SUMMARY
Deletion of self antigen-specific T cells during thymic development provides protection from autoimmunity. However, it is unclear how efficiently this occurs for tissue-restricted self antigens, or how immune tolerance is maintained for self antigen-specific T cells that routinely escape deletion. Here we show that endogenous CD4+ T cells with specificity for a set of tissue-restricted self antigens were not deleted at all. For pancreatic self antigen, this resulted in an absence of steady-state tolerance, while for the lung and intestine, tolerance was maintained by the enhanced presence of thymically-derived antigen-specific Foxp3+ regulatory T (Treg) cells. Unlike deletional tolerance, Treg cell-mediated tolerance was broken by successive antigen challenges. These findings reveal that for some tissue-restricted self antigens, tolerance relies entirely on nondeletional mechanisms that are less durable than T cell deletion. This may explain why autoimmunity is often tissue-specific, and offers a rationale for cancer vaccine strategies targeting tissue-restricted tumor antigens.
INTRODUCTION
To enable recognition of virtually any pathogen, the adaptive immune system harbors a vast diversity of T cell clones, each with a unique T cell antigen receptor (TCR) that is randomly generated during development in the thymus. To prevent autoimmunity, T cell clones with high TCR reactivity to self antigens presented in the thymus are deleted through negative selection, leaving behind a peripheral repertoire of mature T cells that is largely foreign antigen-specific (Hogquist et al., 2005). This basic model of central tolerance applies not only to ubiquitous self antigens, but also to "tissue-restricted" self antigens that are normally absent from the thymus. This is possible due to broad patterns of antigen presentation by the panoply of specialized antigen-presenting cell types found in the thymus (Klein et al., 2009), including medullary thymic epithelial cells that express tissue-restricted genes promiscuously through the activity of the transcriptional regulator Aire (Mathis and Benoist, 2009).
Despite the efficiency of thymic selection and the fact that some self antigen-specific T cells are also deleted in the periphery (Metzger and Anderson, 2011; Redmond and Sherman, 2005), the mature peripheral T cell repertoire is known to harbor significant self antigen specificity (Hogquist and Jameson, 2014), a point made clear by numerous experimental models of autoimmunity that are induced by immunization with self antigens. However, the extent to which central tolerance fails and self antigen-specific T cells escape deletion is unclear, particularly in the case of tissue-restricted self antigens.
The identity of self antigen-specific T cells in the peripheral repertoire is of paramount importance, as these cells are likely involved in autoimmunity as well as anti-tumor immunity. Some of these cells may simply be ignorant of self antigens that are rare or poorly sampled by dendritic cells. Alternatively, these cells may exist in a hyporesponsive state of anergy, the induction of which can constitute a nondeletional mechanism of tolerance (Choi and Schwartz, 2007). In the case of CD4+ T cells, many self antigen-specific T cells are thought to be regulatory T (Treg) cells that suppress rather than promote immunity (Sakaguchi et al., 2008). While some Treg cells differentiate from conventional CD4+ T cells (Tconv) in the periphery, most are generated in the thymus through TCR recognition of self antigens in a process that diverges from negative selection (Josefowicz et al., 2012). Comprehensive analyses of TCR repertoires have shown that the specificities of CD4+ Treg and Tconv cell subsets exhibit limited overlap, suggesting that Treg cell specificity is biased towards self antigens (Hsieh et al., 2012).
Our understanding of self antigen specificity within the T cell repertoire has been hampered by the technical challenges of studying self antigen-specific T cells. The frequency of T cells with specificity to any single foreign peptide:MHC epitope is extremely small, estimated in the range of 1 to 100 per million CD8+ T cells, or 0.1 to 10 per million CD4+ T cells (Jenkins and Moon, 2012). In the case of self antigen-specific T cells, which are presumably impacted by deletional tolerance, these frequencies may be even lower. Hence, most studies of self antigen-specific T cells have involved the use of TCR transgenic mice crossed with mice expressing the cognate antigen (Hogquist et al., 2005; Wirnsberger et al., 2011). While such studies have provided important insights into various mechanisms of T cell tolerance to self antigens, it is now clear that in vivo systems involving abnormally high frequencies of monoclonal T cells often fail to accurately model the development of self antigen-specific T cells (Bautista et al., 2009; Leung et al., 2009). To overcome these limitations, we have developed peptide:MHCII tetramer-based cell enrichment techniques to enable direct characterization of self antigen-specific CD4+ T cells within completely unmanipulated endogenous T cell repertoires (Moon et al., 2007; 2011). Using this approach, we previously revealed that a significant proportion of a CD4+ T cell population specific for a ubiquitous self antigen survives deletion, which raised the question of how efficiently T cell tolerance is established to self antigens with more limited patterns of expression.
In this study, we created an experimental system in which a model self antigen was expressed in mice through different tissue-specific promoters, and the development of the same endogenous antigen-specific CD4+ T cell population was compared across each of these strains. This enabled us to isolate the effects of self antigen tissue localization on CD4+ T cell deletion and Treg cell development in a highly controlled, extremely physiological in vivo context. We found that tissue-restricted expression of this self antigen was met with a profound lack of antigen-specific T cell deletion, which depending on the tissue site of expression, resulted in either an absence of steady-state tolerance, or tolerance maintained by antigen-specific Treg cells.
RESULTS
Absence of deletional T cell tolerance to tissue-restricted self antigens
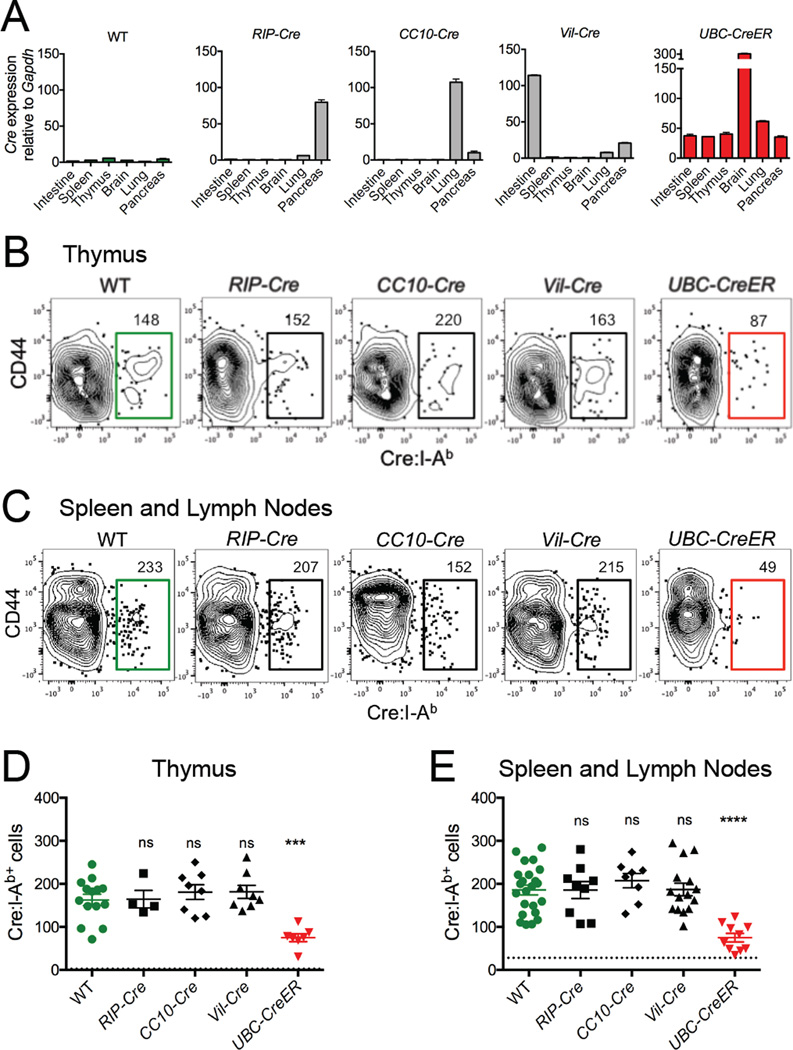
To investigate how T cell tolerance to a self antigen is affected by the tissue environment in which it is expressed, we modeled Cre recombinase as a neo self antigen. Cre is a Bacteriophage P1 protein that is normally absent in laboratory mice (Kasman, 2005), and can thus serve as a tissue-restricted self antigen in various Cre transgenic mouse strains. RIP-Cre (rat insulin promoter), CC10-Cre (Clara cell specific promoter), and Vil-Cre (villin promoter) transgenic mice were used to model self antigen expression in pancreatic β islets, lung epithelium, and intestinal epithelium, respectively, while UbC-CreER transgenic mice served as controls for ubiquitous self antigen expression. Quantitative RT-PCR analysis confirmed tissue-restricted expression patterns of Cre mRNA in these mice (Figure 1A).
