Abstract
Background
Continuous positive airway pressure [CPAP] and supplemental oxygen have become the mainstay of neonatal respiratory support in preterm infants. Although oxygen therapy is associated with respiratory morbidities including bronchopulmonary dysplasia [BPD], the long-term effects of CPAP on lung function are largely unknown. We used a hyperoxia-induced mouse model of BPD to explore the effects of daily CPAP during the first week of life on later respiratory system mechanics.
Objective
To test the hypothesis that daily CPAP in a newborn mouse model of BPD improves longer term respiratory mechanics.
Methods
Mouse pups from C57BL/6 pregnant dams were exposed to room air [RA] or hyperoxia [50% O2, 24hrs/day] for the first postnatal week with or without exposure to daily CPAP [6cmH2O, 3hrs/day]. Respiratory system resistance [Rrs] and compliance [Crs] were measured following a subsequent 2 week period of room RA recovery. Additional measurements included radial alveolar counts and macrophage counts.
Results
Mice exposed to hyperoxia had significantly elevated Rrs, decreased Crs, reduced alveolarization, and increased macrophage counts at three weeks compared to RA treated mice. Daily CPAP treatment significantly improved Rrs, Crs and alveolarization, and decreased lung macrophage infiltration in hyperoxia-exposed pups.
Conclusions
We have demonstrated that daily CPAP had a longer term benefit on baseline respiratory system mechanics in a neonatal mouse model of BPD. We speculate that this beneficial effect of CPAP was the consequence of a decrease in the inflammatory response and resultant alveolar injury associated with hyperoxic newborn lung injury.
Keywords: Hyperoxia, CPAP, bronchopulmonary dysplasia, alveolar development, pulmonary mechanics
INTRODUCTION
Bronchopulmonary dysplasia [BPD] is a chronic respiratory disease that affects over 30% of preterm infants born at less than 30 weeks gestational age [[1,2]. The development of BPD results from neonatal lung injury which is thought to be caused primarily by supplemental oxygen [O2] therapy and mechanical ventilation and remains a major form of respiratory morbidity in former preterm infants [[3,4]. Although lung growth and remodeling may improve over time, lung function abnormalities typically comprising of airway obstruction and wheezing disorders remain throughout childhood and adolescence, and are also observed in preterm infants without the diagnosis of BPD [[4-7]. As a consequence, many preventive and therapeutic interventions have been investigated in this population, but the prevalence of respiratory morbidity remains high.
Although, there is no current consensus on the best respiratory support strategy in preterm patients at risk of developing BPD, there is a substantial increase in the use of continuous positive airway pressure [CPAP] as the primary respiratory modality to avoid intubation and hence, decrease potential lung injury from mechanical ventilation [[8]. Experimental animal studies also demonstrate the benefit of noninvasive respiratory support versus mechanical ventilation in promoting alveolarization and preventing inflammation [9-10]. CPAP given to young ferrets for 2 weeks demonstrated accelerated lung growth and a 40% increase in total lung capacity [11]. The authors suggested mechanical strain [i.e., CPAP] improves lung growth by remodeling the lung parenchyma. To our knowledge, there are no available data on the longer term effects of CPAP on respiratory system mechanics in a neonatal animal model of hyperoxia induced lung injury.
Initial experiments in neonatal mice using 85% O2 showed alveolar simplification and lung dysfunction, creating a lesion similar to human BPD [12]. Recent experiments have shown that even moderate amounts of oxygen can result in long lasting impairment of lung structure and function [13-15] in a rodent model. Therefore, the purpose of this study was to create a BPD phenotype based on exposure to moderate levels of hyperoxia to test the hypothesis that daily CPAP would elicit long lasting beneficial effects on respiratory system mechanics.
