Summary
The skin is a site of constant dialogue between the immune system and commensal bacteria. However, the molecular mechanisms that allow us to tolerate the presence of skin commensals without eliciting destructive inflammation are unknown. Using a model system to study the antigen-specific response to S. epidermidis, we demonstrated that skin colonization during a defined period of neonatal life was required to establish immune tolerance to commensal microbes. This crucial window was characterized by an abrupt influx of highly activated regulatory T (Treg) cells into neonatal skin. Selective inhibition of this Treg cell wave completely abrogated tolerance. Thus, the host-commensal relationship in the skin relied on a unique Treg cell population that mediated tolerance to bacterial antigens during a defined developmental window. This suggests that the cutaneous microbiome composition in neonatal life is crucial in shaping adaptive immune responses to commensals, and disrupting these interactions may have enduring health implications.
Introduction
Our commensal microbiota reside primarily at barrier sites, such as the gastrointestinal tract, respiratory tract, urogenital tract and skin, where they functionally tune our innate and adaptive immune systems (Belkaid and Hand, 2014; Belkaid and Segre, 2014; Hooper et al., 2012; Tremaroli and Bäckhed, 2012). Immune tolerance to these microbes must be established at each of these sites, but to date, the cellular and molecular mechanisms of how this occurs have almost exclusively been studied in the gastrointestinal tract. In this tissue, a simple columnar epithelium is coated by a thick mucus layer that facilitates spatial segregation from luminal bacteria (Vaishnava et al., 2011) and also diminishes the immunogenicity of microbial antigens by delivering tolerogenic signals to resident dendritic cells (Shan et al., 2013). Innate lymphoid cells limit commensal-specific CD4+ T cell responses via an MHC class II-dependent mechanism (Hepworth et al., 2013) and produce interleukin-22, which further promotes anatomical containment of microbes (Sonnenberg et al., 2012). Specialized gut-resident CD103+CD11b+ dendritic cells also play an important role in maintaining intestinal homeostasis by favoring induction of regulatory T (Treg) cells over pro-inflammatory CD4+ subsets (Coombes et al., 2007).
The cellular and molecular mechanisms that mediate tissue immune homeostasis differ between barrier sites (Maloy and Powrie, 2011; Pasparakis et al., 2014; Sather et al., 2007), and it follows that mechanisms to promote tolerance to commensals may also be tissue-specific. The skin is a key barrier site and a rich immunologic organ, with each square centimeter containing over a million lymphocytes and a million commensal bacteria (Clark et al., 2006; Grice et al., 2008). Unlike intestinal or lung mucosa, the skin is a stratified, cornified epithelium with a diverse topography studded by adenexal structures, including hair follicles, sweat ducts and sebaceous glands. As our external body surface, the skin also sustains regular physical trauma that compromises its barrier integrity and facilitates interaction between immune cells and exogenous antigens. These unique properties pose discrete challenges for maintenance of a healthy immune dialogue with commensal microbes. Currently, very little is known about how this process occurs in skin.
Treg cells play a major role in establishing and maintaining immune homeostasis in peripheral tissues, particularly at barrier sites (Powell et al., 1982; Russell et al., 1959) where they stably reside (Burzyn et al., 2013; Cipolletta et al., 2012; Sanchez Rodriguez et al., 2014). In the intestinal lamina propria, Treg cells not only maintain self-tolerance but also play a crucial role in mediating tolerance to commensal organisms. A large percentage of gut-resident Treg cells recognize commensal antigens (Lathrop et al., 2011b), and thymically derived Treg cells support tolerance to intestinal microbes (Cebula et al., 2013). In addition, certain bacterial species expand Treg cells in the lamina propria (Atarashi et al., 2011; Round and Mazmanian, 2010). Despite the clear role that Treg cells play in mediating tolerance to commensal microbes in the gut, it is currently unknown if these cells play a role in establishing tolerance to commensal microbes at other barrier sites. Interestingly, unlike the gut or lung, commensal microbes do not appear to augment the numbers of skin Treg cells (Naik et al., 2012).
In order to dissect the mechanisms by which adaptive immune tolerance is established to skin commensals, we engineered Staphylococcus epidermidis (S. epidermidis) to express a model T cell antigen, allowing us to comprehensively analyze commensal-specific CD4+ T cell responses in the context of both a polyclonal T cell repertoire and a complex microbiota. Using this system, we demonstrated that microbial antigens were continuously detected across an intact skin barrier. Skin colonization of adult animals did not establish tolerance. Instead, colonization during a defined period of neonatal life was required. Examination of neonatal skin revealed an abrupt wave of highly activated Treg cells accumulating in this tissue during the first weeks of life. Selective inhibition of Treg cell migration into skin during this period completely abrogated commensal-specific tolerance. Our results reveal that both a specific cell population and a specific window of time are required to establish a healthy host-commensal relationship in skin.
Results
Establishment of a model system to track the antigen-specific immune response to skin commensal bacteria
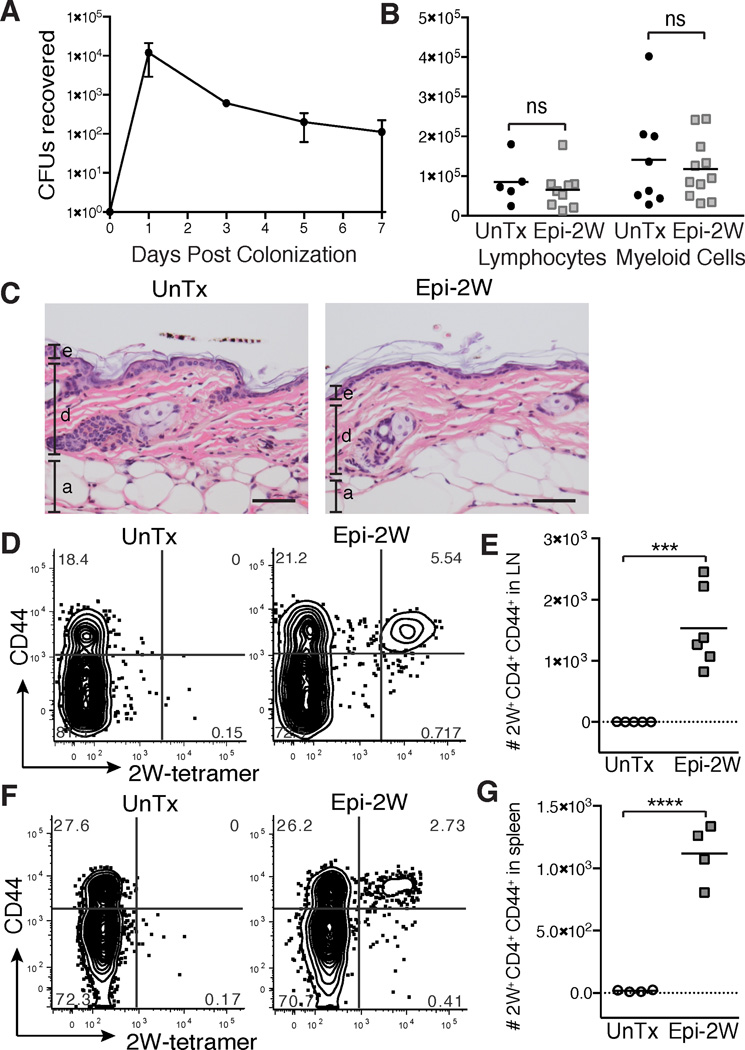
Tools to study the antigen-specific response to commensal microbes residing in the gastrointestinal tract have led to significant advancements in our understanding of the host-commensal relationship at this barrier site (Hand et al., 2012; Yang et al., 2014). To date, similar tools have not been available for skin commensal bacteria, and therefore, work examining the immune response to skin microbes has primarily been limited to observations of bulk polyclonal immune cell populations. Immunologic tolerance is by definition an antigen-specific process. Thus, we developed a murine model of cutaneous commensalism in which T cells specific for a bacterial antigen could be assayed longitudinally in the context of both a complex microbiome and polyclonal immune repertoire. S. epidermidis is a prevalent commensal bacterial species on human skin that can also stably colonize healthy mice (Garcia-Garcerà et al., 2012; Grice et al., 2009; Naik et al., 2015). We engineered S. epidermidis (Augustin and Gotz, 1990) to express the model peptide antigen 2W (Moon et al., 2007) linked to a fluorescent protein (Epi-2W), to allow for standardization of relative antigen expression (Figure S1). A single topical application of Epi-2W to the back skin of adult C57Bl/6 mice resulted in long-term persistence of the strain (Figure 1A). Flow cytometric and histologic evaluation of Epi- 2W colonized skin failed to reveal evidence of tissue inflammation (Figures 1B and C), suggesting that a state of true commensalism was achieved.