Figure 1. Tissue-restricted self antigen-specific CD4+ T cells are not deleted.
(A) Quantitative RT-PCR data depicting Cre mRNA expression levels normalized to GAPDH in indicated tissues of indicated mice. Mean values ±SEM of samples taken from at least two independent experiments are shown. (B) Representative flow cytometry of CD4+ single positive thymocytes following Cre:I-Ab tetramer-based cell enrichment of thymi. (C) Representative flow cytometry of peripheral CD4+ T cells following Cre:I-Ab tetramer-based cell enrichment of pooled spleen and lymph nodes. Plots represent Dump(B220, CD11b, CD11c, F4/80)−CD3+CD4+ events as described in Figure S1E. Numbers above gates indicate total numbers of Cre:I-Ab-specific CD4+T cells calculated for the whole mouse. (D–E) Quantitative summaries of Cre:I-Ab-specific CD4+ (D) thymocyte or (E) peripheral T cell numbers per mouse. Each datapoint represents an individual mouse. Mean values ±SEM are shown. Dotted lines represent the limit of detection as defined by the mean frequency of CD8+tetramer+ events. ns not significant, *** p<0.001, **** p<0.0001 for unpaired t tests between WT and each Cre mouse. See also Figure S1.
We mapped an immunogenic I-Ab-restricted epitope corresponding to residues 61–71 of Cre which is recognized by a relatively large frequency of naive CD4+ T cells in C57BL/6 mice (Figures S1A–S1D), and used this for our analysis of Cre:I-Ab-specific T cells throughout our study. Background levels of Cre:I-Ab tetramer staining were assessed by internal CD8+ T cell staining controls as well as two-color double tetramer staining experiments (Figures S1E and S1F).
Cre:I-Ab tetramer-based magnetic cell enrichment was used to directly measure total numbers of rare endogenous Cre:I-Ab-specific CD4+ T cells in wild type (WT) and Cre transgenic mice. Naive WT mice, in which Cre is a foreign antigen, contained endogenous populations of ~165 Cre:I-Ab-specific CD4+ single positive thymocytes in the thymus (Figures 1B and 1D), and ~185 Cre:I-Ab-specific mature CD4+ T cells in the peripheral lymphoid organs (Figures 1C and 1E). UbC-CreER mice contained ~60% fewer Cre:I-Ab-specific cells in both the thymus and periphery, indicating efficient thymic negative selection of T cells specific for ubiquitously expressed Cre self antigen. However, Cre:I-Ab-specific T cell numbers were not lower in the thymus or periphery of any other Cre transgenic mouse, indicating a marked absence of deletional T cell tolerance against Cre self antigen when expression was restricted to the pancreas, lung, or intestine.
To determine whether Cre:I-Ab self antigen presentation from these tissues was sufficient for T cell recognition, CFSE dye labeled Cre:I-Ab-specific T cells from Cre immunized WT mice were adoptively transferred into WT or Cre mice and monitored for cell division as an indication of antigen-induced activation (Figure S1G). Transferred cells divided in CC10-Cre, Vil-Cre, and UbC-CreER mice, but only showed minimal signs of division in RIP-Cre mice, indicating efficient presentation of Cre:I-Ab complexes from Cre-expressing lung and intestinal tissues, but only poor presentation from pancreatic tissues.
Nondeletional T cell tolerance to lung and intestinal tissue-restricted self antigens
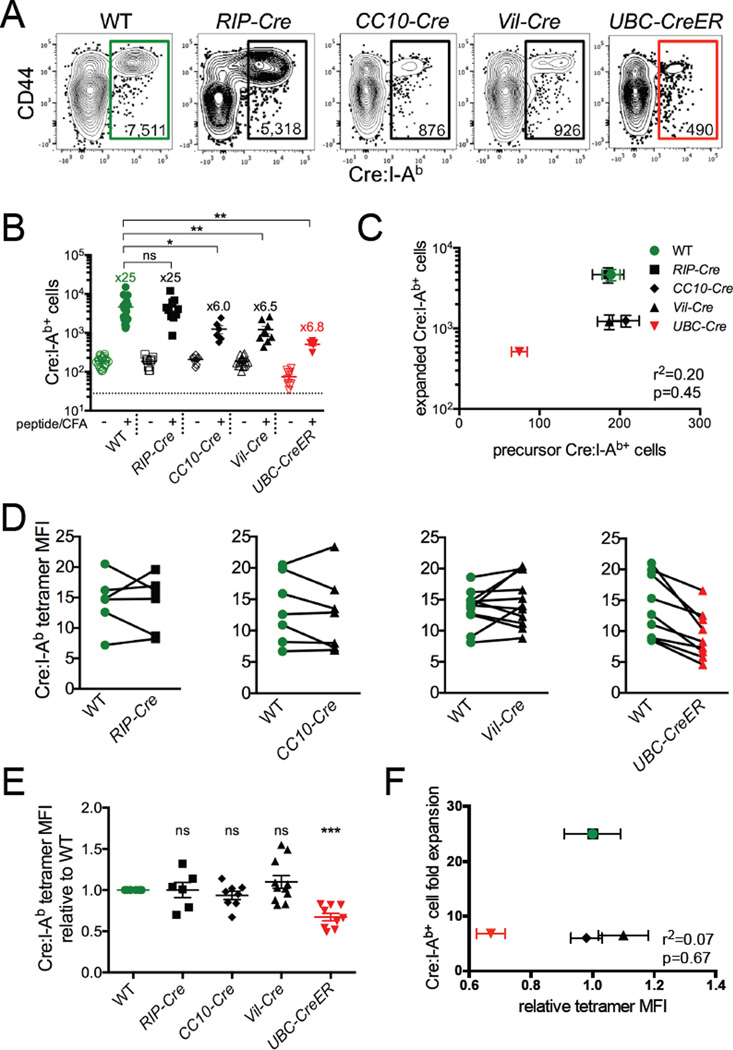
Cre:I-Ab-specific T cells in Cre transgenic mice generally expressed low levels of CD44 that was consistent with a naive phenotype (Figures 1B and 1C), and we did not observe obvious phenotypic markers that distinguished them from their counterparts in WT mice (data not shown). To test the function of these T cells, we subcutaneously immunized each of the Cre transgenic strains with Cre peptide emulsified in complete Freund's adjuvant (CFA) and measured their proliferative response 7 days later. As expected, Cre:I-Ab-specific T cells expanded robustly in WT mice and poorly in UbC-CreER mice (Figures 2A and 2B). T cell expansion in RIP-Cre mice was equivalent to that in WT mice, revealing a lack of pre-established Cre-specific tolerance when Cre expression was restricted to the pancreas. In contrast, T cell expansion in CC10-Cre and Vil-Cre mice was markedly lower than that in WT mice despite the similar frequencies of precursor cells in all these mice. Overall, there was no correlation between Cre:I-Ab-specific precursor and expanded T cell numbers among our panel of mice (Figure 2C), indicating that Cre:I-Ab-specific T cell tolerance was not a result of T cell deletion.
Figure 2. Lung and intestinal self antigen-specific CD4+ T cells exhibit impaired proliferative responses.
Mice were immunized with Cre peptide + CFA and analyzed 7 days later. (A) Representative flow cytometry of CD4+ T cells following Cre:I-Ab tetramer-based cell enrichment of pooled spleen and lymph nodes. Plots represent Dump(B220, CD11b, CD11c, F4/80)−CD3+CD4+ events. Numbers inside gates indicate total numbers of Cre:I-Ab-specific CD4+ T cells calculated for the whole mouse. (B) Quantitative summary of total Cre:I-Ab-specific T cell numbers per mouse with fold expansion values indicated. The dotted line represents the limit of detection. (C) Linear regression analysis of Cre:I-Ab-specific T cell numbers before and after immunization for each mouse strain. SEM, r2, and p values are indicated. (D) Tetramer mean fluorescence intensity (MFI), expressed in arbitrary units, of Cre:I-Ab-specific T cells from individual WT and Cre mice. Lines connect mice analyzed within the same experiment. (E) Quantitative summary of tetramer MFI from individual mice normalized to WT controls analyzed within the same experiment. (F) Linear regression analysis of Cre:I-Ab tetramer staining intensity and Cre:I-Ab-specific T cell expansion for each mouse strain. SEM, r2, and p values are indicated. (B,E) Mean values ±SEM are shown. ns not significant, * p<0.05, ** p<0.01, *** p<0.001 for unpaired t tests between WT and each Cre mouse. See also Figure S2.
