes may especially impact particular endophenotypes (Freedman et al., 2000; Grove, Clementz, Iacono, & Katsanis, 1992), it is unlikely that the genetic basis of all endophenotypes is monogenic rather than polygenic (e.g., Flint & Munafo, 2007; Gottesman & Gould, 2003). Thus, the investigation of the effect of multiple genes on candidate endophenotypes will be important to determine whether the genetic complexity of endophenotypes is indeed reduced relative to the schizophrenia phenotype. Second, because candidate endophenotypes are not necessarily correlated (e.g., Calkins, 2002; Toomey et al., 1998), it is unclear whether different endophenotypes are reflective of the same underlying genetic susceptibility (e.g., Calkins & Iacono, 2000). Genetic overlap among at least some endophenotypes is suggested by apparently shared neurophysiological underpinnings. Consequently, the investigation of gene effects on multiple endophenotypes will be informative regarding the ability of candidate endophenotypes to independently identify unique genetic variants of schizophrenia or in combination form a multivariate endophenotype reflective of shared genetic risk (e.g., Calkins & Iacono, 2000; Iacono, Carlson, & Malone, 2000). Once again, large-scale collaborative investigations will likely be the ideal venue for such analyses.
Conclusions
For more than thirty years, researchers have been writing about the potential for EMD to serve as a tool in the schizophrenia gene hunt. The results of this meta-analytic review suggest that the time is drawing near. Particular aspects of EMD are heritable and evident in schizophrenia families, consistent with the suggestion that they are endophenotypes of schizophrenia. It is possible that oculomotor endophenotypes will be found for other disorders as well (e.g., Bauer, 1997; Van der Stigchel et al., 2007); ideally researchers in these fields will benefit from the lessons learned by schizophrenia researchers over the last 100 years. Despite the promise of endophenotypes, relatively few researchers have attempted to apply EMD endophenotypes in molecular genetic studies. The results of studies that have ventured into this domain have been intriguing but thus far limited to a few specific candidate endophenotypes and genes. Yet, this kind of research can advance schizophrenia research by merging two fields: molecular genetics, which is in need of more genetically homogenous groups, and endophenotype research, which is in need of genes underlying apparently heritable indicators of schizophrenia risk. The actualization of the promise of endophenotypes to assist in the schizophrenia gene hunt, as yet largely unfulfilled, will rest on further research seeking to explicate the relationships among genes and candidate endophenotypes like EMD.
Acknowledgments
This research was supported by R01's MH49738, MH65578, a Neurobehavioral Traineeship (MH17069), a University of Minnesota Graduate School Doctoral Dissertation Fellowship, a Neuropsychiatry Post-Doctoral Traineeship (MH19112), a Scottish Rite Schizophrenia Research Fellowship, and a Mentored Clinical Scientist K Award (K08MH79364). Earlier datasets appearing in this paper were presented at the Society of Biological Psychiatry Annual Meeting, Toronto, Canada, May 1998 (Biological Psychiatry, 43, 126s) and at the Biennial meeting of the International Congress on Schizophrenia Research, Whistler, British Columbia, April 2001 (Schizophrenia Research, 49, s212–s213). We thank the hundreds of researchers and thousands of research participants whose efforts made this work possible, and gratefully acknowledge the effort made by the researchers whom we contacted for additional information about their work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A reference list of excluded and uncodeable articles is available from the authors upon request.
Although it is customary to obtain δ by correcting D for unreliability of measurement (Hunter & Schmidt, 1990), we elected not to report the results of these corrections for four different reasons. First, for some meta-analyses, there was insufficient measurement reliability information provided in the literature to compute δ. Second, for many of the meta-analyses, k was too small to allow for a valid estimate of δ. Third, in a broader set of meta-analyses of EMD in schizophrenia, of 69 meta-analyses in which we were able to correct for unreliability using a distribution of reliability estimates from published reports, the value of D varied from the value of δ by an average of only .04. The difference for the vast majority of meta-analyses (93%) was less than .08. In all cases, the value of δ was slightly higher than the value of D, as expected when correcting for measurement unreliability (Hunter & Schmidt, 1990). These meta-analyses involved the following variables (reliability estimates are provided in parentheses): global qualitative measures (ryy = .85, k = 22), generic saccade rate (ryy = .83, k = 5), square wave jerk rate (ryy = .82, k = 7), catch-up saccade rate (ryy = .93, k = 6), corrective saccade rate (ryy = .93, k = 6), catch-up saccade amplitude (ryy = .94, k = 4) intrusive saccade rate (ryy = .86, k = 16), anticipatory saccade rate (ryy = .89, k = 9), closed-loop gain (ryy = .87, k = 13), time-weighted average gain (ryy = .92, k = 9), and peak gain (ryy = .69, k = 2). Fourth, and most importantly, from a theoretical and applied point of view, we desired no correction for attenuation as our aim was to estimate of the “observed” differences between various groups. That is, unreliability in the measures was not corrected for because our interest was in mean score differences as determined from the actual scores of participants, rather than the theoretical standings of participants on the constructs assessed by the measures. Furthermore, operational decisions, such as determination of affected status, are made on observed scores; therefore, our interest in these meta-analyses is in observed mean scale score differences that are not corrected for attenuation. Similar rationale applied to our choice not to correct for attenuation in our correlational meta-analyses.
Relative v. control analyses were performed without selected subgroups of relatives reported by Thaker et al. (1998), Ross et al. (1998b), and Avila et al. (2002) (as discussed in text).
Results not shown but available from authors upon request.
Analyses with relatives were conducted without the relative subgroups described previously (Ross et al., 1998b; Thaker et al., 1998), and without a similarly identified subgroup of nonschizotypal relatives reported by (Thaker et al., 2000). When all relatives were included, the comparison between relatives and controls yielded: D = .42, Dse = .10, k = 28, n = 2319, .22 < D < .62, file drawer (.1) = 91.
Analyses with relatives were conducted without the relative subgroups described previously (Ross et al., 1998b; Thaker et al., 1998; Thaker et al., 2000). Including all relatives, results were: latency to all trials: D = .35, Dse = .10, k = 12, n = 1133, .11 < D < .57, file drawer (.1) = 24; latency to correct trials: δ = D = .32, Dse = .11, k = 14, n = 1068, .10 < D < .55, file drawer (.1) = 31.
Analysis with relatives was conducted without the relative subgroup described previously (Thaker et al., 1998). Including all relatives, results were: D = −.04, Dse = .18, k = 5, n = 169, −.39 < D < .32, file drawer (.1) = 0.