Figure 1. Antigen-specific recognition of commensal microbes across an intact skin barrier.
(A) Number of Epi-2W colony forming units (CFU) recovered via skin swab from mice colonized with Epi-2W once on day 0. Each data point represents average of 3 mice. Adult mice were colonized with Epi-2W or untreated (UnTx) every three days for three applications and then skin, skin-draining lymph nodes (SDLNs) and spleen were harvested on day 10. (B) Absolute number of lymphocytes (live CD45+CD3+) and myeloid cells (live CD45+CD3neg) per gram of skin and (C) skin histology. Scale bars represent 50 µm. e = epidermis, d = dermis, a = adipose. (D) Flow cytometry plots of CD4+ T cells (gated on live CD45+CD3+CD4+ from tetramer-enriched fraction) and (E) absolute numbers of CD44+CD4+2W+ cells in SDLNs and (F and G) spleen on day 10. Results in B-E representative of three independent experiments and in A, F and G from two independent experiments. See also Figure S1.
To test whether commensal antigens were recognized across an intact skin barrier, we assayed the 2W-specific immune response in mice colonized with Epi-2W. Adult animals were colonized with Epi-2W every three days for one week, and skin, skin-draining lymph nodes (SDLNs) and spleen were examined at day 10. In colonized animals we observed a significant increase in the frequency and absolute number of activated (CD44+) antigen-specific (2W MHC Class II tetramer-positive) CD4+ T cells in both SDLNs (Figures 1D and 1E) and spleen (Figures 1F and 1G). Taken together, these data suggest that we have established a model that closely recapitulates normal cutaneous bacterial commensalism, in that stable colonization occurs without resultant tissue inflammation. The results indicate that skin bacterial antigens were recognized by the adaptive immune system across an intact skin barrier. These findings are consistent with and build upon a recent report showing that skin commensal bacteria influence cutaneous immunity without causing tissue inflammation (Naik et al., 2015).
Bacterial colonization during adult life does not establish tolerance
We hypothesized that the adaptive immune system plays a role in mediating tolerance to skin commensal bacteria. To test this, we colonized the skin of 6 week-old adult mice with Epi-2W. Three to four weeks later, we challenged these mice alongside age-matched control animals (that were not colonized with Epi-2W) by applying Epi-2W in conjunction with light tape-stripping of the epidermis to minimally abrade the skin surface (Figure S2A). We rationalized that this approach was the most physiologically appropriate method of elucidating anti-commensal immune responses because it recapitulated increased exposure to commensal antigens in the setting of frequent incidental skin trauma (e.g. abrasions, scratching), a mildly inflammatory context during which mechanisms of immune tolerance would need to be active.
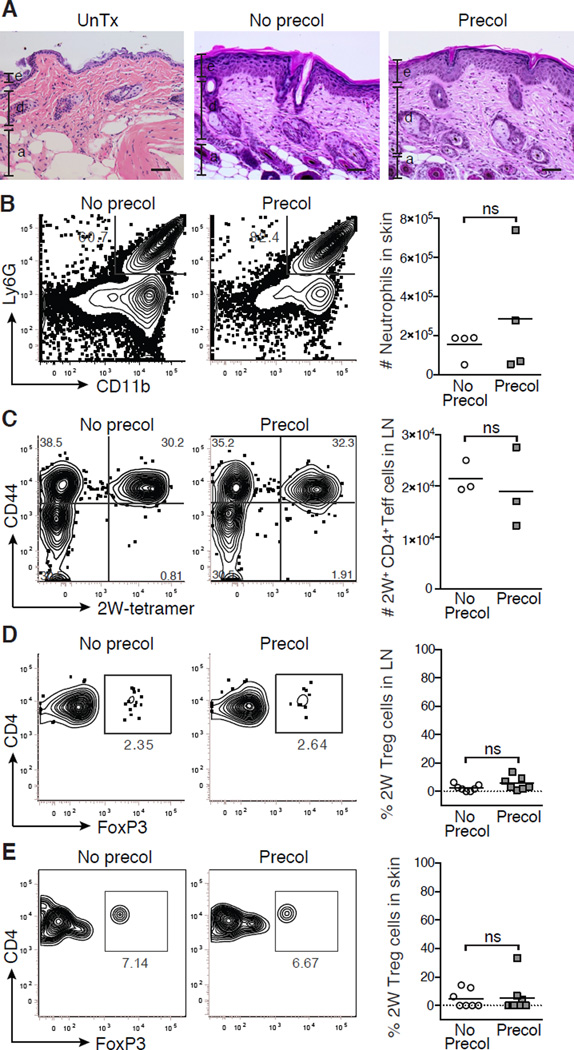
Pre-colonization with Epi-2W did not attenuate skin inflammation upon challenge. Ten days after challenge was initiated, both pre-colonized and control groups had equivalently increased histologic evidence of skin inflammation (Figure 2A) and equivalent numbers and frequency of skin neutrophils (Figure 2B). Consistent with the above observations, pre-colonization did not alter the number of activated antigen-specific CD44+CD4+FoxP3neg effector T (Teff) cells in SDLNs (Figure 2C) nor did it preferentially enrich for antigen-specific Treg cells in either the SDLNs or skin (Figures 2D, 2E and S2B). These results suggest that initial colonization by a skin commensal during adult life is not sufficient to establish tolerance to commensal antigens.
Figure 2. Colonization with commensal bacteria in adult mice does not establish immune tolerance.