Previous studies have shown that thymic negative selection of T cells preferentially affects clones with the highest avidity TCRs (Moon et al., 2011; Zehn and Bevan, 2006). Analysis of Cre:I-Ab tetramer staining intensity, which provided a rough measure of Cre:I-Ab-specific TCR avidity, did not reveal differences in TCR avidity for any of the Cre transgenic mice except UbC-CreER (Figures 2D and 2E), nor were there any differences in overall TCR expression levels as evidenced by CD3 staining (Figure S2). Overall, there was no correlation between tetramer staining intensity and the extent of T cell expansion among the mice (Figure 2F), indicating that the impaired responsiveness of Cre self antigen-specific T cells in CC10-Cre and Vil-Cre mice was not the result of developmental selection against high avidity T cell clones.
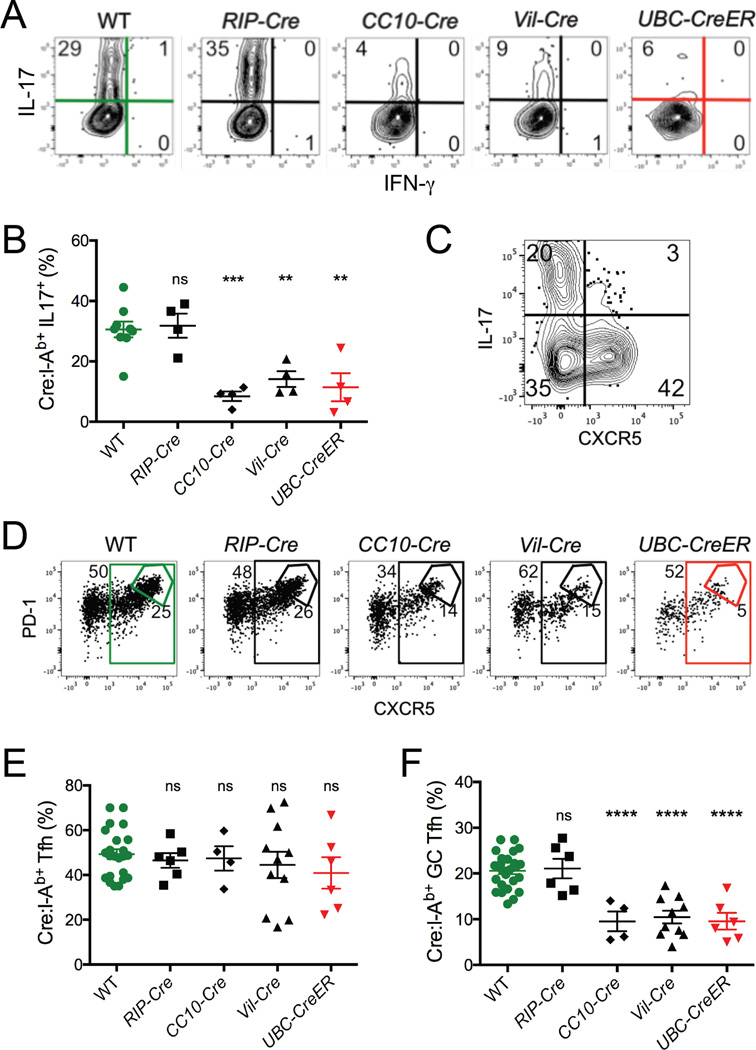
The tolerance of Cre:I-Ab-specific T cells was further evidenced by our analysis of CD4+ T cell effector functions. While 30–35% of the Cre:I-Ab-specific T cells in WT or RIP-Cre mice expressed IL-17 upon in vitro restimulation with PMA plus ionomycin, only 5–15% of these cells did so in CC10-Cre, Vil-Cre, and UbC-CreER mice (Figures 3A and 3B). Analysis of other cytokines did not reveal a diversion into alternative Th subsets (Figure S3). Expression of the chemokine receptor CXCR5, which enables T cells to migrate to lymph node B cell zones (Crotty, 2011), occurred in a subset of Cre:I-Ab-specific T cells distinct from those producing IL-17 (Figure 3C). While the proportion of cells expressing CXCR5 was similar in all of the mice in our panel (Figures 3D and 3E), the development of Cre:I-Ab-specific germinal center follicular helper T cells (GC-Tfh), as defined by the subset of PD-1+CXCR5+ T cells expressing the highest levels of these markers (Crotty, 2011), was strikingly impaired in CC10-Cre, Vil-Cre, and UbC-CreER mice, but not RIP-Cre mice (Figures 3D and 3F). Thus, the reduced proliferative potential of tolerant Cre self antigen-specific T cells was paralleled by functional defects in cytokine production and GC-Tfh development.
Figure 3. Lung and intestinal self antigen-specific CD4+ T cells exhibit impaired effector functions.
Mice were immunized with Cre peptide + CFA and analyzed 7 days later. (A) Representative intracellular flow cytometry of IL-17 and IFNγ cytokine expression by Cre:I-Ab-specific CD4+ T cells from pooled spleen and lymph nodes following in vitro restimulation with PMA + ionomycin. Numbers indicate proportions of cells in each quadrant. (B) Quantitative summary of the proportion of Cre:I-Ab-specific T cells expressing IL-17 in each mouse. (C) Representative flow cytometry of IL-17 and CXCR5 expression by Cre:I-Ab-specific CD4+ T cells from a WT mouse. (D) Representative flow cytometry of PD-1 and CXCR5 expression by Cre:I-Ab-specific CD4+ T cells. Numbers indicate proportions of cells in each gate. (E–F) Quantitative summaries of proportions of Cre:I-Ab-specific T cells exhibiting (E) CXCR5+ or (F) PD-1hiCXCR5hi GC-Tfh cell phenotypes. (B,E–F) Mean values ±SEM are shown. ns not significant, ** p<0.01, *** p<0.001, **** p<0.0001 for unpaired t tests between WT and each Cre mouse. See also Figure S3.
Treg cell-mediated tolerance to lung and intestinal self antigens
It was possible that steady-state presentation of Cre self antigen resulted in the development of Cre:I-Ab-specific T cell populations that are intrinsically hyporesponsive to Cre stimulation, perhaps through the induction of anergy or development into Tregs. Alternatively, the nonresponsiveness of these cells may have been maintained by steady-state interactions with tolerogenic dendritic cells presenting Cre antigen. To determine the role that the Cre antigen expressing environment plays in the tolerance of Cre:I-Ab-specific T cells, whole repertoires of purified CD4+ T cells from WT or Cre-expressing mice were transferred into congenic host mice lacking Cre expression. Smarta TCR transgenic mice were chosen as hosts because they lack endogenous T cell specificity to Cre, yet provide a lymphoreplete environment that limits donor T cell activation from homeostatic proliferation (Sprent and Surh, 2011). As shown in Figure S4A, Cre:I-Ab-specific T cells transferred from CC10-Cre or Vil-Cre mice into Cre antigen-free Smarta mice were still impaired in their ability to expand following immunization, demonstrating that tolerance was intrinsic to them at either the cell or population level.