References
*Studies used in the meta-analyses
- Abel LA, Friedman L, Jesberger LA, Meltzer HY. Relationships between catch-up saccades frequency and smooth pursuit gain in schizophrenics and normals. Society for Neuroscience Abstracts. 1988;14:796. [Google Scholar]
- Abel LA, Friedman L, Lesberger LA, Maliki A, Meltzer HY. Quantitative assessment of smooth pursuit gain and catch-up saccades in schizophrenic and affective disorders. Biological Psychiatry. 1991;29:1063–1072. doi: 10.1016/0006-3223(91)90248-k. [DOI] [PubMed] [Google Scholar]
- Abel LA, Ziegler A. Smooth pursuit eye movements in schizophrenics: What constitutes quantitative assessment? Biological Psychiatry. 1988;24:747–761. doi: 10.1016/0006-3223(88)90250-8. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophrenia Bulletin. 1998;24(2):189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Allen JS, Matsunaga K, Nakamura T, Kitamura T, Furukawam T, Hacisalihzade SS, et al. Schizophrenia, eye movements, and biocultural heterogeneity. Human Biology. 1990;62(3):337–352. [PubMed] [Google Scholar]
- Allen JS, Matsunaja K, Hacisalihzade S, Stark L. Smooth pursuit eye movements of normal and schizophrenic subjects tracking an unpredictable target. Biological Psychiatry. 1990;28:705–720. doi: 10.1016/0006-3223(90)90457-d. [DOI] [PubMed] [Google Scholar]
- Arolt V, Lencer R, Nolte A, Muller-Myhsok B, Purmann S, Schurmann M, et al. Eye tracking dysfunction is a putative phenotypic susceptibility marker of schizophrenia and maps to a locus on chromosome 6p in families with multiple occurrence of the disease. American Journal of Medical Genetics. 1996;67(6):564–579. doi: 10.1002/(SICI)1096-8628(19961122)67:6<564::AID-AJMG10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Arolt V, Lencer R, Nolte A, Pinnow M, Schwinger E. Eye tracking dysfunction in families with multiple cases of schizophrenia. European Archives of Psychiatry & Clinical Neuroscience. 1996;246(4):175–181. doi: 10.1007/BF02188950. [DOI] [PubMed] [Google Scholar]
- Arolt V, Lencer R, Purmann S, Schurmann M, Muller-Myhsok B, Krecker K, et al. Testing for linkage of eye tracking dysfunction and schizophrenia to markers on chromosomes 6, 8, 9, 20, and 22 in families multiply affected with schizophrenia. American Journal of Medical Genetics. 1999;88(6):603–606. [PubMed] [Google Scholar]
- Arolt V, Teichert HM, Steege D, Lencer R, Heide W. Distinguishing schizophrenic patients from healthy controls by quantitative measurement of eye movement parameters. Biological Psychiatry. 1998;44(6):448–458. doi: 10.1016/s0006-3223(97)00479-4. [DOI] [PubMed] [Google Scholar]
- Avanzini G, Villani F. Ocular movements. Current Opinion in Neurology. 1994;7(1):74–80. doi: 10.1097/00019052-199402000-00014. [DOI] [PubMed] [Google Scholar]
- Avila MT, Hong LE, Moates A, Turano KA, Thaker GK. Role of anticipation in schizophrenia-related pursuit initiation deficits. Journal of neurophysiology. 2006;95(2):593–601. doi: 10.1152/jn.00369.2005. [DOI] [PubMed] [Google Scholar]
- Avila MT, McMahon RP, Elliott AR, Thaker GK. Neurophysiological markers of vulnerability to schizophrenia: sensitivity and specificity of specific quantitative eye movement measures. Journal of Abnormal Psychology. 2002;111(2):259–267. [PubMed] [Google Scholar]
- Avila MT, Sherr JD, Hong E, Myers CS, Thaker GK. Effects of nicotine on leading saccades during smooth pursuit eye movements in smokers and nonsmokers with schizophrenia. Neuropsychopharmacology. 2003;28(12):2184–2191. doi: 10.1038/sj.npp.1300265. [DOI] [PubMed] [Google Scholar]
- Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Molecular Psychiatry. 2002;7(4):405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Cherkasova MV, Lindgren K, Goff DC, Intriligator JM, Manoach DS. Antisaccades and task switching: studies of control processes in saccadic function in normal subjects and schizophrenic patients. Annals of the New York Academy of Sciences. 2002;956:250–263. doi: 10.1111/j.1749-6632.2002.tb02824.x. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Smooth pursuit eye movement dysfunction in abstinent cocaine abusers: effects of a paternal history of alcoholism. Alcoholism: Clinical & Experimental Research. 1997;21(5):910–915. [PubMed] [Google Scholar]
- Bell BP, Abel LA, Wei L, Christian JC, Yee RD. Concordance of smooth pursuit and saccadic measures in normal monozygotic twins. Biological Psychiatry. 1994;36:522–526. doi: 10.1016/0006-3223(94)90616-5. [DOI] [PubMed] [Google Scholar]
- Berrettini WH. Genetics of psychiatric disease. Annual Review of Medicine. 2000;51:465–479. doi: 10.1146/annurev.med.51.1.465. [DOI] [PubMed] [Google Scholar]
- Berry N, Jobanputra V, Pal H. Molecular genetics of schizophrenia: a critical review. Journal of Psychiatry & Neuroscience. 2003;28(6):415–429. [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Esposito G, Callicott JH, Mattay VS, Van Horn JD, Frank JA, et al. Specific relationship between prefrontal neuronal N-acetylaspartate and activation of the working memory cortical network in schizophrenia. American Journal of Psychiatry. 2000;157(1):26–33. doi: 10.1176/ajp.157.1.26. [DOI] [PubMed] [Google Scholar]
- Blackwood DHR, St. Clair DM, Muir WJ, Duffy JC. Auditory P300 and eye tracking dysfunction in schizophrenia pedigrees. Archives of General Psychiatry. 1991;48:899–909. doi: 10.1001/archpsyc.1991.01810340031004. [DOI] [PubMed] [Google Scholar]
- Blekher T, Miller K, Yee RD, Christian JC, Abel LA. Smooth pursuit in twins pre and post alcohol. Investigative Opthalmolology and Visual Science. 1997;38:1768–1783. [PubMed] [Google Scholar]
- Bogacki PA, Borkowska A, Wojtanowska-Bogacka M, Rybakowski JK. Relationship between class I and II HLA antigens in schizophrenia and eye movement disturbances: a preliminary study. Neuropsychobiology. 2005;51(4):204–210. doi: 10.1159/000085595. [DOI] [PubMed] [Google Scholar]
- Boudet C, Bocca ML, Chabot B, Delamillieure P, Brazo P, Denise P, et al. Are eye movement abnormalities indicators of genetic vulnerability to schizophrenia? European Psychiatry: the Journal of the Association of European Psychiatrists. 2005;20(4):339–345. doi: 10.1016/j.eurpsy.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Boudet C, Denise P, Bocca ML, Chabot B, Abadie P, Brazo P, et al. Eye tracking disorders in schizophrenic patients and their parents. Encephale. 2001;27(6):551–558. [PubMed] [Google Scholar]
- Braff DL. “Specific measures account for most of the variance in qualitative ratings of smooth pursuit eye movements in schizophrenia:” In reply. Archives of General Psychiatry. 1998;55:185–186. doi: 10.1001/archpsyc.55.2.184. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biological Psychiatry. 1999;46(3):312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Bray NJ, Owen MJ. Searching for schizophrenia genes. Trends in Molecular Science. 2001;7(4):169–174. doi: 10.1016/s1471-4914(01)01950-5. [DOI] [PubMed] [Google Scholar]
- Broerse A, Crawford TJ, den Boer JA. Parsing cognition in schizophrenia using saccadic eye movements: A selective overview. Neuropsychologia. 2001;39(7):742–756. doi: 10.1016/s0028-3932(00)00155-x. [DOI] [PubMed] [Google Scholar]
- Broerse A, Holthausen EA, van den Bosch RJ, den Boer JA. Does frontal normality exist in schizophrenia? A saccadic eye movement study. Psychiatry Research. 2001;103(2–3):167–178. doi: 10.1016/s0165-1781(01)00275-x. [DOI] [PubMed] [Google Scholar]
- Brownstein J, Krastoshevsky O, McCollum C, Kundamal S, Matthysse S, Holzman PS, et al. Antisaccade performance is abnormal in schizophrenia patients but not in their biological relatives. Schizophrenia Research. 2003;63(1–2):13–25. doi: 10.1016/s0920-9964(02)00438-3. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Research - Brain Research Reviews. 2000;31(2–3):138–146. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Light GA, Geyer MA, McDowell JE, Braff DL. Neurobiological measures of schizotypal personality disorder: Defining an inhibitory endophenotype? American Journal of Psychiatry. 2002;159(5):869–871. doi: 10.1176/appi.ajp.159.5.869. [DOI] [PubMed] [Google Scholar]
