Abstract
Background and aims
There is increasing clinical and legal interest in discrepancies between decision-making ability and cognition in old age, a stage of life when decisions have major ramifications. We investigated the frequency and correlates of such discrepancies in non-demented older adults participating in a large community-based cohort study of aging, the Rush Memory and Aging Project.
Methods
Participants [n = 689, mean age 81.8 (SD 7.6), mean education 15.2 (SD 3.1), 76.8 % female and 93.3 % white] completed a measure of financial and healthcare decision making (DM) and a battery of 19 neuropsychological tests from which a composite measure of global cognition (COG) was derived.
Results
Results indicated that 23.9 % of the sample showed a significant discrepancy between DM and COG abilities. Of these, 12.9 % showed DM < COG, while 11.0 % showed DM > COG. Logistic regression models showed older age, being non-white, greater temporal discounting, and greater risk aversion were associated with higher odds of being in the DM < COG group. Being male was associated with higher odds of being in the DM > COG group. Education, income, depressive symptoms, and impulsivity were not associated with a discrepancy. Only demographic associations (age, sex, and race) remained significant in a fully adjusted model with terms included for all factors.
Conclusion
These results support the consideration of decision making and cognition as potentially separate constructs.
Keywords: Decision making, Cognition, Discrepancy
Introduction
Adults over the age of 65 constitute the fastest growing segment of the US population [1]. In old age, adults are routinely faced with important financial and healthcare decisions, such as allocation of retirement funds, intergenerational transfers of wealth, medical treatment decisions, and advanced planning for end-of-life. The decisions older adults make can have a profound impact not only for themselves, but also for their families and society at large. Despite this importance, decision-making ability in old age appears to be poorer than in young or middle age [2, 3], and the reasons for this are poorly understood. A greater understanding of decision-making ability in old age and the factors that influence it is therefore of significant public health importance.
Decision making is a complex process that entails the ability to generate and evaluate multiple possible choices to select an optimal one. While a certain degree of cognitive functioning is necessary for optimal decision making, and older persons with cognitive impairment tend to make poorer decisions [4, 5], adults of all ages with intact cognition often make poor decisions concerning important financial and health matters. Case examples of this have been detailed in clinical and forensic reports [6, 7]. Whereas these case examples support the notion that discrepancies between decision making and cognition can exist at the individual level, it is unclear how frequently such discrepancies occur in late life in particular, and what characteristics are associated with these discrepancies. Discrepancies between decision-making abilities and cognitive abilities in older persons may have important clinical and legal significance.
Recent work suggests that observing performance on measures of function may not be as sensitive to detecting at-risk subgroups as are approaches that examine discrepancies between separate measures of function. For example, a recent study found that difference scores better characterized a preclinical Alzheimer’s disease group from a demographically matched control group despite both groups performing similarly on separate measures of cognition [8]. Another study showed that discrepancy approaches better predicted future cognitive decline among elderly individuals than an established genetic risk factor for dementia, apolipoprotein E genotype [9].
We investigated the frequency of discrepancy between decision-making ability and cognition in a group of non-demented older adults using a Z-transformation discrepancy approach [8–10]. This method allowed us to identify two types of discrepancy: decision making better than cognitive ability, and alternately decision making worse than cognitive ability. We next investigated the association of two major potential determinants of discrepancy, demographic factors (age, education, sex, race, and income) and personality/affective styles known to impact decision making (depressive symptoms, impulsivity, risk aversion, and temporal discounting, which is the tendency to select a smaller immediate reward instead of a larger reward at a later time), with each type of discrepancy using logistic regression.
Based on the prior literature linking decision making and cognition [11], we hypothesized that a subsample of participants has a discrepancy between cognitive and decision-making abilities, and membership in this discrepancy subsample is related to demographic and personality or affective characteristics. While age-related decline in cognitive function has been well-established [12], more recent work indicates that decision-making abilities may decline in old age as well [13]. However, it is unknown whether aging is related to a discrepancy between these two abilities. Research integrating behavior and brain models of cognitive functioning have found that age is associated with additional recruitment of brain regions to perform tasks, and this has been termed “dedifferentiation” [14]. Assuming that consistency between decision making and cognitive abilities is a reflection of neural integration, we hypothesized that older age is associated with a discrepancy between cognition and decision making due to its association with greater neural system dedifferentiation [15–17]. Similarly, we hypothesized that higher education and higher income are associated with lower odds of discrepancy due to their associations with better neural integration [18]. We further hypothesized that being non-white and female is associated with discrepancy due to disparities in financial experiences and access to healthcare [19–23]. Finally, we hypothesized that highly depressed, impulsive, and risk averse older persons, and those who discount future rewards (high temporal discounting), are more likely to show discrepancy between decision-making ability and cognitive ability [24–28]. In the absence of data on what factors are associated with a particular direction of discrepancy, directionality was considered exploratory. To our knowledge, this is the first systematic study of discrepancy between decision-making ability and cognition in old age.
