Abstract
During pubertal development an animal's response to stress changes and sexual differentiation of the brain and behavior continue. We discovered that particular stressors, shipping from suppliers or an immune challenge with lipopolysaccharide, during the prolonged pubertal period of female mice result in long-term changes in behavioral responsiveness of the brain to estradiol assessed in adulthood. All behaviors influenced by estradiol and/or progesterone that we have studied are compromised by a stressor during pubertal development. Depending on the behavior, immune challenge or shipping from suppliers during pubertal development decreases, eliminates, or even reverses the effects of estradiol. Shipping during this period causes changes in the number of estrogen receptor-immunoreactive cells in key brain areas suggesting one cellular mechanism for this remodeling of the brain's response to hormones. We suggest that particular adverse experiences in girls may cause long-term alterations in the brain's response to estradiol and/or progesterone via activation of the immune system. This in turn could lead to an alteration in any aspect of mental health that is influenced by estradiol.
Keywords: Stress, Immune Challenge, Lipopolysaccharide, Depression, Anxiety, Cognitive function, Sexual behavior, Estradiol, Progesterone, Puberty, Females, Mood disorders
1 Introduction
The so-called, ovarian sex steroid hormones1, estradiol and/or progesterone, have profound effects on a wide variety of effects in many species. In the most well characterized species -- rats and mice – they influence female reproductive behaviors, social behaviors, depression-like behavior, anxiety-like behavior, cognitive function, and many other behaviors and physiological end-points. It is well known that developmental events can reprogram an animal's response in adulthood to these hormones. For example, exposure to estradiol via metabolism from testosterone during the perinatal period influences sexual differentiation of the brain altering later neural and behavioral response to estradiol (1). Furthermore, exposure to sex hormones during pubertal development may be responsible for a second period of sexual differentiation again resulting in remodeling of the brain's behavioral response to ovarian hormones (2).
1.1 Puberty and adolescence
An understanding of the enduring influences of events that occur specifically during puberty requires clarification of the meaning of the term “puberty” (3). Puberty refers to the developmental transition to a mature, reproductive state, while adolescence, a period of development distinct from puberty, refers to social and cognitive maturation that accompanies the reproductive maturation (4;5). However, there is a good deal of variability in the way that the term, “puberty,” is used. “Puberty” is sometimes used to refer to the entire period of pubertal development, but often is used in reference to a singular event during this developmental stage. In rodents, some refer to the first stage of pubertal development, vaginal opening, as “puberty,” while others refer to the onset of reproductive cycles. It is more useful to consider puberty as the developmental processes, which begins with the first sign of ovarian activity and terminates with the onset of the first reproductive cycle. It is most clear to refer to “pubertal development” to focus on the concept that puberty is an extended period of development, not a singular event. We have used mice in our studies of pubertal development, because like humans, pubertal development spans an extended period. For example, in humans, the pubertal period begins with the development of pubic hair and breast budding (Tanner Stage II), ends with menarche, and can last two or three years (6). In mice, the first external sign of pubertal development is vaginal opening, although hormonal changes precede this. Pubertal development can take weeks and is heavily influenced by the social environment (7). Unfortunately for our attempts to relate our animal work to humans, the vast majority of work in humans examines events that occur during the less well-defined period of “adolescence.” Adolescence, as discussed above, overlaps, but is not synonymous with, the period of pubertal development (see figure 1: (3)). In our work, we are focusing specifically on the pubertal stage of development, because our interest is in the effects of changes in the hormonal milieu during this period, rather than chronological age per se. Furthermore, this stage of development, unlike adolescence, has discrete markers (e.g., day of vaginal opening, onset of reproductive cycles; (8))
Figure 1.
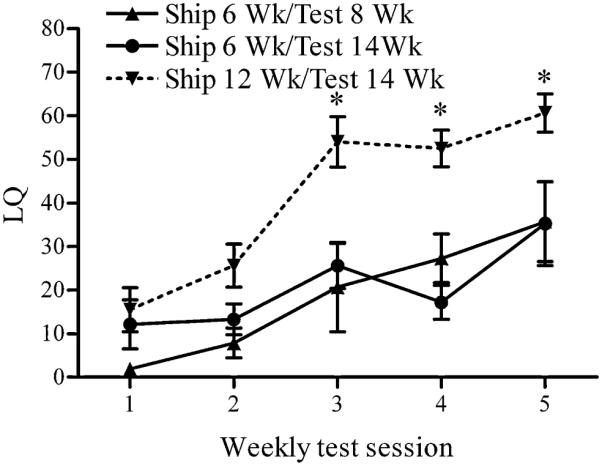
Shipping female mice during pubertal development decreases sex behavioral response to estradiol and progesterone in adulthood. Lordosis quotient (LQ) (mean ± SEM) of C57Bl/6 female mice shipped during or after the peripubertal period. (*, p < 0.05). Reprinted from (16) Copyright 2009, The Endocrine Society.
2 Influence of stressors during pubertal development/adolescence on brain and behavior
Stress during pubertal development and/or adolescence has enduring effects on brain and behavior (8;9); however, the effects of exposure to stressors during this period on subsequent stress reactivity are inconsistent (8), most likely due to the wide variety of classes of stressor used and the variability in age at which exposure occurs. In general, exposure to stressors during pubertal development or adolescence increases anxiety and depression later in life (10), as well as influencing other behaviors (11) and some aspects of learning (12). In rats, binge drinking, which can be considered a stressor, during adolescence affects adult brain and behaviors (13), and some stressors alter later sensitivity to drugs of abuse (14).