- Calkins ME. Unpublished Dissertation. University of Minnesota; Minneapolis, MN: 2002. The co-aggregation of smooth pursuit, saccade and fixation eye movement dysfunction in schizophrenia patients and their relatives. [Google Scholar]
- Calkins ME, Curtis CE, Iacono WG, Grove WM. Antisaccade performance is impaired in medically and psychiatrically healthy biological relatives of schizophrenia patients. Schizophrenia Research. 2004;71(1):167–178. doi: 10.1016/j.schres.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Dobie DJ, Cadenhead KS, Olincy A, Freedman R, Green MF, et al. Under Review. 2007. The Consortium on the Genetics of Endophenotypes in Schizophrenia (COGS): “Model” recruitment, assessment and endophenotyping methods for a multi-site collaboration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Iacono WG. Eye movement dysfunction in schizophrenia: A heritable characteristic for enhancing phenotype definition. American Journal of Medical Genetics. 2000;97:72–76. doi: 10.1002/(sici)1096-8628(200021)97:1<72::aid-ajmg10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Campion D, Thibaut F, Pierre D, Courtin P, Pottier M, Levillain D. SPEM impairment in drug naive schizophrenic patients: Evidence for a trait marker. Biological Psychiatry. 1992;32:891–902. doi: 10.1016/0006-3223(92)90178-3. [DOI] [PubMed] [Google Scholar]
- Cannon TD. The inheritance of intermediate phenotypes for schizophrenia. Current Opinion in Psychiatry. 2005;18(2):135–140. doi: 10.1097/00001504-200503000-00005. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Gottesman II. Twin studies of schizophrenia. American Journal of Medical Genetics (Seminar in Medical Genetics) 2000;97:12–17. [PubMed] [Google Scholar]
- Carpenter WTJ, Buchanan RW, Kirkpatrick B, Tamminga C, Wood F. Strong inference, theory testing, and the neuroanatomy of schizophrenia. Archives of General Psychiatry. 1993;50(10):825–831. doi: 10.1001/archpsyc.1993.01820220081009. [DOI] [PubMed] [Google Scholar]
- Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Psychophysical isolation of a motion-processing deficit in schizophrenics and their relatives and its association with impaired smooth pursuit. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(8):4724–4729. doi: 10.1073/pnas.96.8.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA. Saccades to moving targets in schizophrenia: Evidence for normal posterior cortex functioning. Psychophysiology. 1996;33(6):650–654. doi: 10.1111/j.1469-8986.1996.tb02360.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA. Psychophysiological measures of (dis)inhibition as liability indicators for schizophrenia. Psychophysiology. 1998;35(6):648–668. [PubMed] [Google Scholar]
- Clementz BA, Grove W, Iacono WG, Sweeney Smooth pursuit eye movement dysfunction and liability for schizophrenia: Implications for genetic modeling. Journal of Abnormal Psychology. 1992;101:117–129. doi: 10.1037//0021-843x.101.1.117. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Iacono WG, Grove WM. The construct validity of root-mean-square error for quantifying smooth-pursuit eye tracking abnormalities in schizophrenia. Biological Psychiatry. 1996;39(15):448–450. doi: 10.1016/0006-3223(95)00549-8. [DOI] [PubMed] [Google Scholar]
- Clementz BA, McDowell JE. Smooth pursuit in schizophrenia: Abnormalities of open- and closed-loop responses. Psychophysiology. 1994;31:79–86. doi: 10.1111/j.1469-8986.1994.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, McDowell JE, Zisook S. Saccadic system function among schizophrenia patients and their first-degree biological relatives. Journal of Abnormal Psychology. 1994;103:277–286. [PubMed] [Google Scholar]
- Clementz BA, Reid SA, McDowell JE, Cadenhead KS. Abnormality of smooth pursuit eye movement initiation: Specificity to the schizophrenia spectrum? Psychophysiology. 1995;32(2):130–134. doi: 10.1111/j.1469-8986.1995.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA. Is eye movement dysfunction a biological marker for schizophrenia? A methodological review. Psychological Bulletin. 1990;108:77–92. doi: 10.1037/0033-2909.108.1.77. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hirt M, Haas G. Pursuit gain and saccadic intrusions in first-degree relatives of probands with schizophrenia. Journal of Abnormal Psychology. 1990;99:327–335. doi: 10.1037//0021-843x.99.4.327. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hirt M, Haas G. Phenotypic correlations between oculomotor functioning and schizophrenia-related characteristics in relatives of schizophrenic probands. Psychophysiology. 1991;28(5):570–578. doi: 10.1111/j.1469-8986.1991.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Rev Ed ed Academic Press; New York: 1977. [Google Scholar]
- Craddock N, O'Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32(1):9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford TJ, Haeger B, Kennard C, Reveley MA, Henderson L. Saccadic abnormalities in psychotic patients: I. Neuroleptic-free psychotic patients. Psychological Medicine. 1995;25(3):461–471. doi: 10.1017/s0033291700033389. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Puri BK, Nijran KS, Jones B, Kennard C, S.W. L. Abnormal saccadic distractibility in patients with schizophrenia: a 99mTc-HMPAO SPET study. Psychological Medicine. 1996;26(2):265–277. doi: 10.1017/s0033291700034668. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Sharma T, Puri BK, Murray RM, Berridge DM, Lewis SW. Saccadic eye movements in families multiply affected with schizophrenia: the Maudsley Family Study. American Journal of Psychiatry. 1998;155(12):1703–1710. doi: 10.1176/ajp.155.12.1703. [DOI] [PubMed] [Google Scholar]
- Currie J, Joyce S, Maruff P, Ramsden B, McArthur Jackson C, Malone V. Selective impairment of express saccade generation in patients with schizophrenia. Experimental Brain Research. 1993;97:343–348. doi: 10.1007/BF00228704. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Calkins ME, Grove WM, Feil KJ, Iacono WG. Saccadic disinhibition in acute and remitted schizophrenia patients and their first-degree biological relatives. American Journal of Psychiatry. 2001;158:100–106. doi: 10.1176/appi.ajp.158.1.100. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Calkins ME, Iacono WG. Saccadic disinhibition in schizophrenia patients and their first-degree biological relatives: A parametric study of the effects of increasing inhibitory load. Experimental Brain Research. 2001;137:228–236. doi: 10.1007/s002210000635. [DOI] [PubMed] [Google Scholar]
- de Wilde OM, Bour L, Dingemans P, Boeree T, Linszen D. Antisaccade deficit is present in young first-episode patients with schizophrenia but not in their healthy young siblings. Psychological Medicine. 2008;38(6):871–875. doi: 10.1017/S0033291707001894. [DOI] [PubMed] [Google Scholar]
- Depatie L, O'Driscoll GA, Holahan A-LV, Atkinson V, Thavundayil JX, Kin NNY, et al. Nicotine and behavioral markers of risk for schizophrenia: A double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27(6):1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- Diefendorf AR, Dodge R. An experimental study of the ocular reactions of the insane from photographic records. Brain. 1908;31:451–489. [Google Scholar]
- Dubertret C, Gorwood P, Ades J, Feingold J, Schwartz JC, Sokoloff P. Meta-analysis of DRD3 gene and schizophrenia: ethnic heterogeneity and significant association in Caucasians. American Journal of Medical Genetics. 1998;81(4):318–322. doi: 10.1002/(sici)1096-8628(19980710)81:4<318::aid-ajmg8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Cornblatt B. High-risk research in schizophrenia: a summary of what has been learned. Journal of Psychiatric Research. 1987;21(4):401–411. doi: 10.1016/0022-3956(87)90087-2. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Collier DA, Powell J, Michel T, Zedomi O, et al. Catechol-O-Methyltransferase (COMT) Val-158-Met genotype is associated with BOLD response as a function of task characteristic. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301658. in press. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Crawford TJ, Corr PJ, Das M, Zachariah E, et al. Smooth pursuit and antisaccade eye movements in siblings discordant for schizophrenia. Journal of Psychiatric Research. 2004;38(2):177–184. doi: 10.1016/s0022-3956(03)00105-5. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Picchioni M, Hall M-H, Schulze K, Toulopoulou T, Landau S, et al. Antisaccade performance in monozygotic twins discordant for schizophrenia: the Maudsley twin study. American Journal of Psychiatry. 2006;163(3):543–545. doi: 10.1176/appi.ajp.163.3.543. [DOI] [PubMed] [Google Scholar]