Adult mice were not colonized (No precol) or colonized with Epi-2W (Precol) every three days for one week and then challenged 3–4 weeks later with Epi-2W and superficial skin abrasion. (A) Representative skin histology 10 days post-challenge versus healthy age-matched skin. Scale bars represent 50 µm. e = epidermis, d = dermis, a = adipose. (B) Flow cytometry plots and absolute numbers of neutrophils in skin. Gated on live CD45+CD3neg population. (C) Flow cytometry and absolute numbers of CD44+CD4+2W+FoxP3neg cells in SDLNs. Gated on live DumpnegCD45+CD3+CD4+FoxP3neg population in tetramer-enriched fraction. (D) Flow cytometry and percent 2W-specific Treg cells in SDLNs and (E) skin. Gated on live DumpnegCD45+CD3+CD4+CD44+2W+ population in tetramer-enriched fraction for SDLNs and total unenriched fraction for skin. Each point represents pooled data from 2 mice. Representative data from three independent experiments with ≥6 mice per group. See also Figure S2.
Neonatal colonization is required to establish tolerance to skin commensal bacteria
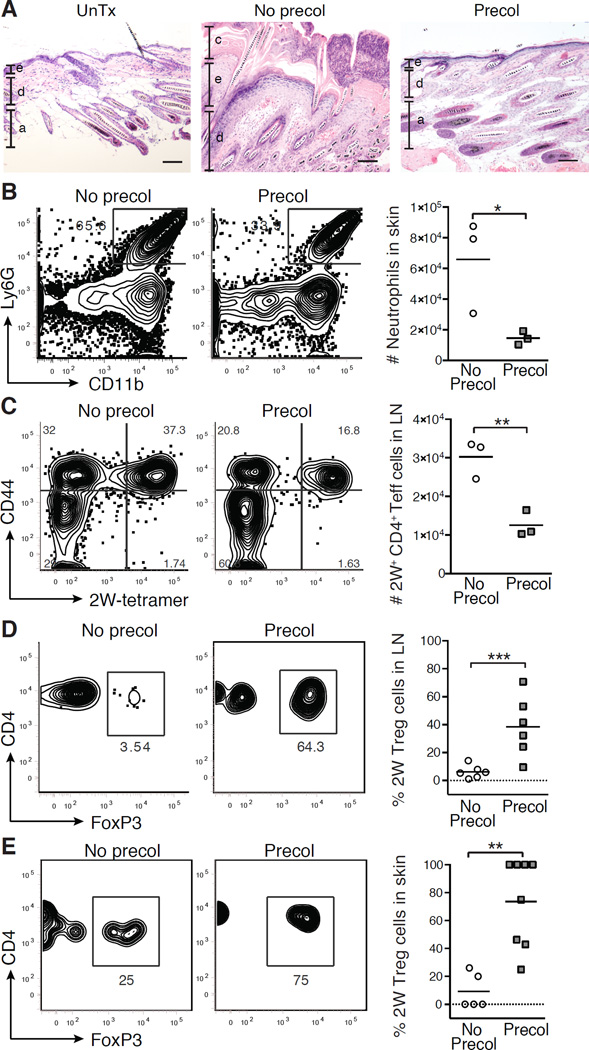
Because the host-commensal relationship is formed immediately after birth (Dominguez-Bello et al., 2010; Rotimi and Duerden, 1981), we hypothesized that mechanisms required to establish tolerance may be preferentially active during this period of time. To test this, we employed the previously outlined experimental approach, but instead colonized mice early in the postnatal period. Neonatal mice were colonized with Epi-2W for one week beginning on postnatal day 7 and challenged alongside Epi- 2W naïve age-matched animals 3–4 weeks later in adult life, using the skin abrasion technique previously described. In contrast to our observations with adult animals, mice colonized with Epi-2W in the neonatal period demonstrated markedly diminished histologic skin inflammation and substantially reduced neutrophilic infiltration upon challenge with Epi-2W (Figures 3A and 3B). This was associated with significantly fewer activated 2W-specific CD44+CD4+ Teff cells in the SDLNs (Figure 3C) and a marked enrichment of 2W-specific FoxP3+ Treg cells within the antigen-specific CD4+ population in the SDLNs (Figures 3D and S2C). Enrichment for antigen-specific Treg cells was also observed in skin (Figure 3E). Notably, bacterial burden of Epi-2W following initial colonization was similar for neonatal and adult mice, suggesting that the distinct effects of Epi-2W exposure in these two windows was not due to differential antigen load or preferential colonization in these different periods of life (Figure S2D). Collectively, these results suggest that skin colonization in the neonatal period uniquely promotes tolerance to commensal bacteria.
Figure 3. Colonization of neonatal mice with commensal bacteria results in antigen-specific immune tolerance.
Neonatal mice were not colonized (No Precol) or colonized (Precol) with Epi-2W on postnatal day 7, then challenged 3–4 weeks later with Epi-2W and superficial skin abrasion. (A) Representative skin histology 10 days post-challenge as compared to untreated age-matched skin. Scale bars represent 50 µm. e = epidermis, d = dermis, a = adipose, c = crust. (B) Flow cytometry and numbers of skin neutrophils. Gated on live CD45+CD3neg population. (C) Flow cytometry and absolute numbers of CD44+CD4+2W+FoxP3neg cells in SDLNs. Gated on live DumpnegCD45+CD3+CD4+FoxP3neg population in tetramer-enriched fraction. (D) Flow cytometry and percent of 2W-specific Treg cells in SDLNs and (E) skin. Gated on live DumpnegCD45+CD3+CD4+CD44+2W+ population in tetramer-enriched fraction for SDLNs and total unenriched fraction for skin. Each point represents pooled data from two mice. Representative data from three independent experiments with ≥6 mice per group. See also Figure S2.
Neonatal skin is characterized by abrupt accumulation of activated Treg cells
The observation that colonization of neonatal but not adult skin results in commensal-specific T cell tolerance prompted us to explore how neonatal and adult skin differ with respect to the major immune cell populations found in this tissue during these periods of life. Consistent with what is known about postnatal thymic development in mice (Adkins et al., 2004), we observed a marked accumulation of T cells expressing the αβ T cell receptor in skin between postnatal day 6 and day 13 (Figures 4A and 4B).