Several years ago we discovered that female mice shipped at six weeks old did not show typical behavioral response in an experiment in which they were compared directly to a group of older mice that responded normally (Figure 1). In a follow-up experiment, we observed that, as assessed on the fifth weekly test, mice shipped at three weeks old were relatively unaffected by shipping compared with mice shipped in adulthood. However, mice shipped at five or six weeks of age exhibited depressed behavioral response to estradiol and progesterone contrasted with mice shipped just one week later (seven weeks old or older). Thus, we began our studies on the enduring effects of stress during pubertal development on behavioral response to ovarian hormones.
3 Other stressors
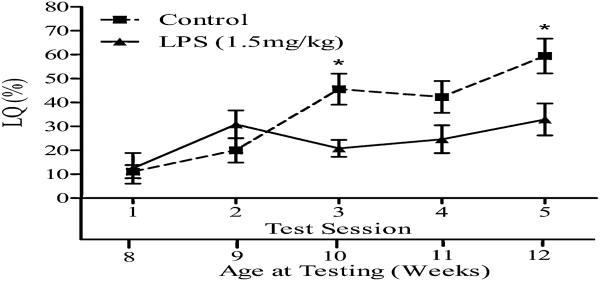
Because we did not consider shipping to be a stressor likely to have great replicability (although we have in fact found it to be quite repeatable), we attempted to develop a laboratory stressor procedure that resulted in a similar loss of behavioral response to estradiol and progesterone in adulthood. Although exposure to all standard stressors used at six weeks of age induced quite high blood corticosterone levels, neither daily restraint, nor a multiple stressor (restraint, heat and light), nor 36 hours of food deprivation resulted in decreased response to estradiol and progesterone in adulthood (15), as was seen with shipping (16). On the other hand, immune challenge with the bacterial endotoxin, lipopolysaccharide (LPS), which results in transient sickness behavior lasting fewer than 48 hours, provided results quite consistent with those obtained with shipping (Figure 2). This suggested that the decrease in adult response to ovarian hormones is not a general response to all peripubertal stressors that increase corticosterone release. Rather it suggested that shipping and immune challenge share some common characteristics. As with shipping, exposure at six weeks old was the optimal age of injection to yield the most consistent effect on adult response to estradiol and progesterone, and four weeks of age was the earliest age at which the LPS was effective in altering response (15).
Figure 2.
Injection of LPS during pubertal development decreases sex behavioral response to estradiol and progesterone in adulthood. Lordosis quotient (LQ) (mean ± SEM) of C57Bl/6 female mice injected with 1.5 mg/kg LPS at 6 wk old. The lower x-axis refers to the animal's age at time of testing. (*= P < 0.05). Reprinted from (15) Copyright 2009, The Endocrine Society.
Interestingly, a social stressor, that is, the stress of social isolation during an extended period of pubertal development (Days 25-60) resulted in a similar enduring decrease in hormone-induced female sexual behavior when tested in adulthood (17). Perhaps the stress of social isolation, the as-yet-undetermined stressors associated with shipping, and the stress of immune challenge share a common mechanism for altering adult behavioral response to ovarian hormones.
4 Extension to an outbred strain of mouse
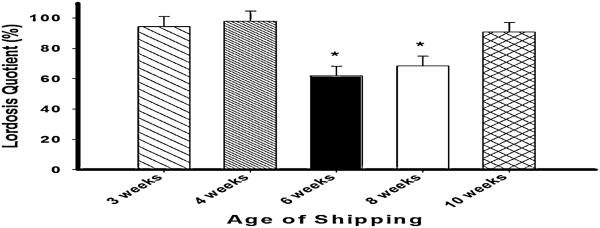
In order to determine the generalizability of this effect of a pubertal immune challenge on adult behavioral response to ovarian hormones, we attempted to extend these findings to the outbred, CD1 strain, of mice. Although the effects of both shipping and LPS on female sexual behavior were confirmed in the CD1 mice (18), the vulnerable period seemed to be extended (Figure 3). Because we have hypothesized that the vulnerable period begins with the first stage of pubertal development (vaginal opening) and terminates with the onset of estrous cycles, it is not surprising that the vulnerable period differs among strains, and it would be expected to be consistent with the peripubertal period in each strain and under particular environmental conditions. In fact pubertal development commences a few days later in CD1 mice than in C57Bl/6 in our laboratory.
Figure 3.
Shipping CD1 strain female mice during pubertal development decreases sex behavioral response to estradiol and progesterone in adulthood. Lordosis quotient (Mean±SEM) on fifth weely test of mice shipped at 3, 4, 6, 8 or 10 weeks. * = p < .05, significantly lower than females shipped at 3, 4 or 10 weeks old. Modified from (18)
5 Extension to rats?
We asked if the finding generalized to females of another rodent species -- rats. Female rats were injected with LPS at either four, six, eight, ten or twelve weeks old, ovariectomized at 14 weeks old and tested for female sexual behavior one week later (H. King and J.D. Blaustein, unpublished observations). LPS did not influence behavioral response in adulthood in rats injected at any age. Does this suggest that this finding is specific to mice? There is another explanation, which is consistent with the results in both rats and mice. The period of pubertal development of mice takes an extended period of time. In our lab, vaginal opening in C57BL/6 mice typically occurs around Day 25 (although this can vary depending on environmental conditions). Estrous cyclicity commences weeks later depending on housing conditions and other environmental factors (7). In contrast, in rats, the latency between the first and last stage of pubertal development can be less than a day (19;20). If the vulnerable period falls between the first and last stage of pubertal development, as we have hypothesized, it is not surprising that we did not duplicate the effect in rats. This lack of effect in rats suggests that mice are a better model for girls in which the stages of pubertal development can span years.