Methods
Participants
Participants for the current study were enrolled in the Rush Memory and Aging Project, a cohort study of aging and dementia [29], which recruits from local residential facilities, including retirement homes, senior housing facilities, and community organizations in the Chicago metropolitan area. Participants are enrolled and evaluated annually. Cognitive impairment was documented with a battery of cognitive performance measures, and determined by a board-certified clinical neuropsychologist with expertise in aging and Alzheimer’s disease who reviewed the cognitive data, information about the participant’s current deficits and background (e.g., education/occupation, sensory and motor deficits), and the clinical evaluation conducted by a clinician with expertise in aging and dementia. Diagnosis of dementia was determined in accordance with NINCDS/ADRDA criteria by the evaluating clinician [30, 31]. Trained technicians under the supervision of a board-certified clinical neuropsychologist assisted in the comprehension of all tasks and measures at administration. All procedures were conducted in accordance with the ethical rules for human experimentation that are stated in the Declaration of Helsinki and were approved by the Institutional Review Board of Rush University Medical Center.
The Rush Memory and Aging Project began in 1997 and assessments of decision making began in 2008. Participants with dementia at the time of decision-making assessment were excluded from the analyses. At the time of these analyses, 1631 participants had completed a baseline evaluation; of those, 552 died and 83 refused further participation in the Memory and Aging Project before the decision-making evaluation began. Of the remaining 996 persons, 125 had not completed their first decision-making or clinical evaluation, and 104 were not asked to participate due to severe difficulties with language, hearing, vision or understanding, or having moved out of the geographical area, leaving 767 participants who had completed the decision-making assessment and had completed clinical evaluations. Fifty of these participants met criteria for dementia and were excluded, and 28 had missing data on the decision-making measure, resulting in a final group of 689 participants for analyses. The first annual decision-making assessment completed and the corresponding cognitive testing for that year were used in these analyses. The mean age of participants was 81.8 years (SD 7.6; range 59.6–100.8), the mean education was 15.2 years (SD 3.1; range 0–28), 76.8 % were women, and 93.3 % were white. The mean score on the Mini-Mental State Examination (MMSE) was 28.3 (SD 1.7; range 21–30). Of the participants, 25.8 % had self-reported annual incomes lower than USD 25,000, 46.4 % had incomes between USD 25,000 and 50,000, and 19.6 % had incomes over USD 50,000.
Assessment of decision making
Decision making was assessed using a modified version of a tool which was specifically designed to measure decision making in older adults [32, 33]. The modified decision-making measure consisted of 12 items, 6 items measuring financial decision making, and 6 items measuring healthcare decision making. Items were developed to represent actual decisions older adults often make and have been described in previous work [32, 34, 35]. The items involved choosing between different mutual funds (financial) and HMOs (healthcare) based on a number of pre-specified preferences. Information on mutual funds and HMOs was presented visually in table format. Questions and visual stimuli regarding mutual funds and HMOs varied in levels of complexity. Modifications to the visual stimuli from the original tool were made so they would be easier to see and simpler to comprehend for older participants. This measure was extensively piloted in a sample of older adults to ensure an appropriate level of comprehension prior to use. The total decision-making score is the number of items answered correctly (range 0–12). In previous research, the decision-making measure has been shown to have appropriate psychometric properties including high inter-rater reliability and short-term temporal stability [32, 33]. Our group has previously reported performance on this measure is associated with cognition [35], personality (i.e., risk aversion preferences [34]), financial and healthcare literacy [36], and risk of mortality [37] in non-demented older adults.
Assessment of cognition
All participants underwent a battery of 21 neuropsychological measures, 19 of which were used to make composite scores of cognitive function [29]. The battery included the word list memory, word list recall, and word list recognition from the CERAD battery, the immediate and delayed recall of Logical Memory Story A and the East Boston Story, verbal fluency, Boston Naming test, a subset of items from the Complex Ideational Material test, the National Adult Reading Test, Digit Span Subtest (forward and backward) of the Wechsler Memory Scale-revised, Digit Ordering, the Symbol Digit Modalities test, Number Comparison, the Judgment of Line Orientation test, Standard Progressive Matrices, Stroop Color Naming, and Stroop Word Reading. Two of the 21 tests, the Mini-Mental Status Examination and the Complex Ideational Material are used for descriptive and clinical diagnostic purposes only. Raw scores across all 19 of the remaining individual measures of cognitive function were converted to z scores using the baseline mean and SD from all subjects, and then were averaged to yield a composite global cognition score as was previously described [38].
Demographic variables
Age was based on date of birth and date of decision-making assessment. Sex, race (white versus non-white), and education (years of schooling) were self-reported. Income was measured using a show card methodology; participants were shown a card with the following 10 possible categories and asked to choose the option that represented their annual income: (1) USD 0–4999, (2) USD 5000–9999, (3) USD 10,000–14,999, (4) USD 15,000–19,999, (5) USD 20,000–24,999, (6) USD 25,000–29,999, (7) USD 30,000–34,999, (8) USD 35,000–49,999, (9) USD 50,000–74,999, (10) USD >75,000.