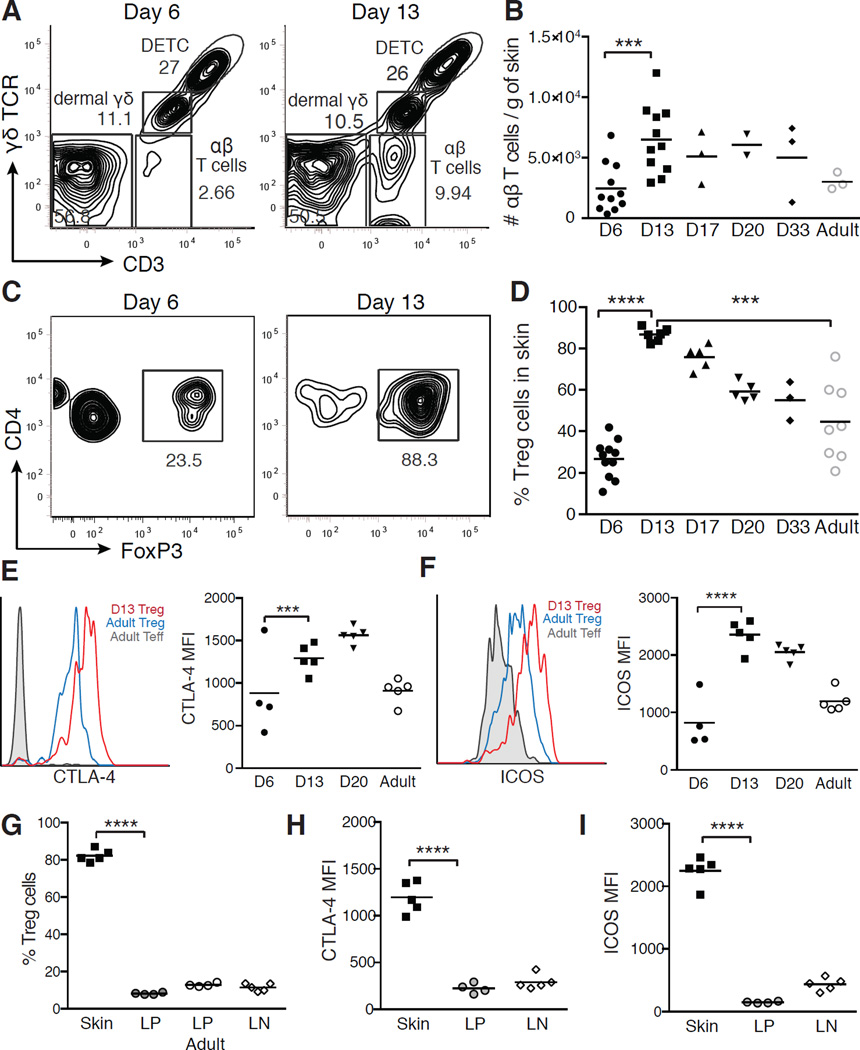
Figure 4. Activated Treg cells abruptly accumulate in neonatal skin.
(A) Representative flow cytometric plots outlining T cell subsets in murine skin at postnatal D6 and D13. Gated on live CD45+ cells. (B) Absolute numbers of skin αβ T cells by age. (C) Flow cytometry of skin CD4+ cells at D6 and D13. (D) Percent Treg cells in skin by age. Expression of (E) total CTLA-4 and (F) ICOS by flow cytometry and mean fluorescent intensity (MFI) on skin Treg cells by age. Teff cells = FoxP3negCD4+ T cells. (G) Percentage of Treg cells, (H) MFI of CTLA-4 and (I) MFI of ICOS in D13 Treg cells from skin, lamina propria (LP) and SDLNs. Each point represents data from an individual mouse. Data in A-D are representative of three independent experiments with ≥3 mice per group. Data in E-G is representative of two independent experiments with ≥3 mice per group. See also Figure S3.
CD4+ Treg cells constituted the vast majority of αβ T cells accumulating in skin during this period (Figures 4C and S3A). By day 13, Treg cells accounted for >80% of CD4+ T cells in skin compared to ~50% found in adult skin (Figure 4D), and their density was more than double that observed in adults (Figure S3A). In contrast, other αβ and γδ T cell subsets did not significantly accumulate in skin during this window (Figures S3BD). Treg cells infiltrating neonatal skin were highly activated, as evidenced by increased expression of CTLA-4 and ICOS when compared to Treg cells in adult skin (Figures 4E and 4F).
To determine whether this wave of activated Treg cells was unique to the skin, we examined Treg cells in the intestinal lamina propria and SDLNs of 13 day-old neonates. In both these sites, Treg cells constituted less than 20 percent of CD4+ T cells at day 13 and were not enriched in neonatal tissues compared to adult tissues (Figures 4G and S3C). Moreover, neonatal Treg cells isolated from the lamina propria and SDLNs expressed significantly lower levels of CTLA-4 and ICOS when compared to Treg cells infiltrating the skin (Figures 4H and 4I), indicating that they were less activated than skin Treg cells. Thus, the influx of highly activated Treg cells seems to be unique to the skin during this postnatal time period.
Neonatal Treg cells are required to establish tolerance to skin commensal microbes
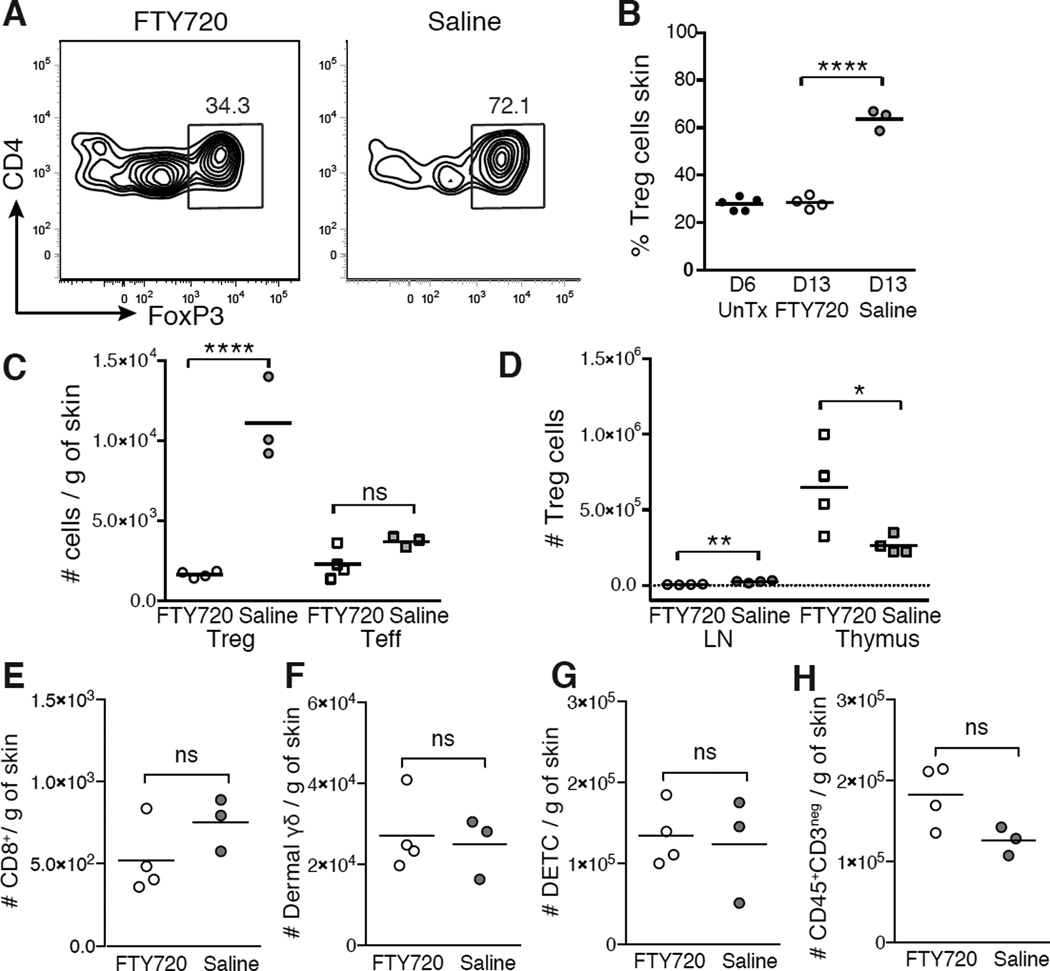
The abrupt accumulation of Treg cells in neonatal skin suggested seeding of the tissue by a migratory wave from developing lymphoid organs. To test this, we treated neonatal mice with the sphingosine 1-phosphate receptor antagonist, FTY720, between postnatal day 5 and 11 to block the egress of lymphocytes from the thymus and SDLNs (Matloubian et al., 2004). On postnatal day 13, FTY720-treated neonates had substantially reduced percentages of Treg cells in skin, with similar percentages to those observed in untreated mice at postnatal day 6 (Figures 5A and 5B). Absolute numbers of skin Treg cells were also markedly reduced in FTY720-treated mice at day 13, whereas CD4+FoxP3neg Teff cells were not significantly changed, consistent with our observation that Treg cells comprise >80% of the CD4+ T cells accumulating in skin in this window (Figure 5C).