MATERIALS AND METHODS
Animal Model and Exposure
Animal protocols were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University. Timed pregnant C57BL/6 mice [Charles River] were purchased and housed in an accredited animal facility at CWRU. Mouse pups from pregnant dams were pooled within 24 hours of birth and randomly assigned into treatment groups as follows: 1] room air [RA]; 2] RA with daily CPAP [+6 cm H2O, 3 hours/day for days 1 to 7]; 3] continuous hyperoxia [50% O2, 24 hours/day for 7 days]; and 4] hyperoxia [50%] with CPAP [+ 6 cm H2O, 3 hours/day for 7 days]. Oxygen exposures and CPAP were initiated the day after birth [P1] and ended on the 7th day of postnatal life [P7]. Animals were maintained on standard 12 hour light-dark cycles with ad libitum standard food and water. Hyperoxia exposed animals were placed in a 38 L Plexiglas chamber with a continuous flow of 50% O2 [4 L/min] for 7 days. Hyperoxia was achieved by mixing air and 100% O2 at appropriate amounts to achieve the desired level of O2, which was monitored daily [MiniOX I; MSA Medical]. Hyperoxia exposed mice that were designated to receive CPAP were removed from the hyperoxia chamber and immediately placed on a mask that delivered 50% O2 while also administering CPAP. CPAP was applied by passing humidified gas [RA or 50% O2 depending on the treatment group] through a custom-made mask fitted to the face of the mouse as previously described [16]. This mask was constructed from a 3ml syringe cut in half to accommodate the entire face of the animal. The “open” end of the syringe allowed for positioning of a latex sleeve containing a hole large enough to accommodate the face of the animal. The latex sleeve was constructed from the finger tip of latex gloves. The level of CPAP was monitored using a manometer connected directly to the mask and could be finely adjusted by altering the size of a leak using an adjustable valve located between the mask and the manometer. At the end of the daily CPAP session, the mice were returned to the mother and the hyperoxic group placed back inside the hyperoxia chamber. CPAP was restricted to 2 hours on the first day to minimize time separated from the nursing dam. On the following 6 days CPAP duration was increased to 3 hours/day. Control animals experienced the same conditions [i.e, mask, humidified air, heat pad and 3 hours of separation from the dam], but without CPAP. On rare occasions the animal was able to maneuver out of the mask, in which case it was immediately re-positioned as the animals were under constant observation. Due to the small size of the neonatal pups, precise measurement of pressures to which various components of the airways were exposed could not be measured. On day 7 mice were returned to the nursing dam in RA and allowed to develop normally for an additional 14 days. At P21 animals were weighed for the measurements of respiratory mechanics or tissue preparation. There was no significant difference between average body weights at 3 weeks and there was approximately equal distribution of females and males between groups.
Pulmonary Function Tests
Mice from the four exposure groups [RA, n=10; RA & CPAP, n=7; 50% O2, n=12; 50% O2 & CPAP, n=7] were anesthetized at P21 with intraperitoneal [i.p.] ketamine [200 mg/kg] and xylazine [20 mg/kg] mixture. Anesthetized mice were placed supine on a heated surgical table, tracheostomized via a 19G blunt tip cannula and ventilated [flexiVent, SCIREQ, Montreal, Canada] at a tidal volume of 10 ml/kg, 150 breaths/min, PEEP of 3 cm H2O, and FiO2 of 50%. Animals were then paralyzed with i.p. pancuronium bromide [10 mg/kg], and respiratory system resistance [Rrs] and compliance [Crs] were calculated with the flexiVent software using a 1.2 second, 2.5 Hz single-frequency forced oscillation maneuver [17].
Lung Alveolarization Assessment
In order to assess lung morphology, additional mice [RA, n=10; RA & CPAP, n=11; 50% O2, n=7; 50% O2 & CPAP, n=7] were removed from litters at postnatal day 21, and euthanized with an intraperitoneal injection of ketamine/xylazine. The trachea was cannulated and lungs were inflation-fixed [25 cm H2O] for 10 min with 10% neutral-buffered formalin. The left lung was removed, post fixed for two days at 4°C and paraffin embedded. Sections were stained with hematoxylin and eosin, and alveolarization was assessed by performing radial alveolar counts [RAC]. Images of each section were captured with a magnified digital camera through a Leica microscope. RAC was determined by counting the number of alveolar septa transected by a perpendicular line drawn from the terminal bronchiole to the nearest connective tissue septum [18], and was averaged from 8-10 sections per animal from each of the four treatment groups. We additionally quantified alveolar-airway attachments. Three to seven randomly selected airways from each animal [5-6 animals/group]. The points where alveolar walls are radially attached to the outer wall of the airways were counted as previously described [14]. They were quantified as the number of attachment points around the margin of airways in relation to the basement membrane perimeter [Pbm]. Only complete airways without alveolar ducts were analyzed.