6 Role of ovarian and other steroid hormones
In order to test the idea that LPS remodels the brain by inducing secretion of either estrogens, progestins, androgens, or glucocorticoids, we (J. Laroche and J.D. Blaustein, unpublished observations) injected each of the following inhibitors prior to LPS injection in independent experiments: CDB 2916, a progestin antagonist; RU 58,668, an estrogen antagonist; formestane, an aromatase inhibitor; RU 38486, a progestin/glucocorticoid antagonist; RU 28318, a mineralocorticoid antagonist; or astressin2-B, a corticotropin releasing factor R2 antagonist. None of these inhibitors blocked the effects of LPS, suggesting that LPS does not induce its long-term effects on behavioral response to estradiol by inducing the secretion of estrogens, progestins, or glucocorticoids. However, injection of the androgen antagonist, flutamide, unexpectedly partially blocked the effects of LPS. It will be interesting to explore the mechanisms whereby blocking androgen receptors blocks the effects of LPS on later behavioral response to estradiol.
In order to determine if the ovaries need to be present in order for peripubertal immune challenge to have its long-term effects on behavioral response to ovarian steroid hormones, the ovaries were removed either one week before LPS injection or at the normal time a week before behavioral tests in adulthood. Ovariectomy prior to LPS injection eliminated the long-term altered response to estradiol and progesterone on female sexual behavior, although it did not influence the severity of sickness behavior resulting from the immune challenge (N. Ismail, B. Rappleyea, H. King and J.D. Blaustein, unpublished observations). While this experiment does not identify the hormone(s) responsible for the permissive effect of LPS, it suggests that it might be estradiol, since estradiol secretion is responsible for the first step in pubertal development, vaginal opening. Experiments are underway to test the notion that estradiol confers vulnerability to the long-term effects of pubertal immune challenge on behavioral response to ovarian hormones in adulthood. We cannot exclude the possibility that LPS has an effect on the ovaries, which in turn influences adult behavioral response to exogenously administered ovarian hormones. However, because all animals are ovariectomized prior to the start of hormonal treatment, this is unlikely.
The idea that estradiol (or another ovarian secretion) confers vulnerability is counter-intuitive, because estradiol is generally considered to be neuroprotective (21;22). However, the ovaries, and estradiol in particular, have been reported to be required for LPS to trigger an inflammatory response in microglia of the brain (23). These authors suggest that the data are consistent with the view that the neuroprotective effect of estradiol is due to its immunosuppressive properties; perhaps the defective, innate immune response in the brain of ovariectomized mice causes the host to be more vulnerable to infection, thus increasing neuronal damage as a result of insult by a pathogen.
7 Mediating mechanisms
In order for peripubertal stressors, but not post-pubertal stressors, to exert their negative, long-term effect on hormone-induced sexual behavior, something must differ between the peripubertal and postpubertal response to the stressor to confer the altered response in adulthood. In addition, some element of the response pathway of the steroid hormones in adulthood must be changed as a consequence of the peripubertal stressor.
With regard to the first question, we focused on potential differences in the function of microglia between the two stages. As a first step, because of the role of microglial activation in brain development (24), we examined microglial morphology in peripubertal and postpubertal period. We observed that peripubertal female mice display a more activated microglial phenotype (e.g., hyper-ramification with long, thick processes) than postpubertal mice, and in some neural areas, such as the hypothalamus and hippocampus, LPS induces a greater degree of microglial activation (M. Holder and J.D. Blaustein, unpublished observations). Perhaps the higher concentration of activated microglia in brain areas like the hypothalamus translates to a long-term change in response to ovarian steroid hormones by altering the trajectory of brain development during the pubertal period.
We have tested two hypotheses of changes in the adult brain as a consequence of the pubertal stressor. The first hypothesis was that the peripubertal stressor induced a change in response of the hypothalamo-pituitary-adrenal (HPA) axis to the behavioral testing, because elevated levels of corticosterone have been reported to inhibit the sex behavioral response to estradiol and progesterone (25). Further, the HPA axis matures as a consequence of pubertal development resulting in robust changes in reactivity to stressors (8;10). For example, after pubertal development is complete, blood levels of ACTH and corticosterone return to baseline levels after a stressor considerably more rapidly than in prepubertal animals (8;26).