- Farber RH, Clementz BA, Swerdlow NR. Characteristics of open- and closed-loop smooth pursuit responses among obsessive-compulsive disorder, schizophrenia, and nonpsychiatric individuals. Psychophysiology. 1997;34(2):157–162. doi: 10.1111/j.1469-8986.1997.tb02126.x. [DOI] [PubMed] [Google Scholar]
- Flechtner KM, Steinacher B, Mackert A. Subthreshold symptoms and vulnerability indicators (e.g., eye tracking dysfunction) in schizophrenia. Comprehensive Psychiatry. 2000;41(2 Suppl 1):86–89. doi: 10.1016/s0010-440x(00)80013-9. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychological Medicine. 2007;37(2):163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Adams CE, Adler LE, Bickford PC, Gault J, Harris JG, et al. Inhibitory neurophysiological deficit as a phenotype for genetic investigation of schizophrenia. American Journal of Medical Genetics. 2000;97(1):58–64. doi: 10.1002/(sici)1096-8628(200021)97:1<58::aid-ajmg8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(2):587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Leonard S, Gault JM, Hopkins J, Cloninger CR, Kaufmann CA, et al. Linkage disequilibrium for schizophrenia at the chromosome 15q13-14 locus of the alpha7-nicotinic acetylcholine receptor subunit gene (CHRNA7) American Journal of Medical Genetics. 2001;105(1):20–22. [PubMed] [Google Scholar]
- Friedman L, Jesberger JA, Siever LJ, Thompson P, Mohs R, Melzer HY. Smooth pursuit performance in patients with affective disorders or schizophrenia and normal controls: Analysis with specific oculomotor measures, RMS error and qualitative ratings. Psychological Medicine. 1995;25:387–403. doi: 10.1017/s003329170003628x. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Fukushima K, Morita N, Yamashita I. Further analysis of the control of voluntary saccadic eye movements in schizophrenic patients. Biological Psychiatry. 1990;28:943–958. doi: 10.1016/0006-3223(90)90060-f. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Morita N, Fukushima K, Chiba T, Tanaka S, Yamashita I. Voluntary control of saccadic eye movements in patients with schizophrenic and affective disorders. Journal of Psychiatry Research. 1990;24(1):9–24. doi: 10.1016/0022-3956(90)90021-h. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: Evidence for mnemonic “scotomas.”. Journal of Neuroscience. 1993;13(4):1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale BW, Abel LA, Christian JC, Sorbel J, Yee RD. Saccadic characteristics of mono and dizygotic twins before and after alcohol administration. Investigative Opthalmology and Visual Science. 1996;37:339–344. [PubMed] [Google Scholar]
- Garver DL. The etiologic heterogeneity of schizophrenia. Harvard Review of Psychiatry. 1997;4(6):317–327. doi: 10.3109/10673229709030559. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud S, Vermersch AI, Pierrot-Deseilligny C. Cortical control of saccades. Experimental Brain Research. 1998;123:159–163. doi: 10.1007/s002210050557. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Niendam TA, Escamilla MA. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disorders. 2004;6(3):171–182. doi: 10.1111/j.1399-5618.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Grabowski JA, Hendershot CS. Fixation stability in schizophrenia, bipolar, and control subjects. Psychiatry Research. 2000;97(2–3):119–128. doi: 10.1016/s0165-1781(00)00226-2. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, et al. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Archives of General Psychiatry. 2007;64(11):1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove WM, Clementz BA, Iacono WG, Katsanis J. Smooth pursuit ocular motor dysfunction in schizophrenia: Evidence for a major gene. American Journal of Psychiatry. 1992;149:1362–1368. doi: 10.1176/ajp.149.10.1362. [DOI] [PubMed] [Google Scholar]
- Grove WM, Lebow B, Clementz BA, Cerri M, Iacono WG. Familial prevalence and co-aggregation of schizotypy indicators: A multitrait family study. Journal of Abnormal Psychology. 1991;100:115–121. doi: 10.1037//0021-843x.100.2.115. [DOI] [PubMed] [Google Scholar]
- Gschwandtner U, Aston J, Borgwardt S, Drewe M, Feinendegen C, Lacher D, et al. Neuropsychological and neurophysiological findings in individuals suspected to be at risk for schizophrenia: preliminary results from the Basel early detection of psychosis study - Fruherkennung von Psychosen (FEPSY) Acta Psychiatrica Scandinavica. 2003;108(2):152–155. doi: 10.1034/j.1600-0447.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Experimental Brain Research. 1985;58(3):455–472. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- Haraldsson HM, Ettinger U, Magnusdottir BB, Sigmundsson T, Sigurdsson E, Ingason A, et al. Catechol-O-Methyltransferase Val-158-Met polymorphism and antisaccade eye movements in schizophrenia. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn064. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Owen MJ. Genes for schizophrenia? Recent findings and their pathophysiological implications. Lancet. 2003;361(9355):417–419. doi: 10.1016/S0140-6736(03)12379-3. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW. In Search of Madness. Oxford University Press; New York: 2001. [Google Scholar]
- Hesselbrock V, Begleiter H, Porjesz B, O'Connor S, Bauer L. P300 event-related potential amplitude as an endophenotype of alcoholism--evidence from the collaborative study on the genetics of alcoholism. Journal of Biomedical Science. 2001;8(1):77–82. doi: 10.1007/BF02255974. [DOI] [PubMed] [Google Scholar]
- Holden C. Neuroscience: Deconstructing schizophrenia. Science. 2003;299(5605):333–335. doi: 10.1126/science.299.5605.333. [DOI] [PubMed] [Google Scholar]
- Holzman PS. Eye movement dysfunction and psychosis. International Review of Neurobiology. 1985;27:179–205. doi: 10.1016/s0074-7742(08)60558-9. [DOI] [PubMed] [Google Scholar]
- Holzman PS. Recent studies of psychophysiology in schizophrenia. Schizophrenia Bulletin. 1987;13:49–75. doi: 10.1093/schbul/13.1.49. [DOI] [PubMed] [Google Scholar]
- Holzman PS. Behavioral markers of schizophrenia useful for genetic studies. Journal of Psychiatric Research. 1992;26(4):427–445. doi: 10.1016/0022-3956(92)90044-o. [DOI] [PubMed] [Google Scholar]
- Holzman PS. Eye movements and the search for the essence of schizophrenia. Brain Research - Brain Research Reviews. 2000;31(2–3):350–356. doi: 10.1016/s0165-0173(99)00051-x. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Kringlen E, Levy DL, Haberman SJ. Deviant eye tracking in twins discordant for psychosis: A replication. Archives of General Psychiatry. 1980;37(6):627–631. doi: 10.1001/archpsyc.1980.01780190025002. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Kringlen E, Levy DL, Proctor LR, Haberman S. Smooth pursuit eye movements in twins discordant for schizophrenia. Journal of Psychiatric Research. 1978;14(1–4):111–120. doi: 10.1016/0022-3956(78)90013-4. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Kringlen E, Levy DL, Proctor LR, Haberman SJ, Yasillo NJ. Abnormal pursuit eye movements in schizophrenia: Evidence for a genetic indicator. Archives of General Psychiatry. 1977;34:803–806. doi: 10.1001/archpsyc.1977.01770190064005. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Kringlen E, Levy DL, Proctor LR, Haberman SJ, Yasillo NJ. Abnormal pursuit eye movements and schizophrenia. In: Grinker R, Harrow M, editors. Clinical Research in Schizophrenia: A Multi-dimensional Approach. Charles Thomas; Springfield, Illinois: 1987. pp. 153–159. [Google Scholar]