Figure 5. FTY720 treatment preferentially blocks migration of Treg cells into neonatal skin.
FTY720 or saline was administered every 48 hours between postnatal D5 and D11 and skin, thymus and SDLNs were harvested on D13. (A) Flow cytometry and (B) percent Treg cells in skin on D13 vs. untreated D6 neonates. Gated on live CD45+CD3+CD4+ cells. (C) Absolute numbers skin of Treg cells and CD4+FoxP3neg (Teff) cells in skin on D13. (D) Absolute numbers of Treg cells in thymus and SDLNs on D13. Absolute numbers of (E) CD8+ T cells, (F) dermal γδ T cells, (G) dendritic epidermal T cells (DETC) and (H) CD45+CD3neg myeloid cells in skin on D13. Data is representative of two independent experiments with ≥3 mice per group.
Preferential accumulation of Treg cells was seen in the thymus rather than SDLNs with FTY720 treatment, suggesting that these cells migrate to the tissue directly from the thymus (Figure 5D). Notably, FTY720 treatment in this window did not significantly alter absolute numbers of other T cell or myeloid populations in the skin at postnatal day 13 (Figures 5E-5H). These data are consistent with our prior observation that these populations did not significantly accumulate in skin between postnatal day 6 and day 13 and demonstrate that FTY720 treatment in this window preferentially blocked migration of Treg cells into skin, while leaving other T cell populations relatively unchanged.
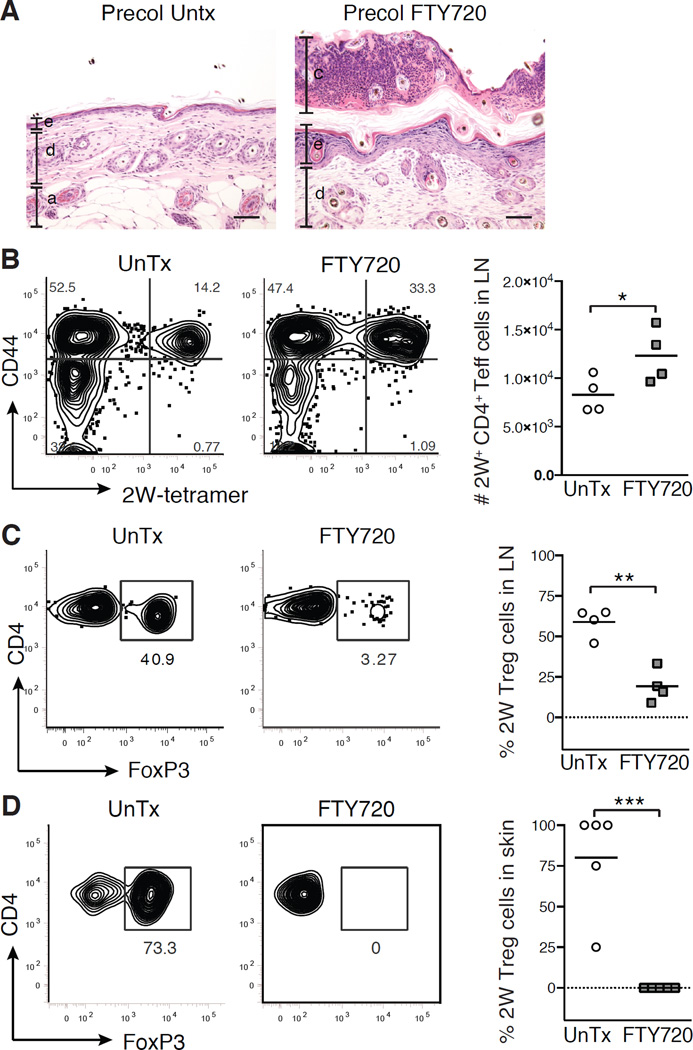
The abrupt accumulation of activated Treg cells in neonatal skin in conjunction with the preferential ability to establish tolerance to Epi-2W in this time period suggested that this population may play a major role in mediating tolerance to skin commensal microbes. To test this, we transiently blocked migration of Treg cells into skin by treating mice with FYT720 on postnatal day 5 and 7, immediately before colonizing with Epi-2W (Figure S4A). These mice were compared to age-matched controls colonized with Epi- 2W but not treated with FTY720. Both groups were then challenged 3–4 weeks later with Epi-2W plus skin abrasion. Blocking migration of skin Treg cells into neonatal skin resulted in increased histologic evidence of skin inflammation upon challenge with Epi- 2W in adult life (Figure 6A). Concomitantly, there were increased numbers of antigenspecific CD4+ Teff cells in the SDLNs (Figure 6B) and a relative reduction of antigen-specific Treg cells in both the SDLNs (Figure 6C) and skin (Figure 6D). Importantly, Treg cells not specific for the 2W antigen (i.e., polyclonal Treg cells) were able to migrate into the skin and SDLNs in the weeks following brief treatment with FTY720 (Figure S4BS4D). This indicates that FTY720 treatment did not permanently impair the migration ability of Treg cells and that the reduced percentages of antigen-specific Treg cells seen in FTY720-treated animals was a consequence of exposure to antigen during this neonatal period of time. Consistent with these results, in SPF mice treated neonatally with FTY720, without addition of Epi-2W, we observed increased frequency of myeloid cells in adult skin (data not shown). This indicates that our observations with the 2W antigen also extended to antigens expressed by native commensal microbes. Collectively, these findings suggest that transiently blocking Treg cell entry into neonatal skin significantly reduced the commensal-specific Treg:Teff cell ratio, resulting in a failure to establish and/or maintain tolerance to these microbes.
Figure 6. Inhibiting Treg cell migration to the skin in neonatal life prevents tolerance to commensal bacteria.