Mac3 immunohistochemistry
Lungs from the four groups [RA, n=4; RA & CPAP, n=4; 50% O2, n=6; and 50% O2 & CPAP, n=6] were inflation-fixed [25 cm H2O] for 10 minutes with 10% neutral-buffered formalin. The 5 μm paraffin embedded tissue was treated with 3% H2O2 after antigen retrieval. Lung sections were incubated 24 hours at 4μC with rat anti-mouse Mac3 monoclonal antibody [1:500; BD Pharmingen, San Jose, CA]. The secondary antibody was biotinylated goat anti-rat [1:500 Jackson immunoResearch West Grove, PA, USA], followed by ABC reagent incubation and visualization by diaminobenzidine [Vectastain, Vector Laboratories, Burlingame, CA, USA]. Finally, lung sections were dehydrated and mounted with permount. Primary antibody was omitted for negative controls. All images were obtained using a Rolera XR CCD camera [Q-Imaging, Canada] mounted on a Leica DMLB microscope [Leica Microsystems, Germany]. Images were analyzed using research-based digital image analysis software [Image-pro Plus 7.0, Media Cybernetics, Silver Spring, MD]. Numbers of labeled macrophages were counted in five random fields at 20x magnification from 4-6 animals per group.
Statistical Analysis
Data are expressed as means ± standard error of the mean [SEM]. Respiratory system resistance [Rrs] and compliance [Crs], radial alveolar counts, alveolar-airway attachments, and macrophage counts were compared using a one-way analysis of variance with Student-Newman-Keul's method. Statistical significance was defined as p<0.05.
RESULTS
Pulmonary Function Tests
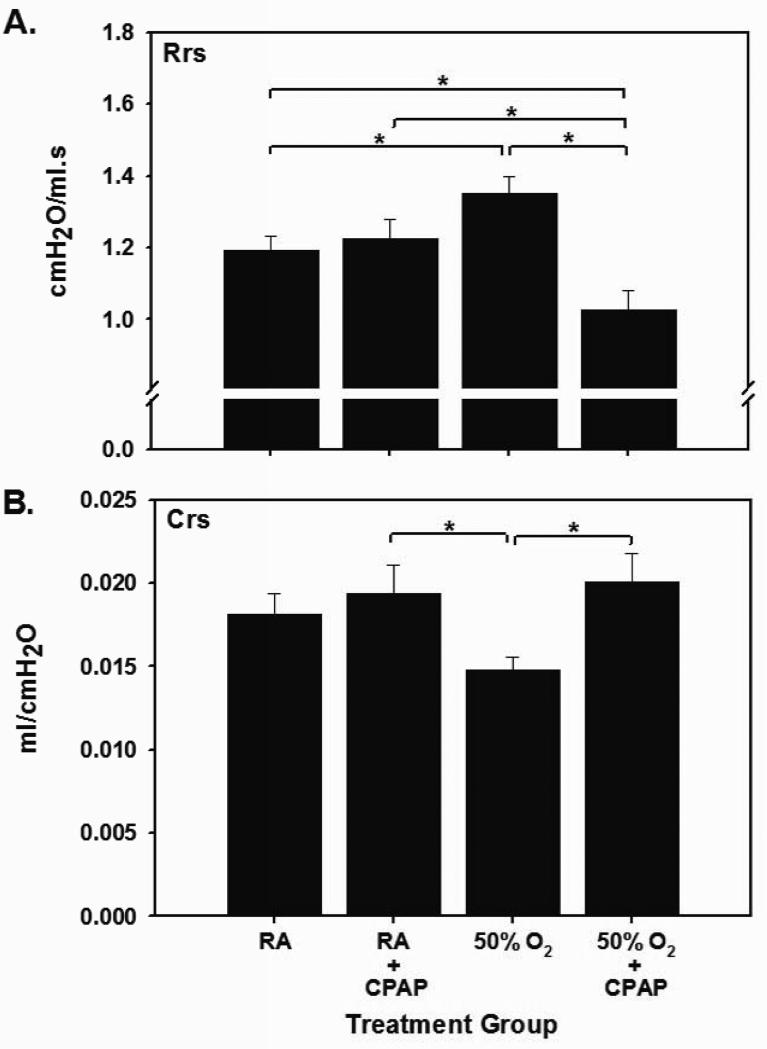