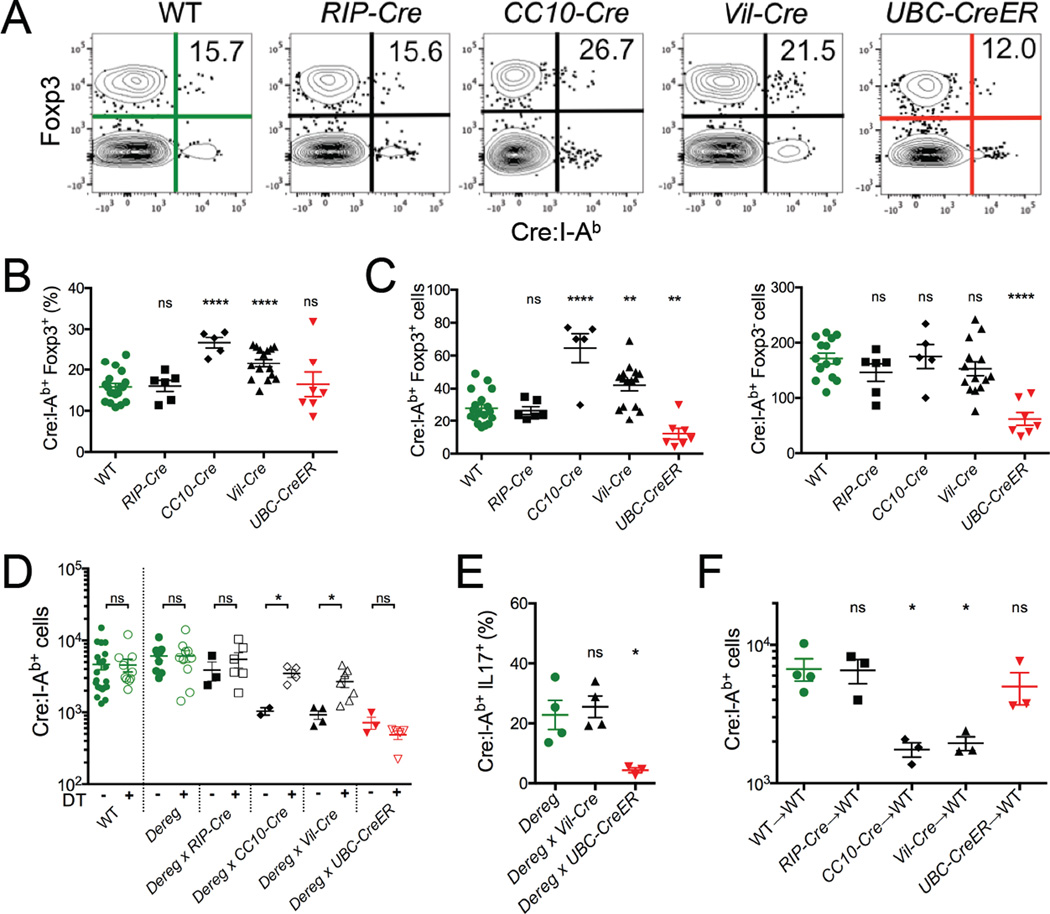
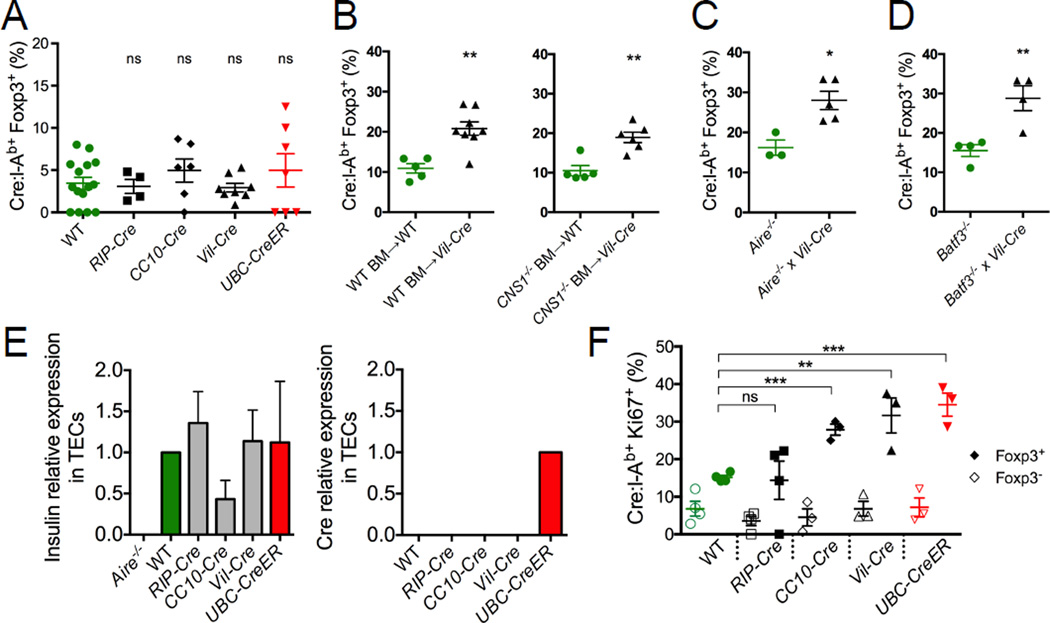
In light of these results, an attractive hypothesis was that many of the Cre:I-Ab-specific T cells in our Cre transgenic mice were Tregs. We have previously shown that Foxp3+ Treg subsets are a general feature of all antigen-specific CD4+ T cell populations, and the frequency of these cells was elevated in a population specific for a ubiquitously expressed self antigen (Moon et al., 2011). Using a Foxp3EGFP reporter gene that was bred into all of our mouse strains (Haribhai et al., 2007), we found that ~15% of the Cre:I-Ab-specific CD4+ T cells in the peripheral lymphoid organs of WT mice expressed Foxp3 (Figures 4A and 4B). This proportion was similar in RIP-Cre mice, but elevated to ~27% and ~22% in CC10-Cre and Vil-Cre mice, respectively, which correlated with their immune tolerance to Cre. Examination of total Foxp3+ and Foxp3− Cre:I-Ab-specific T cell numbers in each mouse revealed that the greater representation of Tregs did not come at the expense of Tconv cells (Figure 4C), albeit the elevated numbers of Tregs was too small to significantly alter the size of the total Cre:I-Ab-specific T cell population (Figure 1E). During immunization, Cre-specific Tregs proliferated along with their conventional counterparts in all mice, but their representation was still only elevated in CC10-Cre and Vil-Cre mice (Figure S4B–S4E).
Figure 4. Tolerance to lung and intestinal self antigen requires antigen-specific Tregs.
(A) Representative flow cytometry depicting Foxp3EGFP reporter expression in CD4+ T cells following Cre:I-Ab tetramer-based cell enrichment of pooled spleen and lymph nodes from indicated naïve mice. Numbers indicate proportions of tetramer+ cells expressing Foxp3. (B) Quantitative summary of the proportion of Cre:I-Ab-specific CD4+ T cells expressing Foxp3 in each indicated mouse strain. (C) Quantitative summary of the total numbers of Foxp3+ and Foxp3− Cre:I-Ab-specific T cells in each indicated mouse strain. (D) Total Cre:I-Ab-specific T cell numbers and (E) proportions of IL-17+ Cre:I-Ab-specific T cells in indicated Foxp3-DEREG mice 7 days following peptide + CFA immunization with (closed symbols) or without (open symbols) concurrent Treg ablation by DT treatment. (F) WT host Cre:I-Ab-specific T cell numbers 7 days after adoptive transfer of ~4 × 106 CD4+CD25+ Tregs from indicated donor mice followed by immunization with Cre peptide + CFA. (B–F) Mean values ±SEM are shown. ns not significant, * p<0.05, ** p<0.01, **** p<0.0001 for unpaired t tests between WT and each Cre mouse. See also Figure S4.
To test the role of Tregs in Cre-specific tolerance, Cre transgenic mice were crossed to Foxp3-DEREG mice, which enabled systemic ablation of Foxp3+ Tregs upon administration of diphtheria toxin (DT) (Lahl et al., 2007). Treg ablation during Cre immunization of CC10-Cre and Vil-Cre mice resulted in partial restoration of Cre:I-Ab-specific T cell expansion and complete restoration of IL-17 production (Figures 4D and 4E). In contrast, Treg ablation did not restore these functions in UbC-CreER mice, where tolerance was mediated by deletion. To test whether Tregs were sufficient in mediating Cre-specific tolerance, we transferred purified populations of total CD4+CD25+ Tregs from WT or Cre transgenic mice into congenically marked WT mice before immunizing them with Cre (Figure 4F). Transferred Tregs from CC10-Cre and Vil-Cre mice, but not WT, RIP-Cre, or UbC-CreER mice, potently suppressed proliferation of host Cre:I-Ab-specific T cells. Therefore, Cre self antigen-specific Tregs from these mice were necessary and sufficient to mediate Cre-specific tolerance.