- Holzman PS, Proctor LR, Levy DL, Yasillo NJ, Meltzer HY, Hurt SW. Eye-tracking dysfunctions in schizophrenic patients and their relatives. Archives of General Psychiatry. 1974;31(2):143–151. doi: 10.1001/archpsyc.1974.01760140005001. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Solomon CM, Levin S, Waternaux CS. Pursuit eye movement dysfunction in schizophrenia patients: Family evidence for specificity. Archives of General Psychiatry. 1984;41:136–139. doi: 10.1001/archpsyc.1984.01790130030004. [DOI] [PubMed] [Google Scholar]
- Hong LE, Avila MT, Adami H, Elliot A, Thaker GK. Components of the smooth pursuit function in deficit and nondeficit schizophrenia. Schizophrenia Research. 2003;63(1–2):39–48. doi: 10.1016/s0920-9964(02)00388-2. [DOI] [PubMed] [Google Scholar]
- Hong LE, Mitchell BD, Avila MT, Adami H, McMahon RP, Thaker GK. Familial aggregation of eye-tracking endophenotypes in families of schizophrenic patients. Archives of General Psychiatry. 2006;63(3):259–264. doi: 10.1001/archpsyc.63.3.259. [DOI] [PubMed] [Google Scholar]
- Hunter JE, Schmidt FL. Methods of meta-analysis: Correcting error and bias in research findings. Sage; Newbury Park, CA: 1990. [Google Scholar]
- Hunter JE, Schmidt FL. Methods of Meta-Analysis: Correcting Error and Bias in Research Findings. 2nd ed Sage; Thousand Oaks, CA: 2004. [Google Scholar]
- Hutton S, Kennard C. Oculomotor abnormalities in schizophrenia: a critical review. Neurology. 1998;50(3):604–609. doi: 10.1212/wnl.50.3.604. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Cuthbert I, Crawford TJ, Kennard C, Barnes TR, Joyce EM. Saccadic hypometria in drug-naive and drug-treated schizophrenic patients: A working memory deficit? Psychophysiology. 2001;38(1):125–132. [PubMed] [Google Scholar]
- Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43(3):302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Joyce EM, Barnes TR, Kennard C. Saccadic distractibility in first-episode schizophrenia. Neuropsychologia. 2002;40(10):1729–1736. doi: 10.1016/s0028-3932(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Hyman SE. The genetics of mental illness: implications for practice. Bulletin of the Whorld Health Organization. 2000;78(4):455–463. [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Lykken DT. Eye tracking and psychopathology: New procedures applied to a sample of normal MZ twins. Archives of General Psychiatry. 1979;36:1361–1369. doi: 10.1001/archpsyc.1979.01780120091011. [DOI] [PubMed] [Google Scholar]
- Iacono WG. Eye tracking in normal twins. Behavior Genetics. 1982;12:517–526. doi: 10.1007/BF01073782. [DOI] [PubMed] [Google Scholar]
- Iacono WG. Psychophysiology and genetics: A key to psychopathology research. Psychophysiology. 1983;20:371–383. doi: 10.1111/j.1469-8986.1983.tb00916.x. [DOI] [PubMed] [Google Scholar]
- Iacono WG. Psychophysiologic markers of psychopathology: A review. Canadian Psychology. 1985;26:96–112. [Google Scholar]
- Iacono WG. Eye movement abnormalities in schizophrenic and affective disorders. In: Johnston CJ, Pirozzolo FJ, editors. Neuropsychology of eye movements. Erlbaum; Hillsdale, NJ: 1988. pp. 115–145. [Google Scholar]
- Iacono WG. Identifying genetic risk for psychopathology. In: Routh DK, DeRubeis RJ, editors. The science of clinical psychology: Accomplishments and future directions. American Psychological Association; Washington, D.C.: 1998. pp. 3–22. [Google Scholar]
- Iacono WG, Carlson SR, Malone SM. Identifying a multivariate endophenotype for substance use disorders using psychophysiological measures. International Journal of Psychophysiology. 2000;38:81–96. doi: 10.1016/s0167-8760(00)00132-x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Clementz BA. A strategy for elucidating genetic influences on complex psychopathological syndromes (with special reference to oculomotor functioning in schizophrenia) In: Chapman LJ, Chapman JP, Fowles DC, editors. Progress in experimental psychopathology research. Vol. 16. Springer; New York: 1992. [PubMed] [Google Scholar]
- Iacono WG, Grove WM. Schizophrenia reviewed: Towards an integrative genetic model. Psychological Science. 1993;4:273–276. [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48(2):147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Moreau M, Beiser M, Fleming JA, Lin T. Smooth pursuit eye tracking in first-episode psychotic patients and their relatives. Journal of Abnormal Psychology. 1992;101:104–116. [PubMed] [Google Scholar]
- Jurewicz I, Owen RJ, O'Donovan MC, Owen MJ. Searching for susceptibility genes in schizophrenia. European Neuropsychopharmacology. 2001;11(6):395–398. doi: 10.1016/s0924-977x(01)00116-x. [DOI] [PubMed] [Google Scholar]
- Karoumi B, Saoud M, d'Amato T, Rosenfeld F, Denise P, Gutknecht C, et al. Poor performance in smooth pursuit and antisaccadic eye-movement tasks in healthy siblings of patients with schizophrenia. Psychiatry Research. 2001;101(3):209–219. doi: 10.1016/s0165-1781(01)00227-x. [DOI] [PubMed] [Google Scholar]
- Kathmann N, Hochrein A, Uwer R, Bondy B. Deficits in gain of smooth pursuit eye movements in schizophrenia and affective disorder patients and their unaffected relatives. American Journal of Psychiatry. 2003;160(4):696–702. doi: 10.1176/appi.ajp.160.4.696. [DOI] [PubMed] [Google Scholar]
- Kato C, Petronis A, Okazaki Y, Tochigi M, Umekage T, Sasaki T. Molecular genetic studies of schizophrenia: challenges and insights. Neuroscience Research. 2002;43(4):295–304. doi: 10.1016/s0168-0102(02)00064-0. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Kortenkamp S, Iacono WG, Grove WM. Antisaccade performance in patients with schizophrenia and affective disorder. Journal of Abnormal Psychology. 1997;106(3):468–472. doi: 10.1037//0021-843x.106.3.468. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Taylor J, Iacono WG, Hammer MA. Heritability of different measures of smooth pursuit eye tracking dysfunction: a study of normal twins. Psychophysiology. 2000;37(6):724–730. [PubMed] [Google Scholar]
- Keefe RS, Silverman JM, Mohs RC, Siever LJ, Harvey PD, Friedman L, et al. Eye tracking, attention, and schizotypal symptoms in nonpsychotic relatives of patients with schizophrenia. Archives of General Psychiatry. 1997;54(2):169–176. doi: 10.1001/archpsyc.1997.01830140081014. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Silverman JM, Siever LJ, Cornblatt BA. Refining phenotype characterization in genetic linkage studies of schizophrenia. Special Section: The genetics of mental illness. Social Biology. 1991;38(3–4):197–218. doi: 10.1080/19485565.1991.9988788. [DOI] [PubMed] [Google Scholar]
- Kennard C, Crawford TJ, Henderson L. A pathophysiological approach to saccadic eye movements in neurological and psychiatric disease. Journal of Neurology, Neurosurgery & Psychiatry. 1994;57(8):881–885. doi: 10.1136/jnnp.57.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Janka Z. Critical evaluation of cognitive dysfunctions as endophenotypes of schizophrenia. Acta Psychiatrica Scandinavica. 2004;110(2):83–91. doi: 10.1111/j.1600-0047.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- Klein C, Heinks T, Andresen B, Berg P, Moritz S. Impaired modulation of the saccadic contingent negative variation preceding antisaccades in schizophrenia. Biological Psychiatry. 2000;47(11):978–990. doi: 10.1016/s0006-3223(00)00234-1. [DOI] [PubMed] [Google Scholar]
- Kringlen E. Twin studies in schizophrenia with special emphasis on concordance figures. American Journal of Medical Genetics. 2000;97:4–11. doi: 10.1002/(sici)1096-8628(200021)97:1<4::aid-ajmg2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kuechenmeister CA, Linton PH, Mueller TV, White HB. Eye tracking in relation to age, sex, and illness. Archives of General Psychiatry. 1977;34:578–579. doi: 10.1001/archpsyc.1977.01770170088008. [DOI] [PubMed] [Google Scholar]
- Landgraf S, Amado I, Bourdel MC, Leonardi S, Krebs MO. Memory-guided saccade abnormalities in schizophrenic patients and their healthy, full biological siblings. Psychological Medicine. 2008;38(6):861–870. doi: 10.1017/S0033291707001912. [DOI] [PubMed] [Google Scholar]