Respiratory system resistance [Rrs] and compliance [Crs] were compared between each experimental group. CPAP alone had no effect on Rrs when compared to RA [Fig 1A]. In contrast, hyperoxia exposure increased Rrs versus RA [1.35±0.17 vs 1.19±0.12, respectively, p<0.05]. In the hyperoxia groups, the addition of CPAP attenuated the increase in Rrs observed with hyperoxia alone [1.35±0.17 vs 1.03±0.14 cmH2O/ml·s, hyperoxia vs hyperoxia+CPAP, p<0.005]. Levels of Rrs in hyperoxia+CPAP pups were also significantly below both RA groups [p<0.05]. As was the case with Rrs, CPAP alone had no effect on Crs when compared to RA [Fig 1B]. Hyperoxia exposure induced a decrease in Crs that did not reach statistical significance compared to RA [p=0.053] but was significantly lower than in pups exposed to CPAP alone [0.015±.003 vs 0.019±0.005 ml/cmH2O, hyperoxia vs RA+CPAP, p=0.04]. In the hyperoxia groups, the addition of CPAP increased Crs [0.015±0.003 vs 0.020±0.005 ml/cmH2O, hyperoxia vs hyperoxia+CPAP, p<0.05]. Levels of Crs in hyperoxia+CPAP pups were comparable to both RA groups.
FIGURE 1.
Effect of exposure to CPAP on respiratory system mechanics in normoxic [RA] and hyperoxic mouse pups. Panel A: Hyperoxic exposure increased respiratory system resistance [Rrs] and this effect was reversed by CPAP in hyperoxic pups. Hyperoxic pups exposed to CPAP also had lower Rrs than both RA control groups. Panel B: Hyperoxic exposure decreased respiratory system compliance [Crs] when compared to both RA and hyperoxic groups treated with CPAP [*p<0.05].
Lung Morphometrics