Peripheral expansion of thymically derived Treg cells
The majority of Foxp3+ Tregs are either derived directly from thymic development (tTreg) or are induced from CD4+ Tconv cells in the periphery (pTreg) (Abbas et al., 2013; Josefowicz et al., 2012). Cre:I-Ab-specific Foxp3+ Tregs in our mice uniformly expressed both Helios and Neuropilin-1, which was consistent with a tTreg phenotype (Figure S5A) (Thornton et al., 2010; Yadav et al., 2012). However, CC10-Cre and Vil-Cre mice did not contain higher frequencies of Foxp3+ Cre:I-Ab-specific thymocytes, suggesting that thymic Treg development was not enhanced in these mice (Figure 5A). To better investigate their origin, we utilized the Foxp3ΔCNS1 mouse model in which peripheral induction of pTregs is impaired due to the deletion of CNS1, a critical TGFβ-responsive regulatory region in the Foxp3 locus (Zheng et al., 2010). Irradiated WT or Vil-Cre mice were reconstituted with congenic WT or Foxp3ΔCNS1 bone marrow, and the extent of Cre:I-Ab-specific Foxp3+ Treg development in the resulting chimeric animals was assessed. As shown in Figure 5B, the proportion of Cre:I-Ab-specific T cells expressing Foxp3 in Vil-Cre host mice was not affected by the absence of Foxp3-CNS1, indicating that these cells were not pTregs, but rather tTregs. Moreover, Vil-Cre mice reconstituted with Foxp3ΔCNS1 bone marrow still exhibited impaired Cre:I-Ab-specific T cell expansion upon immunization, indicating that pTregs in general were dispensable for Cre-specific tolerance (Figure S5B).
Figure 5. Tissue-restricted self antigen-specific Tregs are thymically derived and peripherally expanded.
(A) Quantitative summary of the proportion of Cre:I-Ab-specific CD4+ SP thymocytes expressing Foxp3 in each indicated mouse strain. (B) Proportions of Cre:I-Ab-specific CD4+ T cells expressing Foxp3 in pooled spleen and lymph nodes from lethally irradiated WT or Foxp3ΔCNS1 mice reconstituted with WT or Foxp3ΔCNS1 bone marrow. (C) Proportions of Cre:I-Ab-specific T cells expressing Foxp3 in pooled spleen and lymph nodes from Aire−/− mice. (D) Proportions of Cre:I-Ab-specific T cells expressing Foxp3 in pooled spleen and lymph nodes from Batf3−/− mice. (E) Quantitative RT-PCR analysis of Insulin and Cre mRNA expression relative to β2M in flow sorted thymic epithelial cells (TECs). Mean values ±SEM of samples taken from at least two independent experiments are shown. (F) Proportions of Foxp3− (open symbols) or Foxp3+ (filled symbols) Cre:I-Ab-specific T cells expressing Ki67 in indicated mice. (A–D, F) Mean values ±SEM are shown. ns not significant, * p<0.05, ** p<0.01, *** p<0.001 for unpaired t tests between WT and each Cre mouse. See also Figure S5.
The tTreg phenotype of Cre:I-Ab-specific Tregs in our mice suggested that their development might be driven by ectopic Cre antigen expression in the thymus, possibly via the transcriptional regulator Aire, which has been shown to be required for Treg development to some, but not all, self antigens (Aschenbrenner et al., 2007; Malchow et al., 2013; Perry et al., 2014). However, crossing Vil-Cre mice onto an Aire-deficient background did not alter the development of Cre:I-Ab-specific Tregs (Figure 5C) or their ability to induce tolerance (Figure S5C), arguing against a role for Aire in our experimental system. In addition, Batf3-dependent CD8α+ dendritic cells, which have been shown to help mediate presentation of Aire-regulated genes to developing thymocytes (Perry et al., 2014), were also dispensable for Cre:I-Ab-specific Treg development and tolerance, as shown by our use of Batf3-deficient mice (Figures 5D and S5D). Although we did not detect Cre mRNA in whole thymus preps (Figure 1A), we performed RT-PCR analysis on flow-sorted thymic epithelial cells (TECs) to improve sensitivity to the point where we could reliably detect expression of Ins2, a known Aire-regulated gene (Anderson et al., 2002) (Figure 5E). While Cre mRNA expression was readily detected in TECs from UbC-CreER mice, it was undetectable in WT or any of the other Cre transgenic mice. Therefore, the elevated levels of Cre:I-Ab-specific tTregs in CC10-Cre and Vil-Cre mice were unlikely to be the result of thymic Cre antigen presentation.
Tonic TCR signaling has been implicated in the maintenance and function of mature Tregs (Levine et al., 2014; Moran et al., 2011; Vahl et al., 2014), so an alternative possibility was that Cre:I-Ab-specific Tregs underwent some level of clonal expansion upon antigen encounter in the periphery. In support of this hypothesis, expression analysis of the cellular proliferation marker Ki-67 revealed more steady-state cell cycle activity within the Cre:I-Ab-specific Foxp3+ Tregs of CC10-Cre, Vil-Cre, and UbC-CreER mice than WT or RIP-Cre mice (Figures 5F and S5E). Notably, the Cre:I-Ab-specific Foxp3− T cells of these mice did not express higher levels of Ki-67, suggesting that only Cre:I-Ab-specific Tregs and not Tconv cells were proliferating in response to Cre self antigen.
Limited durability of steady-state Treg cell-mediated tolerance
Tregs have been shown to establish immune memory (Rosenblum et al., 2011), so we asked whether Cre-specific tolerance in CC10-Cre and Vil-Cre mice would be enhanced or diminished upon successive immune challenges. Cre peptide emulsified in CFA is not efficiently cleared after injection, thereby complicating interpretations of immune memory. Therefore, we utilized the bacterium Listeria monocytogenes, which is quickly cleared after infection, as an alternative antigen delivery system to induce acute primary Cre-specific T cell responses (Pamer, 2004). We infected mice systemically with a recombinant strain of Listeria (Lm-Cre) expressing Cre 61–71 peptide embedded in chicken ovalbumin (OVA). This resulted in Cre:I-Ab-specific T cell expansion and GC-Tfh cell development that mirrored the patterns of tolerance seen with peptide immunization (Figures S6A, S6C, and S6E). Notably, T cell responses to the endogenous Listeria Listeriolysin O (LLO) antigen were equivalent in all mice (Figures S6B, S6D, and S6F), indicating that a single Cre:I-Ab epitope-specific population of Tregs was insufficient to mediate bystander suppression of other Listeria antigen-specific T cell responses.