Neonatal mice were colonized for one week with Epi-2W beginning on D7 and FTY720 or saline (UnTx) was administered on postnatal D5 and D7. Mice were then challenged 3–4 weeks later with Epi-2W and superficial skin abrasion. (A) Representative skin histology 10 days post-challenge. Scale bars represent 50 µm. e = epidermis, d = dermis, a = adipose, c = crust. (B) Flow cytometry and absolute numbers of CD44+CD4+2W+FoxP3neg cells in SDLNs. Gated on live DumpnegCD45+CD3+CD4+FoxP3neg population in tetramer-enriched fraction. (C) Flow cytometry and percent of 2W-specific Treg cells in SDLNs and (D) skin. Gated on live DumpnegCD45+CD3+CD4+CD44+2W+ population in tetramer-enriched fraction for SDLNs and total unenriched fraction for skin. Each point represents data pooled from two mice. Data are representative of three independent experiments with ≥6 mice per group. See also Figure S4.
Discussion
We generated a system to study how adaptive immune tolerance is established to commensal bacteria in skin. Using this system, we found that even across an intact skin barrier, commensal antigens were recognized both locally and systemically, as evidenced by the expansion of commensal-specific CD4+ T cells in both the SDLNs and spleen. While colonization of adult animals did not induce immune tolerance, neonatal colonization led to antigen-specific tolerance characterized by enrichment of commensal-specific Treg cells in skin and SDLNs, reduced commensal-specific CD4+ Teff cells and diminished tissue inflammation. Examination of neonatal skin revealed an abrupt accumulation of activated Treg cells, which may preferentially migrate directly to skin from the thymus. Attenuating Treg cell accumulation in neonatal skin prevented the development of commensal-specific tolerance. Utilizing this model system to quantitatively assay commensal-specific T cell responses, we have elucidated a cellular mechanism by which the skin establishes tolerance to commensal microbes. In doing so, we have found that timing of colonization is critical to promote a healthy host-commensal relationship in this tissue.
Our findings illustrate important differences in the mechanisms that support tolerance to commensals in the skin versus the intestine. Although Treg cells are a critical population at both barrier sites, the relative contribution of thymus-derived Treg cells (tTregs) versus peripherally derived Treg cells (pTregs) may be distinct. Both subsets play a role in promoting tolerance to gut commensals (Cebula et al., 2013; Lathrop et al., 2011a). Our ability to prevent commensal-specific tolerance by blocking Treg cell migration to skin (with a concomitant accumulation of Treg cells in the thymus) suggests that the skin may rely primarily on a thymus-derived population during this neonatal window; however, the origin of these cells remains to be definitively determined. Regardless of their ontogeny, our findings highlight that Treg cell accumulation appears unique to skin in this neonatal window and plays an essential role in establishing tolerance to commensals. The specific molecular mechanisms by which skin versus intestinal Treg cells mediate tolerance to commensals may also be a point of divergence for these two barrier sites. Whereas Treg cell production of interleukin-10 is critical to prevent colitis provoked by enteric antigens (Kühn et al., 1993) and is a key immunoregulatory cytokine produced by Treg cells generated in response to B. fragilis (Round and Mazmanian, 2010), interleukin-10 deficiency has minimal impact on skin immune homeostasis (Rubtsov et al., 2008). The molecular mechanisms used by skin Treg cells to promote tolerance to either self or commensal antigens remain poorly defined and is an area of active investigation.
We observed a distinct wave of Treg cells that enters neonatal skin. We did not observe similar enrichment of highly activated Treg cells in the neonatal intestine. Although a transient microbiota-dependent increase in neonatal lung Treg cells was recently reported to be protective in an allergy model, these constitute no more than fifteen percent of lung CD4+ T cells at their peak on postnatal day 8 and have not been shown to mediate tolerance to commensals (Gollwitzer et al., 2014). This suggests that the abrupt accumulation of Treg cells in neonatal skin by day 13 is tissue-specific and not a consequence of a global or systemic increase in thymic efflux. Post-natal hair follicle morphogenesis occurs during the same time frame that this wave of Treg cells migrates into neonatal skin (Paus et al., 1999). Given that Treg cells in both mouse and human skin preferentially localize to hair follicles (Gratz et al., 2013; Sanchez Rodriguez et al., 2014), it is interesting to speculate that a hair-follicle related chemokine is directing them into the tissue, as has been shown for langerhans cells (Nagao et al., 2012). Recognizing that a significant proportion of skin commensal bacteria reside in hair follicles (Lange-Asschenfeldt et al., 2011; Leeming et al., 1984), such a mechanism would have clear evolutionary advantages for establishing and maintaining host-commensal tolerance. Understanding the molecular mechanisms that drive Treg cell accumulation in neonatal skin and determining if the same is true for human skin remains to be determined.
The observation that a defined period of colonization is required to promote antigen-specific tolerance to skin commensals suggests that timing may be critical in inducing tolerance at other barrier sites. Neonatal life also plays a formative role in shaping the host-commensal relationship in the intestine, as altering the intestinal flora during this window can permanently influence host metabolism (Cox et al., 2014), as well as the function of a subset of intestinal natural killer T cells (Cox et al., 2014; Gollwitzer et al., 2014; Olszak et al., 2012). Unique features of the neonatal immune system, specifically a CD71+ erythroid population, have been shown to broadly dampen defense to bacterial infections in this period (Elahi et al., 2013). However, studies examining mechanisms of immune tolerance to gut microbes have not considered the role of timing in exposure to commensal antigens. Scurfy mice succumb to disease early in life and display a pronounced skin phenotype, highlighting a critical role for tissue Treg cells in this developmental window (Lyon et al., 1990). Recent work suggests that Treg cells generated in neonatal life may be endowed with a unique potential to promote self tolerance (Yang et al., 2015). Our data suggests that this principle may extend more broadly to include tolerance to commensal antigens. While the phenomenon of a wave of Treg cells into neonatal tissue appears unique to skin, the functional characteristics and relative contribution of neonatal Treg cells in establishing tolerance to commensals should be carefully examined in other tissues. Further work is also required to clarify whether tolerance to commensals established in this neonatal window persists indefinitely throughout life or if distinct mechanisms are required for its maintenance.
To date, research examining the role of commensal microbes in human health has focused primarily on the intestinal tract. However, mutations in the epidermal protein filaggrin confer risk not only for atopic dermatitis but also asthma (Rodriguez et al., 2009), indicating that skin barrier function influences more than local immunity. We’ve shown that skin colonization results in commensal-specific T cells that are found both locally and systemically, suggesting that maintaining a healthy microbe-host immune dialogue in skin may have implications for systemic as well as tissue-specific immune homeostasis. Recognizing that there is a defined developmental window for establishing tolerance to skin commensal bacteria provides new mechanistic insights into how limiting microbial exposures early in life may contribute to allergic disease via a skinspecific mechanism (Ege et al., 2011). In this context, altering the composition of skin commensal microbiota in the neonatal period may limit the opportunity to establish tolerance to a wide array of microbial antigens, possibly resulting in chronic tissue inflammation. Indeed, many chronic inflammatory diseases of the skin have been postulated to result, at least in part, from abnormal anti-commensal immune responses (Belkaid and Segre, 2014; Nazary et al., 2011; Sanford and Gallo, 2013). Thus, the composition of the cutaneous microbiome in neonatal life may have formative effects on the adaptive immune response to commensals, and disrupting this may have enduring health implications.