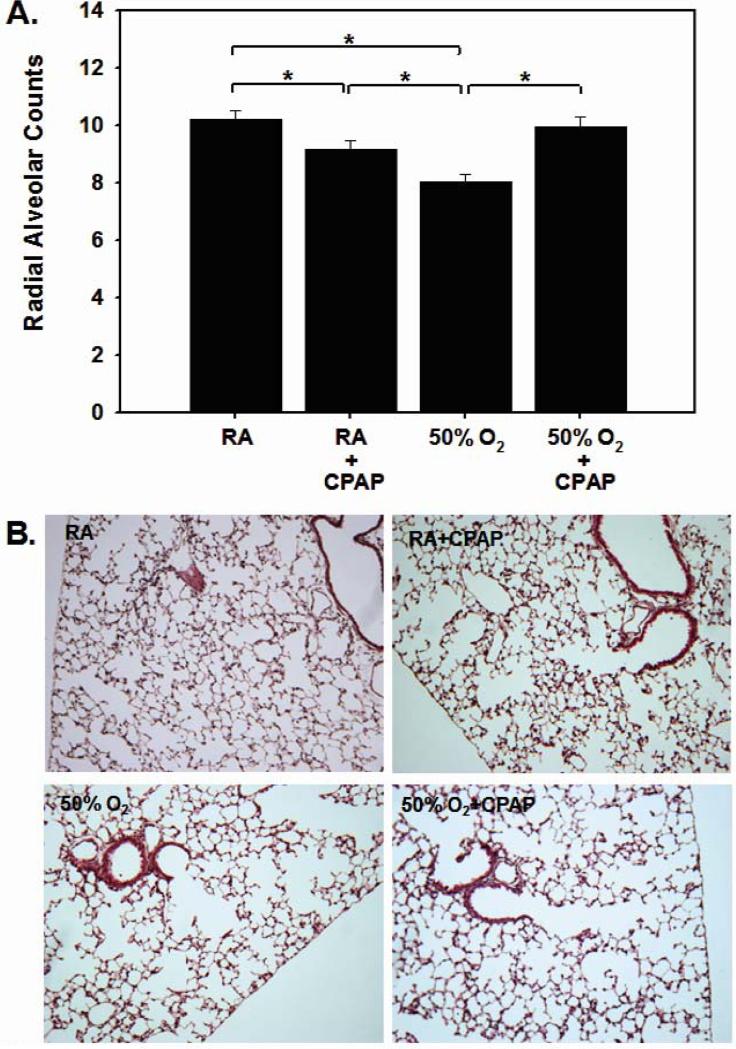
Alveolarization was assessed at 3 weeks of age via morphometric analyses of H&E-stained lung sections. CPAP alone caused a borderline decrease in RAC in RA pups [10.2±1.03 vs 9.15±1.09, p=0.05; Fig 2]. Hyperoxic pups demonstrated significantly lower radial alveolar counts compared to RA-exposed pups [8.07±0.63 vs 10.2±1.03, hyperoxia vs RA, p<0.05]. In the hyperoxia groups, the addition of CPAP prevented the hyperoxia-induced reduction in RAC [8.07±0.63 vs 9.92±0.92, hyperoxia vs hyperoxia+CPAP, p<0.05]. Data for alveolar to airway attachments are consistent with the RAC data, although they did not reach statistical significance [RA 26.4±1.6; RA+CPAP, 25.1±1.6; Hyperoxia 20.6±1.5; Hyperoxia + CPAP 22.4±1.7; p=0.07].
FIGURE 2.
Radial alveolar counts [RAC] from the four groups of pups are summarized in Panel A and representative images shown in Panel B. Hyperoxic exposure decreased RAC and this decrease was reversed when hyperoxic pups were exposed to CPAP [*p<0.05, Hp=0.05].
Macrophage Counts
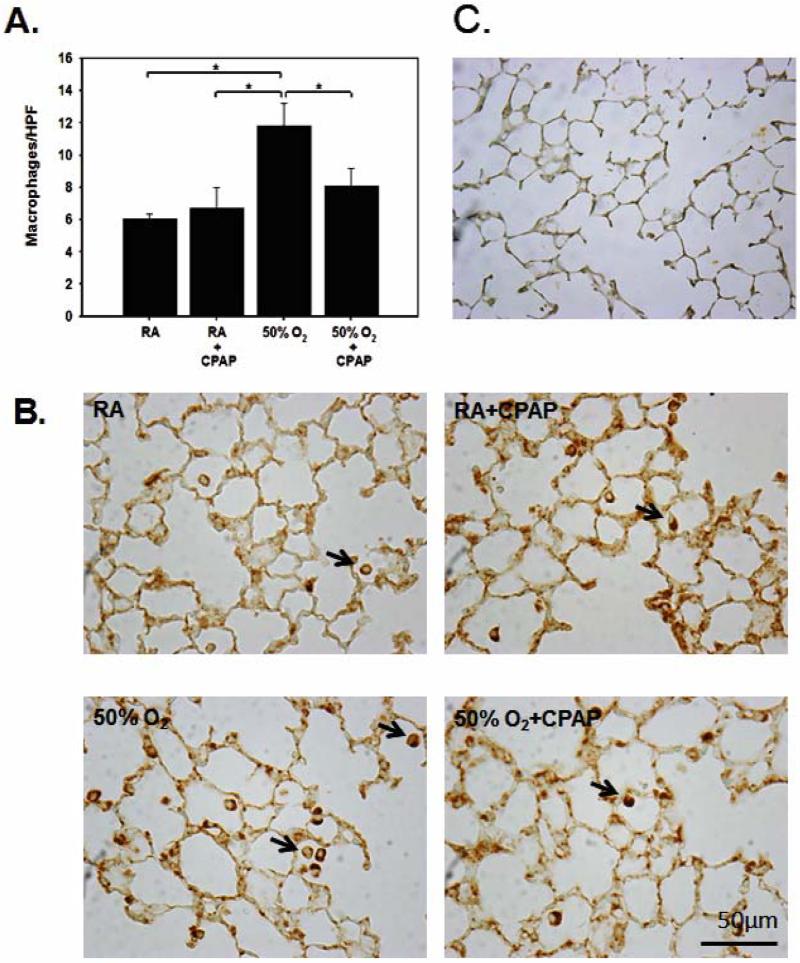
To assess inflammation, pulmonary macrophage numbers were counted on lung tissue sections from mice stained with Mac3 anti-macrophage antibodies [Fig. 3]. RA controls showed very few interstitial Mac3 positive cells as did those in the RA+CPAP group. Hyperoxia alone resulted in a significant increase in Mac3 positive stained parenchymal cells [11.82±3.41] when compared to either RA controls [6.05±0.60, p<.05], RA+CPAP [6.7±2.57, p<0.05] or the hyperoxia + CPAP group [8.06±2.71, p<0.05].