Personality/affective variables
Depressive symptomatology was assessed with a 10-item form of the CES-D scale [39]. For each symptom, a brief item stem was read by the examiner, and the participant indicated whether they had experienced the symptom much of the time during the past week. The score was the total number of symptoms experienced. The CES-D is widely used in epidemiological studies of older persons, and the reliability of this version of the CES-D has been previously demonstrated [40]. A strong benefit of this format is that it minimizes respondent burden by having concise item stems (mean of 4.2 words/item) that are read to the participant and require only a “yes” or “no” response. Impulsiveness was measured from 8 items of the neuroticism subscale of the NEO Personality Inventory-Revised [41]. These items included “I rarely overindulge in anything,” “I have trouble resisting my cravings,” “I have difficulty resisting temptation,” “when I am having my favorite foods, I tend to each too much,” “I seldom give into my impulses,” “I sometimes eat myself sick,” “sometimes I do things on impulse that I later regret,” and “I am always able to keep my feelings under control.” Respondents are given the choice to respond to each item with “strongly agree,” “agree,” “neutral,” “disagree,” or “strongly disagree” with values coded from 0 to 4 each item providing an overall range of 0–32 for the measure. Temporal discounting (alpha) was measured using five binary questions where participants were asked if they would rather have a smaller reward now or a larger reward 1 month later. An example of an item is “Would you prefer $1000 in cash right now or $3000 in a year?” All immediate rewards were $1000 and delayed rewards ranged in value from $1200 to $3000. Our group has shown that temporal discounting is associated with cognition [42] and increased mortality among older adults [43]. Risk aversion (gamma) was assessed with a series of 10 coin toss questions “Would you prefer $15 for sure, OR a coin toss in which you will get $ (an amount greater than $15) if you flip heads or nothing if you flip tails?” [44]. Possible gain amounts varied across questions and ranged from $21.79 to $151.19. Under the expected utility model, any gamble over $30 would be a “preferred” choice. Most of the gambles were over $30. Our group has previously shown risk aversion to be associated with cognition [44] and decision making [34] in older adults.
Statistical analyses
Descriptive statistics were presented on all variables included in the study. Pearson correlations were conducted for all continuous variables. Between-group comparisons were conducted to explore the relation of demographic categorical variables (sex and race) to the main variables of interest. Using an established discrepancy analysis approach [8–10], performances on the decision-making (DM) measure and the cognition (COG) composite were Z-transformed relative to the analytic sample and subtracted from one another to yield a discrepancy measure. Z-transforming the individual scores on the DM and COG measures allowed for standardization of scores relative to the same reference sample so that difference scores could be meaningfully calculated. Dummy variables were then created for discrepancies greater than one Z score (one standard deviation) in either direction. This yielded three groups, decision-making abilities less than one standard deviation of cognitive abilities (DM < COG), decision-making abilities within one standard deviation of (i.e., approximately equivalent to) cognitive abilities (DM ≅ COG), and decision-making abilities greater than one standard deviation of cognitive abilities (DM > COG). Unadjusted logistic regression models were next employed to explore the separate association of age, education, sex, race, income, depressive symptoms, impulsivity, temporal discounting, and risk aversion with discrepancy group status relative to the group with no discrepancy. We then ran an adjusted model that included terms for all of the variables (demographic and personality/affective styles). Significance was interpreted at an alpha of 0.05.
Results
Characterization of the sample
The sample was mostly female (76.8 %) and predominantly white (93.3 %) (Table 1). Performance on the measure of global cognition was correlated with performance on the decision-making measure, and higher age was correlated inversely with global cognition and decision-making performance (Supplemental Table 1). Being female and non-white was associated with worse performance on the decision-making measure (Supplemental Table 2).
Table 1.
Descriptive statistics for the entire sample
| Number | (%) | |
|---|---|---|
| N | 689 | |
| Female sex | 529 | (76.8 %) |
| White race | 643 | (93.3 %) |
| Mean | (SD) | Range | |
|---|---|---|---|
| Age | 81.75 | (7.63) | 59.58 to 100.78 |
| Education | 15.17 | (3.05) | 0 to 28 |
| WMS-R Logical Memory—immediate recall Ia raw score | 12.54 | (4.41) | 0 to 24 |
| WMS-R Logical Memory—delayed recall IIa raw score | 10.98 | (4.81) | 0 to 23 |
| CERAD Word List Memory, trials 1–3, immediate recall raw score | 18.65 | (4.55) | 7 to 30 |
| CERAD Word List Memory, trials 1–3, delayed recall raw score | 5.85 | (2.42) | 0 to 10 |
| CERAD Word List Memory, trials 1–3, recognition memory raw score | 9.63 | (0.96) | 0 to 10 |
| East Boston Memory Test immediate recall raw score | 9.81 | (1.92) | 0 to 12 |
| East Boston Memory Test delayed recall raw score | 9.39 | (2.31) | 0 to 12 |
| Boston Naming Test raw score | 14.10 | (1.12) | 8 to 15 |