- Larrison-Faucher AL, Matorin AA, Sereno AB. Nicotine reduces antisaccade errors in task impaired schizophrenic subjects. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2004;28(3):505–516. doi: 10.1016/j.pnpbp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Bellivier F, Nostenbertrand M, Jouvent R, Pauls D, Mallet J. Psychiatric genetics: Search for phenotypes. Trends in Neurosciences. 1998;21(3):102–105. doi: 10.1016/s0166-2236(97)01187-9. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM. Eye movement dysfunction as a biological marker of risk for schizophrenia. Australian & New Zealand Journal of Psychiatry. 2000;34(Suppl):S91–100. doi: 10.1080/000486700228. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. Neurology of eye movements. 3rd ed FA Davis Company; Philadelphia, PA: 1999. [Google Scholar]
- Lencer R, Malchow CP, Trillenberg-Krecker K, Schwinger E, Arolt V. Eye-tracking dysfunction (ETD) in families with sporadic and familial schizophrenia. Biological Psychiatry. 2000;47(5):391–401. doi: 10.1016/s0006-3223(99)00249-8. [DOI] [PubMed] [Google Scholar]
- Lencer R, Trillenberg-Krecker K, Schwinger E, Arolt V. Schizophrenia spectrum disorders and eye tracking dysfunction in singleton and multiplex schizophrenia families. Schizophrenia Research. 2003;60(1):33–45. doi: 10.1016/s0920-9964(02)00165-2. [DOI] [PubMed] [Google Scholar]
- Leonard S, Breese C, Adams C, Benhammou K, Gault J, Stevens K, et al. Smoking and schizophrenia: abnormal nicotinic receptor expression. European Journal of Pharmacology. 2000;393(1–3):237–242. doi: 10.1016/s0014-2999(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, et al. Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Archives of General Psychiatry. 2002;59(12):1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- Levin S. Frontal lobe dysfunction in schizophrenia-I: Eye movement impairments. Journal of Psychiatric Research. 1984;18:27–55. doi: 10.1016/0022-3956(84)90046-3. [DOI] [PubMed] [Google Scholar]
- Levin S, Luebke A, Zee DS, Hain TC, Robinson DA, Holzman PS. Smooth pursuit eye movements in schizophrenics: Quantitative measurements with the search-coil technique. Journal of Psychiatric Research. 1988;22:255–267. doi: 10.1016/0022-3956(88)90005-2. [DOI] [PubMed] [Google Scholar]
- Levy DL, Holzman PS, Matthysse S, Mendell NR. Eye tracking dysfunction and schizophrenia: A critical perspective. Schizophrenia Bulletin. 1993;19:461–536. doi: 10.1093/schbul/19.3.461. [DOI] [PubMed] [Google Scholar]
- Levy DL, Holzman PS, Matthysse S, Mendell NR. Eye tracking and schizophrenia: A selective review. Schizophrenia Bulletin. 1994;20:47–62. doi: 10.1093/schbul/20.1.47. [DOI] [PubMed] [Google Scholar]
- Levy DL, Lajonchere CM, Dorogusker B, Min D, Lee S, Tartaglini A, et al. Quantitative characterization of eye tracking dysfunction in schizophrenia. Schizophrenia Research. 2000;42(3):171–185. doi: 10.1016/s0920-9964(99)00122-x. [DOI] [PubMed] [Google Scholar]
- Levy DL, O'Driscoll G, Matthysse S, Cook SR, Holzman PS, Mendell NR. Antisaccade performance in biological relatives of schizophrenia patients: a meta-analysis. Schizophrenia Research. 2004;71(1):113–125. doi: 10.1016/j.schres.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Lindsey DT, Holzman PS, Haberman S, Yasillo NJ. Smooth-pursuit eye movements: A comparison of two measurement techniques for studying schizophrenia. Journal of Abnormal Psychology. 1978;87(5):491–496. doi: 10.1037//0021-843x.87.5.491. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Levy DL, Holzman PS, Levin S. Eye movement dysfunctions in psychiatric patients: A review. Schizophrenia Bulletin. 1983;9(1):13–32. doi: 10.1093/schbul/9.1.13. [DOI] [PubMed] [Google Scholar]
- Litman RE, Torrey EF, Hommer DW, Radant AR, Pickar D, Weinberger DR. A quantitative analysis of smooth pursuit eye tracking in monozygotic twins discordant for schizophrenia. Archives of General Psychiatry. 1997;54(5):417–426. doi: 10.1001/archpsyc.1997.01830170035006. [DOI] [PubMed] [Google Scholar]
- Louchart-de la Chapelle S, Nkam I, Houy E, Belmont A, Menard J-F, Roussignol A-C, et al. A concordance study of three electrophysiological measures in schizophrenia. American Journal of Psychiatry. 2005;162(3):466–474. doi: 10.1176/appi.ajp.162.3.466. [DOI] [PubMed] [Google Scholar]
- MacAvoy MG, Bruce CJ. Comparison of the smooth eye tracking disorder of schizophrenics with that of nonhuman primates with specific brain lesions. International Journal of Neuroscience. Special Issue: M. Russell Harter memorial issue: Progress in visual information processing. 1995;80(1–4):117–151. doi: 10.3109/00207459508986097. [DOI] [PubMed] [Google Scholar]
- Maccabe JH, Simon H, Zanelli JW, Walwyn R, McDonald CD, Murray RM. Saccadic distractibility is elevated in schizophrenia patients, but not in their unaffected relatives. Psychological Medicine. 2005;35(12):1727–1736. doi: 10.1017/S0033291705005738. [DOI] [PubMed] [Google Scholar]
- Malone SM, Iacono WG. Error rate on the antisaccade task: heritability and developmental change in performance among preadolescent and late-adolescent female twin youth. Psychophysiology. 2002;39(5):664–673. [PubMed] [Google Scholar]
- Matsue Y, Osakabe K, Saito H, Goto Y, Veno T, Matsuoka H, et al. Smooth pursuit eye movements and express saccades in schizophrenic patients. Schizophrenia Research. 1994;12:121–130. doi: 10.1016/0920-9964(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Matsue Y, Saito H, Osakabe K, Awata S, Ueno T, Matsuoka H, et al. Smooth pursuit eye movements and voluntary control of saccades in the antisaccade task in schizophrenic patients. Japanese Journal of Psychiatry & Neurology. 1994;48(1):13–22. doi: 10.1111/j.1440-1819.1994.tb02991.x. [DOI] [PubMed] [Google Scholar]
- Matthysse S, Holzman PS, Gusella JF, Levy DL, Harte CB, Jorgensen A, et al. Linkage of eye movement dysfunction to chromosome 6p in schizophrenia: Additional evidence. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;128B(1):30–36. doi: 10.1002/ajmg.b.30030. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Brenner CA, Myles-Worsley M, Coon H, Byerley W, Clementz BA. Ocular motor delayed-response task performance among patients with schizophrenia and their biological relatives. Psychophysiology. 2001;38(1):153–156. [PubMed] [Google Scholar]
- McDowell JE, Brown GG, Paulus M, Martinez A, Stewart SE, Dubowitz DJ, et al. Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biological Psychiatry. 2002;51(3):216–223. doi: 10.1016/s0006-3223(01)01204-5. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA. The effect of fixation condition manipulations on antisaccade performance in schizophrenia: studies of diagnostic specificity. Experimental Brain Research. 1997;115(2):333–344. doi: 10.1007/pl00005702. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Myles-Worsley M, Coon H, Byerley W, Clementz BA. Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. Psychophysiology. 1999;36(1) doi: 10.1017/s0048577299980836. [DOI] [PubMed] [Google Scholar]
- Mowry BJ, Nancarrow DJ. Molecular genetics of schizophrenia. Clinical & Experimental Pharmacology & Physiology. 2001;28(1–2):66–69. doi: 10.1046/j.1440-1681.2001.03399.x. [DOI] [PubMed] [Google Scholar]
- Myles-Worsley M, Coon H, McDowell J, Brenner C, Hoff M, Lind B, et al. Linkage of a composite inhibitory phenotype to a chromosome 22q locus in eight Utah families. American Journal of Medical Genetics. 1999;88(5):544–550. [PubMed] [Google Scholar]
- Myles-Worsley M, Dale P, Polloi A, Levy D, Freedman R, Byerley W. Eye tracking abnormalities in two Micronesian families with schizophrenia. Psychiatric Genetics. 1991;2(3):209–212. [Google Scholar]