FIGURE 3.
Representative images of macrophages stained with Mac3 anti-macrophage antibodies from the four groups of pups [B] and a negative control [C] are shown together with summarized data [A]. Hyperoxic exposure increased Mac3 counts in lung parenchyma when compared to both RA groups. This increase was prevented with CPAP exposure in hyperoxic pups [*p<0.05]. The number of macrophages was counted per high power field [HPF, 20X magnification].
DISCUSSION
Hyperoxia model
In this study we employed a hyperoxia-exposed, immature rodent model to test the hypothesis that CPAP might benefit longer term lung injury. Many of the commonly used animal models induce acute lung injury by increasing the amount of supplemental oxygen, exposing immature lungs to mechanical ventilation, and/or inducing pre- or postnatal inflammation [19-21]. While the injurious effects of oxygen can be replicated in many species, the newborn mouse demonstrates stages in lung maturity similar to extremely preterm infants with murine alveolar development beginning on postnatal day 3, and saccular division completed by day 14 [22]. Neonatal rodents exposed to supplemental oxygen are also best suited to characterize longer term effects of neonatal lung injury. As the oxygen concentrations used in many earlier animal models of BPD exceeded the level of oxygen supplementation currently applied to preterm infants, our animal model of BPD employed 50% oxygen to better reflect the modern clinical setting in which high FiO2 is avoided whenever possible. Our results demonstrate that such modest exposure of 50% oxygen for 7 days causes impairment in respiratory function that is characteristic of BPD and similar to previous studies using small animal models exposed to higher oxygen concentrations [10,20,23]. It should be noted, however, that maturation of the murine immune system is a dynamic process which may not parallel the pre- and postnatal human maturational process and may not be in synchrony with lung development.
CPAP model
Many preterm infants only require short periods, if any, of intubation and mechanical ventilation, and instead, spend much longer durations on non-invasive support [i.e., CPAP]. Therefore, there is a need for an animal model of BPD that simulates current clinical practice in the human preterm infant. We have previously reported the ability to successfully administer CPAP to a neonatal mouse [16]. This is the first report of in vivo respiratory function data following CPAP administration to a small rodent and the model has the ability for longer-term follow-up experiments of respiratory function.
Protective effects of CPAP
The results of this study demonstrate daily CPAP protected both respiratory system resistance and compliance from the adverse effects of oxygen exposure. Previous studies in preterm lambs have demonstrated that high frequency nasal ventilation, which is somewhat analogous to CPAP, appears to support normal alveolar development by preserving the balance between mesenchymal cell apoptosis and proliferation, when compared to the injurious effects of intermittent mandatory ventilation [10]. This is consistent with other studies in preterm lambs that demonstrated CPAP or gentler modes of ventilation reduce key markers of lung injury and inflammation [24,25]. In our study, alveolarization was preserved by CPAP in the hyperoxia pups with a resultant improvement in respiratory system compliance.
In pups exposed to CPAP in the absence of hyperoxia, there was a borderline decrease in RAC without significant effect on baseline respiratory mechanics. There was also no clear decrease in alveolar attachments after CPAP exposure in the RA group, which could suggest that CPAP administration alone did not have a clearly defined effect on lung parenchyma. Our observed decrease in RAC with hyperoxic exposure was associated with a trend towards a reduction in alveolar attachments. Fewer alveolar attachments have been observed in mature mice exposed to a higher level of postnatal hyperoxia than used in our study [14]. We have no clear explanation for the significantly lower Rrs after hyperoxia+CPAP exposure when compared to RA controls. Our data on lung morphology do not provide an explanation for this low Rrs after combined hyperoxia and CPAP exposure. We speculate that this combination has a selective effect on airway structures that requires further in vivo and in vitro study.