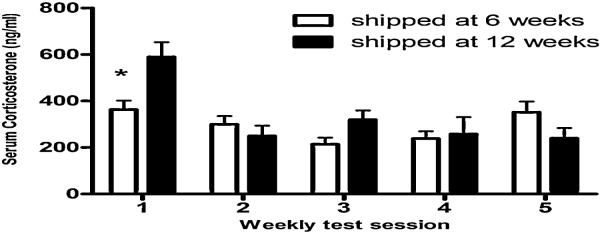
This hypothesis that pubertal stressors induce increases in corticosterone in response to testing or stress was not supported. In the first experiment, mice shipped at six weeks of age actually secreted lower levels of serum corticosterone in response to the first behavioral testing situation than mice shipped at 12 weeks of age (15) (Figure 4). In the second experiment, mice administered LPS at six weeks of age secreted lower levels of serum corticosterone than saline controls in response to moving of the cage from the colony room to the testing room, to exposure to the testing arena, to exposure to the testing arena with a male on the other side of a mesh barrier, or to being tested with a male mouse (J. Laroche and J.D. Blaustein, unpublished observations). Finally, in a general test of response to a stressor, LPS injection resulted in decreased corticosterone release during a restraint-stress test in adulthood (J. Laroche and J.D. Blaustein, unpublished observations), a finding similar to that recently reported in rats after peripubertal immune challenge (27). Therefore, because the pubertal stressors actually result in a blunting of the corticosterone response, the hypothesis that the pubertal stressors result in an exaggerated corticosterone response to the behavioral testing situation or a standard stressor can be rejected.
Figure 4.
Shipping female mice during pubertal development alters corticosterone levels in response to sex behavioral testing in adulthood. Corticosterone levels (mean ± SEM.) in serum taken 15 - 20 minutes in five weekly tests after start of female sexual behavior testing in female mice shipped at 6 weeks or 12 weeks (* = P < 0.01). Reprinted from (16) Copyright 2009, The Endocrine Society.
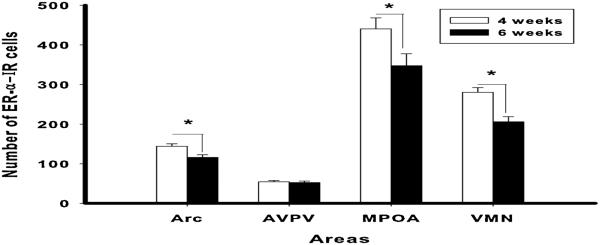
An alternate hypothesis is that the pubertal stressors result in a long-term modification in the level of estrogen receptors (ERs) in areas involved in mediating the effects of estradiol on female sexual behavior. Many of the effects of estradiol on the brain, including female sexual behavior, are mediated via interaction with intracellular estrogen receptors (ERs), and in particular with ERα (28;29). To test the hypothesis that levels of ERα are modified by a peripubertal stressor, CD-1 mice were shipped from the supplier at either four (before the critical period in this strain) or six weeks (during the critical period) old (18). In adulthood, they were tested twice in weekly tests for sexual receptivity, and the effect of shipping during the vulnerable, peripubertal period was confirmed. One week later, they were euthanized, brains immunostained for ERα, and ERα-ir cells counted in four brain areas. Shipping during the vulnerable period resulted in decreased numbers of ERα-ir cells in the arcuate nucleus, medial preoptic area and ventromedial nucleus of the hypothalamus, but not the anteroventral periventricular nucleus (Figure 5). These results suggest that a peripubertal stressor results in long-term change in regulation of ERs in some brain areas associated with reproductive behaviors. In the study discussed earlier in which social isolation induced a similar effect as shipping or LPS on female sexual behavior (17), an increase in ERα-ir cells in the ventromedial hypothalamus was observed. It is difficult to reconcile these two experiments, because the methodology, including treatment of the mice prior to euthanasia, was quite different.
Figure 5.
Shipping female mice during pubertal development results in reduction of ER-α levels in adulthood in some brain areas. Number of ER-α IR cells (mean ± SEM) in CD-1 female mice shipped at 4 or 6 weeks old in the arcuate nucleus (Arc), anteroventral paraventricular nucleus (AVPV), medial preoptic area (MPOA) and ventromedial nucleus of the hypothalamus (VMN). (*= p < 0.05) Reprinted from (18;18) with permission of Elsevier, Copyright 2011.
8 Behavioral specificity of the effect
In a subsequent series of experiments, we tested the hypothesis that the decreased response to ovarian hormones that we reported for female sexual behavior is a result of a general decrease in the brain's behavioral response to the hormones and not specific to sexual receptivity. The effects of either shipping or LPS on the effects of estradiol on depression-like behaviors (30), anxiety-like behaviors (31), and cognitive function (32) were assessed.
Ovarian hormones influence mental health-related behaviors. For example, ovariectomy (OVX) increases depression-like behaviors in rats and mice, and estradiol typically reverses or attenuates these effects (33-42). Consistent with an antidepressive role for estradiol, female aromatase knockout mice that are deficient in estradiol synthesis show greater depression-like symptoms than their wild-type counterparts (43).
In order to test the generality of the effect to other estradiol-influenced behaviors, the effects of peripubertal immune challenge on the antidepression-like effects of estradiol were assessed (30). Although the antidepression-like effects of estradiol were confirmed in two standard tests for depression-like behavior -- the forced swim test and tail suspension tests – estradiol actually increased depression-like behavior in mice that were treated with LPS during pubertal development.