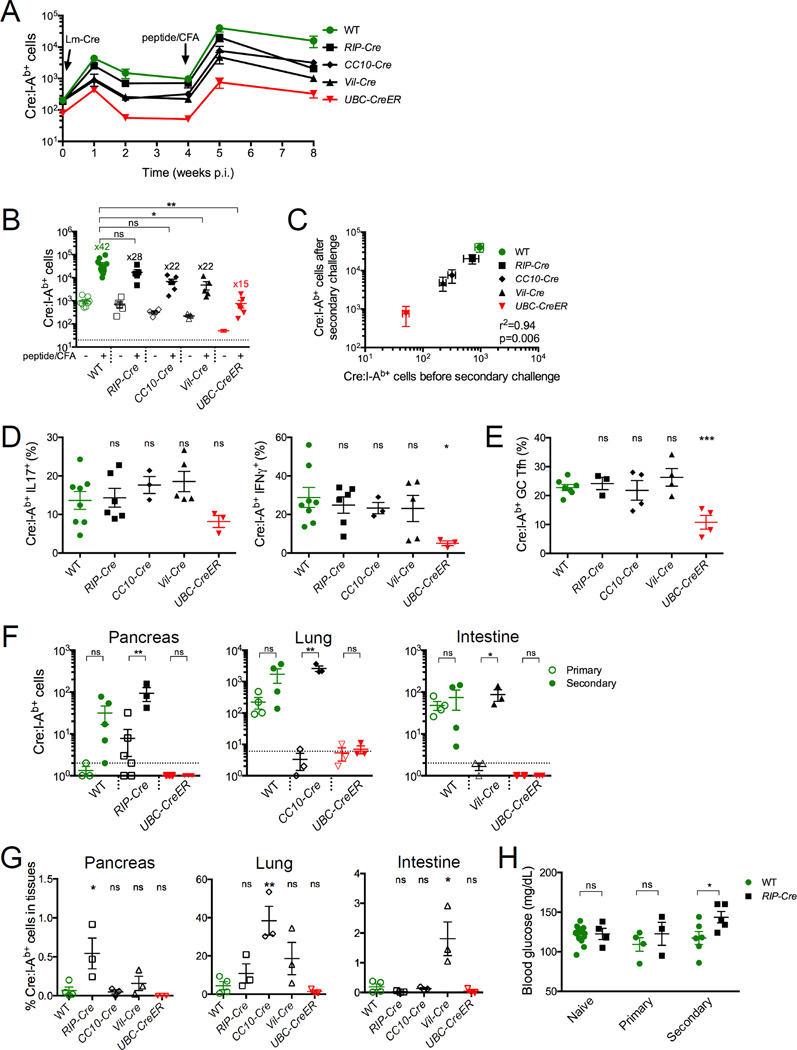
Lm-Cre infected mice were given 4 weeks to recover and were then rechallenged with Cre peptide + CFA (Figure 6A). In contrast to the primary immune response, Cre:I-Ab-specific T cells in rechallenged CC10-Cre and Vil-Cre mice now exhibited robust expansion, cytokine production (IL-17 and IFNγ), and GC-Tfh development that was equivalent to WT controls (Figures 6A, 6B, 6D, 6E, S6G, and S6H). Moreover, the expansion of Cre:I-Ab-specific T cells now correlated directly with the number of Cre:I-Ab-specific precursors before secondary challenge (Figure 6C), indicating a loss of nondeletional tolerance. In contrast, Cre:I-Ab-specific T cells in UbC-CreER mice remained unresponsive as before, demonstrating the greater durability of deletional tolerance.
Figure 6. Treg mediated tolerance to tissue-restricted self antigens is lost upon secondary immune challenge.
(A) Mice were infected with Lm-Cre, and 4 weeks later rechallenged with Cre peptide + CFA. (B) Quantitative summary of total Cre:I-Ab-specific T cell numbers in pooled spleen and lymph nodes before and after secondary challenge with fold expansion values indicated. The dotted line represents the limit of detection. (C) Linear regression analysis of Cre:I-Ab-specific T cell numbers before and after secondary immune challenge. SEM, r2, and p values are indicated. (D–E) Proportions of Cre:I-Ab-specific T cells (D) expressing IL-17 or IFNγ cytokines, or (E) exhibiting a GC-Tfh phenotype 7 days after secondary immune challenge. (F) Cre:I-Ab-specific T cell numbers in the pancreas, lungs, and intestines of indicated mice 7–9 days after primary or secondary immunization. Dotted lines represent the limit of detection. (G) Cre:I-Ab-specific T cell numbers in indicated tissues after secondary immunization, expressed as a percentage of total Cre:I-Ab-specific T cells from the whole mouse. (H) Blood glucose levels in naive WT or RIP-Cre mice 7–10 days after primary or secondary immunization. (B, D–H) Mean values ±SEM are shown. ns not significant, * p<0.05, ** p<0.01, *** p<0.001 for unpaired t tests between WT and each Cre mouse or between indicated conditions. See also Figure S6.
The loss of tolerance and expansion of Cre:I-Ab-specific T cells also resulted in their influx into the pancreas, lungs, and intestines (Figure 6F and S6I). Notably, the extent of Cre:I-Ab-specific T cell accumulation was greater in tissues expressing Cre, concordant with a role for antigen recognition in T cell migration or retention in nonlymphoid tissues (Figure 6G). Although Cre:I-Ab-specific Foxp3+ Tregs were not observed in any of these tissues (data not shown), none of these mice exhibited obvious signs of autoimmunity with the exception of RIP-Cre mice, which experienced a slight and transient increase in blood glucose levels (Figure 6H and data not shown). Therefore, breaking tolerance to a single self antigen epitope resulted in strong epitope-specific effector T cell functions, but this was not enough to initiate sustained autoimmunity.
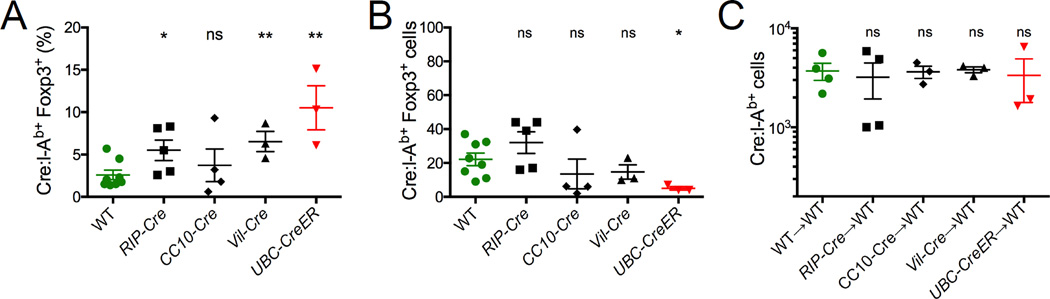
To investigate the mechanism by which Cre:I-Ab-specific tolerance was lost in CC10-Cre and Vil-Cre mice, we compared Cre:I-Ab-specific Treg populations present in these mice just before primary and secondary immunizations. Both the proportions and numbers of Tregs within the Cre:I-Ab-specific T cell population analyzed just before secondary challenge (Figure 7A and 7B) were substantially reduced from that prior to primary immunization (Figure 4B). Furthermore, when purified populations of total CD4+CD25+ Tregs were transferred from these mice into WT recipients, host T cell responses to peptide + CFA immunization were no longer suppressed as before (Figures 7C and 4F). Collectively, these results indicate that the loss of tolerance after immunization was the result of a new equilibrium established between Cre:I-Ab-specific Treg and Tconv subsets.
Figure 7. The balance between Treg and Tconv is altered after immune challenge.
Mice were infected with Lm-Cre and Cre:I-Ab-specific Tregs from the pooled spleen and lymph nodes were analyzed 4 weeks later. (A) Proportions of Cre:I-Ab-specific T cells expressing Foxp3. (B) Cre:I-Ab-specific T cell numbers in indicated mice. (C) WT host Cre:I-Ab-specific T cell numbers 7 days after adoptive transfer of ~4 × 106 CD4+CD25+ Tregs from indicated donor mice followed by immunization with Cre peptide + CFA. (A–C) Mean values ±SEM are shown. ns not significant, * p<0.05, ** p<0.01 for unpaired t tests between WT and each Cre mouse.
DISCUSSION
By studying an endogenous CD4+ T cell population with specificity for a single epitope of a self antigen expressed under different tissue-restricted patterns, we have provided direct evidence that deletional T cell tolerance is minimal to nonexistent for some tissue-restricted self antigens. Rather, mature T cell populations with specificity to such self antigens may be largely ignorant, as we observed in the case of pancreatic expression, or may develop steady-state tolerance through enhanced antigen-specific Treg activity, as observed in the case of lung and intestinal expression. These findings support the concept that T cell tolerance to some tissue-restricted self antigens are shaped entirely by nondeletional processes outside the thymus.
Our conclusion that Cre-specific T cells do not undergo deletion in tissue-restricted Cre-expressing mice is based on our direct measurements of endogenous antigen-specific T cell frequencies in both the thymic and peripheral T cell compartments of WT and Cre-expressing mice. Our findings are congruous with recent tetramer-based studies by Davis and colleagues showing that the frequencies of several self antigen-specific T cell populations in humans are in the same range as frequencies for foreign antigen-specific populations (Su et al., 2013; Yu et al., 2015). While it is formally possible that the presence of Cre antigen caused the deletion of some Cre-specific T cell clones while promoting the development of others, resulting in no net change in frequency, our data shows that any such changes in the Cre-specific T cell repertoire did not result in notable differences in the TCR avidities of these T cells. Moreover, the elevated numbers of Foxp3+ Cre-specific T cells in CC10-Cre and Vil-Cre mice were not offset by lower numbers of Foxp3− cells, suggesting that Tconv cells were not being diverted into a Treg fate in the thymi of these mice.