Experimental Procedures
Mice
Wild-type C57BL/6 mice were used for all experiments. Mice were originally purchased from Jackson Laboratories but all experimental mice were born, bred and maintained in the UCSF specific pathogen-free facility in accordance with the guidelines of the Laboratory Animal Resource Center and Institutional Animal Care and Use Committee of the University of California San Francisco. Animals in experimental groups were matched for gender (and age where appropriate).
Bacterial strains and culture conditions
Staphylococcus epidermidis strain Tu3298 (Allgaier et al., 1986; Augustin and Gotz, 1990) (provided by M.O) and Staphyloccocus aureus strain RN4220 (Nair et al., 2011) were used in this study and grown in tryptic soy broth at 37°C.
Generation of Epi-2W
Plasmid pJL71 (Gauger et al., 2011) was modified to include the 2W peptide sequence optimized for expression in Staphylococcus (DNA2.0, Menlo Park, CA, USA). Specifically primers TS083 (5’- GGATCCGAATTCTTAGGAGGATGATTATTTATGGAAGCATGGGGAGCTTTAGCAAA TTGGGCAGTTGATTCAGCTGGTTCAGGTTCAGGTTCAGGTGTGAGCAAGGGCGAG GAGGATAATATGG-3’) and TS084 (5’- GGATCCGGCGCGCCTTACTTGTACAGCTCGTCCATGCCGCCTGTAGAATGTC-3’) were used with the pJL71 template to generate a construct encoding 2W at the 5’ end of gpmCherry (Gram-positive adapted mCherry) via a 7-mer glycine-serine linker sequence. This 2W-gpmCherry construct was cloned into plasmid pJL71 between the EcoRI and AscI restriction sites downstream of the agr promoter (in place of gpmCherry alone) creating the new plasmid, pJL71-2W-gpmcherry (Genbank KP065813). The restriction-deficient Staphylococcus aureus strain RN4220 was transformed with pJL71- 2W-gpmcherry via electroporation. The plasmid was then recovered via maxi prep and used to transform Staphyloccocus epidermidis Tu3298, using selection with erythromycin, to generate Epi-2W. See also Figure S1.
Epi-2W skin colonization and skin abrasion model
Epi-2W was cultured for 48 hours at 250 rpm in the presence of erythromycin to achieve stationary phase growth which demonstrated consistent and peak mCherry expression by flow cytometry. Cells were washed and re-suspended in PBS, and 108–109 CFU of Epi-2W were applied to the back skin of mice using a pipette and sterile PBS-soaked cotton-tipped swab. This procedure was repeated every three days for a total of three applications to constitute one round of colonization. When mice were in the active stage of hair growth, back hair was shortened with clippers 24 hours prior to colonization to facilitate application of bacteria. To replicate physiologic exposure to commensal skin bacteria in the context of skin abrasion, clippers and depilatory cream were first used to remove the back hair. The upper layers of epidermis were then removed via repeated application and removal of adhesive tape (Shurtape HP-500) and 108–109 CFUs of Epi- 2W were applied as above. This procedure was repeated every three days for a total of three times to constitute one round of challenge. Back skin and back skin-draining lymph nodes (axillary, brachial, inguinal) were harvested 10 days after initiation of the challenge.
Neonatal administration of FTY720
FTY720 (Selleck Chemicals) was dissolved in normal saline and administered to neonates via intraperitoneal injection at a dose of 10mg/kg. For experiments depicted in Figure 5, FTY720 was administered every 48 hours from postnatal day 5 to 11. For experiments depicted in Figures 6 and S4, migration was blocked more transiently with FTY720 treatment only on postnatal days 5 and 7. Control mice (age-matched litters or littermates) were treated with equal volumes of normal saline according to the same schedule.
Tissue processing and histopathology
Isolation of cells from skin and intestinal lamina propria for flow cytometry was performed as previously reported (Broadhurst et al., 2012; Gratz et al., 2014) and is further described in supplemental methods. For histopathology, skin tissue was fixed in 10% formalin and paraffin-imbedded, sectioned and stained with hematoxylin and eosin by the UCSF Mouse Pathology Core. Images were acquired on a Leica microscope using a DS-Ri1 camera and NIS-Elements software (Nikon).
Tetramer staining and enrichment
Phycoerythrin-conjugated MHC class II i-Ab 2W1S55–68 tetramer (provided by J.J.M.), and anti-PE conjugated magnetic beads (Miltenyi Biotec) for enrichment from lymph nodes and enumeration in tissues have been previously reported (Moon et al., 2007). The procedure was adapted for skin as further described in supplemental methods.
Flow cytometry staining
Following isolation from tissues cells were labeled with surface antibodies (see supplementaal methods) in PBS or, for experiments incorporating the 2W-tetramer, in blocking solution with anti-FcγR antibody (24G2 hybridoma supernatant), rat serum (StemCell Technologies) and normal mouse serum (Jackson ImmunoResearch). For intracellular staining, cells were fixed and permeabilized using the FoxP3 staining buffer kit (BD Biosciences). Samples were run on a Fortessa (BD Biosciences) in the UCSF Flow Cytometry Core. For longitudinal experiments comparing mice across time or ages, voltages were standardized using SPHERO Rainbow calibration particles (BD Biosciences). Accucheck counting beads (Invitrogen) were used calculate absolute numbers of cells. Flow cytometry data was analyzed using FlowJo software (Freestar).
Statistics
The number of mice per group used in an experiment is annotated in the corresponding figure legend. Although no prior sample size estimation was performed, we have used as many mice per group as possible. Data followed a Gaussian distribution and variation was similar between groups for each condition analyzed. Significance was assessed using the unpaired (separate groups of mice) Student’s t test. In all figures, the mean value is visually depicted. P values correlate with significance symbols as follows: ns p>0.05, * p≤0.05, ** p≤0.01, *** p≤0.001, **** p≤0.0001. Mice were allocated into experimental groups to match for age and gender. Investigators remained unblinded to group assignments throughout the experiment. No animals were excluded from analysis. Statistical analysis was done using GraphPad Prism software (GraphPad).
Supplementary Material
Acknowledgements
We thank C. Benetiz for assistance with animal husbandry and Creative Commons author Seans Potato Business for use of the mouse image in Figures 2 and 6 under the Attribution-ShareAlike license (creativecommons.org/liscenses/by-sa/3.0/legalcoge).. Flow Cytometry data was generated in the UCSF Parnassus Flow Cytometry Core which is supported by the Diabetes Research Center (DRC) grant, NIH P30 DK063720. Histology was performed with assistance from the UCSF Mouse Pathology Core which is supported by NIH 5P30CA082103-15. T.C.S. is supported by a Dermatology Foundation Career Development Award and the UCSF Department of Dermatology. M.O. is supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health. This work was primarily funded by M.D.R. grants: NIH K08-AR062064, Burroughs Wellcome Fund CAMS-1010934, NIH R21-AR066821, Scleroderma Research Foundation grant, and a National Psoriasis Foundation Translational Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
T.C.S. designed the studies, performed the experiments and analyzed the data. T.C.S. and M.D.R. wrote the manuscript. K.S.V. assisted in mouse experiments, mouse husbandry, data collection and analysis. H.A.T, M.L.P., S.G. and A.N. assisted with mouse experiments. M.O. and J.L. assisted in generation of transgenic S. epidermidis. I.K.G. and J.J.M. assisted in study design. M.D.R. oversaw all study design and data analysis. M.A.F. and A.K.A. were involved in study design and data analysis. All authors discussed results and commented on the manuscript.