- Nakashima Y, Momose T, Sano I, Katayama S, Nakajima T, Niwa S-I, et al. Cortical control of saccade in normal and schizophrenic subjects: A PET study using a task-evoked rCBF paradigm. Schizophrenia Research. 1994;12(3):259–264. doi: 10.1016/0920-9964(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Nieman D, Becker H, van de Fliert R, Plat N, Bour L, Koelman H, et al. Antisaccade task performance in patients at ultra high risk for developing psychosis. Schizophrenia Research. 2007;95(1–3):54–60. doi: 10.1016/j.schres.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr., Foroud T. Chromosome 6 workshop report. American Journal of Medical Genetics. 1999;88(3):233–238. doi: 10.1002/(sici)1096-8628(19990618)88:3<233::aid-ajmg5>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- O'Driscoll GA, Benkelfat C, Florencio PS, Wolff A-LVG, Joober R, Lal S, et al. Neural correlates of eye tracking deficits in first-degree relatives of schizophrenic patients: A positron emission tomography study. Archives of General Psychiatry. 1999;56(12):1127–1134. doi: 10.1001/archpsyc.56.12.1127. [DOI] [PubMed] [Google Scholar]
- Olincy A, Johnson LL, Ross RG. Differential effects of cigarette smoking on performance of a smooth pursuit and a saccadic eye movement task in schizophrenia. Psychiatry Research. 2003;117(3):223–236. doi: 10.1016/s0165-1781(03)00022-2. [DOI] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Young DA, Roath M, Freedman R. Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenic patients. Neuropsychopharmacology. 1998;18(3):175–185. doi: 10.1016/S0893-133X(97)00095-X. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Craddock N, O'Donovan MC. Schizophrenia: genes at last? Trends in Genetics. 2005;21(9):518–525. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49(12):975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Pauler DK, Escobar MD, Sweeney JA, Greenhouse J. Mixture models for eye-tracking data: a case study. Statistics in Medicine. 1996;15(13):1365–1376. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1365::AID-SIM232>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C. Saccade and smooth-pursuit impairment after cerebral hemispheric lesions. European Neurology. 1994;34(3):121–134. doi: 10.1159/000117025. [DOI] [PubMed] [Google Scholar]
- Plomin R, McGuffin P. Psychopathology in the postgenomic era. Annual Review of Psychology. 2003;54:205–228. doi: 10.1146/annurev.psych.54.101601.145108. [DOI] [PubMed] [Google Scholar]
- Price GW, Michie PT, Johnston J, Innes-Brown H, Kent A, Clissa P, et al. A multivariate electrophysiological endophenotype, from a unitary cohort, shows greater research utility than any single feature in the Western Australian family study of schizophrenia. Biological Psychiatry. 2006;60(1):1–10. doi: 10.1016/j.biopsych.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Ramsey NF, Vink M, van den Heuvel MP, Kahn RS. Brain activation during antisaccades in unaffected relatives of schizophrenic patients. Biological Psychiatry. 2006;59(6):530–535. doi: 10.1016/j.biopsych.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Riley B. Linkage studies of schizophrenia. Neurotoxicity Research. 2004;6(1):17–34. doi: 10.1007/BF03033293. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Sweeney JA, Squires-Wheeler E, Keshavan MS, Cornblatt BA, Erlenmeyer-Kimling L. Eye-tracking dysfunction in offspring from the New York High-Risk Project: diagnostic specificity and the role of attention. Psychiatry Research. 1997;66(2–3):121–130. doi: 10.1016/s0165-1781(96)02975-7. [DOI] [PubMed] [Google Scholar]
- Ross DE. The deficit syndrome and eye tracking disorder may reflect a distinct subtype within the syndrome of schizophrenia. Schizophrenia Bulletin. 2000;26(4):855–866. doi: 10.1093/oxfordjournals.schbul.a033500. [DOI] [PubMed] [Google Scholar]
- Ross DE, Ochs AL, Pandurangi AK, Thacker LR, Kendler KS. Mixture analysis of smooth pursuit eye movements in schizophrenia. Psychophysiology. 1996;33(4):390–397. doi: 10.1111/j.1469-8986.1996.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Ross DE, Thaker GK, Buchanan RW, Lahti AC, Conley R, Medoff D. Specific measures account for most of the variance in qualitative ratings of smooth pursuit eye movements in schizophrenia. Archives of General Psychiatry. 1998;55(2):184–186. doi: 10.1001/archpsyc.55.2.184. [DOI] [PubMed] [Google Scholar]
- Ross RG. Early expression of a pathophysiological feature of schizophrenia: saccadic intrusions into smooth-pursuit eye movements in school-age children vulnerable to schizophrenia. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42(4):468–476. doi: 10.1097/01.CHI.0000046818.95464.42. [DOI] [PubMed] [Google Scholar]
- Ross RG, Harris J, Olincy A, Radant A, Adler L, Freedman R. Smooth pursuit eye movements in parents of schizophrenic probands: A most likely carrier approach. Schizophrenia Research. 1997;24:244. [Google Scholar]
- Ross RG, Harris JG, Olincy A, Radant A, Adler LE, Freedman R. Familial transmission of two independent saccadic abnormalities in schizophrenia. Schizophrenia Research. 1998b;30(1):59–70. doi: 10.1016/s0920-9964(97)00133-3. [DOI] [PubMed] [Google Scholar]
- Ross RG, Olincy A, Harris JG, Radant A, Adler LE, Freedman R. Anticipatory saccades during smooth pursuit eye movements and familial transmission of schizophrenia. Biological Psychiatry. 1998a;44(8):690–697. doi: 10.1016/s0006-3223(98)00052-3. [DOI] [PubMed] [Google Scholar]
- Ross RG, Olincy A, Harris JG, Radant A, Hawkins M, Adler LE, et al. Evidence for bilineal inheritance of physiological indicators of risk in childhood-onset schizophrenia. American Journal of Medical Genetics (Neuropsychiatric Genetics) 1999;88:188–199. [PubMed] [Google Scholar]
- Ross RG, Olincy A, Mikulich SK, Radant AD, Harris JG, Waldo M, et al. Admixture analysis of smooth pursuit eye movements in probands with schizophrenia and their relatives suggests gain and leading saccades are potential endophenotypes. Psychophysiology. 2002;39(6):809–819. doi: 10.1111/1469-8986.3960809. [DOI] [PubMed] [Google Scholar]
- Ross RG, Olincy A, Radant A. Amplitude criteria and anticipatory saccades during smooth pursuit eye movements in schizophrenia. Psychophysiology. 1999;36(4):464–468. [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A. Eye movement and neuropsychological studies in first-degree relatives of schizophrenic patients. Schizophrenia Research. 2002;54(1–2):105–110. doi: 10.1016/s0920-9964(01)00357-7. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Dmitrzak-Weglarz M, Hauser J. The study of cytosolic phospholipase A2 gene polymorphism in schizophrenia using eye movement disturbances as an endophenotypic marker. Neuropsychobiology. 2003;47(3):115–119. doi: 10.1159/000070578. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Hauser J. Dopamine D3 receptor (DRD3) gene polymorphism is associated with the intensity of eye movement disturbances in schizophrenic patients and healthy subjects. Molecular Psychiatry. 2001;6(6):718–724. doi: 10.1038/sj.mp.4000927. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Hauser J. Eye movement disturbances in schizophrenia and a polymorphism of catechol-O-methyltransferase gene. Psychiatry Research. 2002;113(1–2):49–57. doi: 10.1016/s0165-1781(02)00245-7. [DOI] [PubMed] [Google Scholar]
- Schlenker R, Cohen R, Berg P, Hubman W, Mohr F, Watzl H, et al. Smooth-pursuit eye movement dysfunction in schizophrenia: The role of attention and general psychomotor dysfunctions. European Archives of Psychiatry & Clinical Neuroscience. 1994;244(3):153–160. doi: 10.1007/BF02191891. [DOI] [PubMed] [Google Scholar]
- Schmidt FL. Statistical significance testing and cumulative knowledge in psychology: Implications for training of researchers. Psychological Methods. 1996;1:115–129. [Google Scholar]
- Schreiber H, Rothmeier J, Becker W, Jurgens R, Born J, Stolz-Born G, et al. Comparative assessment of saccadic eye movements, psychomotor and cognitive performance in schizophrenics, their first-degree relatives and control subjects. Acta Psychiatrica Scandinavica. 1995;91(3):195–201. doi: 10.1111/j.1600-0447.1995.tb09766.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Genetics of the risk for alcoholism. American Journal on Addictions. 2000;9(2):103–112. doi: 10.1080/10550490050173172. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Diaz J, Pilon C, Sokoloff P. Possible implications of the dopamine D(3) receptor in schizophrenia and in antipsychotic drug actions. Brain Research - Brain Research Reviews. 2000;31(2–3):277–287. doi: 10.1016/s0165-0173(99)00043-0. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Monuteaux MC, Weber W, Faraone SV. Neuropsychological functioning in nonreferred siblings of children with attention deficit/hyperactivity disorder. Journal of Abnormal Psychology. 2000;109(2):252–265. doi: 10.1037/0021-843X.109.2.252. [DOI] [PubMed] [Google Scholar]