We have previously documented an increase in airway reactivity in an in vitro lung slice model after neonatal mice were exposed to CPAP under normoxic conditions [16]. Unlike our current in vivo study, the lung slices did not allow measurement of baseline respiratory mechanics. The current data demonstrate that baseline resistance is not affected by prior CPAP in normoxic pups and the beneficial effect of CPAP is limited to our hyperoxia exposed pups. The longer term benefit on respiratory system mechanics was accompanied by a decrease in macrophage mediated inflammation in the hyperoxia exposed pups. A detailed analysis of the effect of CPAP on the time course of other inflammatory markers such as neutrophils and various cytokines will require future study in larger group sizes. However, the association between increased inflammation, as measured by macrophage infiltration elicited by hyperoxia, and decreased radial alveolar counts would be anticipated from prior studies in neonatal rodents [19,20]. Future studies employing our novel rodent model for CPAP administration might also provide clinical guidance for neonatologists who are confronted daily with the dilemma of when to wean infants off CPAP without understanding how this may alter future lung function.
Limitations
Although our daily mask applications with and without added CPAP were surprisingly well tolerated, we chose to only administer CPAP for 3 hours daily in order to minimally disrupt the pups and to avoid longer periods away from maternal care. Even with such minimal exposure to CPAP we found a protective effect for the hyperoxia exposed mice. We only selected one recovery time point at three postnatal weeks to assess our results, however, this was deliberately chosen as a marker for the impaired respiratory function of preterm infants that continues until early childhood [5, 6].
In conclusion, with our model of BPD, CPAP protected against longer term oxygen-induced lung injury, as manifest by improved respiratory system mechanics and alveolar morphology. We speculate that greater understanding of stretch mediated molecular pathways may play an important role in our future understanding of the airway remodeling and alveolar simplification that are associated with longer term respiratory morbidity in preterm infants.
ACKNOWLEDGMENT
Supported by: Rainbow Babies & Children's Foundation Fellowship Research Award, T32-HD060537, and NIH grant HL56470 [YS Prakash & RJ Martin]. In addition, we acknowledge the technical support of Tien Nguyen in generating data for this manuscript.
REFERENCES
- 1.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 2.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 3.Greenough A. Bronchopulmonary dysplasia – Long term follow up. Paediatr Respir Rev. 2006;7S:S189–S191. doi: 10.1016/j.prrv.2006.04.206. [DOI] [PubMed] [Google Scholar]
- 4.Reyburn B, Martin RJ, Prakash YS, MacFarlane PM. Mechanisms of injury to the preterm lung and airway: implications for long-term pulmonary outcome. Neonatology. 2012;101:345–352. doi: 10.1159/000337355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, Thomas S, Stocks J. Lung function and respiratory symptoms at 11 years in children born extremely preterm. Am J Respir Crit Care Med. 2010;182:237–245. doi: 10.1164/rccm.200912-1806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, Sheikh A. Preterm birth and childhood wheezing disorders: A systematic review and meta-analysis. PLOS. 2014;11:1–18. doi: 10.1371/journal.pmed.1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.VollsΦter M, Rρksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax. 2013;58:767–776. doi: 10.1136/thoraxjnl-2012-202980. [DOI] [PubMed] [Google Scholar]
- 8.Carlo WA, Polin RA. American Academy of Pediatrics Committee on Fetus and Newborn. Respiratory support in preterm infants at birth. Pediatrics. 2014;133:171–174. doi: 10.1542/peds.2013-3442. [DOI] [PubMed] [Google Scholar]