The authors declare no competing financial interests.
References
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- Allgaier H, Jung G, Werner RG, Schneider U, Zähner H. Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic. Eur. J. Biochem. 1986;160:9–22. doi: 10.1111/j.1432-1033.1986.tb09933.x. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin J, Gotz F. Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiology Letters. 1990;54:203–207. doi: 10.1016/0378-1097(90)90283-v. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW. Role of the Microbiota in Immunity and Inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346:954–959. doi: 10.1126/science.1260144. [DOI] [PubMed] [Google Scholar]
- Broadhurst MJ, Leung JM, Lim KC, Girgis NM, Gundra UM, Fallon PG, Premenko-Lanier M, McKerrow JH, McCune JM, Loke P. Upregulation of retinal dehydrogenase 2 in alternatively activated macrophages during retinoid-dependent type-2 immunity to helminth infection in mice. PLoS Pathog. 2012;8:e1002883. doi: 10.1371/journal.ppat.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, Bry L, Kraj P, Kisielow P, Ignatowicz L. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258–262. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K-I, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun C-M, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ege MJ, Mayer M, Normand A-C, Genuneit J, Cookson WOCM, Braun-Fahrländer C, Heederik D, Piarroux R, Mutius von E GABRIELA Transregio 22 Study Group. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcerà M, Coscollà M, Garcia-Etxebarria K, Martín-Caballero J, González-Candelas F, Latorre A, Calafell F. Staphylococcus prevails in the skin microbiota of long-term immunodeficient mice. Environ. Microbiol. 2012;14:2087–2098. doi: 10.1111/j.1462-2920.2012.02756.x. [DOI] [PubMed] [Google Scholar]
- Gauger T, Weihs F, Mayer S, Krismer B, Liese J, Kull M, Bertram R. Intracellular monitoring of target protein production in Staphylococcus aureus by peptide tag-induced reporter fluorescence. Microbial Biotechnology. 2011;5:129–134. doi: 10.1111/j.1751-7915.2011.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, Nicod LP, Lloyd CM, Marsland BJ. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med. 2014 doi: 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
- Gratz IK, Rosenblum MD, Maurano MM, Paw JS, Truong H-A, Marshak-Rothstein A, Abbas AK. Cutting edge: Self-antigen controls the balance between effector and regulatory T cells in peripheral tissues. The Journal of Immunology. 2014;192:1351–1355. doi: 10.4049/jimmunol.1301777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz IK, Truong H-A, Yang SH-Y, Maurano MM, Lee K, Abbas AK, Rosenblum MD. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. The Journal of Immunology. 2013;190:4483–4487. doi: 10.4049/jimmunol.1300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISC Comparative Sequencing Program. Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Bouffard GG, Blakesley RW, Murray PR, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISC Comparative Sequencing Program. Grice EA, Kong HH, Renaud G, Young AC, Bouffard GG, Blakesley RW, Wolfsberg TG, Turner ML, Segre JA. A diversity profile of the human skin microbiota. Genome Res. 2008;18:1043–1050. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand TW, Santos Dos, LM, Bouladoux N, Molloy MJ, Pagan AJ, Pepper M, Maynard CL, Elson CO, Belkaid Y. Acute Gastrointestinal Infection Induces Long-Lived Microbiota-Specific T Cell Responses. Science. 2012;337:1553–1556. doi: 10.1126/science.1220961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth MR, Monticelli LA, Fung TC, Ziegler CGK, Grunberg S, Sinha R, Mantegazza AR, Ma H-L, Crawford A, Angelosanto JM, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013 doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10- deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Lange-Asschenfeldt B, Marenbach D, Lang C, Patzelt A, Ulrich M, Maltusch A, Terhorst D, Stockfleth E, Sterry W, Lademann J. Distribution of bacteria in the epidermal layers and hair follicles of the human skin. Skin Pharmacol Appl Skin Physiol. 2011;24:305–311. doi: 10.1159/000328728. [DOI] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW. Peripheral education of the immune system by colonic commensal microbiota: Nature: Nature Publishing Group. Nature. 2011a doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio C-W, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh C-S. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011b;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeming JP, Holland KT, Cunliffe WJ. The microbial ecology of pilosebaceous units isolated from human skinJGen. Microbiol. 1984;130:803–807. doi: 10.1099/00221287-130-4-803. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Peters J, Glenister PH, Ball S, Wright E. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott-Aldrich syndrome. Proc. Natl. Acad. SciU.Sa. 1990;87:2433–2437. doi: 10.1073/pnas.87.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, Ueha S, Horiuchi K, Tanizaki H, Kabashima K, et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol. 2012;13:744–752. doi: 10.1038/ni.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, et al. Compartmentalized Control of Skin Immunity by Resident Commensals. Science. 2012 doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Linehan JL, Han S-J, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015 doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair D, Memmi G, Hernandez D, Bard J, Beaume M, Gill S, Francois P, Cheung AL. Whole-genome sequencing of Staphylococcus aureus strain RN4220, a key laboratory strain used in virulence research, identifies mutations that affect not only virulence factors but also the fitness of the strain. J Bacteriol. 2011;193:2332–2335. doi: 10.1128/JB.00027-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazary M, van der Zee HH, Prens EP, Folkerts G, Boer J. Pathogenesis and pharmacotherapy of Hidradenitis suppurativa. Eur. J. Pharmacol. 2011;672:1–8. doi: 10.1016/j.ejphar.2011.08.047. [DOI] [PubMed] [Google Scholar]
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, et al. Microbial Exposure During Early Life Has Persistent Effects on Natural Killer T Cell Function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14:289–301. doi: 10.1038/nri3646. [DOI] [PubMed] [Google Scholar]
- Paus R, Müller-Röver S, van der Veen C, Maurer M, Eichmüller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B. A Comprehensive Guide for the Recognition and Classification of Distinct Stages of Hair Follicle Morphogenesis. J Investig Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- Powell BR, Buist NR, Stenzel P. An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J. Pediatr. 1982;100:731–737. doi: 10.1016/s0022-3476(82)80573-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, Irvine AD, Weidinger S. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol. 2009;123:1361–70.e1367. doi: 10.1016/j.jaci.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Rotimi VO, Duerden BI. The development of the bacterial flora in normal neonates. J. Med. Microbiol. 1981;14:51–62. doi: 10.1099/00222615-14-1-51. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Russell WL, Russell LB, Gower JS. Exceptional inheritance of a sexlinked gene in the mouse explained on the basis that the X/O sex-chromosome constitution is female. Proc. Natl. Acad. Sci. U.Sa. 1959;45:554–560. doi: 10.1073/pnas.45.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, Yang SH-Y, Anthony BA, Sverdrup FM, Krow-Lucal E, et al. Memory regulatory T cells reside in human skin. J. Clin. Invest. 2014;124:1027–1036. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford JA, Gallo RL. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013;25:370–377. doi: 10.1016/j.smim.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015 doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–156. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.