| Verbal Semantic Fluency raw score | 35.28 | (9.49) | 7 to 70 |
| NART Word Reading raw score | 12.79 | (2.68) | 1 to 15 |
| Digit Span FORWARD raw score | 8.29 | (1.97) | 2 to 12 |
| Digit Span backward raw score | 6.34 | (1.90) | 1 to 12 |
| Digit Ordering raw score | 7.37 | (1.57) | 2 to 13 |
| Symbol Digit raw score | 40.04 | (10.12) | 8 to 77 |
| Number Comparison raw score | 25.15 | (7.11) | 0 to 48 |
| Stroop Color Naming raw score | 19.69 | (7.54) | 0 to 45 |
| Stroop Word Reading raw score | 48.16 | (13.68) | 0 to 80 |
| Judgment of Line Orientation raw score | 10.31 | (3.04) | 0 to 15 |
| Progressive Matrices raw score | 10.52 | (1.83) | 0 to 12 |
| Global Cognition z score | 0.25 | (0.51) | −1.42 to 1.59 |
| Total decision-making raw score | 7.69 | (2.80) | 0 to 12 |
| Income when enrolled | 7.19 | (2.44) | 1 to 10 |
| Depression | 0.94 | (1.53) | 0 to 9 |
| Impulsivity mean | 13.48 | (4.46) | 0 to 28 |
| Risk aversion (gamma) | 0.32 | (0.30) | 0.06 to 0.91 |
| Temporal discounting (alpha) | 0.66 | (0.81) | 0.07 to 2.76 |
WMS-R Logical Memory—immediate recall Ia and delayed recall IIa have a possible range of 0–25; CERAD Word List Memory, trials 1–3, immediate recall has a possible range of 0–30; CERAD Word List Memory, trials 1–3, delayed recall has a possible range of 0–10; CERAD word List Memory, trials 1–3, Recognition Memory has a possible range of 0–10; East Boston Memory Test Immediate Recall and Delayed Recall have a possible range of 0–12; Boston Naming Test has a possible range of 0–15; Verbal Semantic Fluency has a possible range of 0–75; NART Word Reading has a possible range of 0–15; Digit Span forward and Digit Span backward have a possible range of 0–12; digit ordering has a possible range of 0–14; Symbol Digit has a possible range of 0–110; Number Comparison has a range of 0–48; Stroop Color Naming and Stroop Word Reading have a possible range of 0–100; Judgment Of Line Orientation has a possible range of 0–15; Progressive Matrices has a possible range of 0–16; Total decision making has a possible range of 0–12; depression measured by 10-item CES-D sum, impulsivity measured by 8 questions from the NEO Personality Inventory-Revised, income categories: (1) USD 0–4,999, (2) USD 5,000–9,999, (3) USD 10,000–14,999, (4) USD 15,000–19,999, (5) USD 20,000–24,999, (6) USD 25,000–29,999, (7) USD 30,000–34,999, (8) USD 35,000–49,999, (9) USD 50,000–74,999, (10) USD >75,000. Only 2 people had a level of education of less than 9 years
N sample size, SD standard deviation, WMS-R Wechsler Memory Scale-Revised, CERAD Consortium to Establish a Registry for Alzheimer’s Disease, NART National Adult Reading Test
Frequencies of discrepancy between decision making and cognition
After subtracting Z score conversions of global cognition and decision making from one another, 23.9 % of the sample had a discrepancy greater than 1 Z score. Of these, 12.9 % percent had decision-making abilities lower than cognition (DM < COG), while 11.0 % had decision-making abilities higher than cognition (DM > COG). A scatterplot of decision making and cognition Z scores by discrepancy group is presented in Fig. 1. Demographic data and significant bivariate comparisons of the discrepancy groups (DM < COG and DM > COG) with the equivalence group (DM ≅ COG) are presented in Table 2.
Fig. 1.
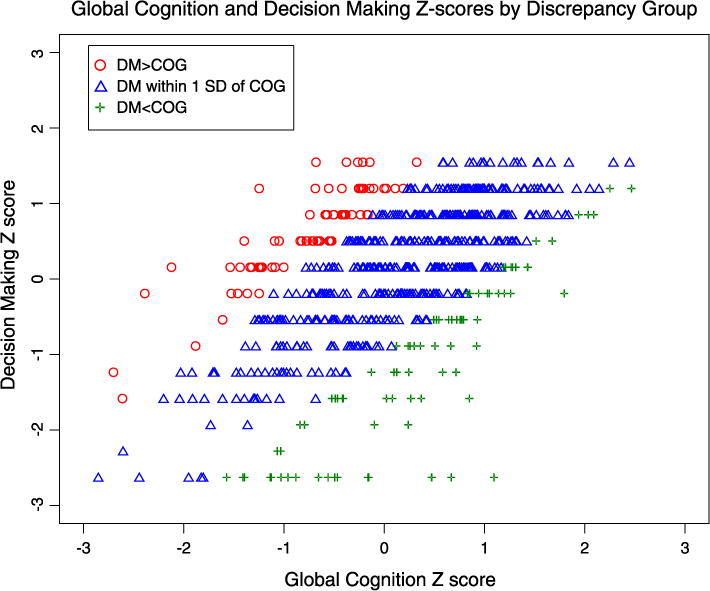
Scatterplot of global cognition and decision-making Z scores by discrepancy group
Table 2.
Descriptive statistics by discrepancy group (N = 689)
| DM < COG | DM ≅ COG | DM > COG | |
|---|---|---|---|
| N (%) | 89 (13.0 %) | 524 (76.3 %) | 76 (11.1 %) |
| Mean age (SD) | 83.36 (8.31)* | 81.34 (7.59) | 82.07 (6.79)* |
| Mean education (SD) | 14.74 (2.75) | 15.22 (3.03) | 15.42 (3.52) |
| Female sex (%) | 76 (85 %) | 410 (78 %) | 43 (57 %)* |
| White race, number participants, (%) | 78 (88 %)* | 495 (94 %) | 70 (92 %) |
| Mean income when enrolled (SD) | 6.88 (2.50) | 7.19 (2.46) | 7.49 (2.22) |
| Depression mean (SD) | 1.09 (1.74) | 0.90 (1.51) | 1.05 (1.39) |
| Impulsivity mean (SD) | 14.01 (4.84) | 13.47 (4.46) | 12.87 (3.96) |
| Risk aversion (gamma) mean (SD) | 0.39 (0.30) | 0.32 (0.30) | 0.23 (0.29) |
| Temporal discounting (alpha) mean (SD) | 0.90 (1.03) | 0.62 (0.77) | 0.69 (0.78) |
Values indicated with a star (*) are significantly different than the DM % COG group at p < 0.05
N sample size, SD standard deviation, DM decision making, COG global cognitive abilities, M male, F female, W white, NW non-white, depression measured by 10-item CES-D sum, impulsivity measured by 8 questions from the NEO Personality Inventory-Revised, income categories: (1) USD 0–4,999, (2) USD 5,000–9,999, (3) USD 10,000–14,999, (4) USD 15,000–19,999, (5) USD 20,000–24,999, (6) USD 25,000–29,999, (7) USD 30,000–34,999, (8) USD 35,000–49,999, (9) USD 50,000–74,999, (10) USD >75,000
Associations of demographics and personality/affective decision-making styles with discrepancy groups
To determine correlates of discrepancies between cognition and decision making, we first examined whether of each of the demographic variables was significantly associated with either discrepancy group (DM < COG, DM > COG) compared to the non-discrepant group (DM ≅ COG) in a series of unadjusted logistic regression models. Older age and being non-white were associated with higher odds of being in the DM < COG group. By contrast, being male was associated with higher odds of being in the DM > COG group. Education and income were not associated with either type of discrepancy (Table 3).