- 9.Null DM, Alvord J, Leavitt W, Wint A, Dahl MJ, Presson AP, Lane RH, DiGeronimo RJ, Yoder BA, Albertine KH. High-frequency nasal ventilation for 21 d maintains gas exchange with lower respiratory pressures and promotes alveolarization in preterm lambs. Pediatr Res. 2014;75(4):507–516. doi: 10.1038/pr.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyburn B, Li M, Metcalfe DB, Kroll NJ, Alvord J, Wint A, Dahl MJ, Sun J, Dong L, Wang ZM, Callaway C, McKnight RA, Moyer-Mileur L, Yoder BA, Null DM, Lane RH, Lane RH, Albertine KH. Nasal ventilation aleters mesenchymal cell turnover and improves alveolarization in preterm lambs. Am J Respir Crit Care Med. 2008;178:407–418. doi: 10.1164/rccm.200802-359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S, Garbutt V, McBride JT. Strained-induced growth of the immature lung. J Appl Physiol. 1996;81(4):1471–1476. doi: 10.1152/jappl.1996.81.4.1471. [DOI] [PubMed] [Google Scholar]
- 12.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275:L110–117. doi: 10.1152/ajplung.1998.275.1.L110. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Jafri A, Martin RJ, Nnanabu J, Farver C, Prakash YS, MacFarlane PM. Severity of neonatal hyperoxia determines structural and functional changes in developing mouse airway. Am J Physiol Lung Cell Mol Physiol. 2014;307:L295–L301. doi: 10.1152/ajplung.00208.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Reilly M, Harding R, Sozo F. Altered airways in aged mice following neonatal exposure to hyperoxic gas. Neonatology. 2014;105:39–45. doi: 10.1159/000355641. [DOI] [PubMed] [Google Scholar]
- 15.Onugha H, MacFarlane PM, Mayer CA, Abrah A, Jafri A, Martin RJ. Airway hyperreactivity is delayed after mild neonatal hyperoxic exposure. Neonatology. 2015;108:65–72. doi: 10.1159/000380758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer CA, Martin RJ, MacFarlane PM. Increased airway reactivity in a neonatal mouse model of continuous positive airway pressure [CPAP]. Pediatr Res. 2015 May 7; doi: 10.1038/pr.2015.90. doi: 10.1038/pr.2015.90. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shalaby KH, Gold LG, Schuessler TF, Martin JG, Robichaud A. Combined forced oscillation and forced expiration measurements in mice for the assessment of airway hyperresponsiveness. Respir Res. 2010;11:82–94. doi: 10.1186/1465-9921-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 2--intrauterine and early postnatal lung growth. Thorax. 1982;37(8):580–583. doi: 10.1136/thx.37.8.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi CW, Kim BI, Mason SN, Potts-Kant EN, Brahmajothi MV, Auten RL. Intraamniotic LPS amplifies hyperoxia-induced airway hyperreactivity in neonatal rats. Pediatr Res. 2013;74:11–18. doi: 10.1038/pr.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Velten M, Heyob KM, Rogers LK, Welty SE. Deficits in lung alveolarization and function after systemic maternal inflammatioin and neonatal hyperoxia exposure. J Apl Physiol. 2010;108:1347–1356. doi: 10.1152/japplphysiol.01392.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almario B, Wu S, Peng J, Alapati D, Chen S, Sosenko IRS. Pentoxifylline and prevention of hyperoxia-induced lung injury in neonatal rats. Pediatr Res. 2012;71:583–589. doi: 10.1038/pr.2012.14. [DOI] [PubMed] [Google Scholar]
- 22.Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM. Postnatal growth of the mouse lung. J Anat. 1977;124:131–151. [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y-J, Markham NE, Balasubramaniam V, Tang J-R, Maxey A, Kinsella JP, Abman SH. Inhaled niric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res. 2005;58:22–29. doi: 10.1203/01.PDR.0000163378.94837.3E. [DOI] [PubMed] [Google Scholar]
- 24.Jobe AH, Kramer BW, Moss TJ, Newnham J, Ikegami M. Decreased indicators of lung injury with continuous positive expiratory pressure in preterm lambs. Pediatr Res. 2002;52:387–392. doi: 10.1203/00006450-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Rehan VK, Fong J, Lee R, Sakuri R, Wang Z-M, Dahl MJ, Lane RH, Albertine KH, Torday JS. Mechanism of reduced lung injury by high frequency nasal ventilation in a preterm lamb model of neonatal chronic lung disease. Pediatr Res. 2011;70:462–466. doi: 10.1203/PDR.0b013e31822f58a1. [DOI] [PMC free article] [PubMed] [Google Scholar]