Abstract
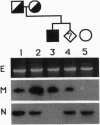
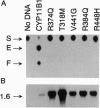
Steroid 11 beta-hydroxylase deficiency (failure to convert 11-deoxycortisol to cortisol) is the second most common cause of congenital adrenal hyperplasia and results in a hypertensive form of the disease. The 11 beta-hydroxylase enzyme is encoded by the CYP11B1 gene on chromosome 8q22. Two mutations in CYP11B1 have previously been reported in patients with 11 beta-hydroxylase deficiency--Arg-448-->His and a 2-bp insertion in codon 394. We now report eight previously uncharacterized mutations causing this disorder. Seven are point mutations (three nonsense and four missense) and one is a single base pair deletion causing a frameshift. We have used an in vitro transfection assay to show that all five known missense mutations causing 11 beta-hydroxylase deficiency abolish enzymatic activity. In principle, deletions of CYP11B1 could be generated by unequal crossing-over between CYP11B1 and the adjacent CYP11B2 gene, but no such deletions were found among the deficiency alleles in this study. Seven of the 10 known mutations are clustered in exons 6-8, a nonrandom distribution within the gene. This may reflect the location of functionally important amino acid residues within the enzyme or an increased tendency to develop mutations within this region of the gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Chua S. C., Szabo P., Vitek A., Grzeschik K. H., John M., White P. C. Cloning of cDNA encoding steroid 11 beta-hydroxylase (P450c11). Proc Natl Acad Sci U S A. 1987 Oct;84(20):7193–7197. doi: 10.1073/pnas.84.20.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- Curnow K. M., Tusie-Luna M. T., Pascoe L., Natarajan R., Gu J. L., Nadler J. L., White P. C. The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol Endocrinol. 1991 Oct;5(10):1513–1522. doi: 10.1210/mend-5-10-1513. [DOI] [PubMed] [Google Scholar]
- Forest M. G., Bétuel H., David M. Prenatal treatment in congenital adrenal hyperplasia due to 21-hydroxylase deficiency: up-date 88 of the French multicentric study. Endocr Res. 1989;15(1-2):277–301. doi: 10.1080/07435808909039101. [DOI] [PubMed] [Google Scholar]
- Helmberg A., Ausserer B., Kofler R. Frame shift by insertion of 2 basepairs in codon 394 of CYP11B1 causes congenital adrenal hyperplasia due to steroid 11 beta-hydroxylase deficiency. J Clin Endocrinol Metab. 1992 Nov;75(5):1278–1281. doi: 10.1210/jcem.75.5.1430088. [DOI] [PubMed] [Google Scholar]
- Kawainoto T., Mitsuuchi Y., Ohnishi T., Ichikawa Y., Yokoyama Y., Sumimoto H., Toda K., Miyahara K., Kuribayashi I., Nakao K. Cloning and expression of a cDNA for human cytochrome P-450aldo as related to primary aldosteronism. Biochem Biophys Res Commun. 1990 Nov 30;173(1):309–316. doi: 10.1016/s0006-291x(05)81058-7. [DOI] [PubMed] [Google Scholar]
- Lifton R. P., Dluhy R. G., Powers M., Rich G. M., Cook S., Ulick S., Lalouel J. M. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992 Jan 16;355(6357):262–265. doi: 10.1038/355262a0. [DOI] [PubMed] [Google Scholar]
- Lifton R. P., Dluhy R. G., Powers M., Rich G. M., Gutkin M., Fallo F., Gill J. R., Jr, Feld L., Ganguly A., Laidlaw J. C. Hereditary hypertension caused by chimaeric gene duplications and ectopic expression of aldosterone synthase. Nat Genet. 1992 Sep;2(1):66–74. doi: 10.1038/ng0992-66. [DOI] [PubMed] [Google Scholar]
- Migeon C. J. Comments about the need for prenatal treatment of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1990 Apr;70(4):836–837. doi: 10.1210/jcem-70-4-836. [DOI] [PubMed] [Google Scholar]
- Mornet E., Crété P., Kuttenn F., Raux-Demay M. C., Boué J., White P. C., Boué A. Distribution of deletions and seven point mutations on CYP21B genes in three clinical forms of steroid 21-hydroxylase deficiency. Am J Hum Genet. 1991 Jan;48(1):79–88. [PMC free article] [PubMed] [Google Scholar]