Table 3.
Association of demographic and personality/affective decision-making styles with discrepancies between decision making and cognition
| Unadjusted models
|
Fully adjusted models
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM < COG
|
DM > COG
|
DM < COG
|
DM > COG
|
|||||||||
| OR | 95 % wald confidence limits | p value | OR | 95 % wald confidence limits | p value | OR | 95 % wald Confidence Limits | p value | OR | 95 % wald confidence limits | p value | |
| Age | 1.05 | 1.02–1.09 | <0.01 | 1.03 | 0.99–1.07 | 0.10 | 1.05 | 1.02–1.09 | 0.01 | 1.03 | 0.99–1.07 | 0.08 |
| Education | 0.98 | 0.90–1.06 | 0.58 | 1.00 | 0.92–1.09 | 0.98 | 0.98 | 0.90–1.07 | 0.99 | 1.00 | 0.92–1.09 | 0.80 |
| Male sex | 0.66 | 0.35–1.25 | 0.20 | 2.63 | 1.56–4.43 | <0.01 | 0.70 | 0.37–1.35 | 0.35 | 2.64 | 1.55–4.51 | <0.01 |
| Race (not white) | 3.13 | 1.36–7.18 | <0.01 | 2.11 | 0.79–5.66 | 0.14 | 2.74 | 1.17–6.43 | 0.01 | 2.07 | 0.76–5.65 | 0.18 |
| Income | 1.00 | 0.90–1.11 | 0.95 | 1.03 | 0.92–1.16 | 0.59 | 1.01 | 0.91–1.12 | 0.75 | 1.03 | 0.92–1.16 | 0.26 |
| Depression | 1.08 | 0.94–1.24 | 0.28 | 1.06 | 0.92–1.23 | 0.41 | 1.00 | 0.85–1.16 | 0.97 | 1.13 | 0.96–1.34 | 0.15 |
| Impulsivity | 1.03 | 0.98–1.08 | 0.30 | 0.97 | 0.92–1.03 | 0.28 | 1.04 | 0.98–1.10 | 0.22 | 0.98 | 0.92–1.04 | 0.56 |
| Risk aversion (gamma) | 2.11 | 1.03–4.33 | 0.04 | 0.67 | 0.29–1.59 | 0.37 | 1.28 | 0.58–2.85 | 0.34 | 0.75 | 0.30–1.88 | 0.66 |
| Temporal discounting (alpha) | 1.44 | 1.13–1.84 | <0.01 | 1.13 | 0.84–1.51 | 0.42 | 1.19 | 0.90–1.56 | 0.14 | 1.17 | 0.84–1.62 | 0.33 |
Estimated from separate logistic regression models, unadjusted models were separate models for each variable, fully adjusted models included all other variables as covariate terms
Next, we examined the association of personality/affective styles with discrepancy groups. In separate models, greater temporal discounting and greater risk aversion were associated with higher odds of being in the DM < COG group (Table 3). Depressive symptoms and impulsivity were not associated with discrepancy groups.
Finally, we conducted fully adjusted models examining all demographic and affective/personality variables together. In this analysis, only the demographic associations (age, sex and race) remained significantly associated with discrepancy groups (Table 3).
Because the results could be influenced by persons with cognitive impairment, we repeated the analyses in a sub-sample of participants who had an MMSE of greater than or equal to 26 and another subsample who had a global cognition score not in the impaired range (this was defined as having a global cognition score above a cutoff of 1.5 standard deviations below the mean score, consistent with clinical neuropsychology practice). Results revealed very similar frequencies of discrepancy according to both methods (Supplemental Table 3). Furthermore, the same demographic variables remained significantly associated with discrepancy in fully adjusted models as were indicated by the full sample.
Discussion
In a large community-based cohort of non-demented older persons, about a quarter of the sample exhibited a significant discrepancy between their decision-making ability and cognitive function. In separate models, older age, being a racial minority (non-white), greater temporal discounting, and greater risk aversion were associated with higher odds of being in the DM < COG group, while unexpectedly, male sex was associated with higher odds of being in the DM > COG group. In a fully adjusted model, however, only demographic variables (age, sex and race) remained significantly associated with discrepancies. In additional analyses of only those with very high cognitive ability, similar proportions of discrepancy were observed in the subsample, and the same variables remained significant in adjusted models as were observed for the whole sample.