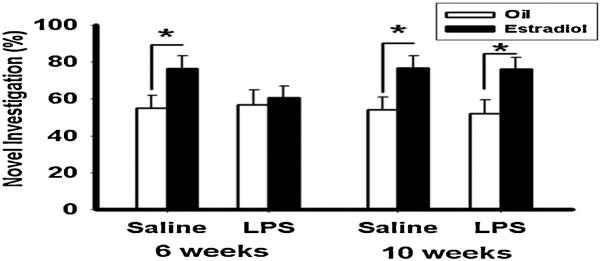
Ovarian steroid hormones also influence anxiety-like behavior in rats. Ovariectomy increases anxiety-like behaviors in rats and mice (44-47), and injection with low doses of hormones have anxiolytic effects (44;45;48-53). Knockout of either the estrogen receptor α (47;54;55) or estrogen receptor β (56;57) gene increases anxiety-like behavior contrasted with wild-types. A combined treatment of estradiol benzoate followed by progesterone regimen has an anxiolytic effect as assessed in the elevated plus maze, light-dark box, and marble burying test in both C57Bl/6 and CD1 strains of mice (31). Injection of LPS at 6 weeks old resulted in the adult hormone treatment having either no effect or an anxiogenic effect (31). There was also a tendency for LPS to reduce anxiety-like behavior in both the light-dark box and the elevated plus maze in the absence of hormone replacement, which agrees with earlier work in which adolescent stressors or prenatal LPS treatment reduced anxiety-like behavior (58;59). Estradiol improves performance on a variety of cognitive tasks, including those that test spatial and recognition memory (60). To test the idea that pubertal stressors influence the pro-cognitive effects of estradiol, mice injected with LPS either during or after pubertal development were assessed for estradiol-influenced cognitive function on four tests – object recognition, object placement recognition (Figure 6), social discrimination, and social recognition. Peripubertal LPS eliminated all of the positive effects of estradiol, suggesting that immune challenge during pubertal development negatively influences the positive effects of estradiol on a variety of tests for cognitive function.
Figure 6.
Estradiol increases the percentage of time spent investigating novel object during the object recognition test in mice treated peripubertally with saline, but not LPS. (Mean ± SEM; *p<0.05) Reprinted from (32) with permission from Elsevier, Inc.
9 Discussion
For all behaviors that were examined, the pubertal stressor had negative consequences on adult behavioral response. The pubertal stressor decreased the effects of estradiol and progesterone on hormone-induced female sexual behavior, reversed the antidepressant-like effects of estradiol, eliminated or reversed the anxiolytic effects of estradiol and progesterone, and eliminated the cognitive enhancing effects of estradiol.
Does a pubertal stressor alter adult behavioral response by an influence on sexual differentiation of the brain? In one study performed in males, shipping at 6 weeks resulted in a decrease in masculine sexual behavior (16). This would suggest that with respect to sexual behavior, the pubertal stressor both defeminized females and demasculinized males. However, it is more parsimonious and more productive to think of the stressors as causing altered responsiveness to steroid hormones.
It is difficult to relate the work discussed in this review to a good deal of the previous work on stressors during the peripubertal or adolescent stages of development for a number of reasons. First, much of the work done previously used males, and the work discussed in this review in on females, which have a very different time course of pubertal development than males. Although we have performed one study on males (16), we have not performed the extensive experiment to determine the optimal age during development to observe an effect of a peripubertal stressor on later response to gonadal hormones, as we have in females. In addition, in much of the previous work, rats were used (8), and puberty has a very different time course in rats than in mice, as discussed above. Also, much of the previous work has used stressors that we have found to be ineffective in inducing this long-term change in response to ovarian hormones (61); thus far, we have identified only shipping and LPS as effective stressors for the long-term alteration in behavioral response to ovarian hormones. Finally, the work discussed in this review is focused specifically on altered response to ovarian hormones in adulthood, an issue that has not been looked at in most other work on developmental stressors.
9.1 Relevance to humans: Some questions and a hypothesis
Estrogens and/or progestins are used in the treatment of depression-related, affective disorders in women, including postpartum depression, premenstrual dysphoric symptoms, and peri- and post-menopausal depression (40;62;63;63-69). We have considered the possibility that the phenomenon of pubertal stress/immune challenge causing long-term changes in response to estradiol and progesterone in mice has relevance to humans.
It is well-known that stress/adverse experiences during pubertal development/adolescence in humans has negative consequences for mental health later in life (70-72), and that this stage of development is a sensitive developmental period for subsequent mental health (10). Inflammation is implicated in the etiology of some depressive disorders (73;74). Further, some stressors during early childhood or adolescence induce acute and enduring changes in the immune system (75-78). Interestingly, adults who have been maltreated in childhood (76) and adult women who experienced sexual abuse during adolescence (79) have elevated CRP and/or interleukin-6 levels in plasma. Further, women with PTSD related to childhood abuse have increased activity in the immune-related, peripheral NF-κB signaling pathway (78). These results collectively suggest that in humans, the immune system responds to particular adverse experiences during critical developmental stages, and that the immune system may remain activated into adulthood. Finally, immune challenge during neonatal development in rodents causes long-term changes in the immune system (80-82). It is tempting to speculate that shipping and LPS treatment during pubertal development have their enduring effects on behavioral response to ovarian steroid hormones by long-term changes in immune function. As discussed earlier, the vast majority of human studies focus on relatively vague independent variables, such as childhood or adolescence (76). It is therefore difficult, if not impossible, to directly correlate the work in humans with the mouse studies discussed in this review. However, can we rely on this mouse model to make predictions about humans? Is there a sensitive period in girls, limited by the first and last stages of pubertal development during which vulnerability to particular stressors that influence the immune system and have long term effects is maximal? It is usually considered that immune changes adversely influence mental health directly through inflammatory pathways (73;74). However, the work in mice suggests that we should consider the hypothesis that adverse peripubertal events exert some of their long-term effects on the brain and mental health indirectly by an intermediary alteration in response to estradiol and/or progesterone. Perhaps exposure to abuse or maltreatment during pubertal development predisposes some individuals to have an atypical response to estradiol later in life. Depression is just one example in which this mechanism could come into play.