- Sereno AB, Holzman PS. Express and antisaccades in schizophrenic, affective disorder, and normal control subjects. Society for Neuroscience Abstracts. 1991;17:858. [Google Scholar]
- Sereno AB, Holzman PS. Antisaccades and smooth pursuit eye movements in schizophrenia. Biological Psychiatry. 1995;37(6):394–401. doi: 10.1016/0006-3223(94)00127-O. [DOI] [PubMed] [Google Scholar]
- Sharpe JA. Neural control of ocular motor systems. In: Hoyt W, editor. Clinical Neuro-opthalmology. 1998. [Google Scholar]
- Sherr JD, Myers C, Avila MT, Elliott A, Blaxton TA, Thaker GK. The effects of nicotine on specific eye tracking measures in schizophrenia. Biological Psychiatry. 2002;52(7):721–728. doi: 10.1016/s0006-3223(02)01342-2. [DOI] [PubMed] [Google Scholar]
- Siegel C, Waldo M, Mizner G, Adler LE, Freedman R. Deficit in sensory gating in schizophrenic patients and their realtives: Evidence obtained with auditory evoked responses. Archives of General Psychiatry. 1984;41:607–612. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Coursey RD. Biological markers for schizophrenia and the biological high-risk approach. Journal of Nervous & Mental Disease. 1985;173(1):4–16. doi: 10.1097/00005053-198501000-00002. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Coursey RD, Alterman IS, Buchsbaum ME, Murphy DL. Impaired smooth pursuit eye movement: Vulnerability marker for schizotypal personality disorder in a normal volunteer population. American Journal of Psychiatry. 1984;141(12):1560–1566. doi: 10.1176/ajp.141.12.1560. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Coursey RD, Alterman IS, Buchsbaum MS, Murphy DL. Psychological and physiological correlates of variation in smooth pursuit eye movements. In: Usdin E, Hanin I, editors. Biological markers in psychiatry and neurology. Pergamon; Elmsford, NY: 1982. pp. 359–370. [Google Scholar]
- Sitskoorn MM, Aleman A, Ebisch SJH, Appels MCM, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophrenia Research. 2004;71(2–3):285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32(1):179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spohn HE, Larson J. Is eye tracking dysfunction specific to schizophrenia? Schizophrenia Bulletin. 1983;9(1):50–55. doi: 10.1093/schbul/9.1.50. [DOI] [PubMed] [Google Scholar]
- Spohn HE, Patterson T. Recent studies of psychophysiology in schizophrenia. Schizophrenia Bulletin. 1979;5:581–611. doi: 10.1093/schbul/5.4.581. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Iacono WG, Thuras PD, Nugent SM, Beiser M. Sensitivity and specificity of select biological indices in characterizing psychotic patients and their relatives. Schizophrenia Research. 2003;63(1–2):27–38. doi: 10.1016/s0920-9964(02)00385-7. [DOI] [PubMed] [Google Scholar]
- Sporn A, Greenstein D, Gogtay N, Sailer F, Hommer DW, Rawlings R, et al. Childhood-onset schizophrenia: smooth pursuit eye-tracking dysfunction in family members. Schizophrenia Research. 2005;73(2–3):243–252. doi: 10.1016/j.schres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, Van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Hantoumi I, et al. Variation in catechol-o-methyltransferase val158 met genotype associated with schizotypy but not cognition: a population study in 543 young men. Biological Psychiatry. 2004;56(7):510–515. doi: 10.1016/j.biopsych.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Haas GL, Keshavan MS, Mann JJ, Thase ME. Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biological Psychiatry. 1999;46(5):671–680. doi: 10.1016/s0006-3223(99)00132-8. [DOI] [PubMed] [Google Scholar]
- Tan H-Y, Callicott JH, Weinberger DR. Intermediate phenotypes in schizophrenia genetics redux: is it a no brainer? Molecular Psychiatry. 2008;13:233–238. doi: 10.1038/sj.mp.4002145. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Avila MT, Hong EL, Medoff DR, Ross DE, Adami HM. A model of smooth pursuit eye movement deficit associated with the schizophrenia phenotype. Psychophysiology. 2003;40(2):277–284. doi: 10.1111/1469-8986.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker GK, Cassady S, Adami H, Moran M, Ross D. Eye movements in spectrum personality disorder: Comparison of community subjects and relatives of schizophrenic patients. American Journal of Psychiatry. 1996;153(3):362–368. doi: 10.1176/ajp.153.3.362. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Buchanan RW, Moran MJ, Lahti A, Kim C, et al. Does pursuit abnormality in schizophrenia represent a deficit in the predictive mechanism? Psychiatry Research. 1996;59(3):221–237. doi: 10.1016/0165-1781(95)02759-9. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Cassady SL, Adami HM, LaPorte D, Medoff DR, et al. Smooth pursuit eye movements to extraretinal motion signals: deficits in relatives of patients with schizophrenia. Archives of General Psychiatry. 1998;55(9):830–836. doi: 10.1001/archpsyc.55.9.830. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Cassady SL, Adami HM, Medoff DR, Sherr J. Saccadic eye movement abnormalities in relatives of patients with schizophrenia. Schizophrenia Research. 2000;45(3):235–244. doi: 10.1016/s0920-9964(99)00193-0. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Wonodi I, Avila MT, Hong LE, Stine OC. Catechol O-methyltransferase polymorphism and eye tracking in schizophrenia: a preliminary report. American Journal of Psychiatry. 2004;161(12):2320–2322. doi: 10.1176/appi.ajp.161.12.2320. [DOI] [PubMed] [Google Scholar]
- Toomey R, Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Tsuang MT. Association of neuropsychological vulnerability markers in relatives of schizophrenic patients. Schizophrenia Research. 1998;31(2–3):89–98. doi: 10.1016/s0920-9964(98)00025-5. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Faraone SV. The frustrating search for schizophrenia genes. American Journal of Medical Genetics. 2000;97(1):1–3. doi: 10.1002/(sici)1096-8628(200021)97:1<1::aid-ajmg1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophrenia Bulletin. 2007;33(1):69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Stigchel S, Rommelse NN, Deijen JB, Geldof CJ, Witlox J, Oosterlaan J, et al. Oculomotor capture in ADHD. Cognitive Neuropsychology. 2007;24(5):535–549. doi: 10.1080/02643290701523546. [DOI] [PubMed] [Google Scholar]
- Venables PH. Overview of psychophysiology in relation to psychopathology with special reference to schizophrenia. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Handbook of Schizophrenia. Vol. 5. Elsevier Press; Amsterdam: 1991. pp. 3–37. [Google Scholar]
- Waterworth DM, Bassett AS, Brzustowicz LM. Recent advances in the genetics of schizophrenia. Cellular & Molecular Life Sciences. 2002;59(2):331–348. doi: 10.1007/s00018-002-8426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland S, Bertrand D, Leonard S. Neuronal nicotinic acetylcholine receptors: from the gene to the disease. Behavioural Brain Research. 2000;113(1–2):43–56. doi: 10.1016/s0166-4328(00)00199-6. [DOI] [PubMed] [Google Scholar]
- Whitener EM. Confusion of confidence intervals and credibility intervals in meta-analysis. Journal of Applied Psychology. 1990;75:315–321. [Google Scholar]
- Williams J, Spurlock G, Holmans P, Mant R, Murphy K, Jones L, et al. A meta-analysis and transmission disequilibrium study of association between the dopamine D3 receptor gene and schizophrenia. Molecular Psychiatry. 1998;3(2):141–149. doi: 10.1038/sj.mp.4000376. [DOI] [PubMed] [Google Scholar]
- Wolf FM. Meta-analysis: Quantitative methods for research synthesis. Sage; Beverly Hills, CA: 1986. [Google Scholar]
- Wong AH, Buckle CE, Van Tol HH. Polymorphisms in dopamine receptors: what do they tell us? European Journal of Pharmacology. 2000;410(2–3):183–203. doi: 10.1016/s0014-2999(00)00815-3. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK. The reliability of meta-analytic review. Psychological Reports. 1998;83:213–222. [Google Scholar]