Our results contrast with our previous findings of robust negative selection against T cells specific for ubiquitously expressed self antigens (Moon et al., 2011), upholding the general assumption that tissue-restricted self antigens elicit less efficient deletional tolerance. The complete absence of T cell deletion in our current study also contrasts with a previous report demonstrating that about 25% of a single chain TCR transgenic CD8+ T cell population with specificity to a pancreas-restricted OVA model self antigen are deleted (Zehn and Bevan, 2006). However, OVA is expressed in the thymi of the RIP-mOVA mice used in this study (Gallegos and Bevan, 2004), while we could not detect any thymic expression of Cre in RIP-Cre mice. It is possible that the discrepancy in thymic expression is due to the small size of the RIP2 promoter used in these mice, which may be influenced by local chromatin effects unique to each transgene insertion site.
Interestingly, the CC10 and villin genes are both Aire-regulated (M. Anderson, personal communication) and expressed in medullary thymic epithelial cells (Derbinski et al., 2005). However, we did not detect thymic expression of Cre in CC10-Cre and Vil-Cre mice either, suggesting that Aire-mediated regulation of transgenic promoters may not accurately reflect that of their corresponding endogenous promoters. Our findings therefore may not be directly applicable to these endogenous self antigens or others that are regulated by Aire. However, it is unclear whether all tissue-restricted genes are ectopically expressed in the thymus through the function of Aire or other mechanisms, and whether such expression always results in physiological antigen presentation to thymocytes. Our colleagues previously showed that in the case of the eye-restricted protein IRBP, thymic expression through Aire results in the tolerance of T cells specific for only some of the epitopes within this protein, and this is due to differences in the efficiency by which these epitopes are presented (Taniguchi et al., 2012). Thus, it is likely that a large number of epitopes in tissue-restricted self antigens are not routinely detected by developing thymocytes. Moreover, neo-epitopes created by genetic mutations in tumor cells would represent another class of self epitopes unrepresented during thymic selection. Our findings reveal the relevant potential outcomes of T cell development and tolerance to those self antigens that are not thymically expressed or presented.
The lack of deletion in RIP-Cre mice resulted in an apparent complete lack of tolerance to the Cre epitope, as Cre-specific T cells in these mice responded to antigen stimulation just as well as those in WT mice. Our data suggest that RIP2-driven Cre expression in the pancreas normally results in a level of Cre:I-Ab presentation that is insufficient to activate Cre-specific T cells under steady-state non-inflammatory conditions. However, after two rounds of immunization, Cre-specific T cells accumulated in the pancreas of RIP-Cre mice and these mice experienced elevated blood glucose levels, suggesting that inflammatory conditions can enhance presentation of this self antigen to a level where it is readily detectable by antigen-specific T cells.
Cre-specific T cells were also spared from deletion in CC10-Cre and Vil-Cre mice, but tolerance to Cre was established by Cre-specific Foxp3+ Tregs that were present at elevated frequencies. Our data indicates that these Tregs were most likely derived from the thymus rather than induced from Cre-specific Tconv cells, which is consistent with the general belief that tTregs are predominantly specific for self antigens (Hsieh et al., 2012; Josefowicz et al., 2012). However, since we did not detect any thymic Cre expression or elevated frequencies of Cre-specific Foxp3+ thymocytes in these mice, we believe that the elevated frequency of Cre-specific Tregs was not the result of increased tTreg output from the thymus, but rather the expansion of these cells in the periphery after antigen encounter. This model is supported by the elevated level of cell cycle activity we found in these cells, and is consistent with studies indicating that Tregs experience more steady-state TCR signaling than Tconv cells (Fisson et al., 2003; Moran et al., 2011), which supports their persistence in the periphery (Levine et al., 2014; Vahl et al., 2014). In our study, only Cre-specific Treg and not Tconv cells exhibited increased cell cycle activity, suggesting that they are more responsive to self antigens. It is also possible that Tconv cells are also sensing self antigen, but are continuously suppressed by their Treg counterparts, as suggested by recent studies of human self antigen-specific CD8+ T cells (Maeda et al., 2014).
Treg-mediated tolerance to Cre in CC10-Cre and Vil-Cre mice was functionally equivalent to deletional tolerance in UbC-CreER mice during primary immune responses, but during a secondary immune challenge, this tolerance was lost while deletional tolerance in UbC-CreER mice remained intact. Our data indicated that the balance of Cre-specific Treg and Tconv cells was altered such that tolerance was no longer a favorable outcome upon secondary challenge. However, it is also possible that the memory Tconv cells stimulated during the secondary antigen challenge were less susceptible to Treg-mediated suppression than their naive precursors. These results contrast with the suppressive Treg memory established in another self antigen-specific T cell study (Rosenblum et al., 2011). However, this earlier study involved sterile expression of self antigen through a drug-inducible promoter, while our study involved antigen challenges in the presence of strong microbial adjuvants. Thus, self antigen-specific T cell memory may be influenced by qualitative features of the priming autoimmune response.
Despite the loss of Treg tolerance, Cre-specific T cell numbers contracted over time and with the exception of some mild hyperglycemia in RIP-Cre mice, there were no signs of autoimmunity in our mice. While strong T cell responses against this single epitope may have initiated damage to Cre-expressing tissue, it is likely that Tregs specific for other epitopes within Cre, as well as the many other self antigens within the tissue environment, would have provided ample bystander suppression to eventually subdue the response. Conversely, during infection of Cre-expressing mice with Cre peptide-expressing Listeria, the single Cre epitope-specific Treg population was unable to suppress T cell responses to immunodominant epitopes from the bacteria. Thus, the breadth of steady-state Treg tolerance to several self antigens in a tissue likely supports a level of robustness that resists the development of autoimmunity from isolated immune responses to one or a few self antigen epitopes.
Our results indicate that self antigen-specific Tregs constitute a less durable, yet more flexible, form of tolerance than T cell deletion. A consequence of this flexibility would be the immune system's ability to recruit these self antigen-specific T cells against crossreactive antigens expressed by pathogens or tumors. The establishment of Treg-mediated tolerance to the large numbers of tissue-restricted self antigens in the body would allow the immune system to retain a more diverse repertoire of TCR specificities than would be possible through deletional tolerance alone. While this could be beneficial for antimicrobial and antitumor immunity, it may come at a cost of higher risks for autoimmunity.
The different levels of T cell tolerance established to different tissue-restricted self antigens are likely attributable to the quantity and quality of self antigen presentation in the respective tissue environments. The lung and intestines are much larger than the pancreas, and moreover, they contain mucosal surfaces from which foreign antigens are continually sampled. As we observed in our panel of Cre mice, presentation of self antigens to circulating T cells may be more efficient in these types of tissues, enabling the antigen recognition that sustains self antigen-specific Tregs. It is also possible that the dendritic cell subsets draining these tissues may be more supportive for the development and maintenance of Tregs. The paucity of steady-state Treg tolerance to self antigens in organs like the pancreas may result in a greater vulnerability to the aberrant initiation of autoimmune responses, which we speculate could be an underlying factor for the high prevalence of autoimmune diseases in these organs.
The enhancement of autoantigen-specific Treg activity is a popular focus for autoimmune disease therapies. The feasibility of this strategy is supported by our discovery that antigen-specific Treg activity constitutes a normal steady-state mechanism of tolerance for some tissue-restricted self antigens. From another standpoint, the lack of deletional tolerance and limited durability of Treg-mediated tolerance to tissue-restricted self antigens offers a strong rationale for the development of cancer vaccines targeting tissue-restricted tumor antigens.
EXPERIMENTAL PROCEDURES
Immunizations and Infections
All peptides were custom ordered from Genscript. For peptide immunizations, mice were injected subcutaneously at the base of the tail with 100 µg peptide in 50 µl of a 1:1 emulsion of PBS and complete Freund’s adjuvant (Sigma). ΔactA Listeria monocytogenes expressing the Cre epitope (Lm-Cre) was generated as previously described (Orr et al., 2007). Mice were infected intravenously at a dose of 108 cfu. For Treg ablation experiments, Foxp3-DEREG mice were injected intraperitoneally with 75 µg/kg diphtheria toxin (List Biologicals) at days 1 and 2 post antigen immunization.