These results suggest three important implications regarding the relationship between decision-making abilities and overall cognitive abilities in old age. First, although optimal decision making requires a certain level of cognitive function, about a quarter of older adults in our sample showed discrepancies between their decision-making and global cognitive abilities of more than a standard unit. There is mounting evidence from behavioral economics and affective science studies that decision making and cognition may be dissociable constructs in old age. For example, our group has shown that risk aversion is associated with poorer decision making after adjusting for the effects of global cognition, executive functions, and non-executive cognitive abilities in older adults without dementia [34]. Our group has also shown a significant association between decision making and mortality in older adults that persisted even after adjusting for cognitive functioning [37]. The fact that we observed a large discrepancy in about a quarter of the sample in the present study supports the notion that decision making is associated with other processes beyond cognition, and bolsters the argument that decision-making ability and cognition are dissociable constructs in old age. Cognitive decline may have a deleterious effect on decision making [35]; however, other factors such as emotional processing and motivation are known to influence decision making as well [45]. For example, older adults tend to show more of a preference for having attention and memory resources directed to emotionally relevant stimuli when making decisions [46].
While we expected to see a portion of the sample exhibits decision-making abilities relatively lower than cognitive abilities based on case studies described in clinical and forensic reports [6, 7], we also observed the opposite pattern of cognitive ability being lower than decision making in a portion of the sample as well. To our knowledge, this has not been previously reported. Participants who exhibit better decision-making abilities relative to their cognitive abilities may do so for a number of reasons. Affective, medical, and lifestyle factors known to impact cognition might selectively contribute to lowering cognitive function relative to other functions [47, 48]; although the mechanisms are unclear. We investigated whether depressive symptomatology and impulsivity, factors previously shown to be associated with lower cognition [49, 50], were associated with discrepancies and found no evidence for these having a significant effect.
The second implication from these results is that specific demographic factors are associated with a discrepancy between decision-making and cognitive abilities. As hypothesized, older age and minority status were associated with higher odds of showing lower decision-making abilities relative to cognitive abilities. The age finding is consistent with the disintegration of neural systems hypothesis of aging, supported by a number of studies using different neuroscience approaches [15–17]. If decision making and cognition are distinguishable constructs, then they will likely have differences in neural representation in the brain. With age, neural processes disassociate [51], and by consequence, abilities that may have been associated early in life may become less associated and more discrepant later in life, as in the example of memory encoding and recognition abilities [52]. Previous studies have identified age-related cognitive decline for persons over age 74 [12], and declines in decision-making abilities have similarly been implicated for older ages [13]. Since the majority of our sample was above the age of 74 (greater than 80 %), our results may be viewed as most relevant to this age group. It is notable that discrepancies between cognitive and decision-making abilities are present for this age group, and further research is needed to determine whether these discrepancies represent age-related changes in abilities or long-term differences that persist from earlier in adulthood. Being non-white was also associated with higher odds of having lower decision-making abilities relative to global cognitive abilities, but there were only 11 minorities in this discrepant group, making interpretation of this finding difficult. In general, there is a lack of research on decision making in older minority populations. But given the well-documented disparities in cognition [53] and racial differences in both economic profiles and healthcare experiences [19–23], further studies with larger samples of minorities are needed to more comprehensively examine the interactions of race, cognition, and decision making. Being male was associated with higher odds of having lower cognitive abilities relative to decision-making abilities. Research has shown that among older adults, males tend to be more financially literate and make more of the household financial decisions regarding retirement [54]. Historically in the US, males tended to be the primary decision makers for the family unit and therefore may have gained more experience with matters that had financial and healthcare implications. For these reasons, older males may make better decisions despite relatively lower cognition due to the benefit of greater experience and exposure. As sex differences in financial provision become less pronounced in society, this association of sex with discrepancy could potentially diminish. Because higher education and income level are commonly associated with better health outcomes, we hypothesized that these would be associated with less discrepancy between decision-making ability and cognition. Contrary to our hypotheses, education and income were not found to be significantly associated with being in a discrepancy group. The reasons for this are unclear, though the implication that discrepancies between decision-making ability and cognition may occur independent of education or income level is interesting, as this suggests high education or income level may not protect against discrepancies that may occur in old age.
The third implication is that certain personality and affective styles known from behavioral economics to have an effect on decision outcomes may not be as sensitive to discrepancies between decision-making and cognitive abilities as demographic factors. Depressive symptoms and impulsivity were not associated with a discrepancy group in unadjusted models. Temporal discounting, the tendency to take a smaller more immediate reward instead of a larger delayed reward, and risk aversion were both associated with being in the DM < COG discrepancy group in unadjusted models; however, these did not remain significant in fully adjusted models. Temporal discounting has been interpreted as a reflection of poor decision-making ability [55], and our group has previously shown temporal discounting to also be associated with poorer cognition [42]. Similarly, greater risk aversion has been associated with poorer decision making [34] as well as lower cognition in older adults [44]. We therefore speculate that one reason for our lack of findings is that these personality and affective styles may not be as sensitive to discrepancies between decision-making and cognitive abilities.