10 Conclusions
In conclusion, exposing female mice to particular stressors during pubertal development has a robust influence on all ovarian steroid hormone-influenced behaviors in adulthood that we have studied. We have also observed changes in the amount of ERα-ir in some brain areas that may mediate in part the altered behavioral response in adulthood. We hypothesize that this phenomenon is present in human females and may be involved in the etiology of some types of mental illness that are linked to ovarian steroid hormones. However, studying this problem in humans will require precise determination of pubertal stage of development rather than more nebulous terms, such as childhood or adolescence, it will require also ascertaining if the disorder in adulthood is linked to changes in ovarian steroid hormones or is independent of them.
Highlights.
Some peripubertal stressors alter behavioral response to estradiol and progesterone in adulthood. Pubertal shipping or immune challenge remodel the brain's behavioral response to estradiol and progesterone.
The authors propose that mice may be a model for studying the etiology of some types of hormone-influenced mental illness in women.
Acknowledgments
This work from the authors' laboratory was supported by grants NS 19327, MH093854, and IOS 1050179 from the National Science Foundation, and an Isis grant from the Society for Women's Health Research. We thank the members of the Stress, Gender, Drugs, and the Brain network of the Society for Women's Research for many thoughtful discussions in the early stages of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Estradiol and progesterone are typically referred to as ovarian steroid hormones or sex steroid hormones. Although we will continue this convention, it should be noted that, although both are secreted from the ovaries, they both can be synthesized de novo by the brain, as well as by the adrenal gland. Referring to them as “ovarian hormones” or “sex hormones” may predispose readers to think of them as exclusively ovarian in origin.
References
- [1].McCarthy MM. A lumpers versus splitters approach to sexual differentiation of the brain. Front Neuroendocrinol. 2011;32:114–123. doi: 10.1016/j.yfrne.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- [3].Holder MK, Blaustein JD. Puberty and adolescence as a time of vulnerability to stressors that alter neurobehavioral processes. Front Neuroendocrinol. 2014;35:89–110. doi: 10.1016/j.yfrne.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Waylen A, Wolke D. Sex 'n' drugs 'n' rock 'n' roll: the meaning and social consequences of pubertal timing. Eur J Endocrinol. 2004;151(Suppl 3):U151–U159. doi: 10.1530/eje.0.151u151. [DOI] [PubMed] [Google Scholar]
- [5].Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- [6].Sizonenko PC. Physiology of puberty. J Endocrinol Invest. 1989;12:59–63. [PubMed] [Google Scholar]
- [7].Vandenbergh JG. Acceleration and inhibition of puberty in female mice by pheromones. J Reprod Fertil Suppl. 1973;19:411–419. [PubMed] [Google Scholar]
- [8].Mccormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- [9].Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- [10].Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- [11].Toledo-Rodriguez M, Sandi C. Stress during Adolescence Increases Novelty Seeking and Risk-Taking Behavior in Male and Female Rats. Front Behav Neurosci. 2011;5:17. doi: 10.3389/fnbeh.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hodes GE, Shors TJ. Distinctive stress effects on learning during puberty. Horm Behav. 2005;48:163–171. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kabbaj M, Isgor C, Watson SJ, Akil H. Stress during adolescence alters behavioral sensitization to amphetamine. Neuroscience. 2002;113:395–400. doi: 10.1016/s0306-4522(02)00188-4. [DOI] [PubMed] [Google Scholar]
- [15].Laroche J, Gasbarro L, Herman JP, Blaustein JD. Enduring influences of peripubertal/adolescent stressors on behavioral response to estradiol and progesterone in adult female mice. Endocrinology. 2009;150:3717–3725. doi: 10.1210/en.2009-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Laroche J, Gasbarro L, Herman JP, Blaustein JD. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology. 2009;150:2351–2358. doi: 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kercmar J, Tobet SA, Majdic G. Social isolation during puberty affects female sexual behavior in mice. Front Behav Neurosci. 2014;8:337. doi: 10.3389/fnbeh.2014.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ismail N, Garas P, Blaustein JD. Long-term effects of pubertal stressors on female sexual receptivity and estrogen receptor-alpha expression in CD-1 female mice. Horm Behav. 2011;59:565–571. doi: 10.1016/j.yhbeh.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ojeda SR, Weaton JE, Jameson HE, McCann SM. The onset of puberty in the female rat: changes in plasma prolactin, gonadotropins, luteinizing hormone-releasing hormone (LHRH), and hypothalamic LHRH content. Endocrinology. 1976;98:630–638. doi: 10.1210/endo-98-3-630. [DOI] [PubMed] [Google Scholar]
- [20].Parker CR, Jr., Mahesh VB. Hormonal events surrounding the natural onset of puberty in female rats. Biol Reprod. 1976;14:347–353. doi: 10.1095/biolreprod14.3.347. [DOI] [PubMed] [Google Scholar]
- [21].Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- [22].Azcoitia I, Arevalo MA, De Nicola AF, Garcia-Segura LM. Neuroprotective actions of estradiol revisited. Trends Endocrinol Metab. 2011;22:467–473. doi: 10.1016/j.tem.2011.08.002. [DOI] [PubMed] [Google Scholar]