- Mornet E., Dupont J., Vitek A., White P. C. Characterization of two genes encoding human steroid 11 beta-hydroxylase (P-450(11) beta). J Biol Chem. 1989 Dec 15;264(35):20961–20967. [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Ogishima T., Shibata H., Shimada H., Mitani F., Suzuki H., Saruta T., Ishimura Y. Aldosterone synthase cytochrome P-450 expressed in the adrenals of patients with primary aldosteronism. J Biol Chem. 1991 Jun 15;266(17):10731–10734. [PubMed] [Google Scholar]
- Pang S. Y., Pollack M. S., Marshall R. N., Immken L. Prenatal treatment of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med. 1990 Jan 11;322(2):111–115. doi: 10.1056/NEJM199001113220207. [DOI] [PubMed] [Google Scholar]
- Pascoe L., Curnow K. M., Slutsker L., Connell J. M., Speiser P. W., New M. I., White P. C. Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8327–8331. doi: 10.1073/pnas.89.17.8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe L., Curnow K. M., Slutsker L., Rösler A., White P. C. Mutations in the human CYP11B2 (aldosterone synthase) gene causing corticosterone methyloxidase II deficiency. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4996–5000. doi: 10.1073/pnas.89.11.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler A., Weshler N., Leiberman E., Hochberg Z., Weidenfeld J., Sack J., Chemke J. 11 Beta-hydroxylase deficiency congenital adrenal hyperplasia: update of prenatal diagnosis. J Clin Endocrinol Metab. 1988 Apr;66(4):830–838. doi: 10.1210/jcem-66-4-830. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Speiser P. W., Dupont J., Zhu D., Serrat J., Buegeleisen M., Tusie-Luna M. T., Lesser M., New M. I., White P. C. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest. 1992 Aug;90(2):584–595. doi: 10.1172/JCI115897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser P. W., Laforgia N., Kato K., Pareira J., Khan R., Yang S. Y., Whorwood C., White P. C., Elias S., Schriock E. First trimester prenatal treatment and molecular genetic diagnosis of congenital adrenal hyperplasia (21-hydroxylase deficiency). J Clin Endocrinol Metab. 1990 Apr;70(4):838–848. doi: 10.1210/jcem-70-4-838. [DOI] [PubMed] [Google Scholar]
- Unger B. P., Gunsalus I. C., Sligar S. G. Nucleotide sequence of the Pseudomonas putida cytochrome P-450cam gene and its expression in Escherichia coli. J Biol Chem. 1986 Jan 25;261(3):1158–1163. [PubMed] [Google Scholar]
- Wada A., Waterman M. R. Identification by site-directed mutagenesis of two lysine residues in cholesterol side chain cleavage cytochrome P450 that are essential for adrenodoxin binding. J Biol Chem. 1992 Nov 15;267(32):22877–22882. [PubMed] [Google Scholar]
- Wagner M. J., Ge Y., Siciliano M., Wells D. E. A hybrid cell mapping panel for regional localization of probes to human chromosome 8. Genomics. 1991 May;10(1):114–125. doi: 10.1016/0888-7543(91)90491-v. [DOI] [PubMed] [Google Scholar]
- White P. C., Dupont J., New M. I., Leiberman E., Hochberg Z., Rösler A. A mutation in CYP11B1 (Arg-448----His) associated with steroid 11 beta-hydroxylase deficiency in Jews of Moroccan origin. J Clin Invest. 1991 May;87(5):1664–1667. doi: 10.1172/JCI115182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C. Genetics of steroid 21-hydroxylase deficiency. Recent Prog Horm Res. 1987;43:305–336. doi: 10.1016/b978-0-12-571143-2.50014-9. [DOI] [PubMed] [Google Scholar]
- White P. C., New M. I. Genetic basis of endocrine disease 2: congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1992 Jan;74(1):6–11. doi: 10.1210/jcem.74.1.1727830. [DOI] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeom Y. I., Abe K., Artzt K. Evolution of the mouse H-2K region: a hot spot of mutation associated with genes transcribed in embryos and/or germ cells. Genetics. 1992 Mar;130(3):629–638. doi: 10.1093/genetics/130.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachmann M., Tassinari D., Prader A. Clinical and biochemical variability of congenital adrenal hyperplasia due to 11 beta-hydroxylase deficiency. A study of 25 patients. J Clin Endocrinol Metab. 1983 Feb;56(2):222–229. doi: 10.1210/jcem-56-2-222. [DOI] [PubMed] [Google Scholar]