This study has limitations and strengths. In regard to limitations, the sample is significantly white; therefore conclusions based on race or ethnicity should be interpreted with caution. Also, measures of risk aversion and temporal discounting involved hypothetical and not real rewards and may therefore not reflect true preferences, though studies indicate that hypothetical paradigms elicit very similar responses as real paradigms [56]. Despite these limitations, there are a number of strengths of the present study. These include the use of a large, well-characterized community-based sample to conduct observations of decision making–cognition discrepancy, an innovative method to investigate discrepancies, a comprehensive battery of cognitive measures to ascertain global cognitive abilities, and established measures of personality/affective factors. Future research is needed to replicate the discrepancy frequency data and the characteristic associations with discrepancy observed in the present study.
Supplementary Material
Acknowledgments
This research was supported by National Institute on Aging grants R01AG017917, R01AG033678, K23AG040625, the American Federation for Aging Research, the Illinois Department of Public Health, and The Marsha K. Dowd Philanthropic Fund. The authors gratefully thank Alysha Kett for her assistance with statistical analyses, and the Rush Memory and Aging Project staff and participants.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s40520-015-0375-7) contains supplementary material, which is available to authorized users.
Conflict of interest None.
Human and Animal Rights All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation.
Informed consent Informed consent was obtained from all patients for being included in the study.
References
- 1.Jacobsen LA, Kent M, Lee M, et al. America’s aging population. Popul Bull. 2011;66(1):1–20. [Google Scholar]
- 2.Tymula A, Rosenberg Belmaker LA, Ruderman L, et al. Like cognitive function, decision making across the life span showed profound age-related changes. PNAS. 2013;110(42):17143–17148. doi: 10.1073/pnas.1309909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal S, Driscoll JC, Gabaix X, et al. The age of reason: financial decisions over the lifecycle with implications for regulation. Brook Papers Econ Act. 2009;2:51–117. [Google Scholar]
- 4.Zamarian L, Weiss EM, Delazer M. The impact of mild cognitive impairment on decision making in two gambling tasks. Psychol Sci. 2010;66B:23–31. doi: 10.1093/geronb/gbq067. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Ruhe G. The cognitive process of decision making. Int J Cogn Inf Nat Intell. 2007;1:73–85. [Google Scholar]
- 6.Templeton VH, Kirkman DN. Fraud, vulnerability, and aging. Alzheimer’s Care Today. 2007;8(3):265–277. [Google Scholar]
- 7.Jackson SL, Hafemeister TL. (US Department of Justice, Document 233613).Financial abuse of elderly people vs other forms of elder abuse: assessing their dynamics, risk factors, and society’s response. 2011 [Google Scholar]
- 8.Jacobson MW, Delis DC, Salmon DP, et al. Do neuropsychological tests detect preclinical Alzheimer’s disease: individual-test versus cognitive-discrepancy score analyses. Neuropsychology. 2002;16(2):132–139. doi: 10.1037//0894-4105.16.2.132. [DOI] [PubMed] [Google Scholar]
- 9.Fine EM, Delis DC, Wetter SR, et al. Cognitive discrepancies versus APOE genotype as predictors of cognitive decline in normal-functioning elderly individuals: a longitudinal study. Am J Geriatr Psychiatry. 2008;16(5):366–374. doi: 10.1097/JGP.0b013e3181629957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delis DC, Lansing A, Houston WS, et al. Creativity lost: the importance of testing higher-level executive functions in school-age children and adolescents. J Psychoeduc Assess. 2007;25:29–40. [Google Scholar]
- 11.Agarwal S, Driscoll JC, Gabaix X, et al. The age of reason: financial decisions over the life cycle with implications for regulation. Brook Papers Econ Act. 2009;2:51–117. [Google Scholar]
- 12.Park DC, Schwartz N. Cognitive aging: a primer. Taylor and Francis Group; New York: 2000. [Google Scholar]
- 13.Qualls SH, Smyer MA. Changes in decision-making capacity in older adults: assessment and intervention. Wiley; Hoboken: 2007. [Google Scholar]
- 14.Park DC, Polk TA, Mikels JA, et al. Cerebral aging: integration of brain and behavioral models of cognitive function. Dialogues Clin Neurosci. 2001;3:151–165. doi: 10.31887/DCNS.2001.3.3/dcpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews-Hanna JR, Snyder AZ, Vincent JL, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raz N, Rodrique KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drzezga A, Becker JA, Van Dijk KRA, et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain. 2011;134:1635–1646. doi: 10.1093/brain/awr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11:651–652. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skarupski KA, Mendes de Leon CF, Barnes LL, et al. Medicare part D enrollment in a biracial community-based population of older adults. Gerontologist. 2009;6:828–838. doi: 10.1093/geront/gnp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin MY, Pisu M, Oster R, et al. Racial variation in willingness to trade financial resources for life prolonging cancer treatment. Cancer. 2011;117(15):3476–3484. doi: 10.1002/cncr.25839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwak J, Haley WE. Current research findings on end-of-life decision making among racially or ethnically diverse groups. Gerontologist. 2005;45:634–641. doi: 10.1093/geront/45.5.634. [DOI] [PubMed] [Google Scholar]
- 22.Krieger N, Chen JT, Waterman PD, et al. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the public health disparities geocoding project. Am J Public Health. 2003;93:1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunlop DD, Manheim LM, Song J, et al. Gender and ethnic/racial disparities in health care utilization among older adults. J Gerontol B Psychol Sci Soc Sci. 2001;57(4):S221–S233. doi: 10.1093/geronb/57.4.s221. [DOI] [PubMed] [Google Scholar]