- [23].Soucy G, Boivin G, Labrie F, Rivest S. Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. J Immunol. 2005;174:6391–6398. doi: 10.4049/jimmunol.174.10.6391. [DOI] [PubMed] [Google Scholar]
- [24].Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33:267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].DeCatanzaro D, Knipping RP, Gorzalka BB. Antagonism of estrogen-induced lordosis by corticosterone in adrenalectomized-ovariectomized female rats and mice. Pharmacol Biochem Behav. 1981;15:761–766. doi: 10.1016/0091-3057(81)90019-8. [DOI] [PubMed] [Google Scholar]
- [26].Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004;79:125–132. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- [27].Traslavina GAA, de Oliveira FL, Franci CR. Early adolescent stress alters behavior and the HPA axis response in male and female adult rats: the relevance of the nature and duration of the stressor. Physiology & Behavior. 2015;133:178–179. doi: 10.1016/j.physbeh.2014.05.031. [DOI] [PubMed] [Google Scholar]
- [28].Rissman EF, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies of estrogen receptor alpha. Brain Res. 1999;835:80–90. doi: 10.1016/s0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- [29].Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci U S A. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ismail N, Kumlin AM, Blaustein JD. A pubertal immune challenge alters the antidepressant-like effects of chronic estradiol treatment in inbred and outbred adult female mice. Neuroscience. 2012;249:43–52. doi: 10.1016/j.neuroscience.2012.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Olesen KM, Ismail N, Merchasin ED, Blaustein JD. Long-term alteration of anxiolytic effects of ovarian hormones in female mice by a peripubertal immune challenge. Horm Behav. 2011;60:318–326. doi: 10.1016/j.yhbeh.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ismail N, Blaustein JD. Pubertal immune challenge blocks the ability of estradiol to enhance performance on cognitive tasks in adult female mice. Psychoneuroendocrinology. 2013;38:1170–1177. doi: 10.1016/j.psyneuen.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bernardi M, Vergoni AV, Sandrini M, Tagliavini S, Bertolini A. Influence of ovariectomy, estradiol and progesterone on the behavior of mice in an experimental model of depression. Physiol Behav. 1989;45:1067–1068. doi: 10.1016/0031-9384(89)90238-2. [DOI] [PubMed] [Google Scholar]
- [34].Okada M, Hayashi N, Kometani M, Nakao K, Inukai T. Influences of ovariectomy and continuous replacement of 17beta-estradiol on the tail skin temperature and behavior in the forced swimming test in rats. Jpn J Pharmacol. 1997;73:93–96. doi: 10.1254/jjp.73.93. [DOI] [PubMed] [Google Scholar]
- [35].Bekku N, Yoshimura H. Animal model of menopausal depressive-like state in female mice: prolongation of immobility time in the forced swimming test following ovariectomy. Psychopharmacology (Berl) 2005;183:300–307. doi: 10.1007/s00213-005-0179-0. [DOI] [PubMed] [Google Scholar]
- [36].Koss WA, Einat H, Schloesser RJ, Manji HK, Rubinow DR. Estrogen effects on the forced swim test differ in two outbred rat strains. Physiol Behav. 2012;106:81–86. doi: 10.1016/j.physbeh.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stoffel EC, Craft RM. Ovarian hormone withdrawal-induced "depression" in female rats. Physiol Behav. 2004;83:505–513. doi: 10.1016/j.physbeh.2004.08.033. [DOI] [PubMed] [Google Scholar]
- [38].Suda S, Segi-Nishida E, Newton SS, Duman RS. A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biol Psychiatry. 2008;64:311–319. doi: 10.1016/j.biopsych.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Estrada-Camarena E, Lopez-Rubalcava C, Fernandez-Guasti A. Facilitating antidepressant-like actions of estrogens are mediated by 5-HT1A and estrogen receptors in the rat forced swimming test. Psychoneuroendocrinology. 2006;31:905–914. doi: 10.1016/j.psyneuen.2006.05.001. [DOI] [PubMed] [Google Scholar]
- [40].Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci U S A. 1998;95:13941–13946. doi: 10.1073/pnas.95.23.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Galea LAN, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- [42].Lagunas N, Calmarza-Font I, Diz-Chaves Y, Garcia-Segura LM. Long-term ovariectomy enhances anxiety and depressive-like behaviors in mice submitted to chronic unpredictable stress. Horm Behav. 2010;58:786–791. doi: 10.1016/j.yhbeh.2010.07.014. [DOI] [PubMed] [Google Scholar]
- [43].Dalla C, Antoniou K, Papadopoulou-Daifoti Z, Balthazart J, Bakker J. Oestrogen-deficient female aromatase knockout (ArKO) mice exhibit depressive-like symptomatology. Eur J Neurosci. 2004;20:217–228. doi: 10.1111/j.1460-9568.2004.03443.x. [DOI] [PubMed] [Google Scholar]
- [44].Diaz-Veliz G, Soto V, Dussaubat N, Mora S. Influence of the estrous cycle, ovariectomy and estradiol replacement upon the acquisition of conditioned avoidance responses in rats. Physiol Behav. 1989;46:397–401. doi: 10.1016/0031-9384(89)90010-3. [DOI] [PubMed] [Google Scholar]
- [45].Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S. Differential effects of exogenous and endogenous estrogen on anxiety as measured by elevated T-maze in relation to the serotonergic system. Behav Brain Res. 2009;198:142–148. doi: 10.1016/j.bbr.2008.10.043. [DOI] [PubMed] [Google Scholar]
- [46].Picazo O, Estrada-Camarena E, Hernandez-Aragon A. Influence of the post-ovariectomy time frame on the experimental anxiety and the behavioural actions of some anxiolytic agents. Eur J Pharmacol. 2006;530:88–94. doi: 10.1016/j.ejphar.2005.11.024. [DOI] [PubMed] [Google Scholar]