Tetramer-Based Cell Enrichment and Flow Cytometry
Single cell suspensions were stained with tetramer at a final concentration of 10 nM for 1 h at room temperature, and then magnetically enriched as described in detail (Legoux and Moon, 2012; Moon et al., 2007). For intracellular cytokine staining, tetramer-based cell enrichment was not used. Instead, total CD4+ T cells were isolated (Miltenyi) from draining lymph nodes of day 7 immunized mice and restimulated in vitro for 2h at 37°C with 50 ng/mL PMA (Sigma), 500 ng/mL ionomycin (Sigma), and 10 µg/mL brefeldin A (Sigma). The cells were then surface stained with tetramer and antibodies, washed, and then fixed, permeabilized, and stained for intracellular cytokines using the Cytofix/Cytoperm kit (BD Biosciences). For Ki67 expression analysis, pooled spleen and lymph node cells from 1 immunized or 2–3 naive mice were fixed and permeabilized using the Foxp3 Fixation/Permeabilization kit (eBioscience) prior to Ki67 antibody staining. Helios staining was performed with the same kit. 7–9 color flow cytometry was performed on an LSRII or Fortessa (BD Immunocytometry Systems). Data was analyzed with FlowJo software (Treestar). All antibodies were purchased from BioLegend, eBioscience, BD Pharmingen, or Life Technologies.
T cell Transfers
Total CD4+ T cells from the pooled spleen and lymph nodes of WT or Cre mice were magnetically purified (Miltenyi) and ~2 × 107 were transferred intravenously into CD45.1 or CD45.2 congenic Smarta mice, where ~3 × 106 cells stably engrafted. For Treg transfer experiments, total CD4+ CD25+ T cells from the pooled spleen and lymph nodes of WT or Cre mice were magnetically purified (Miltenyi) and ~4 × 106 were transferred intravenously into CD45.1 or CD45.2 congenic WT mice, where ~106 stably engrafted. Recipient mice were then immunized the next day, and host Cre:I-Ab-specific CD4+ T cells were isolated and analyzed 7 days later.
Irradiation Bone Marrow Chimeras
Lethally irradiated (950 rads) WT or Vil-Cre mice were reconstituted intravenously with 107 bone marrow cells from CD45.1 or CD45.2 congenic WT or Foxp3ΔCNS1 mice. Lymphoid tissues from the resulting chimeric mice were harvested 7–8 weeks later for analysis of Cre:I-Ab-specific T cells.
Tissue T cell Isolation
Spleen and lymph nodes were mashed over 100 µm nylon mesh to create single cell suspensions. To prepare single cell suspensions from nonlymphoid tissues, euthanized mice were first perfused with 25 ml PBS. Pancreata were injected with 800 µg/ml collagenase P (Roche) and 40 µg/ml DNase I (Roche), incubated 15 min at 37°C, and mashed over 100 µm nylon mesh. Cells were then centrifuged over a 44/67% Percoll gradient (GE Healthcare) to isolate the lymphocyte-containing fraction. Lungs were incubated with 70 µg/ml Liberase TM (Roche) and 10 mM aminoguanidine (Sigma) in HEPES buffer, digested at 37°C for 30 min, and dissociated with a gentleMACS Dissociator (Miltenyi). Pooled Peyer's patches, small intestine and colon lamina proprias were isolated using a Lamina Propria Dissociation Kit (Miltenyi) followed by a 35/70% Percoll gradient to isolate the lymphocyte-containing fraction.
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Yen, A. Han, and J. Im for assistance with mice and tetramers, C. Luo and R. Mylvaganam for assistance with flow cytometry, F. Marangoni for advice on irradiation bone marrow chimeras, M. Byrne for advice on quantitative RT-PCR, M. Jenkins and J. Mclachlan for advice on epitope mapping, B. Fife for advice on pancreas preps, M. Pepper for advice on lung preps, A. Rudensky for support with Foxp3ΔCNS1 mouse experiments, and R. Anthony, T. Mempel, and A. Luster for critical review of the manuscript. This work was supported by the Philippe Foundation (to F.P.L.), the National Institutes of Health (R01 AI100934, R01 AI087830, and R21 AI112186 to S.S.W.; U19 AI095261, P30 DK043351, and R01 AI107020 to J.J.M.), the Burroughs Wellcome Fund (to S.S.W.), and the William F. Milton Fund (to J.J.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information includes six figures and Supplemental Experimental Procedures and can be found with this article online at
AUTHOR CONTRIBUTIONS
F.P.L., J.B.L., and J.J.M. designed the research. F.P.L., J.B.L., A.W.C., S.D., J.E., and J.J.M. performed the experiments. S.D., T.J.M., T.S., and S.S.W. contributed reagents. F.P.L., J.B.L., S.S.W., and J.J.M. analyzed data. F.P.L. and J.J.M. wrote the manuscript.
The authors declare no competing financial interests.
REFERENCES
- Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, Boehmer, von H, Bronson R, Dierich A, Benoist C, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- Bautista JL, Lio C-WJ, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh C-S. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Schwartz RH. Molecular mechanisms for adaptive tolerance and other T cell anergy models. Semin Immunol. 2007;19:140–152. doi: 10.1016/j.smim.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular Helper CD4 T Cells (T(FH)) Annu Rev Immunol. 2011 doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Derbinski J, Gäbler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisson S, Darrasse-Jèze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate “decisions” and effector function. Nat Immunol. 2014;15:815–823. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- Hsieh C-S, Lee HM, Lio C-WJ. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Moon JJ. The Role of Naive T Cell Precursor Frequency and Recruitment in Dictating Immune Response Magnitude. J Immunol. 2012;188:4135–4140. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasman LM. Barriers to coliphage infection of commensal intestinal flora of laboratory mice. Virol. J. 2005;2:34. doi: 10.1186/1743-422X-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legoux FP, Moon JJ. Peptide:MHC Tetramer-based Enrichment of Epitope-specific T cells. JoVE. 2012:e4420. doi: 10.3791/4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung MWL, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. Journal of Experimental Medicine. 2009;206:2121–2130. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Nishikawa H, Sugiyama D, Ha D, Hamaguchi M, Saito T, Nishioka M, Wing JB, Adeegbe D, Katayama I, et al. Detection of self-reactive CD8+ T cells with an anergic phenotype in healthy individuals. Science. 2014;346:1536–1540. doi: 10.1126/science.aaa1292. [DOI] [PubMed] [Google Scholar]
- Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, et al. Aire-Dependent Thymic Development of Tumor-Associated Regulatory T Cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- Metzger TC, Anderson MS. Control of central and peripheral tolerance by Aire. Immunol Rev. 2011;241:89–103. doi: 10.1111/j.1600-065X.2011.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Dash P, Oguin TH, Mcclaren JL, Chu HH, Thomas PG, Jenkins MK. Quantitative impact of thymic selection on Foxp3+ and Foxp3− subsets of self-peptide/MHC class II-specific CD4+ T cells. Proc Natl Acad Sci USA. 2011;108:14602–14607. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. Journal of Experimental Medicine. 2011 doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr MT, Orgun NN, Wilson CB, Way SS. Cutting edge: recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. J Immunol. 2007;178:4731–4735. doi: 10.4049/jimmunol.178.8.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- Perry JSA, Lio C-W, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, Hsieh C-S. Distinct Contributions of Aire and Antigen-Presenting-Cell Subsets to the Generation of Self-Tolerance in the Thymus. Immunity. 2014;41:414–426. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-Specific CD4(+) Memory-Phenotype T Cells Are Abundant in Unexposed Adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi RT, Devoss JJ, Moon JJ, Sidney J, Sette A, Jenkins MK, Anderson MS. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proc Natl Acad Sci USA. 2012;109:7847–7852. doi: 10.1073/pnas.1120607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahl JC, Drees C, Heger K, Heink S, Fischer JC, Nedjic J, Ohkura N, Morikawa H, Poeck H, Schallenberg S, et al. Continuous T Cell Receptor Signals Maintain a Functional Regulatory T Cell Pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunology and Cell Biology. 2011;89:45–53. doi: 10.1038/icb.2010.123. [DOI] [PubMed] [Google Scholar]
- Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. Journal of Experimental Medicine. 2012;209:1713–1722. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Jiang N, Ebert PJR, Kidd BA, Müller S, Lund PJ, Juang J, Adachi K, Tse T, Birnbaum ME, et al. Clonal Deletion Prunes but Does Not Eliminate Self-Specific αβ CD8(+) T Lymphocytes. Immunity. 2015;42:929–941. doi: 10.1016/j.immuni.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25:261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.