- 24.Hindmarch T, Hotopf M, Owen GS. Depression and decision-making capacity for treatment or research: a systematic review. BMC Med Eth. 2013;14:54. doi: 10.1186/1472-6939-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franken IHA, van Strien JW, Nijs I, et al. Impulsivity is associated with behavioral decision-making deficits. Psychiatry Res. 2006;158:155–163. doi: 10.1016/j.psychres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Tom SM, Fox CR, Trepel C, et al. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- 27.Paulsen DJ, Platt ML, Huettel SA, et al. From risk-seeking to risk-averse: the development of economic risk preference from childhood to adulthood. Front Psychol. 2012;3:313. doi: 10.3389/fpsyg.2012.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan BA, Reed DD. Decision processes in choice overload: a product of delay and probability discounting? Behav Processes. 2013;97:21–24. doi: 10.1016/j.beproc.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Bennett DA, Schneider JA, Buchman AS, et al. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer’s Res. 2012;9(6):646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 31.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older person without cognitive impairment from two community-based studies. Neurology. 2006;27:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 32.Finucane ML, Gullion CM. Developing a tool for measuring the decision-making competence of older adults. Psychol Aging. 2010;25:271–288. doi: 10.1037/a0019106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finucane ML, Mertz CK, Slovic P, et al. Task complexity and older adults’ decision-making competence. Psychol Aging. 2005;20:71–84. doi: 10.1037/0882-7974.20.1.71. [DOI] [PubMed] [Google Scholar]
- 34.Boyle PA, Yu L, Buchman AS, et al. Risk aversion is associated with decision making among community-based older persons. Front Psychol. 2012;3:205. doi: 10.3389/fpsyg.2012.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyle PA, Yu L, Wilson RS, et al. Poor decision making is a consequence of cognitive decline among older persons without Alzheimer’s disease or mild cognitive impairment. PLoS One. 2012;7(8):e43647. doi: 10.1371/journal.pone.0043647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James BD, Boyle PA, Bennett JS, et al. The impact of health and financial literacy on decision making in community-based older adults. Gerontology. 2012;58:531–539. doi: 10.1159/000339094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyle PA, Wilson RS, Yu L, et al. Poor decision making is associated with an increased risk of mortality among community-dwelling older persons without dementia. Neuro Epidemiol. 2013;40:247–252. doi: 10.1159/000342781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Psychol. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- 39.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 40.Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult criterion validity of the 10-item Center for Epidemiological Studies Depresssion Scale (CES-D) Arch Intern Med. 1999;159:1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- 41.Costa PT, McCrae RR. Professional manual. Psychol Assess Resour; Lutz, FL: 1992. EO Personality Inventory-revised. [Google Scholar]
- 42.Boyle PA, Yu L, Segawa E. Association of cognition with temporal discounting in community based older persons. BMC Geriatrics. 2012;12:48. doi: 10.1186/1471-2318-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyle PA, Yu L, Gamble K, et al. Temporal discounting is associated with an increased risk of mortality among community-based older persons without dementia. PLoS One. 2013;8(6):e67376. doi: 10.1371/journal.pone.0067376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyle PA, Yu L, Buchman AS, et al. Cognitive function is associated with risk aversion in community-based older persons. BMC Geriatrics. 2011;11:53. doi: 10.1186/1471-2318-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nielsen L, Mather M. Emerging perspectives in social neuroscience and neuroeconomics of aging. SCAN. 2011;6:149–164. doi: 10.1093/scan/nsr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. TRENDS Cogn Sci. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Zelinski EM, Crimmins E, Reynolds S, et al. Do medical conditions affect cognition in older adults? Health Psychol. 1998;17:504–512. doi: 10.1037//0278-6133.17.6.504. [DOI] [PubMed] [Google Scholar]
- 48.Akdag B, Telci EA, Cavlak U. Factors affecting cognitive function in older adults: a Turkish sample. Int J Gerontol. 2013;7:137–141. [Google Scholar]
- 49.Yen Y-C, Rebok GW, Gallo JJ, et al. Depressive symptoms impair everyday problem-solving ability through cognitive abilities in late life. Am J Geriatr Psychiatry. 2011;19:142–150. doi: 10.1097/JGP.0b013e3181e89894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vigil-Colet A, Morales-Vives F. How impulsivity is related to intelligence and academic achievement. Span J Psychol. 2005;8:199–204. doi: 10.1017/s1138741600005072. [DOI] [PubMed] [Google Scholar]
- 51.Goh JOS. Functional dedifferentiation and altered connectivity in older adults: neural accounts of cognitive aging. Aging Dis. 2011;2:30–48. [PMC free article] [PubMed] [Google Scholar]
- 52.Morcom AM, Good CD, Frackowiak RSJ, et al. Age effects on the neural correlates of successful memory encoding. Brain. 2002;26:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- 53.Cisco S, Gross AL, Shih RA, et al. The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. J Gerontol Ser B Psychol Sci Soc Sci. 2014 doi: 10.1093/geronb/gbt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lusardi A, Mitchell OS. Financial literacy around the world: an overview. J Pension Econ Financ. 2011;10(4):497–508. doi: 10.1017/S1474747211000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McHugh L, Wood RL. Using a temporal discounting paradigm to measure decision-making and impulsivity following traumatic brain injury: a pilot study. Brain Inj. 2008;22:715–721. doi: 10.1080/02699050802263027. [DOI] [PubMed] [Google Scholar]
- 56.Locey ML, Jones BA, Rachlin H. Real and hypothetical rewards. Judgm Decis Mak. 2011;6(6):552–564. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