- [47].Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- [48].Diaz-Veliz G, Alarcon T, Espinoza C, Dussaubat N, Mora S. Ketanserin and anxiety levels: influence of gender, estrous cycle, ovariectomy and ovarian hormones in female rats. Pharmacol Biochem Behav. 1997;58:637–642. doi: 10.1016/s0091-3057(97)90004-6. [DOI] [PubMed] [Google Scholar]
- [49].Diaz-Veliz G, Urresta F, Dussaubat N, Mora S. Effects of estradiol replacement in ovariectomized rats on conditioned avoidance responses and other behaviors. Physiol Behav. 1991;50:61–65. doi: 10.1016/0031-9384(91)90498-d. [DOI] [PubMed] [Google Scholar]
- [50].Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- [51].Olivera-Lopez JI, Molina-Hernandez M, Tellez-Alcantara NP, Jaramillo MT. Estradiol and neuropeptide Y (intra-lateral septal) reduce anxiety-like behavior in two animal models of anxiety. Peptides. 2008;29:1396–1403. doi: 10.1016/j.peptides.2008.04.002. [DOI] [PubMed] [Google Scholar]
- [52].Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S. Anxiolytic property of estrogen related to the changes of the monoamine levels in various brain regions of ovariectomized rats. Physiol Behav. 2006;87:828–835. doi: 10.1016/j.physbeh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- [53].Tomihara K, Soga T, Nomura M, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Effect of ER-beta gene disruption on estrogenic regulation of anxiety in female mice. Physiol Behav. 2009;96:300–306. doi: 10.1016/j.physbeh.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: A study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci U S A. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5:528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- [56].Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–163. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- [57].Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta-deficient mice. Proc Natl Acad Sci U S A. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Conrad KL, Winder DG. Altered anxiety-like behavior and long-term potentiation in the bed nucleus of the stria terminalis in adult mice exposed to chronic social isolation, unpredictable stress, and ethanol beginning in adolescence. Alcohol. 2011;45:585–593. doi: 10.1016/j.alcohol.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang H, Meng XH, Ning H, Zhao XF, Wang Q, Liu P, Zhang H, Zhang C, Chen GH, Xu DX. Age- and gender-dependent impairments of neurobehaviors in mice whose mothers were exposed to lipopolysaccharide during pregnancy. Toxicol Lett. 2010;192:245–251. doi: 10.1016/j.toxlet.2009.10.030. [DOI] [PubMed] [Google Scholar]
- [60].Luine VN. Sex steroids and cognitive function. J Neuroendocrinol. 2008;20:866–872. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- [61].Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rubinow DR, Schmidt PJ. The treatment of premenstrual syndrome - forward into the past. New England Journal of Medicine. 1995;332(23, JUN 8):1574–1575. doi: 10.1056/NEJM199506083322309. [DOI] [PubMed] [Google Scholar]
- [63].Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Amer J Psychiat. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- [64].Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44:234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- [65].Harsh V, Meltzer-Brody S, Rubinow DR, Schmidt PJ. Reproductive aging, sex steroids, and mood disorders. Harv Rev Psychiatry. 2009;17:87–102. doi: 10.1080/10673220902891877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Schmidt PJ. Depression, the perimenopause, and estrogen therapy. Ann N Y Acad Sci. 2005;1052:27–40. doi: 10.1196/annals.1347.003. [DOI] [PubMed] [Google Scholar]
- [67].Rocca WA, Grossardt BR, Geda YE, Gostout BS, Bower JH, Maraganore DM, de AM, Melton LJ., III. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050–1059. doi: 10.1097/gme.0b013e318174f155. [DOI] [PubMed] [Google Scholar]
- [68].Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- [69].Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Ann N Y Acad Sci. 2009;1179:70–85. doi: 10.1111/j.1749-6632.2009.04982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ge X, Conger RD, Elder GH., Jr. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol. 2001;37:404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- [71].Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- [73].Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lemieux A, Coe CL, Carnes M. Symptom severity predicts degree of T cell activation in adult women following childhood maltreatment. Brain Behav Immun. 2008;22:994–1003. doi: 10.1016/j.bbi.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun. 2012;26:13–17. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- [79].Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, Rich-Edwards JW. Inflammation and early-life abuse in women. Am J Prev Med. 2012;43:611–620. doi: 10.1016/j.amepre.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Spencer SJ, Field E, Pittman QJ. Neonatal programming by neuroimmune challenge: effects on responses and tolerance to septic doses of lipopolysaccharide in adult male and female rats. J Neuroendocrinol. 2010;22:272–281. doi: 10.1111/j.1365-2826.2010.01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Spencer SJ, Martin S, Mouihate A, Pittman QJ. Early-life immune challenge: defining a critical window for effects on adult responses to immune challenge. Neuropsychopharmacology. 2006;31:1910–1918. doi: 10.1038/sj.npp.1301004. [DOI] [PubMed] [Google Scholar]
- [82].Spencer SJ, Boisse L, Mouihate A, Pittman QJ. Long term alterations in neuroimmune responses of female rats after neonatal exposure to lipopolysaccharide. Brain Behav Immun. 2006;20:325–330. doi: 10.1016/j.bbi.2005.08.004. [DOI] [PubMed] [Google Scholar]